Research on Wide-Temperature Rechargeable Sodium-Sulfur Batteries: Features, Challenges and Solutions
Abstract
1. Introduction
2. Na-S Batteries at High Temperature
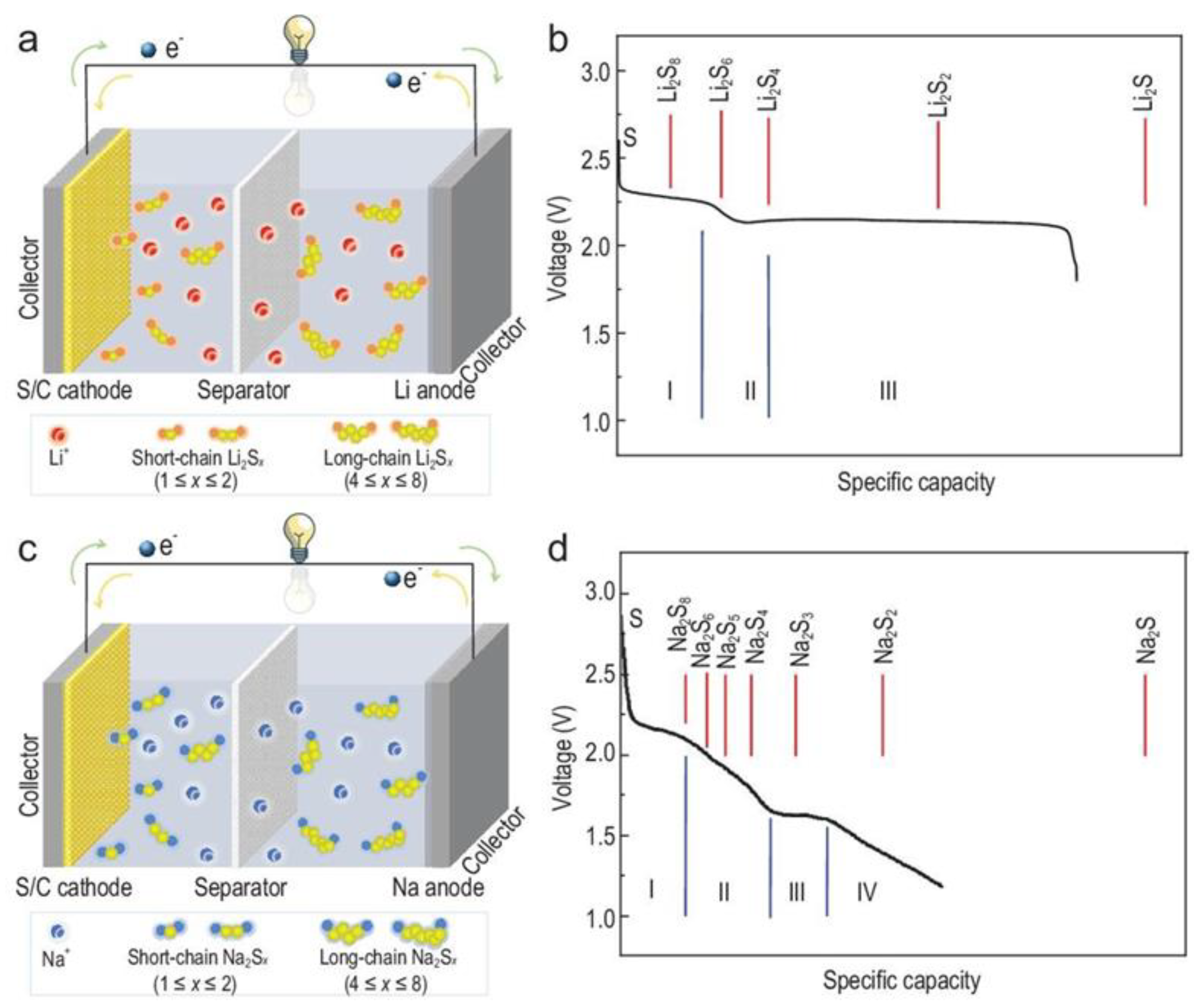
2.1. Reaction Mechanism
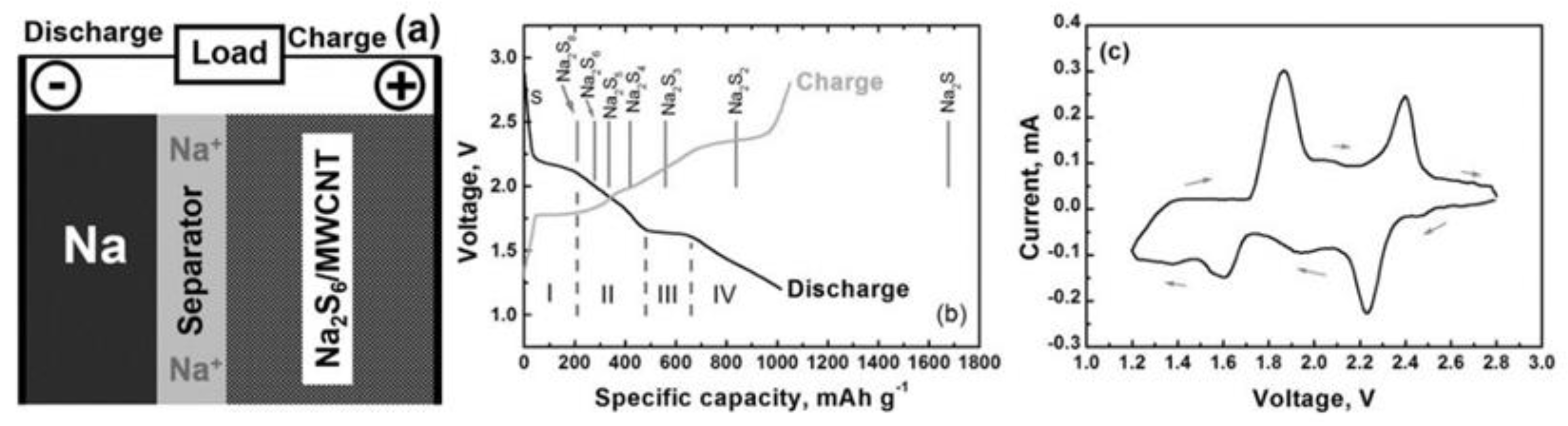
2.2. Features
2.2.1. High Energy Density
2.2.2. Materials
2.3. Challenges
2.3.1. Expenditure
2.3.2. Safety Hazards
2.3.3. Environmental Issues
2.3.4. Instability of Battery Efficiency
2.4. Solutions
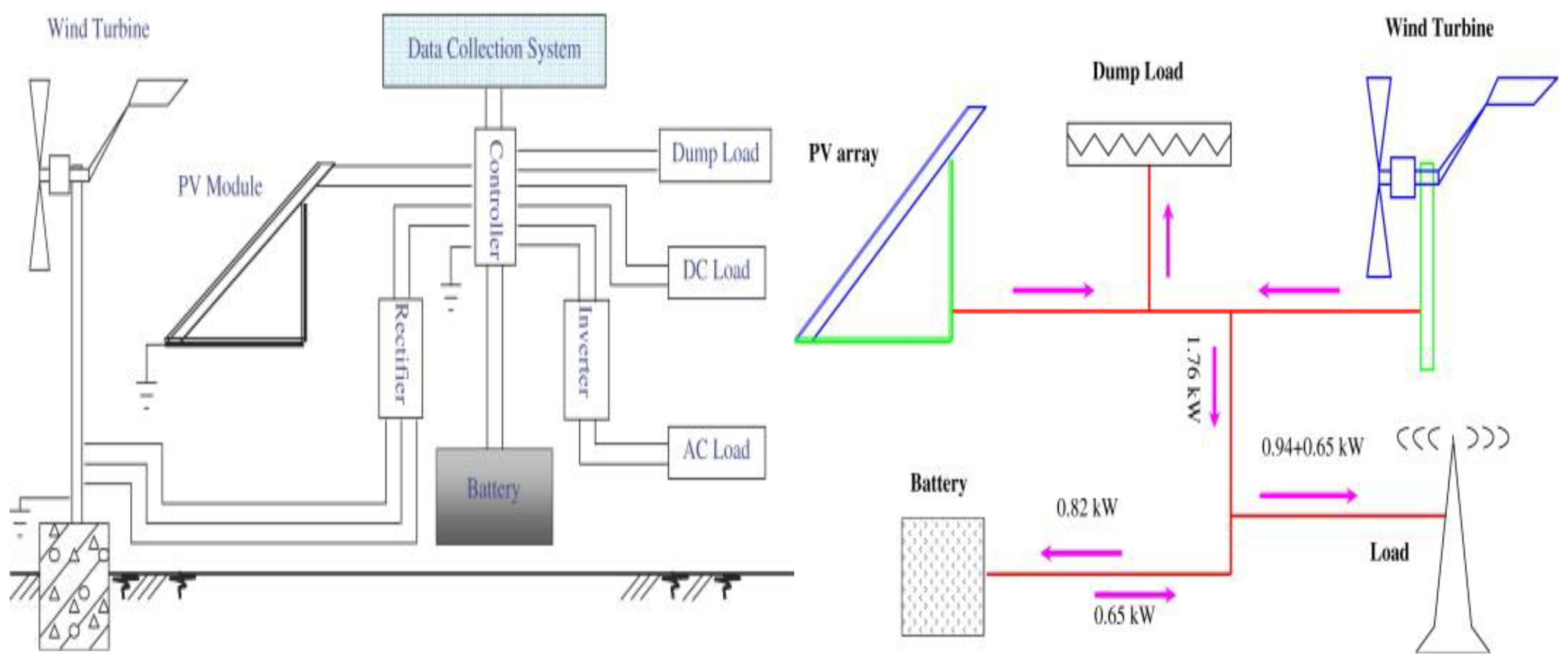
2.4.1. Combining Solar or Wind Power Generation Systems with Batteries
2.4.2. Adopting More Efficient Thermal Dissipation Measures and Utilizing Electrode Materials with Higher Temperature Tolerance
3. Na-S Batteries at Room Temperature
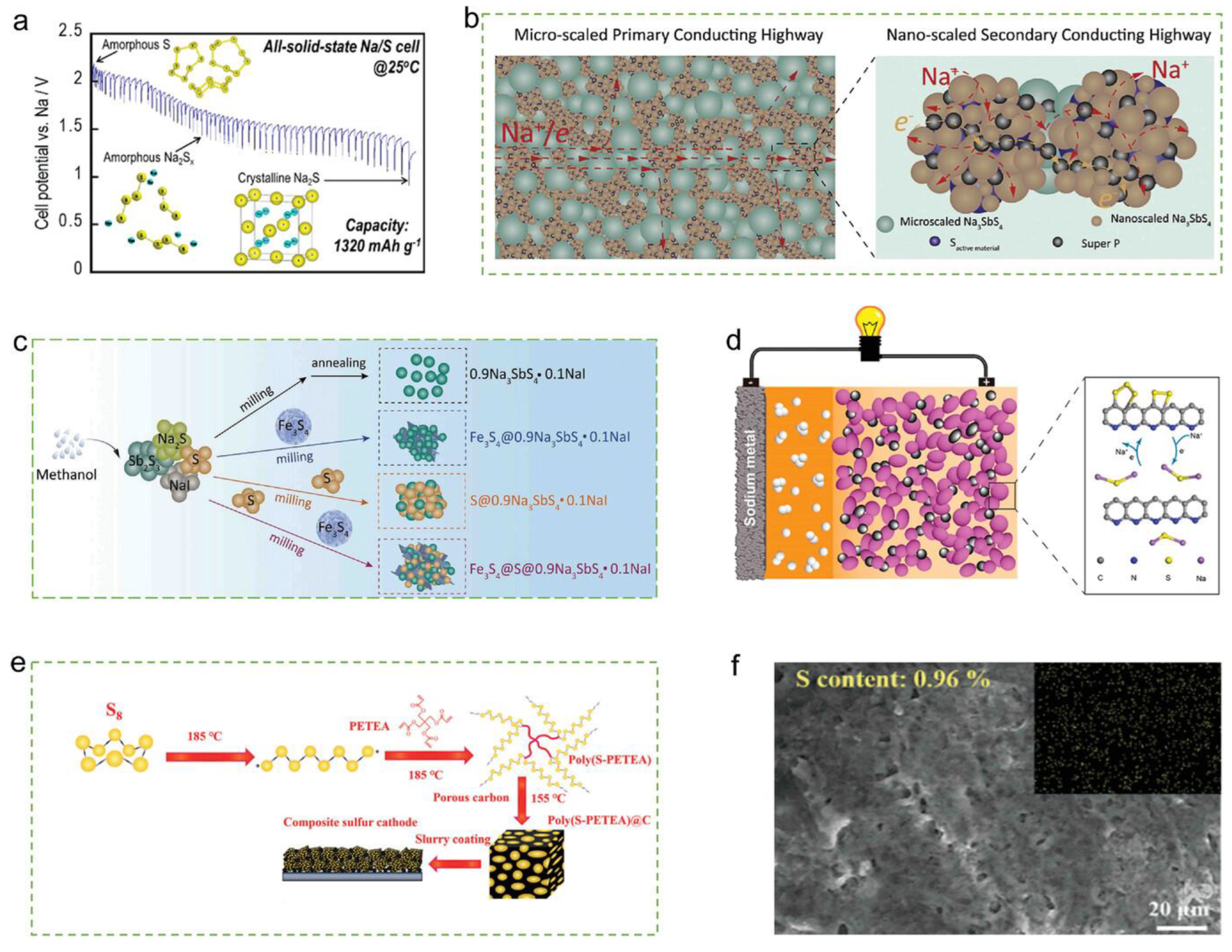
3.1. Reaction Mechanism
3.2. Features
3.2.1. Medium Temperature Flat Sodium Battery
3.2.2. Solid-State Sodium Battery
3.2.3. Sodium Molten Salt Battery
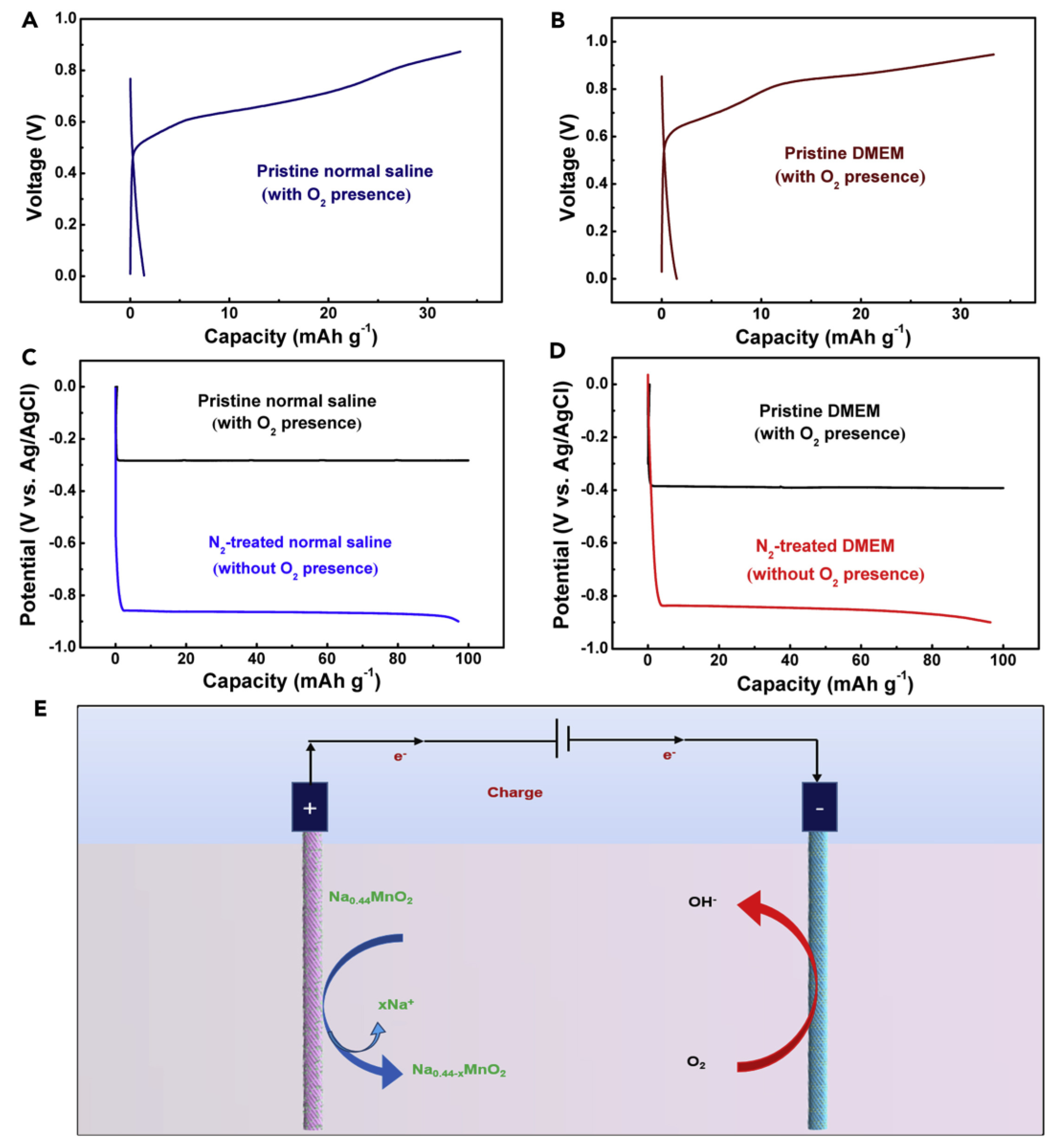
3.2.4. Aqueous Sodium-Ion Battery
3.3. Challenges
3.4. Solutions
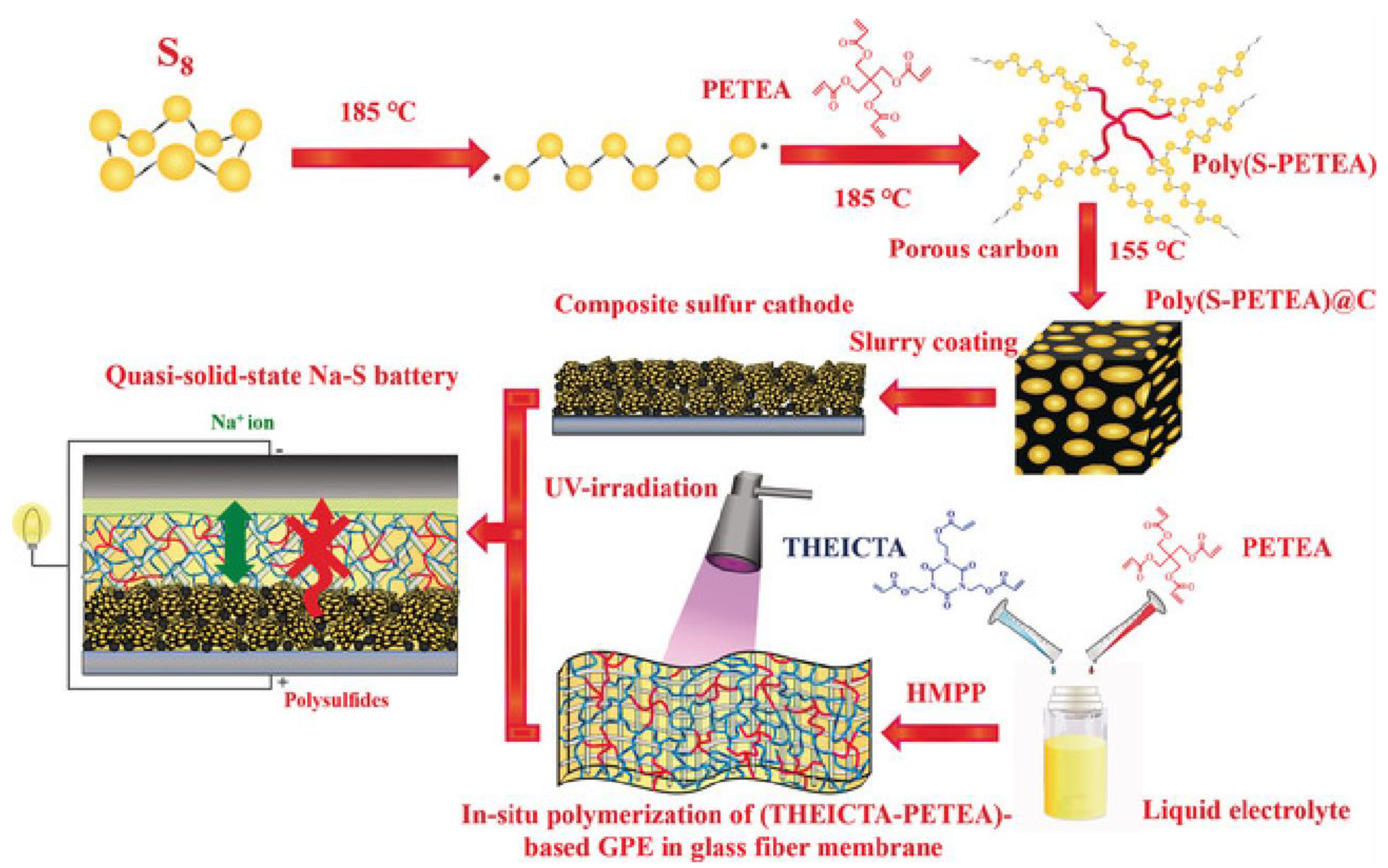
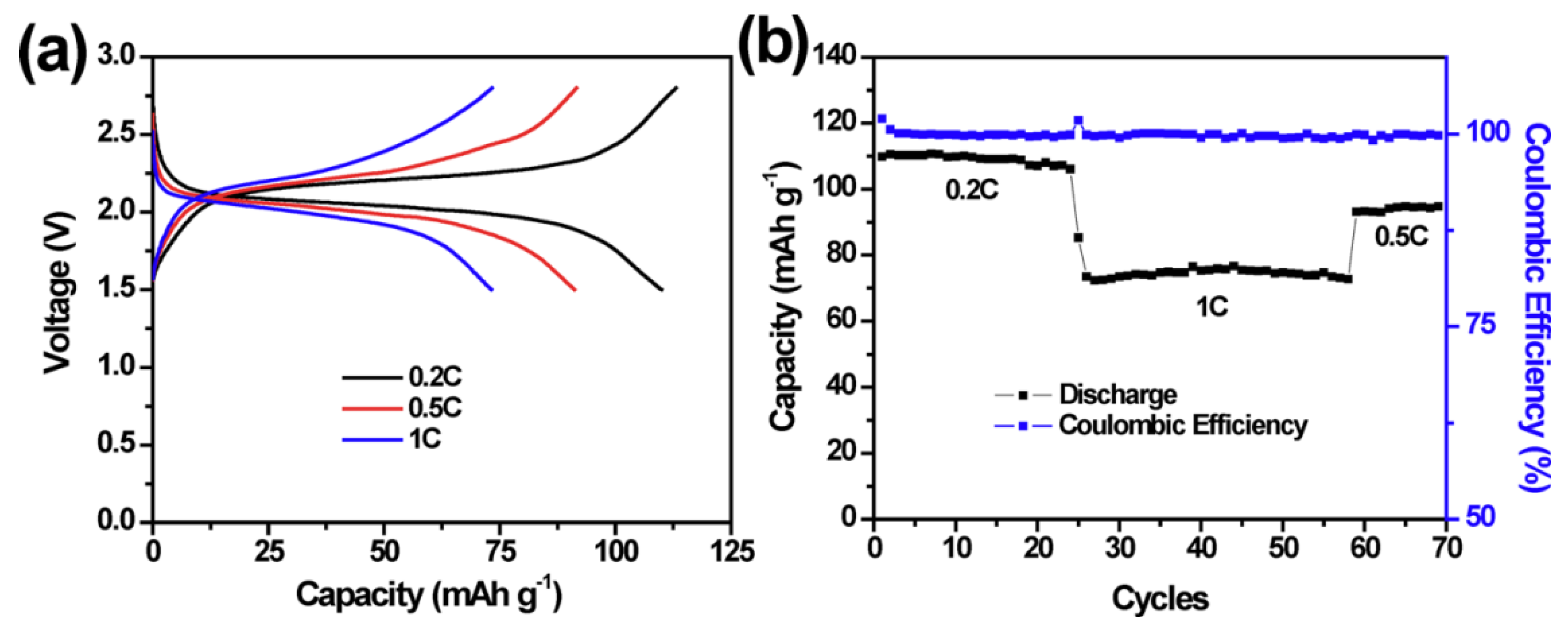
3.4.1. Na2S/C Composites
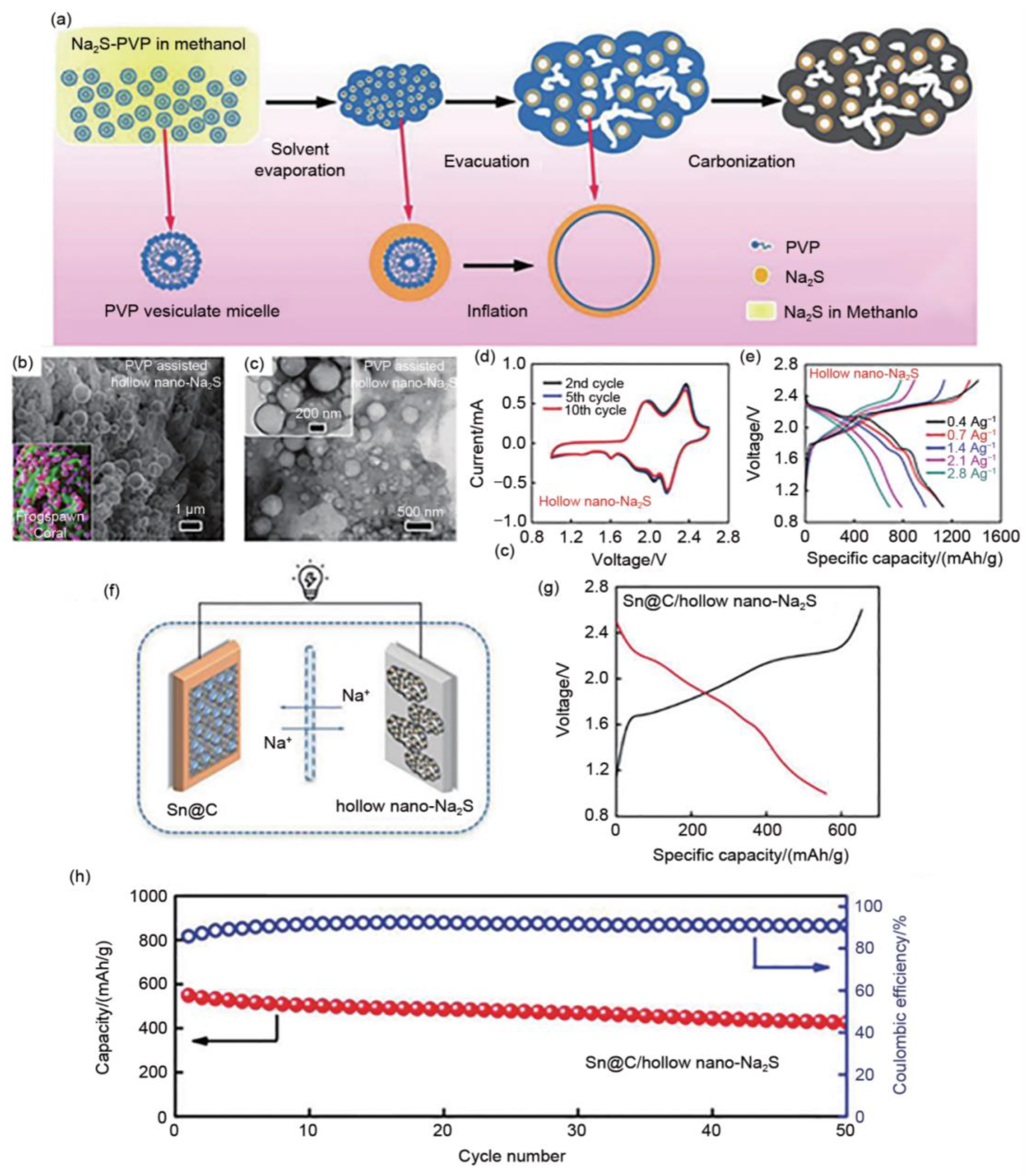
3.4.2. Na2S Morphology Modulation
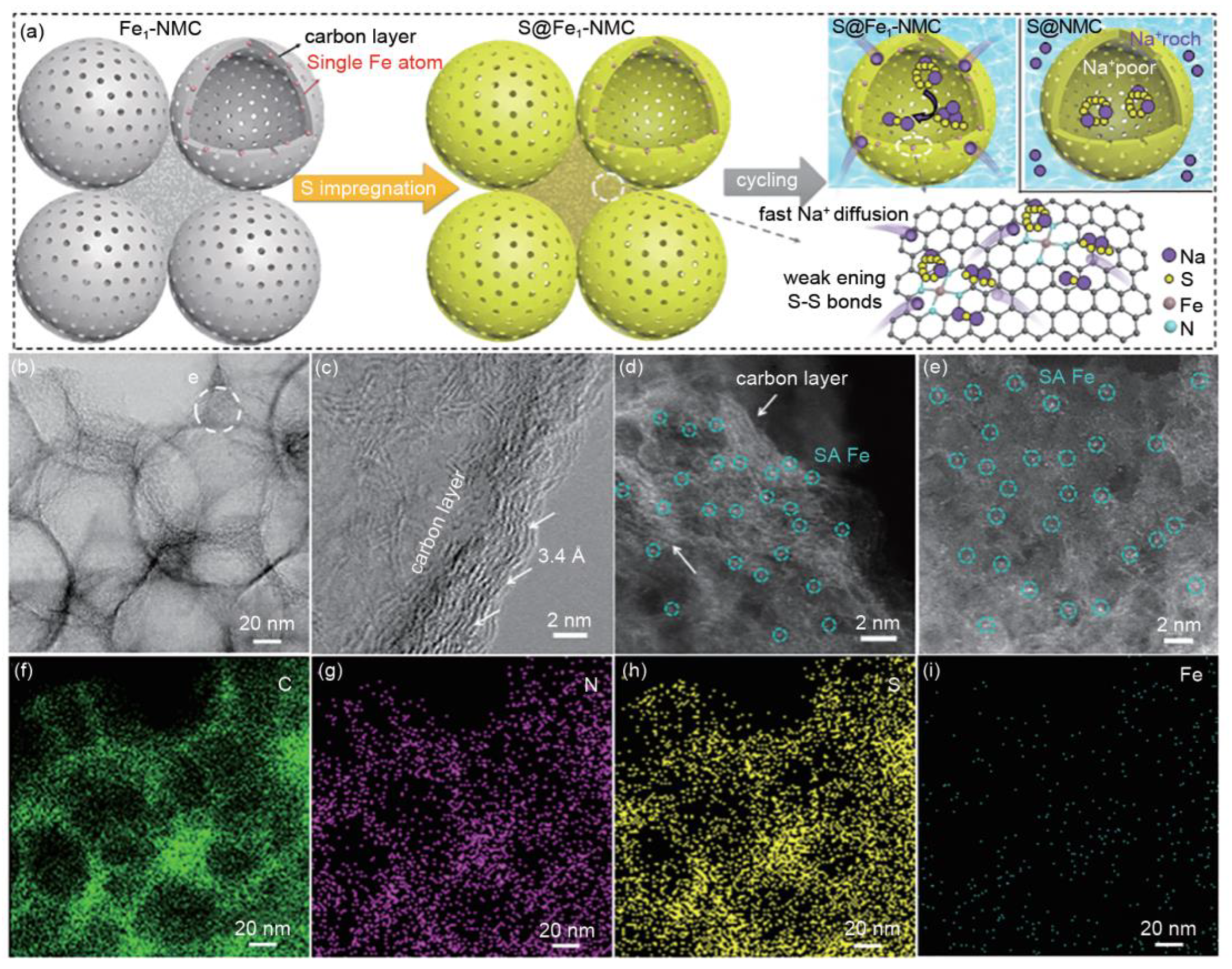
3.4.3. Catalyze Reversible Cycling of Na2S
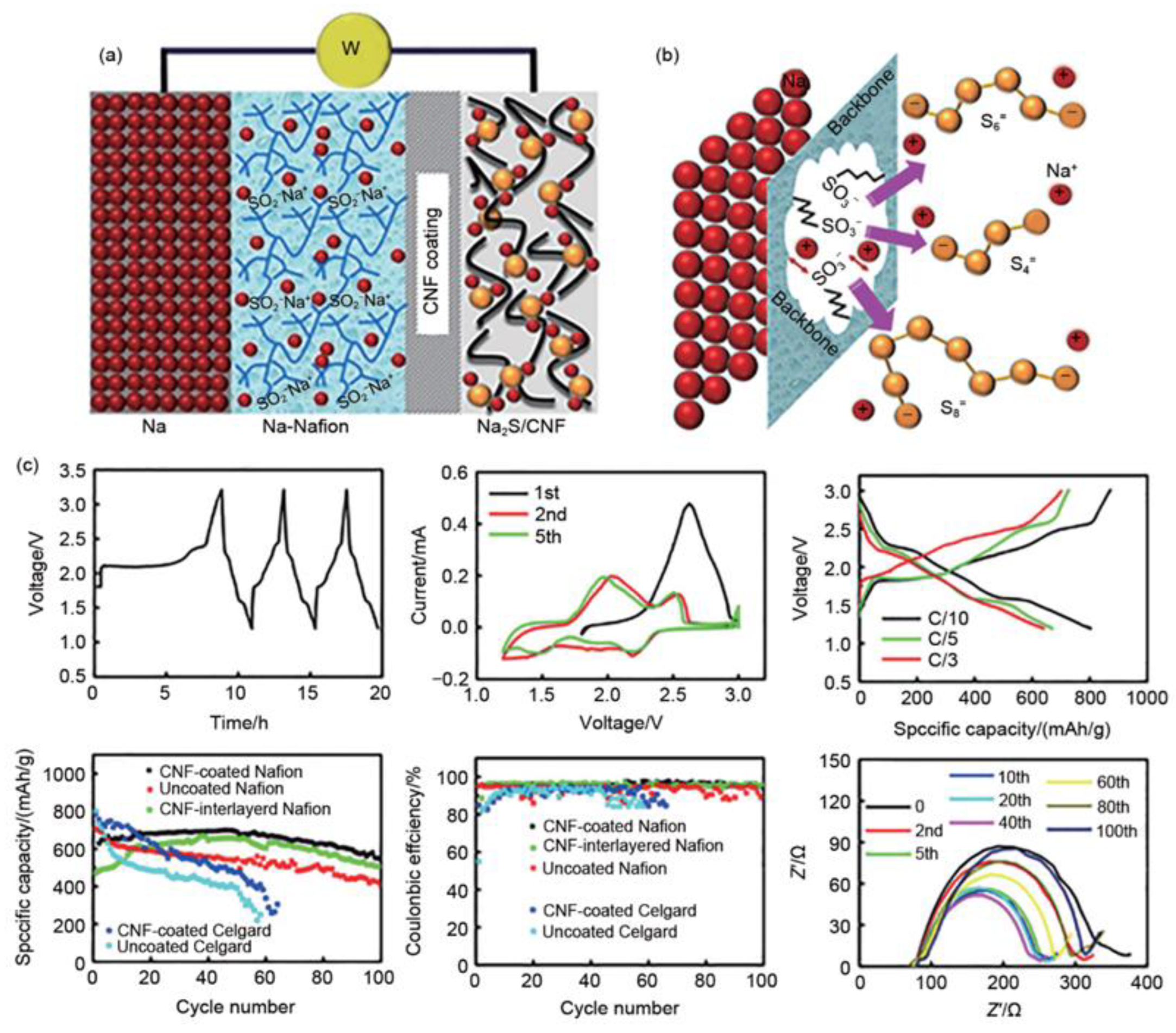
3.4.4. Battery Structure Design
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Chai, J.; Li, Y.; Li, Q.; Du, J.; Chen, Z.; Wang, L.; Tang, B. Strategies to mitigate the shuttle effect in room temperature sodium–sulfur batteries: Improving cathode materials. Dalton Trans. 2023, 52, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Balasankar, A.; Arthiya, S.E.; Ramasundaram, S.; Sumathi, P.; Arokiyaraj, S.; Oh, T.; Aruchamy, K.; Sriram, G.; Kurkuri, M.D. Recent Advances in the Preparation and Performance of Porous Titanium-Based Anode Materials for Sodium-Ion Batteries. Energies 2022, 15, 9495. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, T.; Ali, M.; Meng, Y.; Jin, Y.; Cui, Y.; Chen, W. Rechargeable Batteries for Grid Scale Energy Storage. Chem. Rev. 2022, 122, 16610–16751. [Google Scholar] [CrossRef]
- Du, P.; Cao, L.; Zhang, B.; Wang, C.; Xiao, Z.; Zhang, J.; Wang, D.; Ou, X. Recent progress on heterostructure materials for next-generation sodium/potassium ion batteries. Renew. Sustain. Energy Rev. 2021, 151, 111640. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, M.; Zhang, H.; Shang, Z.; Fu, L.; Zhang, W.; Song, B.; Lu, K. Toward the Advanced Next-Generation Solid-State Na-S Batteries: Progress and Prospects. Adv. Funct. Mater. 2023, 33, 2214430. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, A.; Forsyth, M.; MacFarlane, D.R.; Mitra, S. Free-Radical Catalysis and Enhancement of the Redox Kinetics for Room-Temperature Sodium–Sulfur Batteries. ACS Energy Lett. 2020, 5, 2112–2121. [Google Scholar] [CrossRef]
- Sul, H.; Bhargav, A.; Manthiram, A. Sodium trithiocarbonate cathode for high-performance sodium–sulfur batteries. J. Mater. Chem. A 2023, 11, 130–140. [Google Scholar] [CrossRef]
- Ye, X.; Ruan, J.; Pang, Y.; Yang, J.; Liu, Y.; Huang, Y.; Zheng, S. Enabling a Stable Room-Temperature Sodium–Sulfur Battery Cathode by Building Heterostructures in Multichannel Carbon Fibers. ACS Nano 2021, 15, 5639–5648. [Google Scholar] [CrossRef]
- Jhang, L.-J.; Wang, D.; Silver, A.; Li, X.; Reed, D.; Wang, D. Stable all-solid-state sodium-sulfur batteries for low-temperature operation enabled by sodium alloy anode and confined sulfur cathode. Nano Energy 2023, 105, 107995. [Google Scholar] [CrossRef]
- Bao, W.; Shuck, C.E.; Zhang, W.; Guo, X.; Gogotsi, Y.; Wang, G. Boosting Performance of Na–S Batteries Using Sulfur-Doped Ti3C2Tx MXene Nanosheets with a Strong Affinity to Sodium Polysulfides. ACS Nano 2019, 13, 11500–11509. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lv, W.; Chen, Y.; Wen, K.; Xu, C.; Zhang, W.; Li, Y.; Qin, W.; He, W. Tellurium-Impregnated Porous Cobalt-Doped Carbon Polyhedra as Superior Cathodes for Lithium–Tellurium Batteries. ACS Nano 2017, 11, 8144–8152. [Google Scholar] [CrossRef] [PubMed]
- Nagmani; Pahari, D.; Verma, P.; Puravankara, S. Are Na-ion batteries nearing the energy storage tipping point?—Current status of non-aqueous, aqueous, and solid-sate Na-ion battery technologies for sustainable energy storage. J. Energy Storage 2022, 56, 105961. [Google Scholar] [CrossRef]
- Zhang, B.-W.; Cao, L.; Tang, C.; Tan, C.; Cheng, N.; Lai, W.-H.; Wang, Y.-X.; Cheng, Z.-X.; Dong, J.; Kong, Y.; et al. Atomically Dispersed Dual-Site Cathode with a Record High Sulfur Mass Loading for High-Performance Room-Temperature Sodium–Sulfur Batteries. Adv. Mater. 2023, 35, 2206828. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Yang, J.; Lai, W.; Chou, S.-L.; Gu, Q.-F.; Liu, H.K.; Zhao, D.; Dou, S.X. Achieving High-Performance Room-Temperature Sodium–Sulfur Batteries with S@Interconnected Mesoporous Carbon Hollow Nanospheres. J. Am. Chem. Soc. 2016, 138, 16576–16579. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Sang, P.; Guo, W.; Fu, Y. Organosulfur polymer-based cathode materials for rechargeable batteries. Polym. Chem. 2022, 13, 5676–5690. [Google Scholar] [CrossRef]
- Tang, K.; Peng, X.; Chen, S.; Song, F.; Liu, Z.; Hu, J.; Xie, X.; Wu, Z. Hierarchically porous carbon derived from delignified biomass for high sulfur-loading room-temperature sodium-sulfur batteries. Renew. Energy 2022, 201, 832–840. [Google Scholar] [CrossRef]
- Singsen, S.; Suthirakun, S.; Hirunsit, P.; Balbuena, P.B. Surface Film Formation from Sodium Polysulfide Decomposition on Sodium-Metal Anode Surface. J. Phys. Chem. C 2022, 126, 16615–16626. [Google Scholar] [CrossRef]
- Zhao, Q.; Hu, X.; Zhang, K.; Zhang, N.; Hu, Y.; Chen, J. Sulfur Nanodots Electrodeposited on Ni Foam as High-Performance Cathode for Li–S Batteries. Nano Lett. 2015, 15, 721–726. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cheng, H.; Ni, Z.; Wang, Y.; Xia, G.; Li, X.; Zeng, X. Research Progress toward Room Temperature Sodium Sulfur Batteries: A Review. Molecules 2021, 26, 1535. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, S.; Yu, S.; Ren, J.; Zhang, Z.; Liu, S.; Liu, X.; Wang, Z.; Wu, Y.; Zhang, Y. Lychee seed-derived microporous carbon for high-performance sodium-sulfur batteries. Carbon 2023, 201, 864–870. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, D.; Palomares, V.; Shanmukaraj, D.; Sun, B.; Tang, X.; Wang, C.; Armand, M.; Rojo, T.; Wang, G. Revitalising sodium–sulfur batteries for non-high-temperature operation: A crucial review. Energy Environ. Sci. 2020, 13, 3848–3879. [Google Scholar] [CrossRef]
- Lee, B.; Paek, E.; Mitlin, D.; Lee, S.W. Sodium Metal Anodes: Emerging Solutions to Dendrite Growth. Chem. Rev. 2019, 119, 5416–5460. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. Phosphorus/Phosphide Anodes for Sodium–Ion Batteries on Alloy and Conversion Reactions. In Sodium-Ion Batteries; Wiley Online Library: Hoboken, NJ, USA, 2022; pp. 245–271. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Syali, M.S.; Kumar, D.; Mishra, K.; Kanchan, D.K. Recent advances in electrolytes for room-temperature sodium-sulfur batteries: A review. Energy Storage Mater. 2020, 31, 352–372. [Google Scholar] [CrossRef]
- Zhu, T.; Dong, X.; Liu, Y.; Wang, Y.-G.; Wang, C.; Xia, Y.-Y. An All-Solid-State Sodium–Sulfur Battery Using a Sulfur/Carbonized Polyacrylonitrile Composite Cathode. ACS Appl. Energy Mater. 2019, 2, 5263–5271. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X. Ambient Temperature Sodium–Sulfur Batteries. Small 2015, 11, 2108–2114. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Room-Temperature Sodium–Sulfur Batteries with Liquid-Phase Sodium Polysulfide Catholytes and Binder-Free Multiwall Carbon Nanotube Fabric Electrodes. J. Phys. Chem. C 2014, 118, 22952–22959. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Zeng, Z.; Cheng, S.; Xie, J. Selenium or Tellurium as Eutectic Accelerators for High-Performance Lithium/Sodium–Sulfur Batteries. Electrochem. Energy Rev. 2020, 3, 613–642. [Google Scholar] [CrossRef]
- Li, F.; Wei, Z.; Manthiram, A.; Feng, Y.; Ma, J.; Mai, L. Sodium-based batteries: From critical materials to battery systems. J. Mater. Chem. A 2019, 7, 9406–9431. [Google Scholar] [CrossRef]
- Chung, S.-H.; Manthiram, A. Current Status and Future Prospects of Metal–Sulfur Batteries. Adv. Mater. 2019, 31, 1901125. [Google Scholar] [CrossRef] [PubMed]
- Usiskin, R.; Lu, Y.; Popovic, J.; Law, M.; Balaya, P.; Hu, Y.-S.; Maier, J. Fundamentals, status and promise of sodium-based batteries. Nat. Rev. Mater. 2021, 6, 1020–1035. [Google Scholar] [CrossRef]
- Wu, J.; Tian, Y.; Gao, Y.; Gao, Z.; Meng, Y.; Wang, Y.; Wang, X.; Zhou, D.; Kang, F.; Li, B.; et al. Rational Electrolyte Design toward Cyclability Remedy for Room-Temperature Sodium–Sulfur Batteries. Angew. Chem. Int. Ed. 2022, 61, e202205416. [Google Scholar] [CrossRef]
- Hueso, K.B.; Palomares, V.; Armand, M.; Rojo, T. Challenges and perspectives on high and intermediate-temperature sodium batteries. Nano Res. 2017, 10, 4082–4114. [Google Scholar] [CrossRef]
- Wei, S.; Xu, S.; Agrawral, A.; Choudhury, S.; Lu, Y.; Tu, Z.; Ma, L.; Archer, L.A. A stable room-temperature sodium–sulfur battery. Nat. Commun. 2016, 7, 11722. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Abo-Khalil, A.G.; Maghrabie, H.M.; Elsaid, K.; Abdelkareem, M.A. Battery energy storage systems and SWOT (strengths, weakness, opportunities, and threats) analysis of batteries in power transmission. Energy 2022, 254, 123987. [Google Scholar] [CrossRef]
- Steudel, R.; Chivers, T. The role of polysulfide dianions and radical anions in the chemical, physical and biological sciences, including sulfur-based batteries. Chem. Soc. Rev. 2019, 48, 3279–3319. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Park, J.-Y.; Kim, C.H.; Park, J.-W.; Ahn, J.-P.; Ahn, J.-H.; Kim, K.-W.; Ahn, H.-J. A room temperature Na/S battery using a β″ alumina solid electrolyte separator, tetraethylene glycol dimethyl ether electrolyte, and a S/C composite cathode. J. Power Sources 2016, 301, 332–337. [Google Scholar] [CrossRef]
- Yang, H.; Wei, Z.; Chengzhi, L. Optimal design and techno-economic analysis of a hybrid solar–wind power generation system. Appl. Energy 2009, 86, 163–169. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, H.; Fang, Z. Battery behavior prediction and battery working states analysis of a hybrid solar–wind power generation system. Renew. Energy 2008, 33, 1413–1423. [Google Scholar] [CrossRef]
- Vudata, S.P.; Bhattacharyya, D. Thermal management of a high temperature sodium sulphur battery stack. Int. J. Heat Mass Transf. 2021, 181, 122025. [Google Scholar] [CrossRef]
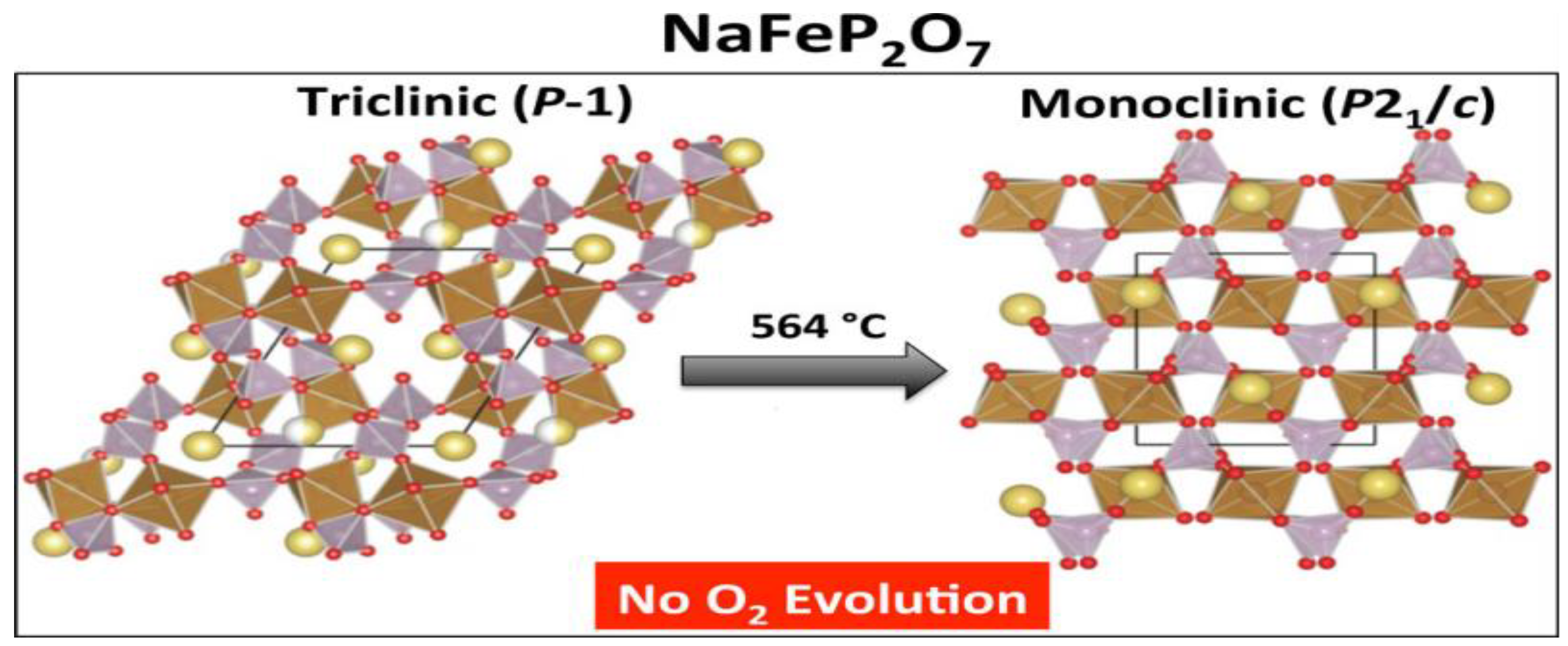
- Barpanda, P.; Liu, G.; Ling, C.D.; Tamaru, M.; Avdeev, M.; Chung, S.-C.; Yamada, Y.; Yamada, A. Na2FeP2O7: A Safe Cathode for Rechargeable Sodium-ion Batteries. Chem. Mater. 2013, 25, 3480–3487. [Google Scholar] [CrossRef]
- Chen, P.; Wang, C.; Wang, T. Review and prospects for room-temperature sodium-sulfur batteries. Mater. Res. Lett. 2022, 10, 691–719. [Google Scholar] [CrossRef]
- Wang, L.; Wang, T.; Peng, L.; Wang, Y.; Zhang, M.; Zhou, J.; Chen, M.; Cao, J.; Fei, H.; Duan, X.; et al. The promises, challenges and pathways to room-temperature sodium-sulfur batteries. Natl. Sci. Rev. 2021, 9, nwab050. [Google Scholar] [CrossRef]
- Ceylan Cengiz, E.; Erdol, Z.; Sakar, B.; Aslan, A.; Ata, A.; Ozturk, O.; Demir-Cakan, R. Investigation of the Effect of Using Al2O3–Nafion Barrier on Room-Temperature Na–S Batteries. J. Phys. Chem. C 2017, 121, 15120–15126. [Google Scholar] [CrossRef]
- Divya, K.C.; Østergaard, J. Battery energy storage technology for power systems—An overview. Electr. Power Syst. Res. 2009, 79, 511–520. [Google Scholar] [CrossRef]
- Wu, C.; Lai, W.-H.; Cai, X.; Chou, S.-L.; Liu, H.-K.; Wang, Y.-X.; Dou, S.-X. Carbonaceous Hosts for Sulfur Cathode in Alkali-Metal/S (Alkali Metal = Lithium, Sodium, Potassium) Batteries. Small 2021, 17, 2006504. [Google Scholar] [CrossRef]
- Vijaya Kumar Saroja, A.P.; Sundara, R. Core–Shell Cathode Design with Molybdenum Trioxide as the Electrocatalytic Trapping Layer for High-Energy Density Room-Temperature Sodium Sulfur Batteries. J. Phys. Chem. C 2020, 124, 7615–7623. [Google Scholar] [CrossRef]
- Scholwin, F.; Held, J. Biomethanebiomethanefrom Anaerobic Processes. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012; pp. 1618–1627. [Google Scholar] [CrossRef]
- Tang, G.; Lin, J.; Yang, J.; Fang, Y.; Liu, H.; Yin, W. R&Dprogress of sodium batteries with high, intermedia and low temperature. Dongfang Electr. Rev. 2017, 31, 12–16. [Google Scholar] [CrossRef]
- Li, M.M.; Lu, X.; Zhan, X.; Engelhard, M.H.; Bonnett, J.F.; Polikarpov, E.; Jung, K.; Reed, D.M.; Sprenkle, V.L.; Li, G. High performance sodium-sulfur batteries at low temperature enabled by superior molten Na wettability. Chem. Commun. 2021, 57, 45–48. [Google Scholar] [CrossRef]
- Medenbach, L.; Hartmann, P.; Janek, J.; Stettner, T.; Balducci, A.; Dirksen, C.; Schulz, M.; Stelter, M.; Adelhelm, P. A Sodium Polysulfide Battery with Liquid/Solid Electrolyte: Improving Sulfur Utilization Using P2S5 as Additive and Tetramethylurea as Catholyte Solvent. Energy Technol. 2020, 8, 1901200. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, Y.; Li, B.; Fan, H.; Cheng, F.; Shanmukaraj, D.; Rojo, T.; Armand, M.; Wang, G. A Stable Quasi-Solid-State Sodium–Sulfur Battery. Angew. Chem. Int. Ed. 2018, 57, 10168–10172. [Google Scholar] [CrossRef] [PubMed]
- Chu, I.-H.; Kompella, C.S.; Nguyen, H.; Zhu, Z.; Hy, S.; Deng, Z.; Meng, Y.S.; Ong, S.P. Room-Temperature All-solid-state Rechargeable Sodium-ion Batteries with a Cl-doped Na3PS4 Superionic Conductor. Sci. Rep. 2016, 6, 33733. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Inazawa, S.J.; Sakai, S.-I.; Fukunaga, A.; Itani, E.; Numata, K.; Hagiwara, R.; Nohira, T. Development of Molten Salt Electrolyte Battery. SEI Tech. Rev. 2013, 76, 33–39. [Google Scholar]
- Cao, C.; Wang, H.; Liu, W.; Liao, X.; Li, L. Nafion membranes as electrolyte and separator for sodium-ion battery. Int. J. Hydrog. Energy 2014, 39, 16110–16115. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, H.; Zhou, Q.; Qu, H.; Dong, T.; Zhang, M.; Tang, B.; Zhang, J.; Cui, G. Safety-Enhanced Polymer Electrolytes for Sodium Batteries: Recent Progress and Perspectives. ACS Appl. Mater. Interfaces 2019, 11, 17109–17127. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, Y.; Ding, Y.; Dong, X.; Chen, L.; Cao, J.; Wang, C.; Xia, Y.; Peng, H.; Wang, Y. Multi-functional Flexible Aqueous Sodium-Ion Batteries with High Safety. Chem 2017, 3, 348–362. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, Z.; Sun, S. The recent progress and future opportunities of Na2S cathode for room temperature sodium sulfur batteries. Energy Storage Sci. Technol. 2022, 11, 2811–2824. [Google Scholar] [CrossRef]
- Li, S.; Zeng, Z.; Yang, J.; Han, Z.; Hu, W.; Wang, L.; Ma, J.; Shan, B.; Xie, J. High Performance Room Temperature Sodium–Sulfur Battery by Eutectic Acceleration in Tellurium-Doped Sulfurized Polyacrylonitrile. ACS Appl. Energy Mater. 2019, 2, 2956–2964. [Google Scholar] [CrossRef]
- Ma, L.; Lv, Y.; Wu, J.; Chen, Y.; Jin, Z. Recent Advances in Emerging Non-Lithium Metal–Sulfur Batteries: A Review. Adv. Energy Mater. 2021, 11, 2100770. [Google Scholar] [CrossRef]
- Yang, T.; Niu, Y.; Liu, Q.; Xu, M. Cathode host engineering for non-lithium (Na, K and Mg) sulfur/selenium batteries: A state-of-the-art review. Nano Mater. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Ghosh, A.; Kumar, A.; Roy, A.; Panda, M.R.; Kar, M.; MacFarlane, D.R.; Mitra, S. Three-Dimensionally Reinforced Freestanding Cathode for High-Energy Room-Temperature Sodium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 14101–14109. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, X.; Wang, X.; Liu, S. Progress in electrolyte materials for sodium-sulfur secondary batteries at room tem-perature. Adv. Mater. Ind. 2018, 2, 60–66. [Google Scholar] [CrossRef]
- Wang, R.; Wang, D.; Dong, Y.; Xie, B.; Wu, X.; Wu, Q.; Zhu, S.; Diao, G.; Chen, M. Recent progress of advanced carbon-based cathode in sodium-selenium batteries. J. Alloy. Compd. 2023, 952, 169980. [Google Scholar] [CrossRef]
- Hao, H.; Hutter, T.; Boyce, B.L.; Watt, J.; Liu, P.; Mitlin, D. Review of Multifunctional Separators: Stabilizing the Cathode and the Anode for Alkali (Li, Na, and K) Metal–Sulfur and Selenium Batteries. Chem. Rev. 2022, 122, 8053–8125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Rajouria, S.K.; Kuhar, S.B.; Kanchan, D.K. Progress and prospects of sodium-sulfur batteries: A review. Solid State Ion. 2017, 312, 8–16. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Hu, X.; Matios, E.; Luo, J.; Zhang, Y.; Lu, X.; Li, W. Frogspawn-Coral-Like Hollow Sodium Sulfide Nanostructured Cathode for High-Rate Performance Sodium–Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1803251. [Google Scholar] [CrossRef]
- Zhang, B.-W.; Li, S.; Yang, H.-L.; Liang, X.; Lai, W.-H.; Zhao, S.; Dong, J.; Chu, S.-Q.; Gu, Q.-F.; Liang, J.; et al. Atomically dispersed S-Fe-N4 for fast kinetics sodium-sulfur batteries via a dual function mechanism. Cell Rep. Phys. Sci. 2021, 2, 100531. [Google Scholar] [CrossRef]
- Tang, W.; Aslam, M.K.; Xu, M. Towards high performance room temperature sodium-sulfur batteries: Strategies to avoid shuttle effect. J. Colloid Interface Sci. 2022, 606, 22–37. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Performance Enhancement and Mechanistic Studies of Room-Temperature Sodium–Sulfur Batteries with a Carbon-Coated Functional Nafion Separator and a Na2S/Activated Carbon Nanofiber Cathode. Chem. Mater. 2016, 28, 896–905. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, L.; Wang, Y.; Zhu, Z.; Chou, S.-L. The Future for Room-Temperature Sodium–Sulfur Batteries: From Persisting Issues to Promising Solutions and Practical Applications. Adv. Funct. Mater. 2022, 32, 2205622. [Google Scholar] [CrossRef]
- Huang, X.L.; Dou, S.X.; Wang, Z.M. Metal-based electrocatalysts for room-temperature Na–S batteries. Mater. Horiz. 2021, 8, 2870–2885. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, X.L.; Liu, H.; Qiu, W.; Feng, C.; Li, C.; Zhang, S.; Liu, H.K.; Dou, S.X.; Wang, Z.M. Nanostructure Engineering Strategies of Cathode Materials for Room-Temperature Na–S Batteries. ACS Nano 2022, 16, 5103–5130. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yue, J.; Han, F.; Chen, J.; Deng, T.; Zhou, X.; Hou, S.; Wang, C. High-Performance All-Solid-State Na–S Battery Enabled by Casting–Annealing Technology. ACS Nano 2018, 12, 3360–3368. [Google Scholar] [CrossRef]
- Ma, P.; Fang, D.; Liu, Y.; Shang, Y.; Shi, Y.; Yang, H.Y. MXene-Based Materials for Electrochemical Sodium-Ion Storage. Adv. Sci. 2021, 8, 2003185. [Google Scholar] [CrossRef]
- Huang, X.L.; Wang, Y.-X.; Chou, S.-L.; Dou, S.X.; Wang, Z.M. Materials engineering for adsorption and catalysis in room-temperature Na–S batteries. Energy Environ. Sci. 2021, 14, 3757–3795. [Google Scholar] [CrossRef]

| Electrochemical Performance of Na-S Batteries | High Temperature | Room Temperature |
|---|---|---|
| Energy density | 200 Wh/kg or higher | 150~240 Wh/kg |
| Cyclic lifespan | 1000~3000 times | 2500~4500 times |
| Power density | 2000 hundred watts per kilogram or above | |
| Li Ion Batteries | Li-S Batteries | Na-S Batteries | |
|---|---|---|---|
| Energy density | 100–265 Wh/kg | 200–300 Wh/kg | 90–150 Wh/kg |
| Cyclic lifespan | Thousands of times more | 100–1000 | Thousands of times more |
| Power density | 1000–3000 W/kg | Below 1000 W/kg | 100–500 W/kg |
| Cost | High | Very high | Low |
| Environmental protection | Both have minimal environmental Impact, but lithium resources are limited. | It has minimal impact on the environment and abundant resources. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Zhang, B.; Shi, Y.; Jiang, R.; Zhang, H. Research on Wide-Temperature Rechargeable Sodium-Sulfur Batteries: Features, Challenges and Solutions. Materials 2023, 16, 4263. https://doi.org/10.3390/ma16124263
Liang Y, Zhang B, Shi Y, Jiang R, Zhang H. Research on Wide-Temperature Rechargeable Sodium-Sulfur Batteries: Features, Challenges and Solutions. Materials. 2023; 16(12):4263. https://doi.org/10.3390/ma16124263
Chicago/Turabian StyleLiang, Yimin, Boxuan Zhang, Yiran Shi, Ruyi Jiang, and Honghua Zhang. 2023. "Research on Wide-Temperature Rechargeable Sodium-Sulfur Batteries: Features, Challenges and Solutions" Materials 16, no. 12: 4263. https://doi.org/10.3390/ma16124263
APA StyleLiang, Y., Zhang, B., Shi, Y., Jiang, R., & Zhang, H. (2023). Research on Wide-Temperature Rechargeable Sodium-Sulfur Batteries: Features, Challenges and Solutions. Materials, 16(12), 4263. https://doi.org/10.3390/ma16124263





