Abstract
Decapod crustaceans have tooth-like denticles on their claw fingers, which come into direct contact with predators and prey. Since the denticles are subject to more frequent and intense stress than other parts of the exoskeleton, they must be especially resistant to wear and abrasion. We clarified the mechanical resistance and tissue structure of the denticles arranged in a line on the fixed finger of the mud crab, which has huge claws. The denticles of the mud crab are small at the fingertip and become larger closer to the palm. The denticles have a twisted-plywood-pattern structure stacked parallel to the surface regardless of size, but the abrasion resistance strongly depends on the size of the denticles. Due to the dense tissue structure and calcification, the abrasion resistance increases as the denticle size increases, reaching its maximum at the denticle surface. The denticles of the mud crab have a tissue structure that prevents them from breaking when pinched. The high abrasion resistance of the large denticle surface is an essential feature for the frequent crushing of shellfish, which is the mud crab’s staple food. The characteristics and tissue structure of the claw denticles on the mud crab may provide ideas for developing stronger, tougher materials.
1. Introduction
The exoskeleton of crustaceans has a unique tissue structure that is not visible in man-made materials [1,2] and is superior in mechanical and functional properties [3,4]. Research on materials with excellent and new properties is being actively advanced by mimicking these structures [5,6,7,8,9,10].
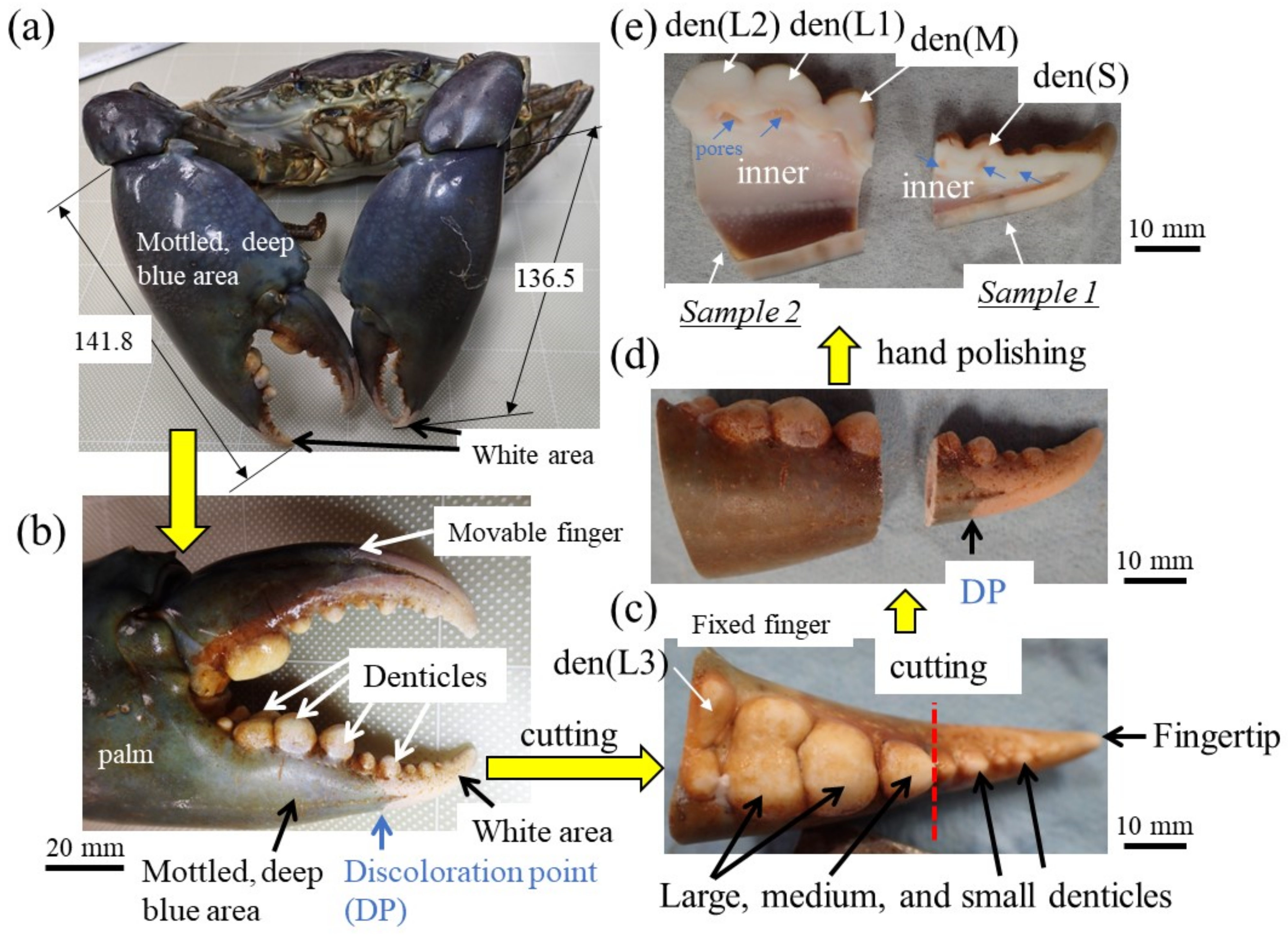
Among crustaceans, the mud crab Scylla serrata has huge claws that exceed its body size [11,12]. The exoskeleton surface of the mud crab is mottled deep blue, while the claw top’s finger surface and the denticles are white (Figure 1a,b). In the mottled, deep-blue exoskeleton, the hard exocuticle layer (exoC) was 1.5–3.0% of the exoskeleton thickness, and the exoskeleton was dominated by the endocuticle layer (endoC) with a twisted-plywood pattern structure (TPS) commonly found in crustacean exoskeletons [13]. The TPS is generally referred to as the Bouligand structure, which is found in many biological materials [1]. On the other hand, in the white exoskeleton at the fingertip, the tissue had a TPS throughout the thickness, but the rigid and dense exoC occupied more than half of the exoskeleton thickness, and the hardness changed gradually at the boundary between the exoC and endoC [14]. The proportion of the exoC in the white exoskeleton was much higher than that in the mottled, deep-blue exoskeleton and in other crustaceans, including the brown crab, Cancer pagurus [15], the Chinese mitten crab, Eriocheir sinensis [16], the Atlantic blue crab, Callinectes sapidus, the Mediterranean green crab, Carcinus aestuarii [17], the coconut crab, Birgus latro [18,19,20], and the American lobster, Homarus americanus [21]. When a mud crab pinches a relatively large prey (such as other crabs), the white part of the fingertip is used to pinch the hard body of the prey and crush it (Figure S1a in the Supplementary Materials). The white parts of the claws are also used to make a loud sound to intimidate enemies (Video S1 in the Supplementary Materials) and to protect the weaker area near the mouth that is not covered by the rigid exoskeleton (Figure S1b,c) [22]. In other words, the white parts play a special role in effectively utilizing the huge claws to enable the mud crab to survive in its habitat. On the other hand, when a mud crab pinches a small prey such as a shellfish, which is its staple food, the denticles on the pinching side are the first point of contact and where a large force acts (Figure S1d). Unlike the case with the exoskeleton of the carapace and the outer side of the claws, there are few studies examining the microstructures and mechanical properties of the denticles of the pinching side [11,23]. In particular, in the case of the mud crab, denticles on the claw finger are characterized by being small (small denticles) at the fingertip and becoming larger (medium and large denticles) closer to the palm, as shown in Figure 1b. The mud crab captures small prey (such as clams) by pinching them with the base of their claws and crushing them (Figure S1d). Since the role of the denticles is different from that of the mottled, deep-blue exoskeleton and the white part of the fingertip, the mechanical properties and tissue structure may also differ and depend on the size of the denticle. In this paper, the mechanical properties, tissue structure, and chemical components of small, medium, and large denticles are investigated, and the results are compared with those of the blue exoskeleton and the fingertip.
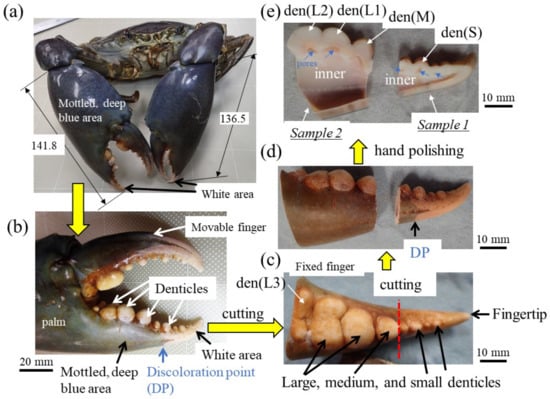
Figure 1.
(a) The mud crab and (b) its right claw used in the present study. Large, medium, and small denticles lined up on the claw finger and the procedure before automatic polishing: (c) the fixed finger, (d) samples 1 and 2, and (e) cross-sectional photos Here, den(S) and den(M) denote small and medium denticles and den(L1), den(L2), and den(L3) denote large denticles.
2. Materials and Methods
A large male mud crab (body weight 1265 g, carapace length 113.4 mm, carapace width 167.3 mm) with a rigid exoskeleton was obtained live from a local market in Naha, Okinawa, Japan. The crab was stored frozen at −18 °C to prevent natural decay processes and transported to our laboratory. The large right claw of 141.8 mm length, 66.5 mm width, and 39.5 mm thickness was removed (Figure 1a,b), and a sample was cut from the fixed finger with a handsaw (Figure 1c). The fixed finger was larger than the maximum mounting cup we have, so it was cut in two (samples 1 and 2) with a handsaw (Figure 1d) and hand polished until the cross sections of all denticles were exposed (Figure 1e). After the samples were dried for 48 h or more, the mounting cup in which the samples were set was filled with epoxy and left to cure at 23 °C for 12 h. To ensure penetration of the epoxy into the sample voids, the samples were placed under a vacuum for 600 s soon after the epoxy was added. After that, each sample was ground with SiC papers, polished with a 9, 3, and 1 μm diamond suspension, and finally polished with a 0.05 μm alumina suspension. The cross-sectional images of samples 1 and 2 were made by merging multiple optical micrograph images. Before observation using a scanning electron microscope (SEM), Vickers hardness and nanoindentation tests were performed.
The Vickers and nanoindentation tests were conducted using a Shimadzu Micro Vickers Hardness Tester, HMV-G31 (SHIMADZU, Kyoto, Japan), and an ENT-NEXUS (ELIONIX, Tokyo, Japan). The Vickers measurement was taken with the application of 98.07 mN for 15 s. Nanoindentation testing was conducted with a Berkovich diamond indenter with an angle of 115°. The loading curve consisted of a 5 s loading to 5 mN, followed by a 5 s hold at that force, and then a 5 s unloading. The tests were performed at an interval of 50 μm from an outer surface to an inner surface. The hardness (HIT) and reduced elastic modulus (Er) were analyzed from the unloading curve using the Oliver–Pharr method [24] employed in biological studies [14,15,19,20,21].
To characterize the microstructure and chemical compositions, two samples were coated with about 2 nm of osmium (Neo Osmium Coater, Meiwafosis Co., Ltd., Tokyo, Japan) before SEM observation. A focused ion beam (FIB)–SEM dual-beam instrument (Scios2, Thermo Fisher Scientific, Waltham, MA, USA) at an accelerating voltage (AC) of 2 kV and a secondary electron detector in a chamber or an in-lens annular back-scattered electron detector was used for the microstructure characterization. An energy-dispersive X-ray spectroscope (EDS) attached to this FIB–SEM instrument was applied for the compositional analysis. A large silicon-drift detector (Ultim Max 170, Oxford Instruments, Abingdon, Oxfordshire, UK) ensured high detecting efficiency and low statistical error in the quantitative analysis. The EDS analysis was used at an AC of 15 kV. Due to the influence of the final alumina suspension in the polishing process, Al remained on the sample surface, so Al was excluded from the EDS quantitative analysis.
3. Results and Discussion
3.1. Mechanical Properties
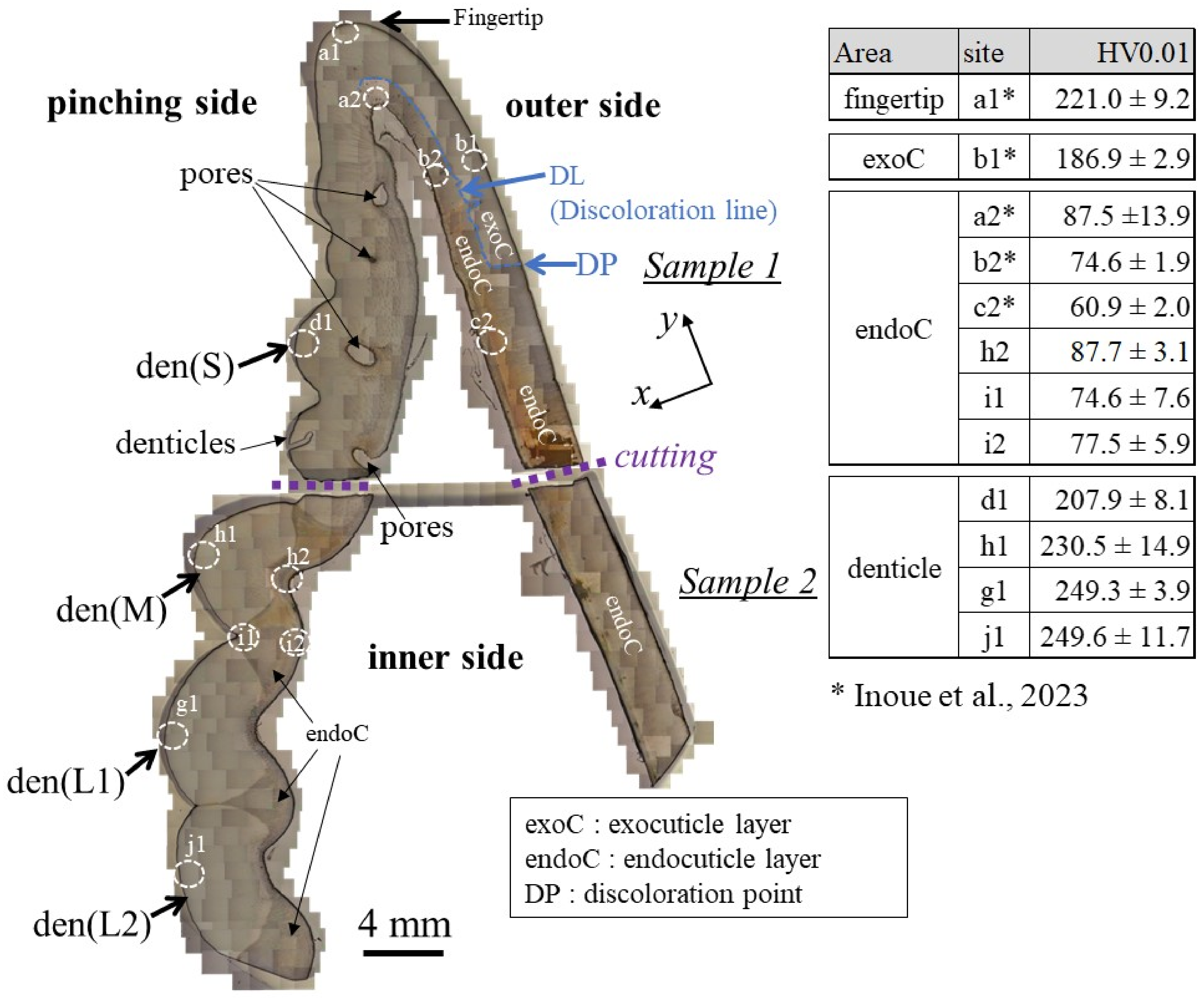
The cross-sections of samples 1 and 2 are shown in Figure 2. The areas of the mineralized claw exoskeleton are classified as the fingertip, exoC, endoC, and denticle. Pores are visible in the exoskeleton with the denticles in sample 1. In sample 2, which has a large denticle, these pores appear as constrictions in the exoskeleton. The pores just below the denticle have also been observed in the coconut crab exoskeleton [20,23]. The results of the Vickers hardness test at characteristic sites for these four areas are represented in the table in Figure 2. Here, the Vickers hardness, HV0.01, is the average value, including the standard deviation. The HV0.01 of the endoC of the pinching side (site h2, i1, and i2) and of the fingertip (site a2) is almost the same as that of the outer side (site b2 and c2). On the other hand, the denticle surfaces were harder than the exoC of the outer side (site b1) and the endoC. The medium denticle (site h1) and large denticles (site g1 and j1) were harder than the fingertip (site a1). The HV0.01 increased with the increase in denticle size. Namely, the hardness depended on the size of the denticle. The value, 250HV0.01, for the two large denticles is comparable to that for the exoC of the coconut crab claw [18] and the maximum value for the exoC of the American lobster claw [21].
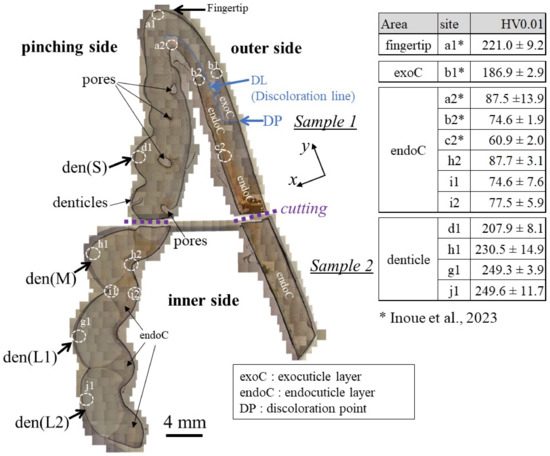
Figure 2.
Optical micrographs of a cross-section of the entire fixed finger of the claw after polishing and the hardness at four characteristic areas were obtained via Vickers tests. The tests were performed more than five times for each site, and the site is 100–200 μm away from the outer and inner surfaces. Here, den(S) and den(M) denote small and medium denticles, and den(L1) and den(L2) denote large denticles [14].
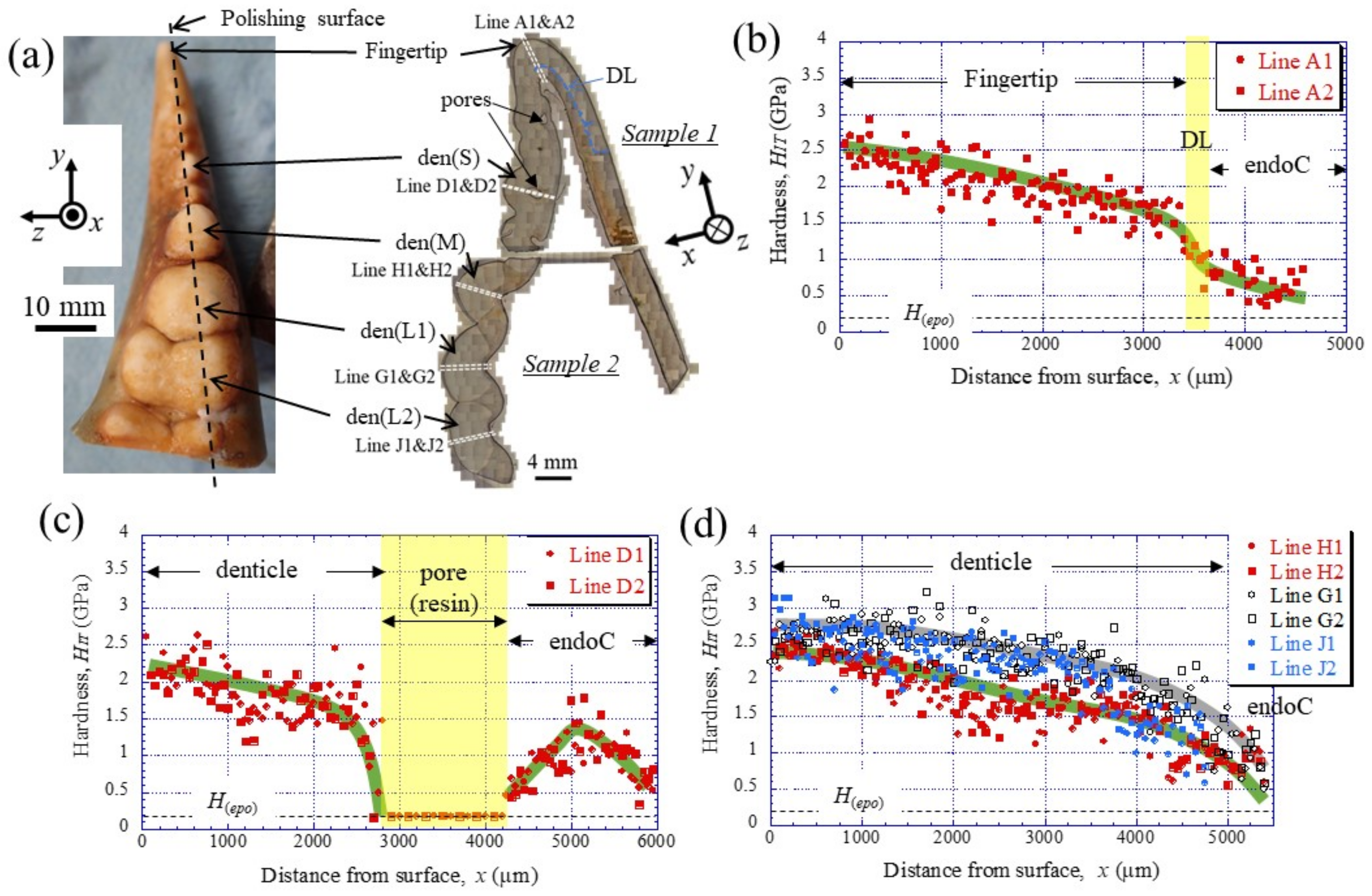
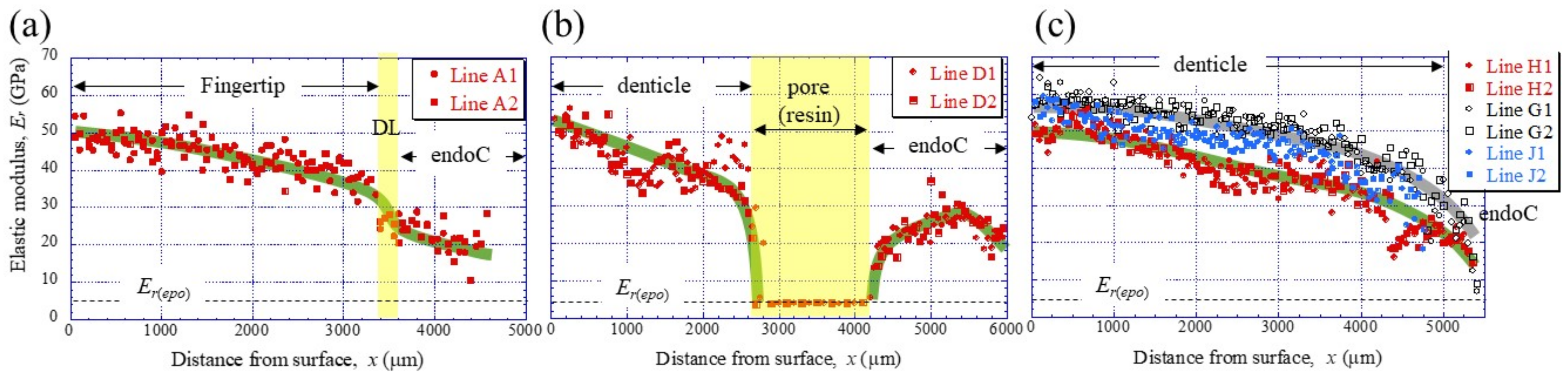
To clarify the denticle size dependence on hardness, the change in mechanical properties, HIT and Er, along the thickness from the outer surface to the inner surface on the pinching side, was investigated through nanoindentation testing. Figure 3c,d shows the distribution of HIT along lines D1, D2 (small denticle), lines H1, H2 (medium denticle), and lines G1, G2 and J1, J2 (large denticles). For comparison, the results for lines A1 and A2 of the fingertip are shown in Figure 3b. Here, the two lines are parallel and separated by more than 50 μm at each thickness. In the fingertip (Figure 3b), the HIT gradually decreased from 2.5 GPa, decreased abruptly at the discoloration line (DL), and then gradually decreased again. The HIT in the small denticle (Figure 3c) decreased gradually from 2–2.5 GPa and then decreased abruptly as it approached the pore. In the medium and large denticles (Figure 3d), the HIT gradually decreased until the inner surface, like the changes, until the DL of the fingertip. The HIT of the two large denticles (lines G1, G2, and J1, J2) was almost the same and larger than that of the medium denticle (lines H1, H2). The results for the Er, which showed changes similar to those for the HIT, are summarized in Figure 4.
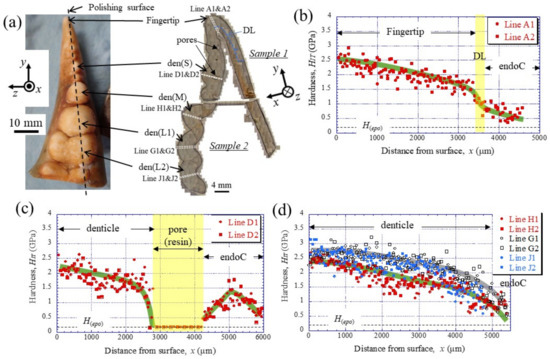
Figure 3.
(a) Appearance of the fixed finger after cutting and cross-sectional micrographs after polishing. Distributions of hardness, HIT, with distance from the outer surface, x, on (b) Line A (fingertip), (c) Line D (small denticle), and (d) Line H (medium denticle) and Lines G and J (large denticles) in the claw cross-section, obtained via nanoindentation tests. Here, H(epo) denotes the hardness of the cold epoxy resin, DL denotes the discoloration line, and endoC denotes the endocuticle.
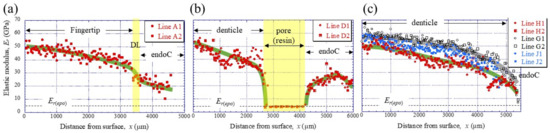
Figure 4.
Distributions of the reduced elastic modulus, Er, with x, on (a) Line A (fingertip), (b) Line D (small denticle), and (c) Line H (medium denticle) and Lines G and J (large denticles) in the claw cross-section, obtained using nanoindentation tests. Each line is shown in Figure 3a. Here, Er(epo) denotes the elastic modulus of the cold epoxy resin, DL denotes the discoloration line, and endoC denotes endocuticle.
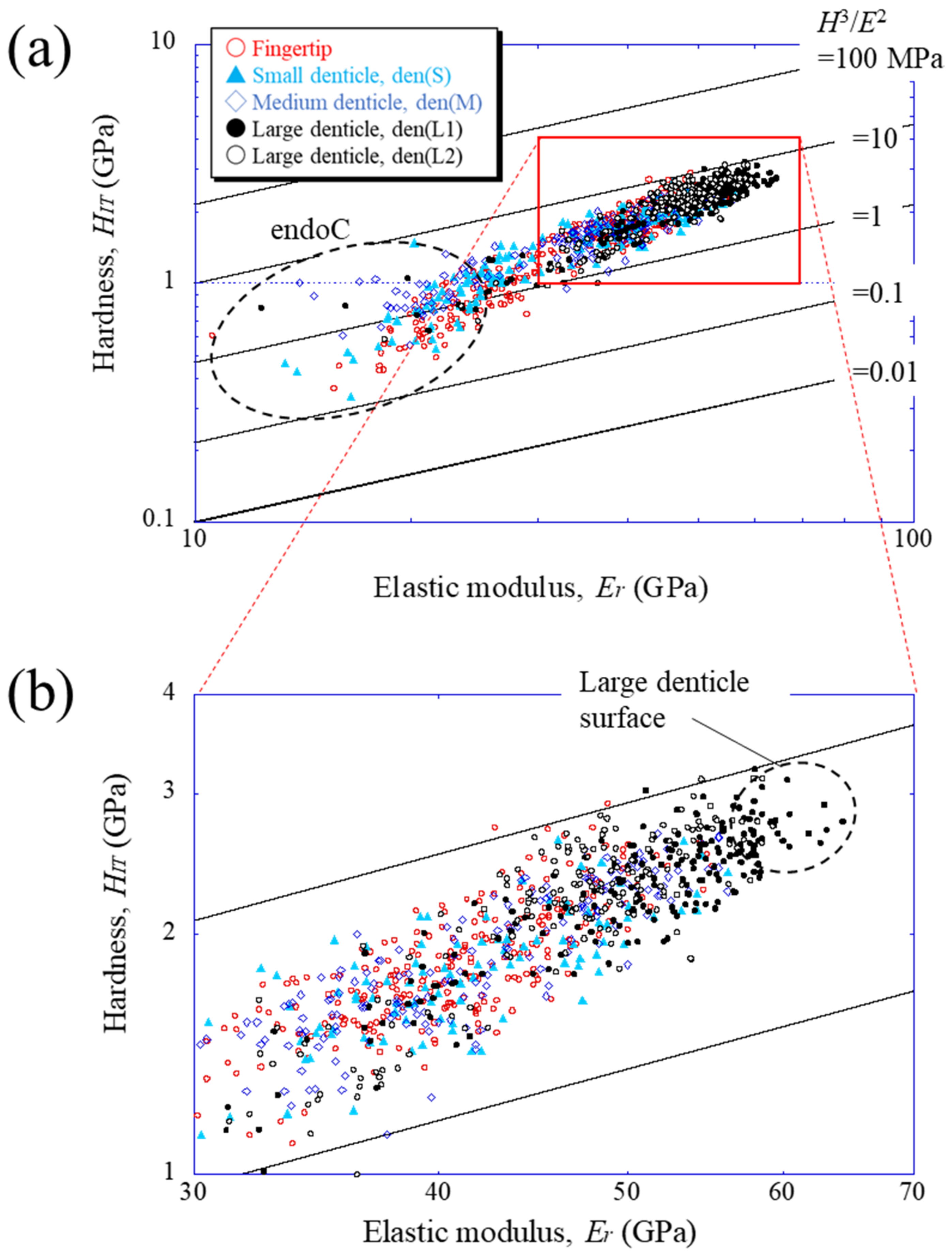
To compare the mechanical properties of each denticle, the results were plotted on a map based on the abrasion resistance [25] of materials. Figure 5 shows the property maps with data for the fingertip and all denticles. The abrasion resistance near the two large denticle surfaces is higher than that in other areas. This means that the two large denticles closer to the palm among the denticles arranged in a line on the claw finger are mainly used when the mud crab pinches and eats small prey, as shown in Figure S1d in the Supplementary Materials. These values, 2.3 < HIT < 3.2 GPa and 56 < Er < 65 GPa, are the limits of the local mechanical properties of the fixed finger of the mud crab’s claw, and the abrasion resistance is classified into materials with 3.3 < H3/E2< 9.0. This is higher than that in the data of all engineering polymers and is comparable to data of the hardest metallic alloys and even some of the softer ceramics [19,25]. The map reveals that the HIT-Er balance decreases from the outer surface to the inner side and approaches the balance of the endocuticle layer.
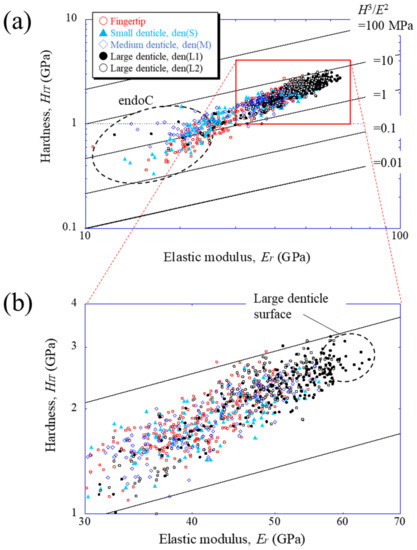
Figure 5.
(a) Property map for abrasion resistance on Line A (fingertip), Line D (small denticle), Line H (medium denticle), and Line G and J (large denticles), shown in Figure 3a, and (b) enlarged map. Here, data lying on a straight line of H3/E2 indicate materials with equivalent performances in abrasion resistance.
3.2. Microstructure
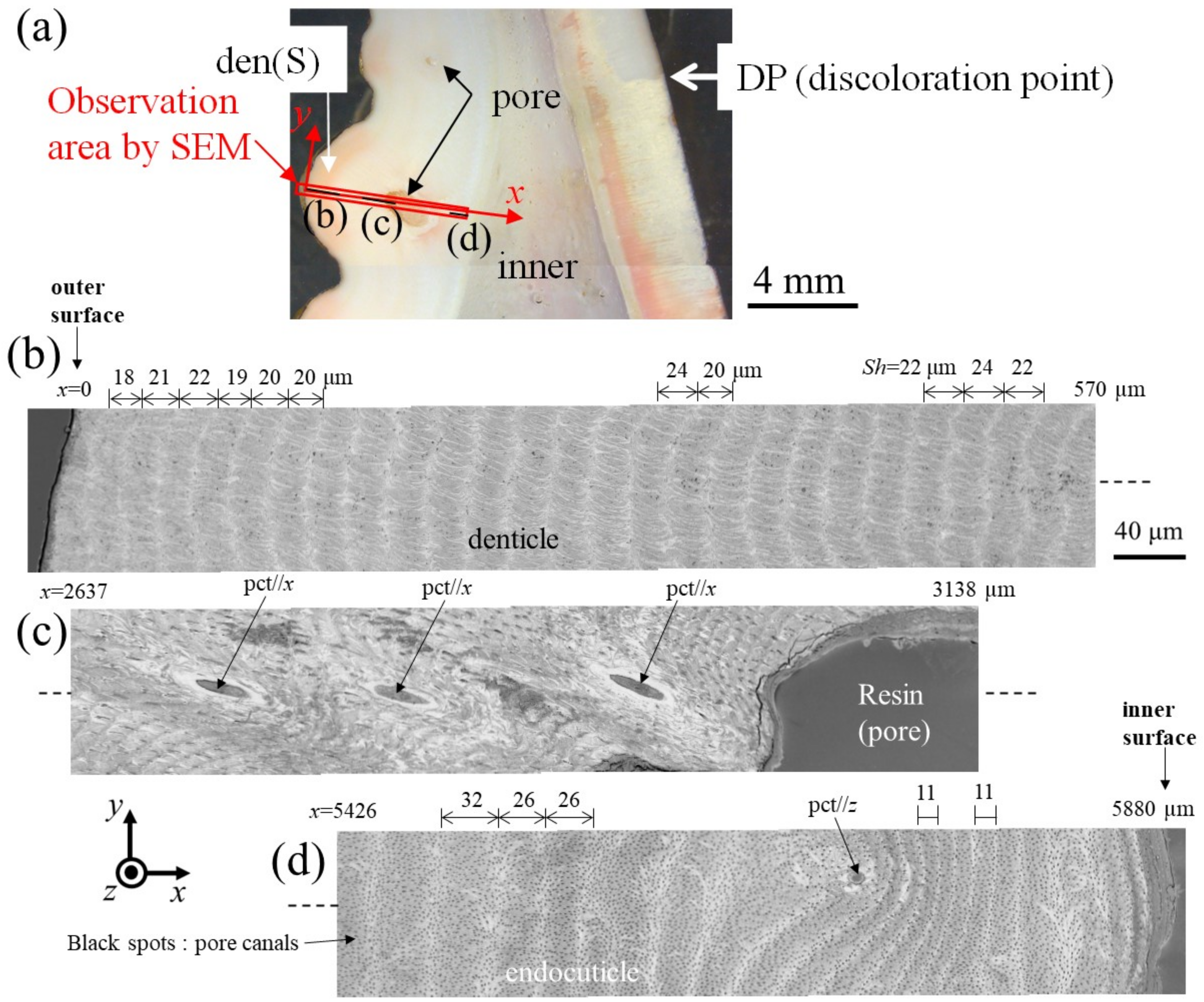
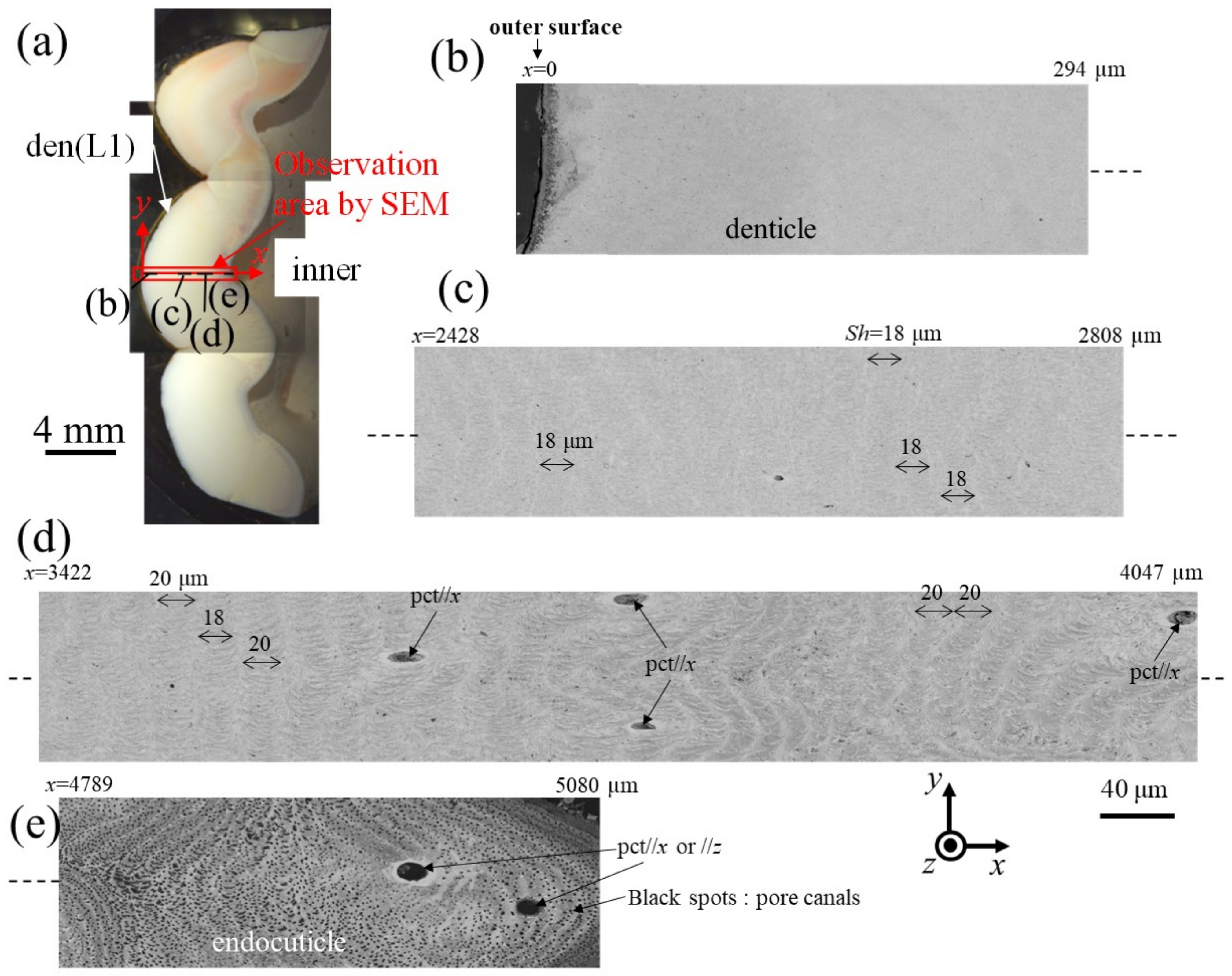
Figure 6 and Figure 7 show SEM micrographs near the outer surface, middle, and inner surface on Line D (small denticle, den(S)) and Line G (large denticle, den(L1)), shown in Figure 2. On Line D in Figure 6, the whole part of the exoskeleton with a 5880 μm thickness is striated parallel to the stacking height (Sh) of the TPS, and the Sh gradually decreases as it approaches the outer and inner surfaces. In particular, the Sh near the inner surface is very low, 11 μm or less. The Sh near the outer surface is 18–24 μm. Some pore-canal tubes (pcts)//x perpendicular to the outer surface were clearly observed near the pore filled with resin. The black spots in the endoC near the inner surface indicate pore canals (pcs). These black spots were also observed on the endoC on the outer side of the claw finger in the previous paper [14] and often can be observed on the polished surface of the exoskeleton with a TPS in crustaceans [1,18,20,23,26]. On the other hand, in Line G, shown in Figure 7, since the large denticle surface had a dense tissue structure, it was not possible to clearly observe the microstructure. The microstructures are visible from the middle to the inner surface and were striated with the Sh (18–20 µm) of the TPS, similar to Line D. Furthermore, many black spots were observed at the endoC near the inner surface.
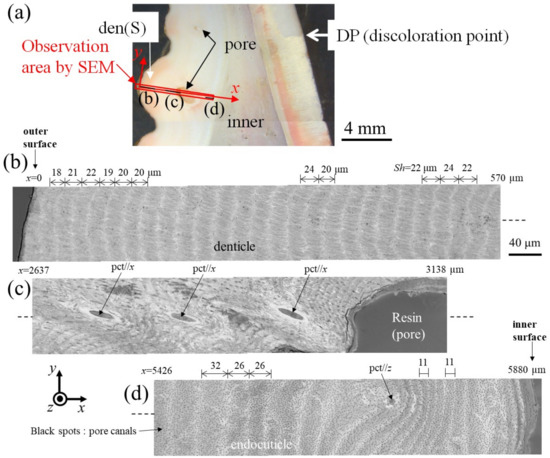
Figure 6.
(a) Stereomicrograph of the claw cross section (sample 1). SEM micrographs of Line D, including the small denticle (den(S)): (b) x = 0–570 μm, (c) x = 2637–3138 μm, and (d) x = 5426–5880 μm. Here, pct//x and pct//z denote pore canal tubules parallel to x and perpendicular to the x-y plane, and Sh denotes the stacking height of the twisted-plywood pattern structure.
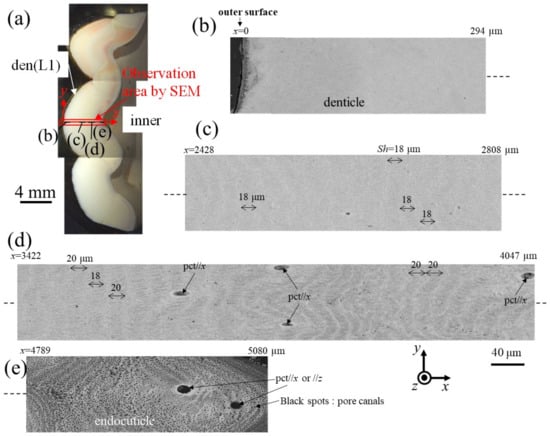
Figure 7.
(a) Stereomicrograph of the claw cross section (sample 2). SEM micrographs of Line G, including the large denticle (den(L1)): (b) x = 0–294 μm, (c) x = 2428–2808 μm, (d) x = 3422–4047 μm, and (e) x = 4789–5080 μm. Here, pct//x and pct//z denote pore canal tubules parallel to x and perpendicular to the x–y plane, and Sh denotes the stacking height of the twisted-plywood pattern structure.
3.3. Chemical Compositions
The EDS results for seven characteristic sites are summarized in Table 1. Calcium (Ca), magnesium (Mg), phosphorus (P), carbon (C), oxygen (O), and sodium (Na) were found to be the main components, and chloride and sulfur were present in minor amounts. This result was the same as that for the exoC in the mottled, deep-blue exoskeleton of the mud crab in the previous paper [13,14]. Here, the crystalline structures of the exoskeleton of the mud crab were calcite [14]. The minor components are found in the exoskeleton of the lobster, edible crab, and coconut crab [19,23,27]. The area scan results for inorganic matter (Ca, Mg, and P) for each site revealed differences in the compositions in the denticle, fingertip, and endoC. Curiously, the Na concentrations were almost identical within the exoskeleton, ranging from 0.7–1.0%. This amount is consistent with the result for the carapace of S. paramamosain [28], which belongs to the same genus. On the other hand, the Ca concentrations at site a1 on the fingertip and sites d1, g1, and g2 in the denticles are higher than those in the endoC (sites a2, d2, and g3), but the Mg concentrations are lower, and the P concentrations are zero. This trend is consistent with the results of Waugh et al. [11], which showed that the denticles contained less P than the surrounding cuticle of the mud crab’s claw and that the amount of MgCO3 present in the calcite was lower in the denticles than in the claw and carapace. The Ca concentration at site g1 of the large denticle surface is larger than that at site d1 of the small denticle surface and site a1 of the fingertip. In the large denticle, the Ca concentration decreases as it approaches the inner side. This reflects the gradual change in mechanical properties from the outer surface to the inner surface, as shown in Figure 3c,d and Figure 4c,d.

Table 1.
EDS area scan results at seven areas of the samples. Here, the results show the average weight % of calcium (Ca), magnesium (Mg), phosphorus (P), carbon (C), oxygen (O), sodium (Na), chlorine (Cl), and sulfur (S).
3.4. Denticles Arranged in a Line on the Claw Finger
The denticles have the same TPS as the endoC [13,14] and are described as a bulge in the endoC. For crustaceans, the denticles on the pinching side exist for the specialized function of pinching and crushing prey like teeth, as compared to the outer exoskeleton that exists to protect the body. Hence, it is necessary for the denticles to have high abrasion resistance and to disperse the force applied to the claw when pinching. In the mud crab, the denticles are arranged in a line on the fixed fingers of the claws, and their sizes become larger closer to the palm (Figure 1). This feature was common to all mud crabs sold at the local market in Naha and to those reported in the literature [11]. The mechanical properties increased with the increase in denticle size and reached their maximum at the denticle surface (Figure 3, Figure 4 and Figure 5). This was due to the dense tissue structure and calcification.
The mud crab inhabits the sandy bottom of relatively shallow inner bays [12,13,22]. The claw with denticles arranged in a line on the finger is found in the blue crab, Callinectes sapidus [11,17], and the green crab, Carcinus maenas [17], of the same Portunidae family and the land crab, Discoplax hirtipes, living on similar sandy bottoms, but not in terrestrial coconut crabs [23]. In the coconut crab, there are many large and small denticles irregularly on the pinching side of the claw, and relatively large denticles are arranged in a line on the front side, with very small denticles randomly arranged on the back side. The difference in these denticles on the claw finger should be associated with biomineralization in the crab’s habitat.
The mud crab, Scylla serrata, is omnivorous but consumes mainly shellfish. The large denticle (den(L2)) is relatively flat and wide compared to the small (den(S)) and medium denticles (den(M)), as shown in Figure 1c. When observed in detail, the top of den(L2) is slightly concave, and the adjacent denticle (den(L3)), opposite to den(L1), is split in two in the middle of its width. When a shellfish is placed on den(L2), it is sandwiched and stable between the convex part of den(L1) and the valley of den(L3). The fixed shellfish is crushed by a larger denticle on the pinching side of the movable finger, and the crabs can consume the contents of the shellfish. The high abrasion resistance of the large denticle surface shown in Figure 5 is an essential feature for the frequent crushing of shellfish. On the other hand, the fingertip of the same white exoskeleton is used to pinch large prey that cannot be captured and crushed by the denticles of the pinching side and to intimidate predators (Figure S1a,b). The curvature of the fingertips toward the pinching side allows the fingertips to pierce prey when pinched. Therefore, the fingertip is as hard as the large denticle surface.
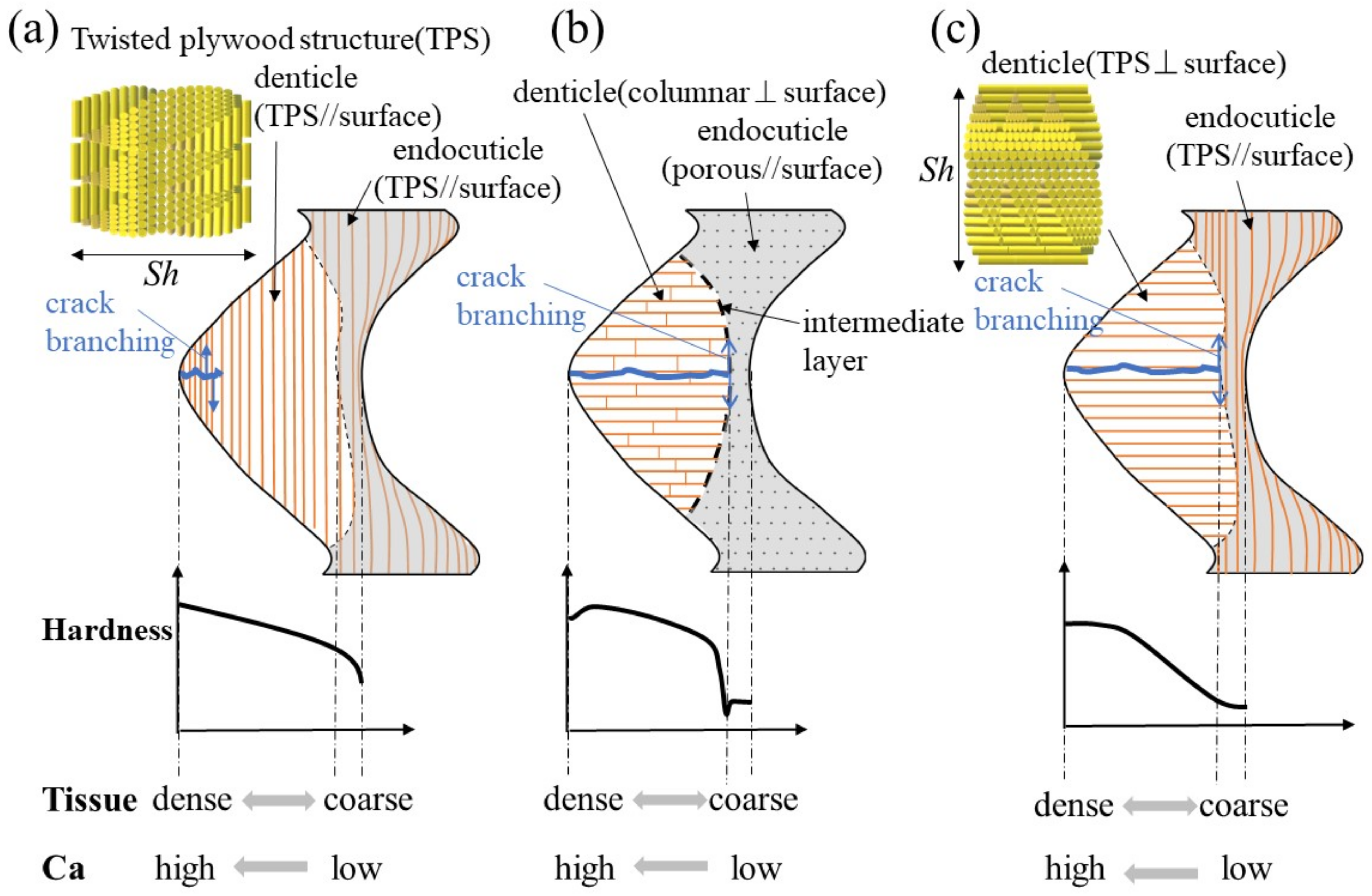
3.5. Twisted–Plywood Structure Stacked Parallel to the Surface in the Denticles
Figure 8 shows a schematic illustration of the exoskeleton tissue of the pinching side in three kinds of crab claws. The mud crab’s white exoskeleton (denticle) tissue is a tough TPS stacked parallel to the surface, and mechanical properties gradually decreased from the outer surface to the inner surface, as shown in Figure 8a. Rosen et al. [26] (two anomuran crabs, Paralithodes camtschaticus and Paralomis birsteini, and three brachyuran crabs, Chionoecetes opilio, Callinectes sapidus, and Cancer borealis) and Inoue et al. [23] (coconut crabs) have investigated the denticle microstructure in detail. Rosen et al. showed that the TPSs run parallel to the exoskeleton surface within the endoC but rotate ~90° (perpendicular to the exoskeleton surface) as they approach the denticle region (Figure 8c). That is, the denticles of the crabs have a TPS stacked perpendicular to the surface. Inoue et al. showed that the denticle in the coconut crab was a columnar structure perpendicular to the surface (Figure 8b). The denticle tissue of all these crustaceans runs perpendicular to the surface, although the tissue patterns are different. With this tissue structure, cracks tend to advance inside, and the claws are more likely to be damaged. However, since the intermediate layer between the denticle and the endoC is soft, only the denticles are considered to be lost or damaged before the claws are fractured. If some denticles are lost, the other denticles maintain the function of the claws, and then new denticles develop by molting.
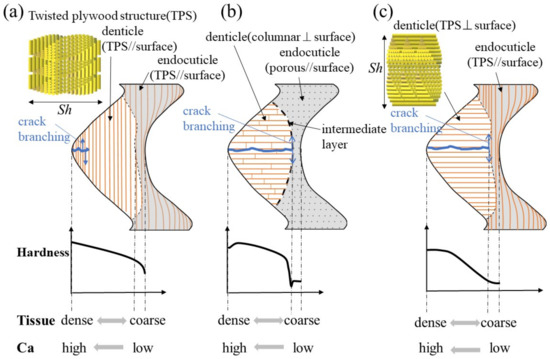
Figure 8.
Schematic illustration of the exoskeleton tissue with denticle in the pinching side of claws and variations of hardness, tissue density, and calcium (Ca): (a) the mud crab, (b) the coconut crab [13,20], and (c) the brachyuran crab [26].
On the other hand, for the mud crab, losing a denticle is directly related to not being able to crush a staple food. Therefore, in the exoskeleton of the pinching side with large denticles, there was almost no endoC (Figure 2), and no rapid decrease in hardness was observed in the intermediate layer (Figure 3b–d). In addition, since the structure of the denticle was a twisted–plywood pattern stacked parallel to the surface, it was tough, making it difficult for cracks to propagate inside, as shown in Figure 8a. In short, the denticles of the mud crab have a tissue structure that is never lost if they crack while pinching. Such a structure has also been observed in the tissue structure of hypermineralized hammer-like dactyl clubs, a weapon for crushing prey [29].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma16114114/s1: Figure S1: Schematic illustrations of predation, attack, and defense of the mud crab, Scylla serrata, with huge claws comparable to its body size; Video S1: The mud crab’s intimidation.
Author Contributions
Conceptualization, T.I.; methodology, T.I.; software, T.I.; validation, T.I.; investigation, T.I.; resources, T.I. and K.N.; data curation, T.I. and Y.H.; writing—original draft preparation, T.I.; writing—review and editing, T.I., Y.H., and K.N.; visualization, T.I. and Y.H.; supervision, T.I.; project administration, T.I.; funding acquisition, T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI Grant Number JP21H04537. The grant is greatly appreciated.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank T. Yoshihama and S. Oka for obtaining some of the samples, T. Hara for advice on microstructural observation, and Y. Kashihara for creating the illustrations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bouligand, Y. Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell 1972, 4, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, B.; Gilman, J.W. Bioinspired Bouligand cellulose nanocrystal composites: A review of mechanical properties. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 376, 20170050. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Song, Z.; Zhang, S.; Ni, Y.; Cai, S.; Gong, X.; He, L.; Yu, S.-H. Discontinuous fibrous Bouligand architecture enabling formidable fracture resistance with crack orientation insensitivity. Proc. Natl. Acad. Sci. USA 2020, 117, 15465–15472. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Hazell, P.J.; Escobedo, J.P.; Wang, H. Biomimetic armour design strategies for additive manufacturing: A review. Mater. Des. 2021, 205, 109730. [Google Scholar] [CrossRef]
- Zhang, B.; Han, Q.; Zhang, J.; Han, Z.; Niu, S.; Ren, L. Advanced bio-inspired structural materials: Local properties determine overall performance. Mater. Today 2020, 41, 177–199. [Google Scholar] [CrossRef]
- Cheng, L.; Thomas, A.; Glancey, J.L.; Karlsson, A.M. Mechanical behavior of bio-inspired laminated composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 211–220. [Google Scholar] [CrossRef]
- Amorim, L.; Santos, A.; Nunes, J.; Viana, J. Bioinspired approaches for toughening of fibre reinforced polymer composites. Mater. Des. 2021, 199, 109336. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, W.; Zhang, T.; Chen, P.; Li, M. Strength and toughness enhancement in 3d printing via bioinspired tool path. Mater. Des. 2020, 185, 108239. [Google Scholar] [CrossRef]
- Yao, H.; Zheng, G.; Li, W.; McDowell, M.T.; Seh, Z.; Liu, N.; Lu, Z.; Cui, Y. Crab shells as sustainable templates from nature for nanostructured battery electrodes. Nano Lett. 2013, 13, 3385–3390. [Google Scholar] [CrossRef]
- Sayekti, P.R.; Fahrunnida, F.; Cerniauskas, G.; Robert, C.; Retnoaji, B.; Alam, P. The Impact Behaviour of Crab Carapaces in Relation to Morphology. Materials 2020, 13, 3994. [Google Scholar] [CrossRef]
- Waugh, D.A.; Schroeder, A.M.; Feldmann, R.M.; Mutel, M.H.E. Differential cuticle architecture and its preservation in fossil and extant callinectes and scylla claws. J. Crustac. Biol. 2006, 26, 271–282. [Google Scholar] [CrossRef]
- Alberts-Hubatsch, H.; Lee, S.Y.; Meynecke, J.-O.; Diele, K.; Nordhaus, I.; Wolff, M. Life-history, movement, and habitat use of Scylla serrata (Decapoda, Portunidae): Current knowledge and future challenges. Hydrobiologia 2016, 763, 5–21. [Google Scholar] [CrossRef]
- Inoue, T.; Kitahara, E.; Hara, Y.; Nakazato, K. Mud crab’s mottled, deep-blue exoskeleton: Surface morphology and internal microstructure. Minerals 2022, 12, 1607. [Google Scholar] [CrossRef]
- Inoue, T.; Hiroto, T.; Hara, Y.; Nakazato, K.; Oka, S.-I. Tissue structure and mechanical properties of the exoskeleton of the huge claws of the mud crab, Scylla serrata. J. Mater. Sci. 2023, 58, 1099–1115. [Google Scholar] [CrossRef]
- Fabritius, H.-O.; Karsten, E.S.; Balasundaram, K.; Hild, S.; Huemer, K.; Raabe, D. Correlation of structure, composition and local mechanical properties in the dorsal carapace of the edible crab Cancer pagurus. Z. Krist. 2012, 227, 766–776. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Li, J.; Qiu, F. Microstructure and mechanical properties of the dactylopodites of the chinese mitten crab (Eriocheir sinensis). Appl. Sci. 2018, 8, 674. [Google Scholar] [CrossRef]
- Nekvapil, F.; Pinzaru, S.C.; Barbu–Tudoran, L.; Suciu, M.; Glamuzina, B.; Tamaș, T.; Chiș, V. Color-specific porosity in double pigmented natural 3d-nanoarchitectures of blue crab shell. Sci. Rep. 2020, 10, 3019. [Google Scholar] [CrossRef]
- Inoue, T.; Oka, S.-I.; Hara, T. Three-dimensional microstructure of robust claw of coconut crab, one of the largest terrestrial crustaceans. Mater. Des. 2021, 206, 109765. [Google Scholar] [CrossRef]
- Inoue, T.; Hara, T.; Nakazato, K.; Oka, S.-I. Superior mechanical resistance in the exoskeleton of the coconut crab, Birgus latro. Mater. Today Bio 2021, 12, 100132. [Google Scholar] [CrossRef]
- Inoue, T.; Oka, S.-I.; Nakazato, K.; Hara, T. Structural changes and mechanical resistance of claws and denticles in coconut crabs of different sizes. Biology 2021, 10, 1304. [Google Scholar] [CrossRef]
- Raabe, D.; Sachs, C.; Romano, P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater. 2005, 53, 4281–4292. [Google Scholar] [CrossRef]
- Yoshihama, T. (Irabu Island, Okinawa, Japan). Private communication. 2022. [Google Scholar]
- Inoue, T.; Oka, S.-I.; Nakazato, K.; Hara, T. Columnar structure of claw denticles in the coconut crab, Birgus latro. Minerals 2022, 12, 274. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Zok, F.; Miserez, A. Property maps for abrasion resistance of materials. Acta Mater. 2007, 55, 6365–6371. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.N.; Baran, K.A.; Sison, J.N.; Steffel, B.V.; Long, W.C.; Foy, R.J.; Smith, K.E.; Aronson, R.B.; Dickinson, G.H. Mechanical resistance in decapod claw denticles: Contribution of structure and composition. Acta Biomater. 2020, 110, 196–207. [Google Scholar] [CrossRef]
- Boßelmann, F.; Romano, P.; Fabritius, H.; Raabe, D.; Epple, M. The composition of the exoskeleton of two crustacea: The American lobster Homarus americanus and the edible crab Cancer pagurus. Thermochim. Acta 2007, 463, 65–68. [Google Scholar] [CrossRef]
- Triajie, H.; Andayani, S.; Yanuhar, U.; Ekawati, A.W. Structure of hard and soft carapace exoskeleton biomaterial through sem-edxrs at various stages of development scylla paramamosain mud crab. Int. J. Biol. Biomed. Eng. 2021, 15, 113–122. [Google Scholar] [CrossRef]
- Weaver, J.C.; Milliron, G.W.; Miserez, A.; Evans-Lutterodt, K.; Herrera, S.; Gallana, I.; Mershon, W.J.; Swanson, B.; Zavattieri, P.; DiMasi, E.; et al. The stomatopod dactyl club: A formidable damage-tolerant biological hammer. Science 2012, 336, 1275–1280. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).