The Constituent Phases and Micromechanical Properties of Steel Corrosion Layers Generated by Hyperbaric-Oxygen Accelerated Corrosion Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Nano-Indentation Tests
2.3. Microstructural Characterization
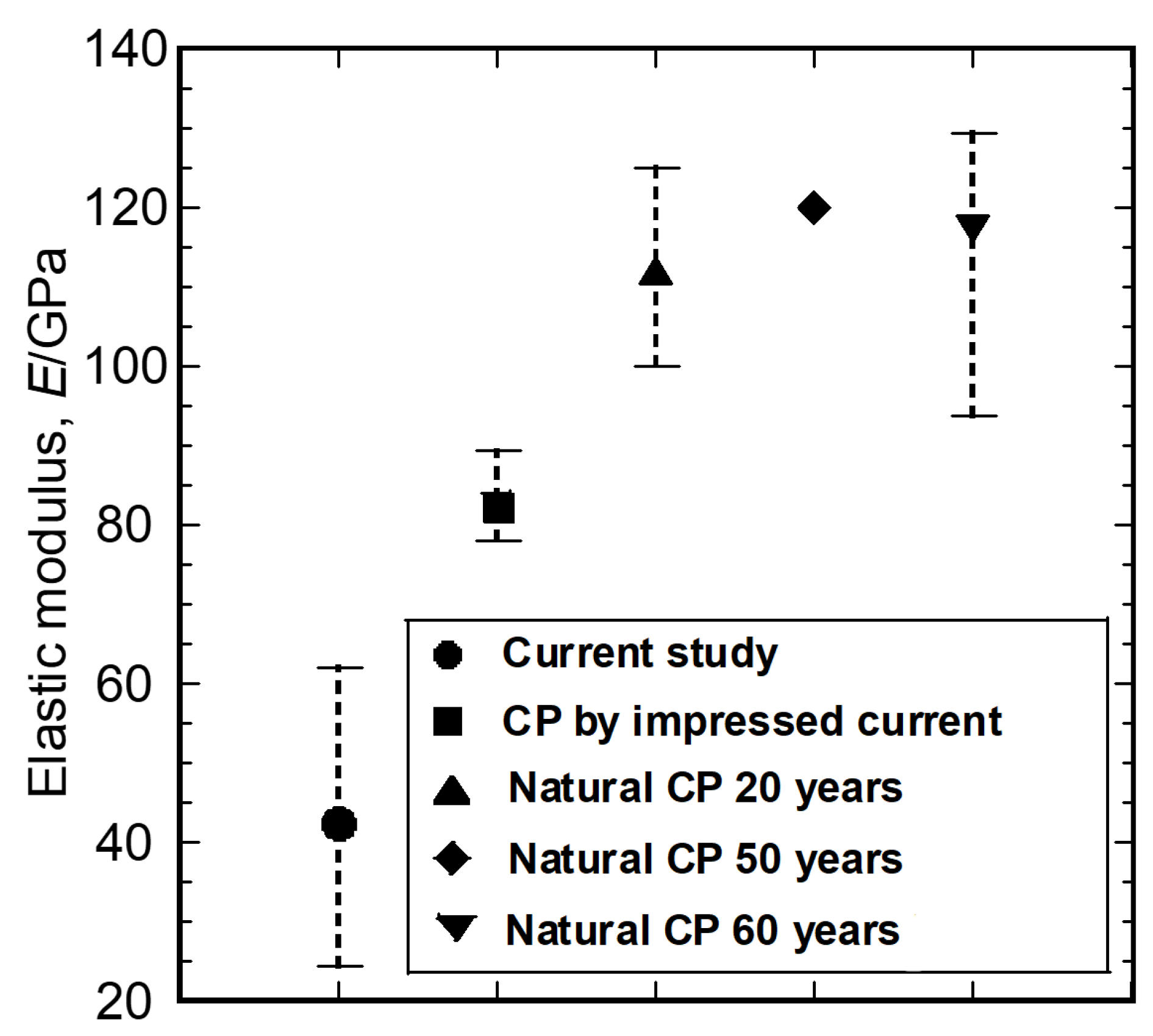
3. Results and Discussion
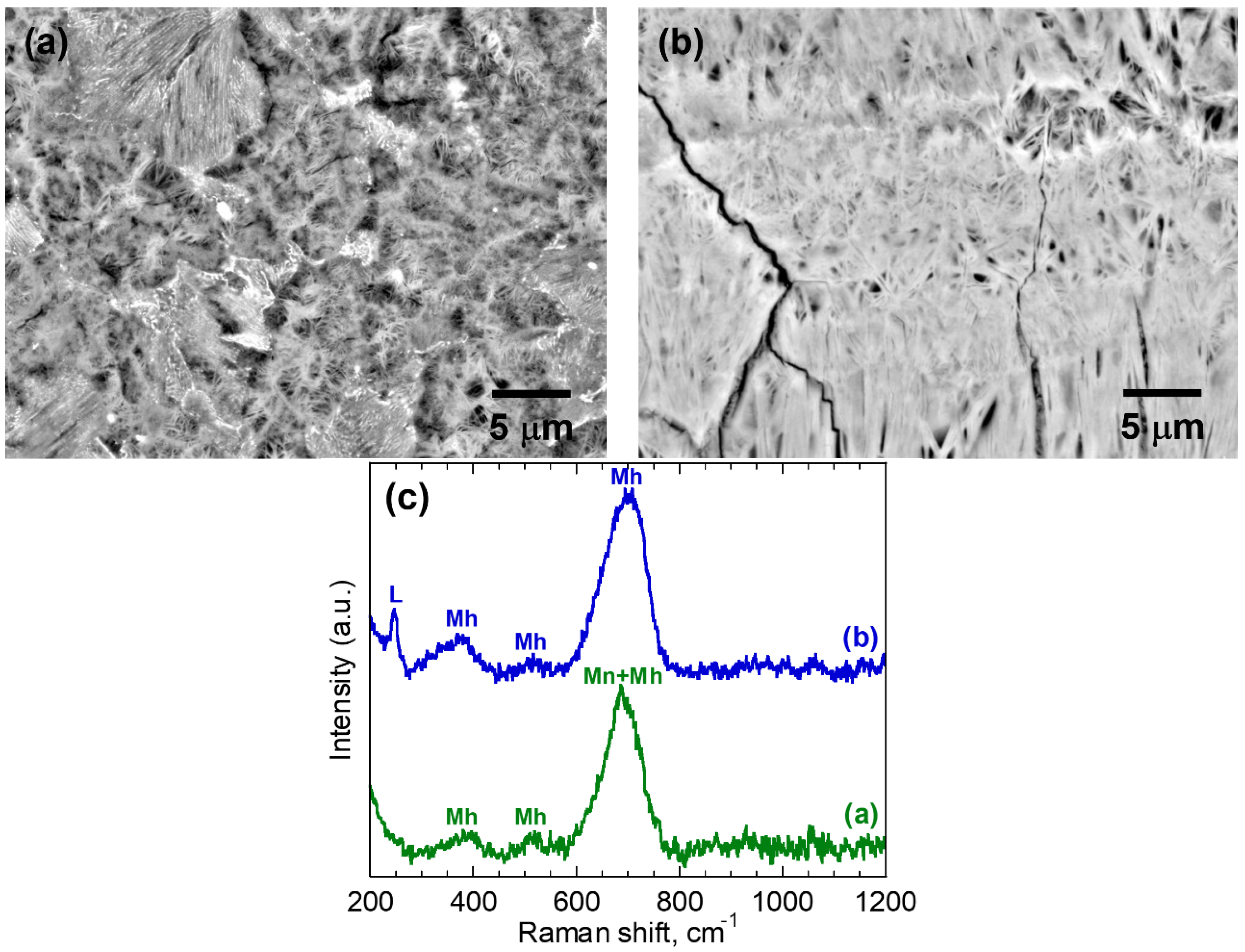
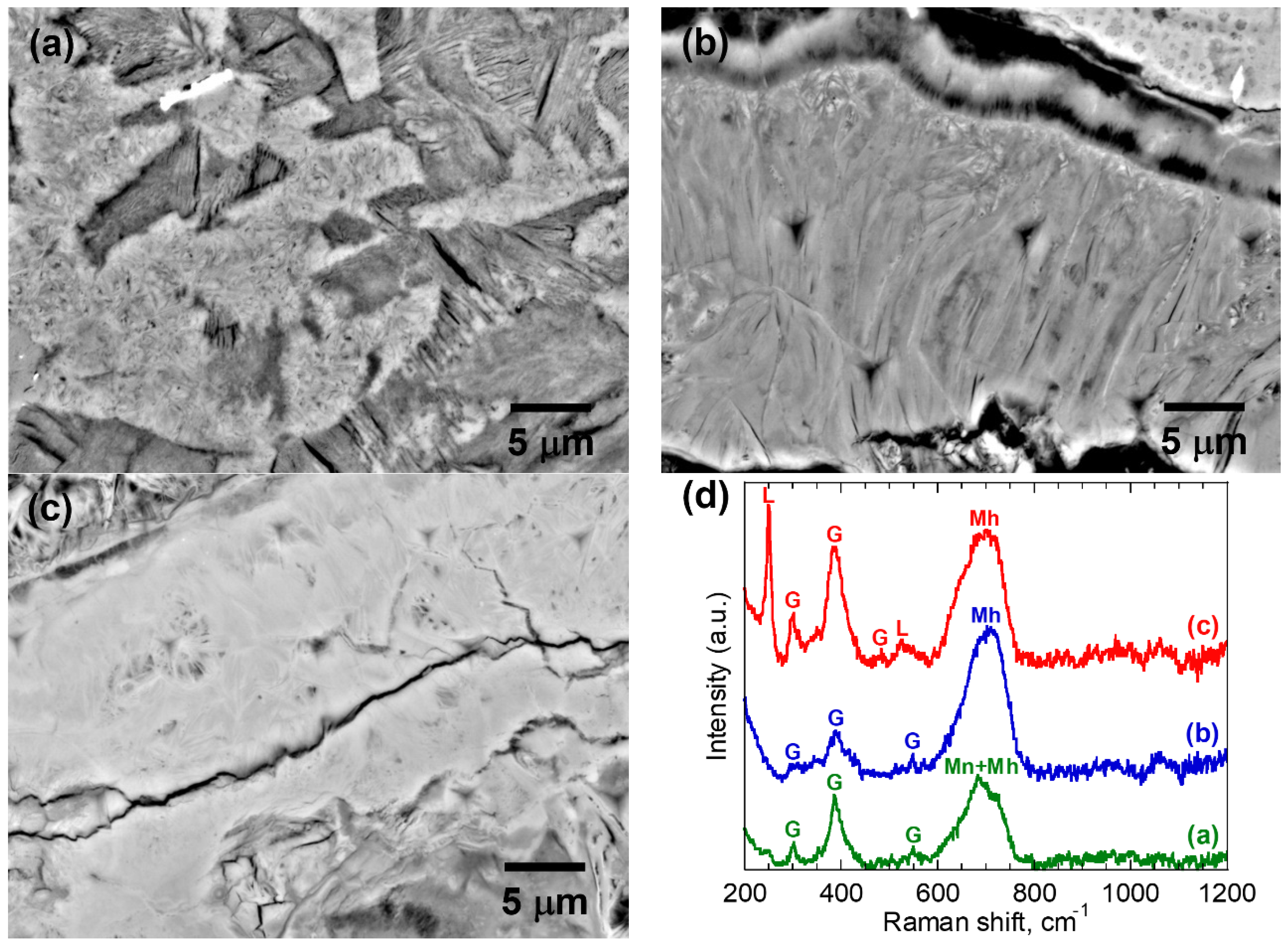
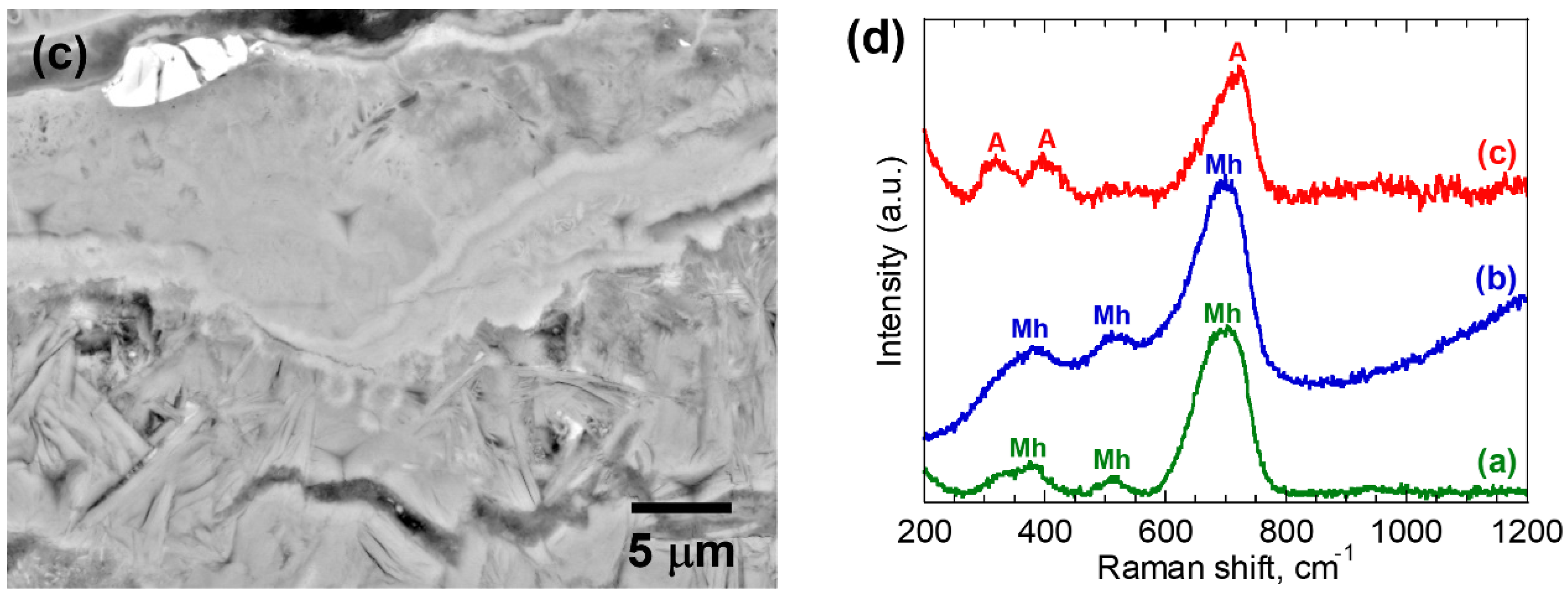
3.1. Microstructural Characterization of Corrosion Products
3.2. Characterization of the Mechanical Properties
3.3. Relationship between Local Microstructures and Mechanical Properties
4. Conclusions
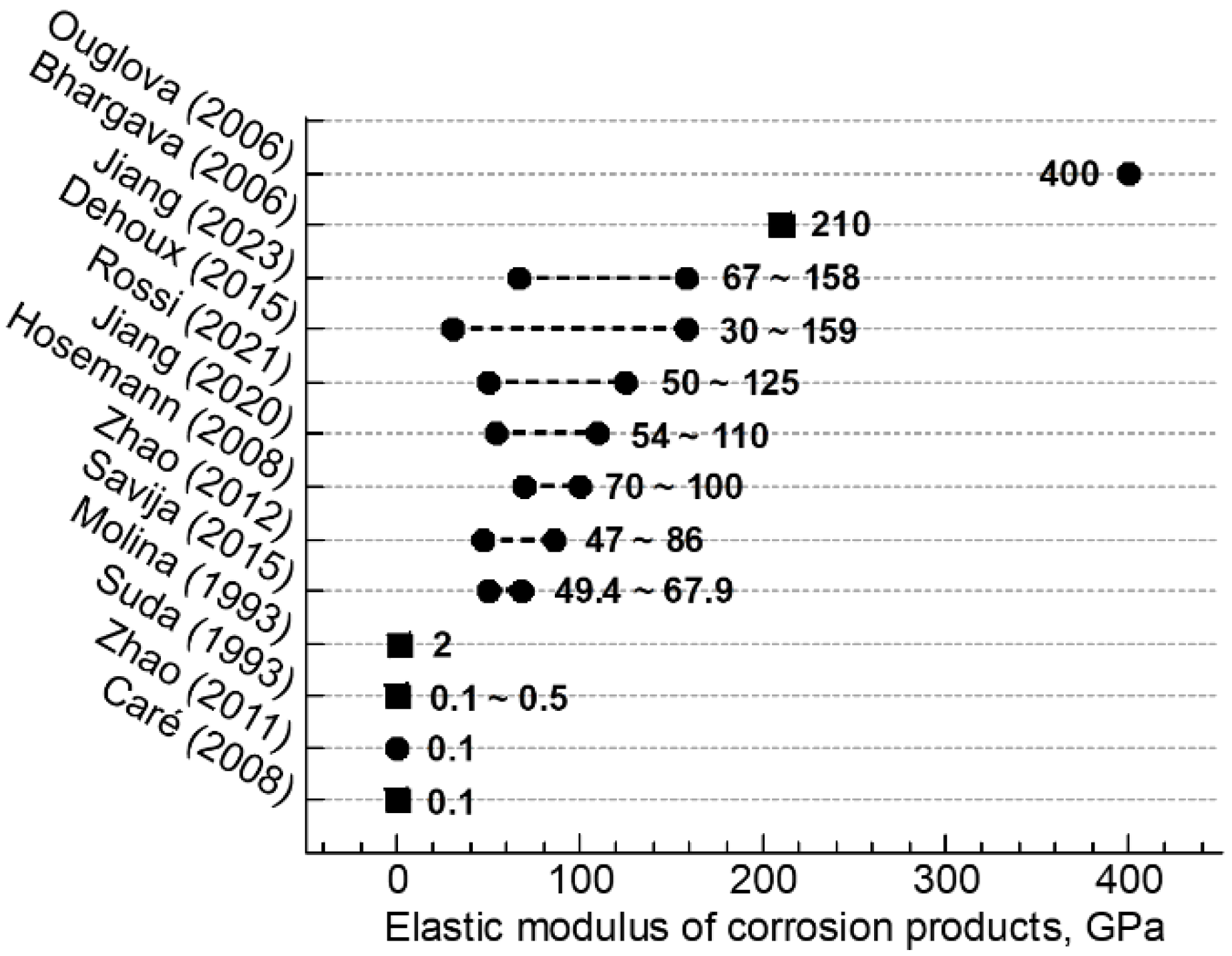
- The phases identified in the corrosion products included goethite, lepidocrocite, maghemite, magnetite, and akageneite, presenting themselves as mixtures in most regions of the corrosion product layer at the microscopic level. Some local regions were composed of a single maghemite or akageneite phrase were also found.
- The micromechanical properties of HOACT-generated corrosion products covered a wide range. The elastic modulus varied between 6.8 and 75.2 GPa, while the nanohardness varied between 0.38 and 4.44 GPa.
- The microstructural porosity greatly influenced the micromechanical properties of the corrosion products. A more porous and looser microstructure usually corresponds to a lower elastic modulus and nanohardness.
- According to the previous studies, as well as the current study of corrosion products, the characteristic mechanical properties per phase should not be considered a constant value. They can change with corrosion conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Redaelli, E.; Polder, R.B. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction—A review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Wong, H.S.; Zhao, Y.X.; Karimi, A.R.; Buenfeld, N.R.; Jin, W.L. On the penetration of corrosion products from reinforcing steel into concrete due to chloride-induced corrosion. Corros. Sci. 2010, 52, 2469–2480. [Google Scholar] [CrossRef]
- Marcotte, T.D. Characterization of Chloride-Induced Corrosion Products that Form in Steel-Reinforced Cementitious Materials. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2001. [Google Scholar]
- Jaffer, S.J.; Hansson, C.M. Chloride-induced corrosion products of steel in cracked-concrete subjected to different loading conditions. Cem. Concr. Res. 2009, 74, 116–125. [Google Scholar] [CrossRef]
- Berrocal, C.G.; Fernandez, I.; Rempling, R. The interplay between corrosion and cracks in reinforced concrete beams with non-uniform reinforcement corrosion. Mater. Struct. 2022, 55, 120. [Google Scholar] [CrossRef]
- Caré, S.; Nguyen, Q.T.; L’Hostis, V.; Berthaud, Y. Mechanical properties of the rust layer induced by impressed current method in reinforced mortar. Cem. Concr. Res. 2008, 38, 1079–1091. [Google Scholar] [CrossRef]
- Suda, K.; Misra, S.; Motohashi, K. Corrosion products of reinforcing bars embedded in concrete. Corros. Sci. 1993, 35, 1543–1549. [Google Scholar] [CrossRef]
- Molina, F.J.; Alonso, C.; Andrade, C. Cover cracking as a function of bar corrosion: Part 2—Numerial model. Mater. Struct. 1993, 26, 532–548. [Google Scholar] [CrossRef]
- Bhargava, K.; Ghosh, A.K.; Mori, Y.; Ramanujam, S. Model for cover cracking due to rebar corrosion in RC structures. Eng. Struct. 2006, 28, 1093–1109. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Dai, H.; Ren, H.Y.; Jin, W.L. Experimental study of the modulus of steel corrosion in a concrete port. Corros. Sci. 2012, 56, 17–25. [Google Scholar] [CrossRef]
- Ouglova, A.; Berthaud, Y.; Francois, M.; Foct, F. Mechanical properties of an iron oxide formed by corrosion in reinforced concrete structures. Corros. Sci. 2006, 48, 3988–4000. [Google Scholar] [CrossRef]
- Savija, B.; Lukovic, M.; Hosseini, S.A.S.; Pacheco, J.; Schlangen, E. Corrosion induced cover cracking studied by X-ray computed tomography, nanoindentation, and energy dispersive X-ray spectrometry (EDS). Mater. Struct. 2015, 48, 2043–2062. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Dai, H.; Jin, W.L. A study of the elastic moduli of corrosion products using nano-indentation techniques. Corros. Sci. 2012, 65, 163–168. [Google Scholar] [CrossRef]
- Hosemann, P.; Swadener, J.G.; Welch, J.; Li, N. Nano-indentation measurement of oxide layers formed in LBE on F/M steels. J. Nucl. Mater. 2008, 377, 201–205. [Google Scholar] [CrossRef]
- Jiang, B.Z.; Doi, K.; Tsuchiya, K.; Kawano, Y.; Kori, A.; Ikushima, K. Micromechanical properties of steel corrosion products in concrete studied by nano-indentation technique. Corros. Sci. 2020, 163, 108304. [Google Scholar] [CrossRef]
- Rossi, E.; Zhang, H.Z.; Garcia, S.J.; Bijleveld, J.; Nijland, T.G.; Copuroglu, O.; Polder, R.B.; Savija, B. Analysis of naturally-generated corrosion products due to chlorides in 20-year old reinforced concrete: An elastic modulus-mineralogy characterization. Corros. Sci. 2021, 184, 109356. [Google Scholar] [CrossRef]
- Dehoux, A.; Bouchelaghem, F.; Berthaud, Y.; Neff, D.; L’Hostis, V. Micromechanical study of corrosion products layer. Part I: Experimental characterization. Corros. Sci. 2012, 54, 52–59. [Google Scholar] [CrossRef]
- Dehoux, A.; Bouchelaghem, F.; Berthaud, Y. Micromechanical and microstructural investigation of steel corrosion layers of variable age developed under impressed current method, atmospheric or saline conditions. Corros. Sci. 2015, 97, 49–61. [Google Scholar] [CrossRef]
- Jiang, B.Z.; Doi, K.; Tsuchiya, K. Characterization of microstructures and micromechanical properties of naturally generated rust layer in 60-year old reinforced concrete. Constr. Build. Mater. 2023, 366, 130203. [Google Scholar] [CrossRef]
- Takaya, S.; Okuno, S.; Honda, M.; Kawakami, K.; Satoh, S.; Hamura, Y.; Yamamoto, Y.; Miyagawa, T. Formation process of steel corrosion products in alkaline environment and corrosion environment of reinforcement in concrete. Zairyo/J. Soc. Mater. Sci. Japan 2017, 66, 545–552. [Google Scholar] [CrossRef]
- Nishikata, A.; Yamashita, Y.; Katayama, H.; Tsuru, T.; Usami, A.; Tanabe, K.; Mabuchi, H. An electrochemical impedance study on atmospheric corrosion of steels in a cyclic wet-dry condition. Corros. Sci. 1995, 37, 2059–2069. [Google Scholar] [CrossRef]
- Doi, K.; Hiromoto, S.; Akiyama, E. Hyperbaric-oxygen accelerated corrosion test for iron in cement paste and mortar. Mater. Trans. 2018, 59, 927–934. [Google Scholar] [CrossRef]
- Doi, K.; Hiromoto, S.; Katayama, H.; Akiyama, E. Effects of oxygen pressure and chloride ion concentration on corrosion of iron in mortar exposed to pressurized humid oxygen gas. J. Electrochem. Soc. 2018, 165, 582–589. [Google Scholar] [CrossRef]
- Doi, K.; Hiromoto, S.; Shinohara, T.; Tsuchiya, K.; Katayama, H.; Akiyama, E. Role of mill scale on corrosion behavior of steel rebars in mortar. Corros. Sci. 2020, 177, 108995. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Maslar, J.E.; Hurst, W.S.; Bowers, W.J.; Hendricks, J.H.; Aquino, M.I. In situ raman spectroscopic investigation of aqueous iron corrosion at elevated temperatures and pressures. J. Electrochem. Soc. 2000, 147, 2532–2542. [Google Scholar] [CrossRef]
- Bellot-Gurlet, L.; Neff, D.; Reguer, S.; Monnier, J.; Saheb, M.; Dillmann, P. Raman studies of corrosion layers formed on archaeological irons in various media. J. Nano Res. 2009, 8, 147–156. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Alcantara, J.; Diaz, I.; Wolthuis, R.; Fuente, D. SEM/Micro-Raman characterization of the morphologies of marine atmospheric corrosion products formed on mild steel. J. Electrochem. Soc. 2016, 163, 426–439. [Google Scholar] [CrossRef]
- Ming, J.; Shi, J.J. Distribution of corrosion products at the steel-concrete interface: Influence of mill scale properties, reinforcing steel type and corrosion inducing method. Constr. Build. Mater. 2019, 229, 116854. [Google Scholar] [CrossRef]
- Alcántara, J.; Chico, B.; Simancas, J.; Díaz, I.; Fuente, D.; Morcillo, M. An attempt to classify the morphologies presented by different rust phases formed during the exposure of carbon steel to marine atmospheres. Mater. Charact. 2016, 118, 65–78. [Google Scholar] [CrossRef]
- Singh, J.K.; Singh, D.D.N. The nature of rusts and corrosion characteristics of low alloy and plain carbon steels in three kinds of concrete pore solution with salinity and different pH. Corros. Sci. 2012, 56, 129–142. [Google Scholar] [CrossRef]
- Song, Y.R.; Jiang, G.M.; Chen, Y.; Zhao, P.; Tian, Y.M. Effects of chloride ions on corrosion of ductile iron and carbon steel in soil environments. Sci. Rep. 2017, 7, 6865. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yuan, R.; Yang, J.H.; Xiao, D.H.; Luo, D.; Zhou, W.H.; Niu, G. Effect of tempering on corrosion behavior and mechanism of low alloy steel in wet atmosphere. J. Mater. Res. Technol. 2022, 20, 4077–4096. [Google Scholar] [CrossRef]
- Gao, M.; Pang, X.; Gao, K. The growth mechanism of CO2 corrosion products films. Corros. Sci. 2011, 53, 557–568. [Google Scholar] [CrossRef]
- Wang, C.L.; Hua, Y.; Nadimi, S.; Taleb, W.; Barker, R.; Li, Y.X.; Chen, X.H.; Neville, A. Determination of thickness and air-void distribution within the iron carbonate layers using X-ray computed tomography. Corros. Sci. 2021, 179, 109153. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhang, X.; Cheng, L.; Liu, J.; Wu, K.M. Role of inclusion and microstructure on corrosion initiation and propagation of weathering steels in marine environment. J. Mater. Res. Technol. 2021, 10, 306–321. [Google Scholar] [CrossRef]
- Mishra, P.; Yavas, D.; Bastawros, A.F.; Hebert, K.R. Electrochemical impedance spectroscopy analysis of corrosion product layer formation on pipeline steel. Electrochim. Acta 2020, 346, 136232. [Google Scholar] [CrossRef]



| Figure | Fe/O Atomic Ratio | Phases | E (GPa) | H (GPa) | |
|---|---|---|---|---|---|
| 3 | (a) | 0.66 ± 0.06 | Mh/Mn | 22.6 ± 4.5 | 0.79 ± 0.25 |
| (b) | 0.60 ± 0.02 | L/Mh | 31.5 ± 5.2 | 1.27 ± 0.40 | |
| 4 | (a) | 0.65 ± 0.02 | G/Mh/Mn | 23.5 ± 3.4 | 1.02 ± 0.27 |
| (b) | 0.62 ± 0.01 | G/Mh | 40.2 ± 3.0 | 2.14 ± 0.29 | |
| (c) | 0.57 ± 0.02 | L/G/Mh | 51.8 ± 5.5 | 3.18 ± 0.41 | |
| 5 | (a) | 0.51 ± 0.02 | A | 35.4 ± 3.3 | 1.55 ± 0.27 |
| (b) | 0.65 ± 0.01 | Mh | 28.3 ± 1.8 | 1.32 ± 0.07 | |
| (c) | 0.65 ± 0.02 | Mh | 54.6 ± 5.2 | 2.79 ± 0.42 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Doi, K.; Tsuchiya, K. The Constituent Phases and Micromechanical Properties of Steel Corrosion Layers Generated by Hyperbaric-Oxygen Accelerated Corrosion Test. Materials 2023, 16, 4521. https://doi.org/10.3390/ma16134521
Jiang B, Doi K, Tsuchiya K. The Constituent Phases and Micromechanical Properties of Steel Corrosion Layers Generated by Hyperbaric-Oxygen Accelerated Corrosion Test. Materials. 2023; 16(13):4521. https://doi.org/10.3390/ma16134521
Chicago/Turabian StyleJiang, Baozhen, Kotaro Doi, and Koichi Tsuchiya. 2023. "The Constituent Phases and Micromechanical Properties of Steel Corrosion Layers Generated by Hyperbaric-Oxygen Accelerated Corrosion Test" Materials 16, no. 13: 4521. https://doi.org/10.3390/ma16134521
APA StyleJiang, B., Doi, K., & Tsuchiya, K. (2023). The Constituent Phases and Micromechanical Properties of Steel Corrosion Layers Generated by Hyperbaric-Oxygen Accelerated Corrosion Test. Materials, 16(13), 4521. https://doi.org/10.3390/ma16134521







