A Kinetic Study on Crystallization in TiO2-SiO2-CaO-Al2O3 Glass under Nucleation Saturation Conditions for the High Value-Added Utilization of CaO-SiO2-Based Solid Wastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Glass Preparation
2.2. Test of Differential Thermal Analysis
2.3. Crystal Identification and Phase Analysis
3. Results and Discussion
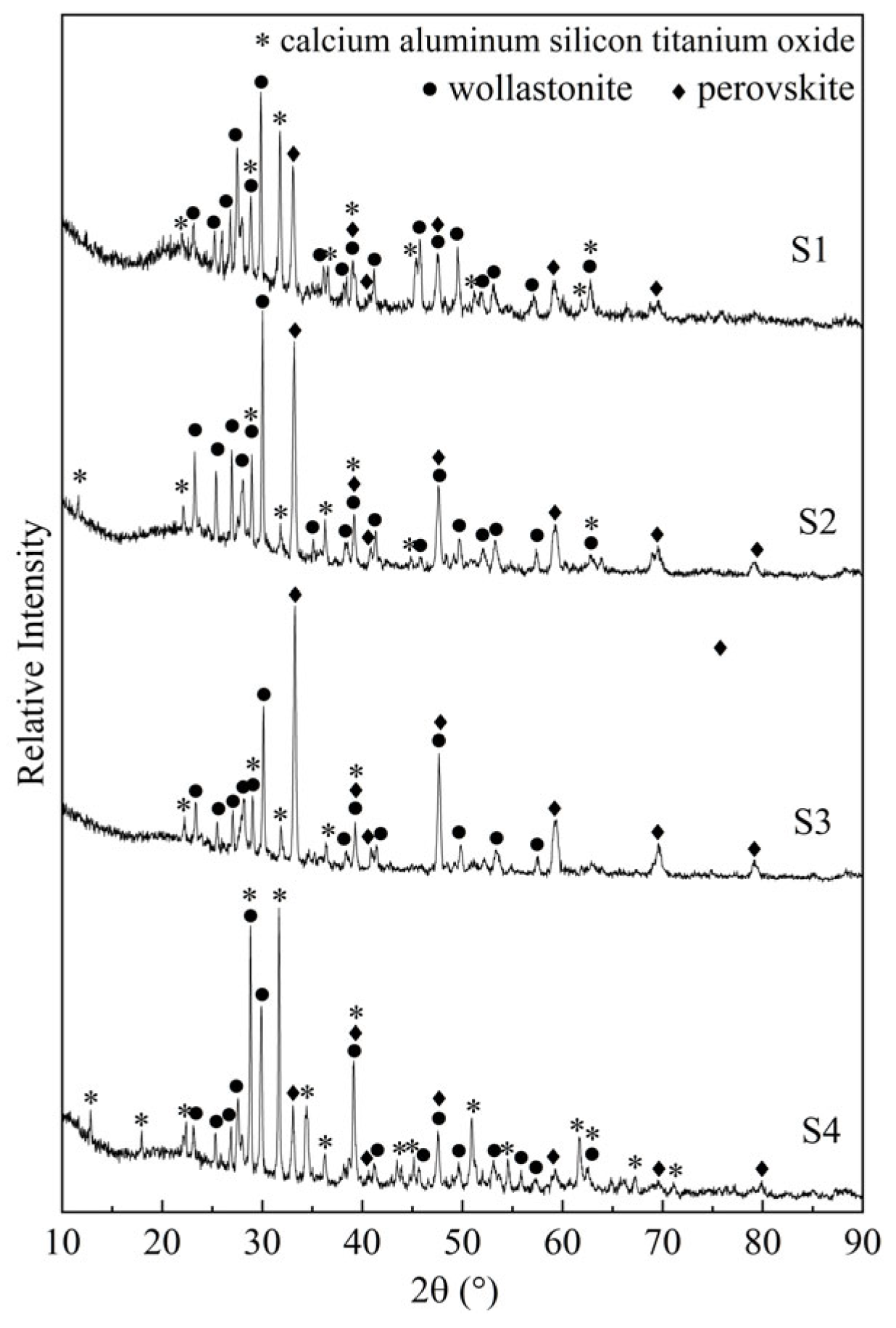
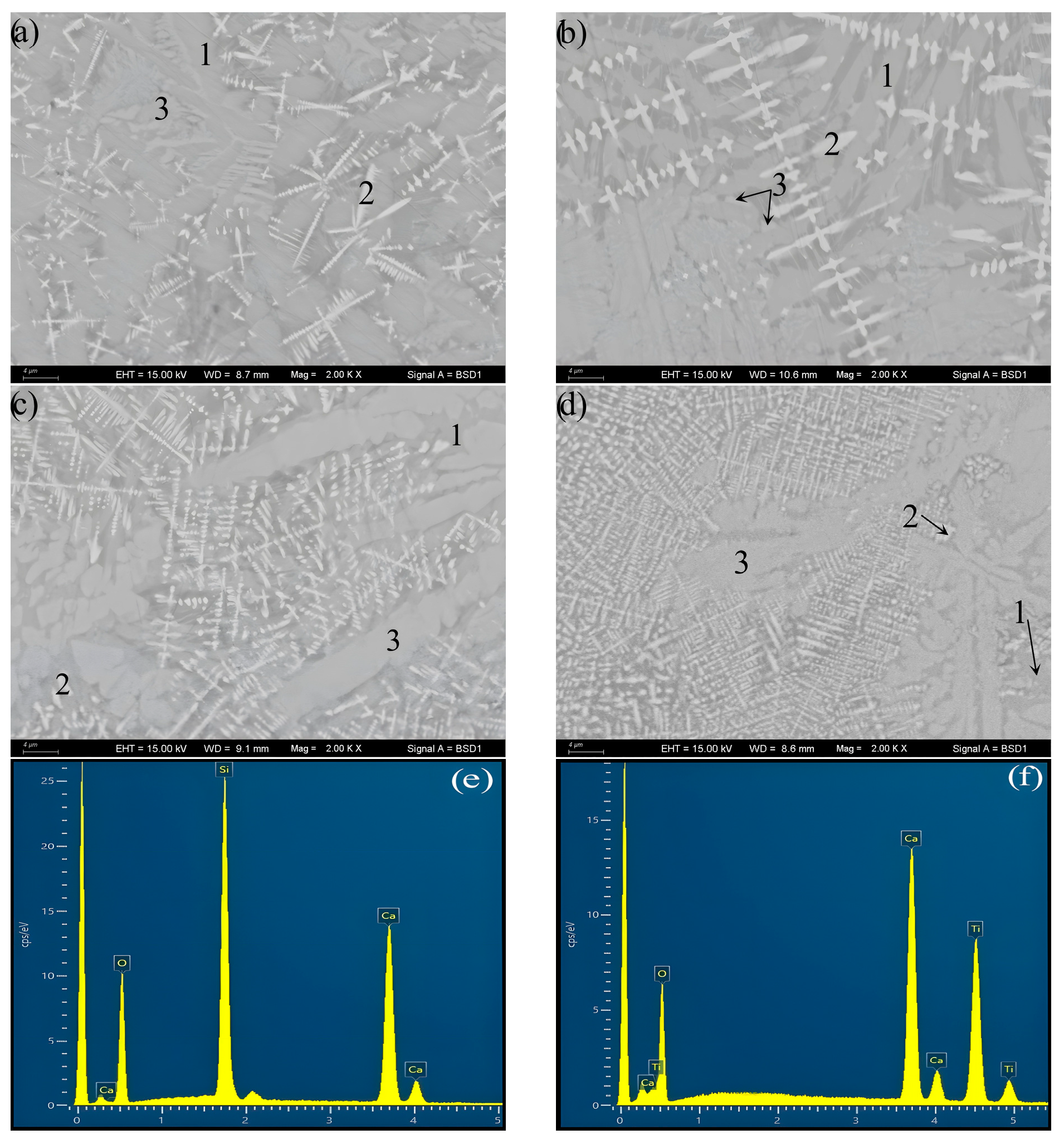
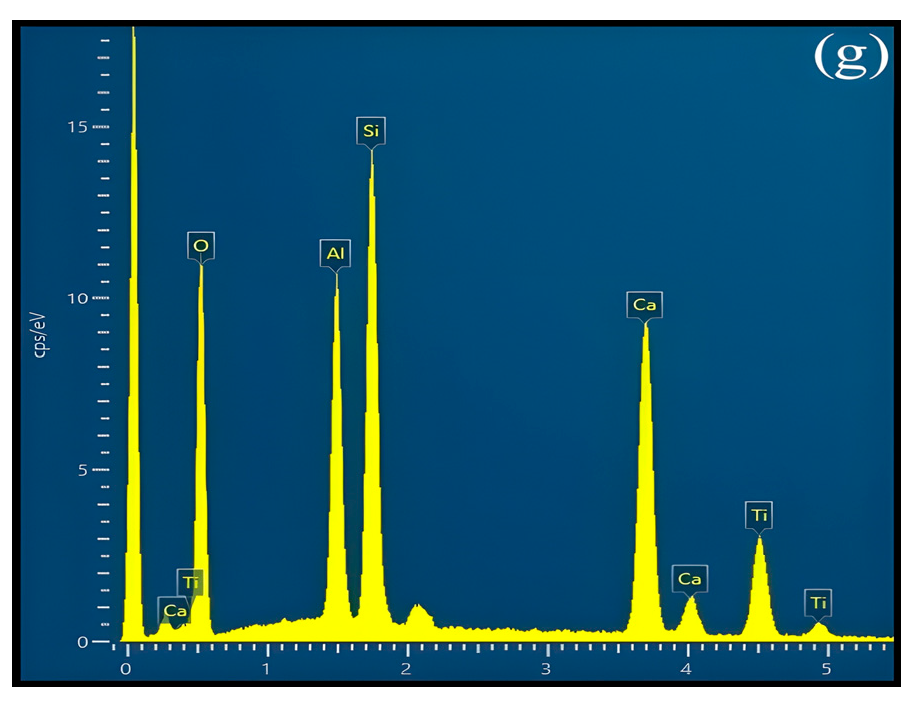
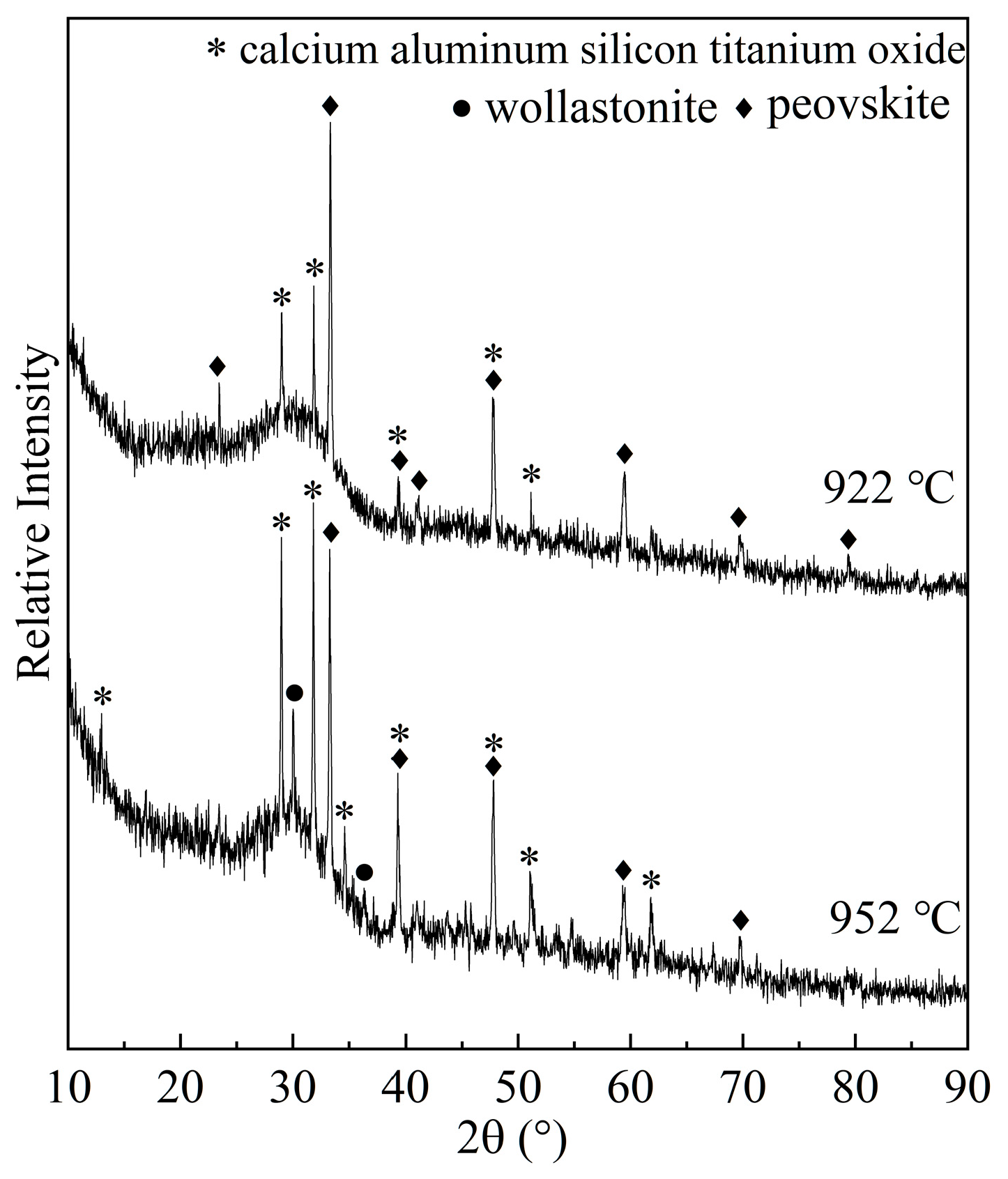
3.1. Crystal Identification
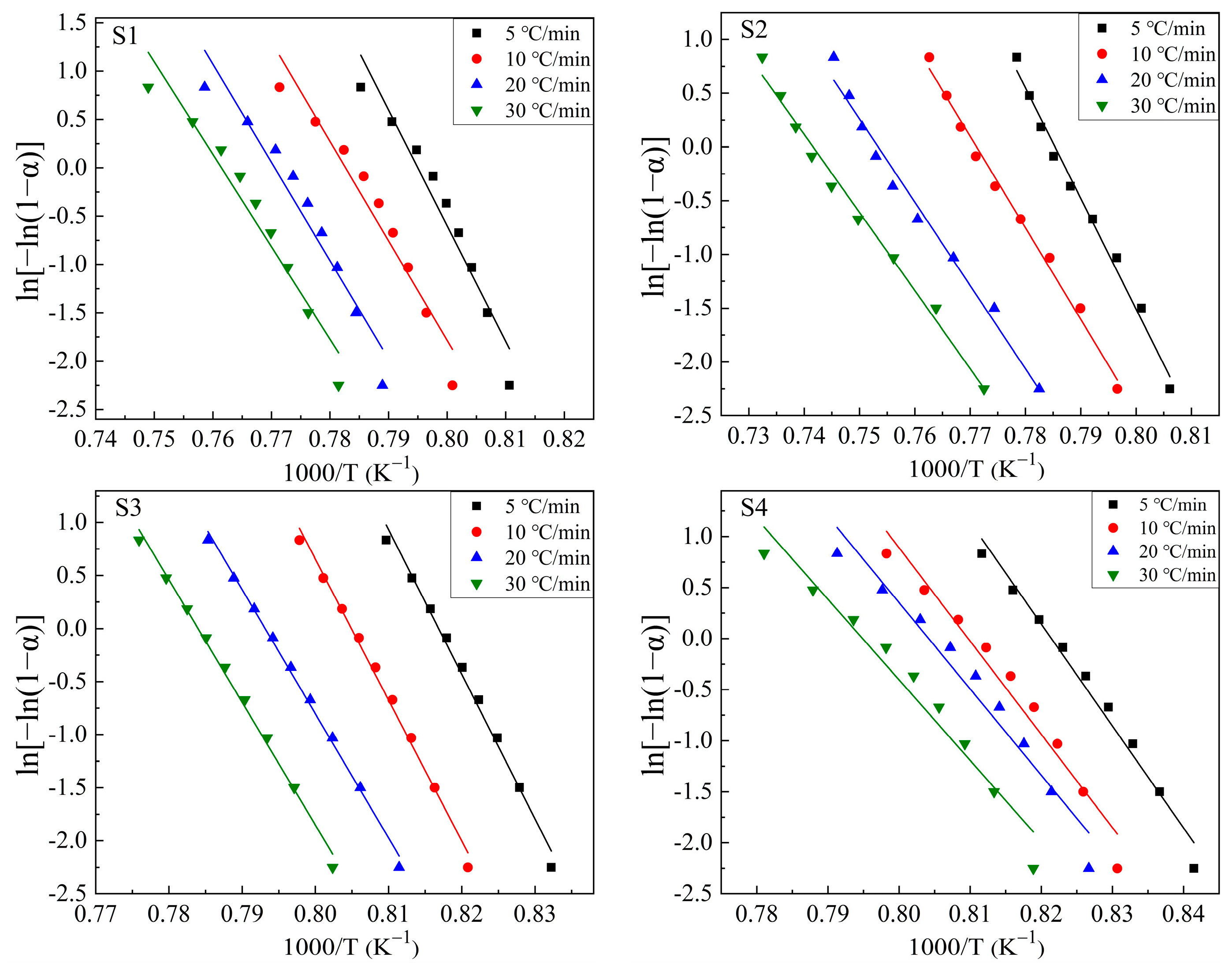
3.2. Crystallization Kinetics
3.2.1. Theoretical Basis
3.2.2. Kinetic Analysis of the Crystallization of CaO-SiO2-Al2O3-TiO2 Glass
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Z.; Leng, H.; Wang, L.; Chou, K. Computational study on various properties of CaO-Al2O3-SiO2 mold flux. Ceram. Int. 2019, 45, 7180–7187. [Google Scholar] [CrossRef]
- Sohn, I.; Min, D.J. A Review of the Relationship between viscosity and the structure of calcium-silicate-based slags in ironmaking. Steel Res. Int. 2012, 83, 611–630. [Google Scholar] [CrossRef]
- Zhang, R.; Min, Y.; Wang, Y.; Zhao, X.; Jia, J.X.; Liu, C.J. Viscosity estimation of multicomponent slags of the CaO-SiO2-Al2O3-FexO system based on microstructure analysis. Energy Fuels 2020, 34, 8129–8138. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, G.; Chou, K.; Fan, D. Mixed alkali effect in viscosity of CaO-SiO2-Al2O3-R2O melts. Metall. Mater. Trans. B 2020, 51, 985–1002. [Google Scholar] [CrossRef]
- Tian, J.; Sun, W.; Zhang, X.; Han, H.; Yu, Z.; Yue, T.; Wang, L.; Yang, Y.; Tang, H.; Li, E. Comprehensive utilization and safe disposal of hazardous arsenic-alkali slag by the combination of beneficiation and metallurgy. J. Clean. Prod. 2021, 295, 126381. [Google Scholar] [CrossRef]
- Brand, A.S.; Fanijo, E.O. A review of the influence of steel furnace slag type on the properties of cementitious composites. Appl. Sci. 2020, 10, 8210. [Google Scholar] [CrossRef]
- Chen, L.; Long, Y.; Zhou, M.; Wang, H. Structure and Crystallization of High-Calcium, CMAS Glass Ceramics Synthesized with a High Content of Slag. Materials 2022, 15, 657. [Google Scholar] [CrossRef]
- Gao, J.; Lan, X.; Guo, Z. Recovery of rutile from Ti-bearing blast furnace slag through phase transformation and super-gravity separation for dielectric material. Ceram. Int. 2020, 46, 9885–9893. [Google Scholar] [CrossRef]
- Gorokhovsky, A.; Escalante-Garcia, J.I.; Gorokhovsky, V.; Mescheryakov, D. Inorganic wastes in the manufacture of glass and glass-ceramics: Quartz-feldspar waste of ore refining, metallurgical slag, limestone dust, and phosphorus slurry. J. Am. Ceram. Soc. 2002, 85, 285–287. [Google Scholar] [CrossRef]
- Hallet, V.; De Belie, N.; Pontikes, Y. The impact of slag fineness on the reactivity of blended cements with high-volume non-ferrous metallurgy slag. Constr. Build. Mater. 2020, 257, 119400. [Google Scholar] [CrossRef]
- Hu, A.; Li, M.; Mao, D. Controlled crystallization of glass-ceramics with two nucleating agents. Mater. Charact. 2009, 60, 1529–1533. [Google Scholar] [CrossRef]
- Pei, F.; Zhu, G.; Li, P.; Guo, H.; Yang, P. Effects of CaF2 on the sintering and crystallisation of CaO-MgO-Al2O3-SiO2 glass-ceramics. Ceram. Int. 2020, 46, 17825–17835. [Google Scholar] [CrossRef]
- Tulyaganov, D.U.; Agathopoulos, S.; Ventura, J.M.; Karakassides, M.A.; Fabrichnaya, O.; Ferreira, J.M.F. Synthesis of glass-ceramics in the CaO-MgO-SiO2 system with B2O3, P2O5, Na2O and CaF2 additives. J. Eur. Ceram. Soc. 2006, 26, 1463–1471. [Google Scholar] [CrossRef]
- Hu, W.; Liu, H.; Hao, H.; Yao, Z.; Cao, M.; Wang, Z.; Song, Z. Influence of TiO2 additive on the microwave dielectric properties of α-CaSiO3-Al2O3 ceramics. Ceram. Int. 2015, 41, S510–S514. [Google Scholar] [CrossRef]
- Teixeira, S.R.; Souza, A.E.; Carvalho, C.L.; Reynoso, V.C.S.; Romero, M.; Rincón, J.M. Characterization of a wollastonite glass-ceramic material prepared using sugar cane bagasse ash (SCBA) as one of the raw materials. Mater. Charact. 2014, 98, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, M.M.; Hamzawy, E.M.A.; Aziz, D.A.A. Effect of TiO2 and Ta2O5 additions on the electrical properties of Li2CaAl4Si4O16 glass-ceramic. Adv. Appl. Ceram. 2017, 116, 260–266. [Google Scholar] [CrossRef]
- Rose, A.; Masin, B.; Sreemoolanadhan, H.; Ashok, K.; Vijayakumar, T. Effects of TiO2 addition on microwave dielectric properties of Li2MgSiO4 ceramics. Mater. Res. Express 2018, 5, 034006. [Google Scholar] [CrossRef]
- Khater, G.A. Influence of Cr2O3, LiF, CaF2 and TiO2 nucleants on the crystallization behavior and microstructure of glass-ceramics based on blast-furnace slag. Ceram. Int. 2011, 37, 2193–2199. [Google Scholar] [CrossRef]
- Rezvani, M.; Eftekhari-Yekta, B.; Solati-Hashjin, M.; Marghussian, V.K. Effect of Cr2O3, Fe2O3 and TiO2 nucleants on the crystallization behaviour of SiO2-Al2O3-CaO-MgO(R2O) glass-ceramics. Ceram. Int. 2005, 31, 75–80. [Google Scholar] [CrossRef]
- Lai, F.; Leng, M.; Li, J.; Liu, Q. The crystallization behaviors of SiO2-Al2O3-CaO-MgO-TiO2 glass-ceramic systems. Crystals 2020, 10, 794. [Google Scholar] [CrossRef]
- Ma, M.; Ni, W.; Wang, Y.; Wang, Z.; Liu, F. The effect of TiO2 on phase separation and crystallization of glass-ceramics in CaO-MgO-Al2O3-SiO2-Na2O system. J. Non-Cryst. Solids 2008, 354, 5395–5401. [Google Scholar] [CrossRef]
- Mukherjee, D.P.; Das, S.K. The influence of TiO2 content on the properties of glass ceramics: Crystallization, microstructure and hardness. Ceram. Int. 2014, 40, 4127–4134. [Google Scholar] [CrossRef]
- Banijamali, S.; Yekta, B.E.; Rezaie, H.R.; Marghussian, V.K. Crystallization and sintering characteristics of CaO-Al2O3-SiO2 glasses in the presence of TiO2, CaF2 and ZrO2. Thermochim. Acta 2009, 488, 60–65. [Google Scholar] [CrossRef]
- Duan, R.G.; Liang, K.M.; Gu, S.R. Effect of changing TiO2 content on structure and crystallization of CaO-Al2O3-SiO2 system glasses. J. Eur. Ceram. Soc. 1998, 18, 1729–1735. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Montazerian, M.; Gulbiten, O.; Mauro, J.C.; Zanotto, E.D.; Yue, Y. Understanding glass through differential scanning calorimetry. Chem. Rev. 2019, 119, 7848–7939. [Google Scholar] [CrossRef]
- Vazquez, J.; Barreda, D.G.; Lopez-Alemany, P.L.; Villares, P.; Jiménez-Garay, R. An alternative method for analyzing the non-isothermal glass-crystal transformation kinetics Application to the crystallization of some alloys of Ge-Sb-Se and Sb-As-Se glassy systems. J. Alloys Compd. 2006, 421, 109–116. [Google Scholar] [CrossRef]
- Erol, M.; Küçükbayrak, S.; Ersoy-Meriçboyu, A. The application of differential thermal analysis to the study of isothermal and non-isothermal crystallization kinetics of coal fly ash based glasses. J. Non-Cryst. Solids 2009, 355, 569–576. [Google Scholar] [CrossRef]
- Matusita, K.; Komatsu, T.; Yokota, R. Kinetics of non-isothermal crystallization process and activation energy for crystal growth in amorphous materials. J. Mater. Sci. 1984, 19, 291–296. [Google Scholar] [CrossRef]
- Vázquez, J.; Wagner, C.; Villares, P.; Jiménez-Garay, R. A theoretical method for determining the crystallized fraction and kinetic parameters by DSC, using non-isothermal techniques. Acta Mater. 1996, 44, 4807–4813. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Málek, J. Kinetic analysis of crystallization processes in amorphous materials. Thermochim. Acta 2000, 355, 239–253. [Google Scholar] [CrossRef]
- López-Alemany, P.L.; Vázquez, J.; Villares, P.; Jiménez-Garay, R. A study on the crystallization kinetics by differential scanning calorimetry using non-isothermal techniques in the case of “site saturation”. Mater. Lett. 2003, 57, 2722–2728. [Google Scholar] [CrossRef]
- Erol, M.; Küçükbayrak, S.; Ersoy-Meriçboyu, A. Influence of particle size on the crystallization kinetics of glasses produced from waste materials. J. Non-Cryst. Solids 2011, 357, 211–219. [Google Scholar] [CrossRef]
- Pustková, P.; Zmrhalová, Z.; Málek, J. The particle size influence on crystallization kinetics of (GeS2)0.1(Sb2S3)0.9 glass. Thermochim. Acta 2007, 466, 13–21. [Google Scholar] [CrossRef]
- Cahn, J.W. The kinetics of grain boundary nucleated reactions. Acta Metall. 1956, 4, 449–459. [Google Scholar] [CrossRef]
- Romero, M.; Kovacova, M.; Rincón, J.M. Effect of particle size on kinetics crystallization of an iron-rich glass. J. Mater. Sci. 2008, 43, 4135–4142. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, R. Non-isothermal crystallization kinetics of CaO-SiO2-B2O3-TiO2 glass studied by differential thermal analysis in the case of “site saturation”. Ceram. Int. 2023, 49, 19346–19354. [Google Scholar] [CrossRef]
- López-Alemany, P.L.; Vázquez, J.; Villares, P.; Jiménez-Garay, R. Crystallization kinetics by differential scanning calorimetry under non-isothermal regime in the case of “site saturation”. J. Mater. Process. Technol. 2003, 143–144, 512–516. [Google Scholar] [CrossRef]
- Matusita, K.; Sakka, S. Study on crystallization kinetics in glass by differential thermal analysis. Thermochim. Acta 1979, 33, 351–354. [Google Scholar] [CrossRef]
- Matusita, K.; Sakka, S. Kinetic study of crystallization of glass by differential thermal analysis-criterion on application of Kissinger plot. J. Non-Cryst. Solids 1980, 38–39, 741–746. [Google Scholar] [CrossRef]
- Málek, J. The application of Johnson-Mehl-Avrami model in the thermal analysis of the crystallization kinetics of glasses. Thermochim. Acta 1995, 267, 61–73. [Google Scholar] [CrossRef]
- McMillan, P.W. Glass-Cremics; Academic Press Inc. (London) Ltd.: London, UK, 1964. [Google Scholar]
- Karamanov, A.; Pelino, M. Induced crystallization porosity and properties of sintereds diopside and wollastonite glass-ceramics. J. Eur. Ceram. Soc. 2008, 28, 555–562. [Google Scholar] [CrossRef]
- Mohammadi, M.; Alizadeh, P.; Atlasbaf, Z. Effect of frit size on sintering, crystallization and electrical properties of wollastonite glass-ceramics. J. Non-Cryst. Solids 2011, 357, 150–156. [Google Scholar] [CrossRef]

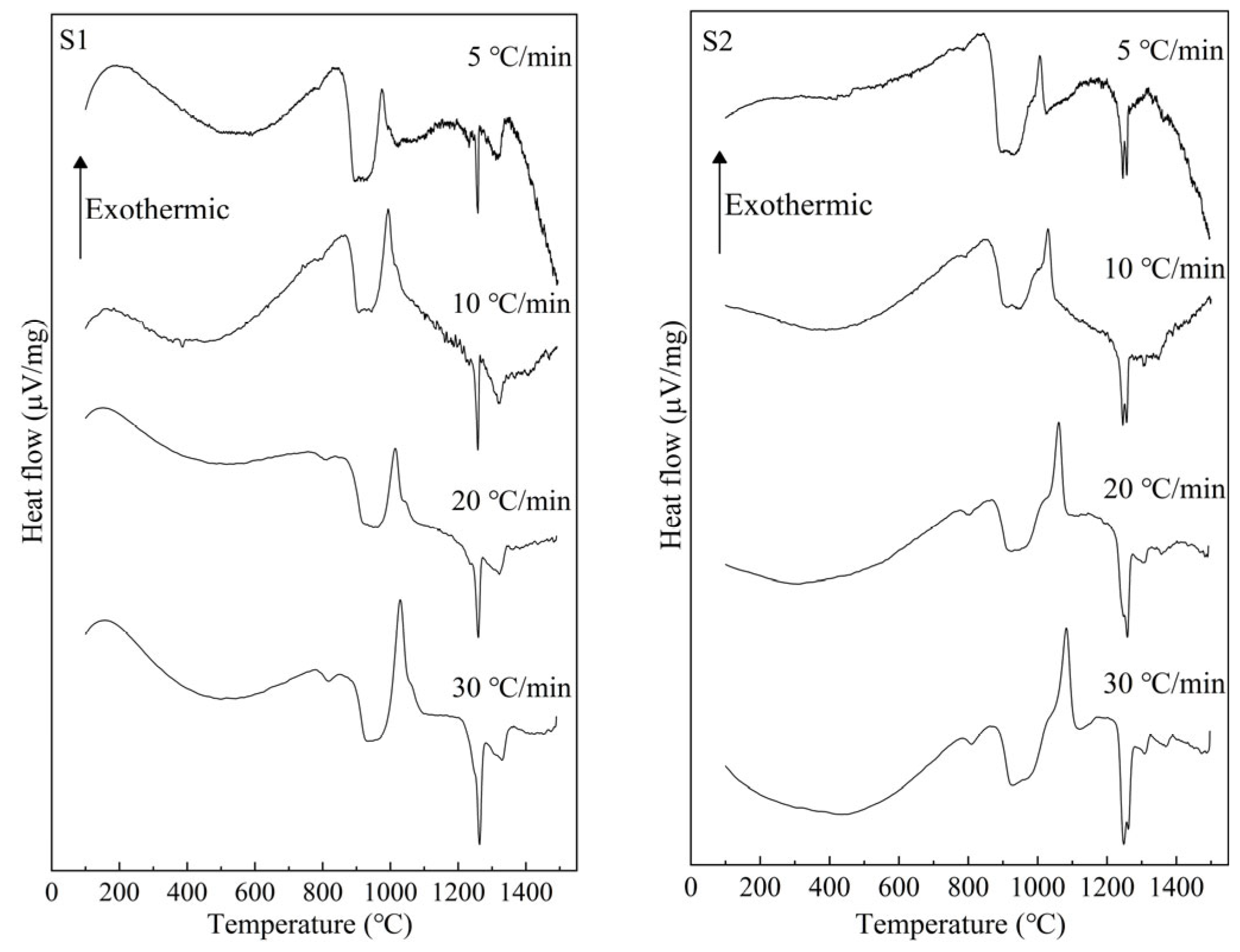
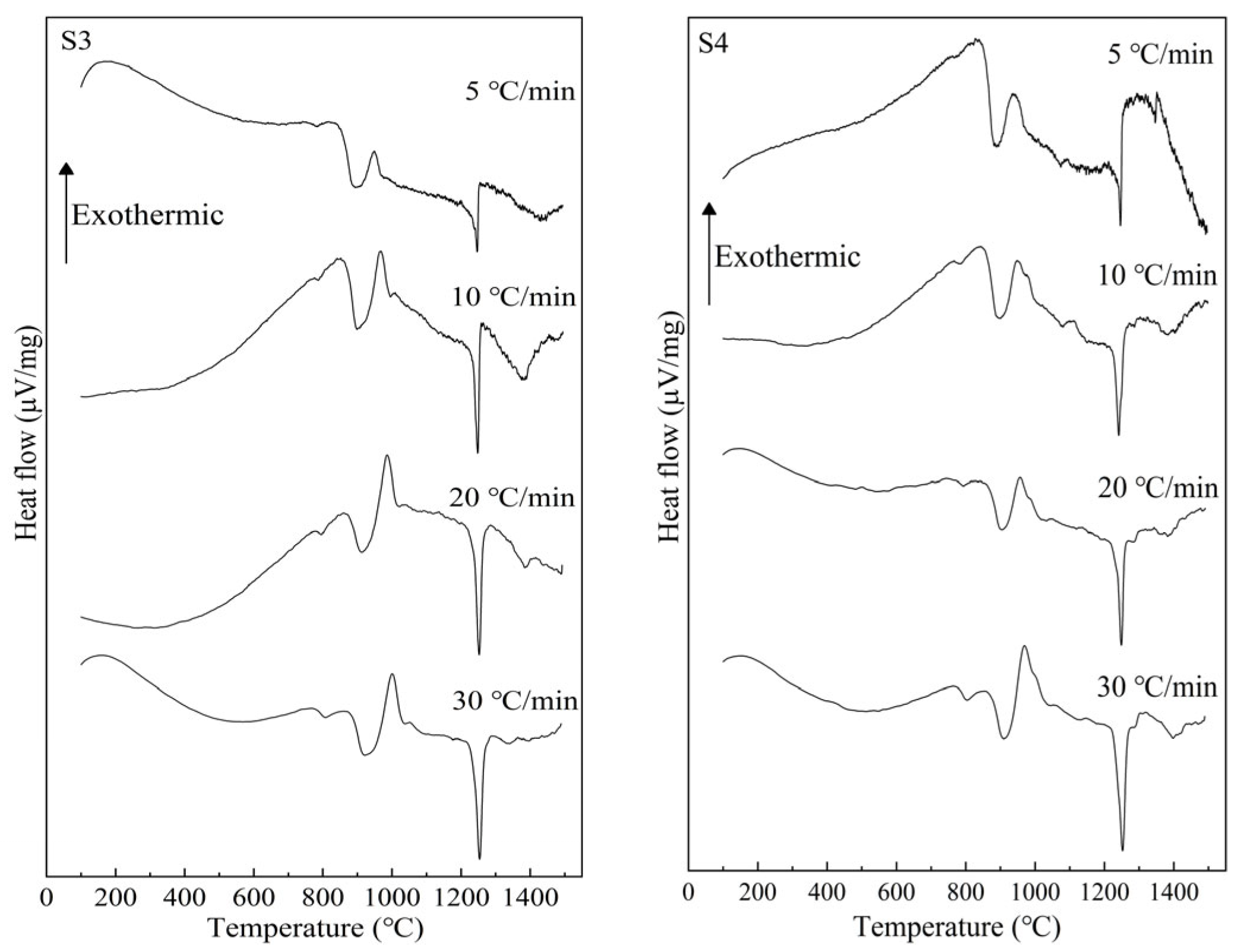
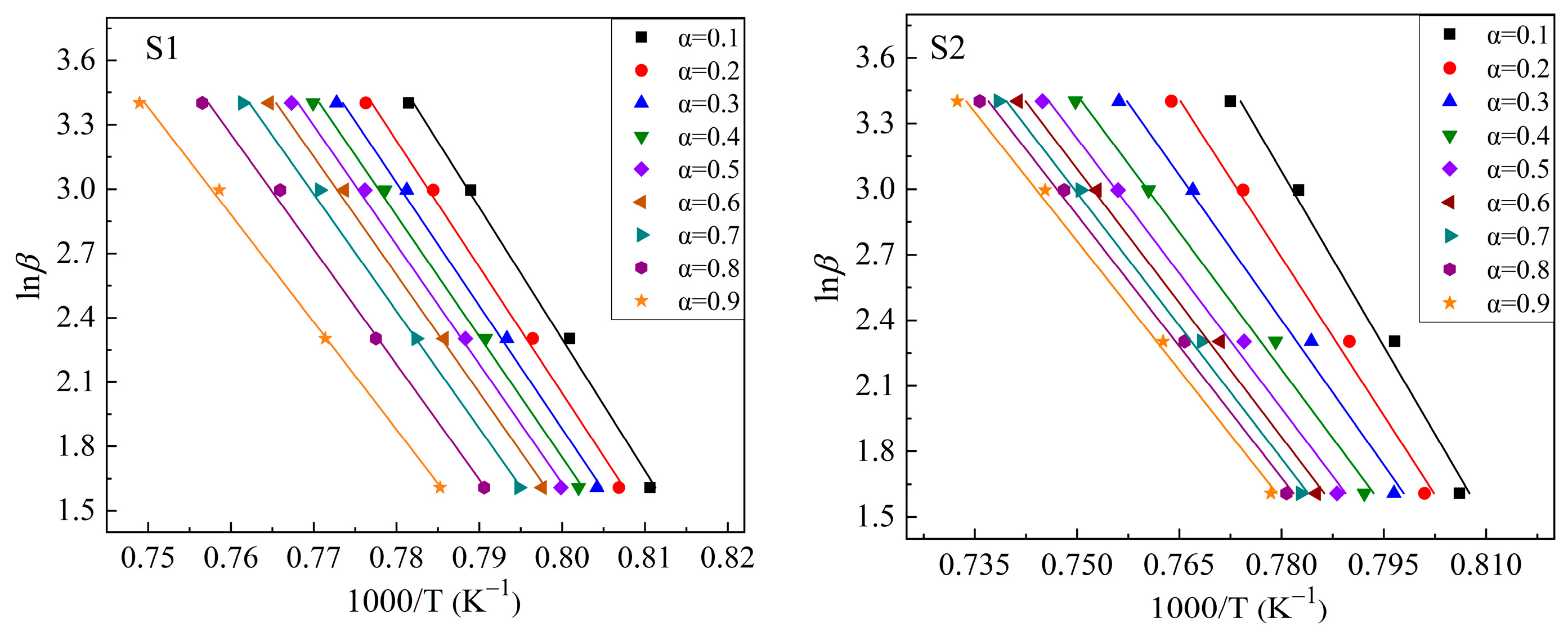
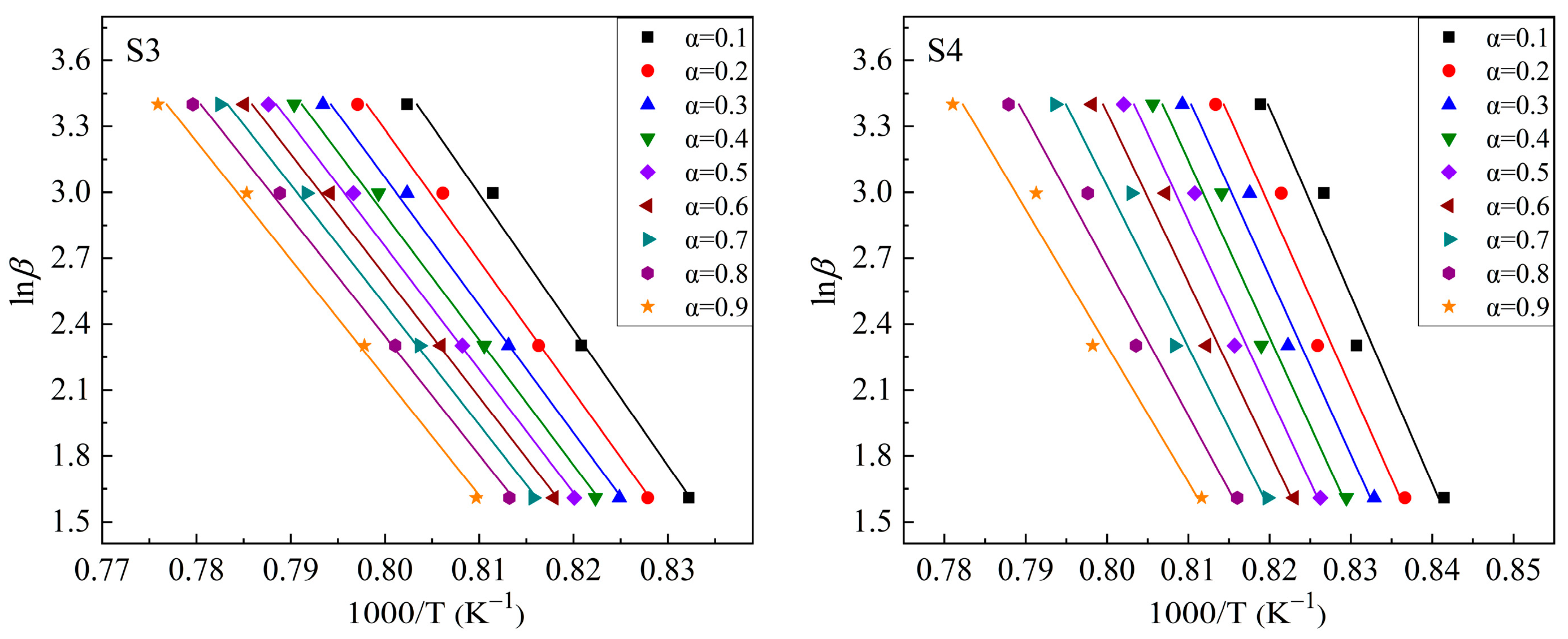


| Sample Number | CaO | SiO2 | Al2O3 | TiO2 | w(CaO)/w(SiO2) |
|---|---|---|---|---|---|
| S1 | 40 | 40 | 10 | 10 | 1 |
| S2 | 38 | 38 | 10 | 14 | 1 |
| S3 | 36 | 36 | 10 | 18 | 1 |
| S4 | 34 | 34 | 10 | 22 | 1 |
| Sample Number | Heating Rate | mEG (kJ/mol) | n | m | EG (kJ/mol) | Crystals |
|---|---|---|---|---|---|---|
| S1-10%TiO2 | 5 °C/min | 946.11 | 2.14 | 2 | 473.06 | CaSiO3, CaTiO3, Ca3TiSi2(AlSiTi)3O14 |
| 10 °C/min | 813.46 | 1.84 | 2 | 406.73 | ||
| 20 °C/min | 803.26 | 1.82 | 2 | 401.63 | ||
| 30 °C/min | 755.63 | 1.71 | 2 | 377.81 | ||
| S2-14%TiO2 | 5 °C/min | 813.35 | 2.38 | 2 | 406.67 | CaSiO3, CaTiO3, Ca3TiSi2(AlSiTi)3O14 |
| 10 °C/min | 673.20 | 1.97 | 2 | 336.60 | ||
| 20 °C/min | 612.08 | 1.79 | 2 | 306.04 | ||
| 30 °C/min | 573.88 | 1.68 | 2 | 286.94 | ||
| S3-18%TiO2 | 5 °C/min | 1077.44 | 2.40 | 2 | 538.72 | Ca3TiSi2(AlSiTi)3O14, CaTiO3, CaSiO3 |
| 10 °C/min | 1050.09 | 2.34 | 2 | 525.04 | ||
| 20 °C/min | 924.80 | 2.06 | 2 | 462.40 | ||
| 30 °C/min | 913.46 | 2.04 | 2 | 456.73 | ||
| S4-22%TiO2 | 5 °C/min | 789.38 | 1.30 | 1 | 789.38 | Ca3TiSi2(AlSiTi)3O14, CaTiO3, CaSiO3 |
| 10 °C/min | 722.00 | 1.19 | 1 | 722.00 | ||
| 20 °C/min | 667.91 | 1.10 | 1 | 667.91 | ||
| 30 °C/min | 623.36 | 1.03 | 1 | 623.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Xu, R. A Kinetic Study on Crystallization in TiO2-SiO2-CaO-Al2O3 Glass under Nucleation Saturation Conditions for the High Value-Added Utilization of CaO-SiO2-Based Solid Wastes. Materials 2023, 16, 4165. https://doi.org/10.3390/ma16114165
Wang Z, Xu R. A Kinetic Study on Crystallization in TiO2-SiO2-CaO-Al2O3 Glass under Nucleation Saturation Conditions for the High Value-Added Utilization of CaO-SiO2-Based Solid Wastes. Materials. 2023; 16(11):4165. https://doi.org/10.3390/ma16114165
Chicago/Turabian StyleWang, Zhen, and Renze Xu. 2023. "A Kinetic Study on Crystallization in TiO2-SiO2-CaO-Al2O3 Glass under Nucleation Saturation Conditions for the High Value-Added Utilization of CaO-SiO2-Based Solid Wastes" Materials 16, no. 11: 4165. https://doi.org/10.3390/ma16114165





