Bactericidal Activity of Graphene Oxide Tests for Selected Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Graphene Oxide (GO)
2.3. Cytotoxicity Assay
2.4. Statistics
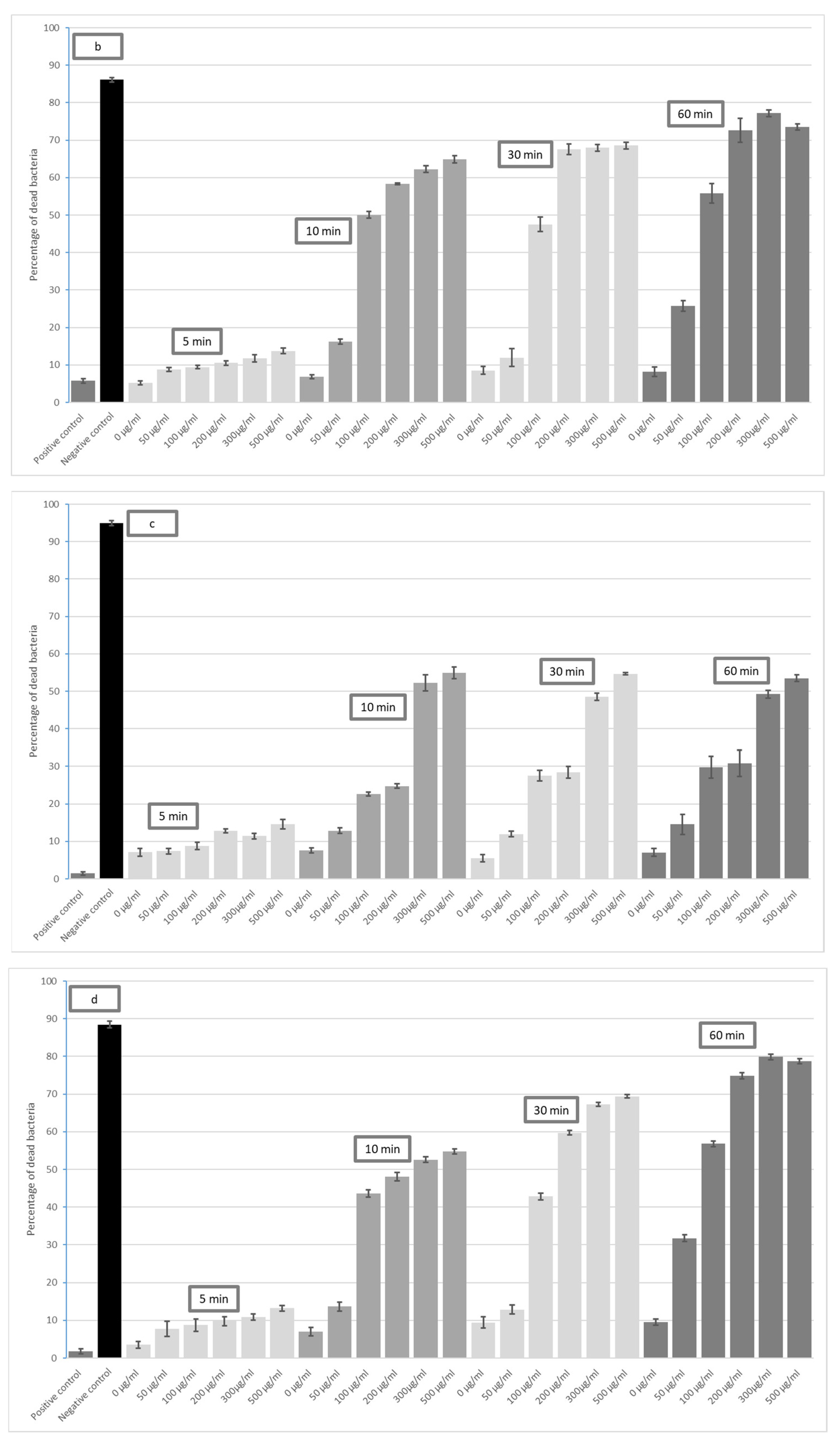
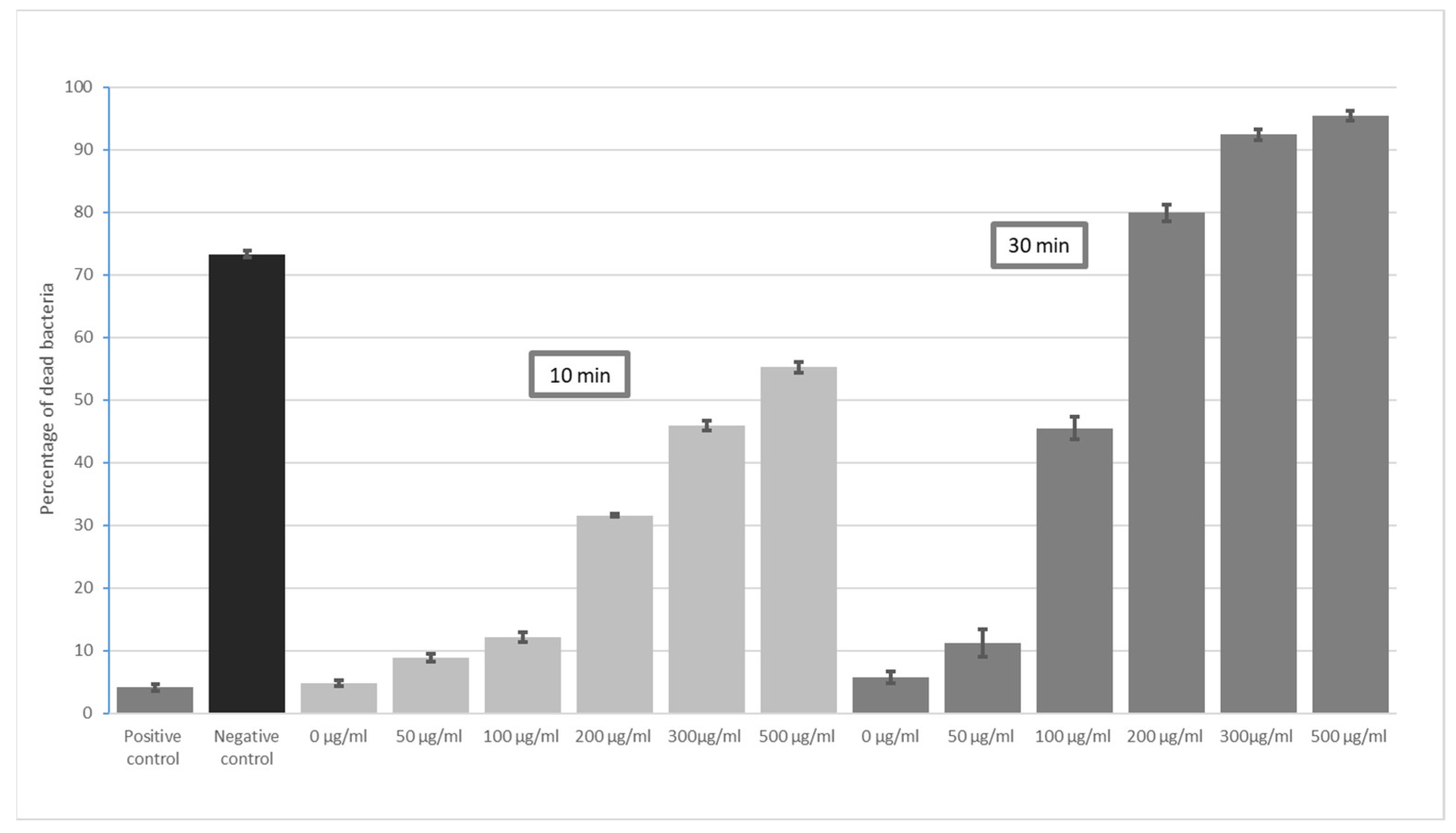
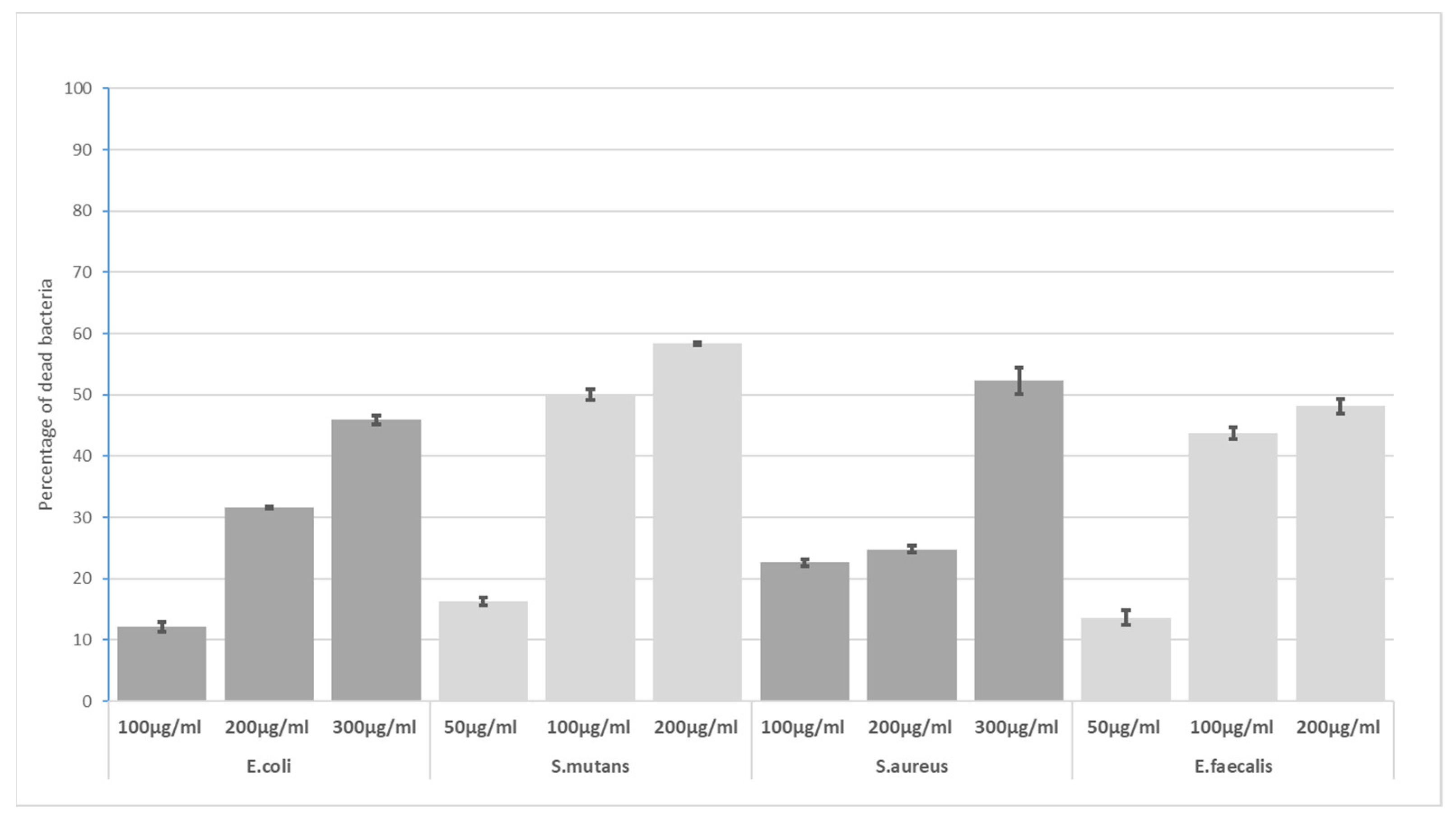
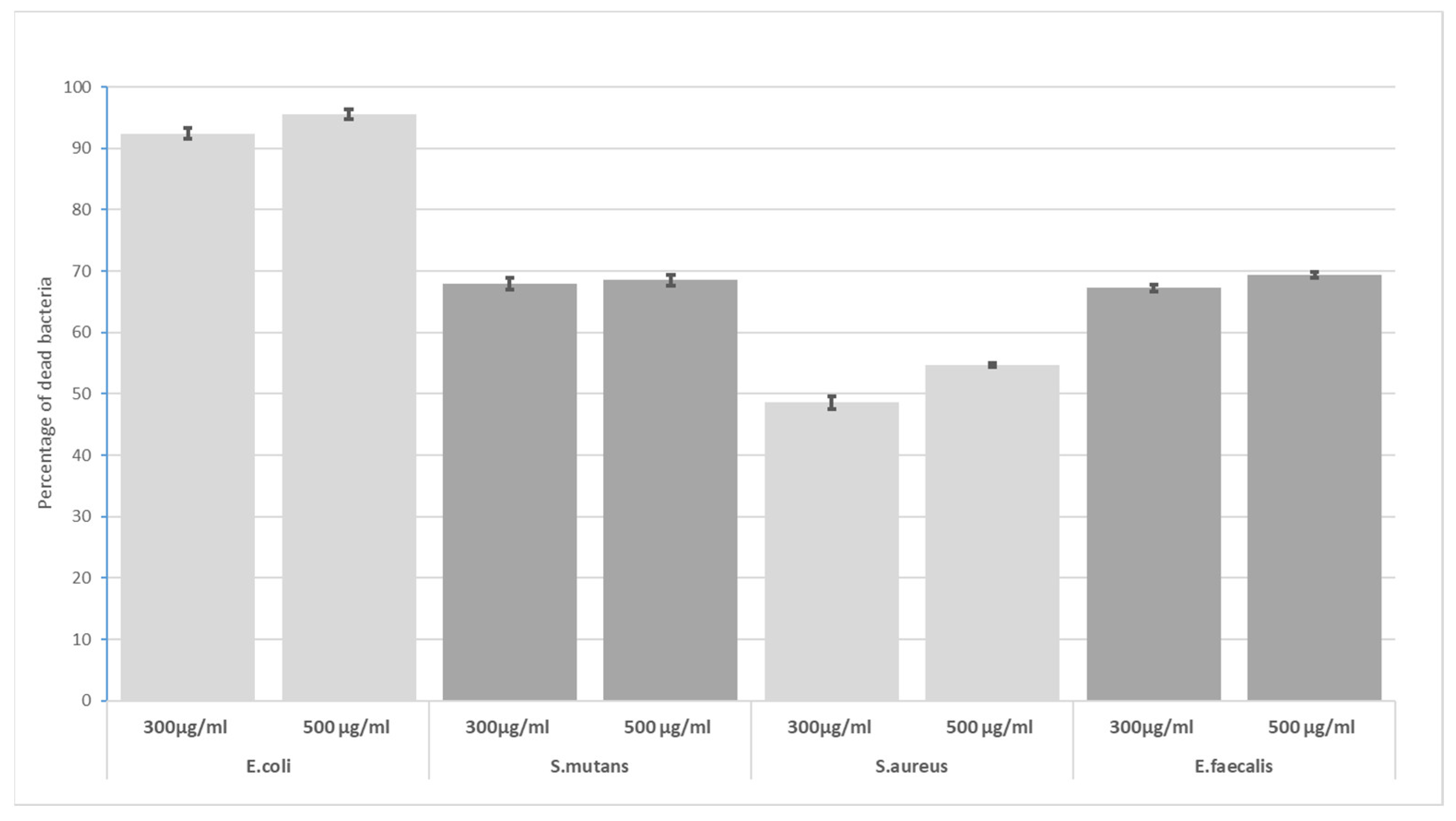
3. Results
4. Discussion
5. Conclusions
- Graphene oxide (GO) has a bactericidal effect on both Gram-positive and Gram-negative bacteria.
- The bactericidal effect of GO on selected bacterial strains depends on its duration of action and concentration.
- The highest bactericidal activity was observed at concentrations of 300 and 500 μg/mL for all incubation times (5, 10, 30 and 60 min). The highest antimicrobial potential was observed for E. coli—after 60 min, the mortality rate was 94% at 300 µg/mL GO and 96% at 500 µg/mL GO; the lowest was found for S. aureus, i.e., 49% (300 µg/mL) and 55% (500 µg/mL).
- GO has high effectiveness in dental applications. As the effectiveness of preparations peaks around 300 µg/mL, there is no need to use higher concentrations.
- Determining the optimal bactericidal concentration of graphene may support the development of dental materials containing similar concentrations of GO.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, C.; Li, Y.; Tjong, S.C. Graphene nanomaterials: Synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randviir, E.P.; Brownson, D.A.C.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Mishra, N.; Boeckl, J.; Motta, N.; Iacopi, F. Graphene growth on silicon carbide: A review. Phys. Status Solidi 2016, 213, 2277–2289. [Google Scholar] [CrossRef]
- Kula, P.; Pietrasik, R.; Kazimierski, D.; Atraszkiewicz, R.; Dybowski, K.; Szymanski, W.; Klimek, L.; Niedzielski, P.; Clapa, M. Resistance-temperature characteristics of CVD and high strength metallurgical graphene. Int. J. Nanotechnol. 2017, 14, 191–203. [Google Scholar] [CrossRef]
- Eluyemi, M.S.; Eleruja, M.A.; Adedeji, A.V.; Olofinjana, B.; Fasakin, O.; Akinwunmi, O.O.; Ilori, O.O.; Famojuro, A.T.; Ayinde, S.A.; Ajayi, E.O.B. Synthesis and Characterization of Graphene Oxide and Reduced Graphene Oxide Thin Films Deposited by Spray Pyrolysis Method. Graphene 2016, 5, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, N.; Eom, H.J.; Choi, J. A systems toxicology approach to the surface functionality control of graphene-cell interactions. Biomaterials 2014, 35, 1109–1127. [Google Scholar] [CrossRef]
- Plachá, D.; Jampilek, J. Graphenic materials for biomedical applications. Nanomaterials 2019, 9, 1758. [Google Scholar] [CrossRef] [Green Version]
- Primo, E.N.; Bollo, S.; Rubianes, M.D.; Rivas, G.A. Immobilization of graphene-derived materials at gold surfaces: Towards a rational design of protein-based platforms for electrochemical and plasmonic applications. Electrochim. Acta 2018, 259, 723–732. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonson, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Seville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [Green Version]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, G.; Qi, X.; Wu, S.; Li, H.; Boey, F.; Zhang, H. A Method for Fabrication of Graphene Oxide Nanoribbons from Graphene Oxide Wrinkles. J. Phys. Chem. C 2009, 113, 19119–19122. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014, 4, 4359. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef]
- Perreault, F.; De Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Abdal Dayem, A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef] [Green Version]
- Mejías Carpio, I.E.; Santos, C.M.; Wei, X.; Rodrigues, D.F. Toxicity of a polymer-graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 2012, 4, 4746–4756. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Wang, G.; Qian, F.; Saltikov, C.W.; Jiao, Y.; Li, Y. Microbial reduction of graphene oxide by Shewanella. Nano Res. 2011, 4, 563–570. [Google Scholar] [CrossRef]
- Salas, E.C.; Sun, Z.; Lüttge, A.; Tour, J.M. Reduction of graphene oxide via bacterial respiration. ACS Nano 2010, 4, 4852–4856. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Cheng, C.; Chen, C. Impact of graphene oxide on the antibacterial activity of antibiotics against bacteria. Environ. Sci. Nano 2017, 4, 1016–1024. [Google Scholar] [CrossRef]
- Di Giulio, M.; Zappacosta, R.; Di Lodovico, S.; Di Campli, E.; Siani, G.; Fontana, A.; Cellini, L. Antimicrobial and antibiofilm efficacy of graphene oxide against chronic wound microorganisms. Antimicrob. Agents Chemother. 2018, 62, e00547-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, F.; Zhou, H.; Jeong, D.Y.; Kwon, J.; Eom, S.U.; Park, T.J.; Hong, S.W.; Lee, J. Wrinkled surface-mediated antibacterial activity of graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, R.R.; Mohammad, M.R.; Hussien, A.M.A. Antibacterial Activity and Morphological Characterization of Synthesis Graphene Oxide Nanosheets by Simplified Hummer’s Method. Biosci. Biotechnol. Res. Asia 2018, 15, 627–633. [Google Scholar] [CrossRef]
- Kim, M.-A.; Rosa, V.; Min, K.-S. Effect of two graphene derivatives on Enterococcus faecalis biofilms and cytotoxicity. Dent. Mater. J. 2023, 42, 211–217. [Google Scholar] [CrossRef]
- Saeed, S.I.; Vivian, L.; Zalati, C.W.S.C.W.; Sani, N.I.M.; Aklilu, E.; Mohamad, M.; Noor, A.A.M.; Muthoosamy, K.; Kamaruzzaman, N.F. Antimicrobial activities of graphene oxide against biofilm and intracellular Staphylococcus aureus isolated from bovine mastitis. BMC Vet. Res. 2023, 19, 10. [Google Scholar] [CrossRef]
- Chandrasekar, V.; Singh, A.V.; Maharjan, R.S.; Dakua, S.P.; Balakrishnan, S.; Dash, S.; Laux, P.; Luch, A.; Singh, S.; Pradhan, M. Perspectives on the Technological Aspects and Biomedical Applications of Virus-Like Particles/Nanoparticles in Reproductive Biology: Insights on the Medicinal and Toxicological Outlook. Adv. NanoBiomed Res. 2022, 2, 2200010. [Google Scholar] [CrossRef]
- Karahan, H.E.; Wiraja, C.; Xu, C.; Wei, J.; Wang, Y.; Wang, L.; Liu, F.; Chen, Y. Graphene Materials in Antimicrobial Nanomedicine: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, 1701406. [Google Scholar] [CrossRef] [Green Version]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossù, M.; Ierardo, G.; Polimeni, A.; et al. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnology 2017, 15, 89. [Google Scholar] [CrossRef]
- Romiszewska, A.; Bombalska, A. Antibacterial properties of graphene and its derivatives. Bull. Mil. Univ. Technol. 2020, 68, 69–84. [Google Scholar] [CrossRef]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E.W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef]
- Prasad, K.; Lekshmi, G.S.; Ostrikov, K.; Lussini, V.; Blinco, J.; Mohandas, M.; Vasilev, K.; Bottle, S.; Bazaka, K.; Ostrikov, K. Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci. Rep. 2017, 7, 1591. [Google Scholar] [CrossRef] [Green Version]
- Spilarewicz-Stanek, K.; Jakimińska, A.; Kisielewska, A.; Batory, D.; Piwoński, I. Understanding the role of silver nanostructures and graphene oxide applied as surface modification of TiO2 in photocatalytic transformations of rhodamine B under UV and vis irradiation. Materials 2020, 13, 4653. [Google Scholar] [CrossRef]
- Yang, F.; Huo, D.; Zhang, J.; Lin, T.; Zhang, J.; Tan, S.; Yang, L. Fabrication of graphene oxide/copper synergistic antibacterial coating for medical titanium substrate. J. Colloid Interface Sci. 2023, 638, 1–13. [Google Scholar] [CrossRef]
- Vanithakumari, S.C.; Jena, G.; Sofia, S.; Thinaharan, C.; George, R.P.; Philip, J. Fabrication of superhydrophobic titanium surfaces with superior antibacterial properties using graphene oxide and silanized silica nanoparticles. Surf. Coatings Technol. 2020, 400, 126074. [Google Scholar] [CrossRef]
- Truong, T.T.V.; Chen, C.C.; Kumar, S.R.; Hu, C.C.; Chen, D.W.; Liu, Y.K.; Lue, S.J. Prismatic Silver Nanoparticles Decorated on Graphene Oxide Sheets for Superior Antibacterial Activity. Pharmaceutics 2022, 14, 924. [Google Scholar] [CrossRef]
- Hajduga, M.B.; Bobinski, R.; Dutka, M.; Bujok, J.; Cwiertnia, M.; Pajak, C.; Kurowska, A.; Rajzer, I. The Influence of Graphene Content on the Antibacterial Properties of Polycaprolactone. Int. J. Mol. Sci. 2022, 23, 10899. [Google Scholar] [CrossRef]
- Miao, J.; Wang, F.; Chen, Y.; Zhu, Y.; Zhou, Y.; Zhang, S. The adsorption performance of tetracyclines on magnetic graphene oxide: A novel antibiotics absorbent. Appl. Surf. Sci. 2019, 475, 549–558. [Google Scholar] [CrossRef]
- Chae, S.Y.; Jeon, S.; Han, D.W.; Hong, S.W. Improved antibacterial activity of 3D wrinkled graphene oxide films implemented with irreversibly shrinkable shape-memory polymer substrates. Environ. Sci. Nano 2023, 10, 732–746. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Aunkor, M.T.H.; Raihan, T.; Prodhan, S.H.; Metselaar, H.S.C.; Malik, S.U.F.; Azad, A.K. Antibacterial activity of graphene oxide nanosheet against multidrug resistant superbugs isolated from infected patients. R. Soc. Open Sci. 2020, 7, 200640. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Tauseef, I.; Ali, M.B.; Yameen, M.A.; Mezni, A.; Hedfi, A.; Haleem, S.K.; Haq, S. In-Vitro and In-Vivo Tolerance and Therapeutic Investigations of Phyto-Fabricated Iron Oxide Nanoparticles against Selected Pathogens. Toxics 2021, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk, B.; Porȩbska, K.; Jakubowski, W.; Miszczak, S. Antibacterial properties of Zn doped hydrophobic SiO2 coatings produced by sol-gel method. Coatings 2019, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Wan, A.K.L.; Seow, W.K.; Walsh, L.J.; Bird, P.S. Comparison of five selective media for the growth and enumeration of Streptococcus mutans. Aust. Dent. J. 2002, 47, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.G.; Foster, S.J.; Richards, R.G.; Lambert, P.; Stickler, D.; Eley, A. An introduction to Staphylococcus aureus, and techniques for identifyingand quantifying S. aureus adhesins in relation to adhesion to biomaterials:Review. Eur. Cells Mater. 2002, 4, 39–60. [Google Scholar] [CrossRef]
- Tran, H.T.; Vo, N.N.; Truong, Q.T. Optimization of medium composition for production of a simulated urine sample containing Enterococcus faecalis. Accredit. Qual. Assur. 2022, 27, 119–124. [Google Scholar] [CrossRef]
- Advanced Graphene Products—Poland Graphene Oxide—Dispersion. Available online: https://advancedgrapheneproducts.com/en/produkt/graphene-oxide-dispersion (accessed on 25 May 2023).
- Singh, A.V.; Kayal, A.; Malik, A.; Maharjan, R.S.; Dietrich, P.; Thissen, A.; Siewert, K.; Curato, C.; Pande, K.; Prahlad, D.; et al. Interfacial Water in the SARS Spike Protein: Investigating the Interaction with Human ACE2 Receptor and In Vitro Uptake in A549 Cells. Langmuir 2022, 38, 7976–7988. [Google Scholar] [CrossRef]
- Pasalic, L.; Williams, R.; Siupa, A.; Campbell, H.; Henderson, M.J.; Chen, V.M.Y. Enumeration of extracellular vesicles by a new improved flow cytometric method is comparable to fluorescence mode nanoparticle tracking analysis. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 977–986. [Google Scholar] [CrossRef]
- Gawad, S.; Schild, L.; Renaud, P. Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing. Lab Chip 2001, 1, 76–82. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its Role in Root Canal Treatment Failure and Current Concepts in Retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef]
- Slade, G.D. Epidemiology of dental pain and dental caries among children and adolescents. Community Dent. Health 2001, 18, 219–227. [Google Scholar]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Sun, L.; Yan, Z.; Duan, Y.; Zhang, J.; Liu, B. Improvement of the mechanical, tribological and antibacterial properties of glass ionomer cements by fluorinated graphene. Dent. Mater. 2018, 34, e115–e127. [Google Scholar] [CrossRef]
- Rojas-Andrade, M.D.; Chata, G.; Rouholiman, D.; Liu, J.; Saltikov, C.; Chen, S. Antibacterial mechanisms of graphene-based composite nanomaterials. Nanoscale 2017, 9, 994–1006. [Google Scholar] [CrossRef]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing dental pathogens using antibacterial graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef]
- Zhao, M.; Shan, T.; Wu, Q.; Gu, L. The Antibacterial Effect of Graphene Oxide on Streptococcus mutans. J. Nanosci. Nanotechnol. 2019, 20, 2095–2103. [Google Scholar] [CrossRef]
- Akbari, T.; Pourhajibagher, M.; Hosseini, F.; Chiniforush, N.; Gholibegloo, E.; Khoobi, M.; Shahabi, S.; Bahador, A. The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagnosis Photodyn. Ther. 2017, 20, 148–153. [Google Scholar] [CrossRef]
- Sharma, D.K.; Bhat, M.; Kumar, V.; Mazumder, D.; Singh, S.V.; Bansal, M. Title: Evaluation of antimicrobial efficacy of graphene silver composite nanoparticles against E. faecalis as root canal irrigant: An ex-vivo study. Int. J. Pharm. Med. Res 2015, 3, 267–272. [Google Scholar]
- Eskandari, F.; Abbaszadegan, A.; Gholami, A.; Ghahramani, Y. The antimicrobial efficacy of graphene oxide, double antibiotic paste, and their combination against Enterococcus faecalis in the root canal treatment. BMC Oral Health 2023, 23, 20. [Google Scholar] [CrossRef]
- Singh, A.A.; Makade, C.S.; Krupadam, R.J. Graphene nanoplatelets embedded polymer: An efficient endodontic material for root canal therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111864. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olczak, K.; Jakubowski, W.; Szymański, W. Bactericidal Activity of Graphene Oxide Tests for Selected Microorganisms. Materials 2023, 16, 4199. https://doi.org/10.3390/ma16114199
Olczak K, Jakubowski W, Szymański W. Bactericidal Activity of Graphene Oxide Tests for Selected Microorganisms. Materials. 2023; 16(11):4199. https://doi.org/10.3390/ma16114199
Chicago/Turabian StyleOlczak, Katarzyna, Witold Jakubowski, and Witold Szymański. 2023. "Bactericidal Activity of Graphene Oxide Tests for Selected Microorganisms" Materials 16, no. 11: 4199. https://doi.org/10.3390/ma16114199








