Assessing the Rheological, Mechanical, and Photocatalytic Properties of Niobium Oxide-Incorporated White Cement Pastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixture Design
2.3. Experimental Methods
3. Results
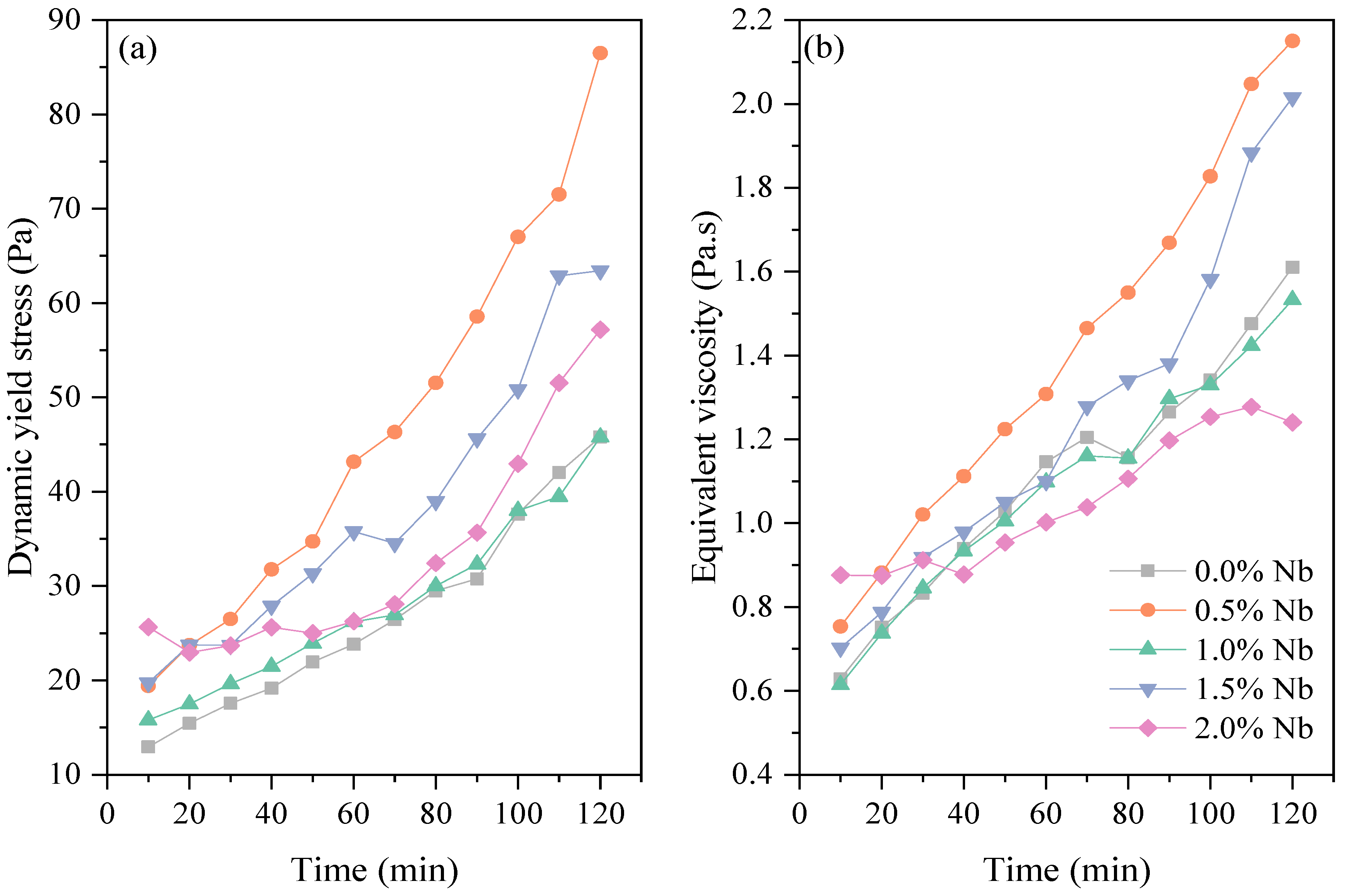
3.1. Rotational Rheometry
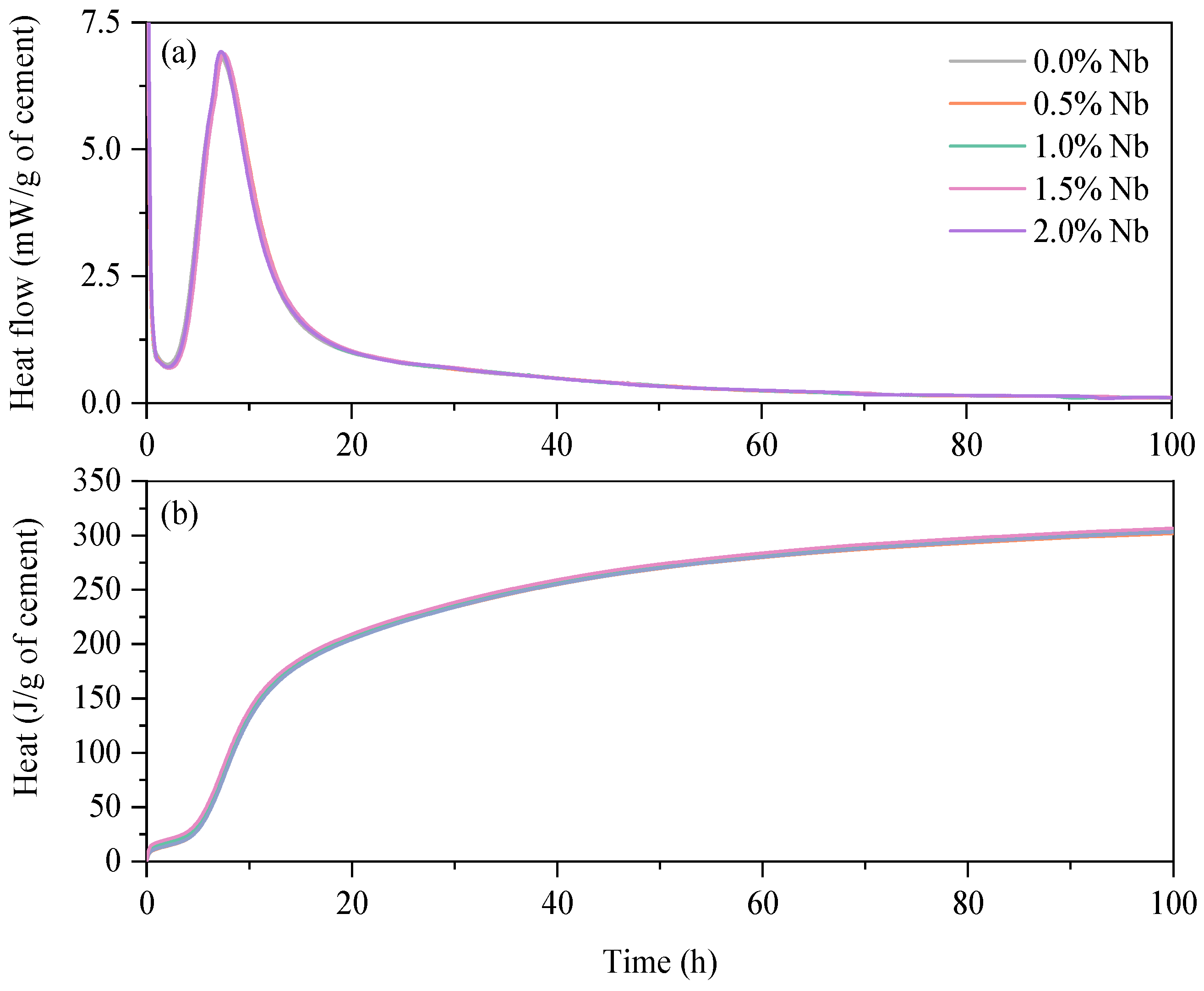
3.2. Isothermal Calorimetry
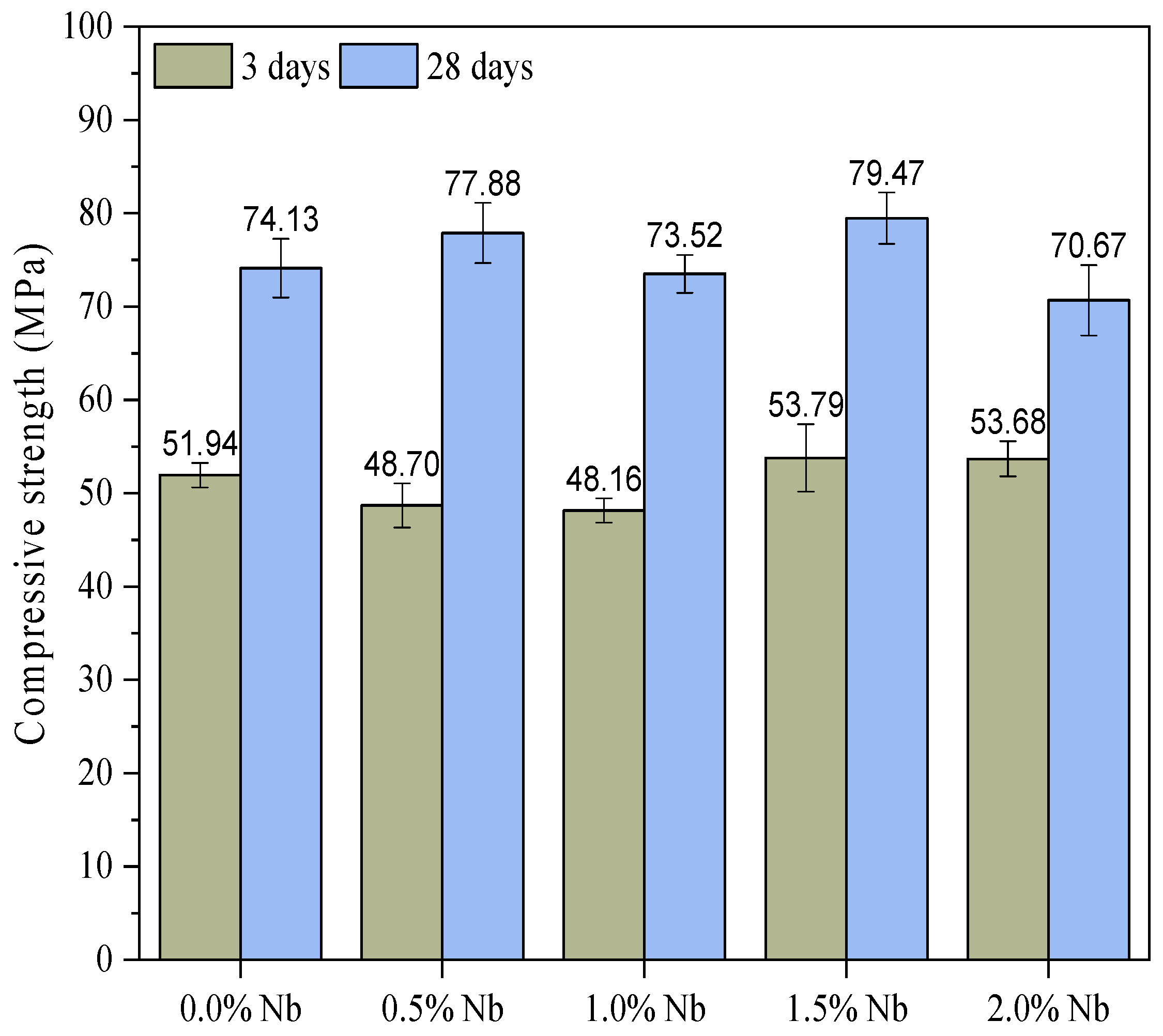
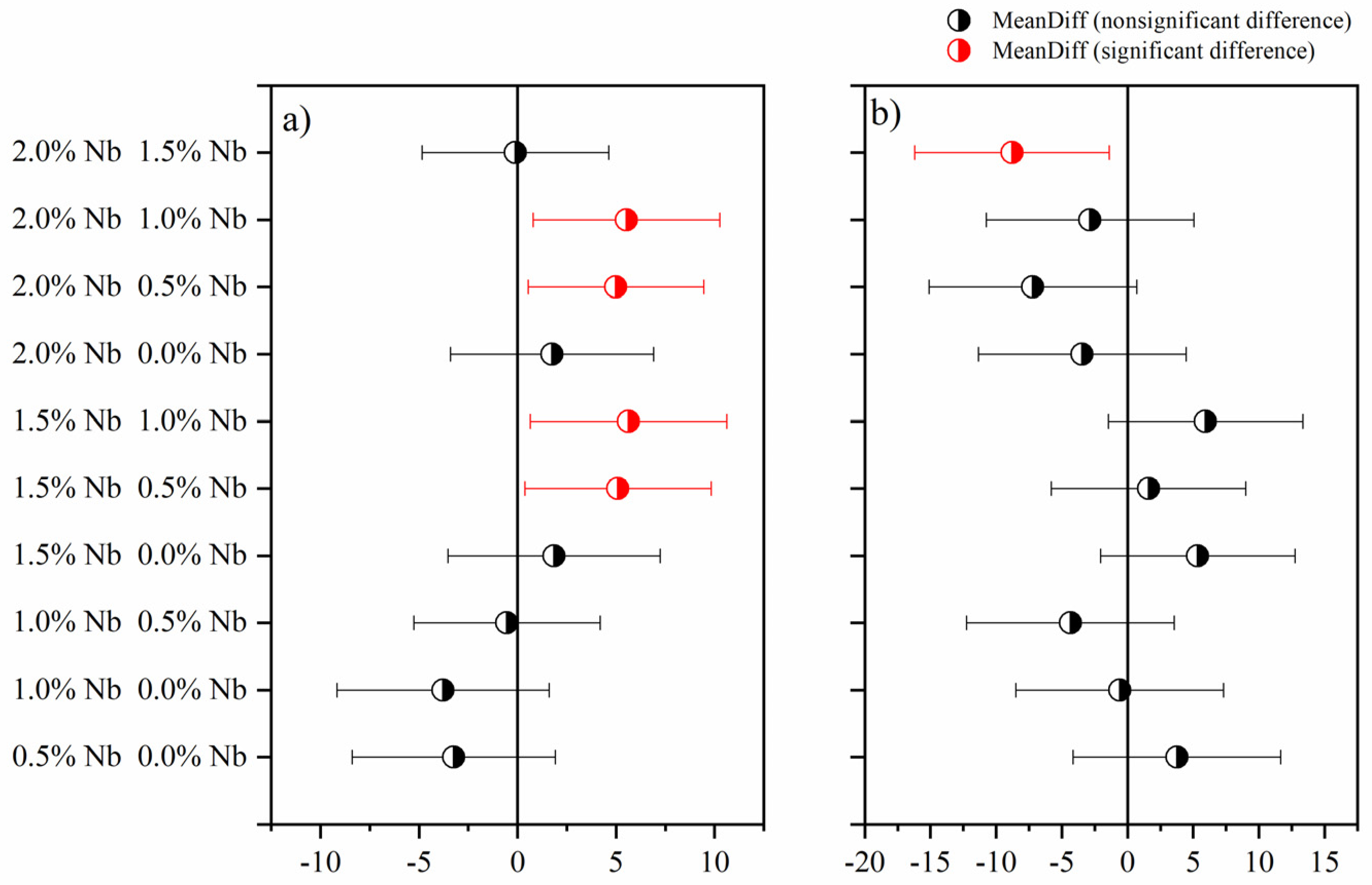
3.3. Compressive Strength
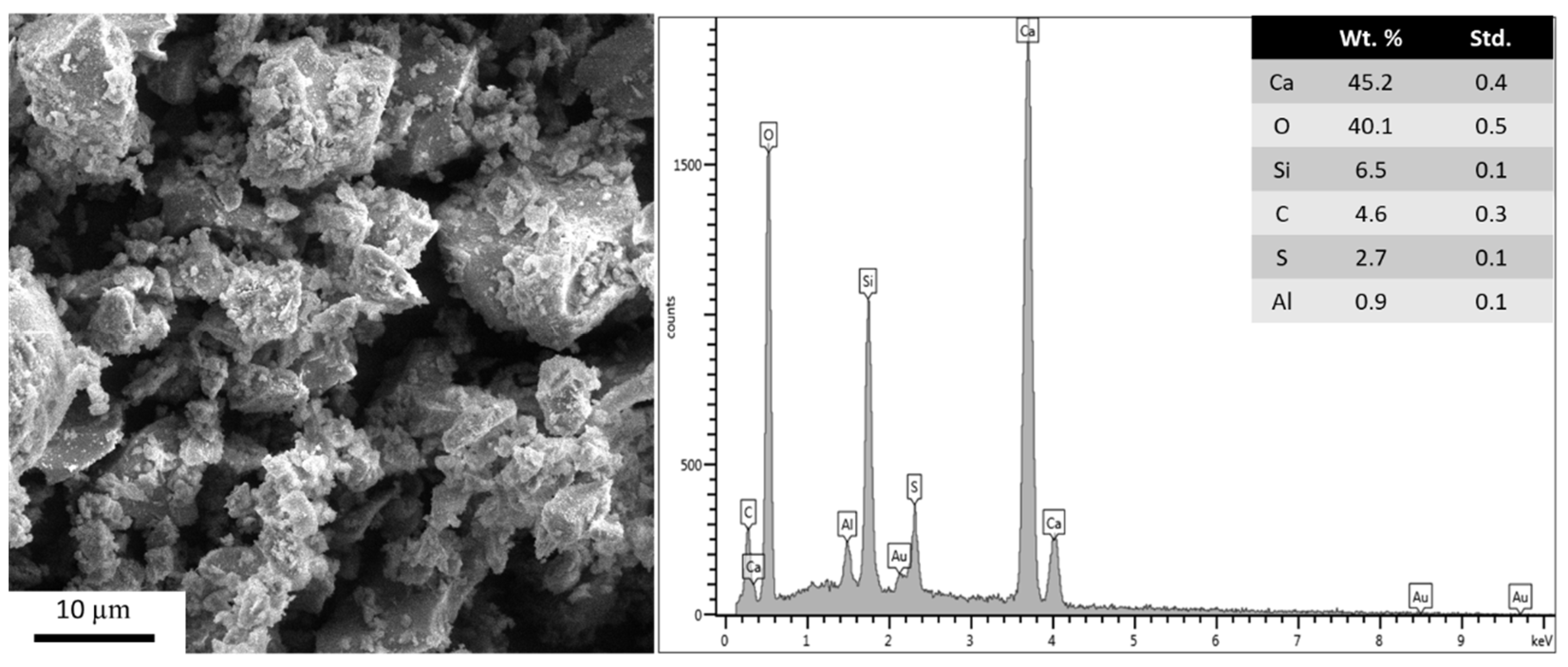
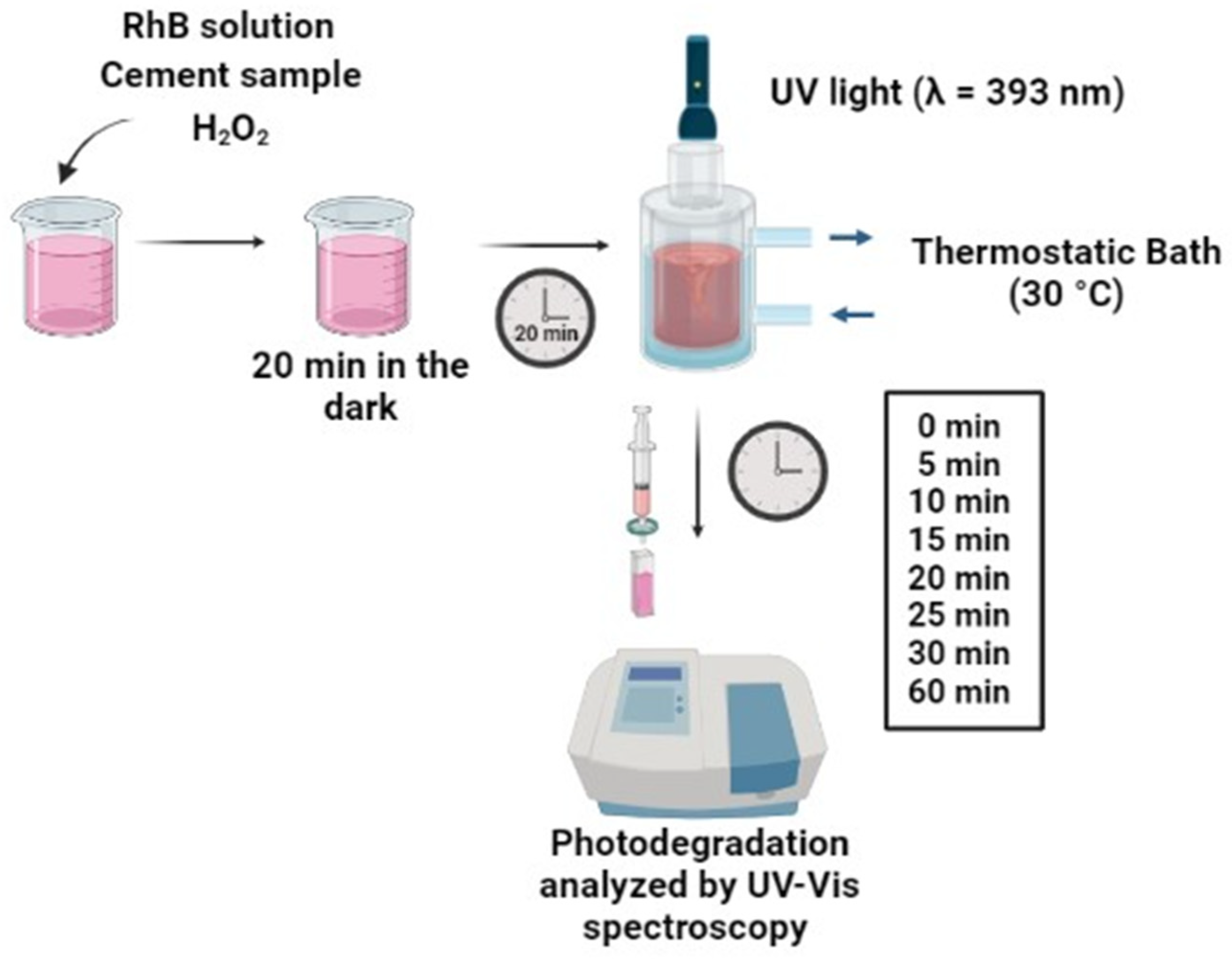
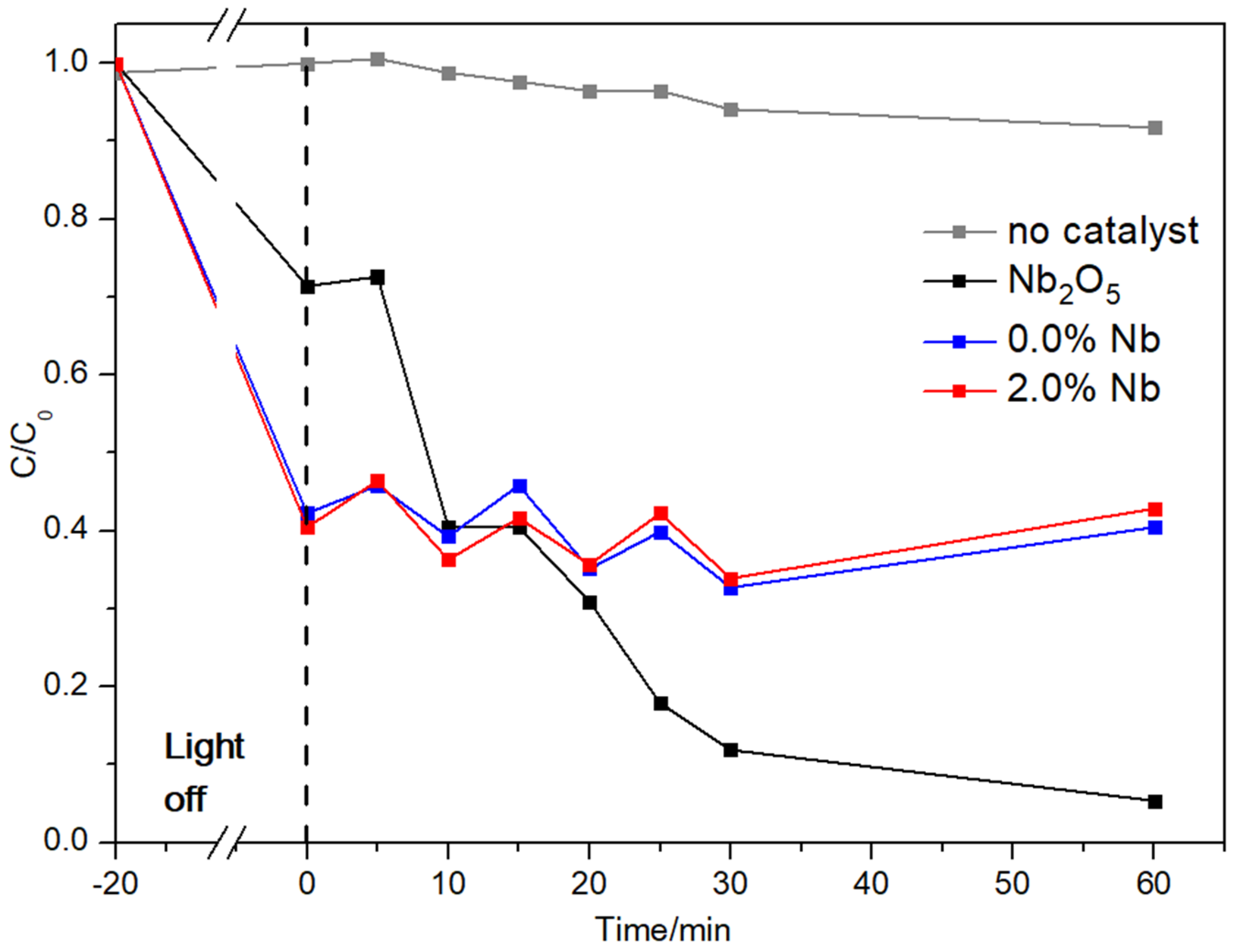
3.4. Photocatalytic Activity
4. Conclusions
- The dynamic yield stress and equivalent viscosity of white cement pastes progressively increased over time (0–120 min). This increase is associated with system flocculation and the formation of a rigid network caused by the precipitation of hydration products. The higher SSA of Nb2O5 nanoparticles contributed to the increase in rheological parameters in cement pastes up to a content of 1.5 wt.%. For a higher content (2.0 wt.%), possibly the Nb2O5 incorporation slightly delayed the initial hydration reactions of white cement, resulting in viscosity values up to 23.0% lower than the plain cement paste.
- The isothermal calorimetry results indicated that the incorporation of Nb2O5 up to a content of 2.0 wt.% did not have a significant impact on the induction period, main heat flow peak, and cumulative heat after 100 h of hydration in white cement pastes.
- Compressive strength results also indicated that the Nb2O5 evaluated contents did not significantly affect white cement paste’s 3-d and 28-d compressive strength. Two possible hypotheses can be suggested: (i) the Nb2O5 contents evaluated are low to produce a noticeable effect, or (ii) the dispersion of the nanomaterial was not adequate, thereby compromising its ability to reinforce the cement matrix.
- A photocatalytic activity was observed for Nb2O5 samples, with RhB concentration C/C0 = 0.05 after 60 min exposure to UV–Vis light. Cement pastes samples exhibited a C/C0 = 0.40 after 60 min. Moreover, the Nb2O5 content of 2.0 wt.% did not enhance cement materials’ photocatalytic activity suggesting the discoloration of the RhB solution.
- An alternative process of RhB degradation was observed, which did not rely on photon activation but rather on the interaction between OH− from CBM and H2O2 in an aqueous medium.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silvestro, L.; Gleize, P.J.P. Effect of carbon nanotubes on compressive, flexural and tensile strengths of Portland cement-based materials: A systematic literature review. Constr. Build. Mater. 2020, 264, 120237. [Google Scholar] [CrossRef]
- Silvestro, L.; Lima, G.T.D.S.; Ruviaro, A.S.; de Matos, P.R.; Mezalira, D.Z.; Gleize, P.J.P. Evaluation of different organosilanes on multi-walled carbon nanotubes functionalization for application in cementitious composites. J. Build. Eng. 2022, 51, 104292. [Google Scholar] [CrossRef]
- Silvestro, L.; Ruviaro, A.S.; de Matos, P.R.; Pelisser, F.; Mezalira, D.Z.; Gleize, P.J.P. Functionalization of multi-walled carbon nanotubes with 3-aminopropyltriethoxysilane for application in cementitious matrix. Constr. Build. Mater. 2021, 311, 125358. [Google Scholar] [CrossRef]
- Goyal, R.; Verma, V.K.; Singh, N. Effect of nano TiO2 & ZnO on the hydration properties of Portland cement. Mater. Today Proc. 2022, 65, 1956–1963. [Google Scholar] [CrossRef]
- Orakzai, M.A. Hybrid effect of nano-alumina and nano-titanium dioxide on Mechanical properties of concrete. Case Stud. Constr. Mater. 2021, 14, e00483. [Google Scholar] [CrossRef]
- Mousavi, M.A.; Sadeghi-Nik, A.; Bahari, A.; Jin, C.; Ahmed, R.; Ozbakkaloglu, T.; de Brito, J. Strength optimization of cementitious composites reinforced by carbon nanotubes and Titania nanoparticles. Constr. Build. Mater. 2021, 303, 124510. [Google Scholar] [CrossRef]
- Liu, J.; Suh, H.; Jee, H.; Xu, J.; Nezhad, E.Z.; Choi, C.-S.; Bae, S. Synergistic effect of carbon nanotube/TiO2 nanotube multi-scale reinforcement on the mechanical properties and hydration process of portland cement paste. Constr. Build. Mater. 2021, 293, 123447. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J. Hydration, reinforcing mechanism, and macro performance of multi-layer graphene-modified cement composites. J. Build. Eng. 2022, 57, 104880. [Google Scholar] [CrossRef]
- Chen, J.; Kou, S.-C.; Poon, C.-S. Hydration and properties of nano-TiO2 blended cement composites. Cem. Concr. Compos. 2012, 34, 642–649. [Google Scholar] [CrossRef]
- Fernandes, C.D.N.; Ferreira, R.L.; Bernardo, R.D.; Avelino, F.; Bertini, A.A. Using TiO2 nanoparticles as a SO2 catalyst in cement mortars. Constr. Build. Mater. 2020, 257, 119542. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Ling, T.-C.; Poon, C.S. Photocatalytic NOx degradation of concrete surface layers intermixed and spray-coated with nano-TiO2: Influence of experimental factors. Cem. Concr. Compos. 2017, 83, 279–289. [Google Scholar] [CrossRef]
- Maggos, T.; Plassais, A.; Bartzis, J.G.; Vasilakos, C.; Moussiopoulos, N.; Bonafous, L. Photocatalytic degradation of NOx in a pilot street canyon configuration using TiO2-mortar panels. Environ. Monit. Assess. 2008, 136, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Linkous, C.A.; Carter, G.J.; Locuson, D.B.; Ouellette, A.J.; Slattery, D.K.; Smitha, L.A. Photocatalytic Inhibition of Algae Growth Using TiO2, WO3, and Cocatalyst Modifications. Environ. Sci. Technol. 2000, 34, 4754–4758. [Google Scholar] [CrossRef]
- Rehman, A.U.; Kim, J.H.; Kim, H.G.; Qudoos, A.; Ryou, J.-S. Effect of leaching on the hardened, microstructural and self-cleaning characteristics of titanium dioxide containing cement mortars. Constr. Build. Mater. 2019, 207, 640–650. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Kumari, N.; Gaurav, K.; Samdarshi, S.; Bhattacharyya, A.; Paul, S.; Rajbongshi, B.; Mohanty, K. Dependence of photoactivity of niobium pentoxide (Nb2O5) on crystalline phase and electrokinetic potential of the hydrocolloid. Sol. Energy Mater. Sol. Cells 2020, 208, 110408. [Google Scholar] [CrossRef]
- Chebanenko, M.; Popkov, V.; Schröettner, H.; Sushnikova, A.; Rempel, A.; Valeeva, A. Sorption-photocatalytic performance of NbOx nanocrystals synthesized via heat-stimulated oxidation of niobium carbide. Appl. Surf. Sci. 2022, 582, 152422. [Google Scholar] [CrossRef]
- Ücker, C.L.; Goetzke, V.; Almeida, S.R.; Moreira, E.C.; Ferrer, M.M.; Jardim, P.L.; Moreira, M.L.; Raubach, C.W.; Cava, S. Photocatalytic degradation of rhodamine B using Nb2O5 synthesized with different niobium precursors: Factorial design of experiments. Ceram. Int. 2021, 47, 20570–20578. [Google Scholar] [CrossRef]
- Tamai, K.; Hosokawa, S.; Teramura, K.; Shishido, T.; Tanaka, T. Synthesis of niobium oxide nanoparticles with plate morphology utilizing solvothermal reaction and their performances for selective photooxidation. Appl. Catal. B Environ. 2016, 182, 469–475. [Google Scholar] [CrossRef]
- Alves, A.R.; Coutinho, A.D.R. The Evolution of the Niobium Production in Brazil. Mater. Res. 2015, 18, 106–112. [Google Scholar] [CrossRef]
- Moreira, M.A.; Heitmann, A.P.; Bezerra, A.C.; Patrício, P.S.; de Oliveira, L.C.; Castro, C.S.; de Souza, P.P. Photocatalytic performance of cementitious materials with addition of red mud and Nb2O5 particles. Constr. Build. Mater. 2020, 259, 119851. [Google Scholar] [CrossRef]
- Reches, Y.; Thomson, K.; Helbing, M.; Kosson, D.S.; Sanchez, F. Agglomeration and reactivity of nanoparticles of SiO2, TiO2, Al2O3, Fe2O3, and clays in cement pastes and effects on compressive strength at ambient and elevated temperatures. Constr. Build. Mater. 2018, 167, 860–873. [Google Scholar] [CrossRef]
- Sun, J.; Cao, X.; Xu, Z.; Yu, Z.; Zhang, Y.; Hou, G.; Shen, X. Contribution of core/shell TiO2@SiO2 nanoparticles to the hydration of Portland cement. Constr. Build. Mater. 2020, 233, 117127. [Google Scholar] [CrossRef]
- Sun, J.; Tian, L.; Yu, Z.; Zhang, Y.; Li, C.; Hou, G.; Shen, X. Studies on the size effects of nano-TiO2 on Portland cement hydration with different water to solid ratios. Constr. Build. Mater. 2020, 259, 120390. [Google Scholar] [CrossRef]
- Chinthakunta, R.; Ravella, D.P.; Chand, M.S.R.; Yadav, M.J. Performance evaluation of self-compacting concrete containing fly ash, silica fume and nano titanium oxide. Mater. Today Proc. 2020, 43, 2348–2354. [Google Scholar] [CrossRef]
- Ratan, J.K.; Saini, A. Enhancement of photocatalytic activity of self-cleaning cement. Mater. Lett. 2019, 244, 178–181. [Google Scholar] [CrossRef]
- Praveenkumar, T.R.; Vijayalakshmi, M.M.; Meddah, M.S. Strengths and durability performances of blended cement concrete with TiO2 nanoparticles and rice husk ash. Constr. Build. Mater. 2019, 217, 343–351. [Google Scholar] [CrossRef]
- Jin, Q.; Saad, E.; Zhang, W.; Tang, Y.; Kurtis, K. Quantification of NOx uptake in plain and TiO2-doped cementitious materials. Cem. Concr. Res. 2019, 122, 251–256. [Google Scholar] [CrossRef]
- Ren, J.; Lai, Y.; Gao, J. Exploring the influence of SiO2 and TiO2 nanoparticles on the mechanical properties of concrete. Constr. Build. Mater. 2018, 175, 277–285. [Google Scholar] [CrossRef]
- Sun, J.; Xu, K.; Shi, C.; Ma, J.; Li, W.; Shen, X. Influence of core/shell TiO2@SiO2 nanoparticles on cement hydration. Constr. Build. Mater. 2017, 156, 114–122. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.; Dionísio, A. Physical-mechanical properties of mortars with addition of TiO2 nanoparticles. Constr. Build. Mater. 2017, 148, 261–272. [Google Scholar] [CrossRef]
- Ma, B.; Li, H.; Li, X.; Mei, J.; Lv, Y. Influence of nano-TiO2 on physical and hydration characteristics of fly ash–cement systems. Constr. Build. Mater. 2016, 122, 242–253. [Google Scholar] [CrossRef]
- Bost, P.; Regnier, M.; Horgnies, M. Comparison of the accelerating effect of various additions on the early hydration of Portland cement. Constr. Build. Mater. 2016, 113, 290–296. [Google Scholar] [CrossRef]
- Jiang, T.; Cui, K.; Chang, J. Development of low-carbon cement: Carbonation of compounded C2S by β-C2S and γ-C2S. Cem. Concr. Compos. 2023, 139, 105071. [Google Scholar] [CrossRef]
- De Larrard, F.; Ferraris, C.F.; Sedran, T. Fresh concrete: A Herschel-Bulkley material. Mater. Struct. 1996, 31, 494–498. [Google Scholar] [CrossRef]
- ASTM C1231/C1231M; Standard Practice for Use of Unbonded Caps in Determination of Compressive Strength of Hardened Cylindrical Concrete Specimens. American Society for Testing and Materials: West Conshohocken, PA, USA, 2015.
- Reddy, G.R.; Chennakesavulu, K. Synthesis and characterization of Nb2O5 supported Pd(II)@SBA15: Catalytic activity towards oxidation of benzhydrol and Rhodamine-B. J. Mol. Struct. 2014, 1075, 406–412. [Google Scholar] [CrossRef]
- Byun, J.M.; Choi, H.R.; Kim, Y.D.; Sekino, T.; Kim, S.H. Photocatalytic activity under UV/Visible light range of Nb-doped titanate nanostructures synthesized with Nb oxide. Appl. Surf. Sci. 2017, 415, 126–131. [Google Scholar] [CrossRef]
- Nussbaum, M.; Shaham-Waldmann, N.; Paz, Y. Synergistic photocatalytic effect in Fe,Nb-doped BiOCl. J. Photochem. Photobiol. A Chem. 2014, 290, 11–21. [Google Scholar] [CrossRef]
- Xu, D.; Ma, H. Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Kirchheim, A.P.; Fernàndez-Altable, V.; Monteiro, P.J.M.; Molin, D.C.C.D.; Casanova, I. Analysis of cubic and orthorhombic C3A hydration in presence of gypsum and lime. J. Mater. Sci. 2009, 44, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Jing, R.; Yan, P. The interaction of sodium citrate and polycarboxylate-based superplasticizer on the rheological properties and viscoelasticity of cement-based materials. Constr. Build. Mater. 2021, 293, 123466. [Google Scholar] [CrossRef]
- Silvestro, L.; Lima, G.T.D.S.; Ruviaro, A.S.; Mezalira, D.Z.; Gleize, P.J.P. Effect of Multiwalled Carbon Nanotube Functionalization with 3-Aminopropyltriethoxysilane on the Rheology and Early-Age Hydration of Portland Cement Pastes. J. Mater. Civ. Eng. 2022, 34, 04022176. [Google Scholar] [CrossRef]
- Roussel, N.; Ovarlez, G.; Garrault, S.; Brumaud, C. The origins of thixotropy of fresh cement pastes. Cem. Concr. Res. 2012, 42, 148–157. [Google Scholar] [CrossRef]
- Jakob, C.; Jansen, D.; Ukrainczyk, N.; Koenders, E.; Pott, U.; Stephan, D. Neubauer, Relating Ettringite Formation and Rheological Changes during the Initial Cement Hydration: Rheological Measurements and Modeling. Materials 2019, 12, 2957. [Google Scholar] [CrossRef]
- Neto, J.D.S.A.; De la Torre, A.G.; Kirchheim, A.P. Effects of sulfates on the hydration of Portland cement—A review. Constr. Build. Mater. 2021, 279, 122428. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Understanding the Filler Effect on the Nucleation and Growth of C-S-H. J. Am. Ceram. Soc. 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- de Matos, P.; Zat, T.; Corazza, K.; Fensterseifer, E.; Sakata, R.; Mohamad, G.; Rodríguez, E. Effect of TiO2 Nanoparticles on the Fresh Performance of 3D-Printed Cementitious Materials. Materials 2022, 15, 3896. [Google Scholar] [CrossRef]
- Silvestro, L.; Ruviaro, A.; Lima, G.; de Matos, P.; de Azevedo, A.R.G.; Monteiro, S.N.; Gleize, P. Influence of Ultrasonication of Functionalized Carbon Nanotubes on the Rheology, Hydration, and Compressive Strength of Portland Cement Pastes. Materials 2021, 14, 5248. [Google Scholar] [CrossRef]
- Senff, L.; Tobaldi, D.M.; Lemes-Rachadel, P.; Labrincha, J.A.; Hotza, D. The influence of TiO2 and ZnO powder mixtures on photocatalytic activity and rheological behavior of cement pastes. Constr. Build. Mater. 2014, 65, 191–200. [Google Scholar] [CrossRef]
- Júnior, L.U.T.; de Matos, P.R.; Lima, G.S.; Silvestro, L.; Rocha, J.C.; Campos, C.E.; Gleize, P.J. Effect of the nanosilica source on the rheology and early-age hydration of calcium sulfoaluminate cement pastes. Constr. Build. Mater. 2022, 327, 126942. [Google Scholar] [CrossRef]
- Lima, G.T.D.S.; Zaleski, A.; Júnior, L.U.D.T.; Rocha, J.C.; Pelisser, F.; Gleize, P.J.P. Evaluation of the effect of nanosilica and recycled fine aggregate in Portland cement rendering mortars. Rev. IBRACON Estruturas Mater. 2022, 15, e15509. [Google Scholar] [CrossRef]
- Sikora, P.; Chung, S.-Y.; Liard, M.; Lootens, D.; Dorn, T.; Kamm, P.H.; Stephan, D.; Elrahman, M.A. The effects of nanosilica on the fresh and hardened properties of 3D printable mortars. Constr. Build. Mater. 2021, 281, 122574. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A Mechanism for Non-Newtonian Flow in Suspensions of Rigid Spheres. Trans. Soc. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Scolaro, T.P.; Silvestro, L.; Ruviaro, A.S.; de Azevedo, A.R.G.; Monteiro, S.N.; Pelisser, F. Effect of Ornamental Stone Waste Incorporation on the Rheology, Hydration, Microstructure, and CO2 Emissions of Ordinary Portland Cement. Materials 2022, 15, 401. [Google Scholar] [CrossRef]
- de Matos, P.R.; Sakata, R.D.; Onghero, L.; Uliano, V.G.; de Brito, J.; Campos, C.E.; Gleize, P.J. Utilization of ceramic tile demolition waste as supplementary cementitious material: An early-age investigation. J. Build. Eng. 2021, 38, 102187. [Google Scholar] [CrossRef]
- Meng, T.; Yu, Y.; Qian, X.; Zhan, S.; Qian, K. Effect of nano-TiO2 on the mechanical properties of cement mortar. Constr. Build. Mater. 2012, 29, 241–245. [Google Scholar] [CrossRef]
- Sobhy, C.S.; Tawfik, T.A.; El Hafez, G.A.; Faried, A.S. Insights on the influence of nano-Titanium dioxide and nano-Zinc oxide on mechanical properties and inhibiting of steel reinforcement. Case Stud. Constr. Mater. 2022, 16, e01017. [Google Scholar] [CrossRef]
- Ren, J.; Luo, X.; Bai, R.; Pan, C.; Zhang, J. Pore characteristics of different phase in nano-modified concrete and their influences on the compressive strength. J. Build. Eng. 2021, 46, 103784. [Google Scholar] [CrossRef]
- Skoog, D.A.; West, D.M.; Holler, F.J.; Crouch, S.R. Fundamentals of Analytical Chemistry; Cengage Learning: Boston, MA, USA, 2014. [Google Scholar]
- Wolski, L.; Walkowiak, A.; Ziolek, M. Photo-assisted activation of H2O2 over Nb2O5—The role of active oxygen species on niobia surface in photocatalytic discoloration of Rhodamine B. Mater. Res. Bull. 2019, 118, 110530. [Google Scholar] [CrossRef]
- Marin, M.L.; Hallett-Tapley, G.L.; Impellizzeri, S.; Fasciani, C.; Simoncelli, S.; Netto-Ferreira, J.C.; Scaiano, J.C. Synthesis, acid properties and catalysis by niobium oxide nanostructured materials. Catal. Sci. Technol. 2014, 4, 3044–3052. [Google Scholar] [CrossRef]
- Skrodczky, K.; Antunes, M.M.; Han, X.; Santangelo, S.; Scholz, G.; Valente, A.A.; Pinna, N.; Russo, P.A. Niobium pentoxide nanomaterials with distorted structures as efficient acid catalysts. Commun. Chem. 2019, 2, 129. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O.; Kukhtik, V.I.; Alekseeva, V.I. Ionization and Tautomerism of Fluorescein, Rhodamine B, N,N-Diethylrhodol and Related Dyes in Mixed and Nonaqueous Solvents. Dyes Pigment. 1994, 24, 11–35. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O.; Vodolazkaya, N.A.; Doroshenko, A. Ionic Equilibria of Fluorophores in Organized Solutions: The Influence of Micellar Microenvironment on Protolytic and Photophysical Properties of Rhodamine B. J. Fluoresc. 2003, 13, 235–248. [Google Scholar] [CrossRef]
- Yousefi, A.; Allahverdi, A.; Hejazi, P. Effective dispersion of nano-TiO2 powder for enhancement of photocatalytic properties in cement mixes. Constr. Build. Mater. 2013, 41, 224–230. [Google Scholar] [CrossRef]
- Verma, P.; Samanta, S.K.; Mishra, S. Photon-independent NaOH/H2O2–based degradation of rhodamine-B dye in aqueous medium: Kinetics, and impacts of various inorganic salts, antioxidants, and urea. J. Environ. Chem. Eng. 2020, 8, 103851. [Google Scholar] [CrossRef]



| Reference | Cementitious Matrix | Dispersion Method | TiO2 | |||
|---|---|---|---|---|---|---|
| Type | w/c | Type | Duration | Type | Content | |
| [5] | concrete | 0.51 | high-speed stirrer | 5 min | replacement | 0.5–2.0% |
| [6] | paste | 0.425 | magnetic stirrer | - | replacement | 0.5–1.5% |
| [7] | paste | 0.3 | sonication | 30 min | addition | 0.1–1.0% |
| [24] | paste | 0.5 | sonication | 3 min | replacement | 1.0% |
| [25] | paste | 0.3–0.5 | - | - | replacement | 0.5–2.0% |
| [10] | mortar | 0.64–0.68 | - | - | replacement | 2.5–10.0% |
| [26] | concrete | 0.38 | sonication | - | addition | 1.0–3.0% |
| [27] | paste | - | - | - | replacement | 3.0% |
| [14] | mortar | 0.4 | sonication | 30 min | replacement | 1.5–6.0% |
| [28] | concrete | 0.41 | - | - | replacement | 1.0–5.0% |
| [29] | paste | 0.4 | handheld electric mixer | 1 min | addition | 5.0% |
| [30] | concrete | 0.4 | sonication | 30 min | replacement | 1.0–5.0% |
| [31] | paste | 0.5 | sonication | 3 min | replacement | 1.0% |
| [32] | mortar | 0.45 | - | - | addition | 1.0–5.0% |
| [33] | mortar | 0.5 | sonication | 30 min | replacement | 1.0–3.0% |
| [34] | paste | 0.45 | - | - | addition | 1.0–10.0% |
| [9] | paste | 0.35 | - | - | addition | 5.0–10.0% |
| WC | |
|---|---|
| Chemical composition (%) | |
| Al2O3 | 2.82 |
| SiO2 | 22.83 |
| Fe2O3 | 0.15 |
| CaO | 68.48 |
| MgO | 0.25 |
| SO3 | 2.37 |
| TiO2 | 0.12 |
| Loss on ignition | 2.98 |
| Physical properties | |
| Specific Gravity (g/cm3) | 3.14 |
| SSA (m2/g) | 1.34 |
| D10 (µm) | 3.30 |
| D50 (µm) | 15.48 |
| D90 (µm) | 34.48 |
| Average diameter (µm) | 16.38 |
| Cement Paste | White Cement (g) | Nb2O5 (g) | Water (g) | Energy (J) |
|---|---|---|---|---|
| 0.0% Nb | 200.00 | 0.00 | 80.00 | - |
| 0.5% Nb | 199.00 | 1.00 | 80.00 | 59,218.00 |
| 1.0% Nb | 198.00 | 2.00 | 80.00 | 58,314.00 |
| 1.5% Nb | 197.00 | 3.00 | 80.00 | 57,283.00 |
| 2.0% Nb | 196.00 | 4.00 | 80.00 | 56,072.00 |
| Degrees of Freedom (DF) | Sum of Squares (SS) | Mean Square (MS) | F Value | p Value | Sig a | |
|---|---|---|---|---|---|---|
| Nb content | 4 | 139.9263 | 34.9815 | 5.1518 | 0.00324 | S |
| Age | 1 | 5063.0015 | 5063.0015 | 745.6459 | 0.00000 | S |
| Interaction | 4 | 156.3748 | 39.0937 | 5.7574 | 0.00175 | S |
| Error | 27 | 183.3323 | 6.7900 | - | - | - |
| Total | 36 | 5793.4570 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestro, L.; Maroli, C.; Koch, B.; Ruviaro, A.S.; Lima, G.; Kempka, M.; Marin, C.F.d.F.; Mezalira, D.Z.; Gleize, P.J.P. Assessing the Rheological, Mechanical, and Photocatalytic Properties of Niobium Oxide-Incorporated White Cement Pastes. Materials 2023, 16, 4090. https://doi.org/10.3390/ma16114090
Silvestro L, Maroli C, Koch B, Ruviaro AS, Lima G, Kempka M, Marin CFdF, Mezalira DZ, Gleize PJP. Assessing the Rheological, Mechanical, and Photocatalytic Properties of Niobium Oxide-Incorporated White Cement Pastes. Materials. 2023; 16(11):4090. https://doi.org/10.3390/ma16114090
Chicago/Turabian StyleSilvestro, Laura, Caroline Maroli, Brenda Koch, Artur Spat Ruviaro, Geannina Lima, Mariane Kempka, Camila Fabiano de Freitas Marin, Daniela Zambelli Mezalira, and Philippe Jean Paul Gleize. 2023. "Assessing the Rheological, Mechanical, and Photocatalytic Properties of Niobium Oxide-Incorporated White Cement Pastes" Materials 16, no. 11: 4090. https://doi.org/10.3390/ma16114090
APA StyleSilvestro, L., Maroli, C., Koch, B., Ruviaro, A. S., Lima, G., Kempka, M., Marin, C. F. d. F., Mezalira, D. Z., & Gleize, P. J. P. (2023). Assessing the Rheological, Mechanical, and Photocatalytic Properties of Niobium Oxide-Incorporated White Cement Pastes. Materials, 16(11), 4090. https://doi.org/10.3390/ma16114090





