Corrosion Risk to Metal-Based Artefacts in a Scientific and Technical Museum: An Assessment of Environmental and Exhibition Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Museums Locations
- 1.
- MUNCYT Alcobendas
- Space–Time showcase (S1): This showcase’s large size extends over an entire room. It contains more than a hundred instruments for navigation, astronomy, topography, etc. It is the location with the greatest variety of material exhibited as a whole, with no separations between the artefacts.
- Cabinet room (R3): This room comprises different showcases, with no ST artefacts outside them.
- Wheels room (R4): In contrast to the previous room, this room exhibits a large collection of cars, motorbikes, bicycles, and carriages. As a result, there is an abundance of tyres, lubricating oils, and plastics.
- 2.
- MUNCYT A Coruña
- Heritage showcase (S5): This showcase is similar to Space–Time, but is divided into several independent parts. The part containing three artefacts is selected, and glass, wood, and copper alloys are identified as the materials composing the artefacts.
- Ex cathedra showcase (S6): This showcase is one of the most complex, as it is divided into several sections, as can be seen in Figure 1b. It contains 19th century physics and chemistry laboratory artefacts made of wood, steel, copper, and brass. For the study, a compartment is selected, in which a binocular microscope and a wooden box of lenses are on display.
- XX Century room (R7): This room is large and is connected to other spaces. It is located in an open area of the museum, which has a glass enclosure system forming the perimeter structure of the building. It contains artefacts made of different materials, both inside and outside showcases (Figure 1c).
- Entrance room (R8): This is the museum’s visitor reception room, where the doors to the outside are open constantly. Hanging from the ceiling is an aluminium Midget Mustang aircraft from the late 1960s. There is also a showcase with temporarily changing artefacts.
2.2. Environmental Conditions: Pollutants, Humidity and Temperature
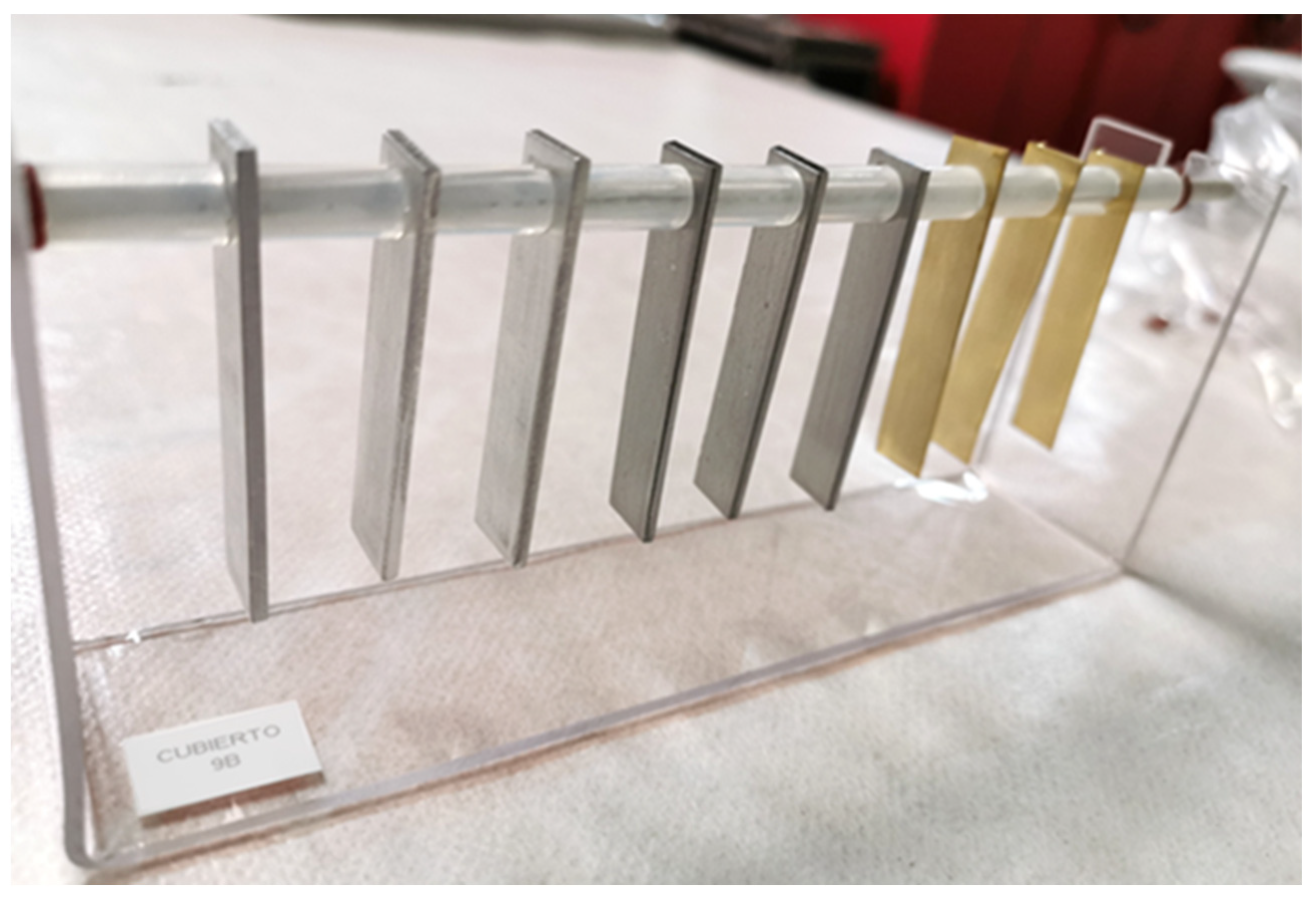
2.3. Sample Preparation: Coupons and Racks
2.4. Calculation of the Rate of Mass Increase
2.5. Characterisation Techniques
3. Results and Discussion
3.1. Environmental Conditions
3.2. Visual Changes and Colour Measurements
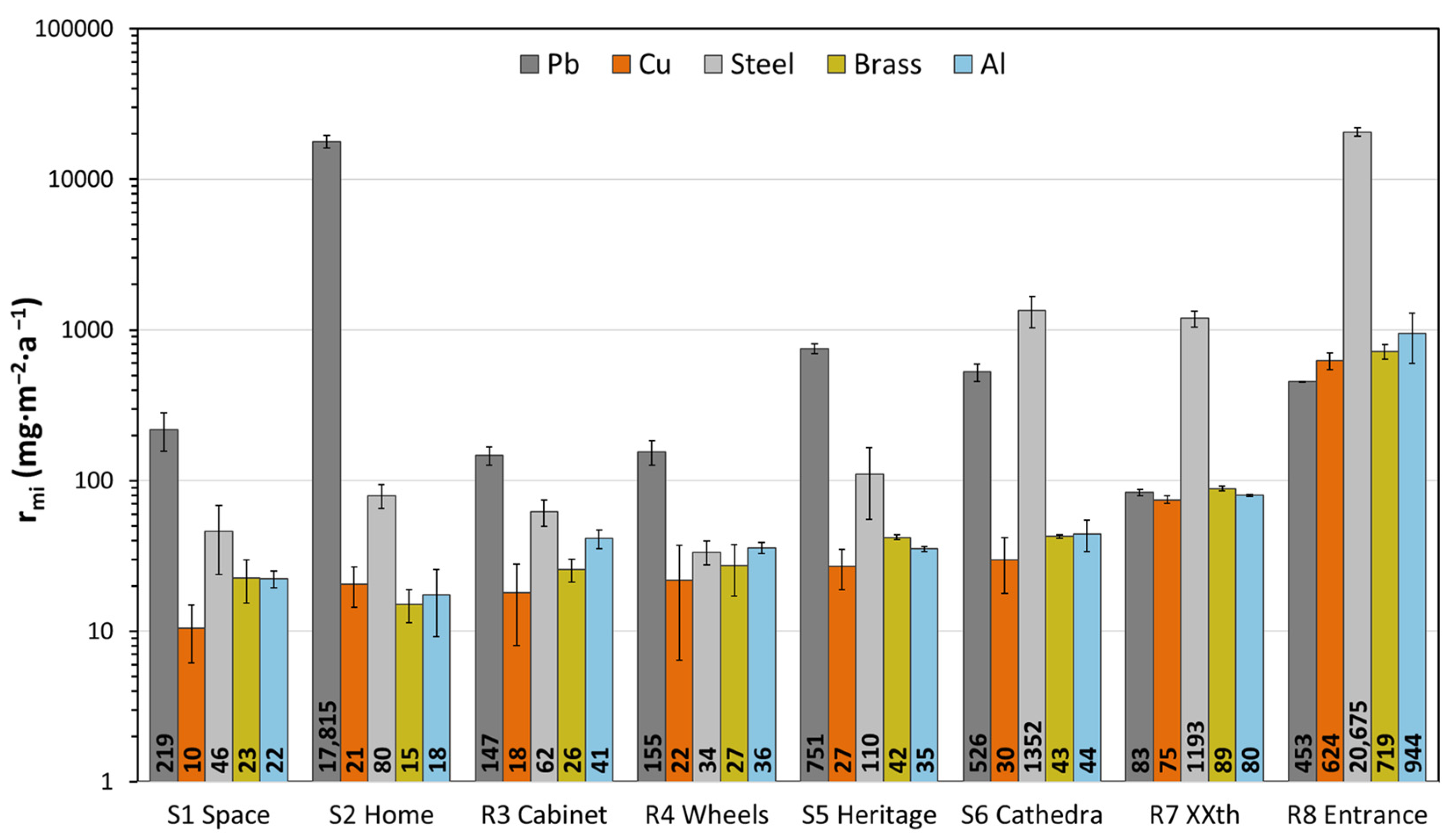
3.3. Corrosion Rate by Mass Increase
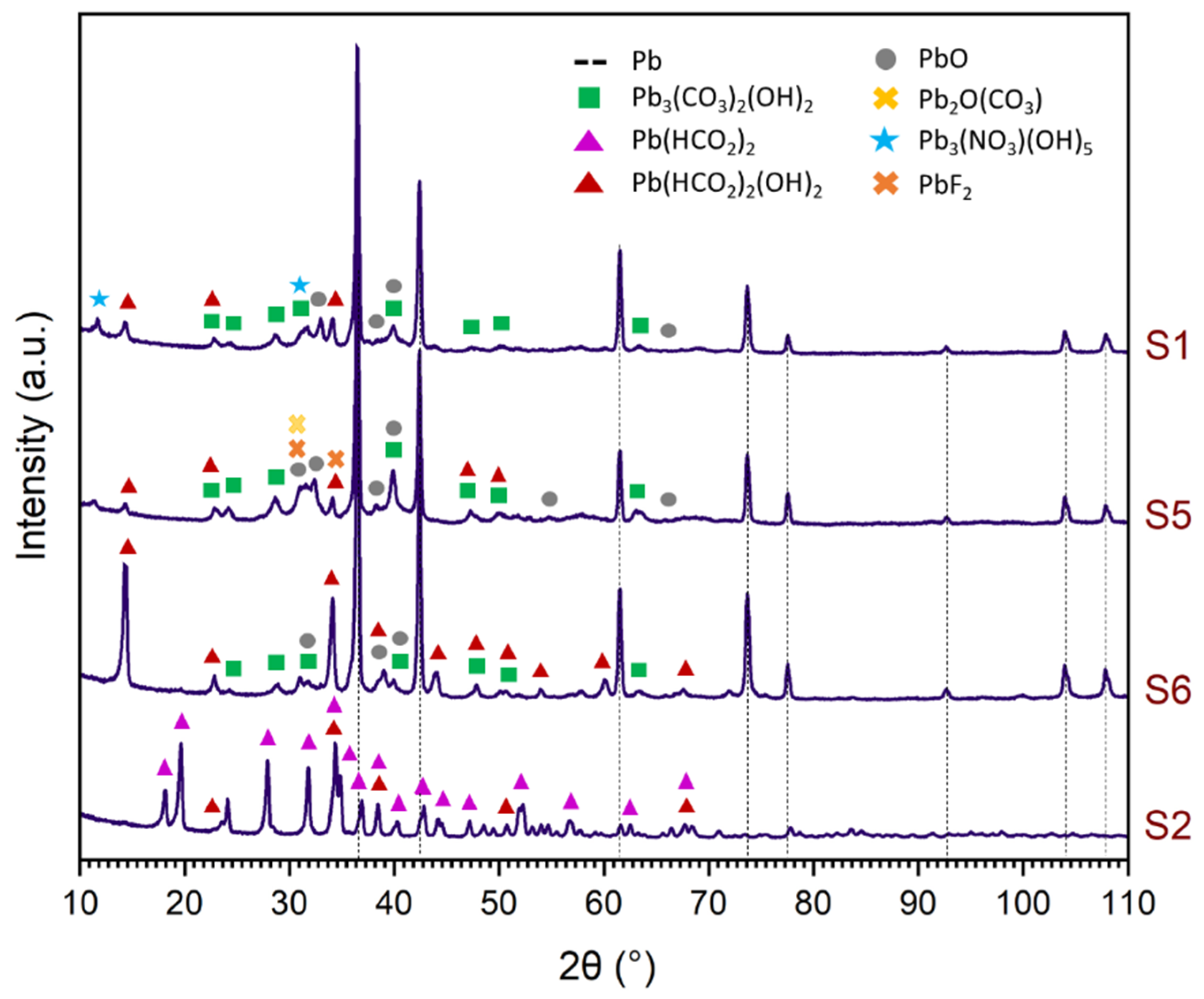
3.4. Corrosion Product Characterisation
3.5. Final Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grzywacz, C.M. Monitoring for Gaseous Pollutants in Museum Environments; The Getty: Los Angeles, CA, USA, 2006; ISBN 978-0-89236-851-8. [Google Scholar]
- Schieweck, A.; Salthammer, T. Emissions from Construction and Decoration Materials for Museum Showcases. Stud. Conserv. 2009, 54, 218–235. [Google Scholar] [CrossRef]
- Chiantore, O.; Poli, T. Indoor Air Quality in Museum Display Cases: Volatile Emissions, Materials Contributions, Impacts. Atmosphere 2021, 12, 364. [Google Scholar] [CrossRef]
- Brimblecombe, P. The Composition of Museum Atmospheres. Atmos. Environ. 1990, 24, 1–8. [Google Scholar] [CrossRef]
- Grzywacz, C.M.; Tennent, N.H. Pollution Monitoring in Storage and Display Cabinets: Carbonyl Pollutant Levels in Relation to Artifact Deterioration. In Proceedings of the Preventive Conservation: Practice, Theory and Research, Ottawa, Canada, 12–16 September 1994; International Institute for Conservation of Historic and Artistic Works: London, UK, 1994; pp. 164–170. [Google Scholar]
- Tetreault, J. Airborne Pollutants in Museums, Galleries and Archives: Risk Assessment, Control Strategies and Preservation Management; Canadian Conservation Institute: Ottawa, ON, Canada, 2003; ISBN 0662340590. [Google Scholar]
- Lemos, M.; Tissot, I. Reflections on the Conservation Challenges of Scientific and Technological Objects. Conserv. Patrim. 2020, 33, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Gual, M. Estudi Sobre La Conservació Preventiva i La Restauració de Les Colleccions de Patrimoni Científic, Tècnic i Industrial Del Sistema Territorial Del Museu Nacional de La Ciència i La Tècnica de Catalunya. Criteris per l’elaboració de Protocols. Master’s Thesis, University of Barcelona, Barcelona, Spain, 2021. [Google Scholar]
- Hallam, D.; Thurrowgood, D.; Otieno-Alego, V.; Creagh, D. An EIS Method for Assessing Thin Oil Films Used in Museums. Proc. Int. Conf. Met. Conserv. 2004, 4, 379–387. [Google Scholar]
- Díaz-Cortés, A.; Barat, B.R.; Leal, J.; Llorente, I.; del Egido, M.; Cano, E. Diagnosis of the Condition of Scientific and Technical Collections: Historical Extinguishers of the MUNCYT. Ge-Conservacion 2020, 18, 7–19. [Google Scholar] [CrossRef]
- Lafuente, D.; Cano, E.; Llorente, I.; Crespo, A.; Künne, J.; Schieweck, A. The Effects of Organic Pollutants on Metals in Museums: Corrosion Products, Synergistic Effects and the Influence of Climatic Parameters. In Proceedings of the Metal 2013: Proceedings of the Interim Meeting of the ICOM-CC, MWG, Edinburgh, Scotland, 16–20 September 2013; Hyslop, E., Gonzalez, V., Troalen, L., Wilson, L., Eds.; Historic Scotland and International Council of Museums–Committee for Conservation: Edinburgh, Scotland, 2013. [Google Scholar]
- Tétreault, J.; Cano, E.; van Bommel, M.; Scott, D.; Dennis, M.; Barthés-Labrousse, M.-G.; Minel, L.; Robbiola, L. Corrosion of Copper and Lead by Formaldehyde, Formic and Acetic Acid Vapours. Stud. Conserv. 2003, 48, 237–250. [Google Scholar] [CrossRef]
- Samide, M.J.; Smith, G.D. Analysis and Quantitation of Volatile Organic Compounds Emitted from Plastics Used in Museum Construction by Evolved Gas Analysis-Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2015, 1426, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Lattuati-Derieux, A.; Egasse, C.; Thao-Heu, S.; Balcar, N.; Barabant, G.; Lavédrine, B. What Do Plastics Emit? HS-SPME-GC/MS Analyses of New Standard Plastics and Plastic Objects in Museum Collections. J. Cult. Herit. 2013, 14, 238–247. [Google Scholar] [CrossRef]
- Robinet, L.; Thickett, D. A New Methodology for Accelerated Corrosion Testing. Stud. Conserv. 2004, 48, 263–268. [Google Scholar] [CrossRef]
- Díaz, I.; Cano, E. Quantitative Oddy Test by the Incorporation of the Methodology of the ISO 11844 Standard: A Proof of Concept. J. Cult. Herit. 2022, 57, 97–106. [Google Scholar] [CrossRef]
- Korenberg, C.; Keable, M.; Phippard, J.; Doyle, A. Refinements Introduced in the Oddy Test Methodology. Stud. Conserv. 2018, 63, 2–12. [Google Scholar] [CrossRef]
- ISO 11844-1; Corrosion of Metals and Alloys—Classification of Low Corrosivity of Indoor Atmospheres—Part 1: Determination and Estimation of Indoor Corrosivity. ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/73253.html (accessed on 27 April 2023).
- ISO 11844-2; Corrosion of Metals and Alloys—Classification of Low Corrosivity of Indoor Atmospheres—Part 2: Determination of Corrosion Attack in Indoor Atmospheres. ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/73254.html (accessed on 27 April 2023).
- MUNCYT Collection. Available online: https://www.muncyt.es/coleccion (accessed on 3 April 2023).
- Molina, M.T.; Cano, E.; Leal, J.; Fort, R.; Álvarez de Buergo, M.; Ramírez-Barat, B. Protective Coatings for Metals in Scientific-Technical Heritage: The Collection of the Spanish National Museum of Science and Technology (MUNCYT). Heritage 2023, 6, 2473–2488. [Google Scholar] [CrossRef]
- ISO 11844-3; Corrosion of Metals and Alloys—Classification of Low Corrosivity of Indoor Atmospheres—Part 3: Measurement of Environmental Parameters Affecting Indoor Corrosivity. ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/73255.html (accessed on 27 April 2023).
- Ferm, M. A Sensitive Diffusional Sampler; Swedish Environmental Research Institute: Göteborg, Sweden, 1991. [Google Scholar]
- Ryhl-Svendsen, M. Corrosivity Measurements of Indoor Museum Environments Using Lead Coupons as Dosimeters. J. Cult. Herit. 2008, 9, 285–293. [Google Scholar] [CrossRef]
- ‘t Hart, L.; Storme, P.; Anaf, W.; Nuyts, G.; Vanmeert, F.; Dorriné, W.; Janssens, K.; de Wael, K.; Schalm, O. Monitoring the Impact of the Indoor Air Quality on Silver Cultural Heritage Objects Using Passive and Continuous Corrosion Rate Assessments. Appl. Phys. A Mater. Sci. Process. 2016, 122, 923. [Google Scholar] [CrossRef]
- Thomson, G. The Museum Environment, 2nd ed.; Butterworths-Heinemann: Oxford, UK, 1986; ISBN 9780750612661. [Google Scholar]
- Bickersteth, J. IIC and ICOM-CC 2014 Declaration on Environmental Guidelines. Stud. Conserv. 2016, 61, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Meteogalicia: Consulta de Datos de Calidade Do Aire. Available online: https://www.meteogalicia.gal/Caire/datos.action?request_locale=gl (accessed on 17 April 2023).
- Selwyn, L. Metals and Corrosion: A Handbook for the Conservation Professional; Canadian Conservation Institute: Ottawa, ON, Canada, 2004. [Google Scholar]
- Ramírez-Barat, B.; Molina, M.T.; Cano, E. Corrosion Detection by Color Change Using Crowdsourced Photographs. Preliminary Results of the MIPAC Project. In Proceedings of the Metal 2022: Proceedings of the Interim Meeting of the ICOM-CC, MWG, Helsinki, Finland, 5–9 September 2022; Mardikian, P., Näsänen, L., Arponen, A., Eds.; ICOM–CC and The National Museum of Finland: Helsinki, Finland, 2022; pp. 153–159. [Google Scholar]
- Chemin, A.; Marques, D.; Bisanha, L.; Motheo, A.d.J.; Bose Filho, W.W.; Ruchert, C.O.F. Influence of Al7Cu2Fe Intermetallic Particles on the Localized Corrosion of High Strength Aluminum Alloys. Mater. Des. 2014, 53, 118–123. [Google Scholar] [CrossRef]
- Barclay, R.L.; Dignard, C.; Selwyn, L. Caring for Metal Objects. Available online: https://www.canada.ca/en/conservation-institute/services/preventive-conservation/guidelines-collections/metal-objects.html (accessed on 21 April 2023).



| Corrosivity Category | Rate of Mass Increase (rmi) (mg·m−2·a−1) | |||
|---|---|---|---|---|
| Steel | Zinc | Copper | ||
| IC 1 | Very low | rmi ≤ 70 | rmi ≤ 50 | rmi ≤ 25 |
| IC 2 | Low | 70 < rmi ≤ 700 | 50 < rmi ≤ 250 | 25 < rmi ≤ 100 |
| IC 3 | Medium | 700 < rmi ≤ 7000 | 250 < rmi ≤ 700 | 100 < rmi ≤ 450 |
| IC 4 | High | 7000 < rmi ≤ 50,000 | 700 < rmi ≤ 2500 | 450 < rmi ≤ 1000 |
| IC 5 | Very high | 50,000 < rmi ≤ 150,000 | 2500 < rmi ≤ 5000 | 1000 < rmi ≤ 2500 |
| Ambient Conditions | Pollutant Concentration (µg/m3) According to STP *1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locations |
T (°C) Average | Tmax | Tmin | RH (%) Average | RHmax | RHmin | SO2 | HF | HCOOH | CH3COOH | HCl | H2S |
| S1 | 23.4 | 26.5 | 21.0 | 35.5 | 52.5 | 19.5 | <0.1 | <0.1 | 54 | <2.0 | <0.3 | 5.3 |
| S2 | 21.4 | 27.3 | 19.2 | 39.4 | 49.5 | 30.5 | <0.1 | <0.1 | >200 *2 | 3.5 | <0.3 | 2.7 |
| R4 | 21.2 | 27.2 | 17.9 | 42.6 | 65.9 | 22.4 | <0.1 | <0.1 | 8.3 | <2.0 | <0.3 | 2.6 |
| S5 | 20.7 | 27.0 | 16.0 | 52.7 | 62.0 | 37.5 | <0.1 | 0.14 | >200 | 107 | <0.3 | 7.0 |
| S6 | 20.5 | 27.0 | 16.5 | 55.9 | 66.0 | 37.5 | <0.1 | <0.1 | 63 | 97 | <0.3 | 1.8 |
| R7 | 22.2 | 30.6 | 16.6 | 70.6 | 91.8 | 48.4 | 0.22 | <0.1 | 3.7 | <2.0 | <0.3 | 2.8 |
| Steel (ISO 11844) | Cu (ISO 11844) | Pb (Proposal) | Brass (Proposal) | |
|---|---|---|---|---|
| S1 Space | IC 1 | IC 1 | IC 2 | IC 1 |
| S2 Home | IC 2 | IC 1 | IC 5 | IC 1 |
| R3 Cabinet | IC 1 | IC 1 | IC 2 | IC 1 |
| R4 Wheels | IC 1 | IC 1 | IC 2 | IC 1 |
| S5 Heritage | IC 2 | IC 2 | IC 4 | IC 2 |
| S6 Cathedra | IC 3 | IC 2 | IC 3 | IC 2 |
| R7 XXth | IC 3 | IC 2 | IC 2 | IC 2 |
| R8 Entrance | IC 4 | IC 4 | IC 3 | IC 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, M.T.; Cano, E.; Llorente, I.; Ramírez-Barat, B. Corrosion Risk to Metal-Based Artefacts in a Scientific and Technical Museum: An Assessment of Environmental and Exhibition Conditions. Materials 2023, 16, 4239. https://doi.org/10.3390/ma16124239
Molina MT, Cano E, Llorente I, Ramírez-Barat B. Corrosion Risk to Metal-Based Artefacts in a Scientific and Technical Museum: An Assessment of Environmental and Exhibition Conditions. Materials. 2023; 16(12):4239. https://doi.org/10.3390/ma16124239
Chicago/Turabian StyleMolina, María Teresa, Emilio Cano, Irene Llorente, and Blanca Ramírez-Barat. 2023. "Corrosion Risk to Metal-Based Artefacts in a Scientific and Technical Museum: An Assessment of Environmental and Exhibition Conditions" Materials 16, no. 12: 4239. https://doi.org/10.3390/ma16124239





