Preparation, Characterization, and Chemically Modified Date Palm Fiber Waste Biomass for Enhanced Phenol Removal from an Aqueous Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.1.1. Preparation of Date Palm Fiber Waste Biomass
2.1.2. Modification of Date Palm Fiber
2.2. Batch Studies
2.3. Normalized Standard Deviation
2.4. Chi-Square Test (χ2)
2.5. Characterization of RDPF and NaOH–CMDPF
3. Results and Discussion
3.1. Characterization of Date Palm Fiber Biomass
3.1.1. Particle Size Data Analysis
3.1.2. Elemental (C, H, N) Analysis
3.1.3. BET Analysis
3.1.4. FESEM-EDX Analysis
3.1.5. FTIR Analysis
3.2. Investigation of Solution pH
3.3. Influence of Sorbent Dosage
3.4. Effect of Contact Time and Initial Concentration
3.5. Adsorption Kinetics
3.6. Equilibrium Adsorption Isotherm Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamad Nor, N.; Lau, L.C.; Lee, K.T.; Mohamed, A.R. Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—A review. J. Environ. Chem. Eng. 2013, 1, 658–666. [Google Scholar] [CrossRef]
- Ho, S. Low-Cost Adsorbents for the Removal of Phenol/Phenolics, Pesticides, and Dyes from Wastewater Systems: A Review. Water 2022, 14, 3203. [Google Scholar] [CrossRef]
- Leong, K.Y.; See, S.; Lim, J.W.; Bashir, M.J.K.; Ng, C.A.; Tham, L. Effect of process variables interaction on simultaneous adsorption of phenol and 4-chlorophenol: Statistical modeling and optimization using RSM. Appl. Water Sci. 2017, 7, 2009–2020. [Google Scholar] [CrossRef]
- Fseha, Y.H.; Shaheen, J.; Sizirici, B. Phenol contaminated municipal wastewater treatment using date palm frond biochar: Optimization using response surface methodology. Emerg. Contam. 2023, 9, 100202. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef]
- Auwal, A.; Hossen, J. Removal of Phenol from Aqueous Solution Using Tamarind Seed Powder As Adsorbent. IOSR J. Environ. Sci. 2018, 12, 41–48. [Google Scholar] [CrossRef]
- Kumar, N.S.; Asif, M.; Al-Hazzaa, M.I. Adsorptive removal of phenolic compounds from aqueous solutions using pine cone biomass: Kinetics and equilibrium studies. Environ. Sci. Pollut. Res. 2018, 25, 21949–21960. [Google Scholar] [CrossRef]
- Dargahi, A.; Samarghandi, M.R.; Shabanloo, A.; Mahmoudi, M.M.; Nasab, H.Z. Statistical modeling of phenolic compounds adsorption onto low-cost adsorbent prepared from aloe vera leaves wastes using CCD-RSM optimization: Effect of parameters, isotherm, and kinetic studies. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Lee, C.G.; Hong, S.H.; Hong, S.G.; Choi, J.W.; Park, S.J. Production of Biochar from Food Waste and its Application for Phenol Removal from Aqueous Solution. Water Air. Soil Pollut. 2019, 230, 70. [Google Scholar] [CrossRef]
- Khan, R.J.; Lau, C.Y.; Guan, J.; Lam, C.H.; Zhao, J.; Ji, Y.; Wang, H.; Xu, J.; Lee, D.J.; Leu, S.Y. Recent advances of lignin valorization techniques toward sustainable aromatics and potential benchmarks to fossil refinery products. Bioresour. Technol. 2022, 346, 126419. [Google Scholar] [CrossRef]
- Goud, V.V.; Mohanty, K.; Rao, M.S.; Jayakumar, N.S. Phenol removal from aqueous solutions by tamarind nutshell activated carbon: Batch and column studies. Chem. Eng. Technol. 2005, 28, 814–821. [Google Scholar] [CrossRef]
- Najafpoor, A.A.; Dousti, S.; Jafari, A.J.; Hosseinzadeh, A. Efficiency in phenol removal from aqueous solutions of pomegranate peel ash as a natural adsorbent. Environ. Health Eng. Manag. J. 2016, 3, 41–46. [Google Scholar]
- Ismail Mustafa, A.; Saiful Alam, M.; Nurul Amin, M.; Mohammad Bahadur, N.; Ahsan Habib, M. Phenol Removal from Aqueous System by Jute Stick. J. Anal. Environ. Chem 2008, 9, 92–95. [Google Scholar]
- Jagwani, D.; Joshi, P. Deportation of Toxic Phenol from Aqueous System by Wheat Husk. Int. J. Plant Anim. Environ. Sci. 2014, 4, 58–64. [Google Scholar]
- Stasinakis, A.S.; Elia, I.; Petalas, A.V.; Halvadakis, C.P. Removal of total phenols from olive-mill wastewater using an agricultural by-product, olive pomace. J. Hazard. Mater. 2008, 160, 408–413. [Google Scholar] [CrossRef]
- Mittal, A.; Kaur, D.; Malviya, A.; Mittal, J.; Gupta, V.K. Adsorption studies on the removal of coloring agent phenol red from wastewater using waste materials as adsorbents. J. Colloid Interface Sci. 2009, 337, 345–354. [Google Scholar] [CrossRef]
- Dursun, A.Y.; Kalayci, Ç.S. Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto chitin. J. Hazard. Mater. 2005, 123, 151–157. [Google Scholar] [CrossRef]
- Abdelkreem, M. Adsorption of Phenol from Industrial Wastewater Using Olive Mill Waste. APCBEE Procedia 2013, 5, 349–357. [Google Scholar] [CrossRef]
- Kulkarni, S.J.; Tapre, R.W.; Patil, S.V.; Sawarkar, M.B. Adsorption of phenol from wastewater in fluidized bed using coconut shell activated carbon. Procedia Eng. 2013, 51, 300–307. [Google Scholar] [CrossRef]
- Rengaraj, S.; Moon, S.H.; Sivabalan, R.; Arabindoo, B.; Murugesan, V. Removal of phenol from aqueous solution and resin manufacturing industry wastewater using an agricultural waste: Rubber seed coat. J. Hazard. Mater. 2002, 89, 185–196. [Google Scholar] [CrossRef]
- Li, J.M.; Meng, X.G.; Hu, C.W.; Du, J. Adsorption of phenol, p-chlorophenol and p-nitrophenol onto functional chitosan. Bioresour. Technol. 2009, 100, 1168–1173. [Google Scholar] [CrossRef]
- Kumar, N.S.; Subbaiah, M.V.; Reddy, A.S.; Krishnaiah, A. Biosorption of phenolic compounds from aqueous solutions onto chitosan-abrus precatorius blended beads. J. Chem. Technol. Biotechnol. 2009, 84, 972–981. [Google Scholar] [CrossRef]
- Nadavala, S.K.; Che Man, H.; Woo, H.-S. Biosorption of Phenolic Compounds from Aqueous Solutions using Pine (Pinus densiflora Sieb) Bark Powder. BioResources 2014, 9, 5155–5174. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Adsorptive removal of p-nitrophenol on microporous activated carbon by FeCl3 activation: Equilibrium and kinetics studies. Desalination Water Treat. 2015, 55, 522–531. [Google Scholar] [CrossRef]
- Altaher, H.; ElQada, E. Investigation of the Treatment of Colored Water Using Efficient Locally Available Adsorbent. Int. J. Energy Ans Environ. 2011, 2, 1113–1124. [Google Scholar]
- Belala, Z.; Jeguirim, M.; Belhachemi, M.; Addoun, F.; Trouvé, G. Biosorption of basic dye from aqueous solutions by Date Stones and Palm-Trees Waste: Kinetic, equilibrium and thermodynamic studies. Desalination 2011, 271, 80–87. [Google Scholar] [CrossRef]
- Alghamdi, A.A. An investigation on the use of date palm fibers and coir pith as adsorbents for Pb(II) ions from its aqueous solution. Desalin. Water Treat. 2016, 57, 12216–12226. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Li, J.; Salamh, Y.; Al-Laqtah, N.; Walker, G.; Ahmad, M.N.M. Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent. J. Hazard. Mater. 2010, 176, 510–520. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Mangwandi, C.; Walker, G.M.; Allen, S.J.; Ahmad, M.N.M.; Khraisheh, M. Influence of solution chemistry on Cr(VI) reduction and complexation onto date-pits/tea-waste biomaterials. J. Environ. Manage. 2013, 114, 190–201. [Google Scholar] [CrossRef]
- Arshad, I.E.; Minerva, E.M.; Hisham, A.; Halim, F.M.A.A. Adsorption of Heavy Metals from Industrial Wastewater using Palm Date Pits as Low Cost Adsorbent. Int. J. Eng. Adv. Technol. 2014, 3, 71–76. [Google Scholar]
- Al-Haidary, A.M.A.; Zanganah, F.H.H.; Al-Azawi, S.R.F.; Khalili, F.I.; Al-Dujaili, A.H. A study on using date palm fibers and leaf base of palm as adsorbents for Pb(II) ions from its aqueous solution. Water. Air. Soil Pollut. 2011, 214, 73–82. [Google Scholar] [CrossRef]
- Al-Mutairi, N.Z. 2,4-Dinitrophenol adsorption by date seeds: Effect of physico-chemical environment and regeneration study. Desalination 2010, 250, 892–901. [Google Scholar] [CrossRef]
- Okasha, A.Y.; Ibrahim, H.G. Phenol removal from aqueous systems by sorption of using some local waste materials. Electron. J. Environ. Agric. Food Chem. 2010, 9, 796–807. [Google Scholar]
- Ahmed, M.J.; Theydan, S.K. Equilibrium isotherms, kinetics and thermodynamics studies of phenolic compounds adsorption on palm-tree fruit stones. Ecotoxicol. Environ. Saf. 2012, 84, 39–45. [Google Scholar] [CrossRef]
- Ye, H.; Zhu, Q.; Du, D. Adsorptive removal of Cd(II) from aqueous solution using natural and modified rice husk. Bioresour. Technol. 2010, 101, 5175–5179. [Google Scholar] [CrossRef]
- Kumar, N.S.; Shaikh, H.M.; Asif, M.; Al-Ghurabi, E.H. Engineered biochar from wood apple shell waste for high-efficient removal of toxic phenolic compounds in wastewater. Sci. Rep. 2021, 11, 2586. [Google Scholar] [CrossRef]
- Dada, A.O.; Adekola, F.A.; Odebunmi, E.O.; Ogunlaja, A.S.; Bello, O.S. Two–three parameters isotherm modeling, kinetics with statistical validity, desorption and thermodynamic studies of adsorption of Cu(II) ions onto zerovalent iron nanoparticles. Sci. Rep. 2021, 11, 16454. [Google Scholar] [CrossRef]
- Kumar, N.S.; Asif, M.; Poulose, A.M.; Suguna, M.; Al-Hazza, M.I. Equilibrium and kinetic studies of biosorptive removal of 2,4,6-trichlorophenol from aqueous solutions using untreated agro-waste pine cone biomass. Processes 2019, 7, 757. [Google Scholar] [CrossRef]
- Daffalla, S.B.; Mukhtar, H.; Shaharun, M.S. Preparation and characterization of rice husk adsorbents for phenol removal from aqueous systems. PLoS ONE 2020, 15, e0243540. [Google Scholar] [CrossRef]
- Kumar, U.; Bandyopadhyay, M. Sorption of cadmium from aqueous solution using pretreated rice husk. Bioresour. Technol. 2006, 97, 104–109. [Google Scholar] [CrossRef]
- Pourhossein, M.; Heravizadeh, O.R.; Omidi, F.; Khadem, M.; Shahtaheri, S.J. Ultrasound-Assisted Emulsified Microextraction Based on Deep Eutectic Solvent for Trace Residue Analysis of Metribuzin in Urine Samples. Methods Objects Chem. Anal. 2021, 16, 153–161. [Google Scholar] [CrossRef]
- de Oliveira Lopes, J.; Garcia, R.A.; de Souza, N.D. Infrared spectroscopy of the surface of thermally-modified teak juvenile wood. Maderas Cienc. Y Tecnol. 2018, 20, 737–746. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Xu, J.; Xu, N.; He, Y. Quantitative visualization of lignocellulose components in transverse sections of moso bamboo based on ftir macro- and micro-spectroscopy coupled with chemometrics. Biotechnol. Biofuels 2018, 11, 1–16. [Google Scholar] [CrossRef]
- Afsharnia, M.; Saeidi, M.; Zarei, A.; Narooie, M.R.; Biglari, H. Phenol Removal from Aqueous Environment by Adsorption onto Pomegranate Peel Carbon. Electron. Physician 2016, 8, 3248–3256. [Google Scholar] [CrossRef]
- Kusmierek, K.; Swiatkowski, A.; Dabek, L. Removal of 2,4,6-Trichlorophenol from Aqueous Solutions Using Agricultural Waste as Low-Cost Adsorbents. Environ. Prot. Eng. 2017, 43, 149–163. [Google Scholar] [CrossRef]
- Aziz, A.S.A.; Manaf, L.A.; Man, H.C.; Kumar, N.S. Equilibrium studies and dynamic behavior of cadmium adsorption by palm oil boiler mill fly ash (POFA) as a natural low-cost adsorbent. Desalination Water Treat. 2015, 54, 1956–1968. [Google Scholar] [CrossRef]
- Suguna, M.; Kumar, N.S.; Sreenivasulu, V.; Krishnaiah, A. Removal of Pb(II) from Aqueous Solutions by using Chitosan Coated Zero Valent Iron Nanoparticles. Sep. Sci. Technol. 2014, 49, 1613–1622. [Google Scholar] [CrossRef]
- Khedri, D.; Hassani, A.H.; Moniri, E.; Ahmad Panahi, H.; Khaleghian, M. Efficient removal of phenolic contaminants from wastewater samples using functionalized graphene oxide with thermo-sensitive polymer: Adsorption isotherms, kinetics, and thermodynamics studies. Surf. Interfaces 2022, 35, 102439. [Google Scholar] [CrossRef]
- Kumar, N.S.; Reddy, A.S.; Boddu, V.M.; Krishnaiah, A. Development of chitosan-alginate based biosorbent for the removal of p-chlorophenol from aqueous medium. Toxicol. Environ. Chem. 2009, 91, 1035–1054. [Google Scholar] [CrossRef]
- Freundlich, H.; Heller, W. The Adsorption of cis- and trans-Azobenzene. J. Am. Chem. Soc. 1939, 301, 2228–2230. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The Equation of the Characteristic Curve of Activated Charcoal. Dokl. Akad. Nauk. SSSR 1947, 55, 327–329. [Google Scholar]
- Alobaidi, D.S.; Alwared, A.I. Role of immobilised Chlorophyta algae in form of calcium alginate beads for the removal of phenol: Isotherm, kinetic and thermodynamic study. Heliyon 2023, 9, e14851. [Google Scholar] [CrossRef]
- Al Bsoul, A.; Hailat, M.; Abdelhay, A.; Tawalbeh, M.; Al-Othman, A.; Al-kharabsheh, I.N.; Al-Taani, A.A. Efficient removal of phenol compounds from water environment using Ziziphus leaves adsorbent. Sci. Total Environ. 2021, 761, 143229. [Google Scholar] [CrossRef]
- Kumar, N.S.; Min, K. Phenolic compounds biosorption onto Schizophyllum commune fungus: FTIR analysis, kinetics and adsorption isotherms modeling. Chem. Eng. J. 2011, 168, 562–571. [Google Scholar] [CrossRef]
- Alves, D.C.S.; Coseglio, B.B.; Pinto, L.A.A.; Cadaval, T.R.S. Development of Spirulina/chitosan foam adsorbent for phenol adsorption. J. Mol. Liq. 2020, 309, 113256. [Google Scholar] [CrossRef]
- Aravindhan, R.; Rao, J.R.; Nair, B.U. Application of a chemically modified green macro alga as a biosorbent for phenol removal. J. Environ. Manag. 2009, 90, 1877–1883. [Google Scholar] [CrossRef]
- Kumar, N.S.; Boddu, V.M.; Krishnaiah, A. Biosorption of phenolic compounds by trametes versicolor polyporus fungus. Adsorpt. Sci. Technol. 2009, 27, 31–46. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, K.; Dixit, U. Adsorption, kinetics and thermodynamics of phenol removal by ultrasound-assisted sulfuric acid-treated pea (Pisum sativum) shells. Sustain. Chem. Pharm. 2021, 22, 100491. [Google Scholar] [CrossRef]
- Makrigianni, V.; Giannakas, A.; Deligiannakis, Y.; Konstantinou, I. Adsorption of phenol and methylene blue from aqueous solutions by pyrolytic tire char: Equilibrium and kinetic studies. J. Environ. Chem. Eng. 2015, 3, 574–582. [Google Scholar] [CrossRef]
- Dehmani, Y.; Sellaoui, L.; Alghamdi, Y.; Lainé, J.; Badawi, M.; Amhoud, A.; Bonilla-Petriciolet, A.; Lamhasni, T.; Abouarnadasse, S. Kinetic, thermodynamic and mechanism study of the adsorption of phenol on Moroccan clay. J. Mol. Liq. 2020, 312, 113383. [Google Scholar] [CrossRef]
- Mandal, A.; Dey, B.B.; Das, S.K. Thermodynamics, kinetics, and isotherms for phenol removal from wastewater using red mud. Water Pract. Technol. 2020, 15, 705–722. [Google Scholar] [CrossRef]
- Mandal, A.; Mukhopadhyay, P.; Das, S.K. Adsorptive removal of phenol from wastewater using guava tree bark. Environ. Sci. Pollut. Res. 2020, 27, 23937–23949. [Google Scholar] [CrossRef]
- Mandal, A.; Bar, N.; Das, S.K. Phenol removal from wastewater using low-cost natural bioadsorbent neem (Azadirachta indica) leaves: Adsorption study and MLR modeling. Sustain. Chem. Pharm. 2020, 17, 100308. [Google Scholar] [CrossRef]
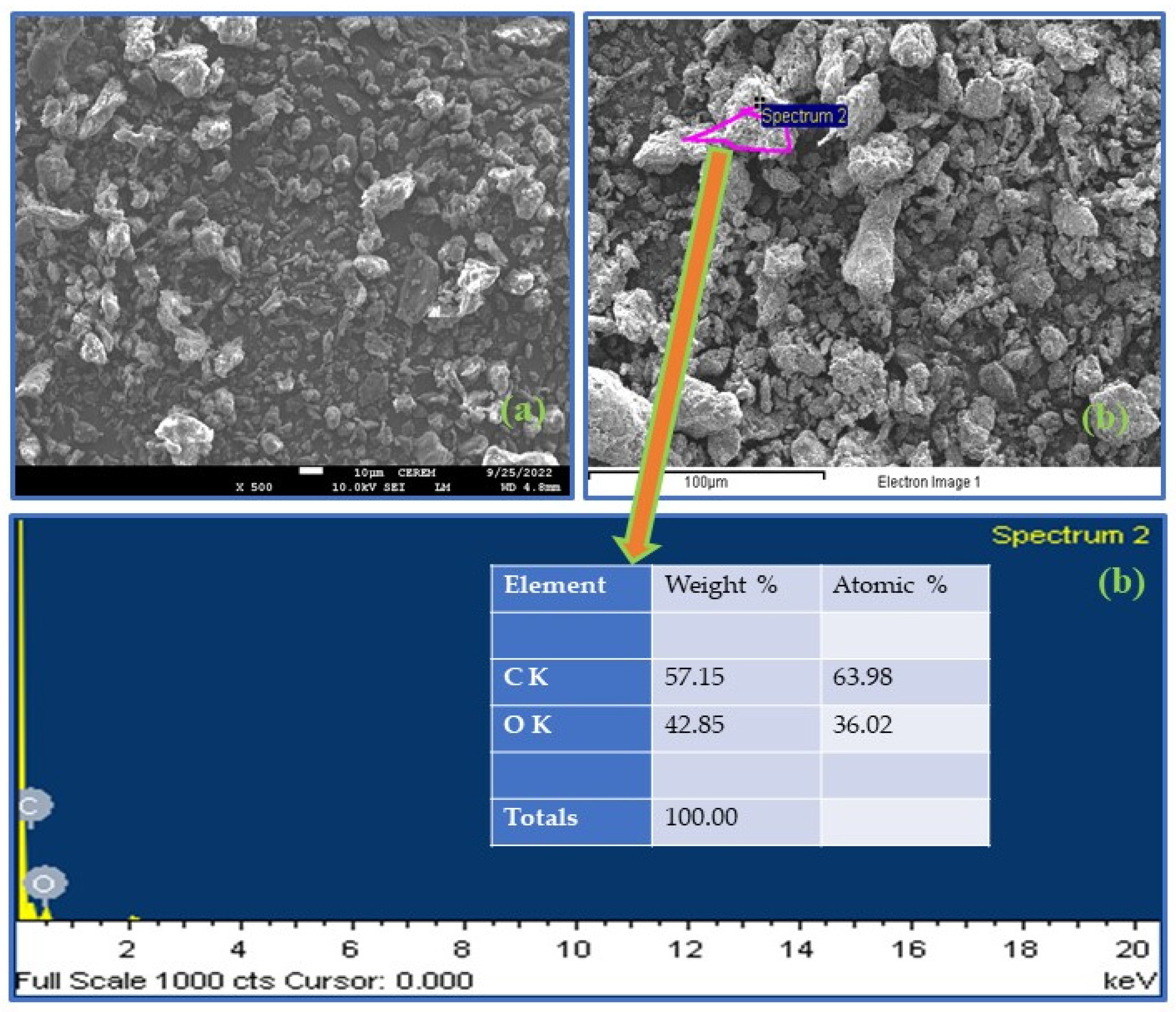
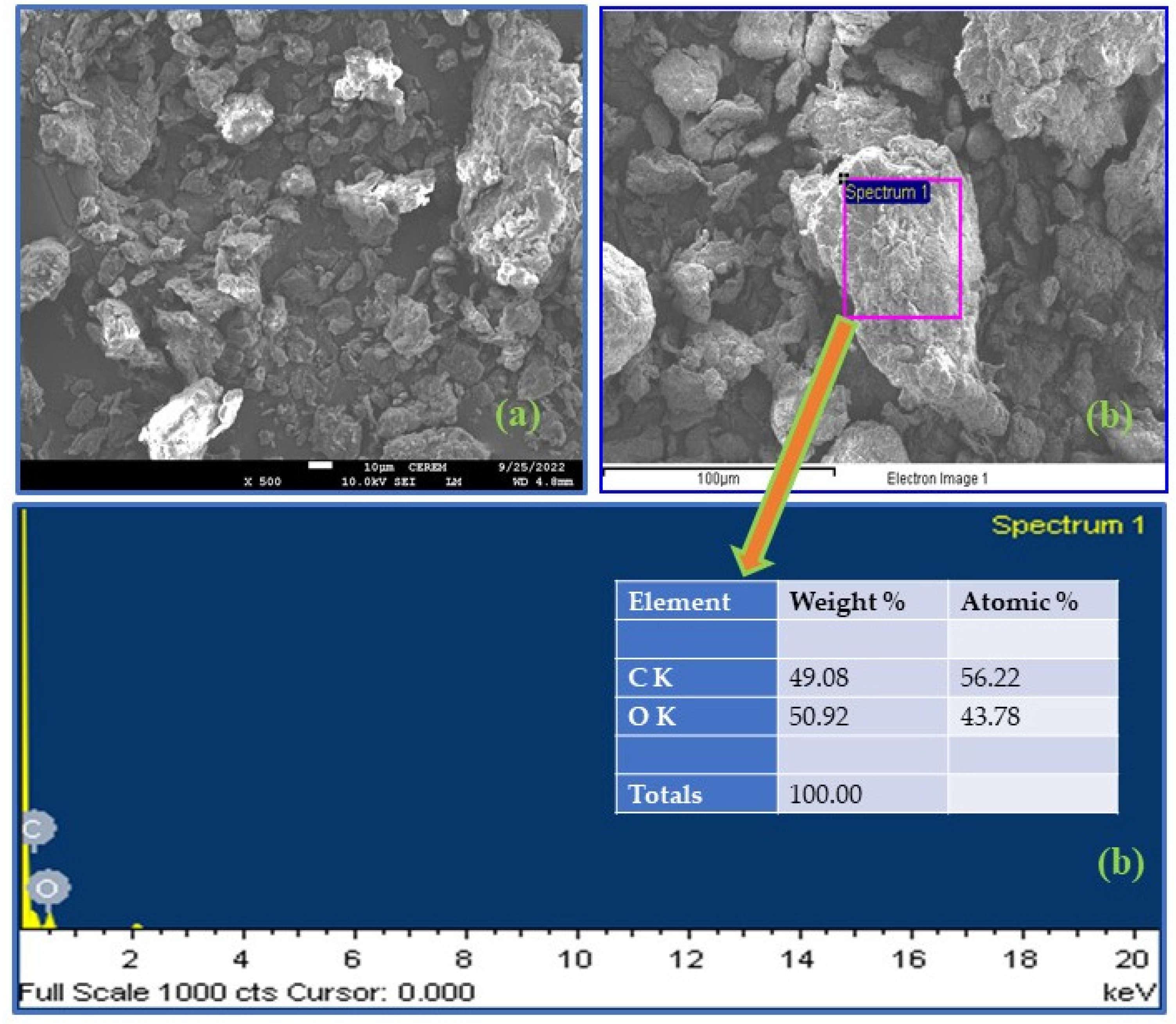
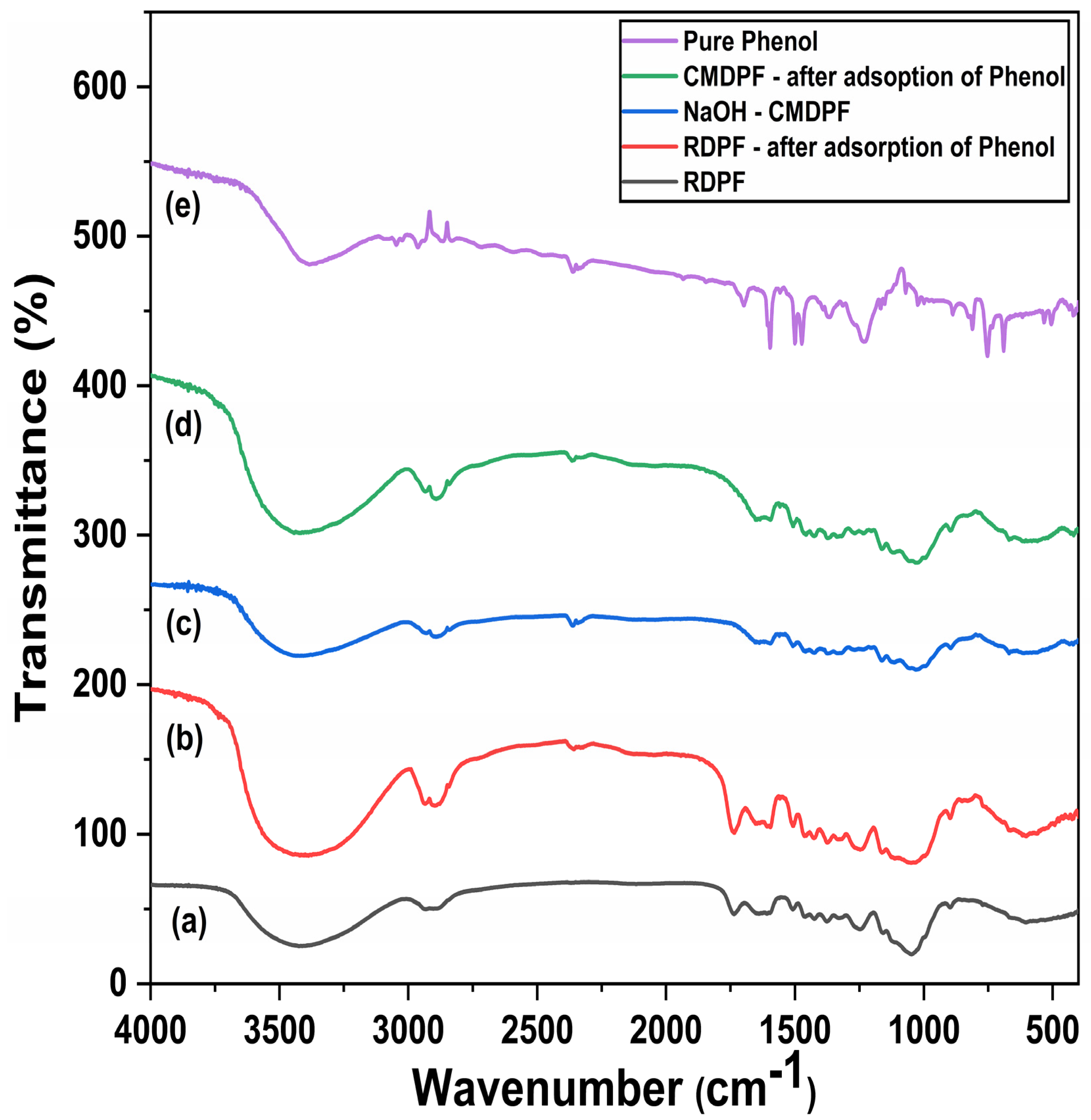

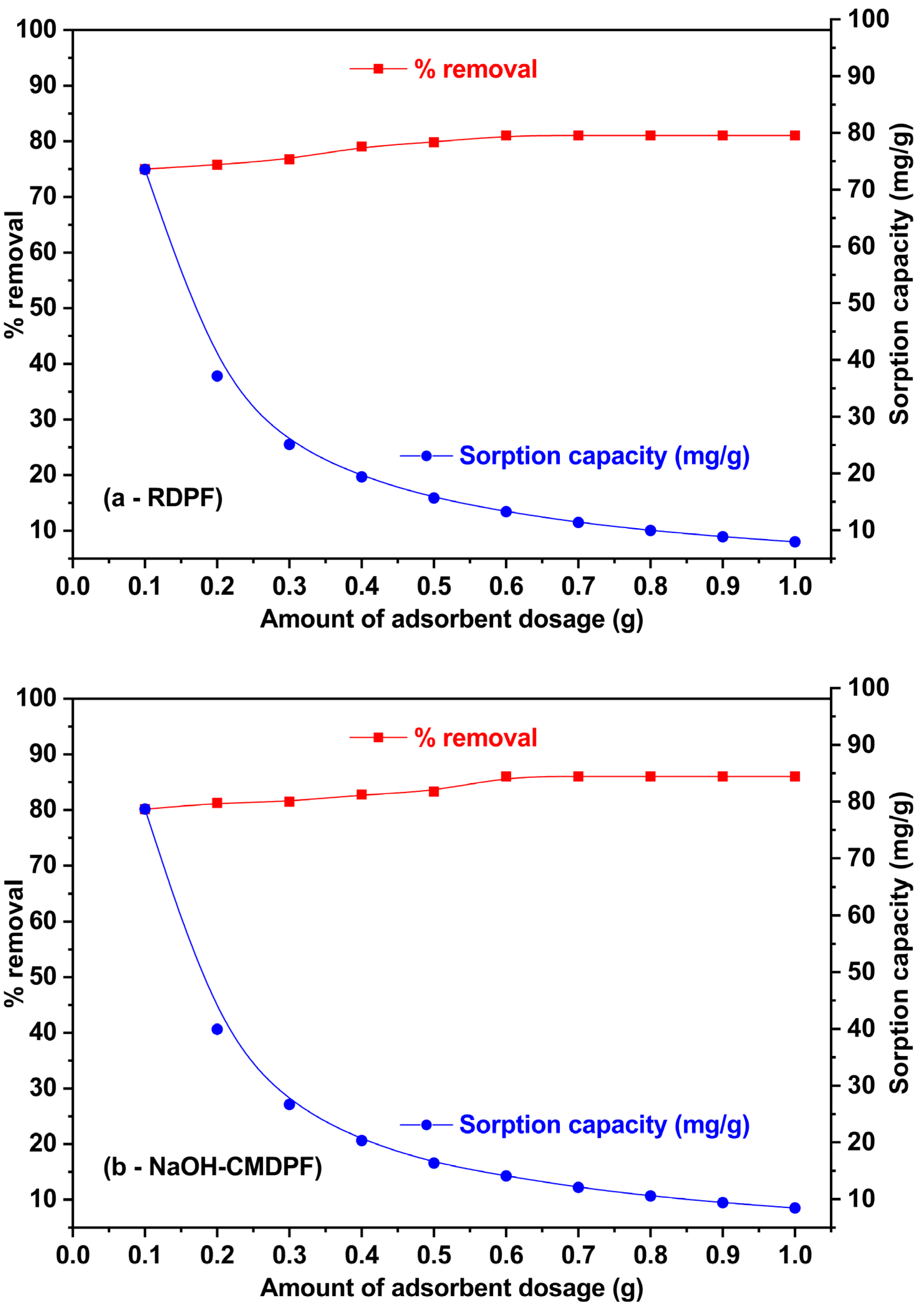

| No. | Sample Name | Carbon (%) | Hydrogen (%) | Nitrogen (%) |
|---|---|---|---|---|
| 1 | RDPF | 46.13 | 5.98 | 0.05 |
| 2 | NaOH–CMDPF | 42.69 | 6.16 | 0.00 |
| Phenol—Raw Date Palm Fiber (RDPF) | |||||||||||
| PFO Kinetic Model | PSO Kinetic Model | ||||||||||
| Conc (mg/L) | qe, exp(mg/g) | qe, cal(mg/g) | k1 (min−1) | R2 | Δqt (%) | χ2 | qe, cal(mg/g) | k2 (g/mg/min) | R2 | Δqt (%) | χ2 |
| 50 | 35.56 | 7.78 | 0.015 | 0.992 | 85.49 | 1756.87 | 35.60 | 0.005 | 0.999 | 3.31 | 0.32 |
| 100 | 76.07 | 12.93 | 0.018 | 0.991 | 87.93 | 4816.63 | 76.67 | 0.003 | 0.999 | 2.11 | 0.28 |
| 150 | 116.03 | 47.32 | 0.014 | 0.889 | 71.72 | 1910.75 | 115.80 | 0.001 | 0.992 | 7.14 | 4.67 |
| 200 | 145.85 | 59.69 | 0.024 | 0.958 | 66.90 | 1835.26 | 149.57 | 0.001 | 0.998 | 4.27 | 2.21 |
| Phenol—NaOH Chemically Modified Date Palm Fiber (NaOH-CMDPF) | |||||||||||
| 50 | 44.62 | 8.59 | 0.017 | 0.960 | 87.66 | 2114.67 | 44.55 | 0.006 | 0.999 | 3.30 | 0.32 |
| 100 | 82.01 | 5.47 | 0.014 | 0.888 | 96.33 | 17238.11 | 81.48 | 0.010 | 0.999 | 1.34 | 0.10 |
| 150 | 128.44 | 43.92 | 0.022 | 0.757 | 74.74 | 2052.09 | 129.34 | 0.001 | 0.992 | 6.04 | 3.09 |
| 200 | 153.19 | 41.67 | 0.027 | 0.858 | 78.94 | 3300.24 | 155.59 | 0.001 | 0.998 | 3.56 | 1.29 |
| Intraparticle Diffusion Model (IDM) | Elovich Kinetic Model (EKM) | ||||||||||||
| Phenol—Raw Date Palm Fiber (RDPF) | |||||||||||||
| Conc (mg/L) | qe, exp (mg/g) | qe, cal (mg/g) | kid | C | R2 | Δqt (%) | χ2 | q(e, cal) (mg/g) | (1/b)ln(ab) (mg/g) | 1/b (mg/g) | R2 | qt (%) | χ2 |
| 50 | 35.56 | 34.87 | 0.67 | 26.97 | 0.975 | 0.82 | 0.02 | 34.52 | 22.54 | 2.44 | 0.968 | 0.98 | 0.03 |
| 100 | 76.07 | 75.71 | 1.12 | 62.59 | 0.976 | 0.60 | 0.02 | 75.15 | 55.01 | 4.10 | 0.993 | 0.31 | 0.01 |
| 150 | 116.03 | 108.87 | 3.41 | 69.19 | 0.966 | 1.56 | 0.22 | 106.78 | 48.41 | 11.89 | 0.902 | 2.67 | 0.64 |
| 200 | 145.85 | 144.73 | 3.38 | 105.38 | 0.994 | 0.39 | 0.02 | 142.89 | 83.56 | 12.09 | 0.976 | 1.04 | 0.13 |
| Phenol—NaOH Chemically Modified Date Palm Fiber (NaOH-CMDPF) | |||||||||||||
| 50 | 44.62 | 43.37 | 0.77 | 35.38 | 0.946 | 0.96 | 0.03 | 43.03 | 31.36 | 2.50 | 0.893 | 1.37 | 0.05 |
| 100 | 82.01 | 80.77 | 0.46 | 75.96 | 0.868 | 0.48 | 0.01 | 80.55 | 73.61 | 1.49 | 0.800 | 0.59 | 0.02 |
| 150 | 128.44 | 122.48 | 2.98 | 91.93 | 0.865 | 2.12 | 0.37 | 120.93 | 77.66 | 9.29 | 0.770 | 2.76 | 0.63 |
| 200 | 153.19 | 150.64 | 2.95 | 120.38 | 0.980 | 0.60 | 0.04 | 149.31 | 105.13 | 9.49 | 0.926 | 1.18 | 0.14 |
| Phenol | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adsorbent | Langmuir | Freundlich | Dubinin-Radushkevich | ||||||||||||
| qm (mg/g) | b (L/mg) | R2 | qe (%) | χ2 | KF ((mg/g)(L/mg)1/n) | n | R2 | qe (%) | χ2 | qs (mmol/g) | E (kJ/mol) | R2 | qe (%) | χ2 | |
| RDPF | 45.62 | 0.034 | 0.998 | 6.96 | 2.36 | 0.938 | 0.680 | 0.976 | 13.38 | 4.43 | 1.86 | 6.55 | 0.967 | 15.79 | 6.22 |
| NaOH-CMDPF | 89.67 | 0.033 | 0.999 | 7.87 | 2.28 | 2.465 | 0.779 | 0.990 | 8.09 | 3.54 | 2.35 | 7.08 | 0.985 | 10.01 | 3.26 |
| Adsorbent | Q0 (mg/g) | Experimental Conditions | References | |
|---|---|---|---|---|
| pH | Contact Time | |||
| Macroalgae/alginate beads | 9.5 | 6 | 120 min | [52] |
| Ziziphus leaves | 15 | 6 | 300 min | [53] |
| Schizophyllum commune fungus | 120 | 5 | 120 min | [54] |
| Spirulina and chitosan foam | 447.6 | 6.5 | 120 min | [55] |
| Modified green macroalga | 20 | 6 | 180 min | [56] |
| Pine cone powder | 164.51 | 5 | 60 min | [7] |
| Trametes versicolor polyporus fungus | 50 | 6 | 240 min | [57] |
| Sulphuric acid-treated pea shells, USAPS | 125.77 | 7 | 180 min | [58] |
| Acid-treated pyrolytic tire char | 51.92 | 6.6 | 60 min | [59] |
| Pine bark powder | 142.85 | 6 | 120 min | [23] |
| Moroccan clay | 15.11 | 4 | 180 min | [60] |
| Red mud | 49.30 | 8 | 480 min | [61] |
| Guava tree bark | 46.76 | 7 | 120 min | [62] |
| Neem leaves | 74.90 | 3 | 240 min | [63] |
| Raw date palm fiber (RDPF) | 45.62 | 6 | 150 min | Present Study |
| NaOH–CMDPF | 89.67 | 6 | 120 min | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siva Kumar, N.; Asif, M.; Poulose, A.M.; Al-Ghurabi, E.H.; Alhamedi, S.S.; Koduru, J.R. Preparation, Characterization, and Chemically Modified Date Palm Fiber Waste Biomass for Enhanced Phenol Removal from an Aqueous Environment. Materials 2023, 16, 4057. https://doi.org/10.3390/ma16114057
Siva Kumar N, Asif M, Poulose AM, Al-Ghurabi EH, Alhamedi SS, Koduru JR. Preparation, Characterization, and Chemically Modified Date Palm Fiber Waste Biomass for Enhanced Phenol Removal from an Aqueous Environment. Materials. 2023; 16(11):4057. https://doi.org/10.3390/ma16114057
Chicago/Turabian StyleSiva Kumar, Nadavala, Mohammad Asif, Anesh Manjaly Poulose, Ebrahim H. Al-Ghurabi, Shaddad S. Alhamedi, and Janardhan Reddy Koduru. 2023. "Preparation, Characterization, and Chemically Modified Date Palm Fiber Waste Biomass for Enhanced Phenol Removal from an Aqueous Environment" Materials 16, no. 11: 4057. https://doi.org/10.3390/ma16114057
APA StyleSiva Kumar, N., Asif, M., Poulose, A. M., Al-Ghurabi, E. H., Alhamedi, S. S., & Koduru, J. R. (2023). Preparation, Characterization, and Chemically Modified Date Palm Fiber Waste Biomass for Enhanced Phenol Removal from an Aqueous Environment. Materials, 16(11), 4057. https://doi.org/10.3390/ma16114057







