Low Temperature Chemoresistive Oxygen Sensors Based on Titanium-Containing Ti2CTx and Ti3C2Tx MXenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Application
- The ability to select a dispenser with the desired channel diameter, which reduces the likelihood of nozzle clogging with solid phase particles.
- High speed and simplicity of refilling the dispenser with inks which are different in composition.
- Variability in the film printing mechanism (discrete or continuous process), etc.
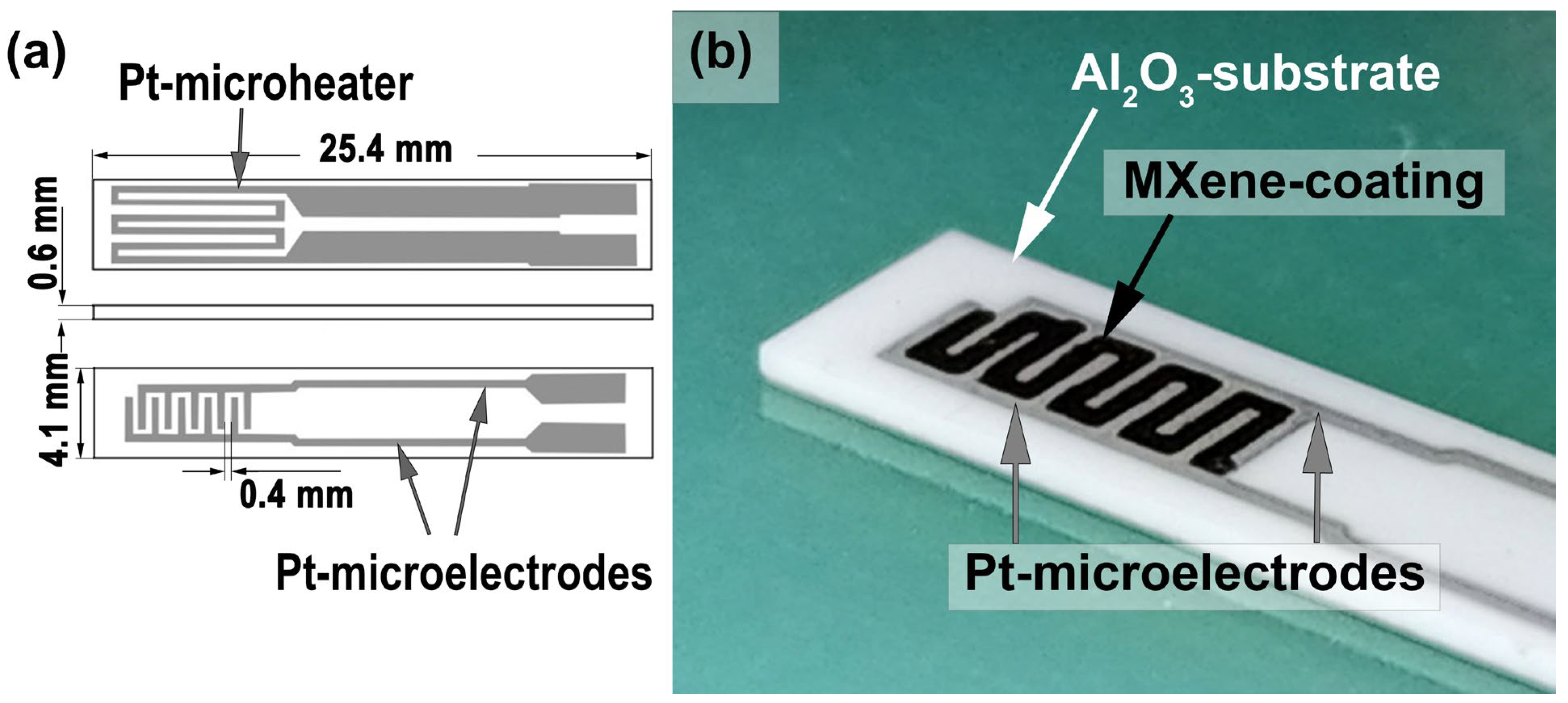
2.2. Instrumentation
3. Results and Discussion
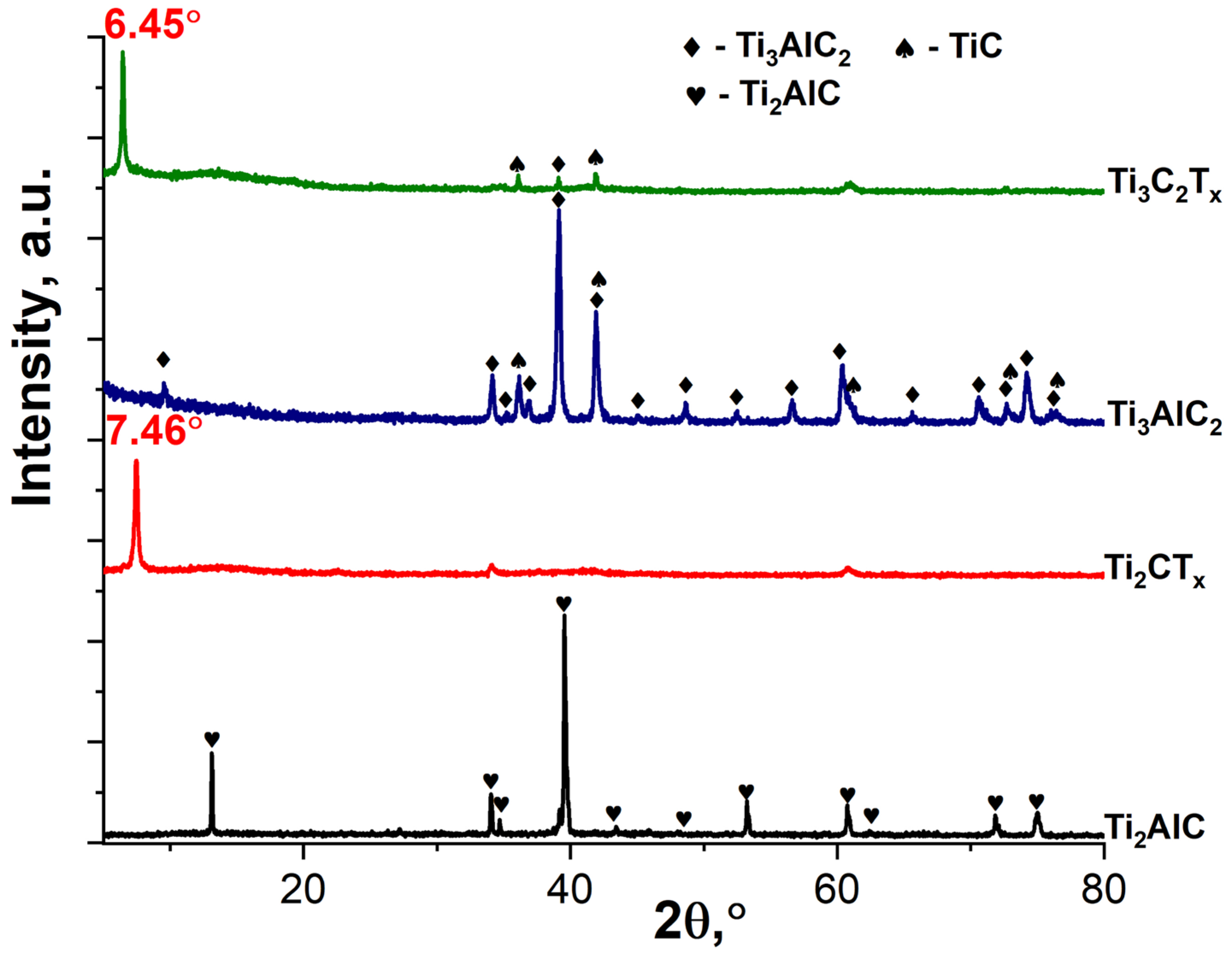
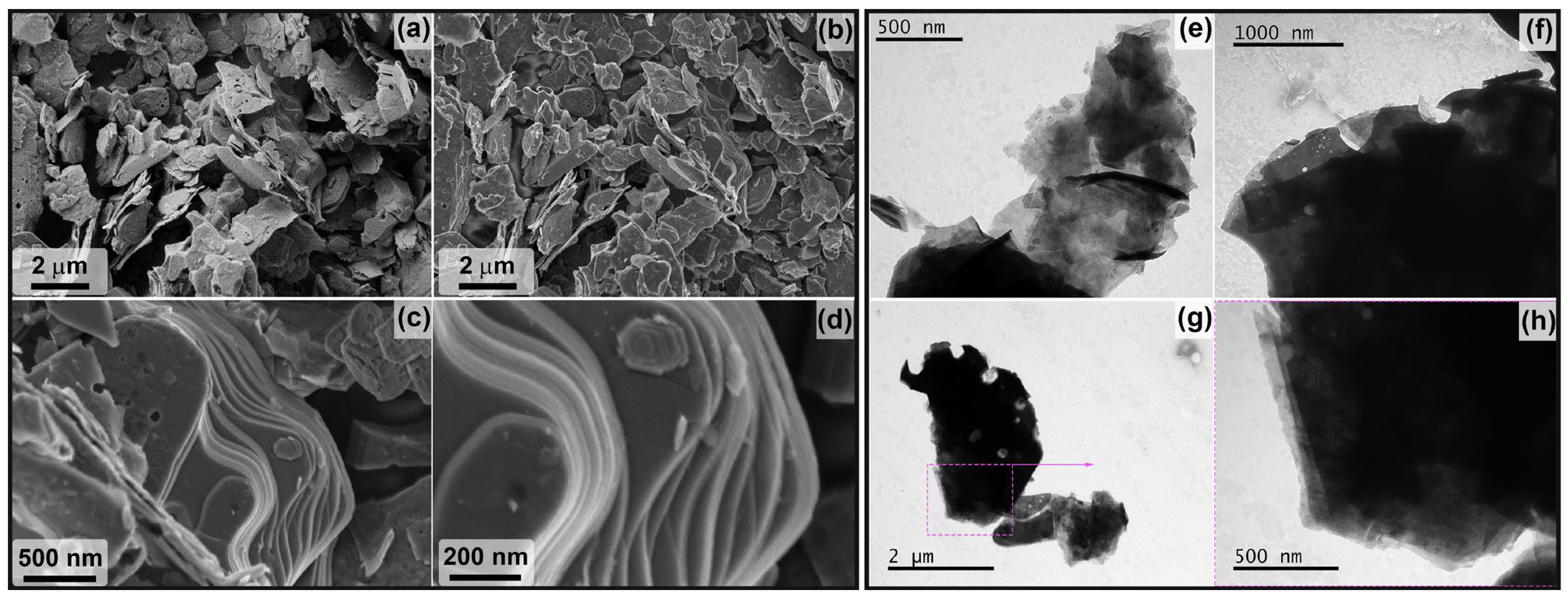
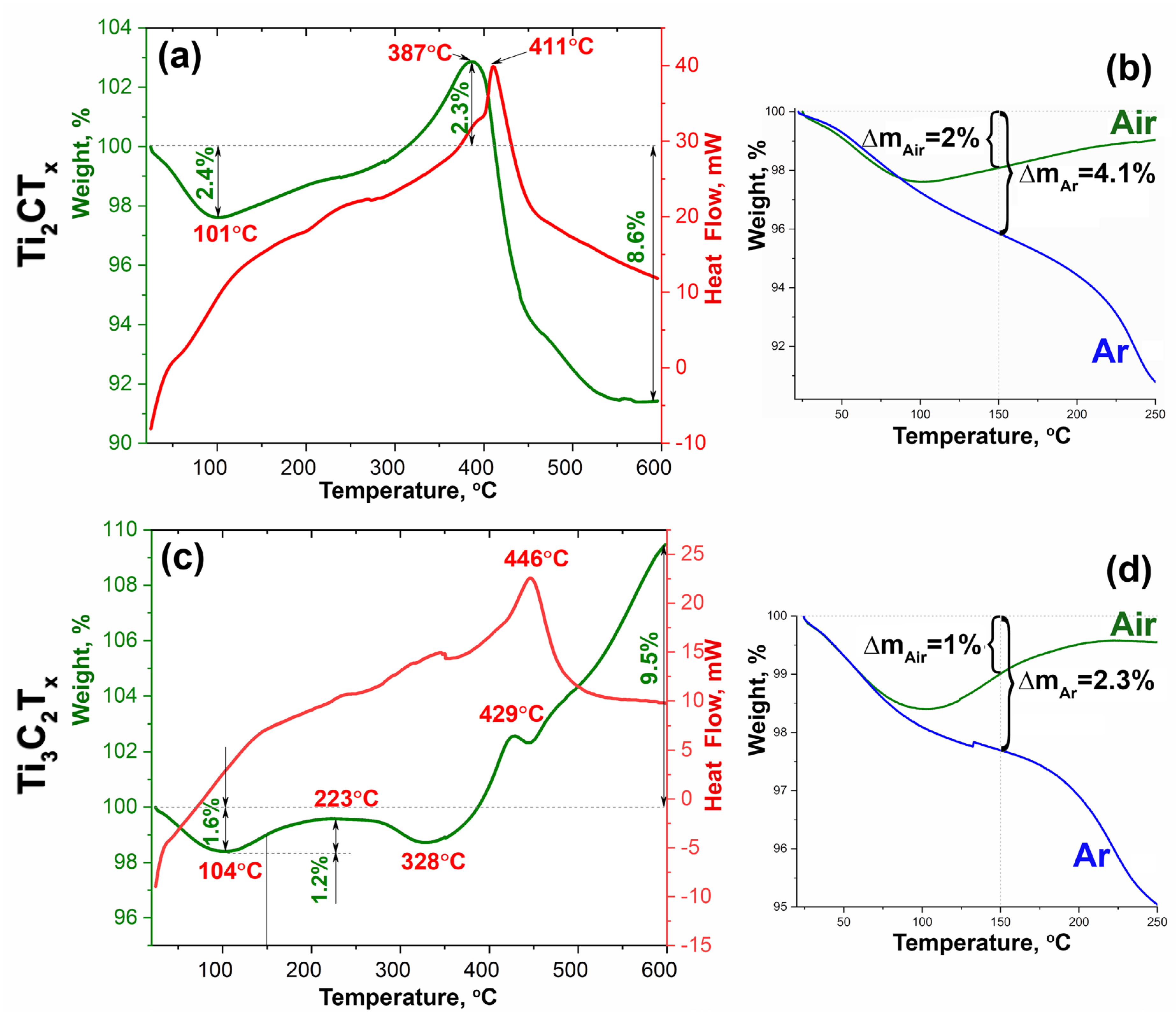
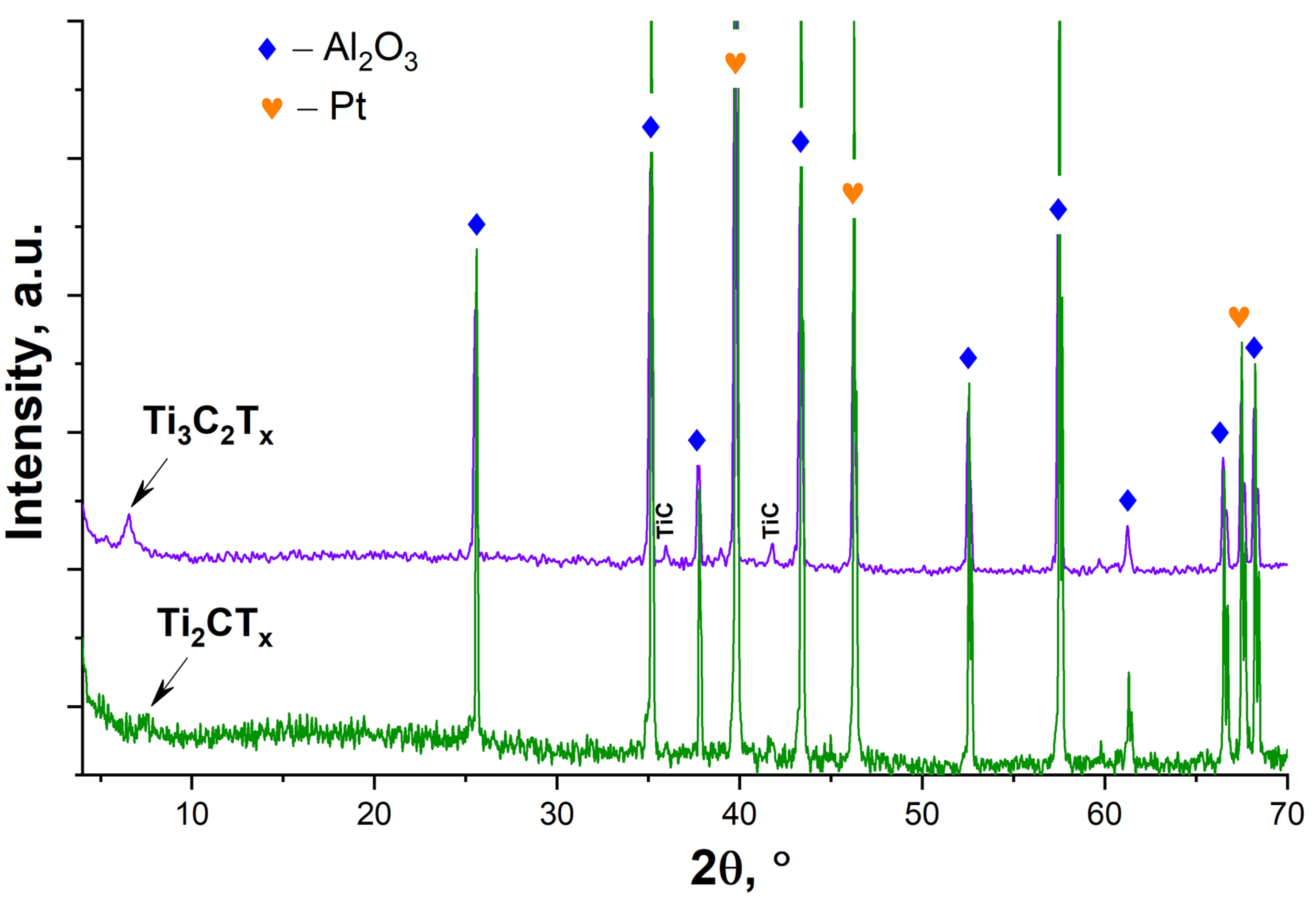
3.1. Study of the Obtained Multilayer Ti2CTx and Ti3C2Tx MXene Powders
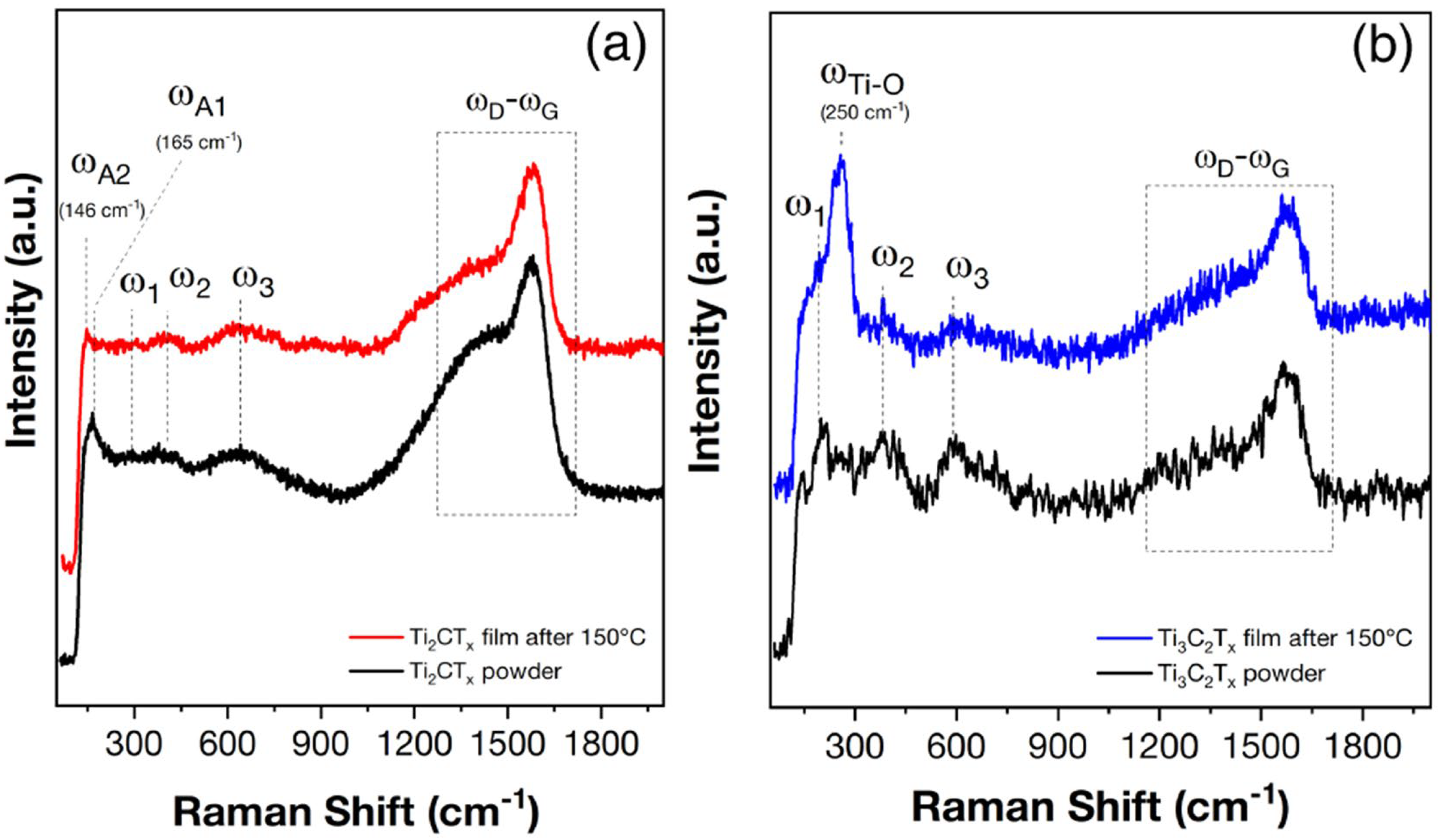
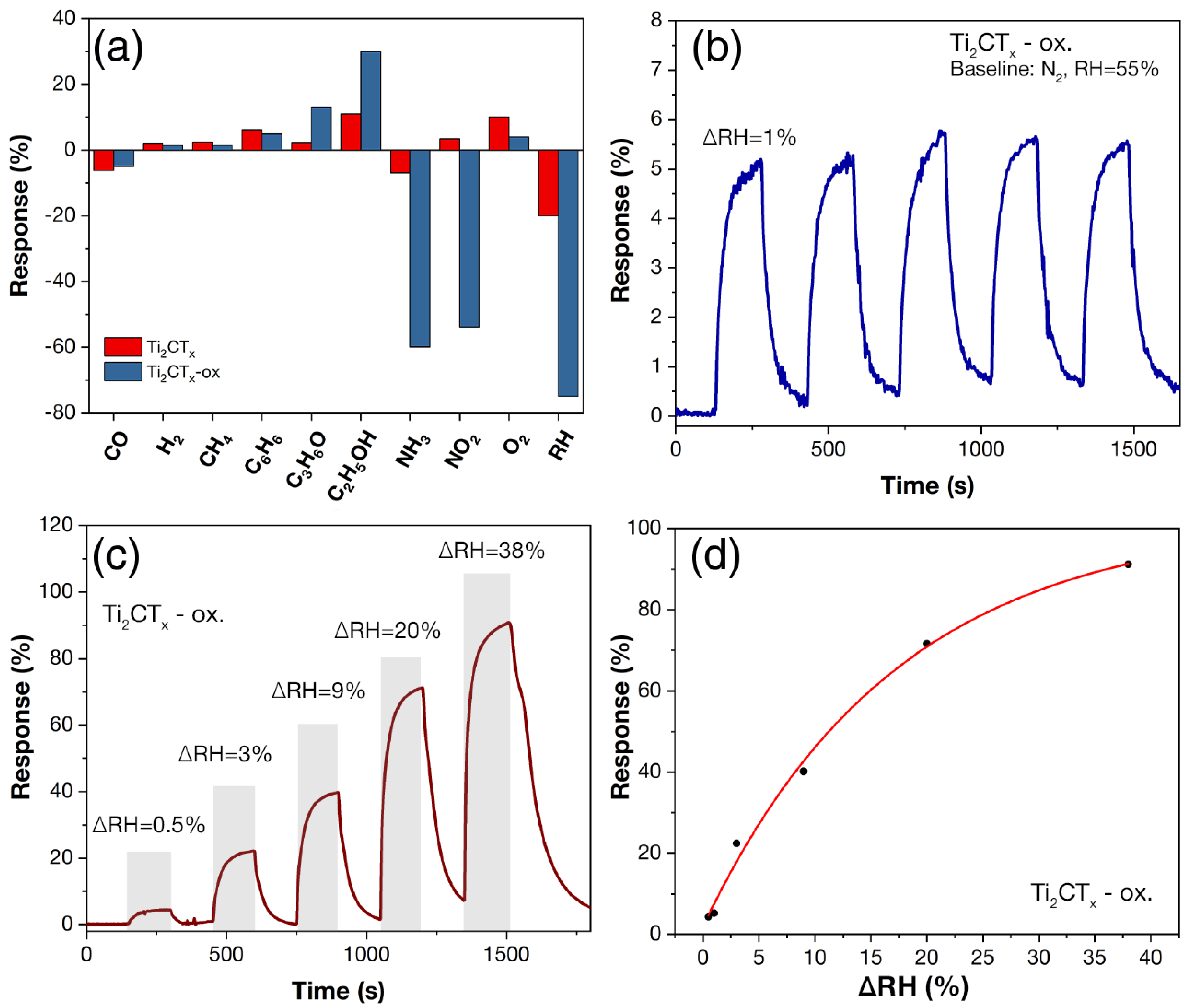
3.2. Study of Multilayer Ti2CTx and Ti3C2Tx MXene Coatings after Partial Oxidation in a Sensor Cell
3.3. Chemoresistive Properties of Ti2CTx and Ti3C2Tx Coatings Deposited by Microplotting
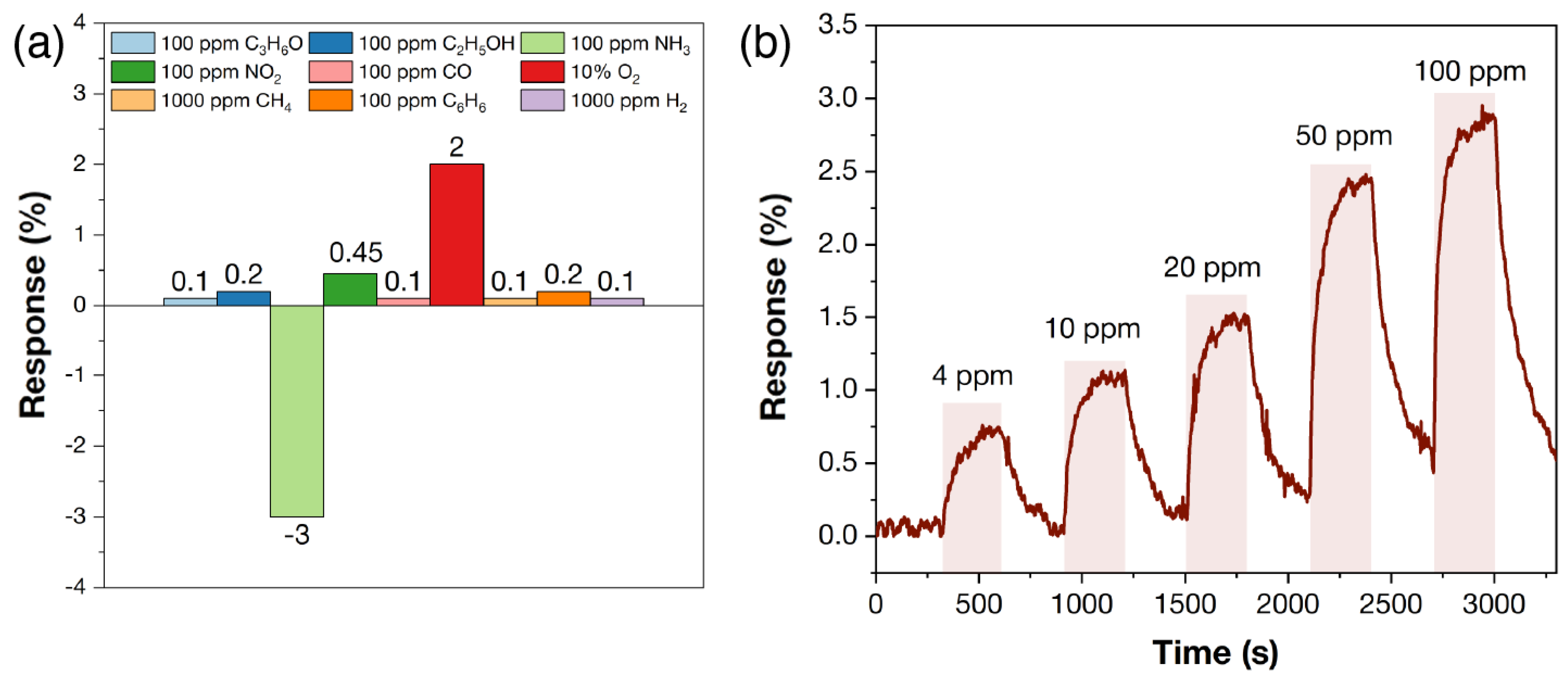
3.4. Chemoresistive Properties of Ti2CTx and Ti3C2Tx Coatings after Partial Oxidation in the Sensor Cell
4. Conclusions
- For the partially oxidized accordion-like Ti2CTx Mxene, there is a sharp increase in sensitivity to humidity and a decrease in the existing sensitivity to oxygen (measurements performed at RT and RH = 55%). The selectivity towards other analytes also changes: the highest sensitivity of the initial Ti2CTx to 100 ppm ethanol (11% response) for the Ti2CTx-ox sample is replaced by high responses to 100 ppm NH3 (S = 60%) and NO2 (S = 54%).
- The Ti3C2Tx multilayer modified with needle-shaped Ti2O3 particles retains high sensitivity to oxygen in dry air, but this requires a slightly higher detection temperature; the optimum operating temperature was found to be 125 °C, at which the response to 10% O2 is 86%. The response to 1% oxygen under these conditions is 33%. Significant sensitivity was also observed to 100 ppm NO2 (21%) and NH3 (15%), with responses to other gases not exceeding 2%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marinković, Z.; Gugliandolo, G.; Latino, M.; Campobello, G.; Crupi, G.; Donato, N. Characterization and Neural Modeling of a Microwave Gas Sensor for Oxygen Detection Aimed at Healthcare Applications. Sensors 2020, 20, 7150. [Google Scholar] [CrossRef]
- Baltes, N.; Beyle, F.; Freiner, S.; Geier, F.; Joos, M.; Pinkwart, K.; Rabenecker, P. Trace Detection of Oxygen—Ionic Liquids in Gas Sensor Design. Talanta 2013, 116, 474–481. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Dutta, P.K.K.; Akbar, S.A.A. Oxygen Sensors: Materials, Methods, Designs. J. Mater. Sci. 2003, 38, 4271–4282. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, E.P.; Simonenko, N.P.; Akkuleva, K.T.; Antipov, V.V.; Zaharova, N.V.; Malygin, A.A.; Bukunov, K.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Oxygen Detection Using Nanostructured TiO2 Thin Films Obtained by the Molecular Layering Method. Appl. Surf. Sci. 2019, 463, 197–202. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Kobune, M.; Gotoh, H. Basic Properties of Rare Earth Oxides. Appl. Catal. A Gen. 2009, 356, 57–63. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Mokrushin, A.S.; Simonenko, E.P.; Glumov, O.V.; Mel’nikova, N.A.; Murin, I.V.; Kalinina, M.V.; Shilova, O.A.; Sevastyanov, V.G.; et al. Microstructural, Electrophysical and Gas-Sensing Properties of CeO2–Y2O3 Thin Films Obtained by the Sol-Gel Process. Ceram. Int. 2020, 46, 121–131. [Google Scholar] [CrossRef]
- Wang, X.; Liu, T.; Yu, J. A limiting Current Oxygen Sensor Constituted of (CeO2)0.95(Y2O3)0.05 as Solid Electrolyte Layer and (CeO2)0.75(ZrO2)0.25 as Dense Diffusion Barrier Layer. Sensors 2019, 19, 3511. [Google Scholar] [CrossRef]
- Ramshanker, N.; Ganapathi, K.L.; Varun, N.; Bhat, M.S.; Mohan, S. Development of CeO2-HfO2 Mixed Oxide Thin Films for High Performance Oxygen Sensors. IEEE Sens. J. 2021, 21, 18326–18333. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Kadyrov, N.C.; Simonenko, E.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Chemoresistive Gas-Sensing Properties of Highly Dispersed Nb2O5 Obtained by Programmable Precipitation. J. Alloys Compd. 2021, 868, 159090. [Google Scholar] [CrossRef]
- Ghom, S.A.; Zamani, C.; Nazarpour, S.; Andreu, T.; Morante, J.R. Oxygen Sensing with Mesoporous Ceria–Zirconia Solid Solutions. Sens. Actuators B Chem. 2009, 140, 216–221. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Mokrushin, A.S.; Simonenko, T.L.; Gorobtsov, P.Y.; Nagornov, I.A.; Korotcenkov, G.; Sysoev, V.V.; Kuznetsov, N.T. Application of Titanium Carbide MXenes in Chemiresistive Gas Sensors. Nanomaterials 2023, 13, 850. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, C.; Liu, R.; Wu, Y.; Li, D.; Ma, X.; Liu, L.; Wang, P.; Wang, Y. Experiment and Simulation Analysis on Thermal Shock Resistance of Laminated Ceramics with Graphite and Boron Nitride Interfaces. Ceram. Int. 2021, 47, 11973–11978. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Jiang, X.; Zhang, H.; Qiu, J.; Zhang, J.C. Perspectives on Solution Processing of Two-Dimensional MXenes. Mater. Today 2021, 48, 214–240. [Google Scholar] [CrossRef]
- Perera, A.A.P.R.; Madhushani, K.A.U.; Punchihewa, B.T.; Kumar, A.; Gupta, R.K. MXene-Based Nanomaterials for Multifunctional Applications. Materials 2023, 16, 1138. [Google Scholar] [CrossRef]
- Jasim, S.A.; Hadi, J.M.; Opulencia, M.J.C.; Karim, Y.S.; Mahdi, A.B.; Kadhim, M.M.; Bokov, D.O.; Jalil, A.T.; Mustafa, Y.F.; Falih, K.T. MXene/Metal and Polymer Nanocomposites: Preparation, Properties, and Applications. J. Alloys Compd. 2022, 917, 165404. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Nan, J.; Guo, X.; Xiao, J.; Li, X.; Chen, W.; Wu, W.; Liu, H.; Wang, Y.; Wu, M.; Wang, G. Nanoengineering of 2D MXene-Based Materials for Energy Storage Applications. Small 2021, 17, 1902085. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, Y.; Gupta, M.; Sharma, S. Study of Quantum Capacitance of Pure and Functionalized Nb 2 c and Ti 2 c Mxenes for Supercapacitor Applications. ECS Trans. 2022, 107, 1751–1760. [Google Scholar] [CrossRef]
- Li, K.; Zhang, S.; Li, Y.; Fan, J.; Lv, K. MXenes as Noble-Metal-Alternative Co-Catalysts in Photocatalysis. Chinese J. Catal. 2021, 42, 3–14. [Google Scholar] [CrossRef]
- Cui, L.; Wen, J.; Deng, Q.; Du, X.; Tang, T.; Li, M.; Xiao, J.; Jiang, L.; Hu, G.; Cao, X.; et al. Improving the Photocatalytic Activity of Ti3C2 MXene by Surface Modification of N Doped. Materials 2023, 16, 2836. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Y.; Wang, X.; Zhi, X. Enhanced Catalytic Effect of Ti2CTx-MXene on Thermal Decomposition Behavior of Ammonium Perchlorate. Materials 2022, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Z.; Liu, X.; Li, C.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Zhu, S.; Hu, W.; et al. Interfacial Engineering of Bi2S3/Ti3C2Tx MXene Based on Work Function for Rapid Photo-Excited Bacteria-Killing. Nat. Commun. 2021, 12, 1224. [Google Scholar] [CrossRef]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Fojtů, M.; Latiff, N.M.; Pumera, M. Two-Dimensional Materials in Biomedical, Biosensing and Sensing Applications. Chem. Soc. Rev. 2021, 50, 619–657. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Rahman, M.H.; Zehravi, M.; Awaji, A.A.; Nasrullah, M.Z.; Gad, H.A.; Bani-Fwaz, M.Z.; Varma, R.S.; Germoush, M.O.; Al-malky, H.S.; et al. MXene (Ti3C2Tx)-Embedded Nanocomposite Hydrogels for Biomedical Applications: A Review. Materials 2022, 15, 1666. [Google Scholar] [CrossRef]
- Yu, S.; Tang, H.; Zhang, D.; Wang, S.; Qiu, M.; Song, G.; Fu, D.; Hu, B.; Wang, X. MXenes as Emerging Nanomaterials in Water Purification and Environmental Remediation. Sci. Total Environ. 2022, 811, 152280. [Google Scholar] [CrossRef]
- Zeng, M.; Chen, M.; Huang, D.; Lei, S.; Zhang, X.; Wang, L.; Cheng, Z. Engineered Two-Dimensional Nanomaterials: An Emerging Paradigm for Water Purification and Monitoring. Mater. Horiz. 2021, 8, 758–802. [Google Scholar] [CrossRef]
- Kumar, J.A.; Prakash, P.; Krithiga, T.; Amarnath, D.J.; Premkumar, J.; Rajamohan, N.; Vasseghian, Y.; Saravanan, P.; Rajasimman, M. Methods of Synthesis, Characteristics, and Environmental Applications of MXene: A Comprehensive Review. Chemosphere 2022, 286, 131607. [Google Scholar] [CrossRef]
- Pacheco-Peña, V.; Hallam, T.; Healy, N. MXene Supported Surface Plasmons on Telecommunications Optical Fibers. Light Sci. Appl. 2022, 11, 22. [Google Scholar] [CrossRef]
- Shang, C.; Zhang, Y.; Wang, G.; Sun, J.; Cheng, Y.; Zhang, Y.-B.; Yao, B.; Fu, B.; Li, J. Nonlinear Optical Properties of MXene and Applications in Broadband Ultrafast Photonics. J. Alloys Compd. 2022, 918, 165580. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, X.; Hui, Z.; Zhou, J.; Huang, X.; Sun, G.; Huang, W. Ti3C2TX MXene for Sensing Applications: Recent Progress, Design Principles, and Future Perspectives. ACS Nano 2021, 15, 3996–4017. [Google Scholar] [CrossRef]
- Devaraj, M.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M. A Review on MXene and Its Nanocomposites for the Detection of Toxic Inorganic Gases. Chemosphere 2022, 302, 134933. [Google Scholar] [CrossRef]
- Riazi, H.; Taghizadeh, G.; Soroush, M. MXene-Based Nanocomposite Sensors. ACS Omega 2021, 6, 11103–11112. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.; Zeng, W. Preparation and Application of 2D MXene-Based Gas Sensors: A Review. Chemosensors 2021, 9, 225. [Google Scholar] [CrossRef]
- Bhati, V.S.; Kumar, M.; Banerjee, R. Gas Sensing Performance of 2D Nanomaterials/Metal Oxide Nanocomposites: A Review. J. Mater. Chem. C 2021, 9, 8776–8808. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, J.; Wang, X.; Dai, J.; Wang, X.; Fan, H.; Wang, Z.; Li, H.; Huang, X.; Huang, W. Treatment-Dependent Surface Chemistry and Gas Sensing Behavior of the Thinnest Member of Titanium Carbide MXenes. Nanoscale 2020, 12, 16987–16994. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Nagornov, I.A.; Averin, A.A.; Simonenko, T.L.; Simonenko, N.P.; Simonenko, E.P.; Kuznetsov, N.T. Chemoresistive Properties of V2CTx MXene and the V2CTx/V3O7 Nanocomposite Based on It. Chemosensors 2023, 11, 142. [Google Scholar] [CrossRef]
- Pazniak, H.; Plugin, I.A.; Loes, M.J.; Inerbaev, T.M.; Burmistrov, I.N.; Gorshenkov, M.; Polcak, J.; Varezhnikov, A.S.; Sommer, M.; Kuznetsov, D.V.; et al. Partially Oxidized Ti3C2Tx MXenes for Fast and Selective Detection of Organic Vapors at Part-per-Million Concentrations. ACS Appl. Nano Mater. 2020, 3, 3195–3204. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Ge, C.; Lei, S.; Hussain, S.; Wang, M.; Qiao, G.; Liu, G. Enhanced Room-Temperature NO2 Sensing Performance of SnO2/Ti3C2 Composite with Double Heterojunctions by Controlling Co-Exposed {221} and {110} Facets of SnO2. Sens. Actuators B Chem. 2022, 365, 131919. [Google Scholar] [CrossRef]
- Fan, C.; Shi, J.; Zhang, Y.; Quan, W.; Chen, X.; Yang, J.; Zeng, M.; Zhou, Z.; Su, Y.; Wei, H.; et al. Fast and Recoverable NO2 Detection Achieved by Assembling ZnO on Ti3C2Tx MXene Nanosheets under UV Illumination at Room Temperature. Nanoscale 2022, 14, 3441–3451. [Google Scholar] [CrossRef]
- Wu, M.; He, M.; Hu, Q.; Wu, Q.; Sun, G.; Xie, L.; Zhang, Z.; Zhu, Z.; Zhou, A. Ti3C2 MXene-Based Sensors with High Selectivity for NH3 Detection at Room Temperature. ACS Sensors 2019, 4, 2763–2770. [Google Scholar] [CrossRef]
- Yan, M.; Yang, L.; Li, C.; Zou, Y. Preparation of Two-Dimensional Ti2CTx by Molten Fluorinated Salt Method. J. Wuhan Univ. Technol. Sci. Ed. 2019, 34, 299–302. [Google Scholar] [CrossRef]
- Lee, J.; Kang, Y.C.; Koo, C.M.; Kim, S.J. Ti3C2Tx MXene Nanolaminates with Ionic Additives for Enhanced Gas-Sensing Performance. ACS Appl. Nano Mater. 2022, 5, 11997–12005. [Google Scholar] [CrossRef]
- Nemufulwi, M.I.; Swart, H.C.; Mdlalose, W.B.; Mhlongo, G.H. Size-Tunable Ferromagnetic ZnFe2O4 Nanoparticles and Their Ethanol Detection Capabilities. Appl. Surf. Sci. 2020, 508, 144863. [Google Scholar] [CrossRef]
- Li, X.; An, Z.; Lu, Y.; Shan, J.; Xing, H.; Liu, G.; Shi, Z.; He, Y.; Chen, Q.; Han, R.P.S.; et al. Room Temperature VOCs Sensing with Termination-Modified Ti3C2Tx MXene for Wearable Exhaled Breath Monitoring. Adv. Mater. Technol. 2022, 7, 2100872. [Google Scholar] [CrossRef]
- Majhi, S.M.; Ali, A.; Greish, Y.E.; El-Maghraby, H.F.; Qamhieh, N.N.; Hajamohideen, A.R.; Mahmoud, S.T. Accordion-like-Ti3C2 MXene-Based Gas Sensors with Sub-Ppm Level Detection of Acetone at Room Temperature. ACS Appl. Electron. Mater. 2022, 4, 4094–4103. [Google Scholar] [CrossRef]
- Shuvo, S.N.; Ulloa Gomez, A.M.; Mishra, A.; Chen, W.Y.; Dongare, A.M.; Stanciu, L.A. Sulfur-Doped Titanium Carbide MXenes for Room-Temperature Gas Sensing. ACS Sensors 2020, 5, 2915–2924. [Google Scholar] [CrossRef]
- Wu, M.; An, Y.; Yang, R.; Tao, Z.; Xia, Q.; Hu, Q.; Li, M.; Chen, K.; Zhang, Z.; Huang, Q.; et al. V2CTx and Ti3C2Tx MXenes Nanosheets for Gas Sensing. ACS Appl. Nano Mater. 2021, 4, 6257–6268. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Nagornov, I.A.; Gorobtsov, P.Y.; Averin, A.A.; Simonenko, T.L.; Simonenko, N.P.; Simonenko, E.P.; Kuznetsov, N.T. Effect of Ti2CTx MXene Oxidation on Its Gas-Sensitive Properties. Chemosensors 2022, 11, 13. [Google Scholar] [CrossRef]
- Wang, J.; Xu, R.; Xia, Y.; Komarneni, S. Ti2CTx MXene: A Novel p-Type Sensing Material for Visible Light-Enhanced Room Temperature Methane Detection. Ceram. Int. 2021, 47, 34437–34442. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Nagornov, I.A.; Simonenko, T.L.; Gorobtsov, P.Y.; Mokrushin, A.S.; Kuznetsov, N.T. Synthesis and Chemoresistive Properties of Single-Layer MXene Ti2CTx. Russ. J. Inorg. Chem. 2022, 67, 1850–1859. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Nagornov, I.A.; Mokrushin, A.S.; Averin, A.A.; Gorban, Y.M.; Simonenko, T.L.; Simonenko, N.P.; Kuznetsov, N.T. Gas-Sensitive Properties of ZnO/Ti2CTx Nanocomposites. Micromachines 2023, 14, 725. [Google Scholar] [CrossRef]
- Badie, S.; Dash, A.; Sohn, Y.J.; Vaßen, R.; Guillon, O.; Gonzalez-Julian, J. Synthesis, Sintering, and Effect of Surface Roughness on Oxidation of Submicron Ti2AlC Ceramics. J. Am. Ceram. Soc. 2021, 104, 1669–1688. [Google Scholar] [CrossRef]
- Roy, C.; Banerjee, P.; Bhattacharyya, S. Molten Salt Shielded Synthesis (MS3) of Ti2AlN and V2AlC MAX Phase Powders in Open Air. J. Eur. Ceram. Soc. 2020, 40, 923–929. [Google Scholar] [CrossRef]
- Luo, W.; Liu, Y.; Wang, C.; Zhao, D.; Yuan, X.; Wang, L.; Zhu, J.; Guo, S.; Kong, X. Molten Salt Assisted Synthesis and Electromagnetic Wave Absorption Properties of (V1−x−yTixCy)2AlC Solid Solutions. J. Mater. Chem. C 2021, 9, 7697–7705. [Google Scholar] [CrossRef]
- Wu, E.; Wang, J.; Zhang, H.; Zhou, Y.; Sun, K.; Xue, Y. Neutron Diffraction Studies of Ti3Si0.9Al0.1C2 Compound. Mater. Lett. 2005, 59, 2715–2719. [Google Scholar] [CrossRef]
- Schuster, J.C.; Nowotny, H.; Vaccaro, C. The Ternary Systems: CrAlC, VAlC, and TiAlC and the Behavior of H-Phases (M2AlC). J. Solid State Chem. 1980, 32, 213–219. [Google Scholar] [CrossRef]
- Habib, I.; Ferrer, P.; Ray, S.C.; Ozoemena, K.I. Interrogating the Impact of Onion-like Carbons on the Supercapacitive Properties of MXene (Ti2CTX). J. Appl. Phys. 2019, 126, 134301. [Google Scholar] [CrossRef]
- Melchior, S.A.; Raju, K.; Ike, I.S.; Erasmus, R.M.; Kabongo, G.; Sigalas, I.; Iyuke, S.E.; Ozoemena, K.I. High-Voltage Symmetric Supercapacitor Based on 2D Titanium Carbide (MXene, Ti2CTX)/Carbon Nanosphere Composites in a Neutral Aqueous Electrolyte. J. Electrochem. Soc. 2018, 165, A501–A511. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kürti, J.; Koltai, J.; Kavan, L. Raman Spectra of Titanium Dioxide (Anatase, Rutile) with Identified Oxygen Isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 2012, 14, 14567. [Google Scholar] [CrossRef]
- Lioi, D.B.; Neher, G.; Heckler, J.E.; Back, T.; Mehmood, F.; Nepal, D.; Pachter, R.; Vaia, R.; Kennedy, W.J. Electron-Withdrawing Effect of Native Terminal Groups on the Lattice Structure of Ti 3 C 2 T x MXenes Studied by Resonance Raman Scattering: Implications for Embedding MXenes in Electronic Composites. ACS Appl. Nano Mater. 2019, 2, 6087–6091. [Google Scholar] [CrossRef]
- Kuang, D.; Wang, L.; Guo, X.; She, Y.; Du, B.; Liang, C.; Qu, W.; Sun, X.; Wu, Z.; Hu, W.; et al. Facile Hydrothermal Synthesis of Ti3C2Tx-TiO2 Nanocomposites for Gaseous Volatile Organic Compounds Detection at Room Temperature. J. Hazard. Mater. 2021, 416, 126171. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Liu, G.; Wan, N.; Ge, C.; Hussain, S.; Meng, H.; Wang, M.; Qiao, G. Enhanced NO2 Gas-Sensing Performance of 2D Ti3C2/TiO2 Nanocomposites by in-Situ Formation of Schottky Barrier. Appl. Surf. Sci. 2021, 567, 150747. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Wang, Y.; Yu, H.; Zhang, R.; Li, J.; Zang, Z.; Li, X. MXene Ti3C2Tx-Derived Nitrogen-Functionalized Heterophase TiO2 Homojunctions for Room-Temperature Trace Ammonia Gas Sensing. ACS Appl. Mater. Interfaces 2021, 13, 56485–56497. [Google Scholar] [CrossRef] [PubMed]
- Tsirelson, V.G.; Antipin, M.Y.; Gerr, R.G.; Ozerov, R.P.; Struchkov, Y.T. Ruby Structure Peculiarities Derived from X-Ray Diffraction Data Localization of Chromium Atoms and Electron Deformation Density. Phys. Status Solidi 1985, 87, 425–433. [Google Scholar] [CrossRef]
- Davey, W.P. Precision Measurements of the Lattice Constants of Twelve Common Metals. Phys. Rev. 1925, 25, 753–761. [Google Scholar] [CrossRef]
- Ma, H.L.; Yang, J.Y.; Dai, Y.; Zhang, Y.B.; Lu, B.; Ma, G.H. Raman Study of Phase Transformation of TiO2 Rutile Single Crystal Irradiated by Infrared Femtosecond Laser. Appl. Surf. Sci. 2007, 253, 7497–7500. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, J. Sol–Gel Preparation and Characterization of Black Titanium Oxides Ti2O3 and Ti3O5. J. Mater. Sci. Mater. Electron. 2014, 25, 1284–1288. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Cheng, J.; Liu, Z.; Li, Q.; Li, W.; Yang, X.; Xiao, B. Monolayer Ti2CO2: A Promising Candidate for NH3 Sensor or Capturer with High Sensitivity and Selectivity. ACS Appl. Mater. Interfaces 2015, 7, 13707–13713. [Google Scholar] [CrossRef]
- Khakbaz, P.; Moshayedi, M.; Hajian, S.; Soleimani, M.; Narakathu, B.B.; Bazuin, B.J.; Pourfath, M.; Atashbar, M.Z. Titanium Carbide MXene as NH3 Sensor: Realistic First-Principles Study. J. Phys. Chem. C 2019, 123, 29794–29803. [Google Scholar] [CrossRef]
- Ludwig, H.R.; Cairelli, S.G.; Whalen, J.J. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs); NOISH: Cincinnati, OH, USA, 1996. [Google Scholar]
- Lee, D.-K.; Jeon, J.-I.; Kim, M.-H.; Choi, W.; Yoo, H.-I. Oxygen Nonstoichiometry (δ) of TiO2−δ-Revisited. J. Solid State Chem. 2005, 178, 185–193. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonenko, E.P.; Nagornov, I.A.; Mokrushin, A.S.; Kashevsky, S.V.; Gorban, Y.M.; Simonenko, T.L.; Simonenko, N.P.; Kuznetsov, N.T. Low Temperature Chemoresistive Oxygen Sensors Based on Titanium-Containing Ti2CTx and Ti3C2Tx MXenes. Materials 2023, 16, 4506. https://doi.org/10.3390/ma16134506
Simonenko EP, Nagornov IA, Mokrushin AS, Kashevsky SV, Gorban YM, Simonenko TL, Simonenko NP, Kuznetsov NT. Low Temperature Chemoresistive Oxygen Sensors Based on Titanium-Containing Ti2CTx and Ti3C2Tx MXenes. Materials. 2023; 16(13):4506. https://doi.org/10.3390/ma16134506
Chicago/Turabian StyleSimonenko, Elizaveta P., Ilya A. Nagornov, Artem S. Mokrushin, Sergey V. Kashevsky, Yulia M. Gorban, Tatiana L. Simonenko, Nikolay P. Simonenko, and Nikolay T. Kuznetsov. 2023. "Low Temperature Chemoresistive Oxygen Sensors Based on Titanium-Containing Ti2CTx and Ti3C2Tx MXenes" Materials 16, no. 13: 4506. https://doi.org/10.3390/ma16134506
APA StyleSimonenko, E. P., Nagornov, I. A., Mokrushin, A. S., Kashevsky, S. V., Gorban, Y. M., Simonenko, T. L., Simonenko, N. P., & Kuznetsov, N. T. (2023). Low Temperature Chemoresistive Oxygen Sensors Based on Titanium-Containing Ti2CTx and Ti3C2Tx MXenes. Materials, 16(13), 4506. https://doi.org/10.3390/ma16134506






