Adaptation of a Standard Method for Water Absorption Testing of Stone Materials: The Case of a Hydrophilic Protective Coating
Abstract
1. Introduction
- ●
- It does not consider the presence of coatings.
- ●
- It does not consider the temperature at which the coatings are no longer stable.
- ●
- It does not specify the composition of the water to be used.
- ●
- It does not specify the type of cloth to be used to collect the water from the test specimens after immersion in water.
- ●
- It does not consider the inherent heterogeneity of the materials to be tested.
2. Materials and Methods
2.1. Materials and Equipment
2.2. Methods
2.2.1. UNE-EN 13755/2008 Standard
2.2.2. UNE-EN 13755/2008 Standard Limitations
- Type of coating and its influence on water or stone;
- Temperature and pressure conditions;
- Suitability of all the materials used during the test;
- Influence of the water used on the rock or on the coating.
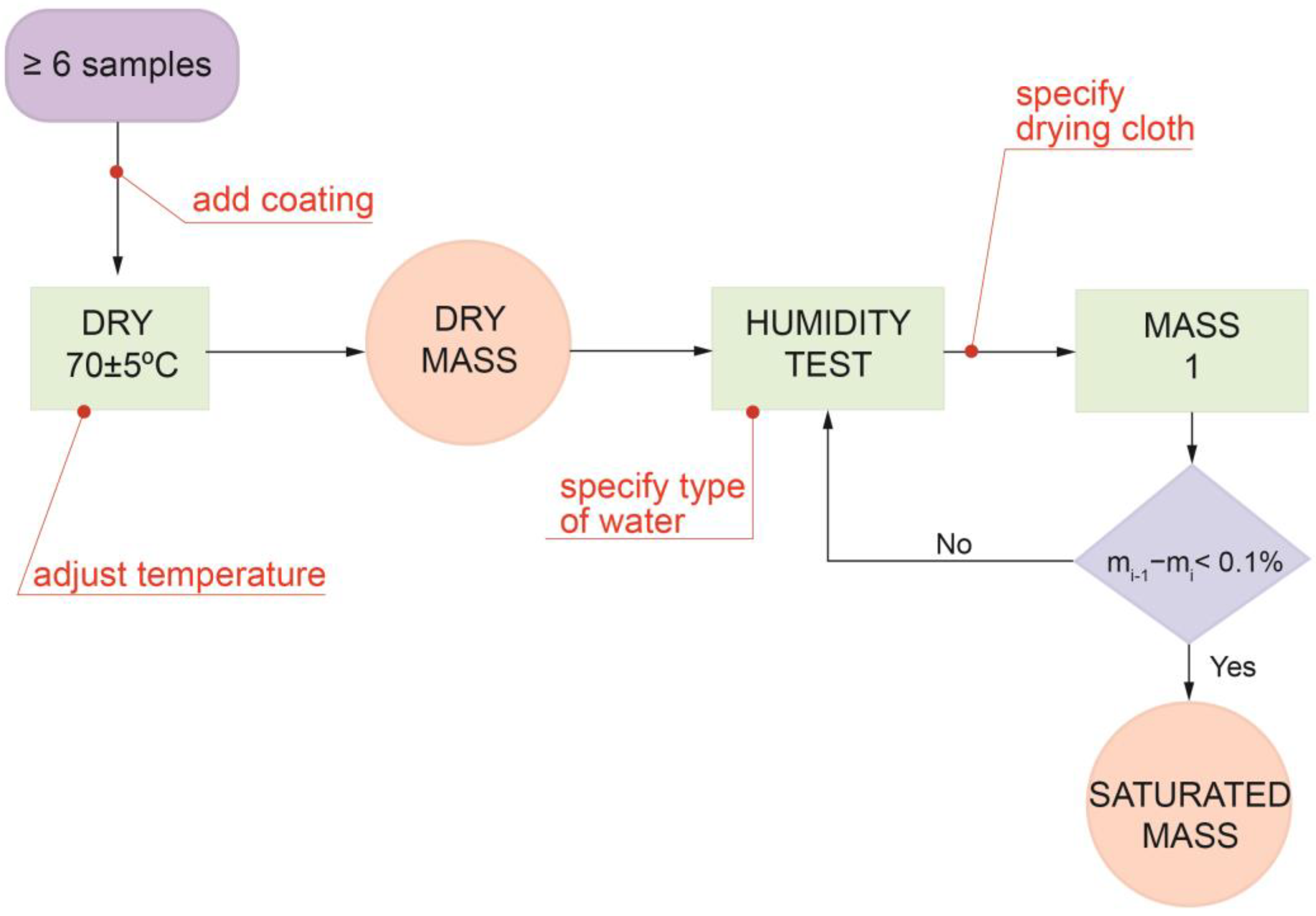
Protective Surface Coatings
Excess Water Removal after Specimen Immersion
Water Composition during Absorption Tests
Overcoming the Native Heterogeneity of Stones
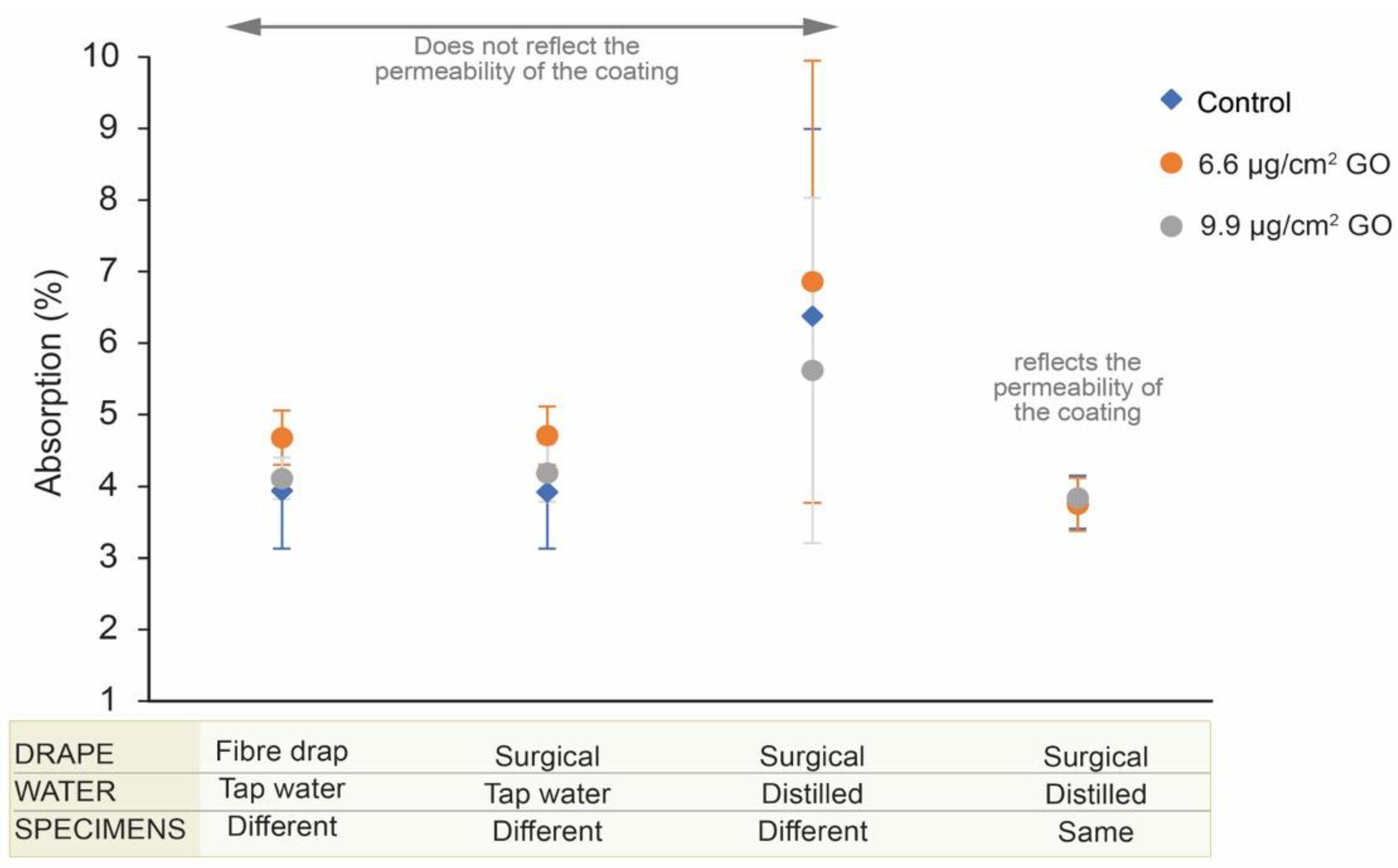
3. Results
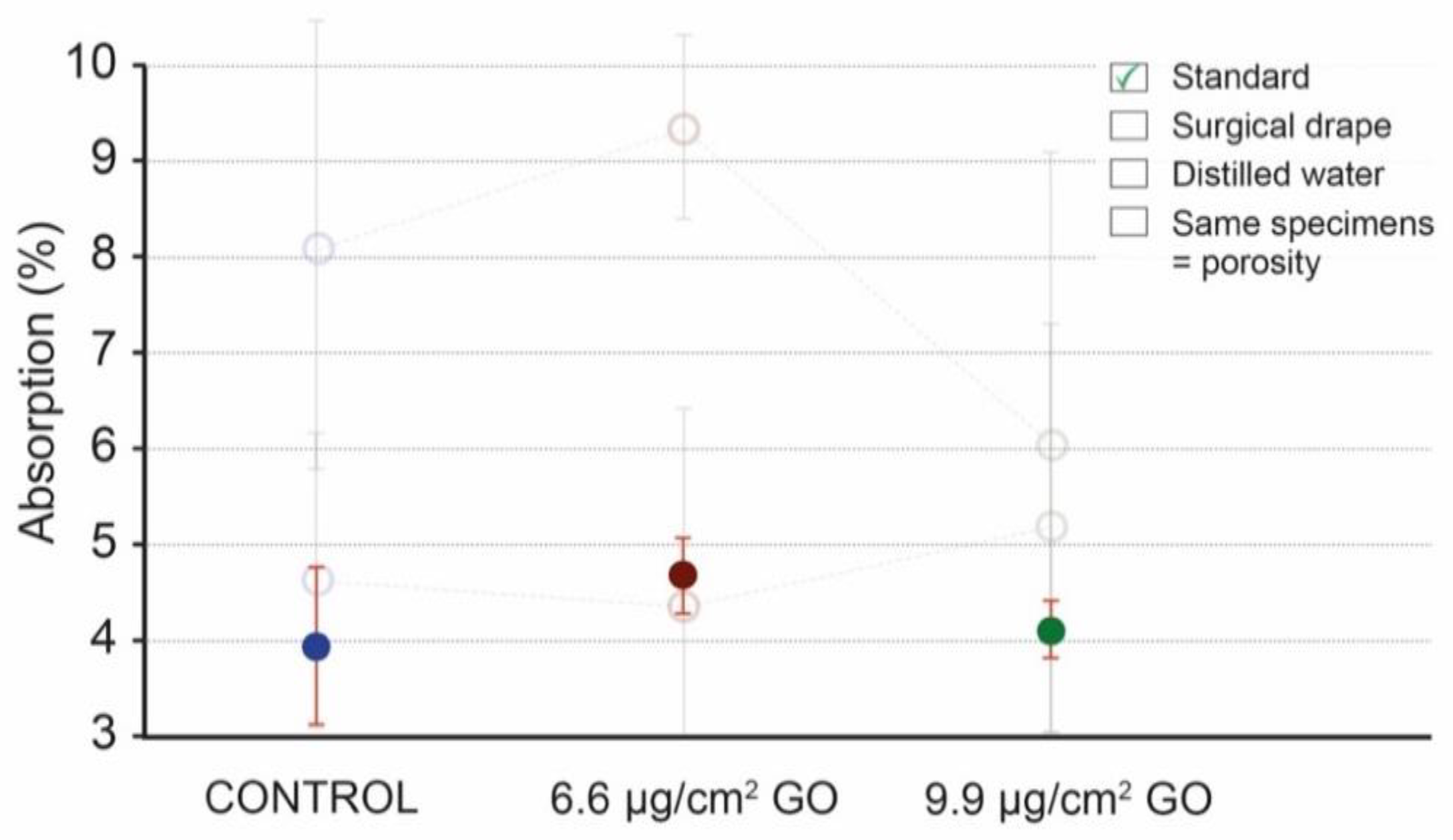
3.1. UNE-EN 13755/2008 Standard
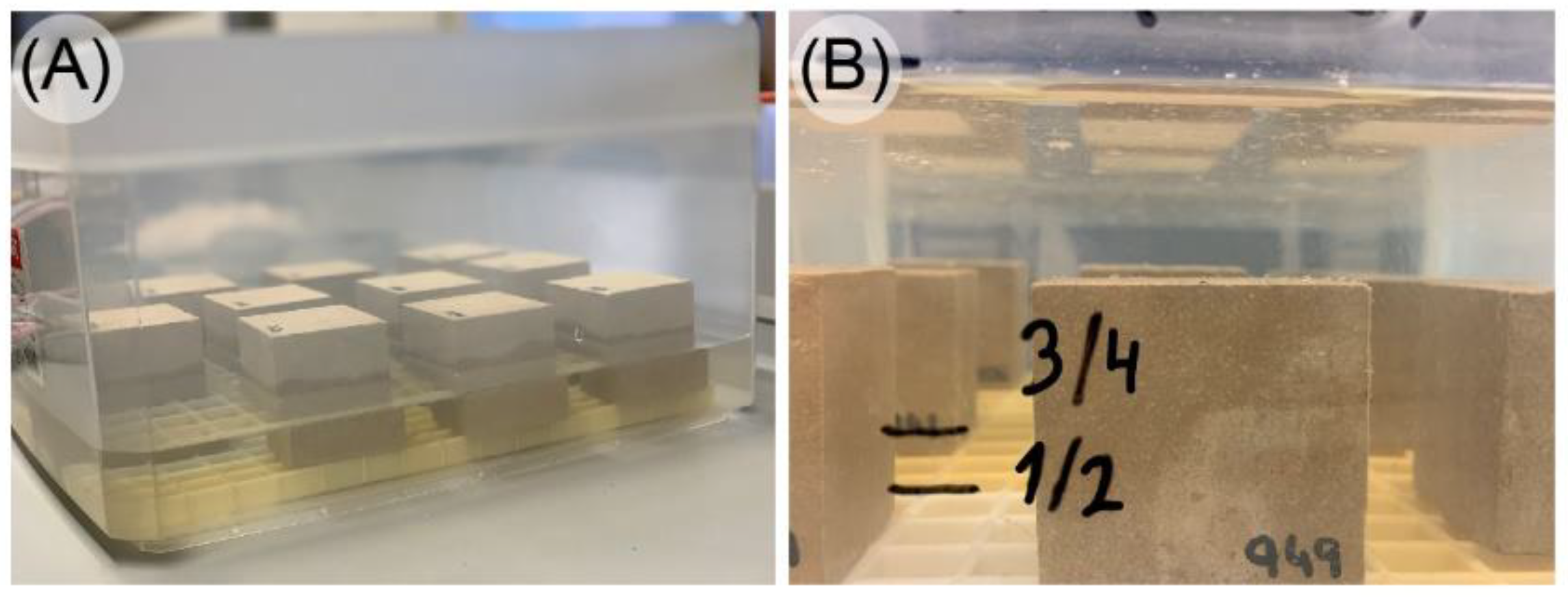
3.2. Use of Surgical Drape to Collect Excess Water after Specimen Immersion
3.3. Use of Distilled or Deionized Water
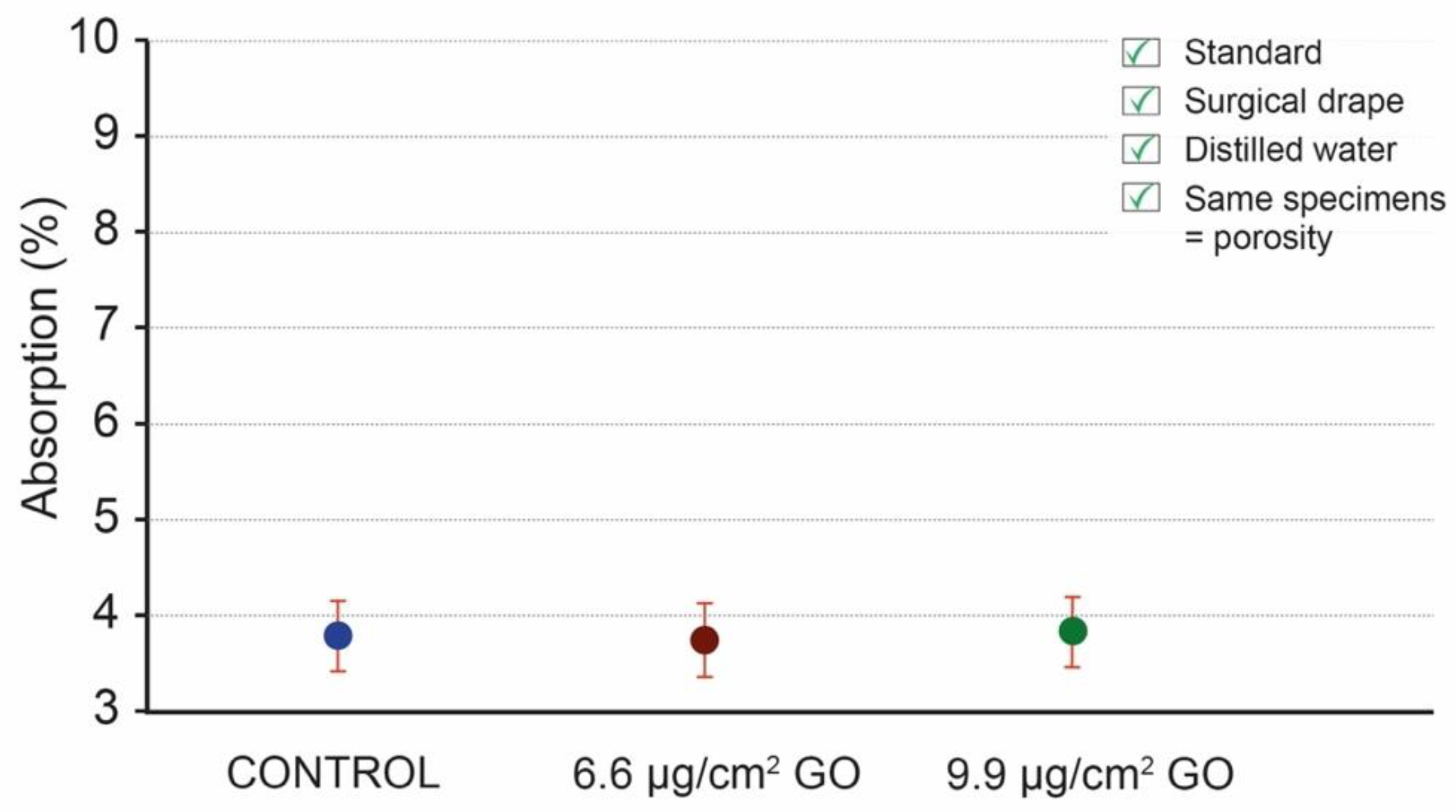
3.4. Use of the Same Specimens for Testing to Overcome the Heterogeneity Problem
4. Discussion
5. Conclusions
- ●
- A specification of the presence of coatings and their quantity (preferably in terms of surface concentration). These may exert physical or chemical properties on stone surfaces that vary when subjected to the test conditions.
- ●
- The temperature at which the coatings are no longer stable. A stipulated temperature for drying the specimens and obtaining the weight of the dry specimen must be below the stability conditions of the coating, if present; otherwise, the test must be modified to ensure a valid result.
- ●
- The composition of the water to be used. We propose to prioritize the use of distilled or deionized water throughout the test, since tap water contains some ions which may react with the rock materials or the coating.
- ●
- The type of cloth to be used to collect the excess water from the test specimens after immersion in water. We propose the use of surgical drapes to collect the excess water present as droplets on the surface of the specimen when it is removed from the water to avoid contamination of the specimen.
- ●
- The heterogeneity of the material to be tested. We recommend to use the same specimens for all comparative analyses if possible when working with natural rocks, since the inherent heterogeneity of certain materials can be an important issue when interpreting the results obtained from the tests. Another compatible possibility could be to increase the number of specimens tested, consequently increasing the variation.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample Code | md (g) | m1 (g) | m2 (g) | ms (g) | A (%) | Amean (%) | sd | |
|---|---|---|---|---|---|---|---|---|
| CONTROL (uncoated) | A1 | 303.80 | 316.60 | 316.90 | 316.90 | 4.31 | 3.94 | 0.81 |
| A2 | 268.80 | 281.70 | 281.90 | 281.90 | 4.87 | |||
| A3 | 271.40 | 284.80 | 284.90 | 284.90 | 4.97 | |||
| A4 | 287.80 | 298.90 | 299.20 | 299.20 | 3.96 | |||
| A5 | 296.10 | 305.90 | 306.10 | 306.10 | 3.38 | |||
| A6 | 311.40 | 320.80 | 321.10 | 321.10 | 3.11 | |||
| A7 | 311.30 | 320.30 | 320.60 | 320.60 | 2.99 | |||
| 6.6 μg/cm2 GO surface concentration | A8 | 289.30 | 300.40 | 300.70 | 300.70 | 3.94 | 4.68 | 0.38 |
| A9 | 265.50 | 278.90 | 279.10 | 279.10 | 5.12 | |||
| A10 | 281.20 | 294.90 | 295.20 | 295.20 | 4.98 | |||
| A11 | 270.30 | 283.10 | 283.40 | 283.40 | 4.85 | |||
| A12 | 269.70 | 281.80 | 282.10 | 282.10 | 4.60 | |||
| A13 | 276.60 | 288.90 | 289.20 | 289.20 | 4.56 | |||
| A14 | 279.80 | 292.70 | 293.00 | 293.00 | 4.72 | |||
| 9.9 μg/cm2 GO surface concentration | A14 | 309.10 | 320.70 | 321.00 | 321.00 | 3.85 | 4.11 | 0.29 |
| A16 | 282.40 | 294.40 | 294.60 | 294.60 | 4.32 | |||
| A17 | 278.90 | 291.10 | 291.40 | 291.40 | 4.48 | |||
| A18 | 293.20 | 306.00 | 306.20 | 306.20 | 4.43 | |||
| A19 | 302.50 | 313.70 | 314.00 | 314.00 | 3.80 | |||
| A20 | 286.40 | 297.50 | 297.80 | 297.80 | 3.98 | |||
| A21 | 300.40 | 311.90 | 312.20 | 312.20 | 3.93 |
| Sample Code | md (g) | m1 (g) | m2 (g) | ms (g) | A (%) | Amean (%) | sd | |
|---|---|---|---|---|---|---|---|---|
| CONTROL (uncoated) | B1 | 304.25 | 316.46 | 316.86 | 316.86 | 4.14 | 3.92 | 0.79 |
| B2 | 269.10 | 281.69 | 282.06 | 282.06 | 4.82 | |||
| B3 | 271.70 | 284.92 | 285.20 | 285.20 | 4.97 | |||
| B4 | 288.16 | 299.24 | 299.61 | 299.61 | 3.97 | |||
| B5 | 296.50 | 306.43 | 306.75 | 306.75 | 3.46 | |||
| B6 | 311.90 | 321.41 | 321.65 | 321.65 | 3.13 | |||
| B7 | 311.86 | 320.89 | 321.08 | 321.08 | 2.96 | |||
| 6.6 μg/cm2 GO surface concentration | B8 | 289.76 | 300.78 | 301.15 | 301.15 | 3.93 | 4.71 | 0.41 |
| B9 | 265.87 | 279.43 | 279.65 | 279.65 | 5.18 | |||
| B10 | 281.66 | 294.94 | 295.95 | 295.95 | 5.07 | |||
| B11 | 270.69 | 283.23 | 283.58 | 283.58 | 4.76 | |||
| B12 | 270.02 | 282.37 | 282.62 | 282.62 | 4.67 | |||
| B13 | 276.91 | 289.53 | 289.61 | 289.61 | 4.59 | |||
| B14 | 280.20 | 293.30 | 293.55 | 293.55 | 4.76 | |||
| 9.9 μg/cm2 GO surface concentration | B15 | 309.48 | 321.28 | 321.61 | 321.61 | 3.92 | 4.19 | 0.41 |
| B16 | 282.77 | 294.43 | 294.86 | 294.86 | 4.28 | |||
| B17 | 270.09 | 282.95 | 283.50 | 283.50 | 4.97 | |||
| B18 | 293.59 | 306.10 | 306.59 | 306.59 | 4.43 | |||
| B19 | 303.00 | 314.08 | 314.37 | 314.37 | 3.75 | |||
| B20 | 286.84 | 297.91 | 298.26 | 298.26 | 3.98 | |||
| B21 | 300.85 | 312.42 | 312.83 | 312.83 | 3.98 |
| Sample Code | md (g) | md (g) + GO | m1 (g) | m2 (g) | ms (g) | A (%) | Amean (%) | sd | |
|---|---|---|---|---|---|---|---|---|---|
| CONTROL (uncoated) | C1 | 284.17 | 284.40 | 297.30 | 297.47 | 297.47 | 4.60 | 6.38 | 2.61 |
| C2 | 277.72 | 277.99 | 294.96 | 295.18 | 295.18 | 6.18 | |||
| C3 | 298.54 | 298.71 | 307.79 | 307.96 | 307.96 | 3.10 | |||
| C4 | 264.59 | 264.81 | 291.95 | 293.39 | 293.39 | 10.80 | |||
| C5 | 286.08 | 286.26 | 305.69 | 306.71 | 306.71 | 7.15 | |||
| C6 | 297.60 | 297.80 | 315.89 | 317.00 | 317.00 | 6.45 | |||
| 6.6 μg/cm2 GO surface concentration | C7 | 302.42 | 302.61 | 312.86 | 312.99 | 312.99 | 3.43 | 6.86 | 3.09 |
| C8 | 319.98 | 320.14 | 329.32 | 329.54 | 329.54 | 2.94 | |||
| C9 | 280.05 | 280.34 | 296.96 | 297.17 | 299.17 | 6.72 | |||
| C10 | 264.88 | 265.06 | 289.21 | 290.82 | 290.82 | 9.72 | |||
| C11 | 285.97 | 286.16 | 308.52 | 309.82 | 309.82 | 8.27 | |||
| C12 | 272.24 | 272.44 | 298.03 | 299.92 | 299.92 | 10.09 | |||
| 9.9 μg/cm2 GO surface concentration | C13 | 304.30 | 304.62 | 323.06 | 323.32 | 323.32 | 6.14 | 5.62 | 2.41 |
| C14 | 310.42 | 310.62 | 319.07 | 319.20 | 319.20 | 2.76 | |||
| C15 | 270.15 | 270.49 | 288.25 | 288.53 | 288.53 | 6.67 | |||
| C16 | 277.73 | 277.92 | 301.93 | 303.46 | 303.46 | 9.19 | |||
| C17 | 306.99 | 307.16 | 324.52 | 325.44 | 325.44 | 5.95 | |||
| C18 | 338.39 | 338.53 | 348.14 | 348.77 | 348.77 | 3.02 |
| Sample Code | md (g) | md (g) + GO | m1 (g) | m2 (g) | ms (g) | A (%) | Amean (%) | sd | |
|---|---|---|---|---|---|---|---|---|---|
| CONTROL (uncoated) | D1 | 320.62 | 320.63 | 330.74 | 330.97 | 330.97 | 3.22 | 3.78 | 0.37 |
| D2 | 298.59 | 298.96 | 310.03 | 310.18 | 310.18 | 3.75 | |||
| D3 | 332.21 | 332.58 | 344.83 | 345.07 | 345.07 | 3.76 | |||
| D4 | 291.67 | 291.97 | 303.20 | 303.40 | 303.40 | 3.91 | |||
| D5 | 317.51 | 317.88 | 331.27 | 331.58 | 331.58 | 4.31 | |||
| D6 | 306.09 | 306.49 | 315.85 | 316.22 | 316.22 | 3.17 | |||
| D7 | 326.94 | 327.27 | 339.04 | 339.27 | 339.27 | 3.67 | |||
| D8 | 299.22 | 299.53 | 311.51 | 311.80 | 311.80 | 4.10 | |||
| D9 | 300.08 | 300.38 | 311.20 | 311.53 | 311.53 | 3.71 | |||
| D10 | 320.28 | 320.63 | 333.77 | 334.00 | 334.00 | 4.17 | |||
| 6.6 μg/cm2 GO surface concentration | D1 | 320.58 | 320.82 | 330.75 | 330.85 | 330.85 | 3.13 | 3.75 | 0.37 |
| D2 | 298.52 | 298.79 | 309.92 | 310.00 | 310.00 | 3.75 | |||
| D3 | 332.16 | 332.44 | 344.59 | 344.65 | 344.65 | 3.67 | |||
| D4 | 291.64 | 291.88 | 303.18 | 303.26 | 303.26 | 3.90 | |||
| D5 | 317.48 | 317.74 | 331.30 | 331.40 | 331.40 | 4.30 | |||
| D6 | 305.98 | 306.28 | 315.97 | 316.22 | 316.22 | 3.25 | |||
| D7 | 326.91 | 327.17 | 339.01 | 339.10 | 339.10 | 3.65 | |||
| D8 | 299.19 | 299.41 | 311.38 | 311.50 | 311.50 | 4.04 | |||
| D9 | 300.03 | 300.27 | 311.19 | 311.28 | 311.28 | 3.67 | |||
| D10 | 320.26 | 320.52 | 333.59 | 333.70 | 333.70 | 4.11 | |||
| 9.9 μg/cm2 GO surface concentration | D1 | 320.56 | 320.82 | 330.73 | 331.58 | 331.58 | 3.35 | 3.84 | 0.36 |
| D2 | 298.51 | 298.79 | 310.00 | 310.29 | 310.29 | 3.85 | |||
| D3 | 332.14 | 332.41 | 344.60 | 344.85 | 344.85 | 3.74 | |||
| D4 | 291.61 | 291.58 | 303.19 | 303.42 | 303.42 | 4.06 | |||
| D5 | 317.45 | 317.73 | 331.28 | 331.65 | 331.65 | 4.38 | |||
| D6 | 305.92 | 306.23 | 315.99 | 316.16 | 316.16 | 3.24 | |||
| D7 | 326.91 | 327.20 | 339.03 | 339.40 | 339.40 | 3.73 | |||
| D8 | 299.18 | 299.42 | 311.50 | 311.58 | 311.58 | 4.06 | |||
| D9 | 300.03 | 300.24 | 311.18 | 311.53 | 311.53 | 3.76 | |||
| D10 | 320.25 | 320.51 | 333.79 | 333.97 | 333.97 | 4.20 |
References
- Gomez-Heras, M.; Smith, B.J.; Viles, H.A. Oxford stone revisited: Causes and consequences of diversity in building limestone used in the historic centre of Oxford, England. In Natural Stone Resources for Historial Monuments; Geological Society: London, UK, 2010; pp. 101–110. [Google Scholar]
- AENOR UNE-EN 12058:2015; Natural Stone Products—Slabs for Floors and Stairs—Requirements. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0055307 (accessed on 19 May 2023).
- AENOR UNE-EN 12057:2005; Natural Stone Products—Modular Tiles—Requirements. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=norma-une-en-12057-2005-n0033981 (accessed on 19 May 2023).
- AENOR UNE-EN 1469:2015; Natural Stone Products—Slabs for Cladding—Requirements. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0055782 (accessed on 19 May 2023).
- AENOR UNE-EN 1341:2013; Slabs of Natural Stone for External Paving—Requirements and Test Methods. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0051551 (accessed on 19 May 2023).
- Vielba-Cuerpo, C.; Hernández Olivares, F. Tests to Characterize the Behaviour of Natural Stone in Contact with Water. Mater. Constr. Cons. Super. Investig. Cient. 2002, 52, 43–54. [Google Scholar] [CrossRef]
- AENOR UNE-EN 13755:2008; Natural Stone Test Methods—Determination of Water Absorption at Atmospheric Pressure. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0042047 (accessed on 29 January 2023).
- Suárez-González, A.; Kovács, T.; Herrero-Hernández, A.; Gómez-Fernández, F. Petrophysical characterization of the Dolomitic Member of the Boñar Formation (Upper Cretaceous; Duero Basin, Spain) as a potential CO2 reservoir. Estud. Geol. 2016, 72, e048. [Google Scholar] [CrossRef]
- Gómez Fernández, F.; Ménderz Cecilia, A.J.; Bahamonde, J.R. La Formación Boñar (Cretácico Superior, Norte de León): Estratigrafía, Geoquímica y Potencial Productor de Roca Ornamental. Rev. Soc. Geol. España 2003, 16, 61–72. [Google Scholar]
- Laboratorio Oficial de Ensayos de Materiales de la Construccion (LOMECO). Estudio de Procesos de Envejecimiento de Monumentos de Piedra. Estudio de Materiales Para Restauración. 1991. Available online: http://info.igme.es/SidPDF/168000/131/168131_0000001.pdf (accessed on 19 May 2023).
- IGME. Interpretación de Los Resultados Obtenidos En El Informe DTT 17/0013. Available online: http://info.igme.es/SidPDF/168000/409/168409_0000003.pdf (accessed on 20 December 2022).
- González-Campelo, D.; Fernández-Raga, M.; Gómez-Gutiérrez, Á.; Guerra-Romero, M.I.; González-Domínguez, J.M. Extraordinary Protective Efficacy of Graphene Oxide over the Stone-Based Cultural Heritage. Adv. Mater. Interfaces 2021, 8, 2101012. [Google Scholar] [CrossRef]
- Kazemi, E.; Ghamari, M.A.K.; Neshanifam, S. The Application of Nanotechnology against Humidity in the Building Preservation of Tabriz Historical and Traditional City, Case Study: Blue Mosque, Tabriz. J. Archit. Urban Des. Urban Plan. 2016, 9, 29–38. [Google Scholar]
- García Martínez, V.; De Oviedo, U.; Rosa, D.; López, M.; Cristina, D.; Velasco, B. Estudio de La Estabilidad Del Óxido de Grafeno Con El Tiempo. Master’s Thesis, Instituto Nacional Del Carbón, Oviedo, Spain, 2013. [Google Scholar]
- Botas, C.; Pérez-Mas, A.M.; Álvarez, P.; Santamaría, R.; Granda, M.; Blanco, C.; Menéndez, R. Optimization of the Size and Yield of Graphene Oxide Sheets in the Exfoliation Step. Carbon 2013, 63, 576–578. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Kim, S.; Zhou, S.; Hu, Y.; Acik, M.; Chabal, Y.J.; Berger, C.; De Heer, W.; Bongiorno, A.; Riedo, E. Room-Temperature Metastability of Multilayer Graphene Oxide Films. Nat. Mater. 2012, 11, 544–549. [Google Scholar] [CrossRef]
- Li, C.; Lu, Y.; Yan, J.; Yu, W.; Zhao, R.; Du, S.; Niu, K. Effect of Long-Term Ageing on Graphene Oxide: Structure and Thermal Decomposition. R. Soc. Open Sci. 2021, 8, 202309. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A.; Enzelberger, M.; Müller, P. Formation and Decomposition of CO2 Intercalated Graphene Oxide. Chem. Mater. 2012, 24, 1276–1282. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, H.-M.; Xu, C.-H.; Zhang, M.-H.; Pan, X.-H.; Gao, Y.-F. A Study of Graphene Oxidation Using Thermal Analysis-Mass Spectrometry Combined with Pulse Thermal Analysis. Acta Phys.-Chim. Sin. 2016, 32, 1634–1638. [Google Scholar] [CrossRef]
- Kauffman, G.B. Rayon: The First Semi-Synthetic Fiber Product. J. Chem. Educ. 1993, 70, 887. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Gardner, D.J.; Shen, W. Contact Angles and Wettability of Cellulosic Surfaces: A Review of Proposed Mechanisms and Test Strategies. Bioresources 2015, 10, 8657–8749. [Google Scholar] [CrossRef]
- Chen, T.C.; Yeung, M.R.; Mori, N. Effect of Water Saturation on Deterioration of Welded Tuff Due to Freeze-Thaw Action. Cold Reg. Sci. Technol. 2004, 38, 127–136. [Google Scholar] [CrossRef]
- Matsuoka, N. Mechanisms of Rock Breakdown by Frost Action: An Experimental Approach. Cold Reg. Sci. Technol. 1990, 17, 253–270. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, S.; Zheng, H.; Chen, Y. Elastoplastic Integration Method of Mohr-Coulomb Criterion. Geotechnics 2022, 2, 599–614. [Google Scholar] [CrossRef]
- BOE-A-2003-3596 Real Decreto 140/2003, de 7 de Febrero, Por El Que Se Establecen Los Criterios Sanitarios de La Calidad Del Agua de Consumo Humano. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2003-3596 (accessed on 19 May 2023).
- Ellis, A.J. The Solubility of Calcite in Carbon Dioxide Solutions. Am. J. Sci. 1959, 257, 354–365. [Google Scholar] [CrossRef]
- Real Decreto 314/2016, de 29 de Julio, Por El Que Se Modifican El Real Decreto 140/2003, de 7 de Febrero, Por El Que Se Establecen Los Criterios Sanitarios de La Calidad Del Agua de Consumo Humano. Available online: https://www.boe.es/eli/es/rd/2016/07/29/314 (accessed on 17 April 2023).
- Vergès-Belmin, V. Towards a Definition of Common Evaluation Criteria for the Cleaning of Porous Building Materials: A Review. Sci. Technol. Cult. Herit. 1996, 5, 69–83. [Google Scholar]
- Topal, T.; Doyuran, V. Engineering Geological Properties and Durability Assessment of the Cappadocian Tuff. Eng. Geol. 1997, 47, 175–187. [Google Scholar] [CrossRef]
- Gisbert Aguilar, J.; Buj Fandos, O.; Bauluz Lázaro, B.; Peddis, F.; Cuccuru, F. Deterioration Caused by Dimensional Change in Stone (EBD Pathology): The Role of the Organic Matter—Pore Network—Salt Combination. J. Cult. Herit. 2018, 34, 198–207. [Google Scholar] [CrossRef]
- Coelho, G.B.A.; Entradas Silva, H.; Henriques, F.M.A. Impact of Climate Change in Cultural Heritage: From Energy Consumption to Artefacts’ Conservation and Building Rehabilitation. Energy Build. 2020, 224, 110250. [Google Scholar] [CrossRef]
- Esbert, R.M.; Marcos, R.M.; Ordaz, J.; Montoto, M.; del Rio, L.M.S.; de Argandoña, V.G.R.; Calleja, L.; Alonso, F.J.; Rodríguez-Rey, Á. Petrografía, Propiedades Físicas y Durabilidad de Algunas Rocas Utilizadas En El Patrimonio Monumental de Catalunya, España. Mater. Constr. 1989, 39, 37–47. [Google Scholar] [CrossRef]
- Graham, L.E. Propiedades geofísicas de rocas y suelos calcáreos. Mediciones de laboratorio en especímenes pequeños. Ingeniería 2002, 6, 23–32. [Google Scholar]
- Bonazza, A.; Messina, P.; Sabbioni, C.; Grossi, C.M.; Brimblecombe, P. Mapping the Impact of Climate Change on Surface Recession of Carbonate Buildings in Europe. Sci. Total Environ. 2009, 407, 2039–2050. [Google Scholar] [CrossRef]
- Huerto-Cardenas, H.E.; Aste, N.; Del Pero, C.; Della Torre, S.; Leonforte, F. Effects of Climate Change on the Future of Heritage Buildings: Case Study and Applied Methodology. Climate 2021, 9, 132. [Google Scholar] [CrossRef]
- Franceschi, S.; Germani, L. Manuale Operativo per il Restauro Architettonico: Metodologie Di Intervento per il Restauro e la Conservazione del Patrimonio Storico, 4th ed.; Tipografia del Genio Civile: Roma, Italy, 2010. [Google Scholar]
- Martínez-García, R.; González-Campelo, D.; Fraile-Fernández, F.J.; Castañón, A.M.; Caldevilla, P.; Giganto, S.; Ortiz-Marqués, A.; Zelli, F.; González-Domínguez, J.M.; Fernández-Raga, M. Performance Study of Graphene Oxide as an Antierosion Coating in Ornamental and Heritage Dolostone. Adv. Mater. Technol. 2023, 2300486. [Google Scholar] [CrossRef]
- Rodríguez, I.; Ortiz, A.; Caldevilla, P.; Giganto, S.; Búrdalo, G.; Fernández-Raga, M. Comparison between the Effects of Normal Rain and Acid Rain on Calcareous Stones under Laboratory Simulation. Hydrology 2023, 10, 79. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Búrdalo-Salcedo, G.; Rodríguez, I.; Fernández-Raga, M.; Fernández-Raga, S.; Rodríguez-Fernández, C.; González-Domínguez, J.M. Adaptation of a Standard Method for Water Absorption Testing of Stone Materials: The Case of a Hydrophilic Protective Coating. Materials 2023, 16, 4228. https://doi.org/10.3390/ma16124228
Búrdalo-Salcedo G, Rodríguez I, Fernández-Raga M, Fernández-Raga S, Rodríguez-Fernández C, González-Domínguez JM. Adaptation of a Standard Method for Water Absorption Testing of Stone Materials: The Case of a Hydrophilic Protective Coating. Materials. 2023; 16(12):4228. https://doi.org/10.3390/ma16124228
Chicago/Turabian StyleBúrdalo-Salcedo, Gabriel, Indira Rodríguez, María Fernández-Raga, Sagrario Fernández-Raga, Carlos Rodríguez-Fernández, and José Miguel González-Domínguez. 2023. "Adaptation of a Standard Method for Water Absorption Testing of Stone Materials: The Case of a Hydrophilic Protective Coating" Materials 16, no. 12: 4228. https://doi.org/10.3390/ma16124228
APA StyleBúrdalo-Salcedo, G., Rodríguez, I., Fernández-Raga, M., Fernández-Raga, S., Rodríguez-Fernández, C., & González-Domínguez, J. M. (2023). Adaptation of a Standard Method for Water Absorption Testing of Stone Materials: The Case of a Hydrophilic Protective Coating. Materials, 16(12), 4228. https://doi.org/10.3390/ma16124228











