Al–Al3Ni In Situ Composite Formation by Wire-Feed Electron-Beam Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
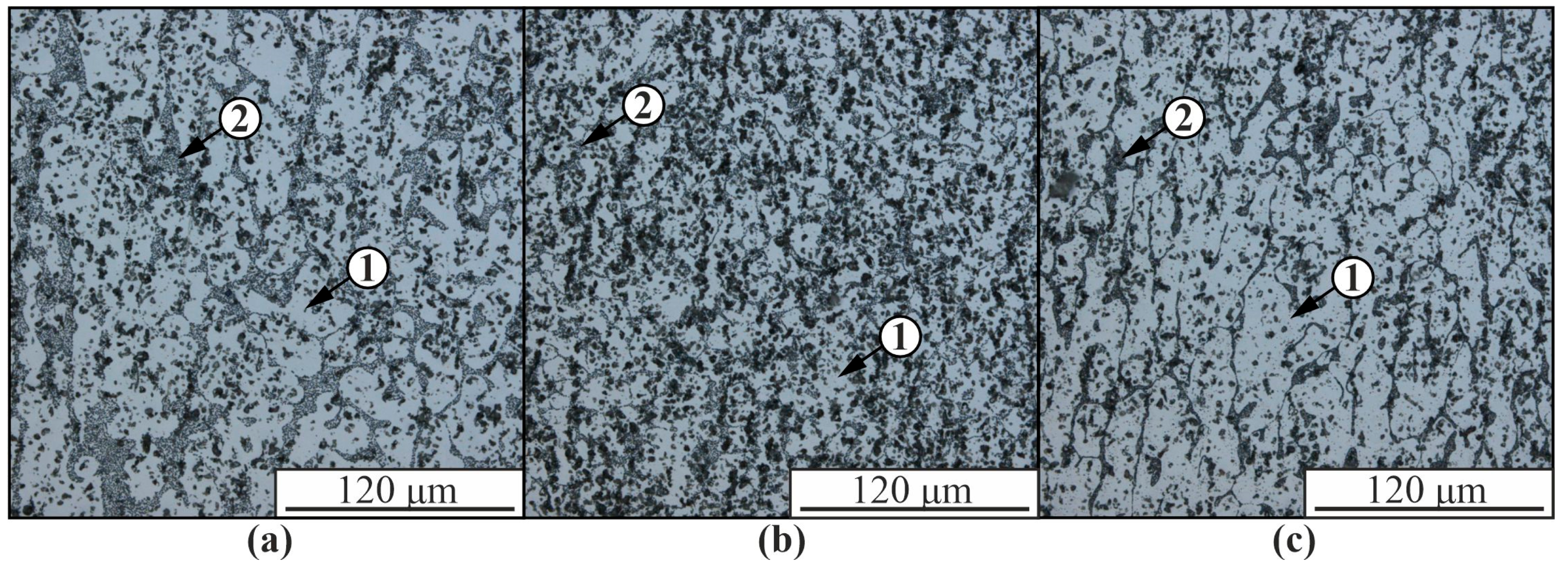
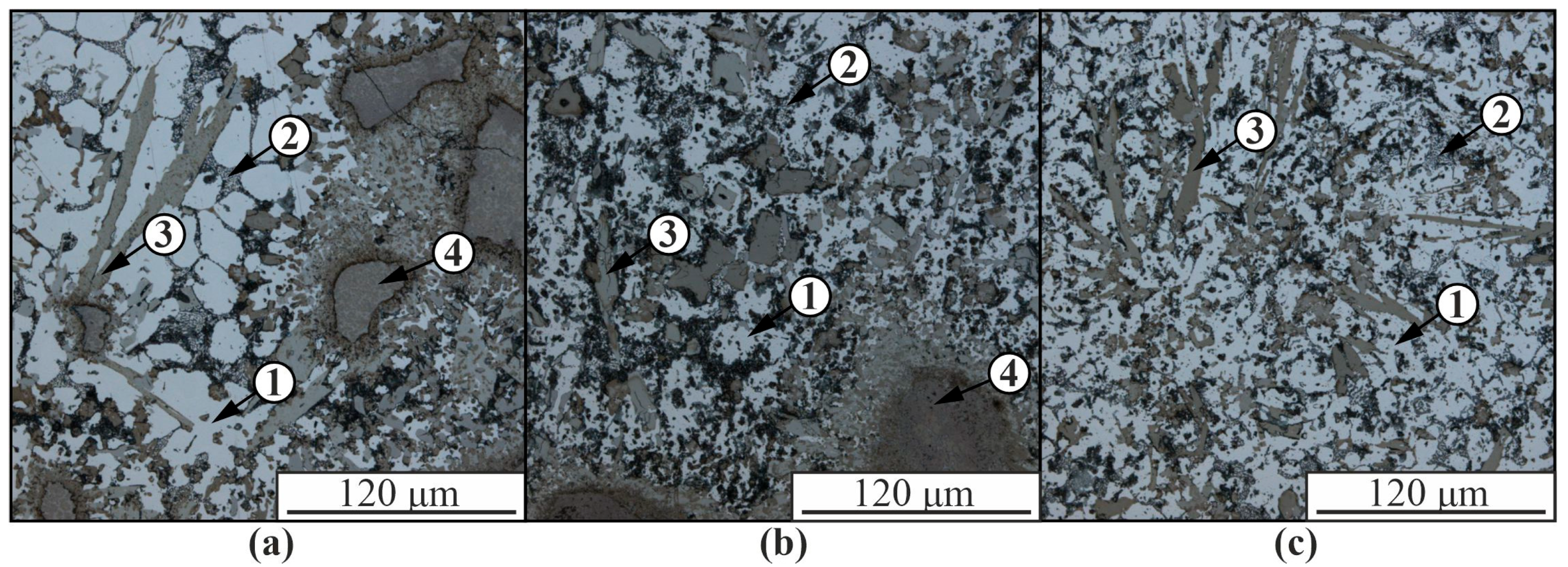
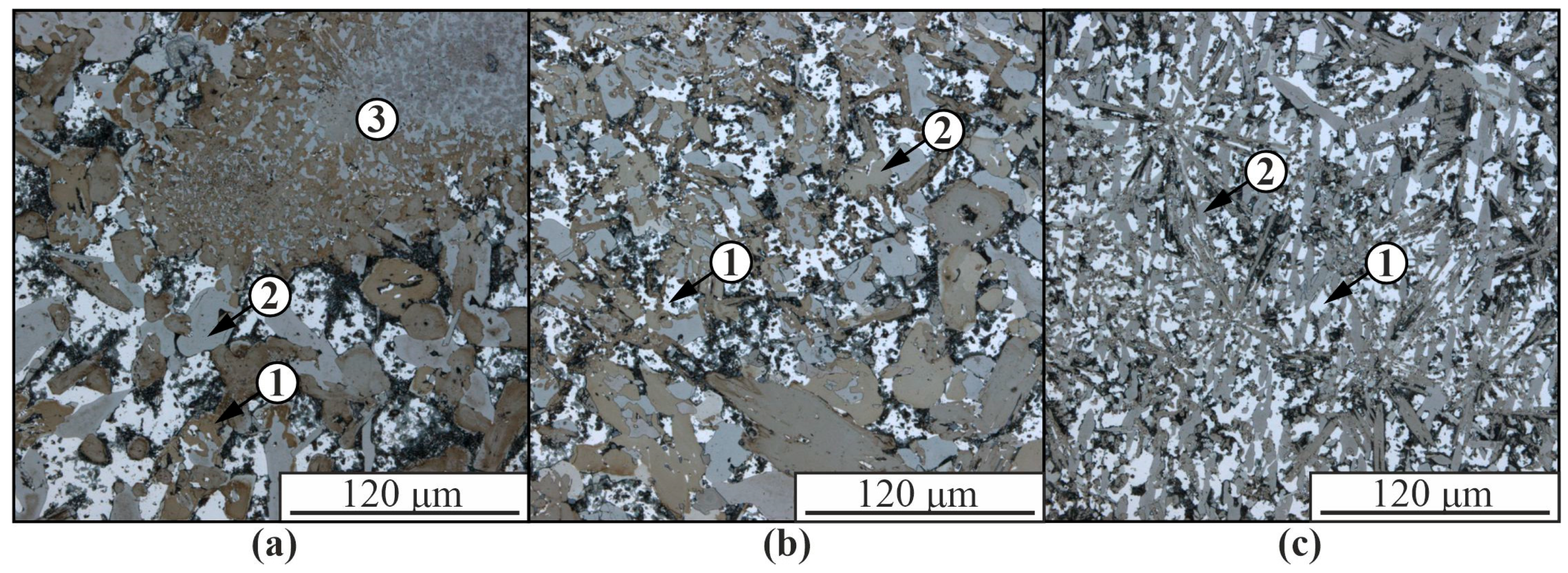
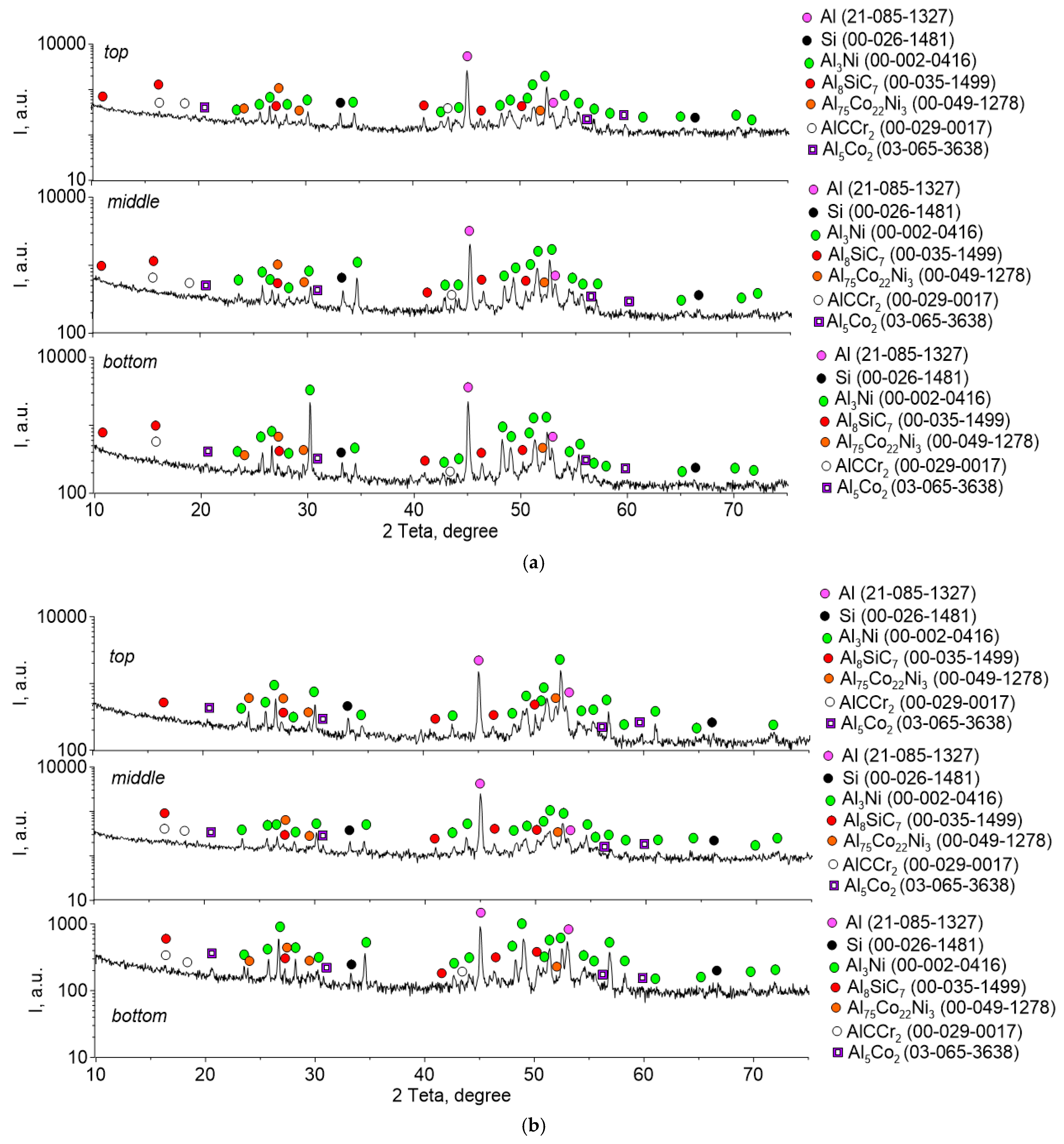
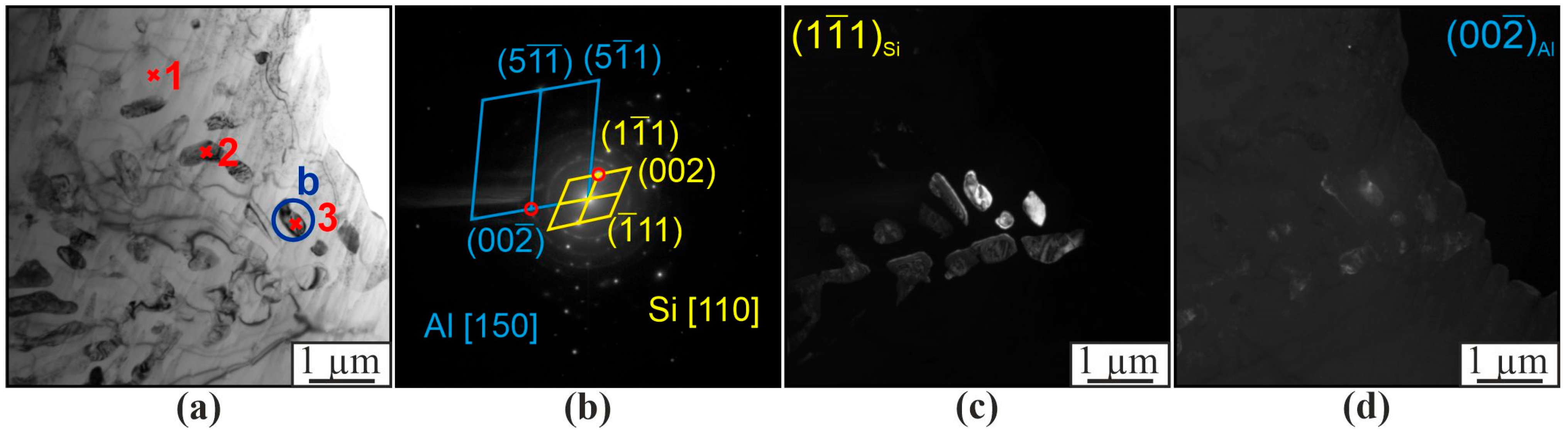
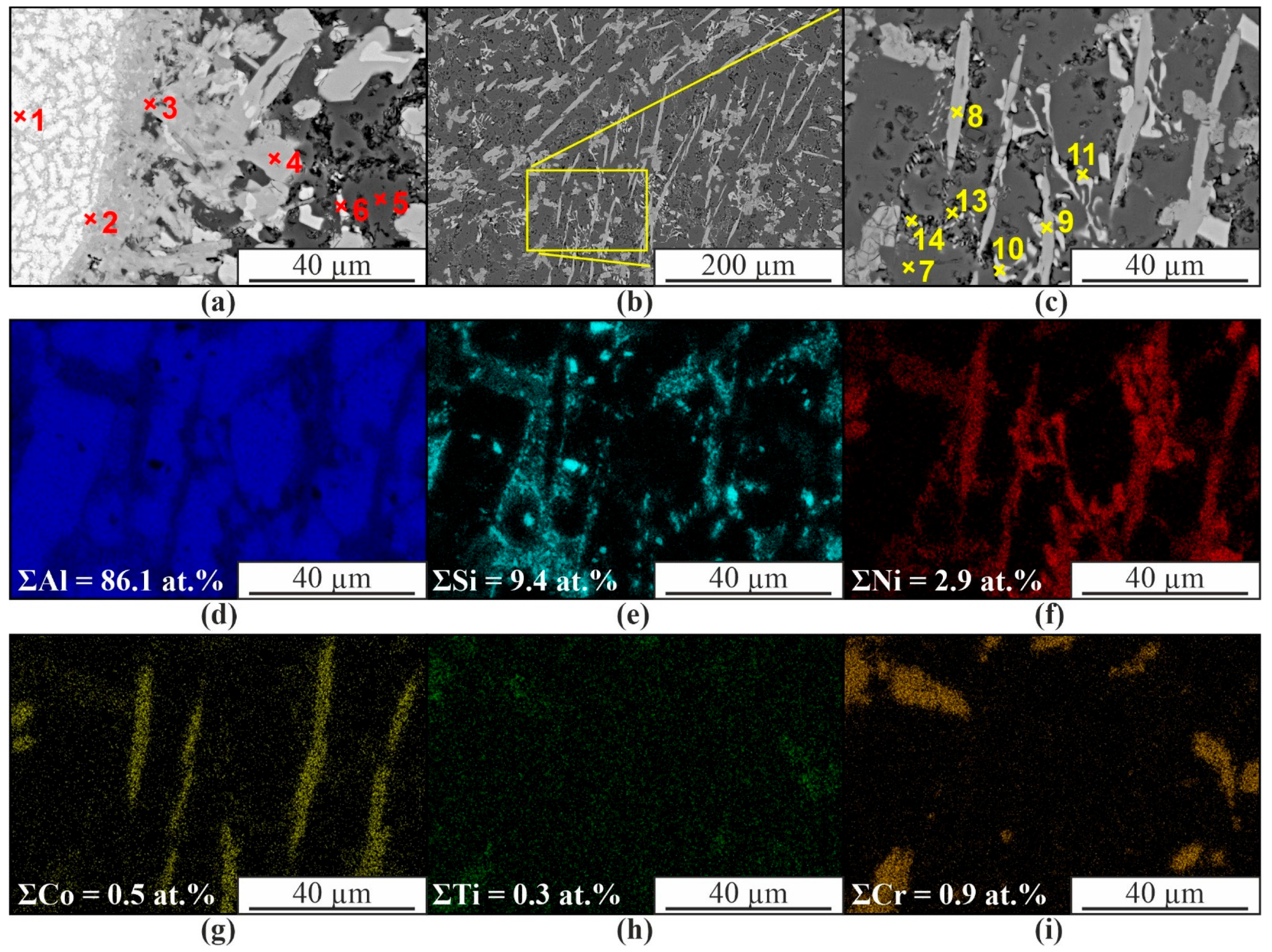
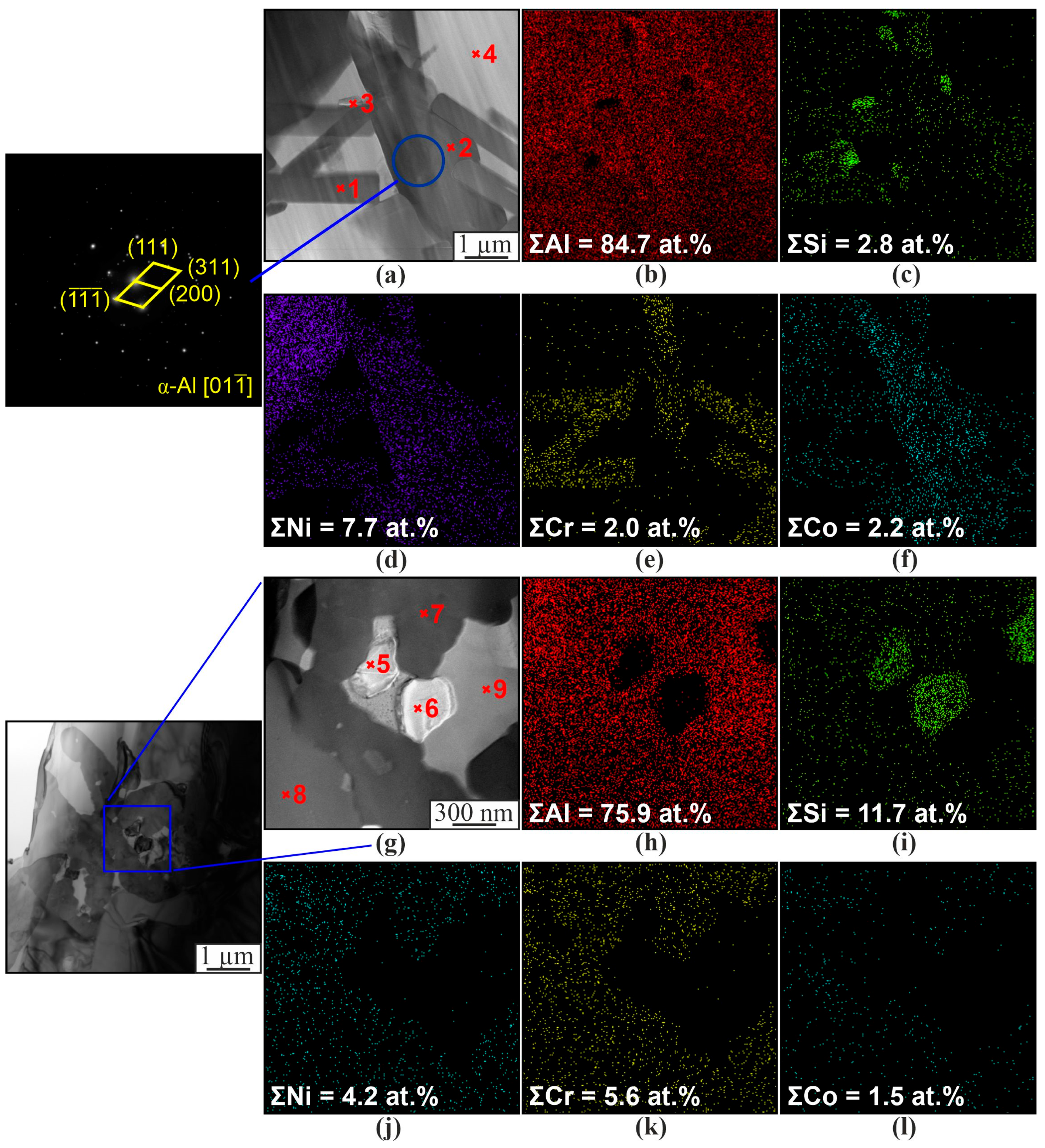
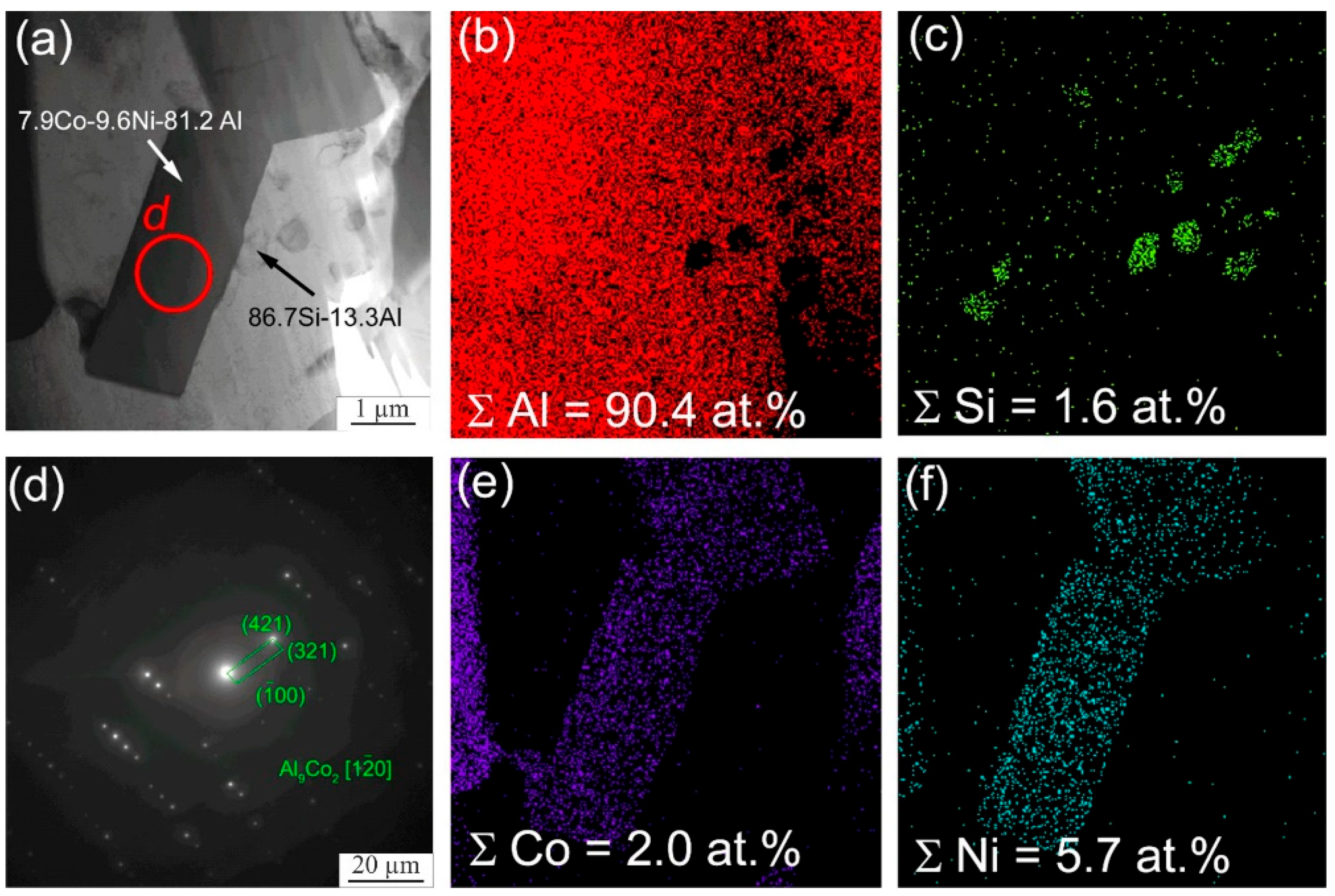
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lukach, I.; Shlesar, M.; Khrokh, P. Structure and mechanical properties of Silumin. Met. Sci. Heat Treat. 1976, 18, 624–626. [Google Scholar] [CrossRef]
- Hernandez, F.C.R.; Ramírez, J.M.H.; Mackay, R. Al-Si Alloys: Automotive, Aeronautical, and Aerospace Applications; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Abdel-Jaber, G.T.; Omran, A.M.; Khalil, K.A.; Fujii, M.; Seki, M.; Yoshida, A. An investigation into solidification and mechanical properties behavior of Al-Si casting alloys. Int. J. Mech. Mechatron. Eng. IJMME-IJENS 2010, 10, 30–35. [Google Scholar]
- Kobayashi, T. Strength and fracture of aluminum alloys. Mater. Sci. Eng. A 2000, 280, 8–16. [Google Scholar] [CrossRef]
- Caceres, C. Microstructure Design and Heat Treatment Selection for Casting Alloys Using the Quality Index. J. Mater. Eng. Perform. 2000, 9, 215–221. [Google Scholar] [CrossRef]
- Perrin, C.; Rainforth, W.M. Work hardening behaviour at the worn surface of Al-Cu and Al-Si alloys. Wear 1997, 203, 171–179. [Google Scholar] [CrossRef]
- Sigworth, G.K. The Modification of Al-Si Casting Alloys: Important Practical and Theoretical Aspects. Int. J. Met. 2008, 2, 19–40. [Google Scholar] [CrossRef]
- Heusler, L.; Schneider, W. Influence of alloying elements on the thermal analysis results of Al-Si cast alloys. J. Light Met. 2002, 2, 17–26. [Google Scholar] [CrossRef]
- Morri, A.; Ceschini, L.; Messieri, S.; Cerri, E.; Toschi, S. Mo addition to the a354 (Al-Si-Cu-Mg) casting alloy: Effects on microstructure and mechanical properties at room and high temperature. Metals 2018, 8, 393. [Google Scholar] [CrossRef] [Green Version]
- Shaha, S.K.; Czerwinski, F.; Kasprzak, W.; Friedman, J.; Chen, D.L. Effect of Zr, V and Ti on hot compression behavior of the Al–Si cast alloy for powertrain applications. J. Alloys Compd. 2014, 615, 1019–1031. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, H.W.; Lee, J.M.; Kim, M.S. A new approach to the design of a low Si-added Al-Si casting alloy for optimising thermal conductivity and fluidity. J. Mater. Sci. 2015, 50, 7271–7281. [Google Scholar] [CrossRef]
- Rana, R.S.; Purohit, R.; Das, S. Reviews on the influences of alloying elements on the microstructure and mechanical properties of aluminum alloys and aluminum alloy composites. Int. J. Sci. Res. Publ. 2012, 2, 1–7. [Google Scholar]
- Saini, N.; Pandey, C.; Thapliyal, S.; Dwivedi, D.K. Mechanical Properties and Wear Behavior of Zn and MoS2 Reinforced Surface Composite Al-Si Alloys Using Friction Stir Processing. Silicon 2018, 10, 1979–1990. [Google Scholar] [CrossRef]
- Shaha, S.K.; Czerwinski, F.; Kasprzak, W.; Friedman, J.; Chen, D.L. Microstructure and mechanical properties of Al–Si cast alloy with additions of Zr-V-Ti. Mater. Des. 2015, 83, 801–812. [Google Scholar] [CrossRef]
- El-Labban, H.F.; Abdelaziz, M.; Mahmoud, E.R. Preparation and characterization of squeeze cast-Al–Si piston alloy reinforced by Ni and nano-Al2O3 particles. J. King Saud Univ.-Eng. Sci. 2016, 28, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Mohan, R.R.; Venkatraman, R.; Raghuraman, S.; Kumar, P.M.; Rinawa, M.L.; Subbiah, R.; Arulmurugan, B.; Rajkumar, S. Processing of Aluminium-Silicon Alloy with Metal Carbide as Reinforcement through Powder-Based Additive Manufacturing: A Critical Study. Scanning 2022, 2022, 5610333. [Google Scholar] [CrossRef]
- Rometsch, P.A.; Zhu, Y.; Wu, X.; Huang, A. Review of high-strength aluminium alloys for additive manufacturing by laser powder bed fusion. Mater. Des. 2022, 219, 10779. [Google Scholar] [CrossRef]
- Hernández-Méndez, F.; Altamirano-Torres, A.; Miranda-Hernández, J.G.; Térres-Rojas, E.; RochaRangel, E. Effect of nickel addition on microstructure and mechanical properties of aluminum-based alloys. Mater. Sci. Forum 2011, 691, 10–14. [Google Scholar] [CrossRef]
- Suwanpreecha, C.; Pandee, P.; Patakham, U.; Limmaneevichitr, C. New generation of eutectic Al-Ni casting alloys for elevated temperature services. Mater. Sci. Eng. A 2018, 709, 46–54. [Google Scholar] [CrossRef]
- Deng, J.; Chen, C.; Liu, X.; Li, Y.; Zhou, K.; Guo, S. A high-strength heat-resistant Al−5.7Ni eutectic alloy with spherical Al3Ni nano-particles by selective laser melting. Scr. Mater. 2021, 203, 114034. [Google Scholar] [CrossRef]
- Gxowa-Penxa, Z.; Daswa, P.; Modiba, R.; Mathabathe, M.; Bolokang, A. Development and characterization of Al-Al3Ni-Sn metal matrix composite. Mater. Chem. Phys. 2020, 259, 124027. [Google Scholar] [CrossRef]
- Zuo, L.; Ye, B.; Feng, J.; Zhang, H.; Kong, X.; Jiang, H. Effect of ε-Al3Ni phase on mechanical properties of Al-Si-Cu-Mg-Ni alloys at elevated temperature. Mater. Sci. Eng. A 2020, 772, 138794. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Dinaharan, I.; Kalaiselvan, K.; Palanivel, R. Friction stir processing of Al3Ni intermetallic particulate reinforced cast aluminum matrix composites: Microstructure and tensile properties. J. Mater. Res. Technol. 2020, 9, 4356–4367. [Google Scholar] [CrossRef]
- Pariyar, A.; Perugu, C.S.; Dash, K.; Kailas, S.V. Microstructure and mechanical behavior of high toughness Al-based metal matrix composite reinforced with in-situ formed nickel aluminides. Mater. Charact. 2020, 171, 110776. [Google Scholar] [CrossRef]
- Khaki-Davoudi, S.; Nourouzi, S.; Aval, H.J. Microstructure and mechanical properties of AA7075/Al3Ni composites produced by compocasting. Mater. Today Commun. 2021, 28, 102537. [Google Scholar] [CrossRef]
- Czerwinski, F.; Aniolek, M.; Li, J. Strengthening retention and structural stability of the Al-Al3Ni eutectic at high temperatures. Scr. Mater. 2022, 214, 114679. [Google Scholar] [CrossRef]
- Wang, X.; Ran, Z.; Wei, Z.; Zou, C.; Wang, H.; Gouchi, J.; Uwatoko, Y. The formation of bulk β-Al3Ni phase in eutectic Al-5.69wt%Ni alloy solidified under high pressure. J. Alloys Compd. 2018, 742, 670–675. [Google Scholar] [CrossRef]
- Gonzalez, G.; Sagarzazu, A.; Bonyuet, D.; D’angelo, L.; Villalba, R. Solid state amorphisation in binary systems prepared by mechanical alloying. J. Alloys Compd. 2009, 483, 289–297. [Google Scholar] [CrossRef]
- Zhang, Z.; Akiyama, E.; Watanabe, Y.; Katada, Y.; Tsuzaki, K. Effect of α-Al/Al3Ni microstructure on the corrosion behaviour of Al-5.4wt% Ni alloy fabricated by equal-channel angular pressing. Corros. Sci. 2007, 49, 2962–2972. [Google Scholar] [CrossRef]
- Uan, J.; Chen, L.; Lui, T. On the extrusion microstructural evolution of Al-Al3Ni in situ composite. Acta Mater. 2001, 49, 313–320. [Google Scholar] [CrossRef]
- Song, C.-J.; Xu, Z.-M.; Li, J.-G. In-situ Al/Al3Ni functionally graded materials by electromagnetic separation method. Mater. Sci. Eng. A 2007, 445–446, 148–154. [Google Scholar] [CrossRef]
- Qian, J.; Li, J.; Xiong, J.; Zhang, F.; Lin, X. In situ synthesizing Al3Ni for fabrication of intermetallic-reinforced aluminum alloy composites by friction stir processing. Mater. Sci. Eng. A 2012, 550, 279–285. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Frederick, A.; Wang, Y.; Wang, Y.; Allard, L.; Koehler, M.; Shin, S.; Hu, A.; Feng, Z. In-situ formation of Al3Ni nano particles in synthesis of Al 7075 alloy by friction stir processing with Ni powder addition. J. Mater. Process. Technol. 2023, 311, 117803. [Google Scholar] [CrossRef]
- Dinaharan, I. Liquid metallurgy processing of intermetallic matrix composites. In Intermetallic Matrix Composites: Properties and Applications; Mitra, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 167–202. [Google Scholar]
- Mitra, R. Composites of Nickel Aluminides, Intermetallic Matrix Composites: Properties and Applications; Woodhead Publishing Limited: Sawston, UK, 2018; pp. 37–59. [Google Scholar]
- Camagu, S.; Mathabathe, M.; Motaung, D.; Muller, T.; Arendse, C.; Bolokang, A. Investigation into the thermal behaviour of the B2–NiAl intermetallic alloy produced by compaction and sintering of the elemental Ni and Al powders. Vacuum 2019, 169, 108919. [Google Scholar] [CrossRef]
- Fuchs, J.; Schneider, C.; Enzinger, N. Wire-based additive manufacturing using an electron beam as heat source. Weld. World 2018, 62, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Osipovich, K.S.; Astafurova, E.G.; Chumaevskii, A.V.; Kalashnikov, K.N.; Astafurov, S.V.; Maier, G.G.; Kolubaev, E.A. Gradient transition zone structure in “steel–copper” sample produced by double wire-feed electron beam additive manufacturing. J. Mater. Sci. 2020, 55, 9258–9272. [Google Scholar] [CrossRef]
- Osipovich, K.; Kalashnikov, K.; Chumaevskii, A.; Gurianov, D.; Kalashnikova, T.; Vorontsov, A.; Zykova, A.; Utyaganova, V.; Panfilov, A.; Nikolaeva, A.; et al. Wire-Feed Electron Beam Additive Manufacturing: A Review. Metals 2023, 13, 279. [Google Scholar] [CrossRef]
- Ding, D.; Pan, Z.; Cuiuri, D.; Li, H. Wire-feed additive manufacturing of metal components: Technologies, developments and future interests. Int. J. Adv. Manuf. Technol. 2015, 81, 465–481. [Google Scholar] [CrossRef]
- Pu, Z.; Du, D.; Wang, K.; Liu, G.; Zhang, D.; Zhang, H.; Xi, R.; Wang, X.; Chang, B. Study on the NiTi shape memory alloys in-situ synthesized by dual-wire-feed electron beam additive manufacturing. Addit. Manuf. 2022, 56, 102886. [Google Scholar] [CrossRef]
- Pu, Z.; Chang, B. Control of droplet transfer during in-situ synthesis of NiTi alloys by dual-wire electron beam additive manufacturing. J. Phys. Conf. Ser. 2022, 2369, 012011. [Google Scholar] [CrossRef]
- Kolubaev, E.A.; Rubtsov, V.E.; Chumaevsky, A.V.; Astafurova, E.G. Micro-, Meso- and Macrostructural Design of Bulk Metallic and Polymetallic Materials by Wire-Feed Electron-Beam Additive Manufacturing. Phys. Mesomech. 2022, 25, 479–491. [Google Scholar] [CrossRef]
- Li, Z.; Chang, B.; Cui, Y.; Zhang, H.; Liang, Z.; Liu, C.; Wang, L.; Du, D.; Chang, S. Effect of twin-wire feeding methods on the in-situ synthesis of electron beam fabricated Ti-Al-Nb intermetallics. Mater. Des. 2022, 215, 110509. [Google Scholar] [CrossRef]
- Zykova, A.; Chumaevskii, A.; Vorontsov, A.; Kalashnikov, K.; Gurianov, D.; Gusarova, A.; Kolubaev, E. Evolution of microstructure and properties of Fe-Cu, manufactured by electron beam additive manufacturing with subsequent friction stir processing. Mater. Lett. 2021, 307, 131023. [Google Scholar] [CrossRef]
- Lei, S.; Li, X.; Deng, Y.; Xiao, Y.; Chen, Y.; Wang, H. Microstructure and mechanical properties of electron beam freeform fabricated TiB2/Al-Cu composite. Mater. Lett. 2020, 277, 128273. [Google Scholar] [CrossRef]
- Chen, G.; Shu, X.; Liu, J.; Zhang, B.; Feng, J. A new coating method with potential for additive manufacturing: Premelting electron beam-assisted freeform fabrication. Addit. Manuf. 2020, 33, 101118. [Google Scholar] [CrossRef]
- Zykova, A.; Chumaevskii, A.; Panfilov, A.; Vorontsov, A.; Nikolaeva, A.; Osipovich, K.; Gusarova, A.; Chebodaeva, V.; Nikonov, S.; Gurianov, D.; et al. Aluminum Bronze/Udimet-500 Composites Prepared by Electron-Beam Additive Double-Wire-Feed Manufacturing. Materials 2022, 15, 6270. [Google Scholar] [CrossRef]
- Kalashnikov, K.; Kalashnikova, T.; Semenchuk, V.; Knyazhev, E.; Panfilov, A.; Cheremnov, A.; Chumaevskii, A.; Nikonov, S.; Vorontsov, A.; Rubtsov, V.; et al. Development of a Multimaterial Structure Based on CuAl9Mn2 Bronze and Inconel 625 Alloy by Double-Wire-Feed Additive Manufacturing. Metals 2022, 12, 2048. [Google Scholar] [CrossRef]
- Filippov, A.; Utyaganova, V.; Shamarin, N.; Vorontsov, A.; Savchenko, N.; Gurianov, D.; Chumaevskii, A.; Rubtsov, V.; Kolubaev, E.; Tarasov, S. Microstructure and Corrosion Resistance of AA4047/AA7075 Transition Zone Formed Using Electron Beam Wire-Feed Additive Manufacturing. Materials 2021, 14, 6931. [Google Scholar] [CrossRef]


| Material | Elements, wt. % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Mn | Fe | Zn | Cr | Co | Mo | Ti | Si | Zr | Ni | V | |
| ER4043 (AlSi5) | bal. | 0.032 | 0.09 | 0.07 | - | - | - | 0.01 | 5.1 | - | - | 0.021 |
| Udimet-500 | 1.6 | 0.1 | 0.4 | 0.04 | 17.3 | 13.2 | 4.3 | 2.7 | 0.3 | 0.04 | bal. | - |
| Al–5% Ni | 82.18 | - | 0.127 | - | 1.88 | 1.33 | - | 0.324 | 7.36 | - | 6.31 | 0.035 |
| Al-10%Ni | 66.53 | - | 1.08 | - | 5.16 | 3.44 | - | 0.654 | 5.35± | - | 16.29 | 0.06 |
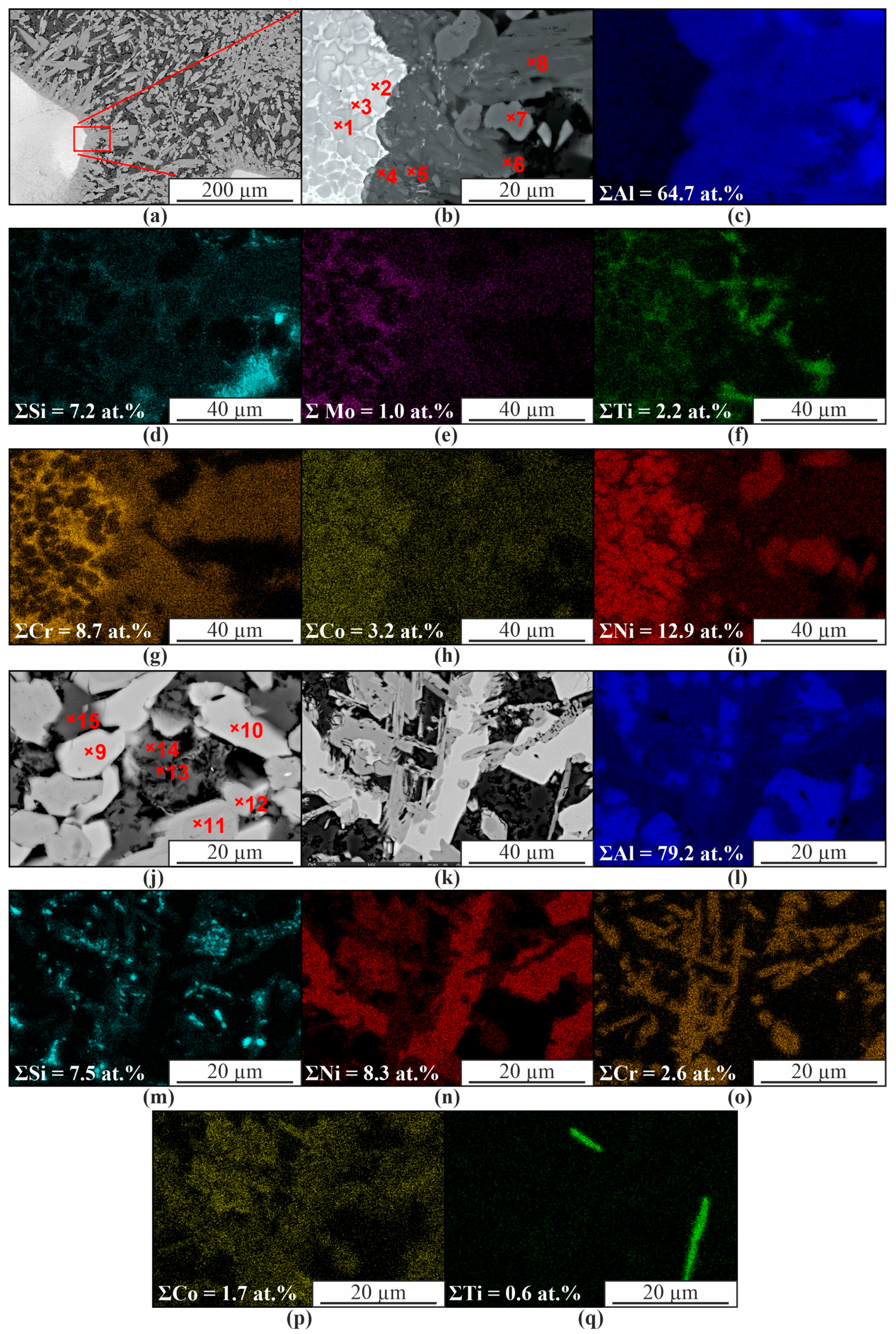
| Spectrum | Chemical Composition, at.% | Possible Phase | |||||
|---|---|---|---|---|---|---|---|
| Al | Si | Ti | Cr | Co | Ni | ||
| 1 | 54.5 | 1.3 | 1.0 | 4.6 | 6.7 | 31.9 | AlNi |
| 2 | 63.5 | 5.4 | 1.0 | 4.6 | 5.0 | 20.5 | Al3Ni |
| 3 | 75.8 | 5.1 | 1.0 | 6.6 | 3.2 | 8.2 | Al3(Ni,Cr,Si) |
| 4 | 82.8 | 2.4 | 0.3 | 1.4 | 6.4 | 6.8 | Al(Co,Ni) |
| 5 | 97.9 | 1.2 | 0.4 | 0.3 | 0.1 | 0.2 | α-Al |
| 6 | 74.8 | 6.1 | 0.1 | 0.4 | 0.5 | 18.0 | Al3Ni |
| 7 | 98.8 | 0.7 | 0.2 | 0.1 | - | 0.1 | α-Al |
| 8 | 81.3 | 4.7 | 0.1 | 0.1 | 4.3 | 9.6 | Al(Co,Ni, Si) |
| 9 | 80.5 | 1.7 | 0.1 | 0.2 | 5.2 | 12.2 | Al(Co,Ni, Si) |
| 10 | 78.4 | 1.2 | 0.1 | - | 0.6 | 19.8 | Al3Ni |
| 11 | 80.6 | 3.6 | 0.1 | - | 0.5 | 15.3 | Al3Ni |
| 12 | 84.9 | 1.2 | 0.1 | 0.1 | 0.3 | 13.4 | Al3Ni |
| 13 | 72.0 | 18.3 | 0.1 | - | 0.3 | 9.4 | α-Al, β-Si |
| 14 | 73.0 | 18.7 | 0.1 | 0.1 | 0.2 | 7.8 | α-Al, β-Si |
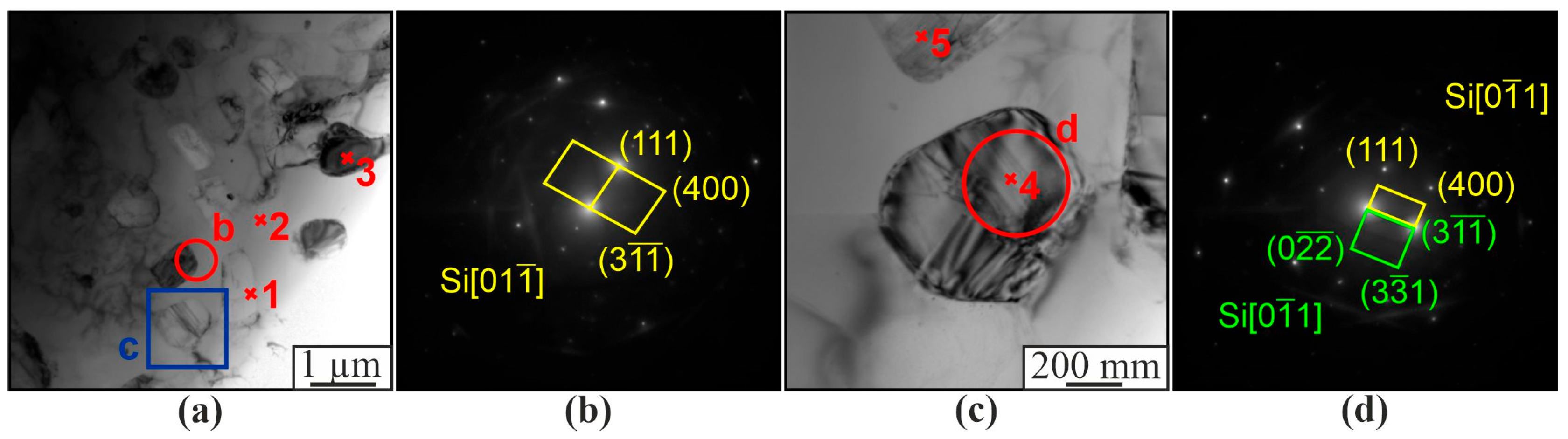
| Spectrum | Chemical Composition, at.% | Possible Phase | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Al | Si | Ti | Cr | Co | Ni | Fe | Mo | ||
| 1 | 77.8 | 5.6 | 0.6 | 7.5 | 1.9 | 5.2 | 0.1 | 0.3 | Al3(Ni,Cr,Si) |
| 2 | 80.8 | 1.6 | 0.03 | 1.6 | 6.7 | 8.9 | 0.1 | 0.1 | Al(Co,Ni) |
| 3 | 2.0 | 98.0 | - | - | - | - | - | - | β-Si |
| 4 | 99.0 | 0.1 | - | - | - | - | - | - | α-Al |
| 5 | 1.7 | 98.3 | - | - | - | - | - | - | β-Si |
| 6 | 3.1 | 96.9 | - | - | - | - | - | - | β-Si |
| 7 | 76.1 | 7.2 | 0.2 | 7.3 | 1.9 | 6.3 | - | - | Al3(Ni,Cr,Si) |
| 8 | 77.3 | 5.5 | 0.3 | 7.6 | 2.1 | 5.6 | - | - | Al3(Ni,Cr,Si) |
| 9 | 99.9 | 0.1 | - | - | - | - | - | - | α-Al |
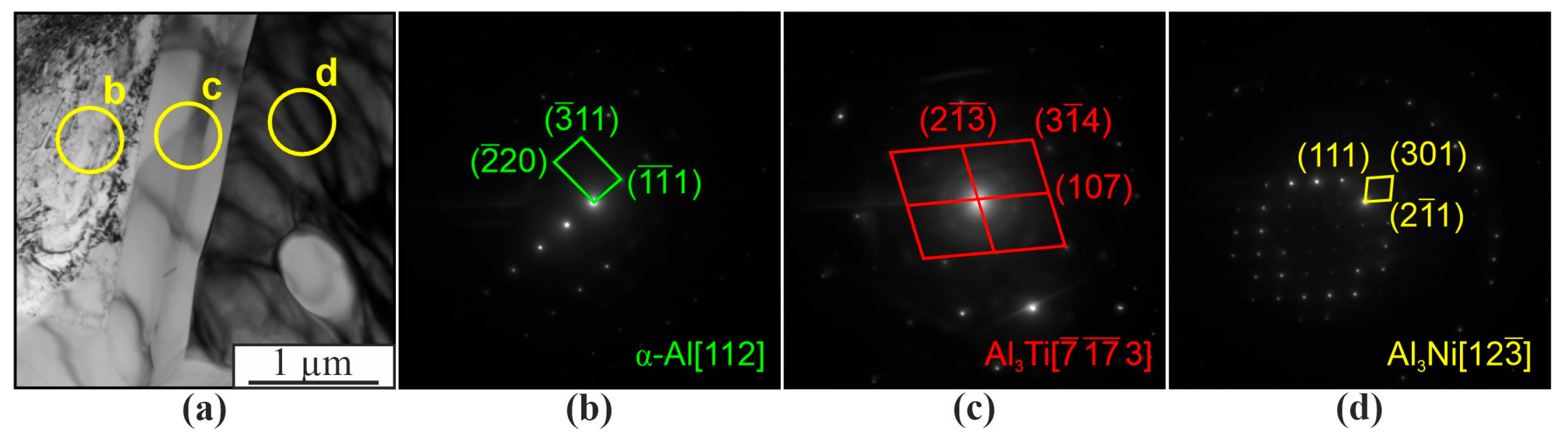
| Spectrum | Chemical Composition, at.% | Possible Phase | ||||||
|---|---|---|---|---|---|---|---|---|
| Al | Si | Ti | Cr | Co | Ni | Mo | ||
| 1 | 42.8 | 1.0 | 1.8 | 7.3 | 7.9 | 39.2 | - | AlNi(Cr,Co) |
| 2 | 40.0 | 0.4 | 1.8 | 6.2 | 8.0 | 43.7 | - | AlNi(Cr,Co) |
| 3 | 26.9 | 6.0 | 7.0 | 39.2 | 5.1 | 11.6 | 4.3 | AlCCr2 |
| 4 | 53.5 | 4.0 | 2.9 | 10.2 | 5.2 | 23.4 | 0.8 | Al3Ni |
| 5 | 73.1 | 4.7 | 0.5 | 9.6 | 3.0 | 8.1 | 1.0 | Al3(Ni,Cr,Si) |
| 6 | 73.7 | 5.4 | 0.5 | 9.9 | 2.8 | 6.6 | 1.1 | Al3(Ni,Cr,Si) |
| 7 | 72.6 | 0.7 | 0.2 | 0.5 | 4.0 | 22.0 | - | Al3Ni |
| 8 | 74.0 | 6.2 | 0.4 | 9.4 | 2.3 | 6.7 | 0.9 | Al3(Ni,Cr,Si) |
| 9 | 73.6 | 0.6 | 0.1 | 0.2 | 3.4 | 22.1 | - | Al3Ni |
| 10 | 74.0 | 0.5 | 0.2 | 0.3 | 2.6 | 22.4 | - | Al3Ni |
| 11 | 75.4 | 4.5 | 1.0 | 9.4 | 2.8 | 5.9 | 1.0 | Al3(Ni,Cr,Si) |
| 12 | 75.9 | 5.5 | 0.2 | 8.8 | 2.3 | 6.4 | 1.0 | Al3(Ni,Cr,Si) |
| 13 | 32.5 | 58.8 | 0.3 | 2.2 | 0.8 | 5.3 | - | β-Si |
| 14 | 96.7 | 2.6 | 0.2 | 0.1 | 0.1 | 0.3 | - | α-Al |
| 15 | 96.4 | 1.5 | 0.3 | 0.8 | 0.2 | 0.8 | - | α-Al |
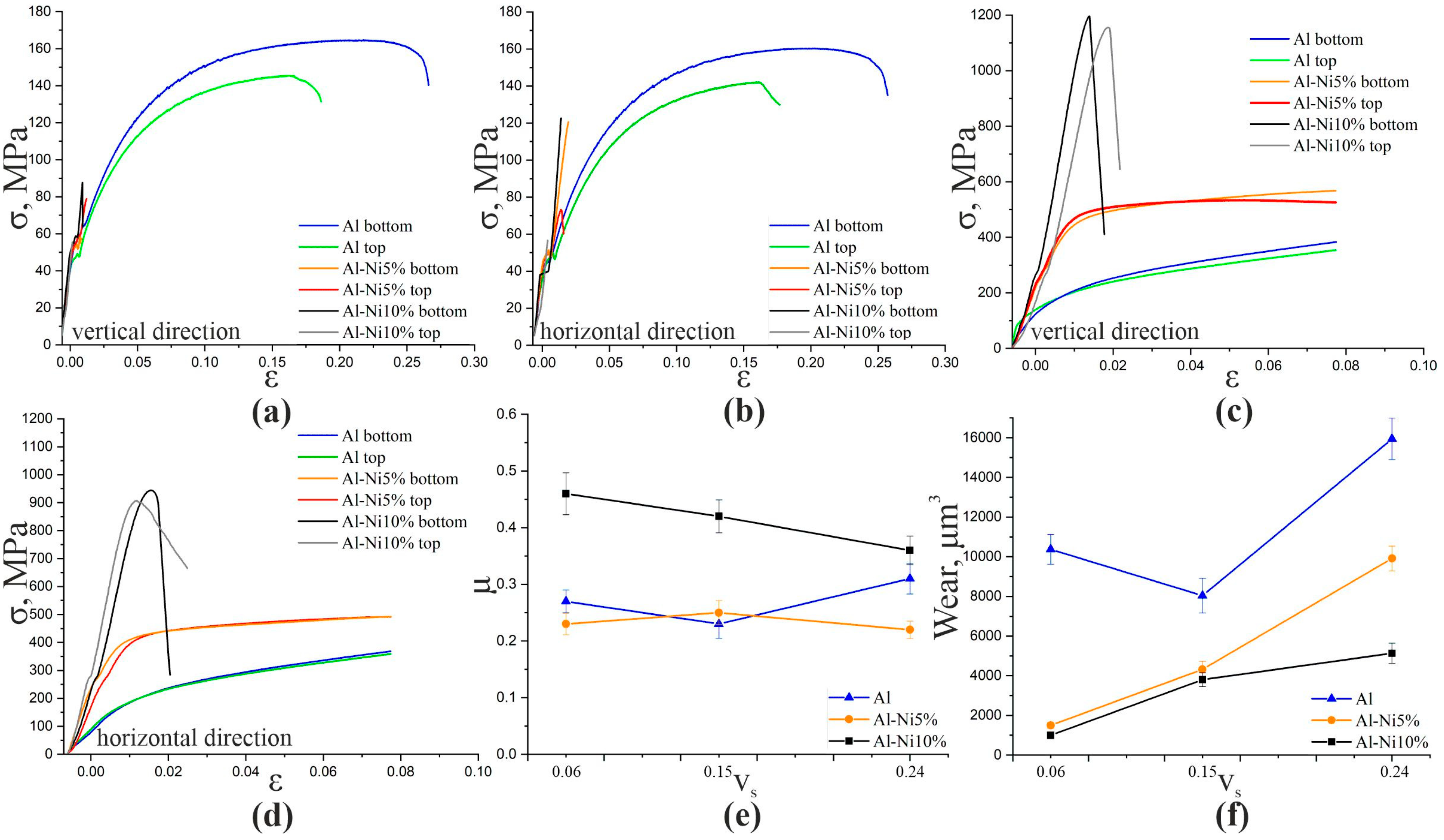
| Material | Test | Al | Al-Ni5% | Al-Ni10% | |||
|---|---|---|---|---|---|---|---|
| Top | Bottom | Top | Bottom | Top | Bottom | ||
| Vertical | Tension | 142 | 143 | 74 | 121 | 57 | 123 |
| Horizontal | 145 | 164 | 79 | 59 | 55 | 88 | |
| Vertical | Compression | - | - | 530 | 570 | 1150 | 1200 |
| Horizontal | - | - | 490 | 500 | 905 | 945 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrovolskii, A.; Chumaevskii, A.; Zykova, A.; Savchenko, N.; Gurianov, D.; Nikolaeva, A.; Semenchuk, N.; Nikonov, S.; Sokolov, P.; Rubtsov, V.; et al. Al–Al3Ni In Situ Composite Formation by Wire-Feed Electron-Beam Additive Manufacturing. Materials 2023, 16, 4157. https://doi.org/10.3390/ma16114157
Dobrovolskii A, Chumaevskii A, Zykova A, Savchenko N, Gurianov D, Nikolaeva A, Semenchuk N, Nikonov S, Sokolov P, Rubtsov V, et al. Al–Al3Ni In Situ Composite Formation by Wire-Feed Electron-Beam Additive Manufacturing. Materials. 2023; 16(11):4157. https://doi.org/10.3390/ma16114157
Chicago/Turabian StyleDobrovolskii, Artem, Andrey Chumaevskii, Anna Zykova, Nikolay Savchenko, Denis Gurianov, Aleksandra Nikolaeva, Natalia Semenchuk, Sergey Nikonov, Pavel Sokolov, Valery Rubtsov, and et al. 2023. "Al–Al3Ni In Situ Composite Formation by Wire-Feed Electron-Beam Additive Manufacturing" Materials 16, no. 11: 4157. https://doi.org/10.3390/ma16114157
APA StyleDobrovolskii, A., Chumaevskii, A., Zykova, A., Savchenko, N., Gurianov, D., Nikolaeva, A., Semenchuk, N., Nikonov, S., Sokolov, P., Rubtsov, V., & Kolubaev, E. (2023). Al–Al3Ni In Situ Composite Formation by Wire-Feed Electron-Beam Additive Manufacturing. Materials, 16(11), 4157. https://doi.org/10.3390/ma16114157









