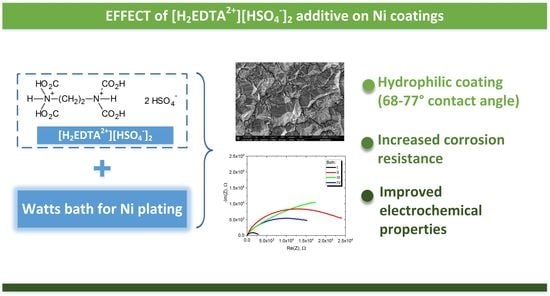
Effect of Versenium Hydrogensulfate on Properties of Nickel Coatings
Abstract
1. Introduction
2. Materials and Methods
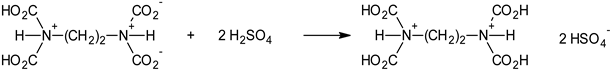
3. Results
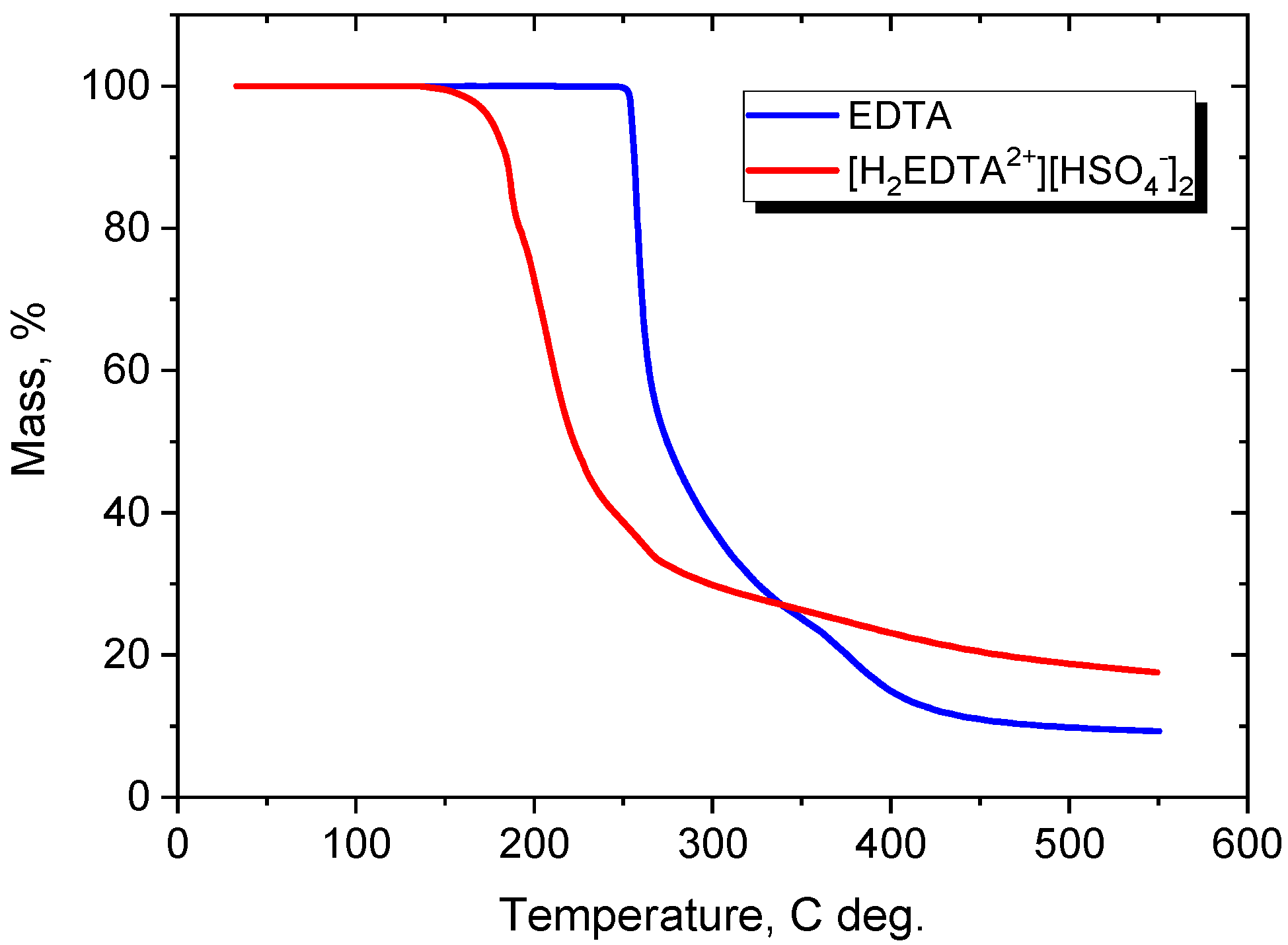
3.1. Characterization of the Synthesized Versenium Salt
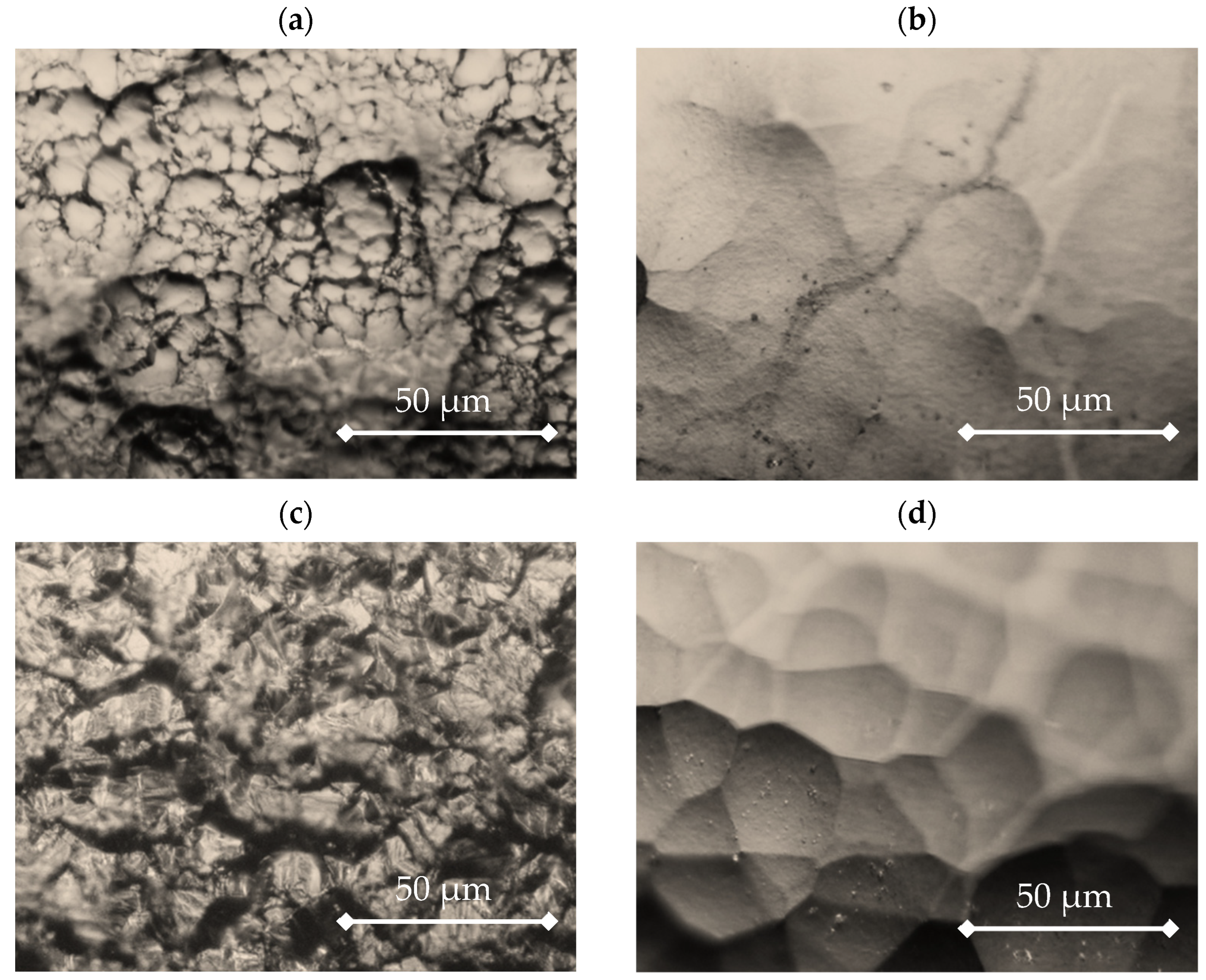
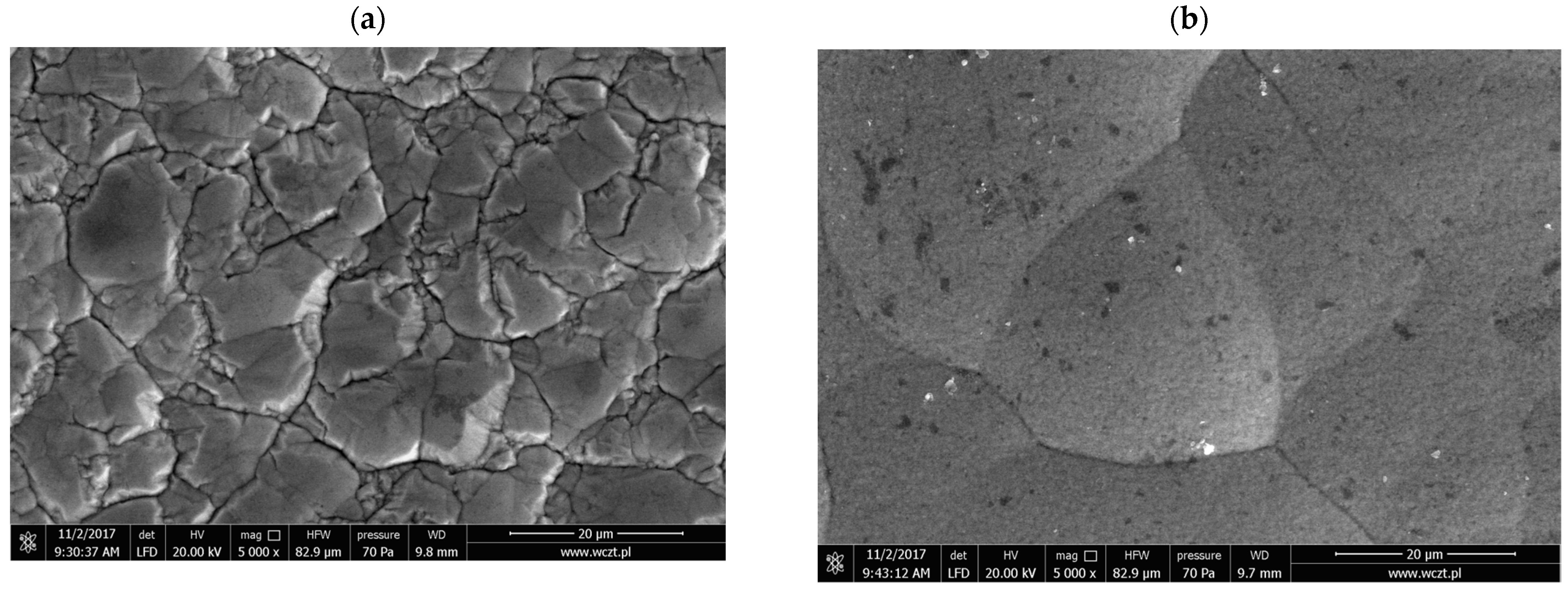
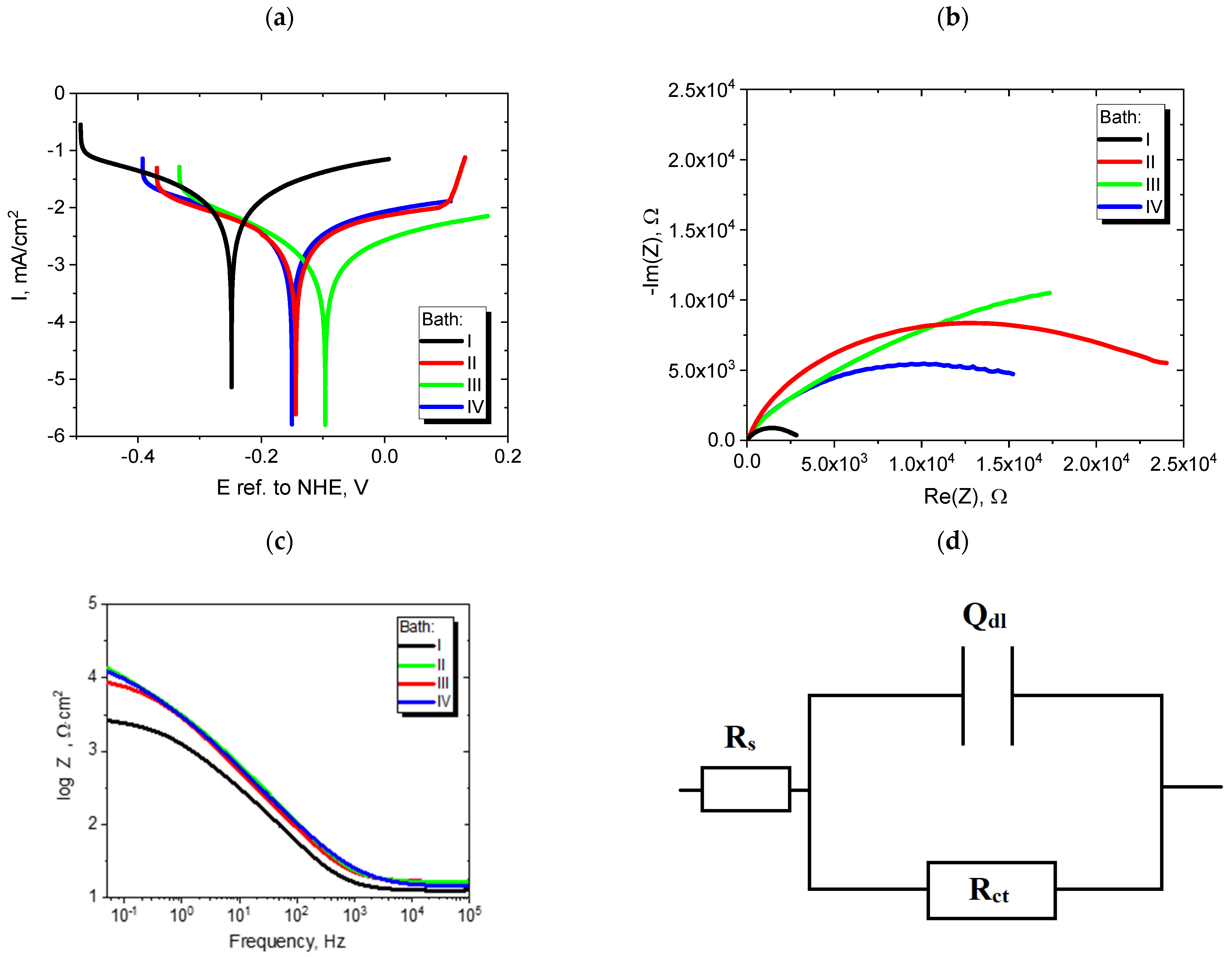
3.2. Nickel Plating from Modified Baths
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubel, E.; Tomassi, P.; Ziółkowski, J. Best Available Techniques (BAT)—Guidelines for the Surface Treatment of Metals and Plastics (Najlepsze Dostępne Techniki (BAT)—Wytyczne Dla Powierzchniowej Obróbki Metali i Tworzyw Sztucznych); Ministry of the Environment: Warsaw, Poland, 2009.
- Schlesinger, M.; Paunovic, M. (Eds.) Modern Electroplating; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; ISBN 9780470602638. [Google Scholar]
- Rose, I.; Whittington, C. Nickel Plating Handbook; Nickel Institute: Durham, NC, USA, 2013; ISBN 9783527813964. [Google Scholar]
- Ciszewski, A.; Posluszny, S.; Milczarek, G.; Baraniak, M. Effects of Saccharin and Quaternary Ammonium Chlorides on the Electrodeposition of Nickel from a Watts-Type Electrolyte. Surf. Coat. Technol. 2004, 183, 127–133. [Google Scholar] [CrossRef]
- Wojciechowski, J.; Baraniak, M.; Pernak, J.; Lota, G. Nickel Coatings Electrodeposited from Watts Type Baths Containing Quaternary Ammonium Sulphate Salts. Int. J. Electrochem. Sci. 2017, 12, 3350–3360. [Google Scholar] [CrossRef]
- DuRose, A.H.; Heights, R.; Stern, R.L. Alkaline Nickel Plating Solutions. U.S. Patent 3,718,549A, 27 February 1973. [Google Scholar]
- Brown, H.; Tomaszewski, T.W. Article Coated with a Coelectrodeposit of Nickel and Plastic Particles, an Overlayer Thereon, and Method of Making Said Article. U.S. Patent 3,356,467A, 5 December 1967. [Google Scholar]
- Zhou, Z.; Ma, B.; Zhang, X.; Tang, L.; Lin, X.; Hu, C.; Ren, K. Enhanced Corrosion Resistance and Self-Cleaning Properties of Superhydrophobic Nickel Coating Fabricated by One-Step Electrodeposition. Ceram. Int. 2023, 49, 13109–13118. [Google Scholar] [CrossRef]
- Xiang, T.; Ding, S.; Li, C.; Zheng, S.; Hu, W.; Wang, J.; Liu, P. Effect of Current Density on Wettability and Corrosion Resistance of Superhydrophobic Nickel Coating Deposited on Low Carbon Steel. Mater. Des. 2017, 114, 65–72. [Google Scholar] [CrossRef]
- Naziri Mehrabani, S.A.; Ahmadzadeh, R.; Abdian, N.; Taghizadeh Tabrizi, A.; Aghajani, H. Synthesis of Ni-GO Nanocomposite Coatings: Corrosion Evaluation. Surf. Interfaces 2020, 20, 100546. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, L.; Tang, W.; Li, J. Effect of Graphene on Tribological Properties of Ni Based Composite Coatings Prepared by Oxidation Reduction Method. J. Mater. Res. Technol. 2020, 9, 3796–3804. [Google Scholar] [CrossRef]
- Tabish, M.; Malik, M.U.; Khan, M.A.; Yasin, G.; Asif, H.M.; Anjum, M.J.; Khan, W.Q.; Ibraheem, S.; Nguyen, T.A.; Slimani, Y.; et al. Construction of NiCo/Graphene Nanocomposite Coating with Bulges-like Morphology for Enhanced Mechanical Properties and Corrosion Resistance Performance. J. Alloys Compd. 2021, 867, 159138. [Google Scholar] [CrossRef]
- Makarova, I.; Dobryden, I.; Kharitonov, D.; Kasach, A.; Ryl, J.; Repo, E.; Vuorinen, E. Nickel-Nanodiamond Coatings Electrodeposited from Tartrate Electrolyte at Ambient Temperature. Surf. Coat. Technol. 2019, 380, 125063. [Google Scholar] [CrossRef]
- Nowak, M.; Mizera, J.; Klyszewski, A.; Dobkowska, A.; Boczkal, S.; Kozik, A.; Koprowski, P. Effect of Various Organic Additives in Galvanic Bath on Properties of Ni-SiC Composite Coatings. Arch. Metall. Mater. 2019, 64, 1327–1333. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ballantyne, A.; Harris, R.C.; Juma, J.A.; Ryder, K.S.; Forrest, G. A Comparative Study of Nickel Electrodeposition Using Deep Eutectic Solvents and Aqueous Solutions. Electrochim. Acta 2015, 176, 718–726. [Google Scholar] [CrossRef]
- Chu, C.M. The Effect of Complexing Agents on the Electrodeposition of Fe-Ni Powders. J. Chin. Inst. Chem. Eng. 2003, 34, 689–695. [Google Scholar]
- El Boraei, N.F.; Ibrahim, M.A.M. Catalytic Effect of L-Proline on the Reduction of Ni(II) Ions during Nickel Electrodeposition from a Watts-Type Nickel Bath. Surf. Coat. Technol. 2018, 347, 113–122. [Google Scholar] [CrossRef]
- El Sayed, M.A. Improving the Characteristics of Nickel Coatings Produced on Copper from Watts Bath in the Presence of Ascorbic Acid—Combined Experimental and Theoretical Study. Int. J. Electrochem. Sci. 2022, 17, 22044. [Google Scholar] [CrossRef]
- Cruz, A.C.; Hernández, L.S.; Gutiérrez, E.J. Cyanide-Free Copper-Silver Electroplated Coatings on Carbon Steel Exposed to 5% NaClO Bleacher. J. Mater. Eng. Perform. 2023, 32, 2432–2444. [Google Scholar] [CrossRef] [PubMed]
- Amer, J.; Al-Khawaja, S. Nickel Coated on Beech Wood by Electroless Nickel Coating: Effects of Complexing Agent Concentrations on Nickel Film Properties. Compos. Interfaces 2014, 21, 659–669. [Google Scholar] [CrossRef]
- Casella, I.G.; Spera, R. Electrochemical Deposition of Nickel and Nickel–Thallium Composite Oxides Films from EDTA Alkaline Solutions. J. Electroanal. Chem. 2005, 578, 55–62. [Google Scholar] [CrossRef]
- Mech, K. Influence of Organic Ligands on Electrodeposition and Surface Properties of Nickel Films. Surf. Coat. Technol. 2017, 315, 232–239. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, L.; Hu, C.; Liu, H.; Qu, J. Simultaneous Destruction of Nickel (II)-EDTA with TiO2/Ti Film Anode and Electrodeposition of Nickel Ions on the Cathode. Appl. Catal. B Environ. 2014, 144, 478–485. [Google Scholar] [CrossRef]
- Bordas, F.; Bourg, A.C.M. Effect of Complexing Agents (EDTA and ATMP) on the Remobilization of Heavy Metals from a Polluted River Sediment. Aquat. Geochem. 1998, 4, 201–214. [Google Scholar] [CrossRef]
- Abbott, A.P.; Frisch, G.; Ryder, K.S. Electroplating Using Ionic Liquids. Annu. Rev. Mater. Res. 2013, 43, 335–358. [Google Scholar] [CrossRef]
- Qibo, Z.; Yixin, H. Ionic Liquids as Electrodeposition Additives and Corrosion Inhibitors. In Progress and Developments in Ionic Liquids; Handy, S., Ed.; IntechOpen: Rijeka, Croatia, 2017; Ch. 7; ISBN 978-953-51-2902-8. [Google Scholar]
- Ohno, H. Electrochemical Aspects of Ionic Liquids; Wiley-Interscience: Hoboken, NJ, USA, 2011; ISBN 978-0-471-76252-2. [Google Scholar]
- Pernak, J.; Czepukowicz, A.; Poźniak, R. New Ionic Liquids and Their Antielectrostatic Properties. Ind. Eng. Chem. Res. 2001, 40, 2379–2383. [Google Scholar] [CrossRef]
- Omar, I.M.A.; Aziz, M.; Emran, K.M. Impact of Ionic Liquid [FPIM]Br on the Electrodeposition of Ni and Co from an Aqueous Sulfate Bath. J. Mater. Res. Technol. 2021, 12, 170–185. [Google Scholar] [CrossRef]
- Omar, I.M.A.; Al-Fakih, A.M.; Aziz, M.; Emran, K.M. Part II: Impact of Ionic Liquids as Anticorrosives and Additives on Ni-Co Alloy Electrodeposition: Experimental and DFT Study. Arab. J. Chem. 2021, 14, 102909. [Google Scholar] [CrossRef]
- Biegun, M.; Kaniewski, M.; Klem-Marciniak, E.; Hoffmann, J. Thermal Decomposition Characterization and Kinetics of Copper, Iron, Manganese and Zinc Chelates of Ethylenediaminetetraacetic Acid in Air Atmosphere. Thermochim. Acta 2022, 716, 179307. [Google Scholar] [CrossRef]
- Socha, J.; Weber, J.A. The Basics of Electrolytic Deposition of Metal Alloys (Podstawy Elektrolitycznego Osadzania Stopów Metali); Wydawnictwo Instytutu Mechaniki Precyzyjnej: Warsaw, Poland, 2001. [Google Scholar]
- Zhou, X.; Wang, Y.; Liang, Z.; Jin, H. Electrochemical Deposition and Nucleation/Growth Mechanism of Ni–Co–Y2O3 Multiple Coatings. Materials 2018, 11, 1124. [Google Scholar] [PubMed]
- Kung, C.H.; Sow, P.K.; Zahiri, B.; Mérida, W. Assessment and Interpretation of Surface Wettability Based on Sessile Droplet Contact Angle Measurement: Challenges and Opportunities. Adv. Mater. Interfaces 2019, 6, 1900839. [Google Scholar] [CrossRef]
- Thomas, R.R.; Brusic, V.A.; Rush, B.M. Correlation of Surface Wettability and Corrosion Rate for Benzotriazole-Treated Copper. J. Electrochem. Soc. 1992, 139, 678–685. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, M.; Mbugua, N.S.; Gbenontin, B.V.; Jin, M.; Zhu, J. Wettability, Microhardness, Wear and Corrosion Resistance of Ni–Co–P–BN(h)–Al2O3 Binary Nanocomposite Coatings Surface with Varying Long-Pulse Laser Parameters. Coatings 2021, 11, 1467. [Google Scholar] [CrossRef]

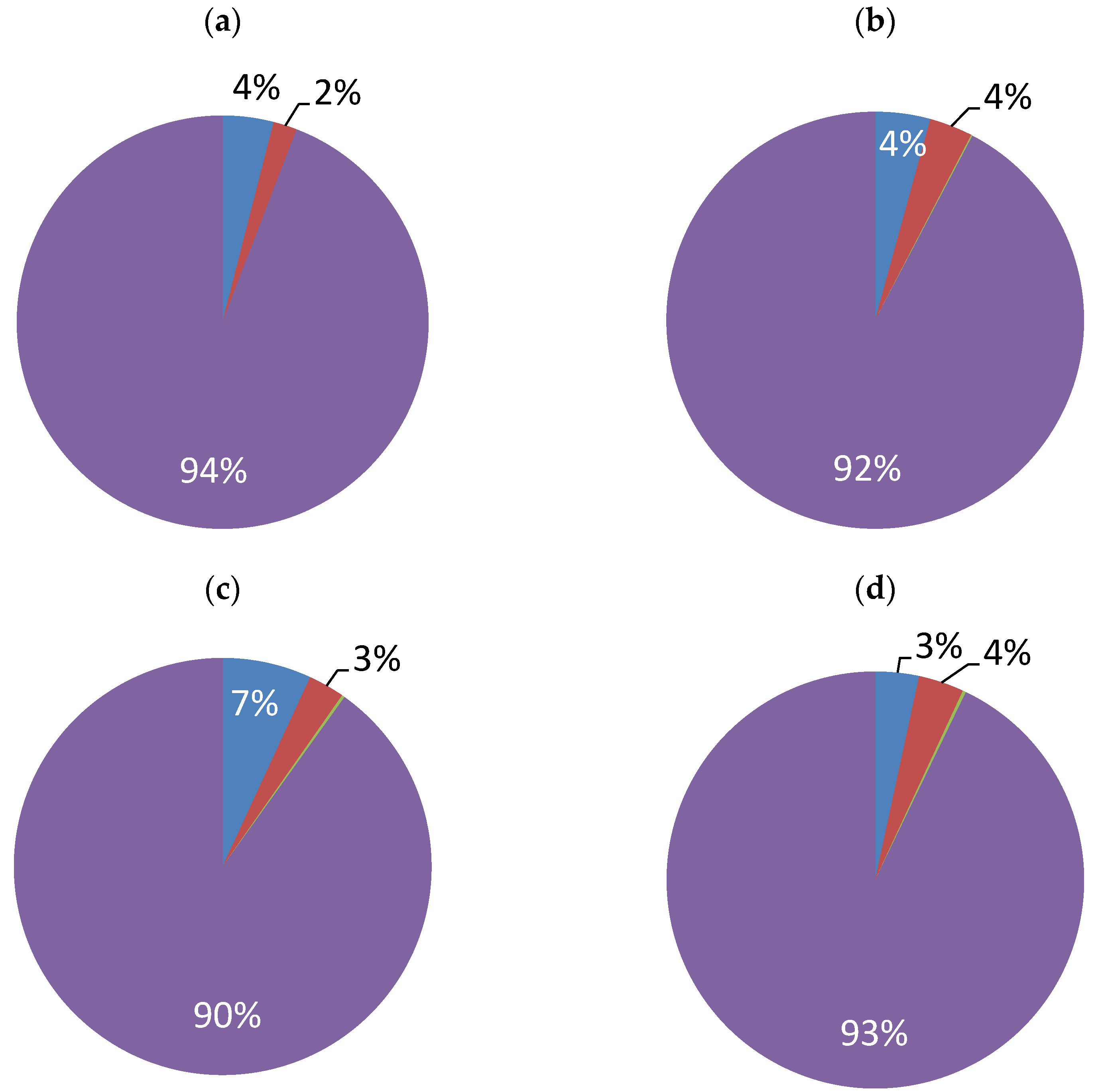
| Techniques/Parameter | Ni Coating from | ||||
|---|---|---|---|---|---|
| Bath I | Bath II | Bath III | Bath IV | ||
| Open circuit voltage | |||||
| E ref. to NHE | (mV) | −183 | −87 | −57 | −84 |
| Voltamperometry | |||||
| Ecor ref. to NHE | (mV) | −250 | −144 | −100 | −150 |
| Icor | (µA/cm2) | 12.89 | 2.70 | 1.35 | 2.94 |
| |βa| | (mV/dec) | 304 | 370 | 324 | 371 |
| |βc| | (mV/dec) | 283 | 272 | 201 | 271 |
| Corrosimetry | |||||
| Ecor ref. to NHE | (mV) | −244.0 | −89.5 | −78.6 | −134.5 |
| Icor | (µA/cm2) | 9.02 | 1.10 | 0.88 | 1.50 |
| Rp | (Ω∙cm2) | 2891 | 23,721 | 29,600 | 17,402 |
| Electrochemical impedance spectroscopy | |||||
| Rct | (Ω∙cm2) | 2997 | 27,299 | 42,193 | 19,454 |
| Qdl∙10−6 | (F∙s(α−1)) | 69.6 | 33.9 | 43.1 | 36.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baraniak, M.; Lota, G.; Wojciechowski, J.; Walkiewicz, F.; Regel-Rosocka, M. Effect of Versenium Hydrogensulfate on Properties of Nickel Coatings. Materials 2023, 16, 4101. https://doi.org/10.3390/ma16114101
Baraniak M, Lota G, Wojciechowski J, Walkiewicz F, Regel-Rosocka M. Effect of Versenium Hydrogensulfate on Properties of Nickel Coatings. Materials. 2023; 16(11):4101. https://doi.org/10.3390/ma16114101
Chicago/Turabian StyleBaraniak, Marek, Grzegorz Lota, Jarosław Wojciechowski, Filip Walkiewicz, and Magdalena Regel-Rosocka. 2023. "Effect of Versenium Hydrogensulfate on Properties of Nickel Coatings" Materials 16, no. 11: 4101. https://doi.org/10.3390/ma16114101
APA StyleBaraniak, M., Lota, G., Wojciechowski, J., Walkiewicz, F., & Regel-Rosocka, M. (2023). Effect of Versenium Hydrogensulfate on Properties of Nickel Coatings. Materials, 16(11), 4101. https://doi.org/10.3390/ma16114101