Biological Response of Human Gingival Fibroblasts to Zinc-Doped Hydroxyapatite Designed for Dental Applications—An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Hydroxyapatite and Zinc-Doped Hydroxyapatite
2.1.1. Zinc-Doped Hydroxyapatite
2.1.2. Physiochemical Characterization
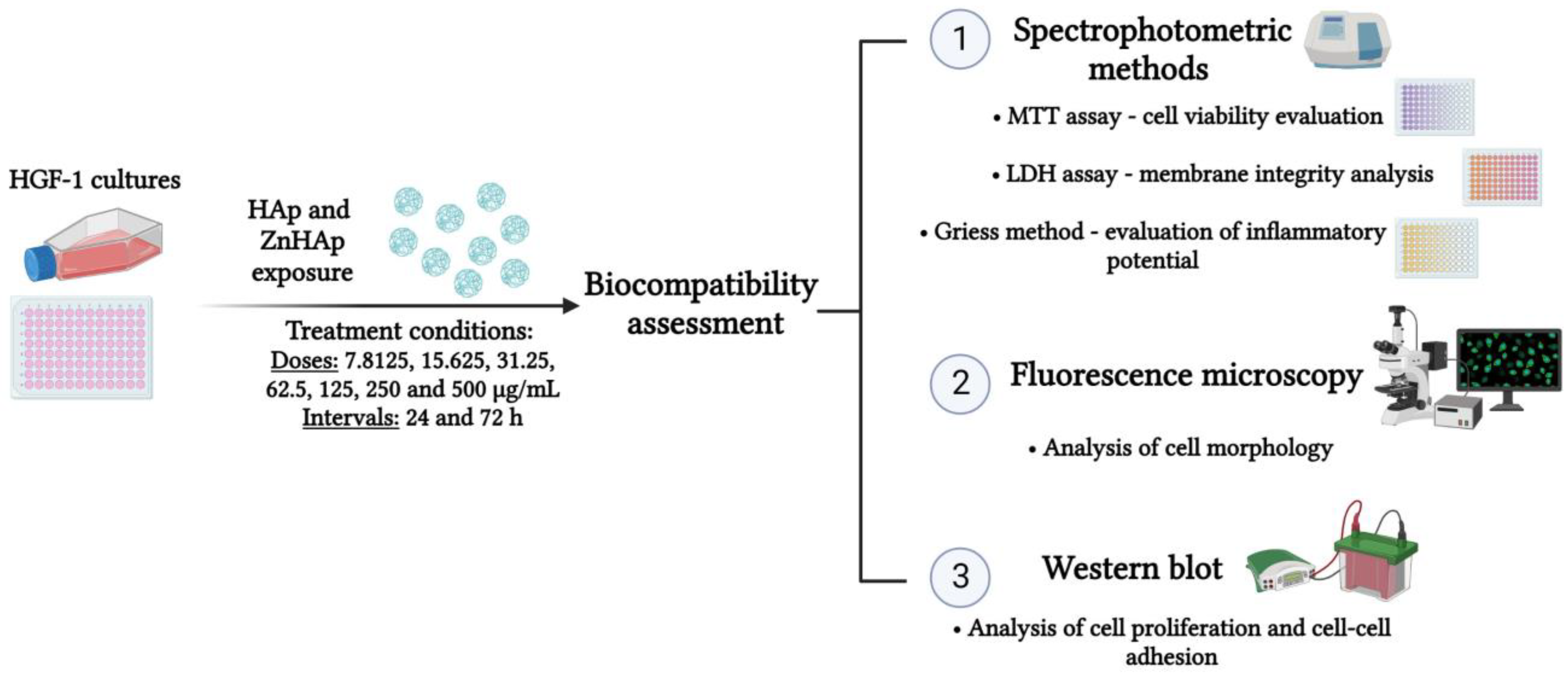
2.2. In Vitro Biocompatibility Assessment
2.2.1. Cell Culture Conditions
2.2.2. Treatment Conditions
2.2.3. Cell Viability Evaluation
2.2.4. Lactate Dehydrogenase (LDH) Activity Assessment
2.2.5. Measurement of Nitric Oxide (NO) Production
2.2.6. Analysis of F-Actin Cytoskeleton Organization
2.2.7. Western Blot
2.2.8. Statistical Analysis
2.3. Antimicrobial Activity Evaluation
3. Results
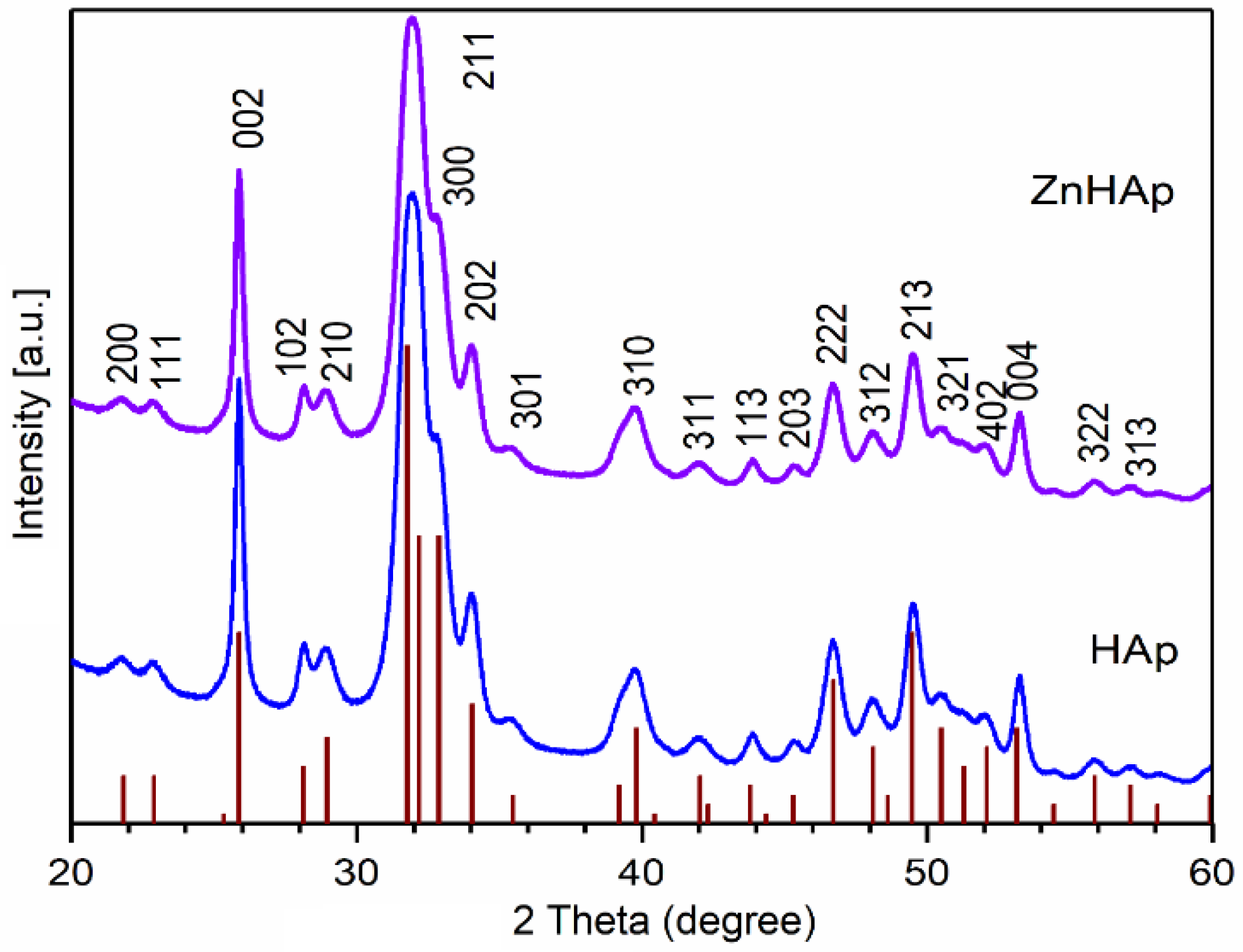
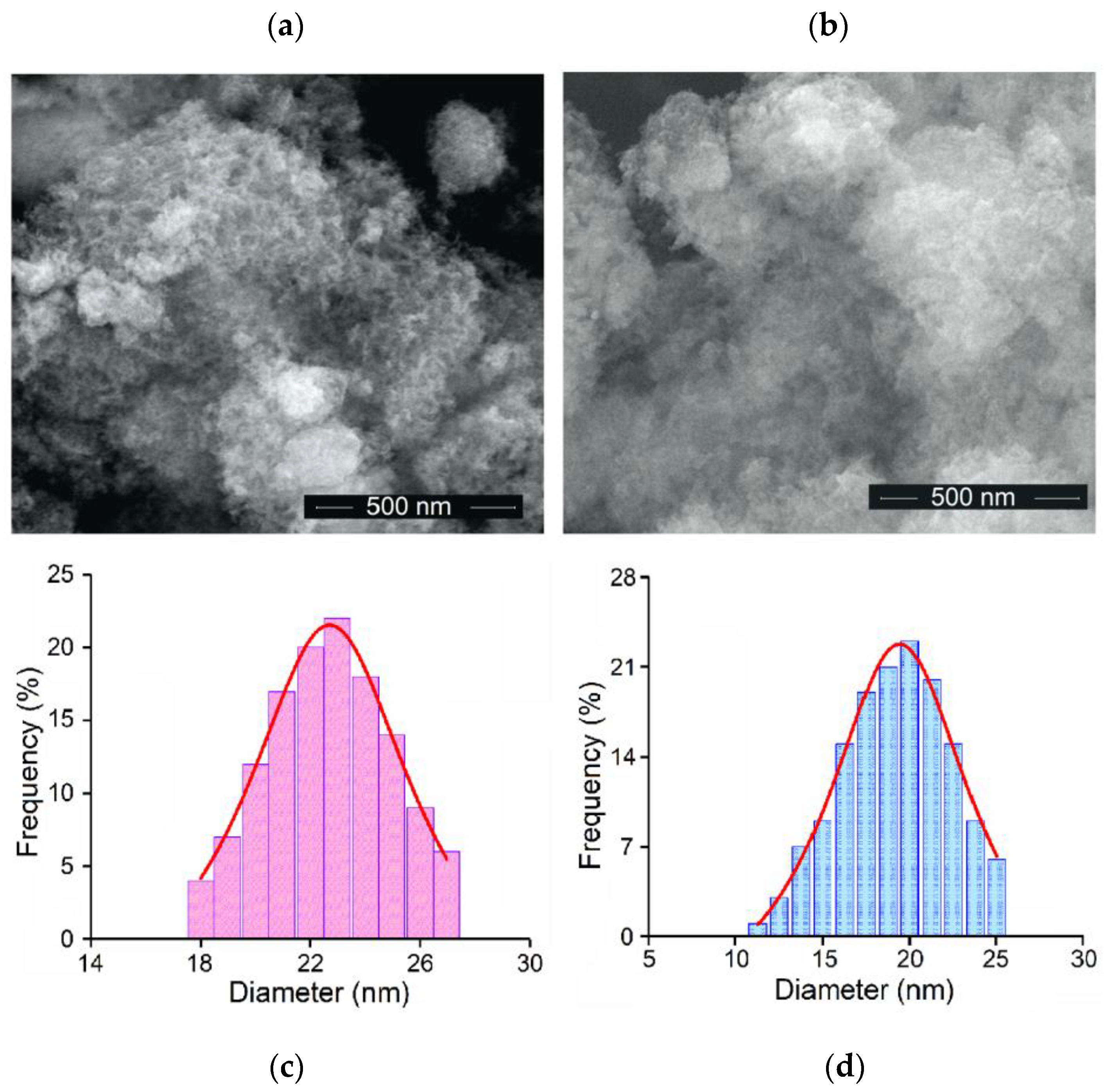
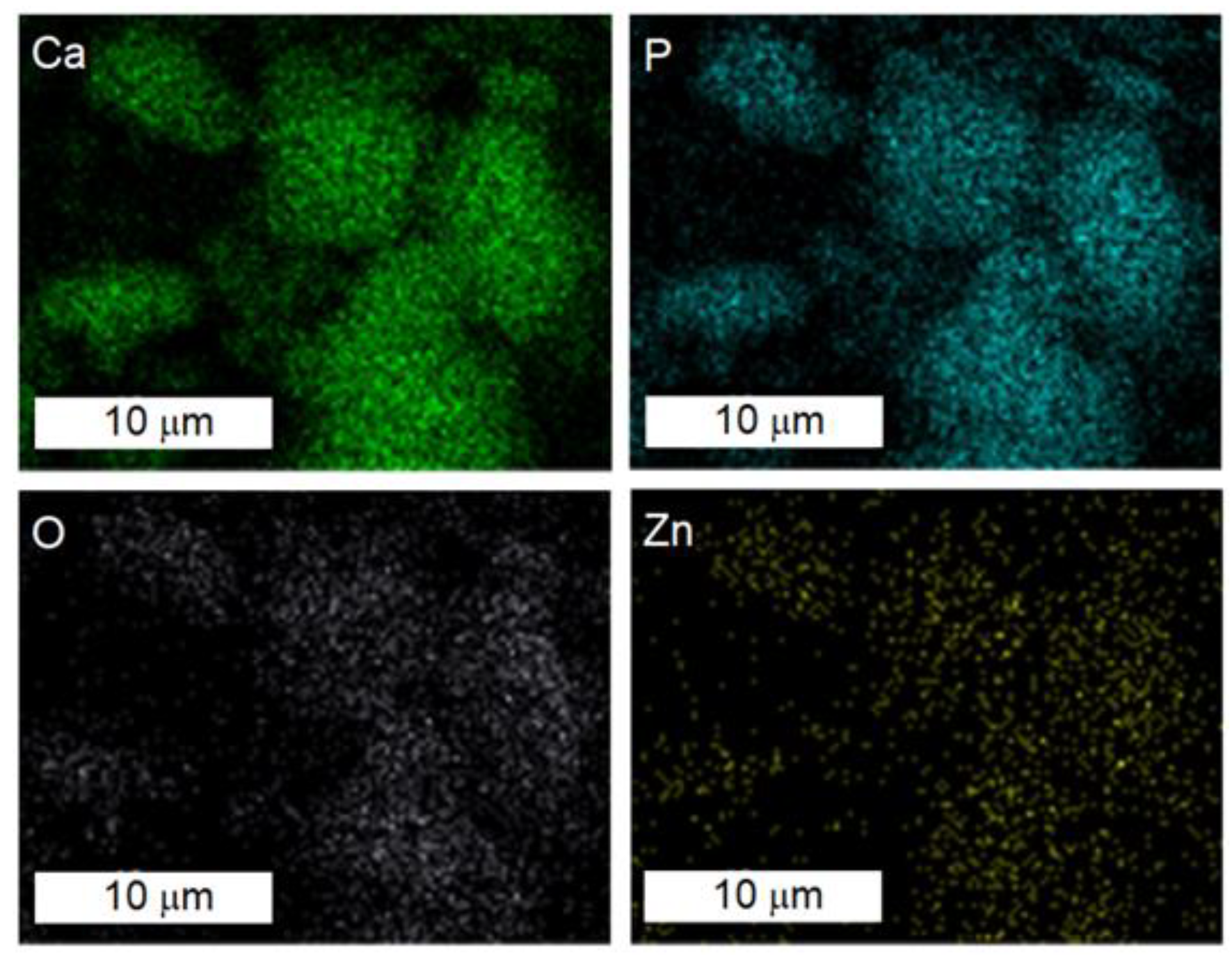
3.1. Characterization of Hydroxyapatite and Zinc-Doped Hydroxyapatite
3.2. Biological Studies
3.2.1. Biocompatibility Assessment
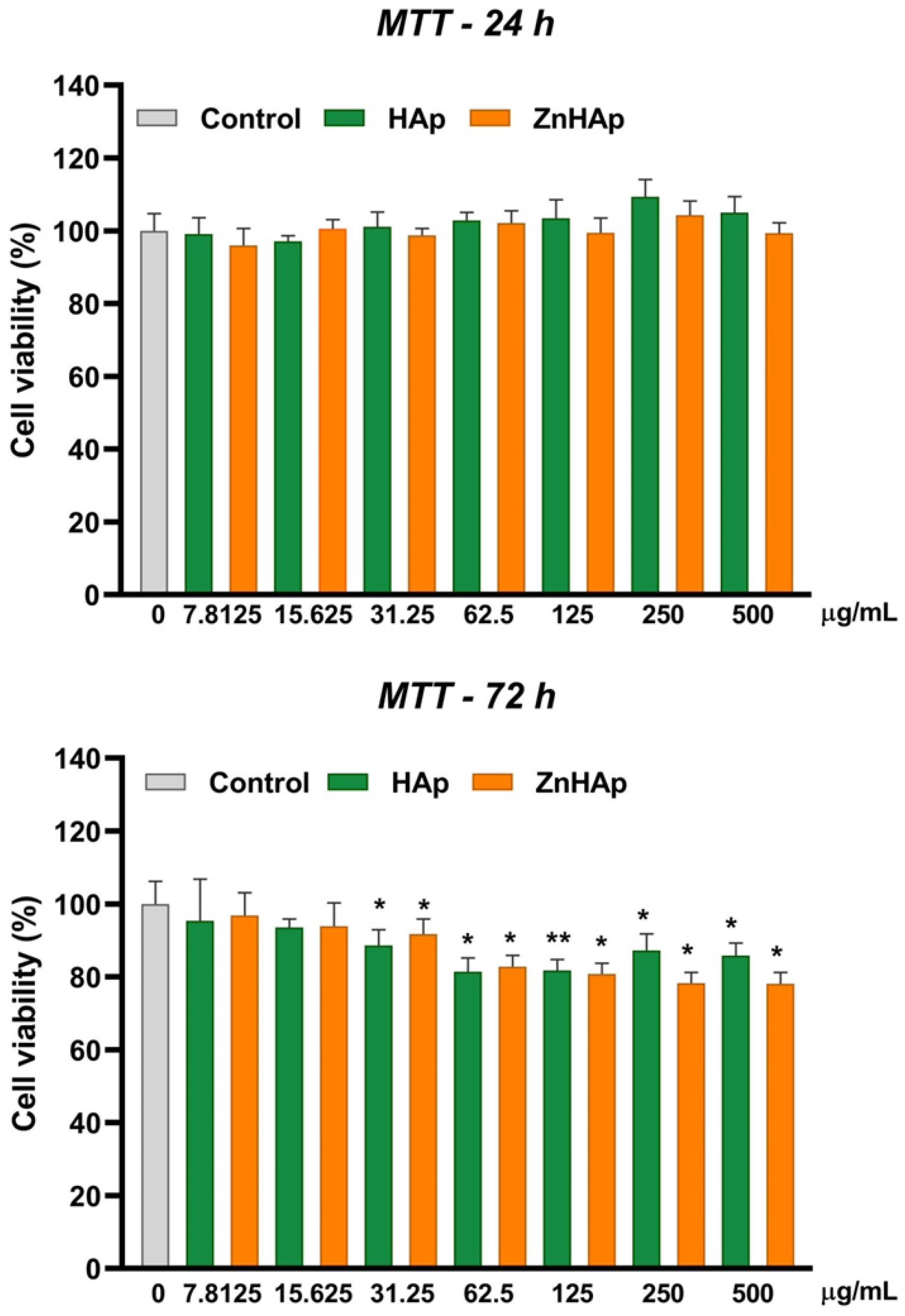
Cell Viability
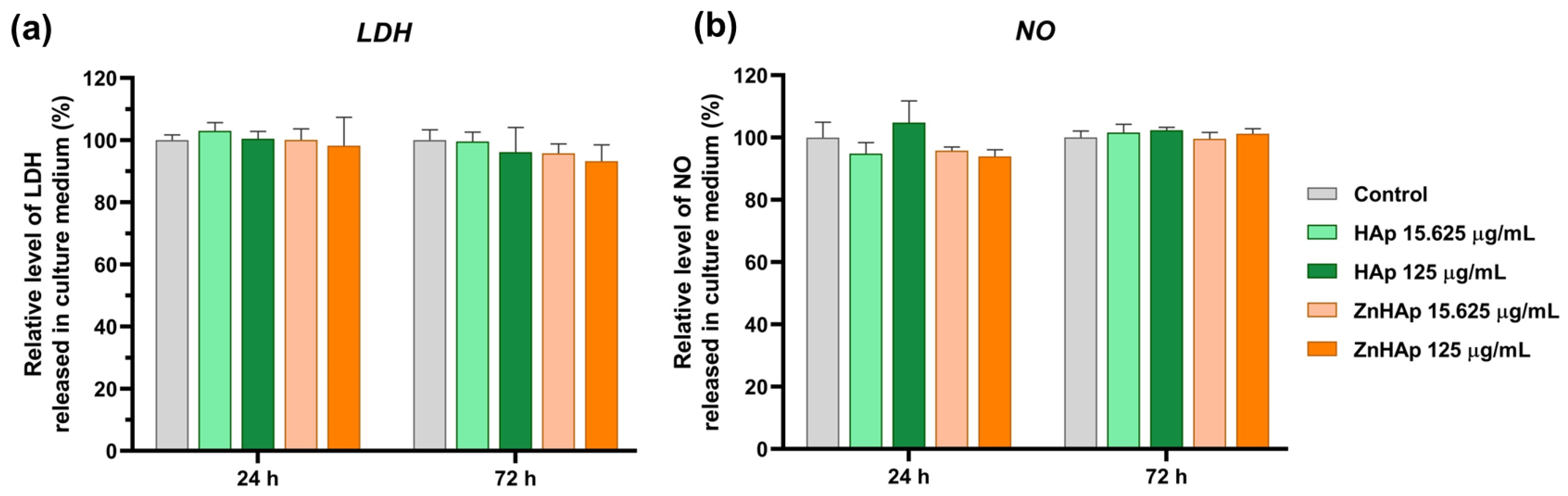
Cell Membrane Integrity and Inflammatory Response
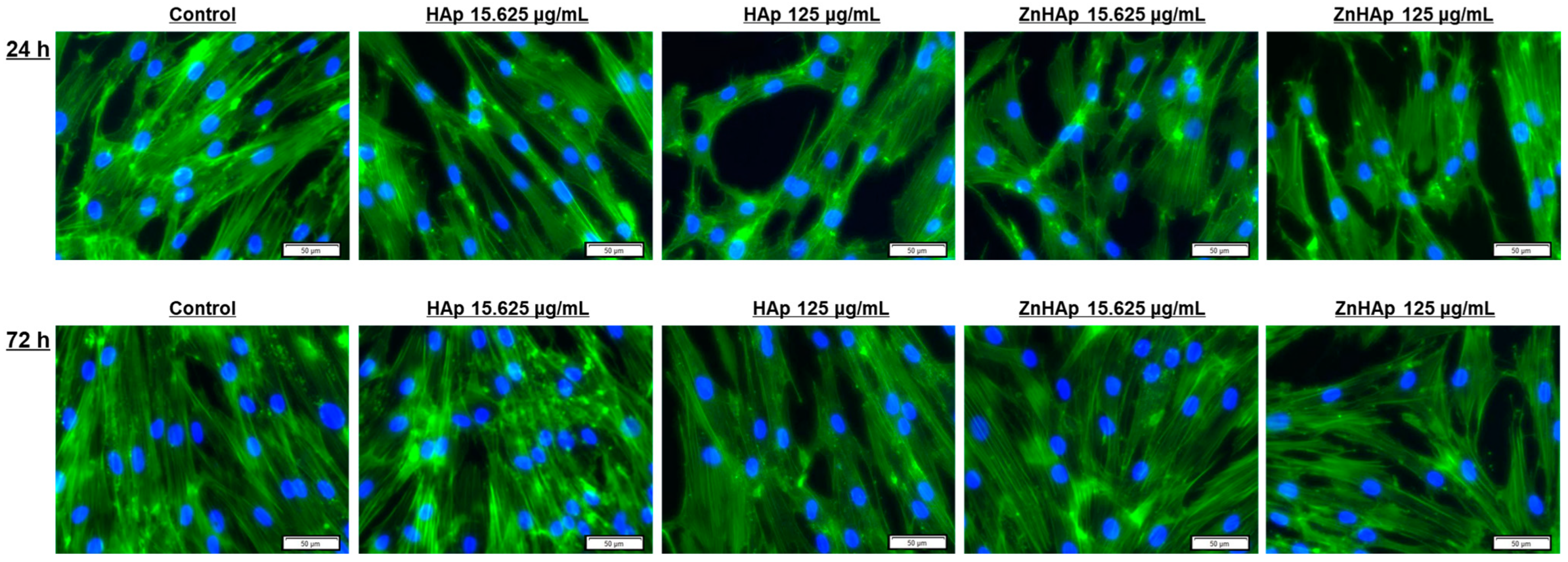
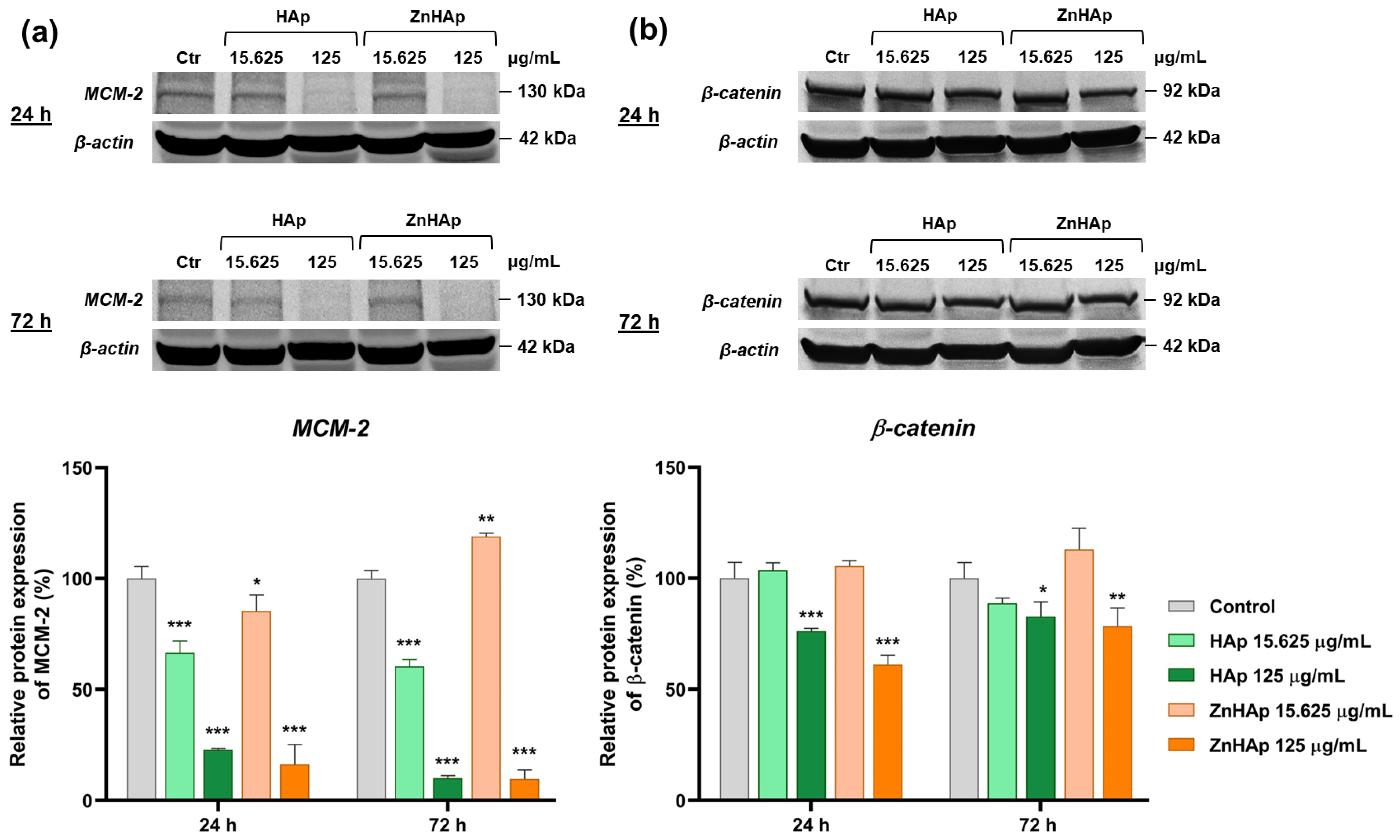
Cell Morphological Alterations and Protein Expression of Cell Proliferation and Adhesion-Related Markers
3.2.2. Antimicrobial Evaluation
4. Discussion
5. Conclusions
- -
- Cell viability remained at the control level after 24 h of treatment with 7.8125–500 µg/mL HAp and ZnHAp; after 72 h, a slight decrease in cell viability was registered starting with a dose of 31.25 µg/mL HAp and ZnHAp;
- -
- Cells maintained their membrane integrity and no inflammatory response was triggered in the presence of HAp and ZnHAp;
- -
- A dose of 15.625 µg/mL HAp and ZnHAp presented good biocompatibility, as indicated by the lack of alteration of F-actin cytoskeleton organization and unchanged protein expression of β-catenin cell adhesion marker;
- -
- A dose of 125 µg/mL promoted the decrease in β-catenin protein expression and changed the architecture of F-actin filaments after 24 and 72 h;
- -
- Cell rate proliferation was decreased in all experimental conditions, except for the dose of 15.625 µg/mL ZnHAp at 72 h, when a slight increase was induced, proving an improvement in HAp properties provided by zinc substitution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haider, A.; Haider, S.; Han, S.S.; Kang, I.-K. Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: A review. RSC Adv. 2017, 7, 7442–7458. [Google Scholar] [CrossRef]
- Chen, L.; Al-Bayatee, S.; Khurshid, Z.; Shavandi, A.; Brunton, P.; Ratnayake, J. Hydroxyapatite in Oral Care Products—A Review. Materials 2021, 14, 4865. [Google Scholar] [CrossRef] [PubMed]
- Balhuc, S.; Campian, R.; Labunet, A.; Negucioiu, M.; Buduru, S.; Kui, A. Dental Applications of Systems Based on Hydroxyapatite Nanoparticles—An Evidence-Based Update. Crystals 2021, 11, 674. [Google Scholar] [CrossRef]
- Munir, M.U.; Salman, S.; Ihsan, A.; Elsaman, T. Synthesis, Characterization, Functionalization and Bio-Applications of Hydroxyapatite Nanomaterials: An Overview. Int. J. Nanomed. 2022, 17, 1903–1925. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.C.J.; Moore, T.; Banik, B.; Alexis, F. Biomedical Applications of Hydroxyapatite Nanoparticles. Curr. Pharm. Biotechnol. 2010, 11, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite Based Materials for Bone Tissue Engineering: A Brief and Comprehensive Introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef]
- Habibah, T.U.; Amlani, D.V.; Brizuela, M. Hydroxyapatite Dental Material; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Izzetti, R.; Gennai, S.; Nisi, M.; Gulia, F.; Miceli, M.; Giuca, M.R. Clinical Applications of Nano-Hydroxyapatite in Dentistry. Appl. Sci. 2022, 12, 10762. [Google Scholar] [CrossRef]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Băciuț, M.; Băciuț, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Compos. B Eng. 2021, 215, 108790. [Google Scholar] [CrossRef]
- Mazumder, S.; Nayak, A.K.; Ara, J.T.; Hasnain, S. Hydroxyapatite Composites for Dentistry. In Applications of Nanocomposite Materials in Dentistry; Woodhead Publishing: Cambridge, UK, 2019; pp. 123–143. [Google Scholar]
- Okada, M.; Matsumoto, T. Synthesis and modification of apatite nanoparticles for use in dental and medical applications. Jpn. Dent. Sci. Rev. 2015, 51, 85–95. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Deshpande, J.D.; Joshi, M.M.; Giri, P.A. Zinc: The trace element of major importance in human nutrition and health. Int. J. Med. Sci. Public Health 2013, 2, 1–6. [Google Scholar] [CrossRef]
- Doğan, M.S. Relation of trace elements on dental health. In Trace Elements. Human Health and Environment; Saleh, H.E.-D.M., El-Adham, E., Eds.; IntechOpen: London, UK, 2018; p. 75899. [Google Scholar]
- Uwitonze, A.M.; Ojeh, N.; Murererehe, J.; Atfi, A.; Razzaque, M.S. Zinc Adequacy Is Essential for the Maintenance of Optimal Oral Health. Nutrients 2020, 12, 949. [Google Scholar] [CrossRef]
- Poggio, C.; Gulino, C.; Mirando, M.; Colombo, M.; Pietrocola, G. Protective effect of zinc-hydroxyapatite toothpastes on enamel erosion: An in vitro study. J. Clin. Exp. Dent. 2017, 9, e118–e122. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Quintini, M.; Lelli, M.; Tarterini, F.; Foltran, I.; Scribante, A. Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review. Biomimetics 2023, 8, 71. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and characterization of Zn-Doped hydroxyapatite: Scaffold application, antibacterial and bioactivity studies. Heliyon 2019, 5, e01716. [Google Scholar] [CrossRef]
- Anwar, A.; Akbar, S.; Sadiqa, A.; Kazmi, M. Novel continuous flow synthesis, characterization and antibacterial studies of nanoscale zinc substituted hydroxyapatite bioceramics. Inorganica Chim. 2016, 453, 16–22. [Google Scholar] [CrossRef]
- Ijaz, K.; Khalid, H.; Chaudhry, A.A. Zinc-Substituted Hydroxyapatite. In Handbook of Ionic Substituted Hydroxyapatites; Khan, A.S., Chaudhry, A.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 217–236. [Google Scholar]
- Pu’ad, N.M.; Haq, R.A.; Noh, H.M.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Popa, C.L.; Predoi, D. Sm:HAp Nanopowders Present Antibacterial Activity against Enterococcus faecalis. J. Nanomater. 2014, 2014, 6. [Google Scholar] [CrossRef]
- Predoi, S.A.; Ciobanu, S.C.; Chifiriuc, M.C.; Motelica-Heino, M.; Predoi, D.; Iconaru, S.L. Hydroxyapatite Nanopowders for Effective Removal of Strontium Ions from Aqueous Solutions. Materials 2023, 16, 229. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 613. [Google Scholar] [CrossRef]
- Sudarsanan, K.; Young, R.A. Significant precision in crystal structural details. Holly Springs hydroxyapatite. Acta Crystallogr. 1969, 25, 1534–1543. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, L.; An, J. Crystallographic characteristics of hydroxylapatite in hard tissues of Cololabis saira. Crystals 2017, 7, 103. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Deniaud, A.; Chevallet, M.; Michaud-Soret, I.; Buton, N.; Prodan, A.M. Textural, Structural and Biological Evaluation of Hydroxyapatite Doped with Zinc at Low Concentrations. Materials 2017, 10, 229. [Google Scholar] [CrossRef]
- Popa, C.L.; Deniaud, A.; Michaud-Soret, I.; Guégan, R.; Motelica-Heino, M.; Predoi, D. Structural and Biological Assessment of Zinc Doped Hydroxyapatite Nanoparticles. J. Nanomater. 2016, 2016, 1062878. [Google Scholar] [CrossRef]
- Hontsu, S.; Yoshikawa, K. Ultra-Thin Hydroxyapatite Sheets for Dental Applications. In Hydroxyapatite (Hap) for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2015; pp. 129–142. [Google Scholar]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.C.; Jamshidi, P.; Grover, L.M.; Mallick, K.K. Preparation and characterisation of nanophase Sr, Mg, and Zn substituted hydroxyapatite by aqueous precipitation. Mater. Sci. Eng. C 2014, 35, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Lytkina, D.; Gutsalova, A.; Fedorishin, D.; Korotchenko, N.; Akhmedzhanaov, R.; Kozik, V.; Kurzina, I. Synthesis and Properties of Zinc-Modified Hydroxyapatite. J. Funct. Biomater. 2020, 11, 10. [Google Scholar] [CrossRef]
- Razzaghi, R.; Pidar, F.; Momen-Heravi, M.; Bahmani, F.; Akbari, H.; Asemi, Z. Magnesium supplementation and the effects on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res. 2018, 181, 207–215. [Google Scholar] [CrossRef]
- Wang, X.H.; Ni, J.S.; Cao, N.L.; Yu, S.; Chen, Y.G.; Zhang, S.X.; Gu, B.J.; Yan, J. In Vivo evaluation of Mg-6Zn and titanium alloys on collagen metabolism in the healing of intestinal anastomosis. Sci. Rep. 2017, 7, 44919. [Google Scholar] [CrossRef] [PubMed]
- Rokosz, K.; Hryniewicz, T.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Matysek, D.; Dudek, L.; Pietrzak, K. Novel porous Phosphorus-Calcium-Magnesium coatings on titanium with copper or zinc obtained by DC plasma electrolytic oxidation: Fabrication and characterization. Materials 2018, 11, 1680. [Google Scholar] [CrossRef] [PubMed]
- Kamadjaja, M.J.K.; Abraham, J.F.; Laksono, H. Biocompatibility of Portunus pelagicus hydroxyapatite graft on human gingival fibroblast cell culture. Med Arch. 2019, 73, 378–381. [Google Scholar] [CrossRef]
- Manoj, M.; Subbiah, R.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C.; Park, W. Influence of Growth Parameters on the Formation of Hydroxyapatite (HAp) Nanostructures and Their Cell Viability Studies. Nanobiomedicine 2015, 2, 2. [Google Scholar] [CrossRef]
- Grenho, L.; Salgado, C.L.; Fernandes, M.H.; Monteiro, F.J.; Ferraz, M.P. Antibacterial activity and biocompatibility of three-dimensional nanostructured porous granules of hydroxyapatite and zinc oxide nanoparticles—An in vitro and in vivo study. Nanotechnology 2015, 26, 315101. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, C.; Qian, J.; Wang, J.; Zhang, Y. Size-mediated cytotoxicity and apoptosis of hydroxyapatite nanoparticles in human hepatoma HepG2 cells. Biomaterials 2010, 31, 730–740. [Google Scholar] [CrossRef]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.T.Y.; Ng, K.W.; Lo, S.C.J. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2013, 87, 1037–1052. [Google Scholar] [CrossRef]
- Zhao, X.; Heng, B.C.; Xiong, S.; Guo, J.; Tan, T.T.-Y.; Boey, F.Y.C.; Ng, K.W.; Loo, J.S.C. In vitro assessment of cellular responses to rod-shaped hydroxyapatite nanoparticles of varying lengths and surface areas. Nanotoxicology 2011, 5, 182–194. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, 465–468. [Google Scholar] [CrossRef]
- Albrecht, C.; Scherbart, A.M.; van Berlo, D.; Braunbarth, C.M.; Schins, R.P.F.; Scheel, J. Evaluation of cytotoxic effects and oxidative stress with hydroxyapatite dispersions of different physicochemical properties in rat NR8383 cells and primary macrophages. Toxicol. Vitr. 2009, 23, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Velard, F.; Laurent-Maquin, D.; Braux, J.; Guillaume, C.; Bouthors, S.; Jallot, E.; Nedelec, J.-M.; Belaaouaj, A.; Laquerriere, P. The effect of zinc on hydroxyapatite-mediated activation of human polymorphonuclear neutrophils and bone implant-associated acute inflammation. Biomaterials 2010, 31, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Grandjean-Laquerriere, A.; Laquerriere, P.; Jallot, E.; Nedelec, J.-M.; Guenounou, M.; Laurent-Maquin, D.; Phillips, T.M. Influence of the zinc concentration of sol–gel derived zinc substituted hydroxyapatite on cytokine production by human monocytes in vitro. Biomaterials 2006, 27, 3195–3200. [Google Scholar] [CrossRef]
- Acharya, S.; Arnold, D.; Prabhu, P.; Niranjan, K.C.; Hallikeri, K. MCM-2 an alternative to Ki-67 for assessing cell proliferation in odontogenic pathologies. J. Oral Maxillofac. Surg. Med. Pathol. 2019, 31, 52–58. [Google Scholar] [CrossRef]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Oshita, M.; Kataoka, M.; Azuma, Y.; Furuzono, T. Shareability of antibacterial and osteoblastic-proliferation activities of zinc-doped hydroxyapatite nanoparticles in vitro. J. Biomed. Mater. Res. 2022, 110, 799–805. [Google Scholar] [CrossRef]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of β-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef] [PubMed]
- Brembeck, F.H.; Rosário, M.; Birchmeier, W. Balancing cell adhesion and Wnt signaling, the key role of β-catenin. Curr. Opin. Genet. Dev. 2006, 16, 51–59. [Google Scholar] [CrossRef]
- Lymburner, S.; McLeod, S.; Purtzki, M.; Roskelley, C.; Xu, Z. Zinc inhibits magnesium-dependent migration of human breast cancer MDA-MB-231 cells on fibronectin. J. Nutr. Biochem. 2013, 24, 1034–1040. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hieda, Y.; Kogai, Y. Effect of hydroxyapatite surface morphology on cell adhesion. Mater. Sci. Eng. C 2016, 69, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nihonmatsu, S.; Okawara, T.; Onuki, H.; Sakagami, H.; Nakajima, H.; Takeishi, H.; Shimada, J. Adhesion and Proliferation of Osteoblastic Cells on Hydroxyapatite-dispersed Ti-based Composite Plate. Vivo 2019, 33, 1067–1079. [Google Scholar] [CrossRef]
- Mehta, J.S.; Futter, C.E.; Sandeman, S.R.; Faragher, R.G.A.F.; Hing, K.A.; Tanner, K.E.; Allan, B.D.S. Hydroxyapatite promotes superior keratocyte adhesion and proliferation in comparison with current keratoprosthesis skirt materials. Br. J. Ophthalmol. 2005, 89, 1356–1362. [Google Scholar] [CrossRef]
- Chung, R.-J.; Hsiesh, M.-F.; Huang, C.-W.; Perng, L.-H.; Wen, H.-W.; Chin, T.-S. Antimicrobial effects and human gingival biocompatibility of hydroxyapatite sol–gel coatings. J. Biomed. Mater. 2005, 76B, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, N.; Kakuchi, Y.; Ohtsuki, T. Antibacterial effect of zinc oxide/hydroxyapatite coatings prepared by chemical solution deposition. Appl. Surf. Sci. 2018, 445, 596–600. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Q.-L.; Zhu, Y.-J.; Zhu, C.-L.; Feng, X.-P. Synthesis and antibacterial property of zinc loaded hydroxyapatite nanorods. Mater. Lett. 2012, 89, 233–235. [Google Scholar] [CrossRef]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of Hydroxyapatite with Antibacterial Properties Using a Microwave-Assisted Combustion Method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef] [PubMed]
- Yanovska, A.; Bolshanina, S. Composite materials based on hydroxyapatite embedded in biopolymer matrices: Ways of synthesis and application. In Materials for Biomedical Engineering. Hydrogels and Polymer-Based Scaffolds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 403–440. [Google Scholar]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef] [PubMed]
- Bartmański, M.; Rościszewska, M.; Wekwejt, M.; Ronowska, A.; Nadolska-Dawidowska, M.; Mielewczyk-Gryń, A. Properties of New Composite Materials Based on Hydroxyapatite Ceramic and Cross-Linked Gelatin for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 9083. [Google Scholar] [CrossRef] [PubMed]
| Compound | S. aureus | E. faecalis | E. coli | P. aeruginosa | ||||
|---|---|---|---|---|---|---|---|---|
| HAp (mg/mL) | 4.625 | 4.625 | No | No | 37 | 9.25 | 9.25 | 9.25 |
| ZnHAp (mg/mL) | 0.962 | 0.481 | 3.848 | 3.848 | No | No | 3.848 | 3.848 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badea, M.A.; Balas, M.; Popa, M.; Borcan, T.; Bunea, A.-C.; Predoi, D.; Dinischiotu, A. Biological Response of Human Gingival Fibroblasts to Zinc-Doped Hydroxyapatite Designed for Dental Applications—An In Vitro Study. Materials 2023, 16, 4145. https://doi.org/10.3390/ma16114145
Badea MA, Balas M, Popa M, Borcan T, Bunea A-C, Predoi D, Dinischiotu A. Biological Response of Human Gingival Fibroblasts to Zinc-Doped Hydroxyapatite Designed for Dental Applications—An In Vitro Study. Materials. 2023; 16(11):4145. https://doi.org/10.3390/ma16114145
Chicago/Turabian StyleBadea, Madalina Andreea, Mihaela Balas, Marcela Popa, Teodora Borcan, Anamaria-Cristina Bunea, Daniela Predoi, and Anca Dinischiotu. 2023. "Biological Response of Human Gingival Fibroblasts to Zinc-Doped Hydroxyapatite Designed for Dental Applications—An In Vitro Study" Materials 16, no. 11: 4145. https://doi.org/10.3390/ma16114145
APA StyleBadea, M. A., Balas, M., Popa, M., Borcan, T., Bunea, A.-C., Predoi, D., & Dinischiotu, A. (2023). Biological Response of Human Gingival Fibroblasts to Zinc-Doped Hydroxyapatite Designed for Dental Applications—An In Vitro Study. Materials, 16(11), 4145. https://doi.org/10.3390/ma16114145










