Elimination of a Mixture of Microplastics Using Conventional and Detergent-Assisted Coagulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Coagulation Experiments
2.3. Measurement of Microplastics
2.4. Characteristics of Microplastics
3. Results and Discussion
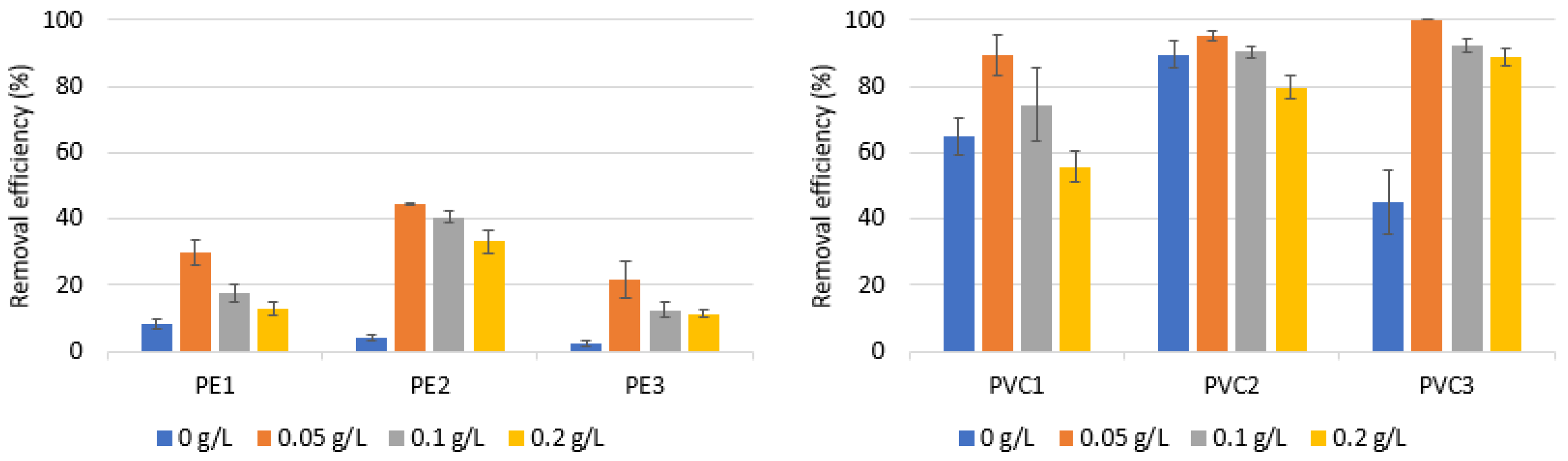
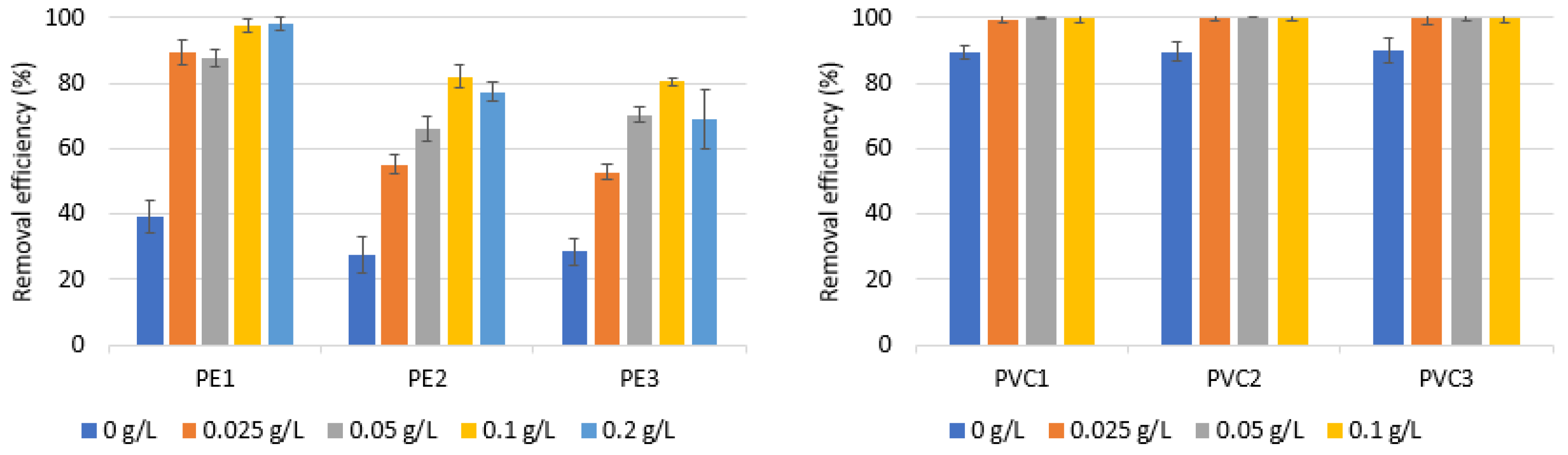
3.1. Performance of Coagulation to Remove PE and PVC—Impact of Coagulant Dose and Microplastics Type
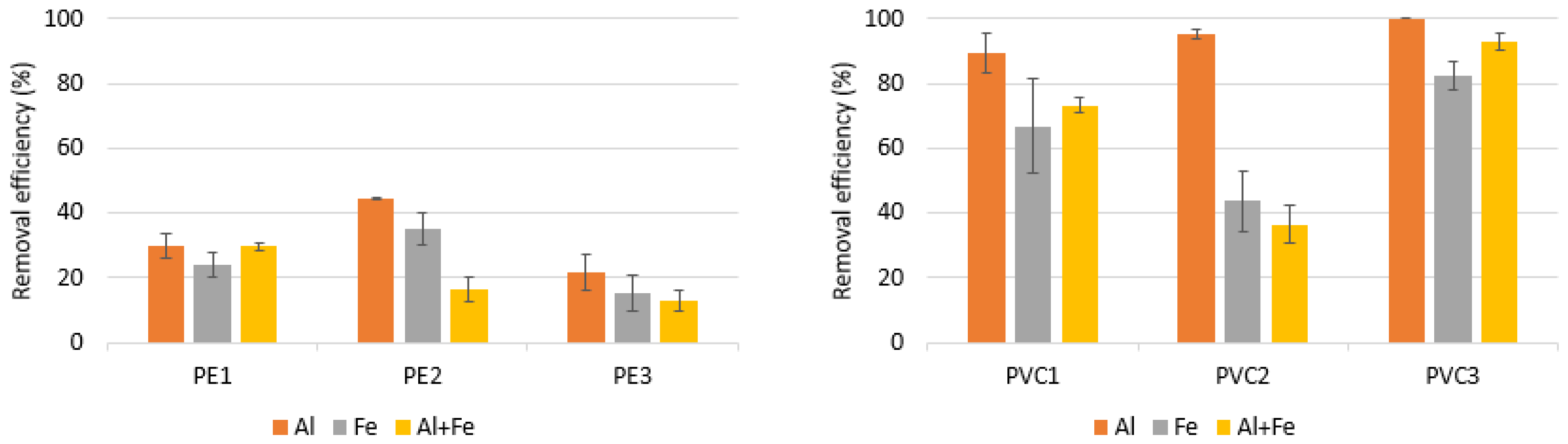
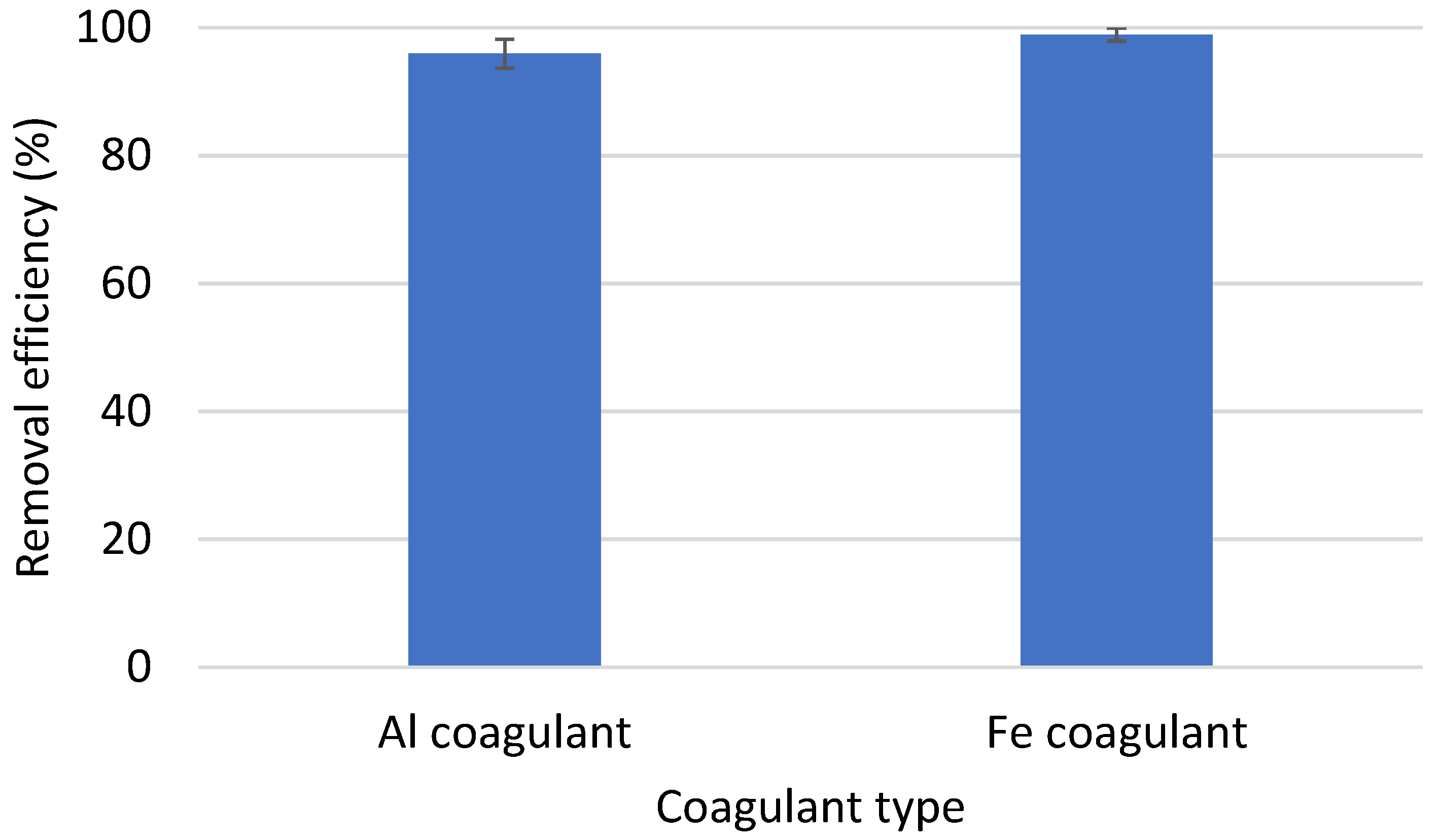
3.2. Performance of Coagulation to Remove PE and PVC—Impact of Coagulant Type
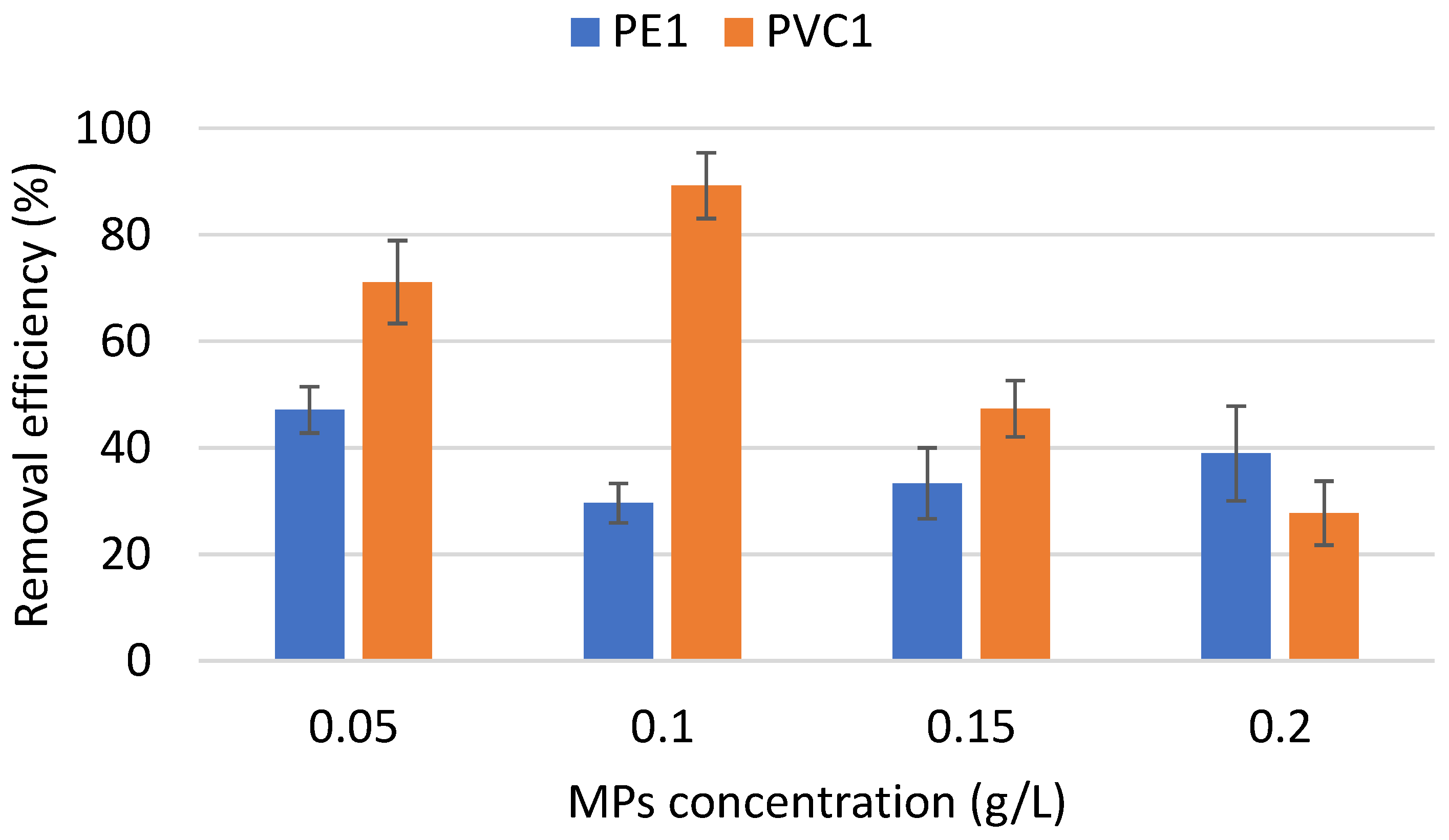
3.3. Performance of Coagulation to Remove PE and PVC—Impact of pH and MP Initial Concentration
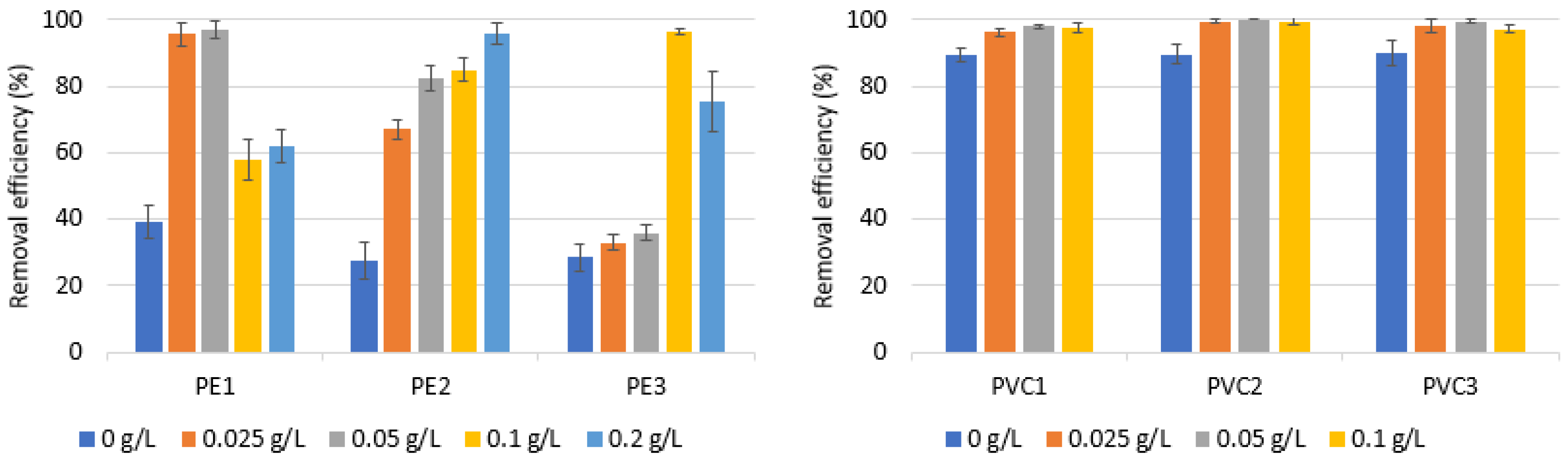
3.4. Performance of Coagulation to Remove PE and PVC—Impact of SDBS Addition
3.5. Performance of Coagulation to Remove Mixture of PE and PVC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.; Trushna, T.; Kalyanasundaram, M.; Tamhankar, A.J.; Diwan, V. Microplastics in drinking water: A macro issue. Water Supply 2022, 22, 5650–5674. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Vara Prasad, P.V. Impacts of plastic pollution on ecosystem services. sustainable development goals and need to focus on circular economy and policy interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- PlasticEurope. Plastics—The Facts. 2020. Available online: https://www.plasticseurope.org/application/files/5716/0752/4286/AF_Plastics_the_facts-WEB-2020-ING_FINAL.pdf (accessed on 24 May 2021).
- Kumar, R.; Sharma, P.; Manna, C.; Jain, M. Abundance interaction ingestion ecological concerns and mitigation policies of microplastic pollution in riverine ecosystem: A review. Sci. Total Environ. 2021, 782, 146695. [Google Scholar] [CrossRef]
- Wayman, C.; Niemann, H. The fate of plastic in the ocean environment—A minireview. Environ. Sci. Process. Impacts 2021, 23, 198–212. [Google Scholar] [CrossRef]
- Gerritse, J.; Leslie, H.A.; de Tender, C.A.; Devriese, L.I.; Vethaak, A.D. Fragmentation of plastic objects in a laboratory seawater microcosm. Sci. Rep. 2020, 10, 10945. [Google Scholar] [CrossRef]
- El Hadri, H.; Gigault, J.; Maxit, B.; Grassl, B.; Reynaud, S. Nanoplastic from mechanically degraded primary and secondary microplastics for environmental assessments. Nanoimpact 2020, 17, 100206. [Google Scholar] [CrossRef]
- Krystynik, P.; Strunakova, K.; Syc, M.; Kluson, P. Notes on Common Misconceptions in Microplastics Removal from Water. Appl. Sci. 2021, 11, 5833. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Kida, M.; Ziembowicz, S.; Koszelnik, P. CH4 and CO2 Emissions from the decomposition of microplastics in the bottom sediment-preliminary studies. Environments 2022, 9, 91. [Google Scholar] [CrossRef]
- Frias, J.P.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss floodplain soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, P. Microplastics pollution pathways to groundwater in India. Curr. Sci. 2021, 120, 249. [Google Scholar]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, P.; Bandyopadhyay, S. Evidence of microplastics in wetlands: Extraction and quantification in Freshwater and coastal ecosystems. J. Water Process. Eng. 2021, 40, 101966. [Google Scholar] [CrossRef]
- Lusher, A.L.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and subsurface samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef]
- La Daana, K.K.; Gårdfeldt, K.; Lyashevska, O.; Hassellöv, M.; Thompson, R.C.; O’Connor, I. Microplastics in sub-surface waters of the Arctic Central Basin. Mar. Pollut. Bull. 2018, 130, 8–18. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef]
- Panno, S.V.; Kelly, W.R.; Scott, J.; Zheng, W.; McNeish, R.E.; Holm, N.; Baranski, E.L. Microplastic contamination in karst groundwater systems. Groundwater 2019, 57, 189–196. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.Y. Emerging concerns about microplastic pollution on groundwater in South Korea. Sustainability 2020, 12, 5275. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M. Atmospheric microplastics: A review on current status and perspectives. Earth-Sci. Rev. 2020, 203, 103118. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Edo, C.; Aguilera, Á.; Viúdez-Moreiras, D.; Pulido-Reyes, G.; González-Toril, E.; Rosal, R. Occurrence and transport of microplastics sampled within and above the planetary boundary layer. Sci. Total Environ. 2021, 761, 143213. [Google Scholar] [CrossRef]
- Tibbetts, J.; Krause, S.; Lynch, I.; Sambrook Smith, G.H. Abundance. distribution. and drivers of microplastic contamination in urban river environments. Water 2018, 10, 597. [Google Scholar] [CrossRef]
- Bujaczek, T.; Kolter, S.; Locky, D.; Ross, M.S. Characterization of microplastics and anthropogenic fibers in surface waters of the North Saskatchewan River, Alberta, Canada. Facets 2021, 6, 26–43. [Google Scholar] [CrossRef]
- Barcelo, D.; Pico, Y. Case studies of macro-and microplastics pollution in coastal waters and rivers: Is there a solution with new removal technologies and policy actions? Case Stud. Chem. Environ. Eng. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Xie, Y.; Zhong, S.; Yang, B.; Lu, D.; Zhong, Q. Distribution of microplastics in surface water and sediments of Qin river in Beibu Gulf, China. Sci. Total Environ. 2020, 708, 135176. [Google Scholar] [CrossRef] [PubMed]
- Amelia, T.S.M.; Khalik, W.M.; Afiq, W.M.; Ong, M.C.; Shao, Y.T.; Pan, H.J.; Bhubalan, K. Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog. Earth Planet. Sci. 2021, 8, 12. [Google Scholar] [CrossRef]
- Pauna, V.H.; Buonocore, E.; Renzi, M.; Russo, G.F.; Franzese, P.P. The issue of microplastics in marine ecosystems: A bibliometric network analysis. Mar. Pollut. Bull. 2019, 149, 110612. [Google Scholar] [CrossRef]
- Shen, M.; Ye, S.; Zeng, G.; Zhang, Y.; Xing, L.; Tang, W.; Liu, S. Can microplastics pose a threat to ocean carbon sequestration? Mar. Pollut. Bull. 2020, 150, 110712. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Pivokonský, M.; Pivokonská, L.; Novotná, K.; Čermáková, L.; Klimtová, M. Occurrence and fate of microplastics at two different drinking water treatment plants within a river catchment. Sci. Total Environ. 2020, 741, 140236. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef] [PubMed]
- Adib, D.; Mafigholami, R.; Tabeshkia, H. Identification of microplastics in conventional drinking water treatment plants in Tehran. J. Environ. Health Sci. Eng. 2021, 19, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Jiang, Q.; Hu, X.; Zhong, X. Occurrence and identification of microplastics in tap water from China. Chemosphere 2020, 252, 126493. [Google Scholar] [CrossRef] [PubMed]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics. Chemosphere 2018, 193, 567–573. [Google Scholar] [CrossRef]
- Li, X.; Mei, Q.; Chen, L.; Zhang, H.; Dong, B.; Dai, X. Enhancement in adsorption potential of microplastics in sewage sludge for metal pollutants after the wastewater treatment process. Water Res. 2019, 157, 228–237. [Google Scholar] [CrossRef]
- Liu, S.; Huang, J.; Zhang, W.; Shi, L.; Yi, K.; Yu, H.; Li, J. Microplastics as a vehicle of heavy metals in aquatic environments: A review of adsorption factors mechanisms and biological effects. J. Environ. Manag. 2022, 302, 113995. [Google Scholar] [CrossRef]
- Kida, M.; Ziembowicz, S.; Pochwat, K.; Koszelnik, P. Experimental and computational hazard prediction associated with reuse of recycled car tire material. J. Hazard. Mater. 2022, 438, 129489. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Hadibarata, T. Microplastics removal through water treatment plants: Its feasibility efficiency future prospects and enhancement by proper waste management. Environ. Chall. 2021, 5, 100264. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Ding, Y.; Hu, C.; Liu, H.; Qu, J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 2019, 78, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, G.; Yue, J.; Xing, X.; Yang, Z.; Wang, X.; Zhang, J. Enhanced removal of polyethylene terephthalate microplastics through polyaluminum chloride coagulation with three typical coagulant aids. Sci. Total Environ. 2021, 800, 149589. [Google Scholar] [CrossRef] [PubMed]
- Skaf, D.W.; Punzi, V.L.; Rolle, J.T.; Kleinberg, K.A. Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. Chem. Eng. J. 2020, 386, 123807. [Google Scholar] [CrossRef]
- Xia, Y.; Xiang, X.M.; Dong, K.Y.; Gong, Y.Y.; Li, Z.J. Surfactant stealth effect of microplastics in traditional coagulation process observed via 3-D fluorescence imaging. Sci. Total Environ. 2020, 729, 138783. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M.; Koszelnik, P. Efficient removal of polyethylene and polyvinyl chloride microplastics from water using a modified coagulation process supported by the addition of a surfactant. Desalin. Water Treat. 2023, 288, 51–59. [Google Scholar] [CrossRef]
- Monira, S.; Bhuiyan, M.A.; Haque, N.; Pramanik, B.K. Assess the performance of chemical coagulation process for microplastics removal from stormwater. Process. Saf. Environ. Prot. 2021, 155, 11–16. [Google Scholar] [CrossRef]
- Wu, K.; Wang, H.; Liu, R.; Zhao, X.; Liu, H.; Qu, J. Arsenic removal from a high-arsenic wastewater using in situ formed Fe–Mn binary oxide combined with coagulation by poly-aluminum chloride. J. Hazard. Mater. 2011, 185, 990–995. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef]
- Xiangli, Q.; Zhenjia, Z.; Nongcun, W.; Wee, V.; Low, M.; Loh, C.S.; Hing, N.T. Coagulation pretreatment for a large-scale ultra-filtration process treating water from the Taihu River. Desalination 2008, 230, 305–313. [Google Scholar] [CrossRef]
- Regulation of the Minister of Health Dated 7 December 2017 on the Quality of Water Intended for Human Consumption (Journal of Laws, Item 2294). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20170002294 (accessed on 9 January 2023).
- Muhonja, C.N.; Makonde, H.; Magoma, G.; Imbuga, M. Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS ONE 2018, 13, e0198446. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Ma-laysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Almatrafi, E.; Hu, T.; Zhou, C.; Song, B.; Zeng, G. Efficient removal of microplastics from wastewater by an electrocoagulation process. Chem. Eng. J. 2022, 428, 131161. [Google Scholar] [CrossRef]
- IRO Coating Additive. Available online: https://www.irocoatingadditive.com (accessed on 26 August 2022).
- Shahi, N.K.; Maeng, M.; Kim, D.; Dockko, S. Removal behavior of microplastics using alum coagulant and its enhancement using polyamine-coated sand. Process. Saf. Environ. Prot. 2020, 141, 9–17. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Abdiyev, K.Z.; Maric, M.; Orynbayev, B.Y.; Toktarbay, Z.; Zhursumbaeva, M.B.; Seitkaliyeva, N.Z. Flocculating properties of 2-acrylamido-2-methyl-1-propane sulfonic acid-co-allylamine polyampholytic copolymers. Polym. Bull. 2022, 79, 10741–10756. [Google Scholar] [CrossRef]
- Kerimkulova, A.R.; Azat, S.; Velasco, L.; Mansurov, Z.A.; Lodewyckx, P.; Tulepov, M.I.; Imangazy, A. Granular rice husk-based sorbents for sorption of vapors of organic and inorganic matters. J. Chem. Technol. Metall. 2019, 54, 578–584. [Google Scholar]

| Parameters | Unit | Average Value |
|---|---|---|
| Color | mg Pt/L | <5 |
| Turbidity | NTU | <0.20 |
| pH | - | 7.70 |
| Conductivity | µS/cm | 647 |
| NO3− | mg/L | 6.3 |
| NO2− | mg/L | <0.05 |
| Cl− | mg/L | 38 |
| SO42− | mg/L | 36 |
| Total organic carbon | mg/L | 1.50 |
| Total hardness | mg CaCO3/L | 260 |
| MPs | Characteristics |
|---|---|
| PE1 | Ultra-high molecular weight, surface-modified, powder, 15–97 μm particle size * |
| PE2 | Ultra-high molecular weight, surface-modified, powder, 20–241 μm particle size * |
| PE3 | Ultra-high molecular weight, average Mw of 3,000,000–6,000,000, 69–250 μm particle size * |
| PVC1 | Low molecular weight, 50–162 μm particle size * |
| PVC2 | High molecular weight, 51–165 μm particle size * |
| PVC3 | Average Mw of ~233,000, average Mn of ~99,000, 19–331 μm particle size * |
| Removal Efficiency (%) | ||||||
|---|---|---|---|---|---|---|
| MPs | PE1 | PE2 | PE3 | |||
| Coagulant | Al | Fe | Al | Fe | Al | Fe |
| pH = 3 | 4.2 ± 0.6 | 5.81 ± 0.9 | 20.2 ± 4.2 | 9.6 ± 3.1 | 20.2 ± 3.9 | 8.2 ± 2.1 |
| pH = 5 | 19.5 ± 2.7 | 5.88 ± 0.6 | 40.9 ± 2.5 | 10.2 ± 3 | 25.3 ± 4 | 6.9 ± 3 |
| pH = 7 | 29.6 ± 3.7 | 18.31 ± 6.8 | 44.3 ± 4 | 31.7 ± 5.1 | 28.3 ± 4.2 | 15.4 ± 2.2 |
| pH = 9 | 14.9 ± 1.9 | 9.0 ± 1 | 19 ± 2 | 23 ± 4 | 20.3 ± 5 | 12.1 ± 2.3 |
| Removal Efficiency (%) | ||||||
| MPs | PVC1 | PVC2 | PVC3 | |||
| Coagulant | Al | Fe | Al | Fe | Al | Fe |
| pH = 3 | 51.8 ± 7.8 | 38.4 ± 5.7 | 52 ± 3.8 | 52.62 ± 4.9 | 42 ± 5.5 | 31.72 ± 3.2 |
| pH = 5 | 49.1 ± 8.9 | 41.8 ± 10 | 63 ± 4 | 35.54 ± 4.4 | 65 ± 7.2 | 80.63 ± 3.1 |
| pH = 7 | 89.2 ± 6.2 | 66.8 ± 14 | 95.5 ± 4 | 43.72 ± 5.2 | 100 ± 2 | 82.42 ± 3.4 |
| pH = 9 | 46.6 ± 3.5 | 39 ± 3.5 | 63 ± 5 | 40 ± 7.1 | 80 ± 7.1 | 63 ± 3.2 |
| MIX | MPs | Percentages (%) | Diameter Range (µm) | Mean Diameter (µm) | Mean Area (µm²) | Mean Perimeter (µm) | Mean Circularity | Mean Solidity |
|---|---|---|---|---|---|---|---|---|
| MIX1 | PE1 before | 53 | 15–97 | 51.83 | 3126.68 | 225.6 | 0.65 | 0.900 |
| PE1 after | 86 | 14–201 | 53.21 | 2962.05 | 221.91 | 0.66 | 0.904 | |
| PVC1 before | 47 | 50–162 | 136.79 | 17,993.89 | 653.06 | 0.53 | 0.86 | |
| PVC1 after | 14 | 23–416 | 165.91 | 26,350.19 | 769.83 | 0.76 | 0.95 | |
| MIX2 | PE2 before | 58 | 20–241 | 124.92 | 10,021.49 | 379.57 | 0.68 | 0.93 |
| PE2 after | 91 | 31–415 | 156.35 | 22,810.99 | 628.61 | 0.78 | 0.97 | |
| PVC2 before | 42 | 51–165 | 120.2 | 45,202.94 | 968.75 | 0.56 | 0.85 | |
| PVC2 after | 9 | 103–422 | 216.11 | 41,527.94 | 925.45 | 0.57 | 0.89 | |
| MIX3 | PE3 before | 50 | 69–250 | 156.72 | 14,735.44 | 434.29 | 0.68 | 0.92 |
| PE3 after | 44 | 18–484 | 191.79 | 24,536.79 | 620.73 | 0.75 | 0.95 | |
| PVC3 before | 50 | 19–331 | 179.72 | 31,508.76 | 745.49 | 0.59 | 0.89 | |
| PVC3 after | 56 | 25–497 | 225.5 | 46,824.47 | 958.53 | 0.67 | 0.93 |
| Particle Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Coagulation Process | Percentages (Remaining in the Water) | Diameter Range (µm) | Mean Diameter (µm) | Mean Area (µm²) | Mean Perimeter (µm) | Mean Circularity | Mean Solidity |
| PE1 | |||||||
| Before | 53% | 15–97 | 51.83 | 3127 | 225.6 | 0.65 | 0.900 |
| AlCl3·6H2O | 90.3% | 14–253 | 43.45 | 2316 | 165.95 | 0.75 | 0.94 |
| FeCl3·6H2O | 79.6% | 24–187 | 73.34 | 4297 | 256.77 | 0.74 | 0.95 |
| PVC1 | |||||||
| Before | 47% | 50–162 | 136.79 | 17,994 | 653.06 | 0.53 | 0.86 |
| AlCl3·6H2O | 9.7% | 25–238 | 123.88 | 13,074 | 476.64 | 0.71 | 0.94 |
| FeCl3·6H2O | 20.4% | 81–258 | 137.95 | 13,899 | 492.41 | 0.72 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziembowicz, S.; Kida, M.; Koszelnik, P. Elimination of a Mixture of Microplastics Using Conventional and Detergent-Assisted Coagulation. Materials 2023, 16, 4070. https://doi.org/10.3390/ma16114070
Ziembowicz S, Kida M, Koszelnik P. Elimination of a Mixture of Microplastics Using Conventional and Detergent-Assisted Coagulation. Materials. 2023; 16(11):4070. https://doi.org/10.3390/ma16114070
Chicago/Turabian StyleZiembowicz, Sabina, Małgorzata Kida, and Piotr Koszelnik. 2023. "Elimination of a Mixture of Microplastics Using Conventional and Detergent-Assisted Coagulation" Materials 16, no. 11: 4070. https://doi.org/10.3390/ma16114070






