Application and Development of Silicon Anode Binders for Lithium-Ion Batteries
Abstract
:1. Background
2. Positive and Negative Characteristics of a Si-Based Anode
3. Suggestions for Enhancing Si Anode Performance
4. Si-Based Anode Polymer Binders
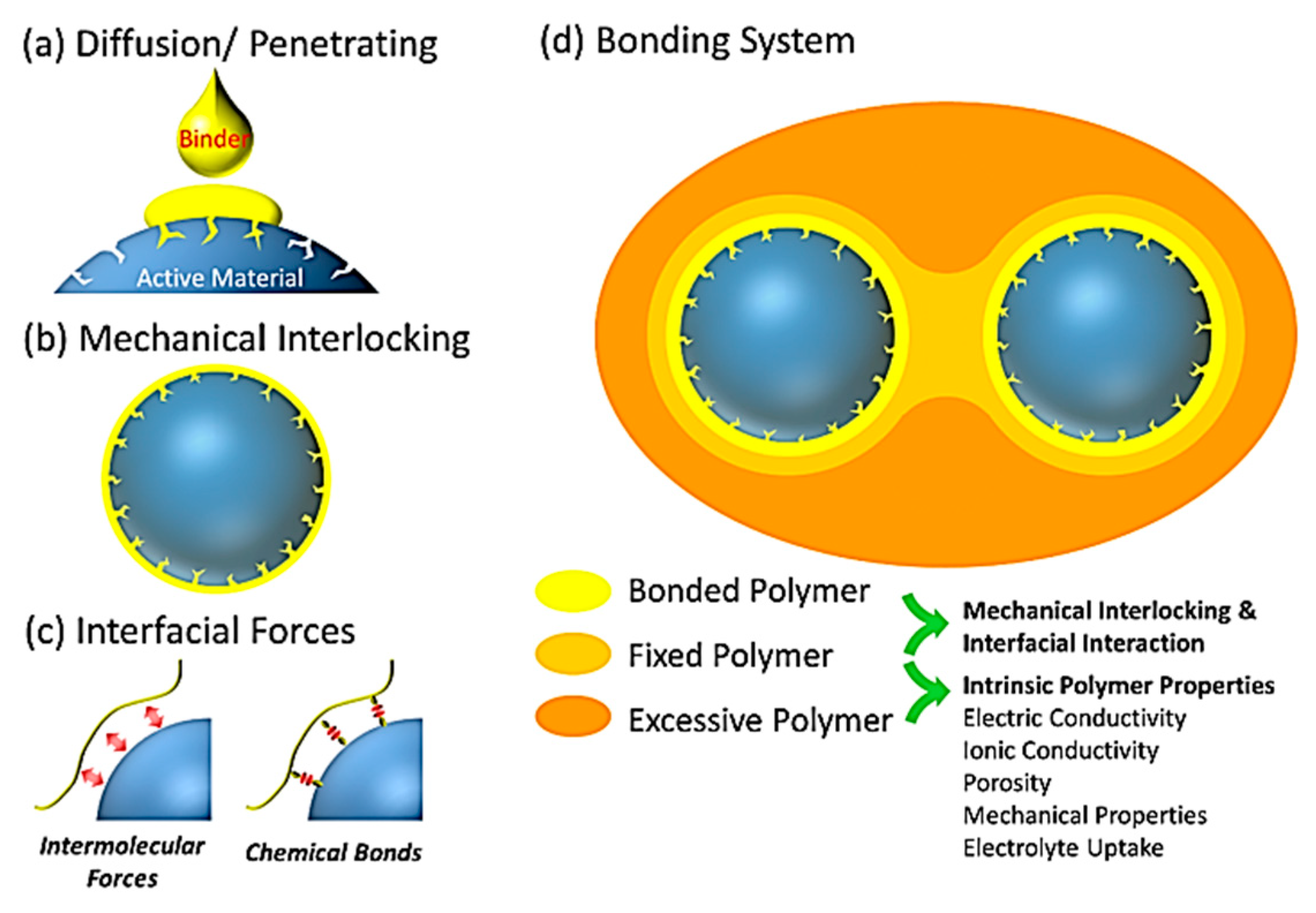
4.1. Bonding Mechanism of the Binders
4.2. Physical Interaction between Binder and Si Anode Surface
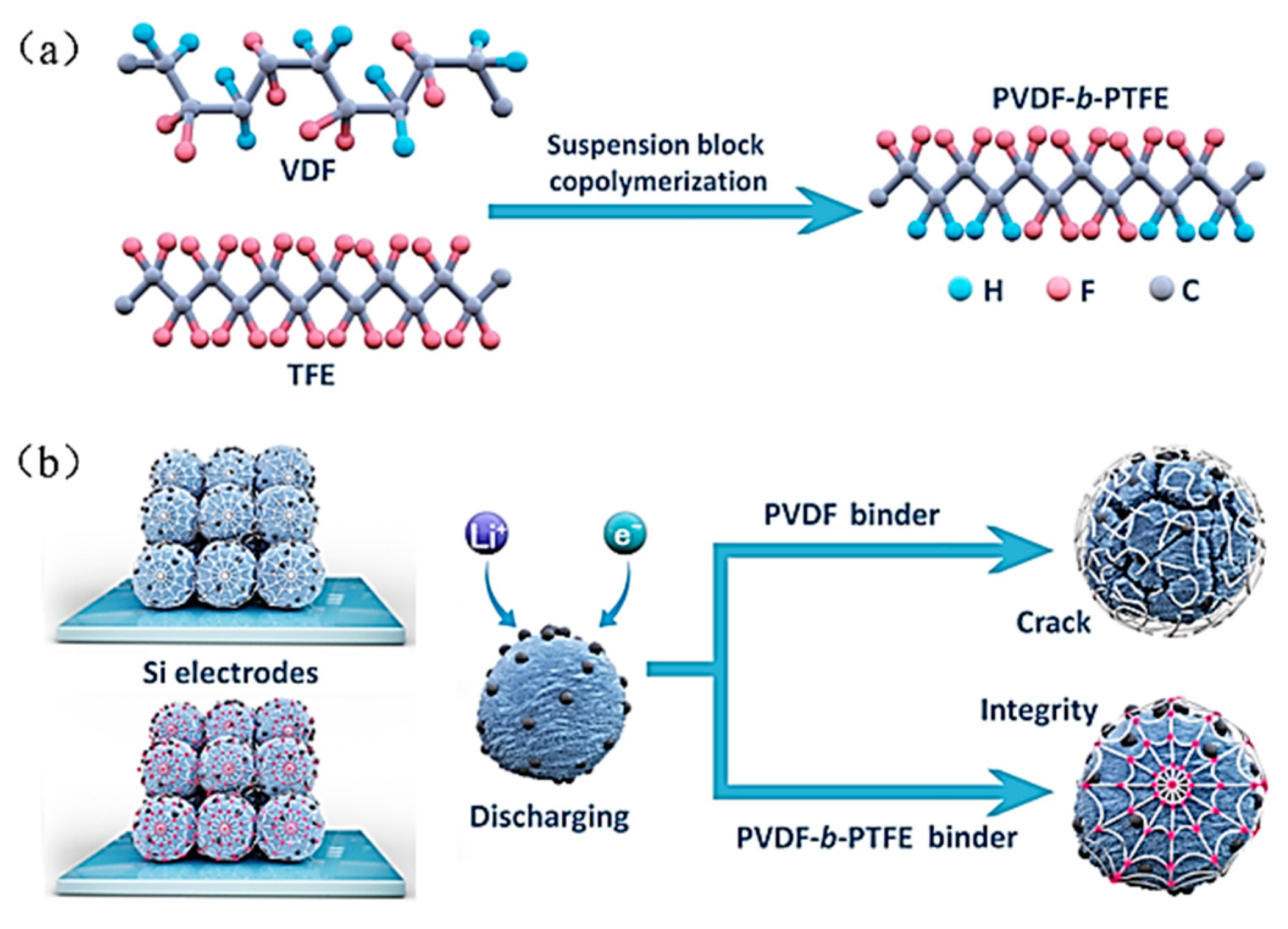
4.3. Chemical Bonding of Binder to Si Anode Surface
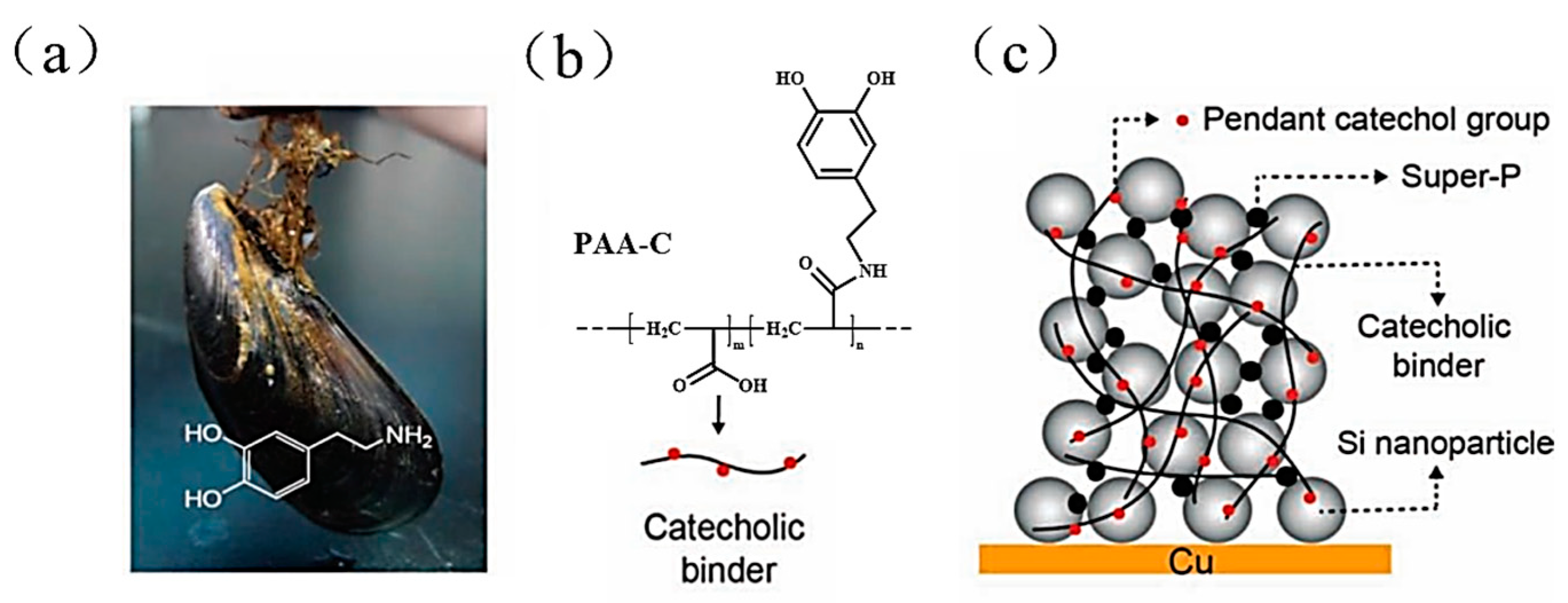
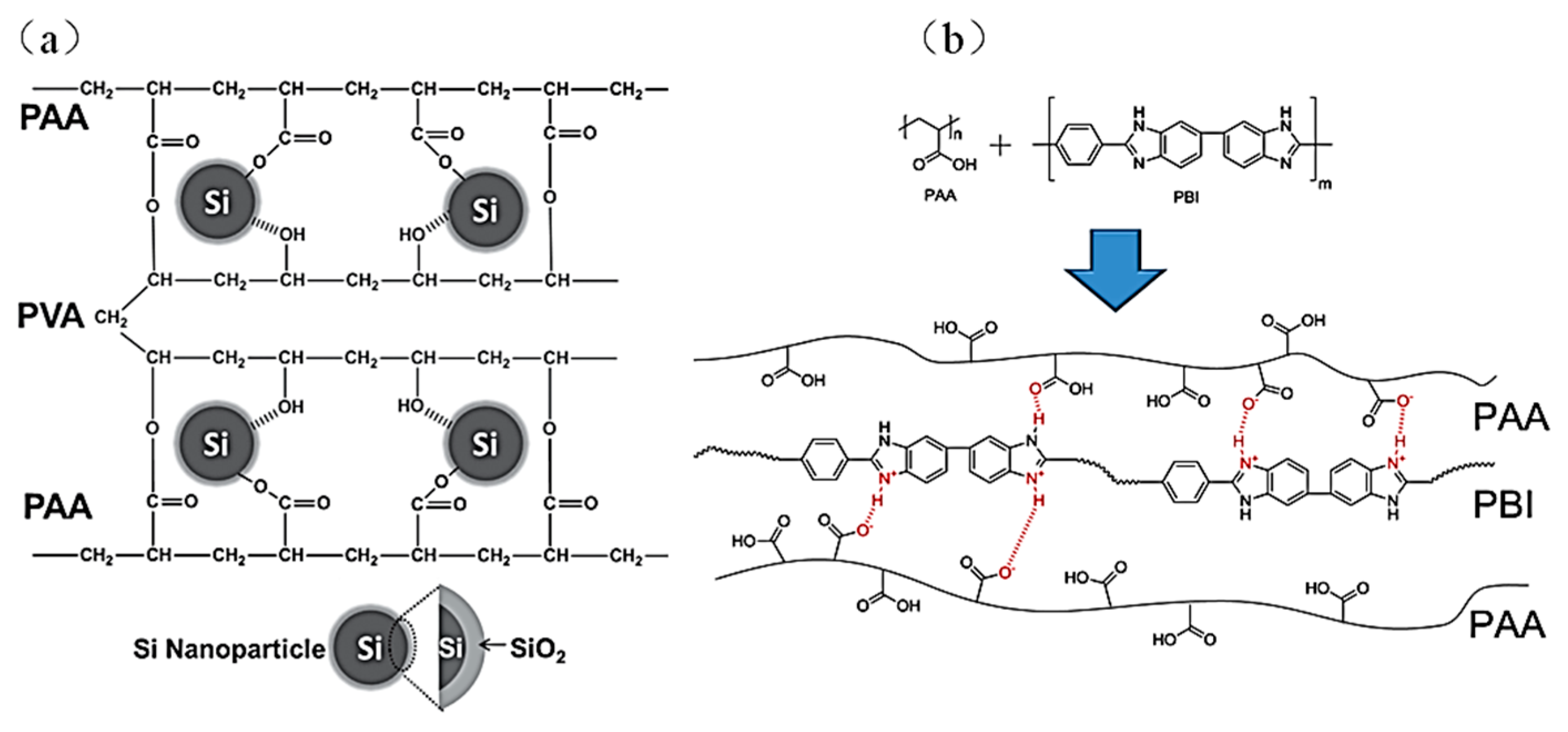
4.3.1. Polyacrylic Acid (PAA) Binder
4.3.2. Sodium Carboxymethyl Cellulose (CMC) Binder
4.3.3. Natural Polymer Binder
- I.
- Alginate (Alg) binder
- II.
- Chitosan (CS) binder
- III.
- β-cyclodextrin polymer (β-CDp)-based binders
- IV.
- Guar Gum (GG) binder
- V.
- Other natural binders
4.3.4. Functional Binder
- I.
- Self-healing binder
- II.
- Conductive binder
4.4. Summary
5. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Cai, Y.; Wu, H.; Yu, Z.; Yan, X.; Zhang, Q.; Gao, T.Z.; Liu, K.; Jia, X.; Bao, Z. Polymers in lithium-ion and lithium metal batteries. Adv. Energy Mater. 2021, 11, 2003239. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of lithium-ion batteries. Adv. Mater. 2018, 30, e1800561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Yang, S.; Luo, L.; Pan, Q.; Zhang, P.; Huang, Y.; Zheng, F.; Wang, H.; Li, Q. From spent graphite to recycle graphite anode for high-performance lithium ion batteries and sodium ion batteries. Electrochim. Acta 2020, 356, 136856. [Google Scholar] [CrossRef]
- Wang, C.S.; Wu, G.T.; Li, W.Z. Lithium insertion in ball-milled graphite. Power Sources 1998, 76, 1–10. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.E.; Su, L.; Huang, S.S.; Fang, C.C.; Wu, Y.S.; Chou, J.; Wu, N.L. A proof-of-concept graphite anode with a lithium dendrite suppressing polymer coating. J. Power Sources 2018, 406, 63–69. [Google Scholar] [CrossRef]
- Kwon, T.W.; Choi, J.W.; Coskun, A. The emerging era of supramolecular polymeric binders in silicon anodes. Chem. Soc. Rev. 2018, 47, 2145–2164. [Google Scholar] [CrossRef] [Green Version]
- Baris, K.; Mathieu, M.; Jean-Marie, T.; Grey, C.P. Pair distribution function analysis and solid state NMR studies of silicon electrodes for lithium Ion batteries: Understanding the (de)lithiation mechanisms. J. Am. Chem. Soc. 2010, 133, 503–512. [Google Scholar]
- Zhang, C.; Wang, F.; Han, J.; Bai, S.; Tan, J.; Liu, J.; Li, F. Challenges and Recent Progress on Silicon-Based Anode Materials for Next-Generation Lithium-Ion Batteries. Small Struct. 2021, 2, 2100009. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.J.; Wu, S.X.; Zhang, Y.P.; Liu, C.B.; Wei, X.G.; Luo, D.; Lin, Z. Towards efficient binders for silicon based lithium-ion battery anodes. Chem. Eng. J. 2021, 406, 126807. [Google Scholar] [CrossRef]
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G. Lithium batteries and the solid electrolyte interphase (SEI)—Progress and outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Kumar, P.; Berhaut, C.L.; Zapata Dominguez, D.; De Vito, E.; Tardif, S.; Pouget, S.; Lyonnard, S.; Jouneau, P.H. Nano-Architectured Composite Anode Enabling Long-Term Cycling Stability for High-Capacity Lithium-Ion Batteries. Nano Micro. Small 2020, 16, e1906812. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, J.; Zhang, T.; Nuli, Y.; Wang, J.; Hirano, S.-I. Silicon Microparticle Anodes with Self-Healing Multiple Network Binder. Joule 2018, 2, 950–961. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.M.; Yue, F.S.; Li, S.C.; Zhang, Y.; Tian, Z.R.; Xu, Q.; Xin, S.; Guo, Y.G. Advances of polymer binders for silicon-based anodes in high energy density lithium-ion batteries. InfoMat 2021, 3, 460–501. [Google Scholar] [CrossRef]
- Choi, N.S.; Yew, K.H.; Lee, K.Y.; Sung, M.; Kim, H.; Kim, S.S. Effect of fluoroethylene carbonate additive on interfacial properties of silicon thin-film electrode. J. Power Sources 2006, 161, 1254–1259. [Google Scholar] [CrossRef]
- Chen, L.; Wang, K.; Xie, X.; Xie, J. Effect of vinylene carbonate (VC) as electrolyte additive on electrochemical performance of Si film anode for lithium ion batteries. J. Power Sources 2007, 174, 538–543. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Mei, D.; Feng, J.; Hu, M.Y.; Hu, J.; Engelhard, M.; Zheng, J.; Xu, W.; Xiao, J.; et al. Reduction mechanism of fluoroethylene carbonate for stable solid-electrolyte interphase film on silicon anode. ChemSusChem 2014, 7, 549–554. [Google Scholar] [CrossRef]
- Xu, C.; Lindgren, F.; Philippe, B.; Gorgoi, M.; Björefors, F.; Edström, K.; Gustafsson, T. Improved performance of the silicon anode for Li-Ion batteries: Understanding the surface modification mechanism of fluoroethylene carbonate as an effective electrolyte additive. Chem. Mater. 2015, 27, 2591–2599. [Google Scholar] [CrossRef]
- Li, F.; Xu, J.; Hou, Z.; Li, M.; Yang, R. Silicon Anodes for High-Performance Storage Devices: Structural Design, Material Compounding, Advances in Electrolytes and Binders. ChemNanoMat 2020, 6, 720–738. [Google Scholar] [CrossRef]
- Vorauer, T.; Kumar, P.; Berhaut, C.L.; Chamasemani, F.F.; Jouneau, P.H.; Aradilla, D.; Tardif, S.; Pouget, S.; Fuchsbichler, B.; Helfen, L.; et al. Multi-scale quantification and modeling of aged nanostructured silicon-based composite anodes. Commun. Chem. 2020, 3, 141. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Shao, R.; Wu, J.; Jiang, R.; Jin, Z. Recent progress and future perspective on practical silicon anode-based lithium ion batteries. Energy Storage Mater. 2022, 46, 482–502. [Google Scholar] [CrossRef]
- Son, I.H.; Hwan Park, J.; Kwon, S.; Park, S.; Rummeli, M.H.; Bachmatiuk, A.; Song, H.J.; Ku, J.; Choi, J.W.; Choi, J.M.; et al. Silicon carbide-free graphene growth on silicon for lithium-ion battery with high volumetric energy density. Nat. Commun. 2015, 6, 7393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Sun, M.; Yuan, Y.; Wu, Q.; Wang, H.; He, Y.; Lin, Z.; Zhou, F.; Ling, M.; Qian, C.; et al. Accommodation of silicon in an interconnected copper network for robust Li-ion storage. Adv. Funct. Mater. 2020, 30, 1910249. [Google Scholar] [CrossRef]
- Lai, Y.; Li, H.; Yang, Q.; Li, H.; Liu, Y.; Song, Y.; Zhong, Y.; Zhong, B.; Wu, Z.; Guo, X. Revisit the progress of binders for a silicon-based anode from the perspective of designed binder structure and special sized silicon nanoparticles. Ind. Eng. Chem. Res. 2022, 61, 6246–6268. [Google Scholar] [CrossRef]
- Cui, L.F.; Ruffo, R.; Chan, C.K.; Peng, H.; Cui, Y. Crystalline-amorphous core-shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett. 2009, 9, 491–495. [Google Scholar] [CrossRef]
- Choi, J.W.; McDonough, J.; Jeong, S.; Yoo, J.S.; Chan, C.K.; Cui, Y. Stepwise nanopore evolution in one-dimensional nanostructures. Nano Lett. 2010, 10, 1409–1413. [Google Scholar] [CrossRef]
- Song, T.; Xia, J.; Lee, J.H.; Lee, D.H.; Kwon, M.S.; Choi, J.M.; Wu, J.; Doo, S.K.; Chang, H.; Park, W.I.; et al. Arrays of sealed silicon nanotubes as anodes for lithium ion batteries. Nano Lett. 2010, 10, 1710–1716. [Google Scholar] [CrossRef]
- Yan, L.; Liu, J.; Wang, Q.; Sun, M.; Jiang, Z.; Liang, C.; Pan, F.; Lin, Z. In situ wrapping Si nanoparticles with 2D carbon nanosheets as high-areal-capacity anode for lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 38159–38164. [Google Scholar] [CrossRef]
- Xu, Q.; Li, J.Y.; Sun, J.K.; Yin, Y.X.; Wan, L.J.; Guo, Y.G. Watermelon-inspired Si/C microspheres with hierarchical buffer structures for densely compacted lithium-Ion battery anodes. Adv. Energy Mater. 2017, 7, 1601481. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, J.; Zhang, F.; Li, T.; Yang, Y.; Yin, J.; Tian, Z.; Li, W.; Lai, Y.; Yang, L. Sustainable silicon micro-dendritic anodes integrated by a moderately cross-linked polymer binder with superior elasticity and adhesion. J. Alloy. Compd. 2022, 926, 166858. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Tu, J.; Chen, F.; Xie, J.; Zhu, T.; Zhao, X. Cross-linked binder enables reversible volume changes of Si-based anodes from sustainable photovoltaic waste silicon. Mater. Today Sustain. 2022, 19, 100178. [Google Scholar] [CrossRef]
- Di, S.; Zhang, D.; Weng, Z.; Chen, L.; Zhang, Y.; Zhang, N.; Ma, R.; Chen, G.; Liu, X. Crosslinked polymer binder via phthalic acid for stabilizing SiOx anodes. Macromol. Chem. Phys. 2022, 223, 2200068. [Google Scholar] [CrossRef]
- Liu, J.; Ma, R.; Zheng, W.; Wang, M.; Sun, T.; Zhu, J.; Tang, Y.; Wang, J. Cross-linking network of soft-rigid dual chains to effectively suppress volume change of silicon anode. J. Phys. Chem. Lett. 2022, 13, 7712–7721. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, W.; Wang, Y.; Qu, Q.; Jin, C.; Zheng, H. Overcoming the fundamental challenge of PVDF binder use with silicon anodes with a super-molecular nano-layer. J. Mater. Chem. A 2021, 9, 1541–1551. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, T.; Wang, L.; Zhao, Y.; Li, Z.; Zhang, M.; Wang, K.; Xue, S.; Fang, J.; Ji, Y.; et al. Bio-inspired binder design for a robust conductive network in silicon-based anodes. Small Methods 2022, 6, e2101591. [Google Scholar] [CrossRef]
- Tong, Y.; Jin, S.; Xu, H.; Li, J.; Kong, Z.; Jin, H.; Xu, H. An energy dissipative binder for self-tuning silicon anodes in lithium-ion batteries. Adv. Sci. 2023, 10, e2205443. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Chen, C.; Huang, T.; Yu, A. Natural sesbania gum as an efficient biopolymer binder for high-performance Si-based anodes in lithium-ion batteries. J. Power Sources 2022, 539, 231604. [Google Scholar] [CrossRef]
- Malik, Y.T.; Shin, S.Y.; Jang, J.I.; Kim, H.M.; Cho, S.; Do, Y.R.; Jeon, J.W. Self-repairable silicon anodes using a multifunctional binder for high-performance lithium-ion batteries. Nano Micro. Small 2022, 19, e2206141. [Google Scholar] [CrossRef]
- Zheng, F.; Tang, Z.; Lei, Y.; Zhong, R.; Chen, H.; Hong, R. PAAS-β-CDp-PAA as a high-performance easily prepared and water-soluble composite binder for high-capacity silicon anodes in lithium-ion batteries. J. Alloys Compd. 2023, 932, 167666. [Google Scholar] [CrossRef]
- Wu, S.; Huang, L.; Tian, H.; Geng, Y.; Wang, F. LiCl-Promoted Chain Growth Kumada Catalyst-Transfer Polycondensation of the “Reversed” Thiophene Monomer. Macromolecules 2011, 44, 7558–7567. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Figgemeier, E. Confronting the Challenges of Next-Generation Silicon Anode-Based Lithium-Ion Batteries: Role of Designer Electrolyte Additives and Polymeric Binders. Chem. Eur. 2019, 12, 2515–2539. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Mackanic, D.G.; Cui, Y.; Bao, Z. Designing polymers for advanced battery chemistries. Nat. Rev. Mater. 2019, 4, 312–330. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shao, R.; Jiang, R.; Song, X.; Jin, Z.; Sun, L. A review of existing and emerging binders for silicon anodic Li-ion batteries. Nano Res. 2023, 16, 6736–6752. [Google Scholar] [CrossRef]
- Li, J.T.; Wu, Z.Y.; Lu, Y.Q.; Zhou, Y.; Huang, Q.S.; Huang, L.; Sun, S.G. Water soluble binder, an electrochemical performance booster for electrode materials with high energy density. Adv. Energy Mater. 2017, 7, 1701185. [Google Scholar] [CrossRef]
- Li, Z.; Wu, G.; Yang, Y.; Wan, Z.; Zeng, X.; Yan, L.; Wu, S.; Ling, M.; Liang, C.; Hui, K.N.; et al. An ion-conductive grafted polymeric binder with practical loading for silicon anode with high interfacial stability in lithium-ion batteries. Adv. Energy Mater. 2022, 12, 2201197. [Google Scholar] [CrossRef]
- Deng, L.; Zheng, Y.; Zheng, X.; Or, T.; Ma, Q.; Qian, L.; Deng, Y.; Yu, A.; Li, J.; Chen, Z. Design criteria for silicon-based anode binders in half and full cells. Adv. Energy Mater. 2022, 12, 2200850. [Google Scholar] [CrossRef]
- Miranda, A.; Sarang, K.; Gendensuren, B.; Oh, E.S.; Lutkenhaus, J.; Verduzco, R. Molecular design principles for polymeric binders in silicon anodes. Mol. Syst. Des. Eng. 2020, 5, 709–724. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring chemical, mechanical, and electrical functionalities of binders for advanced energy-storage devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef]
- Chou, S.L.; Pan, Y.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef]
- Chen, Z.; Christensen, L.; Dahn, J.R. Mechanical and electrical properties of poly(vinylidene fluoride-tetrafluoroethylene-propylene)/Super-S carbon black swelled in liquid solvent as an electrode binder for lithium-ion batteries. J. Appl. Polym. Sci. 2004, 91, 2958–2965. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Zhang, Y.; Wang, H.; Aboalhassan, A.A.; Li, G.; Xu, G.; Xue, C.; Yu, J.; Yan, J.; et al. Highly elastic block copolymer binders for silicon anodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 38132–38139. [Google Scholar] [CrossRef] [PubMed]
- Ryou, M.H.; Kim, J.; Lee, I.; Kim, S.; Jeong, Y.K.; Hong, S.; Ryu, J.H.; Kim, T.S.; Park, J.K.; Lee, H.; et al. Mussel-inspired adhesive binders for high-performance silicon nanoparticle anodes in lithium-ion batteries. Adv. Mater. 2013, 25, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhou, M.; Yi, R.; Xu, T.; Gordin, M.L.; Tang, D.; Yu, Z.; Regula, M.; Wang, D. Interpenetrated gel polymer binder for high-performance silicon anodes in lithium-ion batteries. Adv. Funct. Mater. 2014, 24, 5904–5910. [Google Scholar] [CrossRef]
- Lim, S.; Chu, H.; Lee, K.; Yim, T.; Kim, Y.J.; Mun, J.; Kim, T.H. Physically cross-linked polymer binder induced by reversible acid-base interaction for high-performance silicon composite anodes. ACS Appl. Mater. Interfaces 2015, 7, 23545–23553. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kwon, T.-W.; Ali, C.; Jang, W.C. Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 2017, 357, 279–283. [Google Scholar] [PubMed] [Green Version]
- Wei, L.; Chen, C.; Hou, Z.; Wei, H. Poly (acrylic acid sodium) grafted carboxymethyl cellulose as a high performance polymer binder for silicon anode in lithium ion batteries. Sci. Rep. 2016, 6, 19583. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Cheng, S.; Xie, M.; Zheng, Y.; Xu, G.; Gao, S.; Li, J.; Liu, Z.; Liu, X.; Liu, J.; et al. A delicately designed functional binder enabling in situ construction of 3D cross-linking robust network for high-performance Si/graphite composite anode. J. Polym. Sci. 2021, 60, 1835–1844. [Google Scholar] [CrossRef]
- Pan, H.; Xu, Z.; Wei, Z.; Liu, X.; Xu, M.; Zong, C.; Li, W.; Cui, G.; Cao, L.; Wang, Q. Synergistic double cross-linked dynamic network of epoxidized natural rubber/glycinamide modified polyacrylic acid for silicon anode in lithium ion battery: High peel strength and super cycle stability. ACS Appl. Mater. Interfaces 2022, 14, 33315–33327. [Google Scholar] [CrossRef]
- Liu, Y.; Tai, Z.; Zhou, T.; Sencadas, V.; Zhang, J.; Zhang, L.; Konstantinov, K.; Guo, Z.; Liu, H.K. An all-Integrated anode via interlinked chemical bonding between double-shelled-yolk-structured silicon and binder for lithium-Ion batteries. Adv. Mater. 2017, 29, 1703028. [Google Scholar] [CrossRef]
- Lee, D.; Park, H.; Goliaszewski, A.; Byeun, Y.K.; Song, T.; Paik, U. In situ cross-linked carboxymethyl cellulose-polyethylene glycol binder for improving the long-term cycle life of silicon anodes in Li ion batteries. Ind. Eng. Chem. Res. 2019, 58, 8123–8130. [Google Scholar] [CrossRef]
- Tang, B.; He, S.; Deng, Y.; Shan, Y.; Qin, H.; Noor, H.; Hou, X. Advanced binder with ultralow-content for high performance silicon anode. J. Power Sources 2023, 556, 232237. [Google Scholar]
- Liu, J.; Zhang, Q.; Wu, Z.Y.; Wu, J.H.; Li, J.T.; Huang, L.; Sun, S.G. A high-performance alginate hydrogel binder for the Si/C anode of a Li-ion battery. Chem. Commun. 2014, 50, 6386–6389. [Google Scholar]
- Wu, Z.Y.; Deng, L.; Li, J.T.; Huang, Q.S.; Lu, Y.Q.; Liu, J.; Zhang, T.; Huang, L.; Sun, S.G. Multiple hydrogel alginate binders for Si anodes of lithium-ion battery. Electrochim. Acta 2017, 245, 371–378. [Google Scholar]
- Lee, S.H.; Lee, J.H.; Nam, D.H.; Cho, M.; Kim, J.; Chanthad, C.; Lee, Y. Epoxidized natural rubber/chitosan network binder for silicon anode in lithium-ion battery. ACS Appl. Mater. Interfaces 2018, 10, 16449–16457. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liu, N.; He, W.; Long, J.; Dou, H.; Zhang, X. Three-dimensional cross-linked binder based on ionic bonding for a high-performance SiOx anode in lithium-ion batteries. ACS Appl. Energy Mater. 2022, 5, 4788–4795. [Google Scholar]
- Kwon, T.W.; Jeong, Y.K.; Deniz, E.; AlQaradawi, S.Y.; Wook Choi, J.; Coskun, A. Dynamic cross-linking of polymeric binders based on host guest interactions for silicon anodes in lithium Ion batteries. ACS Nano 2015, 9, 11317–11324. [Google Scholar]
- Yang, J.; Zhang, L.; Zhang, T.; Wang, X.; Gao, Y.; Fang, Q. Self-healing strategy for Si nanoparticles towards practical application as anode materials for Li-ion batteries. Electrochem. Commun. 2018, 87, 22–26. [Google Scholar]
- Jiao, X.; Yin, J.; Xu, X.; Wang, J.; Liu, Y.; Xiong, S.; Zhang, Q.; Song, J. Highly energy-dissipative, fast self-healing binder for stable Si anode in lithium-Ion batteries. Adv. Funct. Mater. 2020, 31, 2005699. [Google Scholar] [CrossRef]
- Lee, K.; Lim, S.; Tron, A.; Mun, J.; Kim, Y.-J.; Yim, T.; Kim, T.-H. Polymeric binder based on PAA and conductive PANI for high performance silicon-based anodes. RSC Adv. 2016, 6, 101622–101625. [Google Scholar] [CrossRef]
- Higgins, T.M.; Park, S.H.; King, P.J.; Zhang, C.J.; McEvoy, N.; Berner, N.C.; Daly, D.; Shmeliov, A.; Khan, U.; Duesberg, G.; et al. A commercial conducting polymer as both binder and conductive additive for silicon nanoparticle-based lithium-Ion battery negative electrodes. ACS Nano 2016, 10, 3702–3713. [Google Scholar]
- Wang, L.; Liu, T.; Peng, X.; Zeng, W.; Jin, Z.; Tian, W.; Gao, B.; Zhou, Y.; Chu, P.K.; Huo, K. Highly stretchable conductive glue for high-performance silicon anodes in advanced lithium-ion batteries. Adv. Funct. Mater. 2018, 28, 1704858. [Google Scholar] [CrossRef]
- Su, Y.; Feng, X.; Zheng, R.; Lv, Y.; Wang, Z.; Zhao, Y.; Shi, L.; Yuan, S. Binary network of conductive elastic polymer constraining nanosilicon for a high-performance lithium-ion battery. ACS Nano 2021, 15, 14570–14579. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Wang, D.; Zhu, J.; Guo, H.; Hou, Y.; Gao, X.; Lu, J.; Zhan, X.; He, X.; Zhang, Q. A self-healable polyelectrolyte binder for highly stabilized sulfur, silicon, and silicon oxides electrodes. Adv. Funct. Mater. 2021, 31, 2104433. [Google Scholar] [CrossRef]
- Wang, H.; Wu, B.; Wu, X.; Zhuang, Q.; Liu, T.; Pan, Y.; Shi, G.; Yi, H.; Xu, P.; Xiong, Z.; et al. Key Factors for Binders to Enhance the Electrochemical Performance of Silicon Anodes through Molecular Design. Nano Micro. Small 2022, 18, e2101680. [Google Scholar] [CrossRef] [PubMed]
- Mazouzi, D.; Karkar, Z.; Reale Hernandez, C.; Jimenez Manero, P.; Guyomard, D.; Roué, L.; Lestriez, B. Critical roles of binders and formulation at multiscales of silicon-based composite electrodes. J. Power Sources 2015, 280, 533–549. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward efficient binders for Li-ion battery Si-based anodes: Polyacrylic acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- Komaba, S.; Yabuuchi, N.; Ozeki, T.; Han, Z.J.; Shimomura, K.; Yui, H.; Katayama, Y.; Miura, T. Comparative study of sodium polyacrylate and poly(vinylidene fluoride) as binders for high capacity Si–graphite composite negative electrodes in Li-Ion batteries. J. Phys. Chem. C 2011, 116, 1380–1389. [Google Scholar] [CrossRef]
- Wei, D.; Mao, J.; Zheng, Z.; Fang, J.; Luo, Y.; Gao, X. Achieving a high loading Si anode via employing a triblock copolymer elastomer binder, metal nanowires and a laminated conductive structure. J. Mater. Chem. A 2018, 6, 20982–20991. [Google Scholar] [CrossRef]
- Liu, T.; Chu, Q.; Yan, C.; Zhang, S.; Lin, Z.; Lu, J. Interweaving 3D network binder for high-areal-capacity Si anode through combined hard and soft polymers. Adv. Energy Mater. 2018, 9, 1802645. [Google Scholar] [CrossRef]
- Liu, Z.; Han, S.; Xu, C.; Luo, Y.; Peng, N.; Qin, C.; Zhou, M.; Wang, W.; Chen, L.; Okada, S. In situ crosslinked PVA–PEI polymer binder for long-cycle silicon anodes in Li-ion batteries. RSC Adv. 2016, 6, 68371–68378. [Google Scholar] [CrossRef]
- Yook, S.H.; Kim, S.H.; Park, C.H.; Kim, D.W. Graphite–silicon alloy composite anodes employing cross-linked poly(vinyl alcohol) binders for high-energy density lithium-ion batteries. RSC Adv. 2016, 6, 83126–83134. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; He, S.; Huang, C.; Gan, L.; Gong, Z.; Long, M. A novel high-performance 3D polymer binder for silicon anode in lithium-ion batteries. J. Phys. Chem. Solids 2019, 135, 109113. [Google Scholar] [CrossRef]
- Li, J.; Lewis, R.B.; Dahna, J.R. Sodium carboxymethyl cellulose: A potential binder for Si negative electrodes for Li-Ion batteries. Electrochem. Solid-State Lett. 2007, 10, A17–A20. [Google Scholar] [CrossRef]
- Buqa, H.; Holzapfel, M.; Krumeich, F.; Veit, C.; Novák, P. Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries. J. Power Sources 2006, 161, 617–622. [Google Scholar] [CrossRef]
- Hochgatterer, N.S.; Schweiger, M.R.; Koller, S.; Raimann, P.R.; Wöhrle, T.; Wurm, C.; Winter, M. Silicon/graphite composite electrodes for high-capacity anodes: Influence of binder chemistry on cycling stability. Electrochem. Solid-State Lett. 2008, 11, A76–A80. [Google Scholar] [CrossRef]
- Mazouzi, D.; Lestriez, B.; Roué, L.; Guyomard, D. Silicon composite electrode with high capacity and long cycle life. Electrochem. Solid-State Lett. 2009, 12, A215–A218. [Google Scholar] [CrossRef]
- Bridel, J.S.; Azaïs, T.; Morcrette, M.; Tarascon, J.M.; Larcher, D. Key parameters governing the reversibility of Si/carbon/CMC electrodes for Li-Ion batteries. Chem. Mater. 2009, 22, 1229–1241. [Google Scholar] [CrossRef]
- Munao, D.; van Erven, J.W.M.; Valvo, M.; Garcia-Tamayo, E.; Kelder, E.M. Role of the binder on the failure mechanism of Si nano-composite electrodes for Li-ion batteries. J. Power Sources 2011, 196, 6695–6702. [Google Scholar] [CrossRef]
- Yue, L.; Zhang, L.; Zhong, H. Carboxymethyl chitosan: A new water soluble binder for Si anode of Li-ion batteries. J. Power Sources 2014, 247, 327–331. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Kwon, T.W.; Lee, I.; Kim, T.S.; Coskun, A.; Choi, J.W. Hyperbranched beta-cyclodextrin polymer as an effective multidimensional binder for silicon anodes in lithium rechargeable batteries. Nano Lett. 2014, 14, 864–870. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Zhang, T.; Li, J.T.; Huang, L.; Sun, S.G. A robust ion-conductive biopolymer as a binder for Si anodes of lithium-ion batteries. Adv. Funct. Mater. 2015, 25, 3599–3605. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Kwon, T.W.; Lee, I.; Kim, T.S.; Coskun, A.; Choi, J.W. Millipede-inspired structural design principle for high performance polysaccharide binders in silicon anodes. Energy Environ. Sci. 2015, 8, 1224–1230. [Google Scholar] [CrossRef]
- Choi, D.; Choy, K.L. Spider silk binder for Si-based anode in lithium-ion batteries. Mater. Des. 2020, 191, 108669. [Google Scholar] [CrossRef]
- Ma, L.; Niu, S.L.; Zhao, F.F.; Tang, R.X.; Zhang, Y.; Su, W.D.; Wei, L.M.; Tang, D.; Wang, Y.; Pang, A.M.; et al. A High-Performance Polyurethane-Polydopamine Polymeric Binder for Silicon Microparticle Anodes in Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 7571–7581. [Google Scholar] [CrossRef]
- Huang, S.; Ren, J.; Liu, R.; Yue, M.; Huang, Y.; Yuan, G. The progress of novel binder as a non-ignorable part to improve the performance of Si-based anodes for Li-ion batteries. Int. J. Energy Res. 2018, 42, 919–935. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, S.; Sun, F.; Yang, H.; Cao, P.F. Polymer Binders Constructed through Dynamic Noncovalent Bonds for High-Capacity Silicon-Based Anodes. Chem. Eur. 2019, 25, 10976–10994. [Google Scholar] [CrossRef]
- Wu, H.; Yu, G.; Pan, L.; Liu, N.; McDowell, M.T.; Bao, Z.; Cui, Y. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 2013, 4, 1941–1943. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liu, Y.M.; Zhang, Y.C.; Song, Y.; Wang, G.K.; Liu, Y.X.; Wu, Z.G.; Zhong, B.H.; Zhong, Y.J.; Guo, X.D. A review of rational design and investigation of binders applied in silicon-based anodes for lithium-ion batteries. J. Power Sources 2021, 485, 229331. [Google Scholar] [CrossRef]
- Choi, N.S.; Ha, S.Y.; Lee, Y.; Jang, J.Y.; Jeong, M.H.; Shin, W.C.; Ue, M. Recent Progress on Polymeric Binders for Silicon Anodes in Lithium-Ion Batteries. J. Electrochem. Sci. Technol. 2015, 6, 35–49. [Google Scholar] [CrossRef]
- Taskin, O.S.; Hubble, D.; Zhu, T.; Liu, G. Biomass-derived polymeric binders in silicon anodes for battery energy storage applications. Green Chem. 2021, 23, 7890–7901. [Google Scholar] [CrossRef]
- Song, Z.; Wang, L.; Yang, K.; Gong, Y.; Yang, L.; Liu, X.; Pan, F. Intermolecular chemistry for designing functional binders in silicon/carbon composite anodes. Mater. Today Energy 2022, 30, 101153. [Google Scholar] [CrossRef]
- Liu, Y.; Su, M.Y.; Gu, Z.Y.; Zhang, K.Y.; Wang, X.T.; Du, M.; Guo, J.Z.; Wu, X.L. Advanced lithium primary batteries: Key materials, research progresses and challenges. Chem. Rev. 2022, 22, e202200081. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, J.; Xiang, S.; Bian, X.; Yin, J.; Jiang, J.; Yang, L. Progress of binder structures in silicon-based anodes for advanced lithium-ion batteries: A mini review. Front. Chem. 2021, 9, 712225. [Google Scholar] [CrossRef]
- Preman, A.N.; Lee, H.; Yoo, J.; Kim, I.T.; Saito, T.; Ahn, S.K. Progress of 3D network binders in silicon anodes for lithium ion batteries. J. Mater. Chem. A 2020, 8, 25548–25570. [Google Scholar] [CrossRef]
- Ezeigwe, E.R.; Dong, L.; Manjunatha, R.; Tan, M.; Yan, W.; Zhang, J. A review of self-healing electrode and electrolyte materials and their mitigating degradation of Lithium batteries. Nano Energy 2021, 84, 105907. [Google Scholar] [CrossRef]
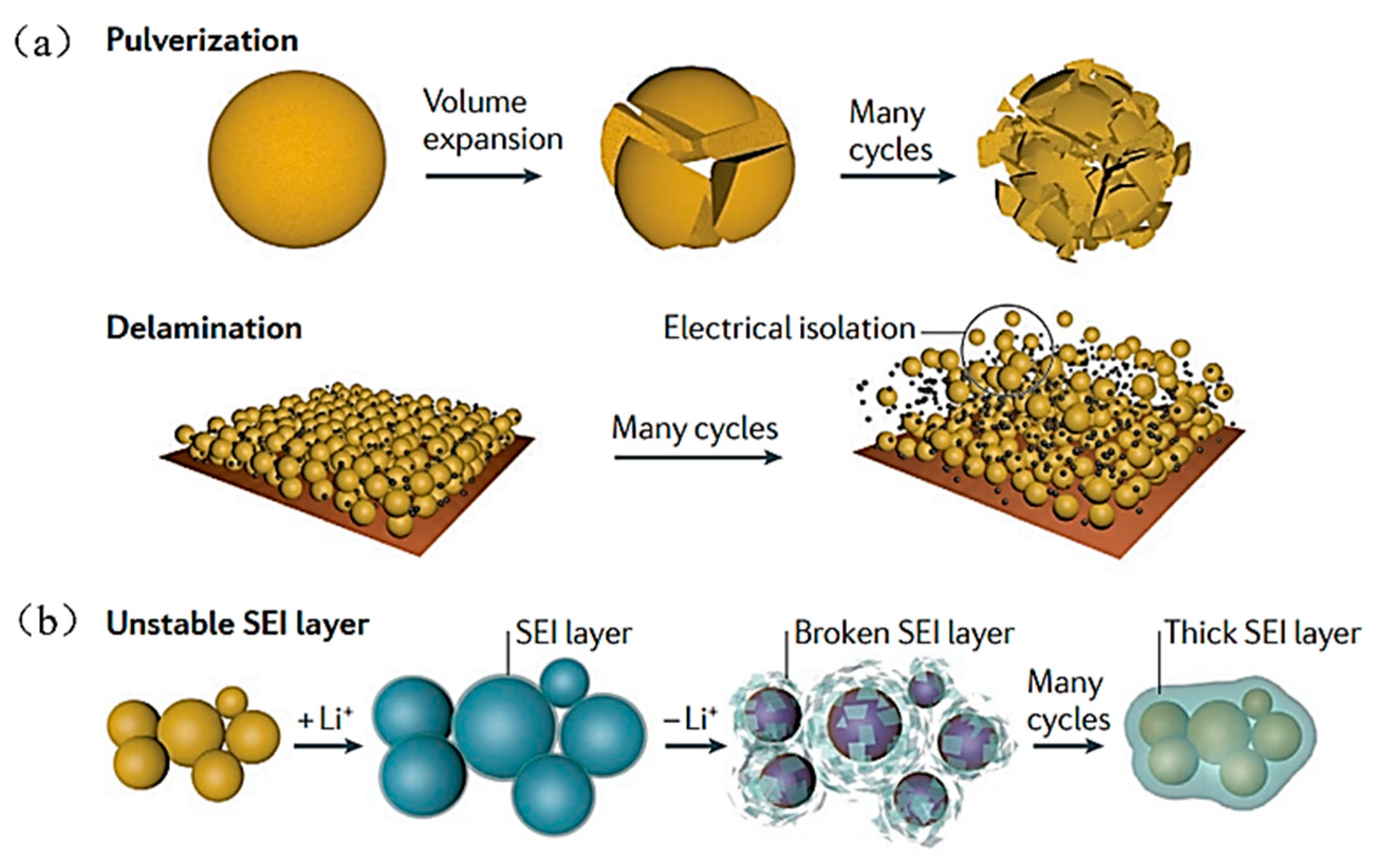
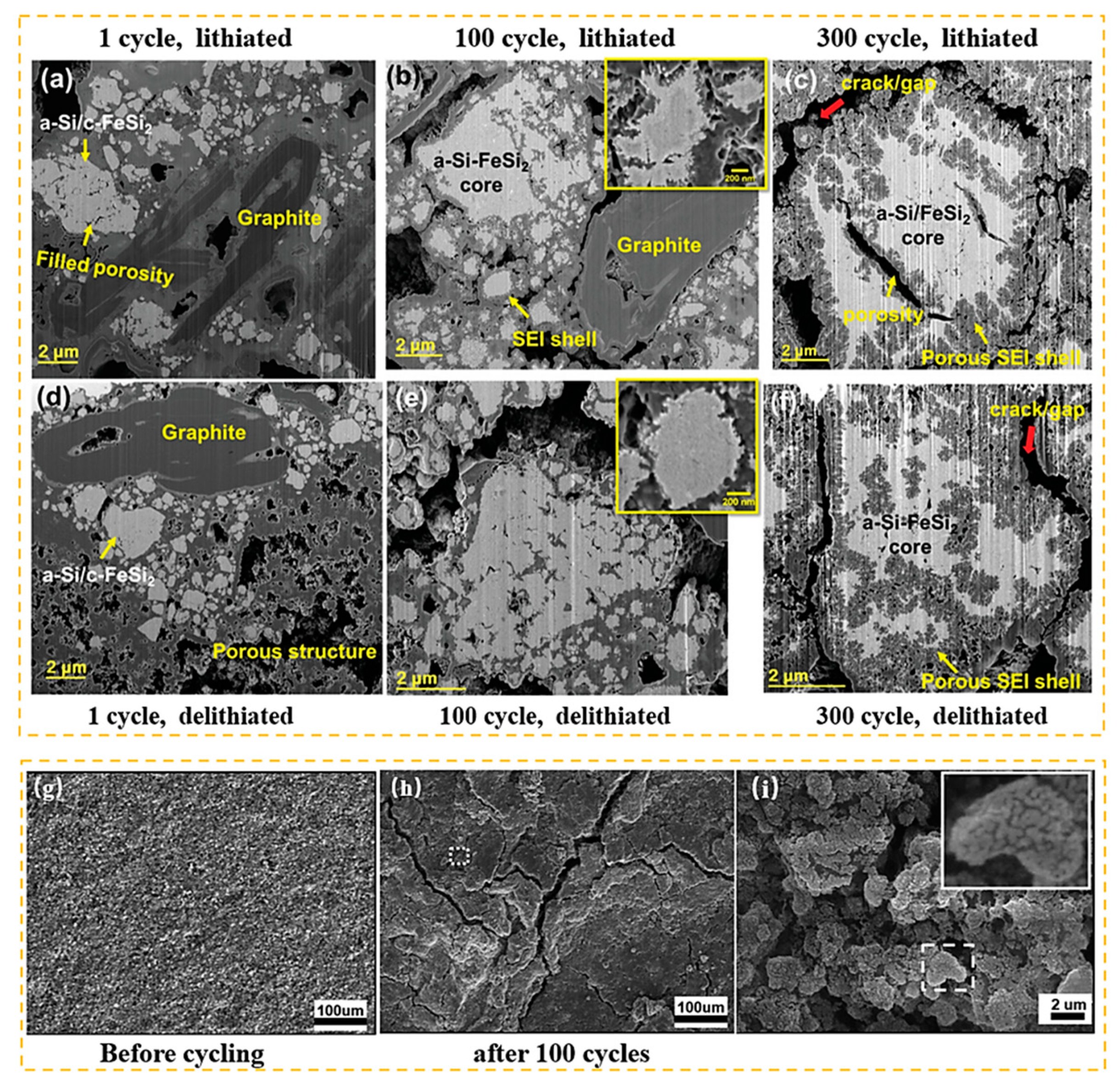
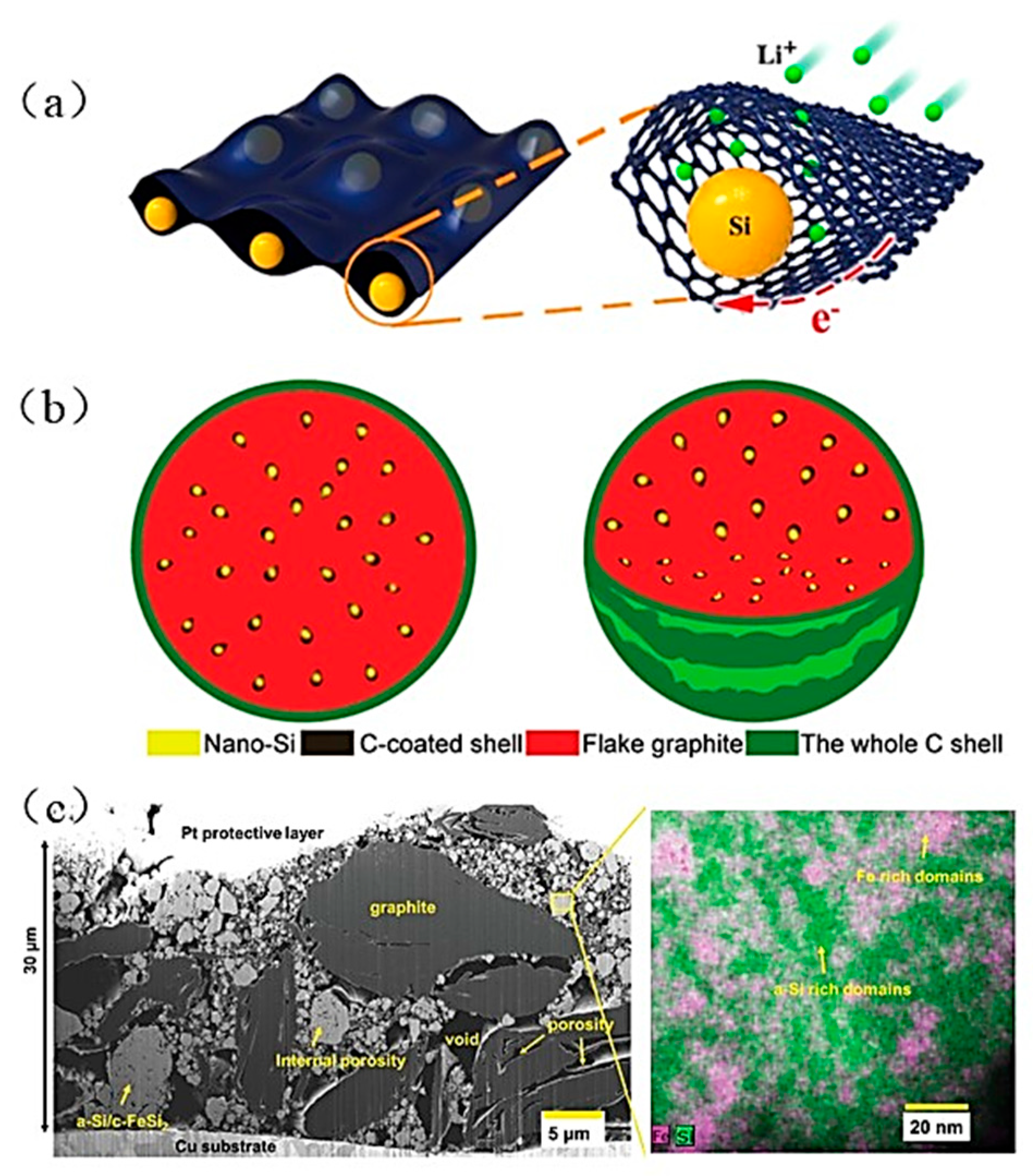

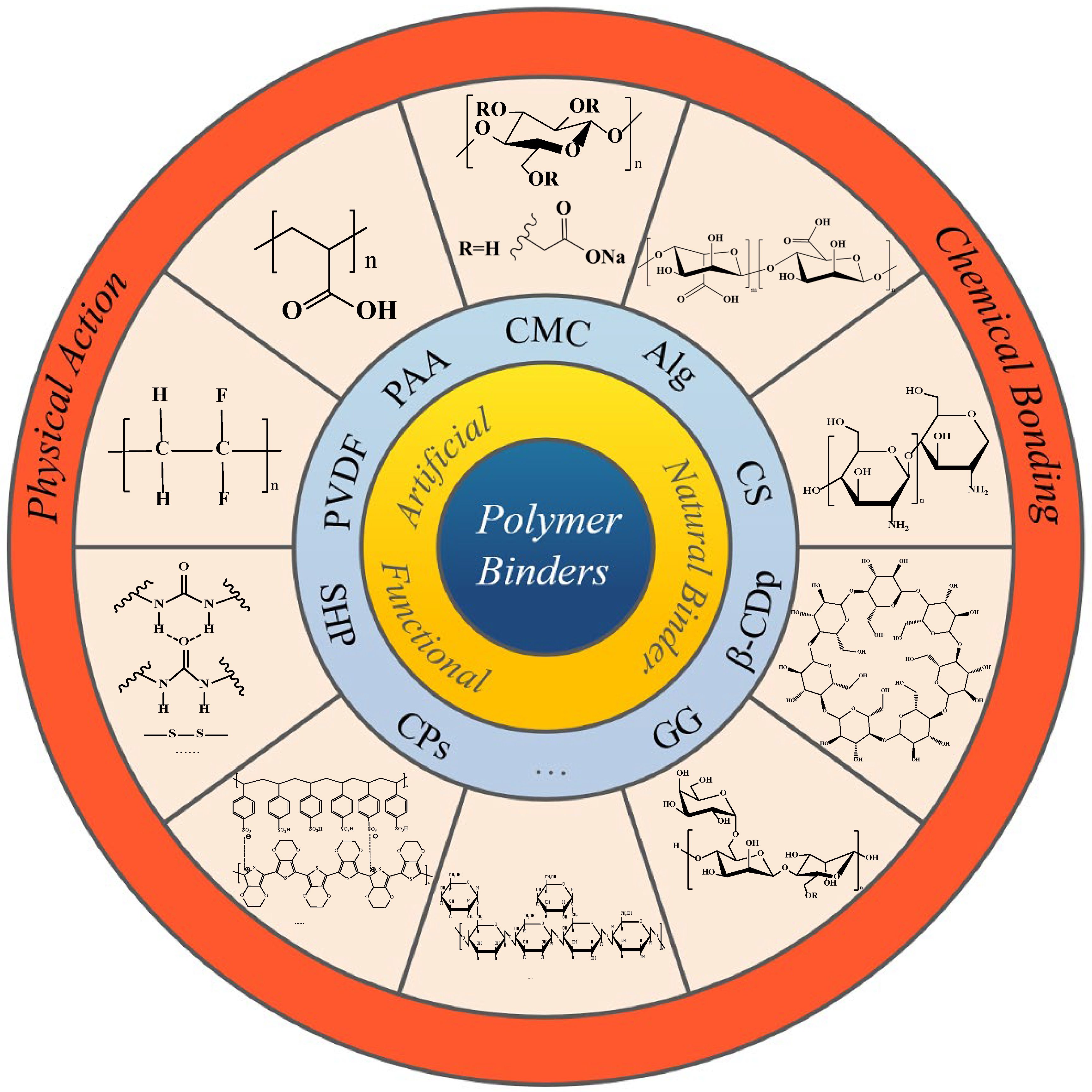
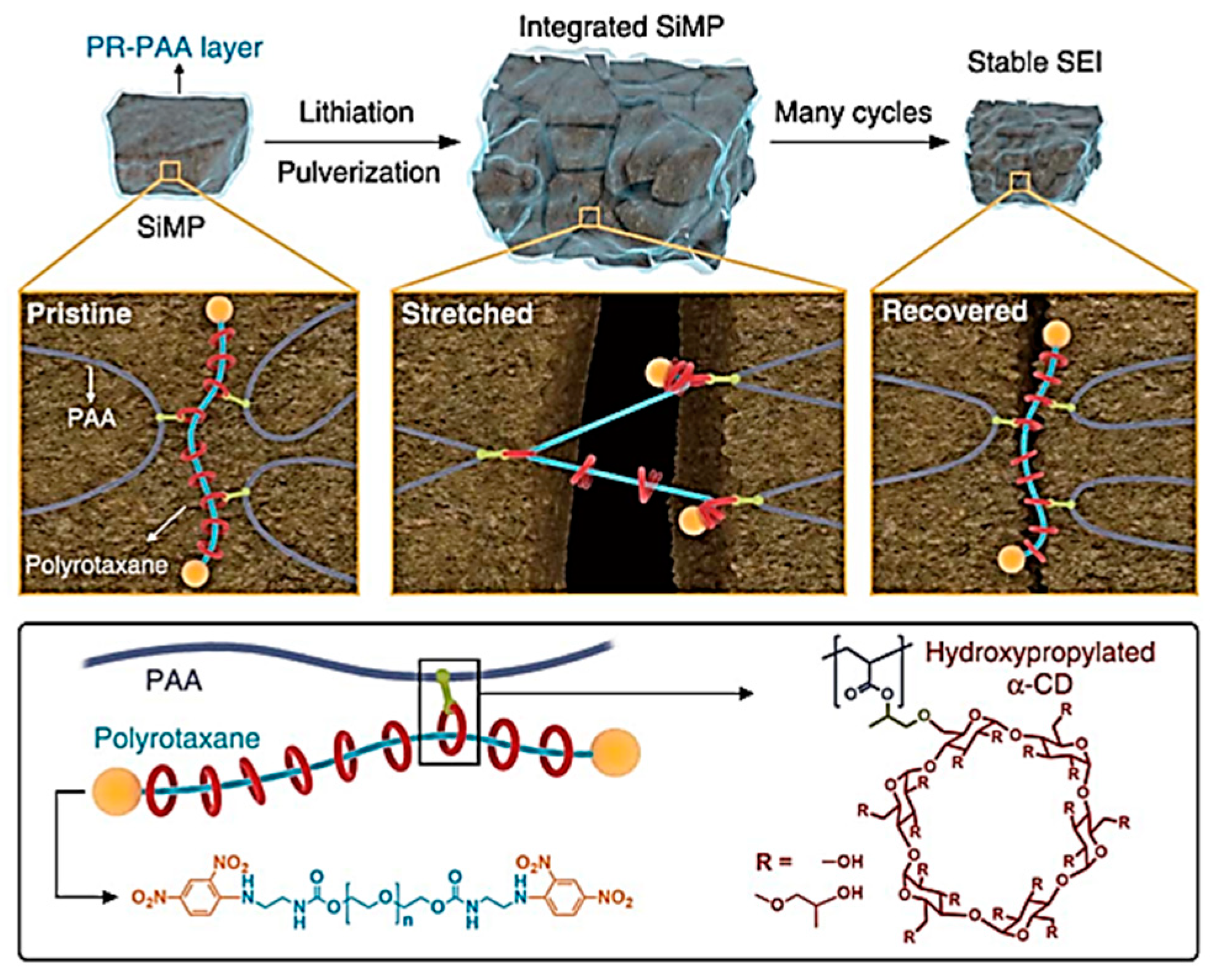
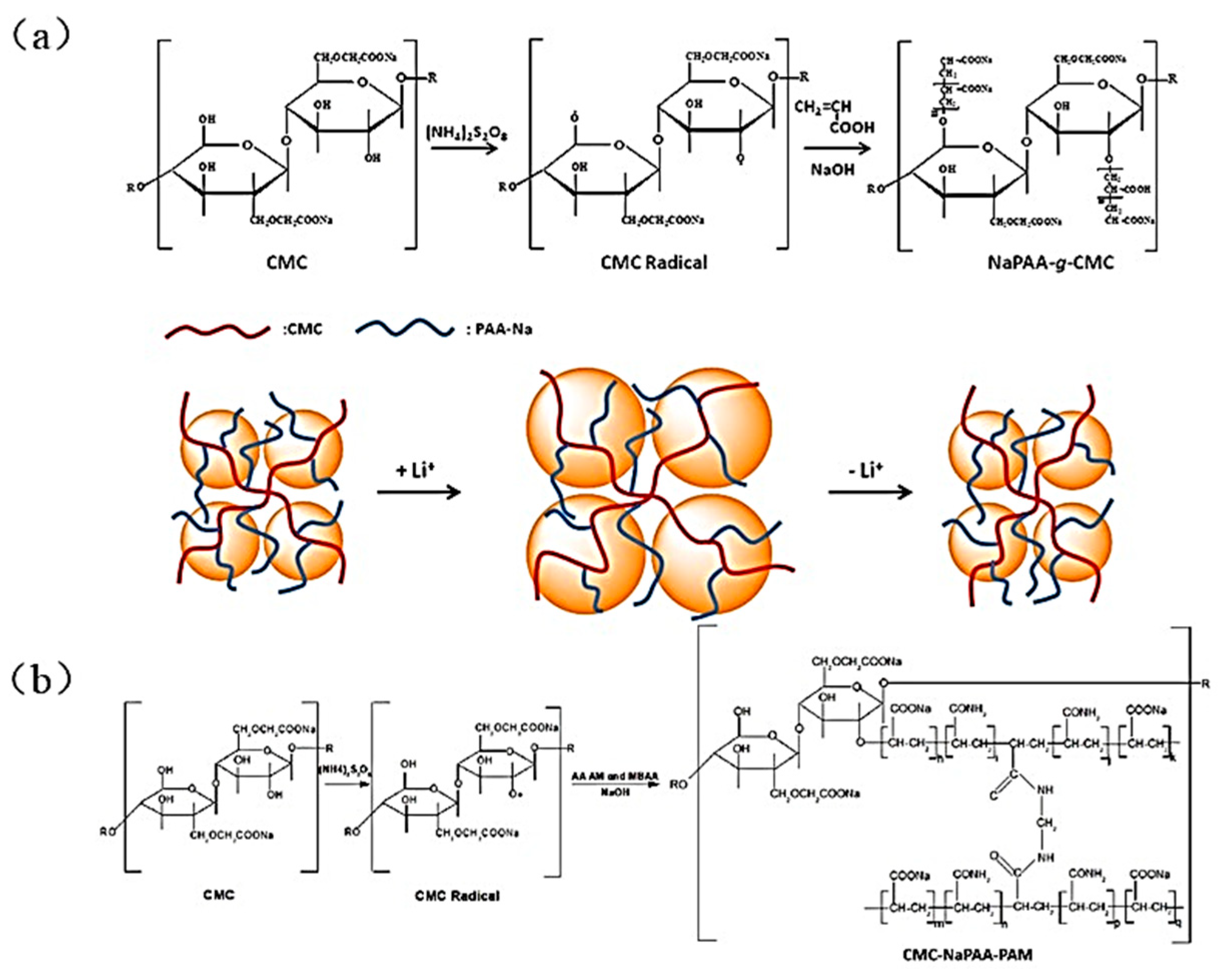
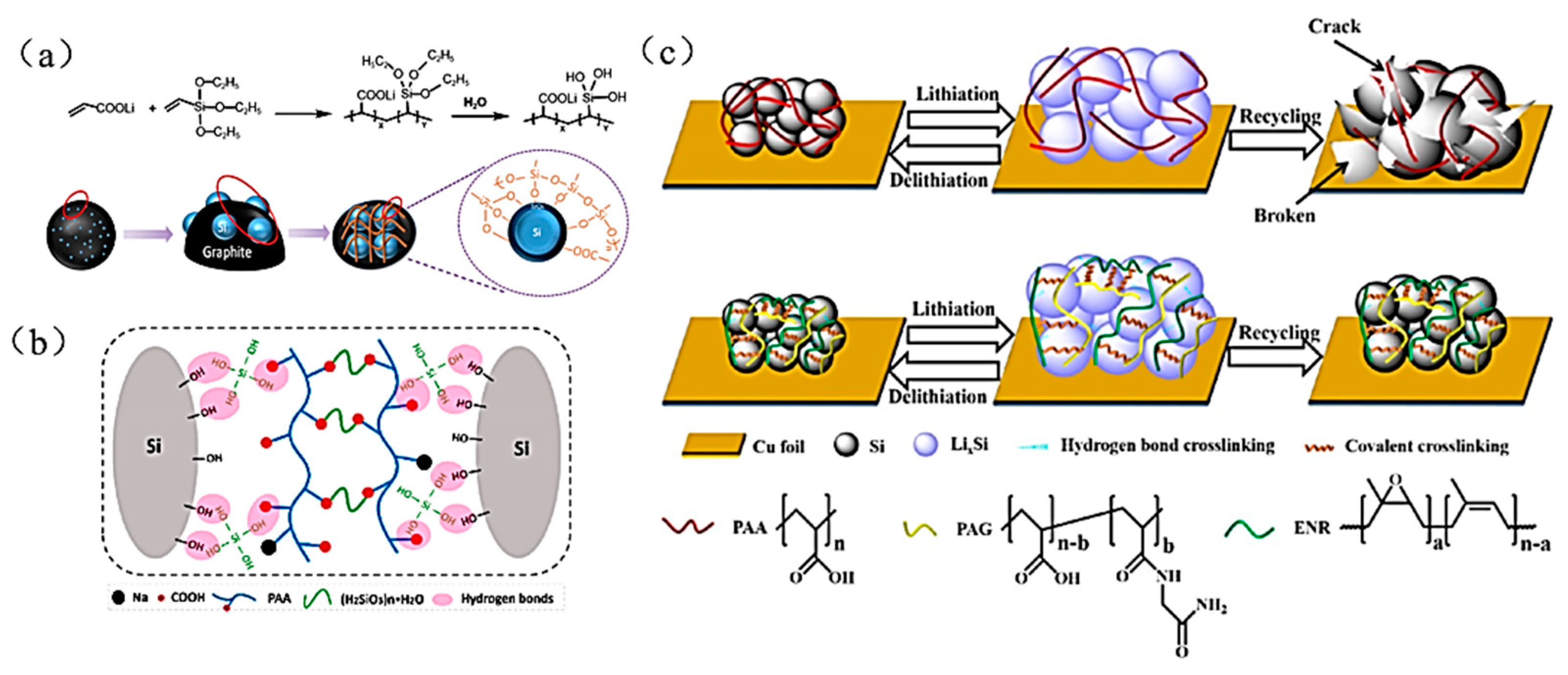
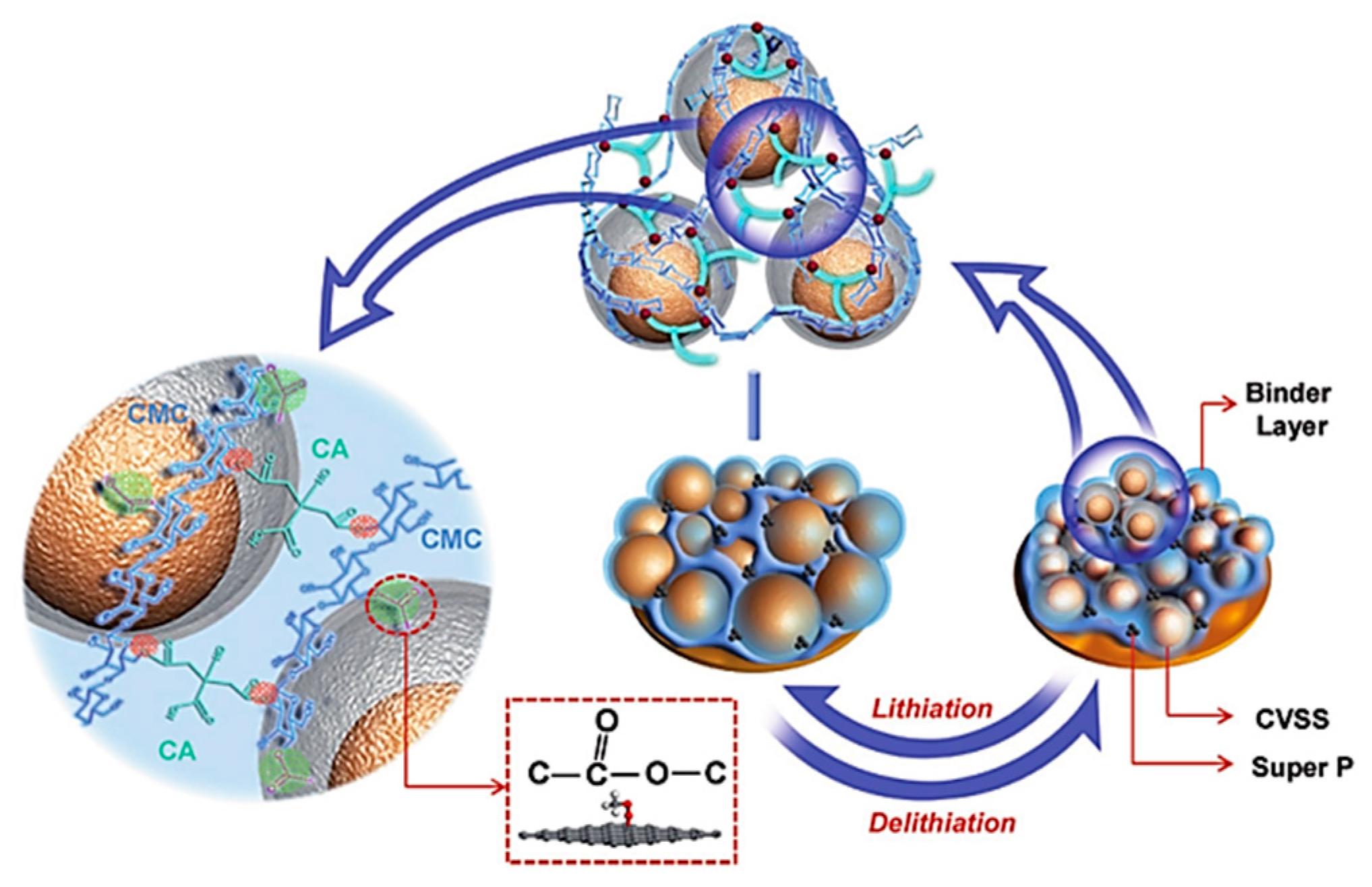
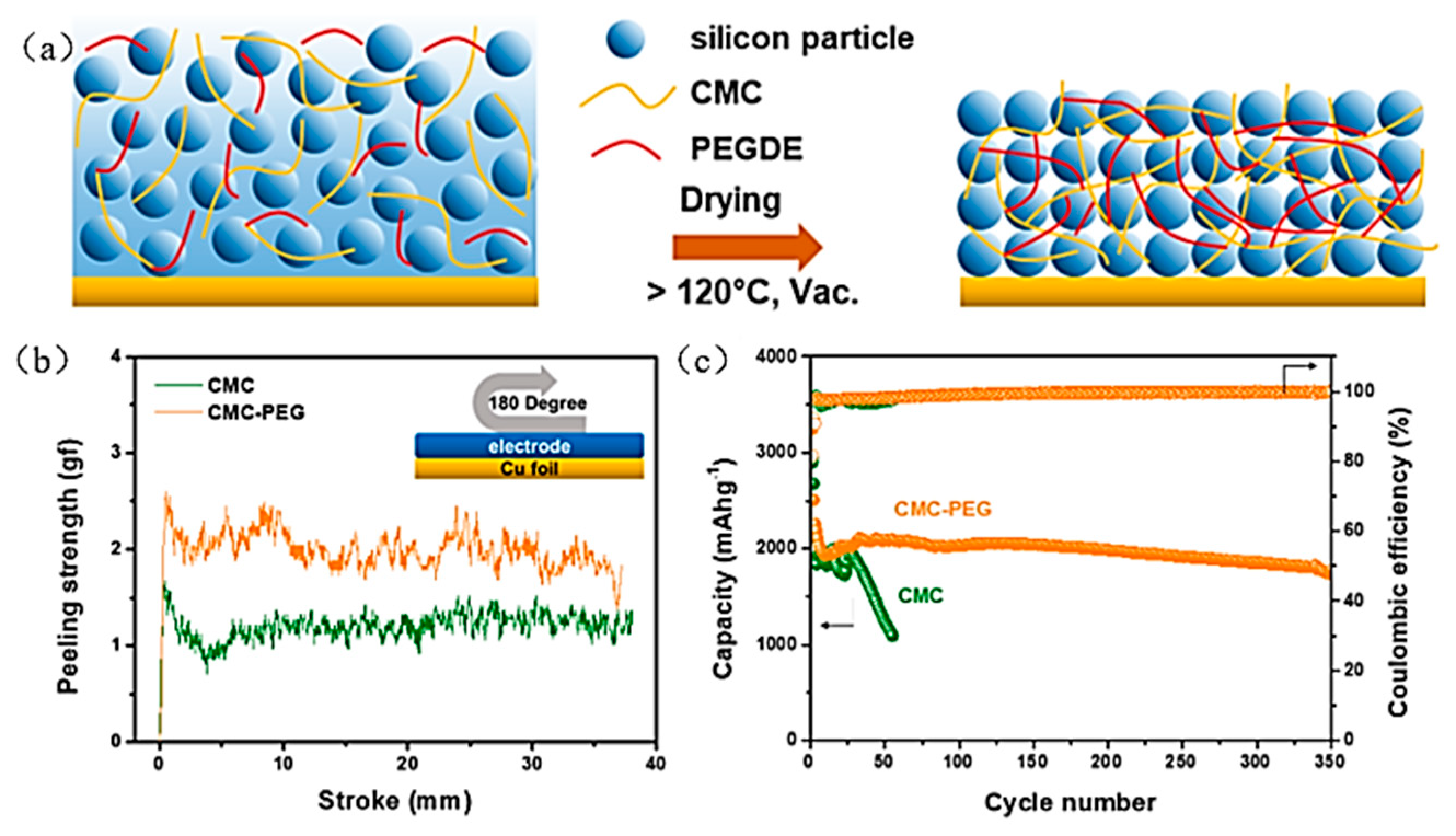
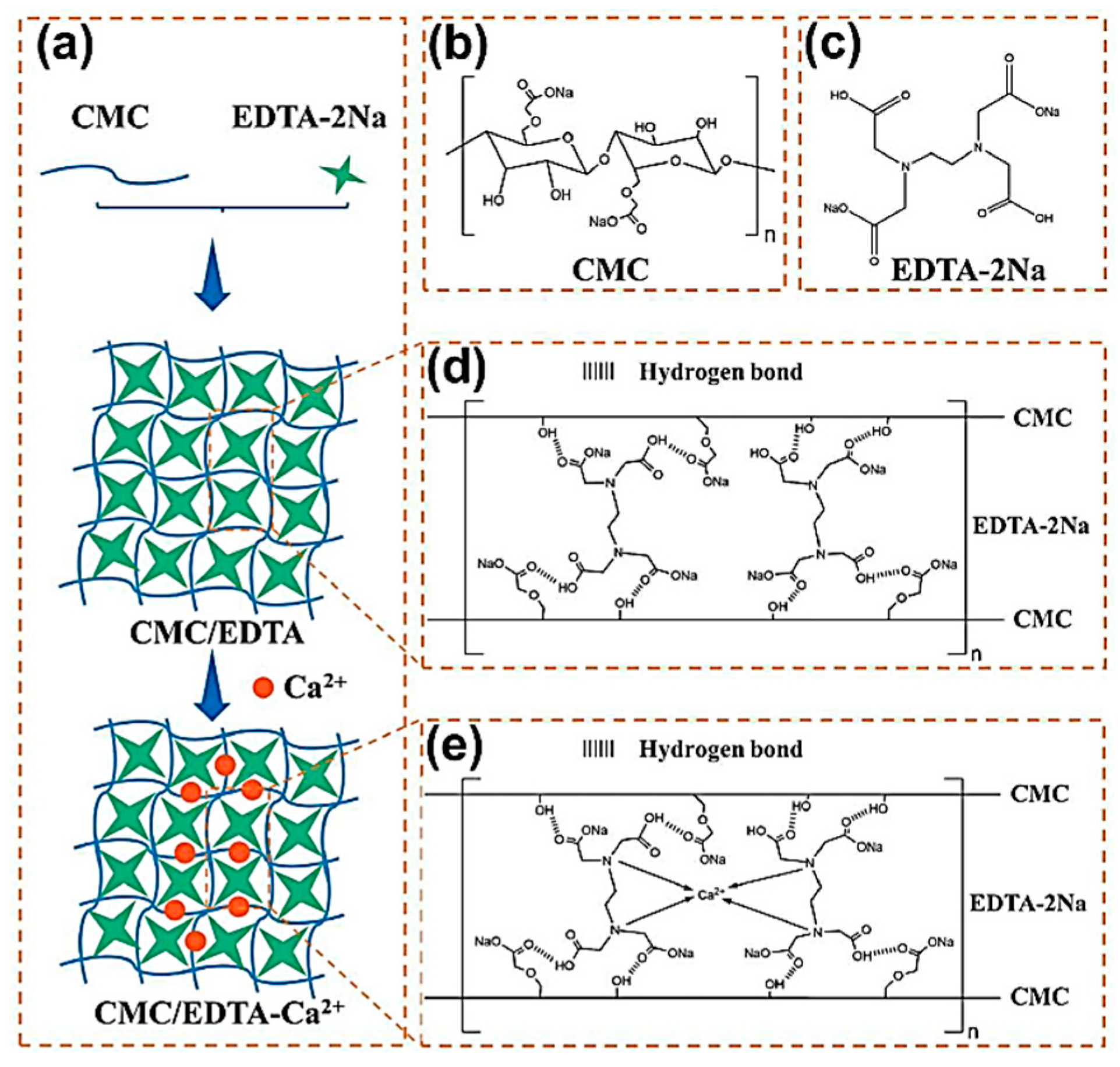
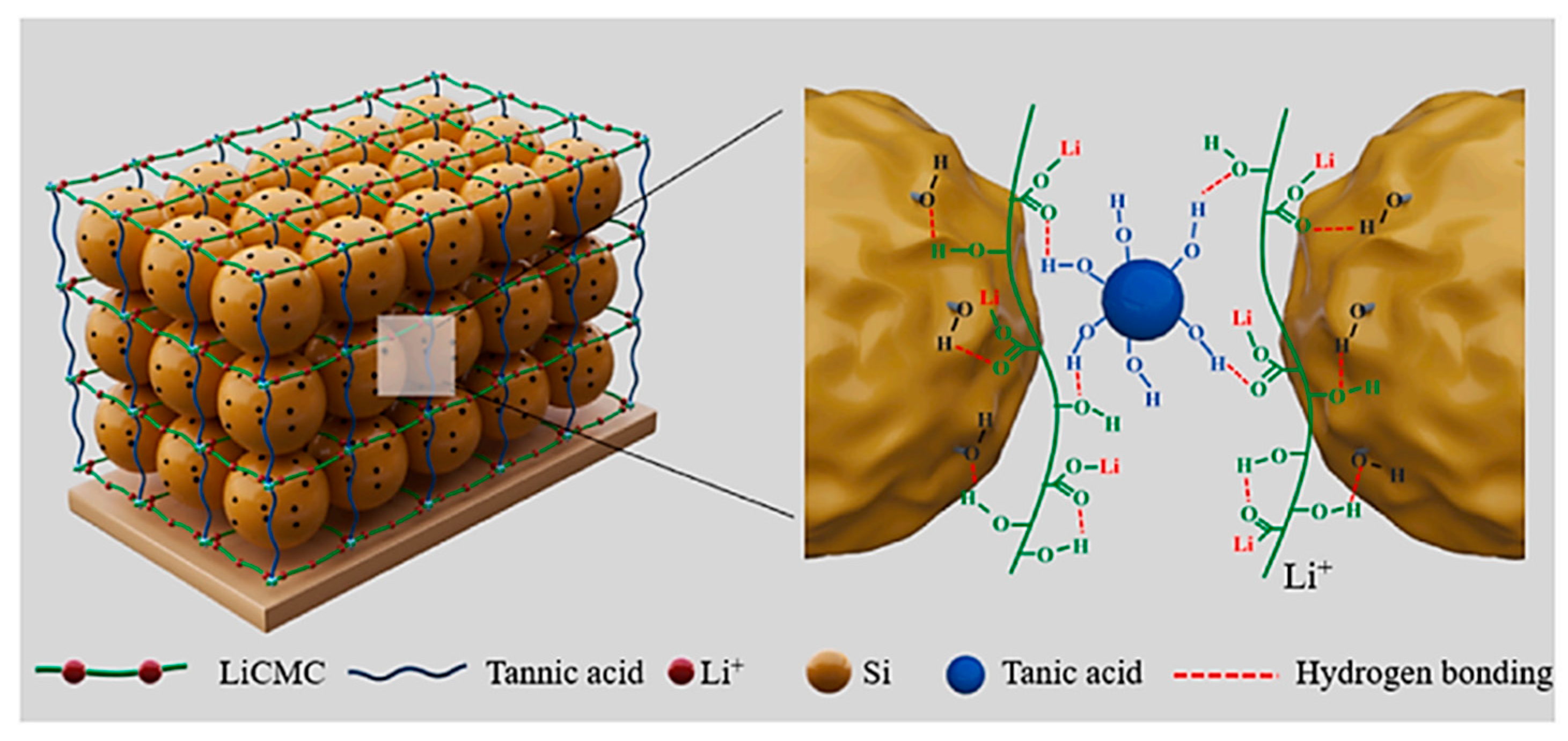
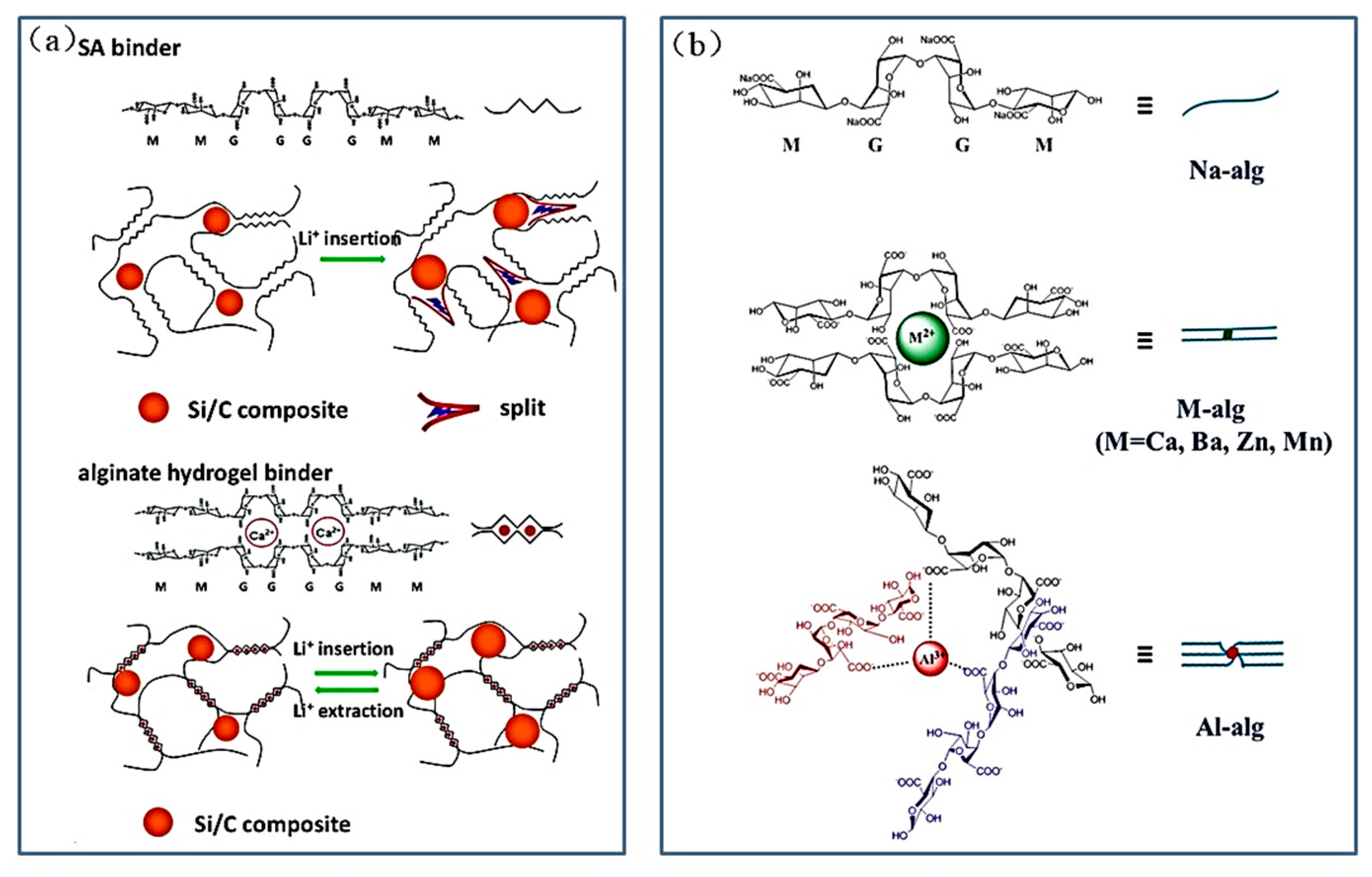
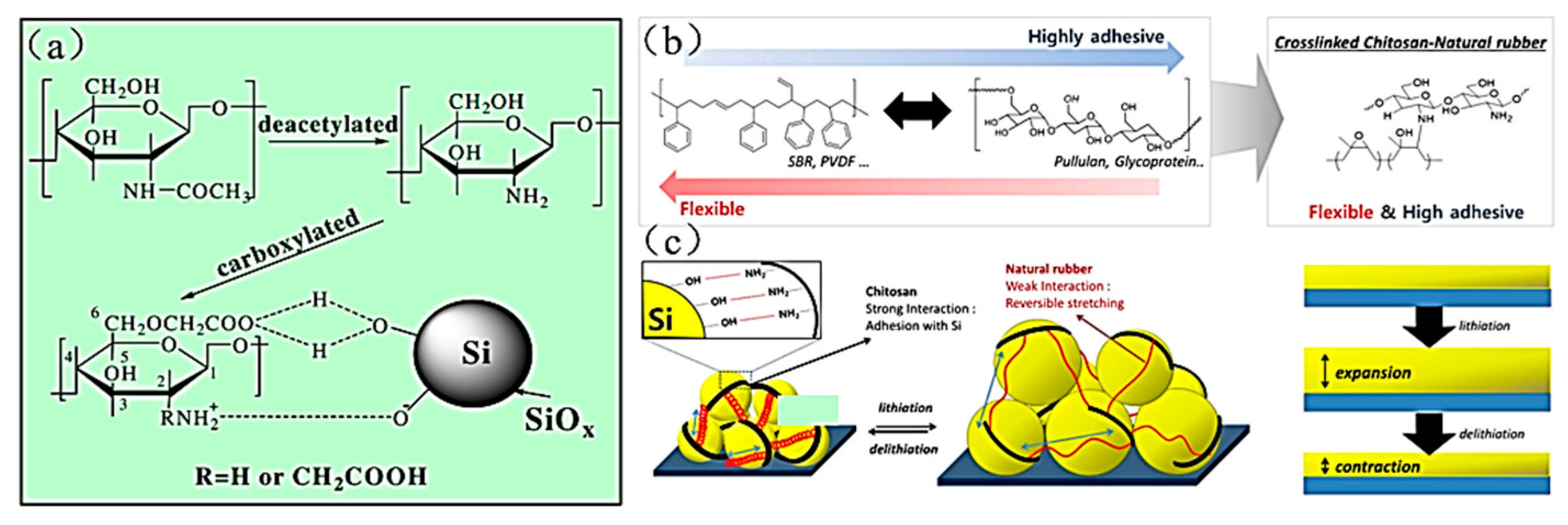
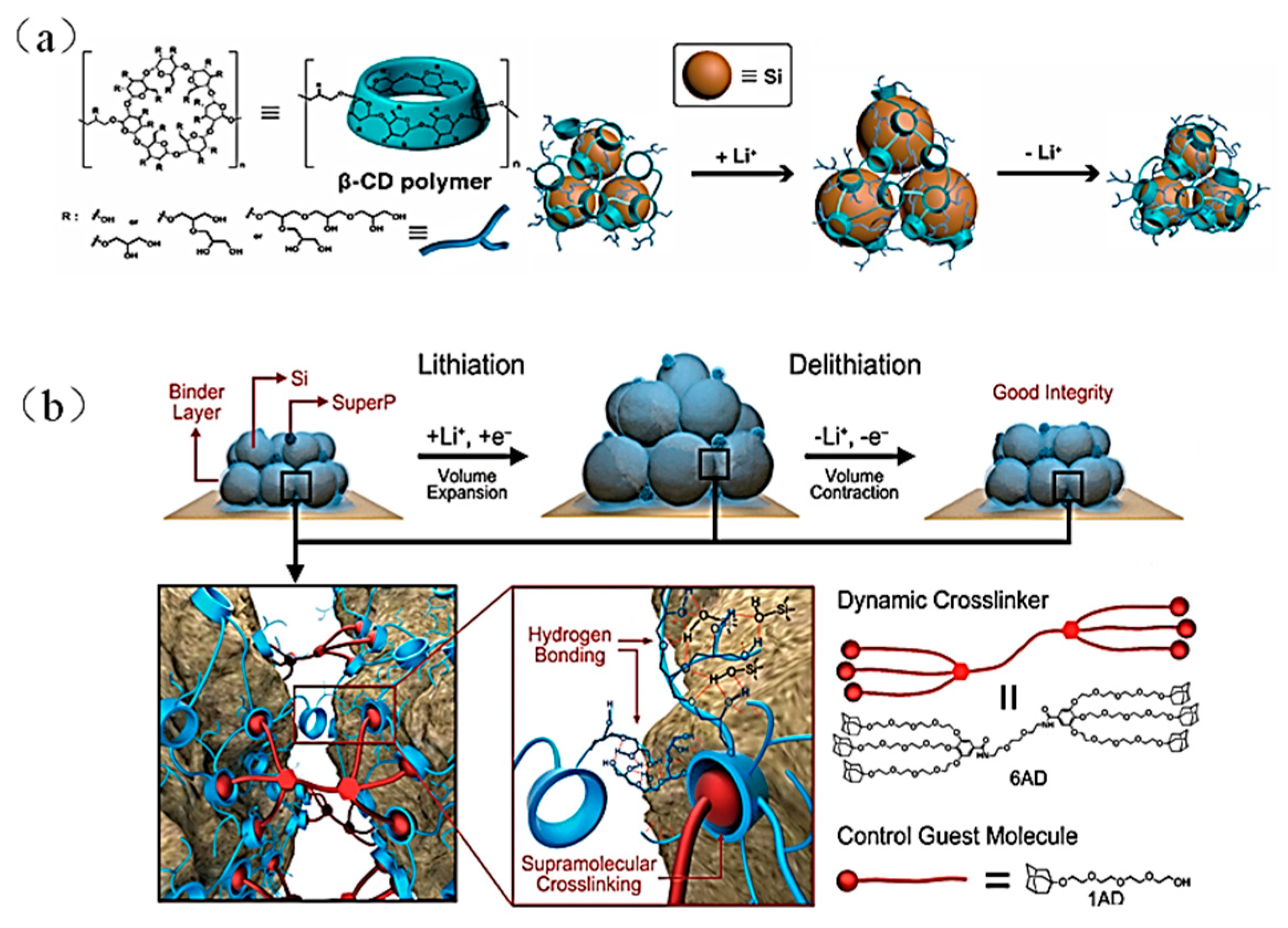

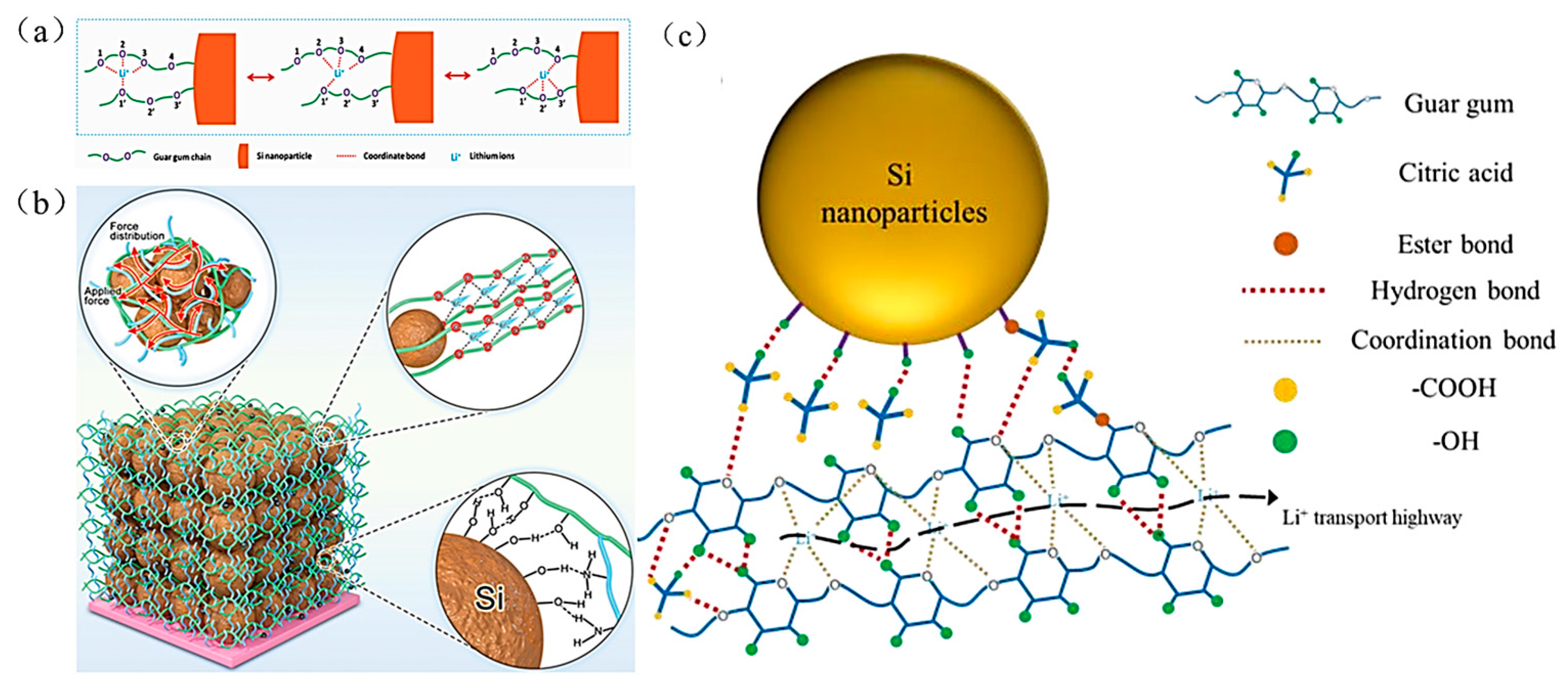
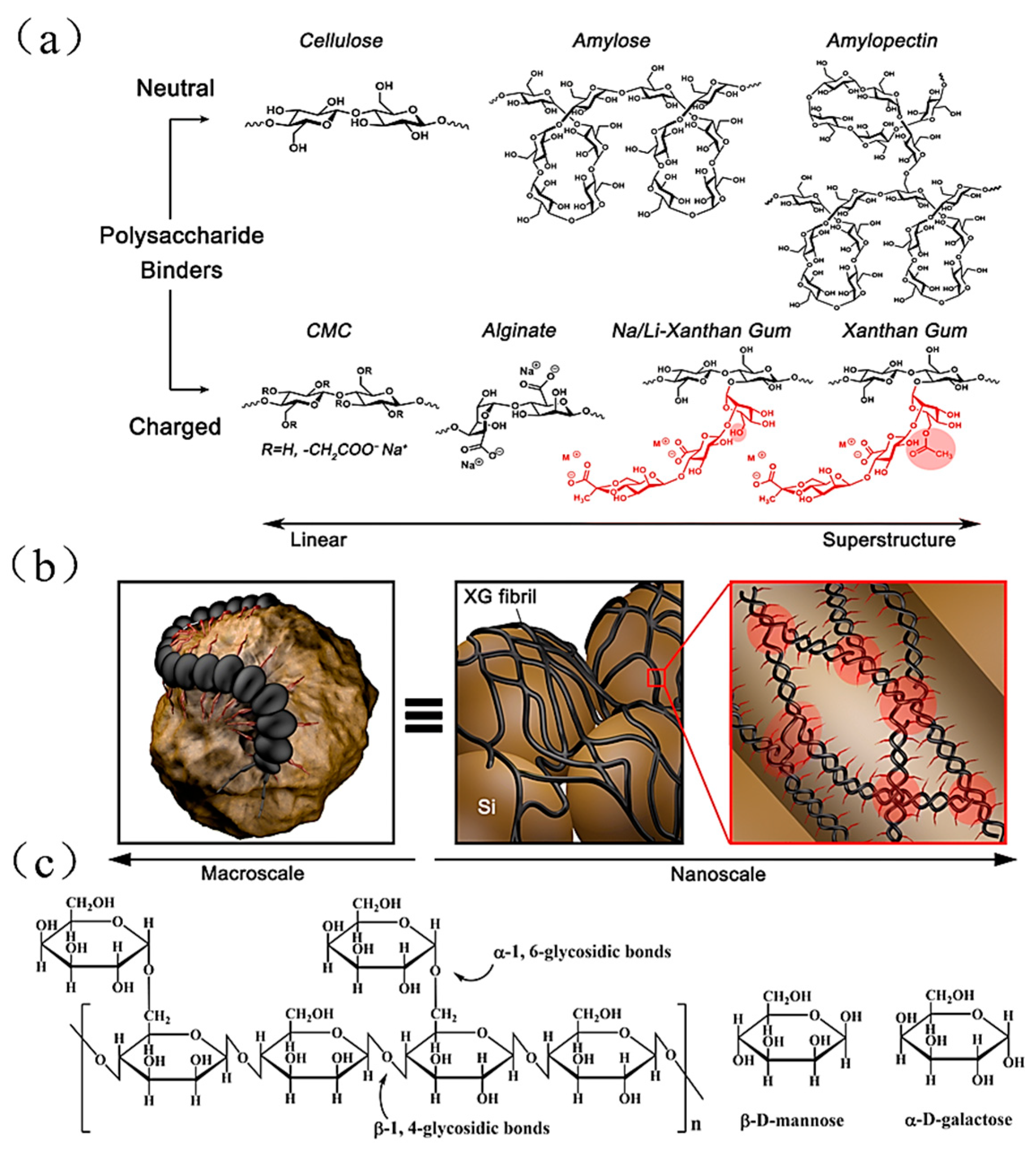
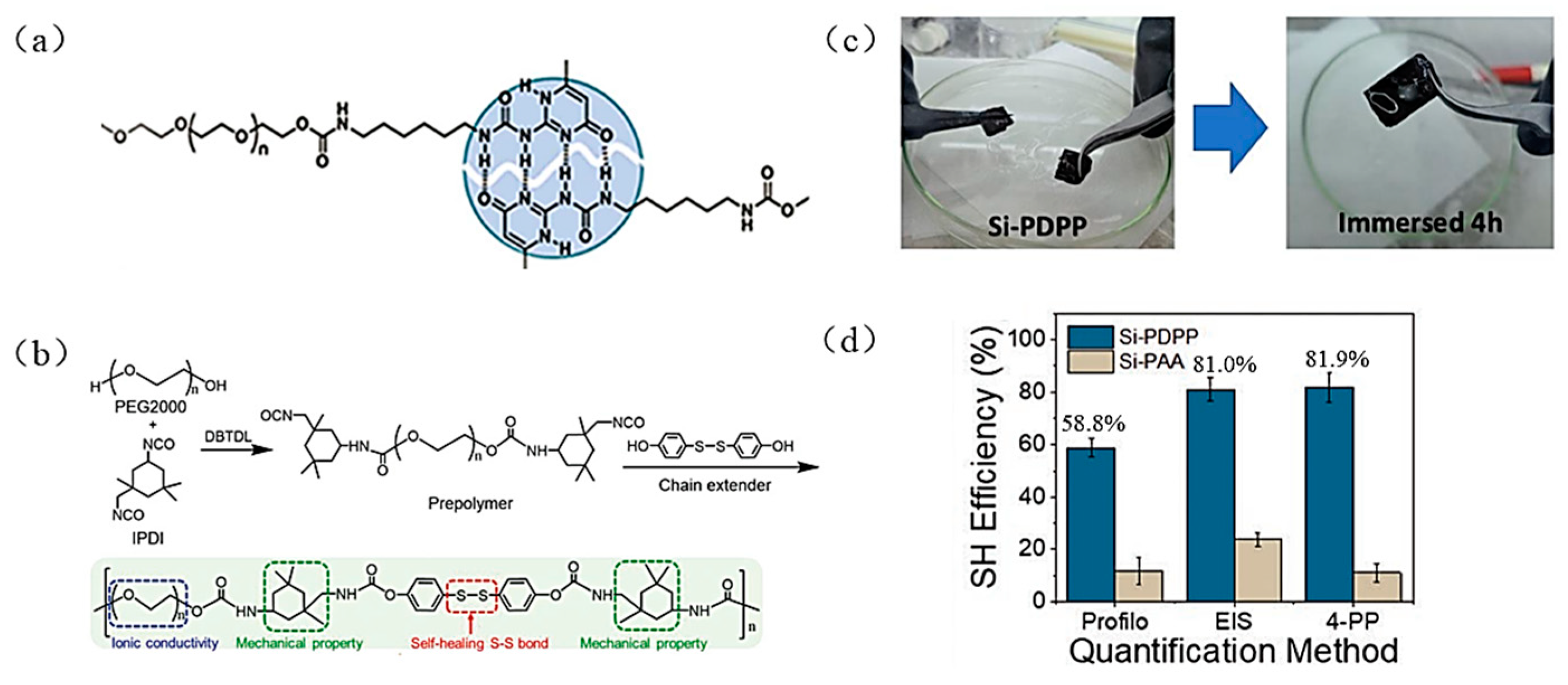
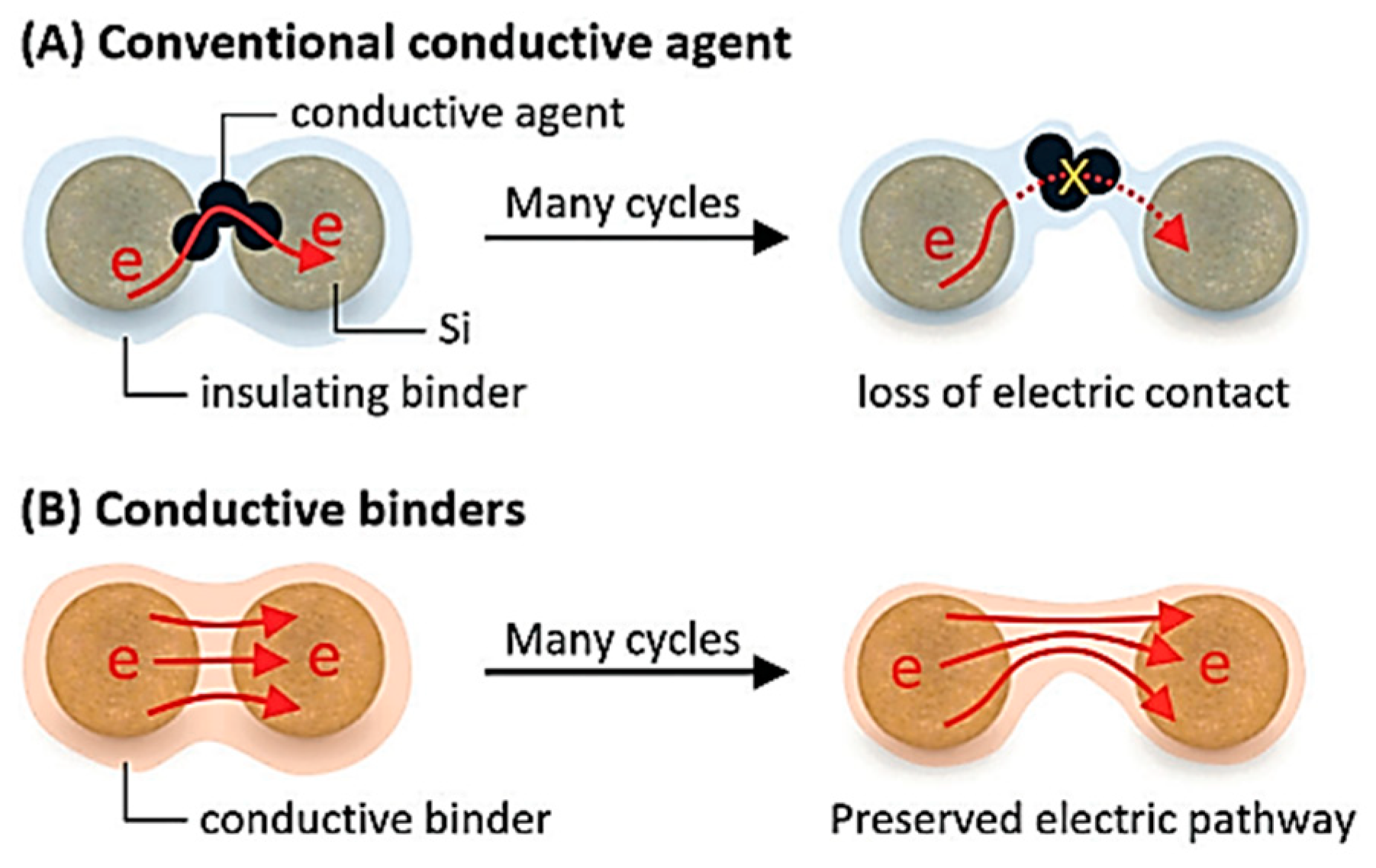
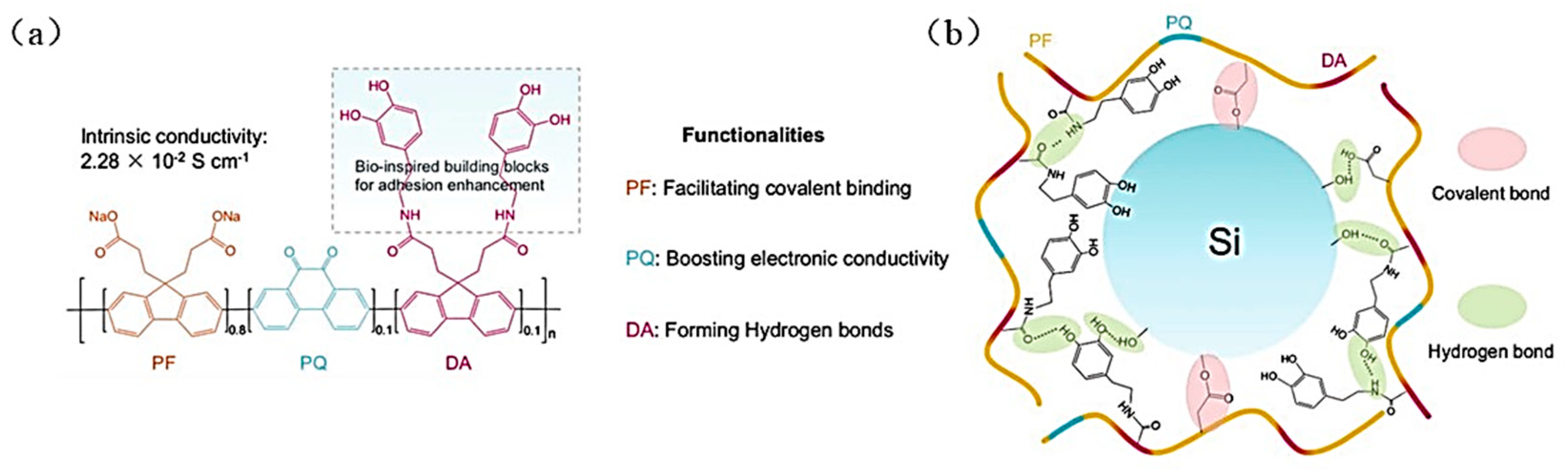
| Suggestion | Specific Implement Scheme | Literature |
|---|---|---|
| Active-inactive alloy system, Silicon nanoparticles, Silicon nanowires, Silicon nanotubes, Si/C composites, SiOx materials, etc. | [12,20,22,23,24,25,26,27,28,29] |
| Fluoroethylene carbonate (FEC), Phosphorous-containing compounds, etc. | [17,18] |
| Polyvinylidene fluoride (PVDF), Poly (acrylic acid) (PAA), Sodium carboxymethylcellulose (CMC), Alginate (Alg), Chitosan (CS), β-cyclodextrin polymer (β-CDp), Guar gum (GG), Self-healing polymer, Conducting polymers, etc. | [30,31,32,33,34,35,36,37,38,39] |
| Type of Binder | Active Material | Solvent | Binder: Conductive Agent: Active Material | Initial Coulomb Efficiency | Current Density (mA g−1) | Cycling Performance | Capacity Retention | Ref. |
|---|---|---|---|---|---|---|---|---|
| PVDF-b-PTFE | Si powder (1–5 um) | NMP | 5:15:80 | 82.70% | 4200 | ~1000 mAh g−1 after 250 cycles | - | [51] |
| Si-PAA-C | Si nanoparticles | Tris buffer solution | 20:20:60 | 71.20% | 2100 | ~1700 mAh g−1 after 400 cycles | 48.57% | [52] |
| PAA-PVA | Si nanoparticles (30–100 nm) | Water | 20:20:60 | 83.90% | 400 | 2283 mAh g−1 after 100 cycles | 63.14% | [53] |
| PAA-PEGPBI | Si nanoparticles /graphite (3:7) | NMP | 10:10:80 | 87.30% | 130 | 751.0 mAh g−1 after 100 cycles | 58.13% | [54] |
| PR-PAA | Si micron particles (~2 um) | DMSO | 10:10:80 | 91.22% | 600 | 2.43 mAh cm−2 after 150 cycles | 91% | [55] |
| NaPAA-g-CMC | Si nanoparticles (50–100 nm) | Water | 20:20:60 | 84% | 840 | 1816 mAh g−1 after 100 cycles | 79.30% | [56] |
| PAA-VTEO | Si/graphite | Water | 3:7:90 | 89.40% | 47 | 466 mAh g−1 after 100 cycles | 99.19% | [57] |
| PAA-SS | Si nanoparticles | Water | 20:20:60 | 93.20% | 4200 | 1559 mAh g−1 after 150 cycles | - | [33] |
| PE55 | Si nanoparticles (30–50 nm) | Water | 20:20:60 | 89.70% | 1000 | 2322.2 mAh g−1 after 100 cycles | - | [58] |
| c-CMC-CA | Core–Shell Carbon/Si | Water | 20:20:60 | 74% | 1000 | 1640 mAh g−1 after 100 cycles | 87.70% | [59] |
| CMC-PEG | Silicon powder (≤50 nm) | Water | 10:5:85 | 81% | 1786 | ~2000 mAh g−1 after 350 cycles | - | [60] |
| CMC/EDTA-Ca2+ | Si/graphite | Water | 15:7.5:77.5 | 81.10% | 500 | 776 mAh g−1 after 200 cycles | 87.00% | [31] |
| LiCMC-TA | Si nanoparticles (~100 nm) | Water | 1:9:90 | 80.65% | 1000 | 1701 mAh g−1 after 150 cycles | 80.00% | [61] |
| Ca-Alg | Si/C composite | Water | 29:18:53 | - | 420 | 1822 mAh g−1 after 120 cycles | 82.30% | [62] |
| Al/Ba-alg | Si nanoparticles | Water | 20:20:60 | - | 420 | ~2100 mAh g−1 after 300 cycles | - | [63] |
| PAA-SA | Si-Al alloys (Si:Al = 8:92) | Water | 22.5:7.5:70 | 80.10% | 1000 | ~1419.8 mAh g−1 after 200 cycles | 48.46% | [30] |
| CS/ENR | Si nanoparticles (~100 nm) | Water | 15:15:70 | 81.40% | 8000 | 1350 mAh g−1 after 1600 cycles | - | [64] |
| CS-EDTA | SiOx (Si:SiO2 = 1:2) | Water | 10:20:70 | 62.80% | 1000 | 721 mAh g−1 after 200 cycles | 78% | [65] |
| β-CDp/6AD | Si nanoparticles (~50 nm) | Water | 20:20:60 | 84% | 1500 | ~1500 mAh g−1 after 150 cycles | 90% | [66] |
| PAAS-β-CDp-PAA | Si nanoparticles | Water | 20:20:60 | 89.79% | 200 | 2513 mAh g−1 After 100 cycles | 71.10% | [39] |
| GG-g-PAM | Si nanoparticles | Water | 10:10:80 | 87.50% | 1000 | 1687 mAh g−1 after 200 cycles | 71.80% | [45] |
| GCA13 | Si nanoparticles (~100 nm) | Water | 15:5:80 | 93% | 2000 | 1184 mAh g−1 after 740 cycles | - | [36] |
| SG | Si nanoparticles (80–100 nm) | Water | 20:20:60 | 90.92% | 1000 | 2023 mAh g−1 after 120 cycles | - | [37] |
| UPy-PEG-UPy | Si nanoparticles (50–70 nm) | Tetrachloroethane | 15:25:60 | 81% | - | 1454 mAh g−1 after 400 cycles | 84% | [67] |
| PAA-BFPU | SiOx material | Water | 15:15:70 | ~89% | 1200 | ~3000 mAh g−1 after 100 cycles | 97% | [68] |
| PDPP | Si powder (<100 nm) | Water | 15:10:75 | ~94% | 2415 | 2312 mAh g−1 after 100 cycles | 84% | [38] |
| PAA–PANI | Si nanoparticles (~100 nm) | Water | 25:75 (no conductive agent) | - | 4200 | ~1118 mAh g−1 after 300 cycles | 56.50% | [69] |
| PEDOT:PSS | Si nanoparticles (50–70 nm) | Water | 10:10:80 | ~78% | 1000 | 1950 mAh g−1 after 100 cycles | ~75% | [70] |
| CG | Si nanoparticles | Water | 10:90 (no conductive agent) | 80% | 840 | 1500 mAh g−1 after 700 cycles | - | [71] |
| PPTU | Si nanoparticles | Water | 10:80 (no conductive agent) | 80.10% | 1000 | 2081 mAh g−1 after 300 cycles | ~76.7% | [72] |
| PFPQDA | Si nanoparticles (~50 nm) | Water | 10:20 (no conductive agent) | 72.30% | 420 | 2523 mAh g−1 after 150 cycles | 96% | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Wang, Q.; Chen, Z.; Rong, C.; Chao, D. Application and Development of Silicon Anode Binders for Lithium-Ion Batteries. Materials 2023, 16, 4266. https://doi.org/10.3390/ma16124266
Shen H, Wang Q, Chen Z, Rong C, Chao D. Application and Development of Silicon Anode Binders for Lithium-Ion Batteries. Materials. 2023; 16(12):4266. https://doi.org/10.3390/ma16124266
Chicago/Turabian StyleShen, Huilin, Qilin Wang, Zheng Chen, Changru Rong, and Danming Chao. 2023. "Application and Development of Silicon Anode Binders for Lithium-Ion Batteries" Materials 16, no. 12: 4266. https://doi.org/10.3390/ma16124266
APA StyleShen, H., Wang, Q., Chen, Z., Rong, C., & Chao, D. (2023). Application and Development of Silicon Anode Binders for Lithium-Ion Batteries. Materials, 16(12), 4266. https://doi.org/10.3390/ma16124266






