Modified Natural Diatomite with Various Additives and Its Environmental Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Diatomite
2.2. Materials Used as Additives
2.3. Procedure for Preparing Test Mixtures
2.4. Chemical Analyses in Designed Materials
2.5. Physical Analyses in Designed Materials
- —the volume of micropores
- —the volume of mesopores
2.6. Ecotoxicity Analyses in Designed Materials
2.7. Statistical Analysis
3. Results
3.1. pH and Electrical Conductivity of DTs and Mixtures
3.2. Content and Mobility of Selected Heavy Metals in DTs and Mixtures
3.3. Specific Surface Area and Porosity of DTs and Mixtures
3.4. Ecotoxicity of DTs and Mixtures
4. Discussion
5. Conclusions
- The use of unenriched DTs poses the risk of releasing heavy metals into the environment.
- Enrichment of the DTs with BC and DL resulted in a reduction or absence of Cd, Zn, Pb and Ni in aqueous extracts.
- It was found that for SBET values, the type of DT additive used was of crucial importance. The results of nitrogen sorption/desorption experiments on samples of DTs and their mixtures with BC, DL and BN showed no significant differences depending on the DT grain fraction used.
- The reduction in DT toxicity has been proven under the influence of various additives. Taking into account the response of all organisms, DTs + DL and DTs + BN mixtures are classified as not posing an environmental risk. DT2 + BC and DT3 + BC mixtures were the most toxic to test organisms. These samples were classified as class IV (very toxic).
- The proposed experiment confirms the legitimacy of diatomite enrichment before its environmental use.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Li, G.; Chen, D.; Gao, K.; Cao, Y.; Zhou, Y.; Mao, Y.; Fan, S.; Tang, L.; Jia, H. The Effects of Diatomite as an Additive on the Macroscopic Properties and Microstructure of Concrete. Materials 2023, 16, 1833. [Google Scholar] [CrossRef] [PubMed]
- Akoto, O.; Gyimah, E.; Zhan, Z.; Xu, H.; Nimako, C. Evaluation of health risks associated with trace metal exposure in water from the Barekese reservoir in Kumasi, Ghana. Hum. Ecol. Risk Assess. 2020, 26, 1134–1148. [Google Scholar] [CrossRef]
- Bashir, S.; Adeel, M.; Gulshan, A.B.; Iqbal, J.; Khan, S.; Rehman, M.; Azeem, M. Effects of organic and inorganic passivators on the immobilization of cadmium in contaminated soils. A review. Environ. Eng. Sci. 2019, 36, 986–998. [Google Scholar] [CrossRef]
- Ye, X.; Kang, S.; Wang, H.; Li, H.; Zhang, Y.; Wang, G.; Zhao, H. Modified natural diatomite and its enhanced immobilization of lead, copper and cadmium in simulated contaminated soil. J. Hazard. Mater. 2015, 289, 210–218. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Bajda, T.; Kopeć, M. Zastosowanie biowęgla i zeolitu jako adsorbentów zanieczyszczeń mineralnych. Przemysł Chem. 2019, 98, 1969–1972. (In Polish) [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Hayyat, A.; Javed, M.; Rasheed, I.; Ali, S.; Shahid, M.J.; Rizwan, M.; Javed, M.T.; Ali, Q. Role of biochar in remediating heavy metals in soil. In Phytoremediation; Springer: Cham, Switzerland, 2016; pp. 421–437. [Google Scholar]
- Kaleta, J.; Papciak, D.; Puszkarewicz, A. Clinoptylolite and diatomite respect of their usefulness for water conditioning and wastewater purification. Miner. Resour. Manag. 2007, 23, 21–34. [Google Scholar]
- Ikusika, O.O.; Mpendulo, C.T.; Zindove, T.J.; Okoh, A.I. Fossil shell flour in livestock production: A review. Animals 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Aksakal, E.L.; Angin, I.; Oztas, T. Effects of diatomite on soil physical properties. Catena 2012, 88, 1–5. [Google Scholar] [CrossRef]
- Song, X.; Li, C.; Zhu, Y.; Yang, Y.; Chen, M.; Ma, R.; Ling, X.; Wu, J. Application of diatomite for gallic acid removal from molasses wastewater. Sci. Total Environ. 2021, 765, 142711. [Google Scholar] [CrossRef]
- Sanad, M.S.; Gaber, S.E.; El-Aswar, E.I.; Farahat, M. Graphene-magnetite functionalized diatomite for efficient removal of organochlorine pesticides from aquatic environment. J. Environ. Manag. 2023, 330, 117145. [Google Scholar] [CrossRef]
- Lin, A.J. Sustainable in-situ remediation of heavy metal contaminated soils: Recent advances in biomass-based remediation materials. Chin. J. Environ. Eng. 2019, 13, 2025–2026. [Google Scholar]
- Gao, L.D.; Wu, C.Y.H. Remediation technology and research progress of heavy metal contaminated soil. J. Environ. Dev. 2020, 32, 38–39. [Google Scholar]
- Zhang, Y.P.; Tan, X.X.; Chen, X.Y.; Liang, J.H.; Ma, C.J.; Guo, Y.J.; Zhou, J.B. Study on the passivation effect of Ca-Si soil conditioner on heavy metal absorption by rice. J. Agric. Biotechnol. 2020, 9, 104–106. [Google Scholar]
- Li, Z.; Chen, L.; Chen, Z.; Chen, G.; Zhou, J.; Liu, X. Study of effect on Mn, Pb, and Zn solidification in soil by a Mied curing agent of modified diatomite. ACS Omega 2022, 4, 25229–25238. [Google Scholar] [CrossRef]
- Gondek, K.; Mierzwa-Hersztek, M.; Kopeć, M.; Bajda, T. Phytostabilization on post-flotation sediment waste: Mobility of heavy metals and stimulation of biochemical processes by mineral-organic mixtures. J. Soils Sediments 2020, 20, 3502–3513. [Google Scholar] [CrossRef]
- Gondek, K.; Mierzwa-Hersztek, M.; Kopeć, M. Mobility of heavy metals in sandy soil after application of composts produced from maize straw, sewage sludge and biochar. J. Environ. Manag. 2018, 210, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1060, 60, 235–241. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances II. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Phytotoxkit. Seed Germination and Early Growth Microbiotest with Higher Plants. Standard Operational Procedure; MicroBioTest Inc.: Nazareth, Belgium, 2004; p. 24. [Google Scholar]
- Ostracodtoxkit, F. Direct Contact Toxicity Test for Freshwater Sediments. Standard Operational Procedure; MicroBioTest Inc.: Nazareth, Belgium, 2001; p. 35. [Google Scholar]
- Microbics Corporation. Microtox Manual Toxicity Testing Handbook; U.S. Microbics, Inc.: Carlsbad, CA, USA, 1992. [Google Scholar]
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nalecz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L.; et al. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef]
- Singh, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas solid systems with special reference to the determination of surface-area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Perez-Ramirez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mat. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Alsar, Z.; Duskinova, B.; Insepov, Z. New sorption properties of diatomaceous earth for water desalination and reducing salt stress of palnts. Eurasian Chem. Technol. J. 2020, 22, 89–97. [Google Scholar] [CrossRef]
- Danil de Namor, A.F.; El Gamouz, A.; Frangie, S.; Martinez, V.; Valiente, L.; Webb, O.A. Turning the Volume Down on Heavy Metals Using Tuned Diatomite. A Review of Diatomite and Modified Diatomite for the Extraction of Heavy Metals from Water. J. Hazard. Mater. 2012, 241–242, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Lamastra, F.R.; De Angelis, R.; Antonucci, A.; Salvatori, D.; Prosposito, P.; Casalboni, M.; Congestri, R.; Melino, S.; Nanni, F. Polymer Composite Random Lasers Based on Diatom Frustules as Scatterers. RSC. Adv 2014, 4, 61809. [Google Scholar] [CrossRef]
- Uthappa, U.; Brahmkhatri, V.; Sriram, G.; Jung, H.-Y.; Yu, J.; Kurkuri, N.; Aminabhavi, T.M.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Nature Engineered Diatom Biosilica as Drug Delivery Systems. Artic. J. Control. Release 2018, 281, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhang, J.; Tan, B.; Wang, Q.; Liu, N.; Xue, Q. New insight into the removal of Cd(II) from aolution by diatomite. Environ. Sci. Pollut. Res. Int. 2020, 27, 9882–9890. [Google Scholar] [CrossRef] [PubMed]
- Dobor, J.; Perényi, K.; Varga, I.; Varga, M. A new carbon-diatomite earth composite adsorbent for removal of heavy metals from aqueous solution and novel application idea. Microporous Mesoporous Mater. 2015, 217, 63–70. [Google Scholar] [CrossRef]
- Hanna, A.A.; Sherif, M.; Aboelenin, R. Removal of some heavy metals from wastewater by using doiatomaceous earth. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 198–205. [Google Scholar]
- Andrunik, M.; Bajda, T. Modification of bentonite with cationic and nonionic surfactants: Structural and textural features. Materials 2019, 12, 3772. [Google Scholar] [CrossRef] [Green Version]
- Casado-Martinez, M.C.; Burga-Perez, K.F.; Bebon, R.; Ferard, J.-F.; Vermeirssen, E.L.M.; Werner, I. The sediment-contact test using the ostracod Heterocypris incongruens: Effect of fine sediments and determination of toxicity thresholds. Chemosphere 2016, 151, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Cooman, W.; Blaise, C.; Janssen, C.R.; Detemmerman, L.; Elst, R.; Persoone, G. History and sensitivity comparison of two standard whole-sediment toxicity tests with crustaceans: The amphipod Hyalella azteca and the ostracod Heterocypris incongruens microbiotest. Knowl. Manag. Aquat. Ecosyst. 2015, 416, 15. [Google Scholar] [CrossRef] [Green Version]
- Mariani, L.; Grenni, P.; Caracciolo, A.; Pescatore, T.; Spataro, F.; Rauseo, J.; Narciso, A.; Rolando, L.; Patrolecco, L. Use of the Heterocypris incongruens bioassay for assessing ecotoxicity of soils containing the anionic surfactant sodium lauryl ether sulphate (SLES). Ecol. Indic. 2022, 145, 109597. [Google Scholar] [CrossRef]
- Korunic, Z. Diatomaceous earths, a group of natural insecticides. J. Stored Prod. Res. 1998, 34, 87–97. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Peteinatos, G.G.; Boukouvala, M.C.; Benelli, G. Insecticidal effect and impact of fitness of three diatomaceous earths on different maize hybrids for the eco-friendly control of the invasive storedproduct pest Prostephanus truncatus (Horn). Environ. Sci. Pollut. Res. 2018, 25, 10407–10417. [Google Scholar] [CrossRef] [PubMed]
- Barroso, P.M.; Vaverková, M.D.; Elbl, J. Assessing the ecotoxicity of soil affected by wildfire. Environments 2021, 8, 3. [Google Scholar] [CrossRef]
- Scherer, C.; Wolf, R.; Völker, J.; Stock, F.; Brennhold, N.; Reifferscheid, G.; Wagner, M. Toxicity of microplastics and natural particles in the freshwater dipteran Chironomus riparius: Same but different? Sci. Total Environ. 2020, 711, 134604. [Google Scholar] [CrossRef]
- Visioli, G.; Conti, F.D.; Menta, C.; Bandiera, M.; Malcevschi, A.; Jones, D.L.; Vamerali, T. Assessing biochar ecotoxicology for soil amendment by root phytotoxicity bioassays. Environ. Monit. Assess. 2016, 188, 166. [Google Scholar] [CrossRef] [Green Version]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef]
- Heise, S.; Babut, M.; Casado, C.; Feiler, U.; Ferrari, B.J.D.; Marziali, L. Ecotoxicological testing of sediments and dredged material: An overlooked opportunity? J. Soils Sediments 2020, 20, 4218–4228. [Google Scholar] [CrossRef]
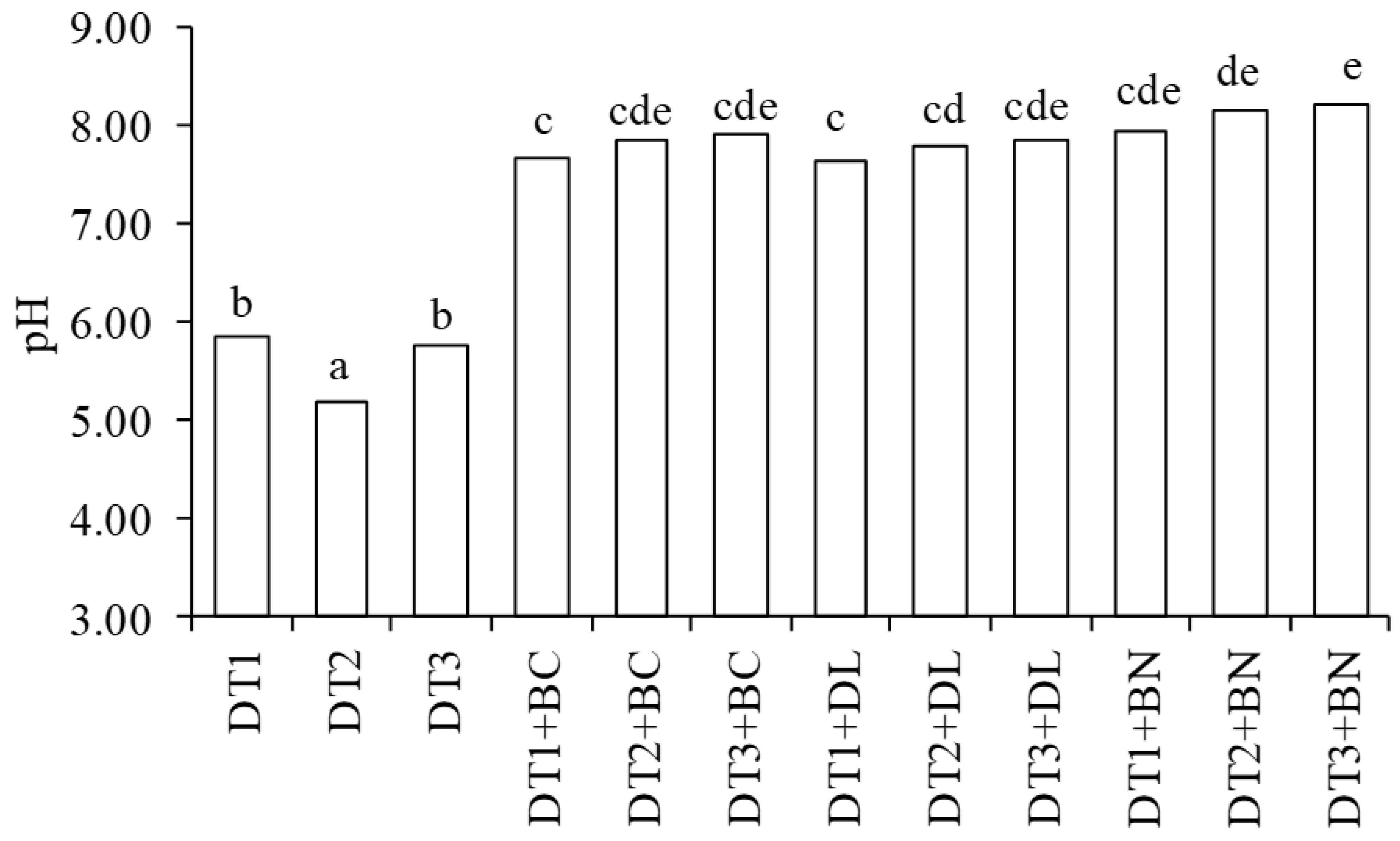
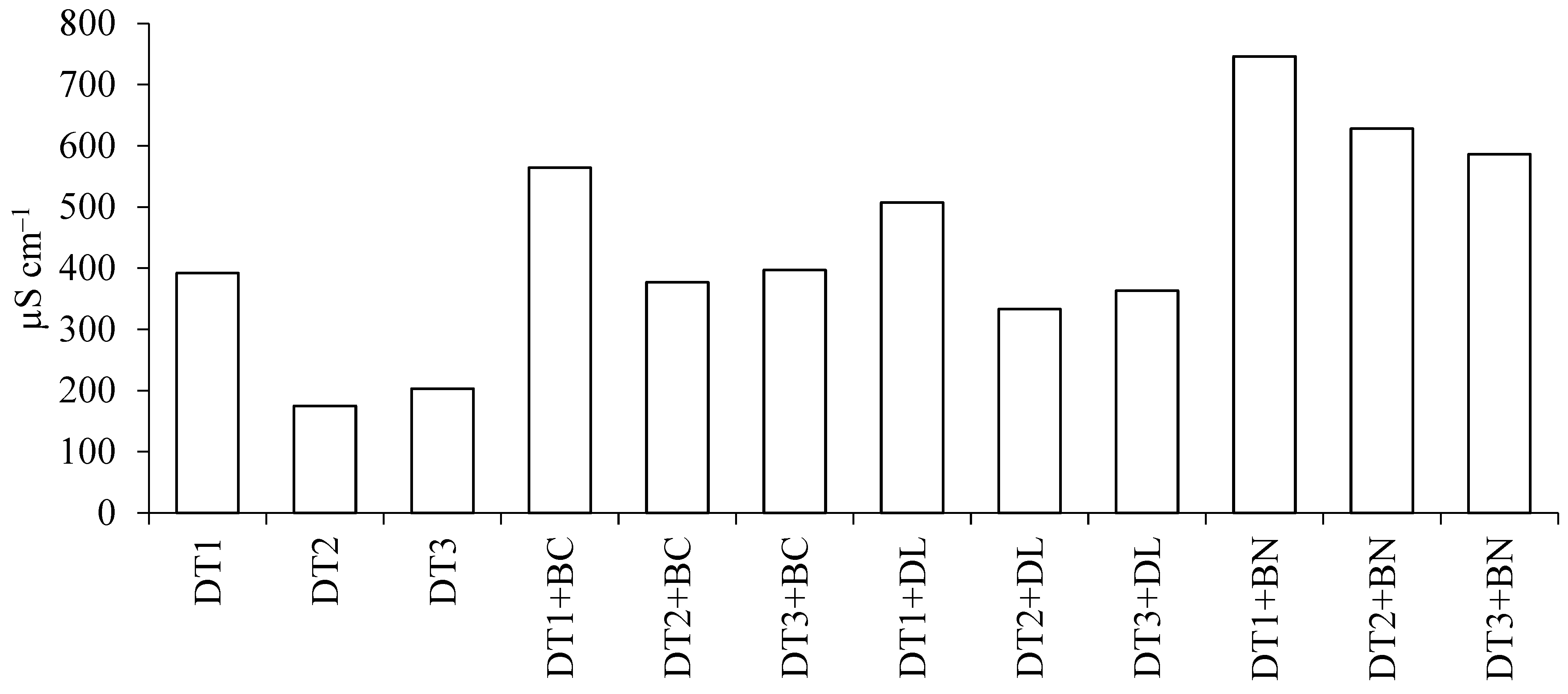
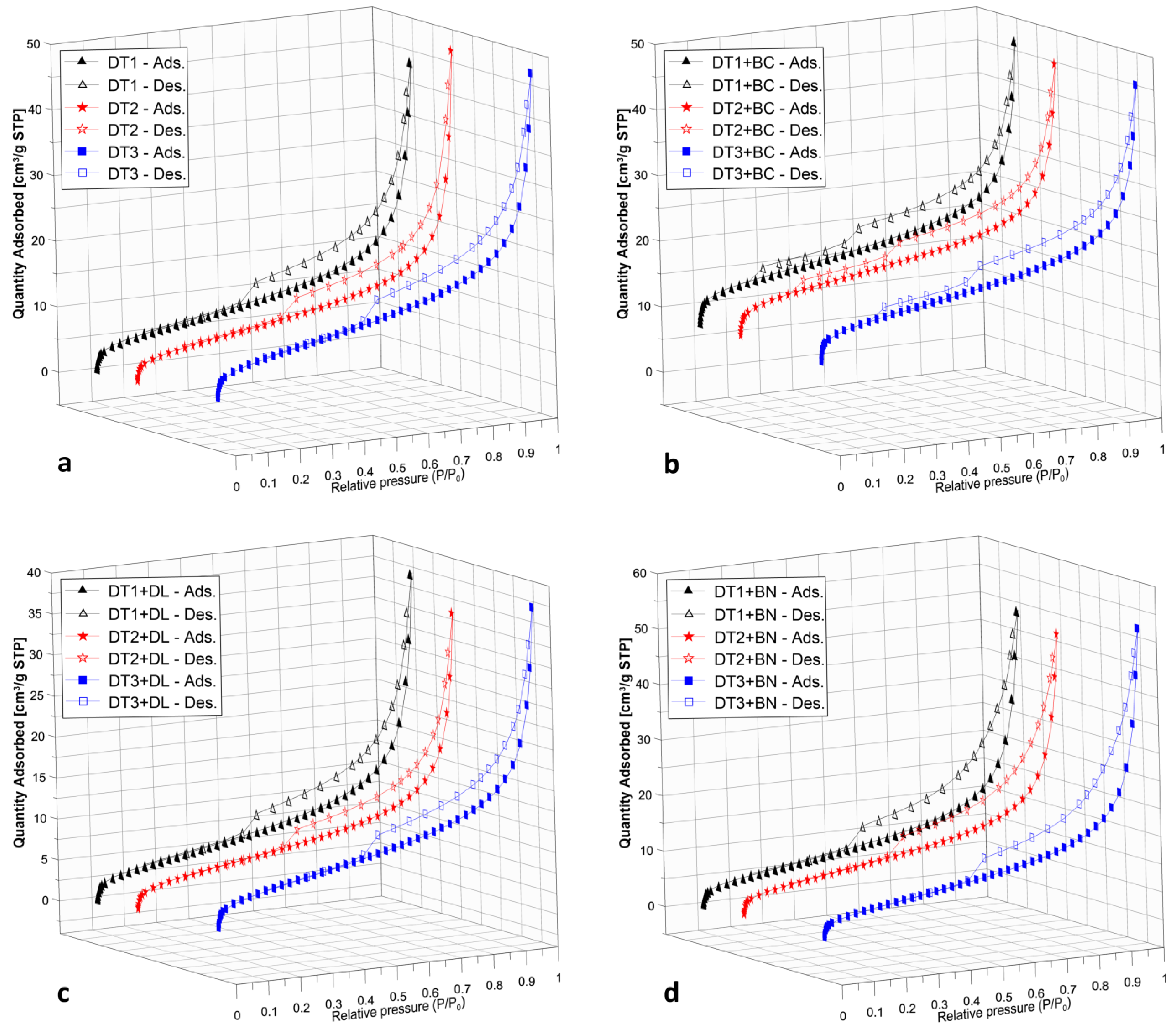
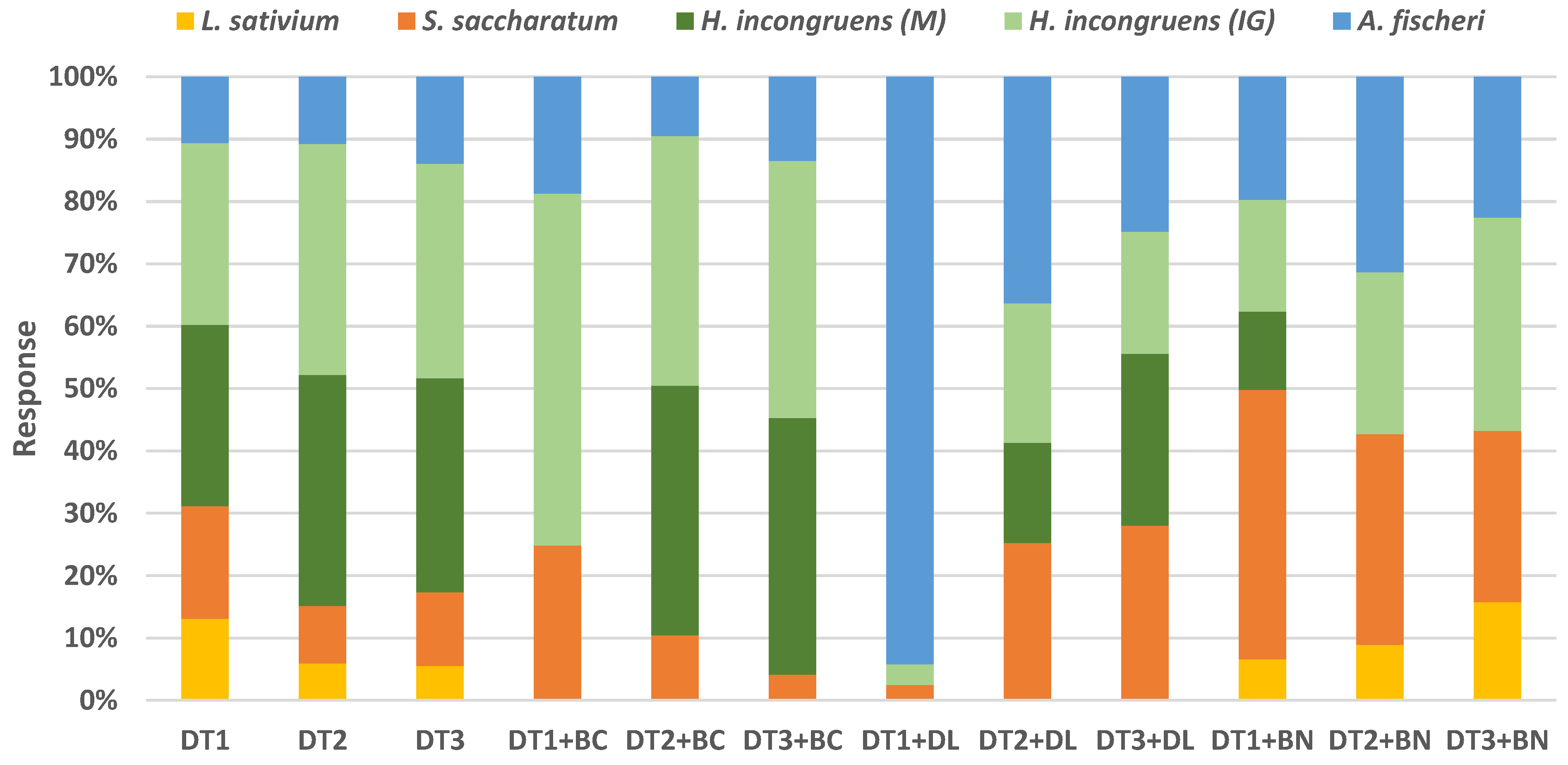
| Material * | Dry Matter g·kg−1 | Ash g·kg−1 | Loss on Ignition g·kg−1 | pH H2O | EC ** µS·cm−1 | BET Surface Area m2·g−1 | Total Pore Volume cm3·g−1 |
|---|---|---|---|---|---|---|---|
| DT1 | 855 ± 3 | 808 ± 3 | 192 ± 3 | 5.84 ± 0.05 | 392 ± 1 | 25.9 ± 0.9 | 0.064 ± 0.002 |
| DT2 | 937 ± 4 | 891 ± 4 | 109 ± 4 | 5.19 ± 0.11 | 175 ± 2 | 24.6 ± 0.9 | 0.062 ± 0.002 |
| DT3 | 932 ± 4 | 879 ± 2 | 121 ± 2 | 5.76 ± 0.17 | 203 ± 6 | 31.3 ± 1.1 | 0.067 ± 0.002 |
| BC | 952 ± 4 | 99 ± 0 | 901 ± 0 | 7.74 ± 0.02 | 330 ± 2 | 185.6 ± 6.6 | 0.088 ± 0.003 |
| DL | 942 ± 4 | 915 ± 3 | 85 ± 3 | 7.87 ± 0.04 | 107 ± 1 | 3.0 ± 0.1 | 0.009 ± 0.000 |
| BN | 949 ± 4 | 881 ± 3 | 119 ± 3 | 9.31 ± 0.01 | 842 ± 8 | 36.2 ± 1.3 | 0.115 ± 0.004 |
| Material | Zn | Pb | Cd | Cu | Ni |
|---|---|---|---|---|---|
| mg·kg−1 | |||||
| DT1 | 40.7 ± 1.2 | 14.06 ± 0.08 | 0.34 ± 0.06 | 45.3 ± 0.8 | 21.67 ± 0.73 |
| DT2 | 38.9 ± 1.1 | 12.57 ± 0.05 | 0.14 ± 0.00 | 44.4 ± 0.4 | 17.38 ± 0.58 |
| DT3 | 36.8 ± 0.7 | 11.43 ± 0.04 | 0.16 ± 0.01 | 47.6 ± 1.9 | 17.52 ± 0.57 |
| BC | 30.0 ± 1.3 | 2.26 ± 0.40 | 4.95 ± 0.01 | 14.4 ± 2.3 | 18.77 ± 3.02 |
| DL | 38.8 ± 1.9 | 11.40 ± 0.07 | 1.89 ± 0.19 | 13.2 ± 0.7 | 1.61 ± 0.15 |
| BN | 28.6 ± 2.3 | 14.56 ± 0.05 | 1.06 ± 0.03 | 21.6 ± 2.1 | 1.13 ± 0.03 |
| Material | Treatment |
|---|---|
| DT1 | 100 g·kg−1 |
| DT2 | |
| DT3 | |
| DT1 + BC | DT1 (75 g·kg−1) + BC (25 g·kg−1) |
| DT2 + BC | DT2 (75 g·kg−1) + BC (25 g·kg−1) |
| DT3 + BC | DT3 (75 g·kg−1) + BC (25 g·kg−1) |
| DT1 + DL | DT1 (75 g·kg−1) + DL (25 g·kg−1) |
| DT2 + DL | DT2 (75 g·kg−1) + DL (25 g·kg−1) |
| DT3 + DL | DT3 (75 g·kg−1) + DL (25 g·kg−1) |
| DT1 + BN | DT1 (75 g·kg−1) + BN (25 g·kg−1) |
| DT2 + BN | DT2 (75 g·kg−1) + BN (25 g·kg−1) |
| DT3 + BN | DT3 (75 g·kg−1) + BN (25 g·kg−1) |
| Material | Cd | Zn | Pb | Cu | Ni |
|---|---|---|---|---|---|
| mg·kg−1 | |||||
| DT1 | 0.085 a | 3.802 c | 0.276 a | 0.137 b | 3.654 f |
| DT2 | 0.013 b | 0.746 a | 0.206 a | 0.005 a | 0.551 e |
| DT3 | nd | 0.271 a | 0.204 a | 0.018 ab | 0.206 bc |
| DT1 + BC | nd | 0.094 a | 0.201 a | 0.009 a | 0.177 ab |
| DT2 + BC | nd | 0.103 a | 0.188 a | 0.015 a | 0.108 a |
| DT3 + BC | nd | 0.107 a | 0.175 a | 0.025 ab | 0.110 a |
| DT1 + DL | nd | 0.164 a | 0.192 a | 0.007 a | 0.294 c |
| DT2 + DL | nd | 0.157 a | 0.190 a | 0.032 ab | 0.179 ab |
| DT3 + DL | nd | 0.165 a | 0.178 a | 0.036 ab | 0.173 ab |
| DT1 + BN | nd | 2.565 b | 0.910 b | 0.473 c | 0.585 e |
| DT2 + BN | nd | 4.222 c | 1.416 c | 0.792 d | 0.450 d |
| DT3 + BN | nd | 3.679 c | 1.250 c | 0.698 d | 0.411 d |
| Material | Cd | Zn | Pb | Cu | Ni | |
|---|---|---|---|---|---|---|
| DT1 | Share of total (%) | 25.20 | 9.35 bc | 1.96 ab | 0.30 b | 16.85 d |
| IM (%) | - | - | - | - | - | |
| DT2 | Share of total (%) | 8.94 | 1.92 | 1.64 | 0.01 | 3.17 bc |
| IM (%) | - | - | - | - | - | |
| DT3 | Share of total (%) | - | 0.74 a | 1.79 ab | 0.04a | 1.18 ab |
| IM (%) | - | - | - | - | - | |
| DT1 + BC | Share of total (%) | - | 0.25 a | 1.95 ab | 0.02 a | 0.77 a |
| IM (%) | - | 98 | 27 | 93 | 95 | |
| DT2 + BC | Share of total (%) | - | 0.28 a | 2.03 b | 0.04 a | 0.56 a |
| IM (%) | - | 86 | 8 | −201 | 80 | |
| DT3 + BC | Share of total (%) | - | 0.30 a | 1.95 ab | 0.07 a | 0.57 a |
| IM (%) | - | 61 | 14 | −42 | 47 | |
| DT1 + DL | Share of total (%) | - | 0.15 a | 0.60 a | 0.02 a | 2.07 b |
| IM (%) | - | 96 | 30 | 95 | 92 | |
| DT2 + DL | Share of total (%) | - | 0.12 a | 0.57 a | 0.09 a | 1.44 ab |
| IM (%) | - | 79 | 7 | −540 | 67 | |
| DT3 + DL | Share of total (%) | - | 0.13 a | 0.53 a | 0.10 a | 1.38 ab |
| IM (%) | - | 39 | 13 | −104 | 16 | |
| DT1 + BN | Share of total (%) | - | 6.67 b | 6.00 c | 1.30 c | 3.44 c |
| IM (%) | - | 33 | −230 | −246 | 84 | |
| DT2 + BN | Share of total (%) | - | 10.71 c | 10.59 d | 2.26 d | 3.14 bc |
| IM (%) | - | −466 | −589 | −15814 | 18 | |
| DT3 + BN | Share of total (%) | - | 10.64 c | 10.20 d | 2.00 d | 3.03 bc |
| IM (%) | - | −1259 | −512 | −3845 | −100 | |
| Material | SBET [m2 g−1] | [cm3 g−1] | [cm3 g−1] | [cm3 g−1] | [cm3 g−1] |
|---|---|---|---|---|---|
| DT1 | 25.9 ± 0.23 | 0.064 | 0.011 | 0.037 | 0.016 |
| DT2 | 24.6 ± 0.25 | 0.062 | 0.010 | 0.034 | 0.018 |
| DT3 | 31.3 ± 0.25 | 0.067 | 0.013 | 0.041 | 0.013 |
| DT1 + BC | 56.7 ± 0.83 | 0.067 | 0.022 | 0.035 | 0.010 |
| DT2 + BC | 55.6 ± 0.81 | 0.066 | 0.022 | 0.033 | 0.011 |
| DT3 + BC | 52.2 ± 0.83 | 0.064 | 0.021 | 0.035 | 0.008 |
| DT1 + DL | 19.4 ± 0.18 | 0.053 | 0.008 | 0.031 | 0.004 |
| DT2 + DL | 19.7 ± 0.22 | 0.046 | 0.008 | 0.027 | 0.011 |
| DT3 + DL | 23.5 ± 0.19 | 0.052 | 0.009 | 0.032 | 0.011 |
| DT1 + BN | 25.5 ± 0.22 | 0.072 | 0.010 | 0.043 | 0.019 |
| DT2 + BN | 27.1 ± 0.27 | 0.072 | 0.011 | 0.041 | 0.020 |
| DT3 + BN | 26.1 ± 0.23 | 0.076 | 0.011 | 0.045 | 0.020 |
| Material * | L. sativum | S. saccharatum | H. incongruens | A. fischeri | Class of Toxicity | |
|---|---|---|---|---|---|---|
| IGR% * | IGR% | M% | IG% | IL% | ||
| DT1 | 45 f | 62 f | 100 c | 100 e | 37 f | IV |
| DT2 | 16 de | 25 bcd | 100 c | 100 e | 29 d | IV |
| DT3 | 16 de | 35 cde | 100 c | 100 e | 41 g | IV |
| DT1 + BC | −5 ab | 20 abc | 0 a | 44 d | 15 a | II |
| DT2 + BC | −12 a | 26 bcd | 100 c | 100 e | 24 c | IV |
| DT3 + BC | −4 abc | 10 a | 100 c | 100 e | 33 de | IV |
| DT1 + DL | −14 a | 1 a | 0 a | 1 a | 19 b | I |
| DT2 + DL | −1 abc | 11 a | 7 ab | 9 ab | 15 a | I |
| DT3 + DL | −4 abc | 17 ab | 17 b | 12 ab | 15 a | I |
| DT1 + BN | 7 bcd | 46 e | 13 b | 19 bc | 21 b | II |
| DT2 + BN | 9 cde | 34 bcde | 0 a | 26 c | 32 de | II |
| DT3 + BN | 22 e | 38 de | 0 a | 47 d | 31 de | II |
| Parameter | Ls IGR ** | Ss IGR% | Hi M% | Hi IG% | Af IL% |
|---|---|---|---|---|---|
| Ss IGR | 0.74 * | ||||
| Hi M | 0.29 | 0.22 | |||
| Hi IG | 0.40 | 0.32 | 0.92 | ||
| Af IL | 0.62 | 0.50 | 0.63 | 0.70 | |
| pH | −0.54 | −0.32 | −0.66 | −0.61 | −0.51 |
| EC | −0.11 | 0.16 | −0.68 | −0.55 | −0.27 |
| Cd H2O | 0.25 | 0.11 | 0.58 | 0.44 | 0.08 |
| Cu H2O | 0.31 | 0.41 | −0.46 | −0.29 | 0.23 |
| Fe H2O | 0.23 | 0.35 | −0.48 | −0.31 | 0.20 |
| Mn H2O | 0.66 | 0.56 | 0.09 | 0.15 | 0.28 |
| Ni H2O | 0.75 | 0.65 | 0.31 | 0.30 | 0.39 |
| Pb H2O | 0.26 | 0.37 | −0.47 | −0.30 | 0.21 |
| Zn H2O | 0.66 | 0.67 | −0.19 | −0.05 | 0.43 |
| Cr H2O | 0.19 | 0.39 | −0.44 | −0.32 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gondek, K.; Micek, P.; Baran, A.; Bajda, T.; Kowal, J.; Lis, M.; Wyrobisz-Papiewska, A.; Wojtysiak, D.; Smoroń, K. Modified Natural Diatomite with Various Additives and Its Environmental Potential. Materials 2023, 16, 4494. https://doi.org/10.3390/ma16124494
Gondek K, Micek P, Baran A, Bajda T, Kowal J, Lis M, Wyrobisz-Papiewska A, Wojtysiak D, Smoroń K. Modified Natural Diatomite with Various Additives and Its Environmental Potential. Materials. 2023; 16(12):4494. https://doi.org/10.3390/ma16124494
Chicago/Turabian StyleGondek, Krzysztof, Piotr Micek, Agnieszka Baran, Tomasz Bajda, Jerzy Kowal, Marcin Lis, Anna Wyrobisz-Papiewska, Dorota Wojtysiak, and Krzysztof Smoroń. 2023. "Modified Natural Diatomite with Various Additives and Its Environmental Potential" Materials 16, no. 12: 4494. https://doi.org/10.3390/ma16124494
APA StyleGondek, K., Micek, P., Baran, A., Bajda, T., Kowal, J., Lis, M., Wyrobisz-Papiewska, A., Wojtysiak, D., & Smoroń, K. (2023). Modified Natural Diatomite with Various Additives and Its Environmental Potential. Materials, 16(12), 4494. https://doi.org/10.3390/ma16124494








