Abstract
Two crystalline phases, which are analogues of common secondary uranyl minerals, namely, becquerelite (Ca[(UO2)6O4 (OH)6]·8H2O) and phurcalite (Ca2[(UO2)3O2 (PO4)2]·7H2O) were identified on the surface of a Chernobyl corium-containing sample affected by hydrothermal alteration in distilled water at 150 °C for one year. Phases were characterized using Single-Crystal X-ray Diffraction Analysis (SCXRD) as well as optical and scanning electron microscopy. Features of the structural architecture of novel phases, which come from the specific chemical composition of the initial fragment of Chernobyl sample, are reported and discussed. Precise identification of these phases is important for modelling of severe nuclear accidents and their long-term consequences, including expected corium–water interaction processes at three damaged Units of the Nuclear Power Plant Fukushima Daiichi.
1. Introduction
A severe nuclear accident at the 4-th Unit of the Chernobyl Nuclear Power Plant (ChNPP) on 26 April 1986 was characterized with high-temperature interactions between U-oxide nuclear fuel, zircaloy cladding, and construction materials such as steel, serpentine and concrete [1]. Products of corium formation and solidification in the form of solid solutions “UO2-ZrO2” with different U/Zr ratio were identified in the matrices of so-called Chernobyl “lava” and “hot” particles [2,3]. In addition, corium products were discovered recently in the matrix of an unusual material which consisted of mainly molten and oxidized steel [4]. Such a material was formed during an initial very high-temperature (at least 2400–2600 °C) stage of the accident and it was injected into room 305/2 (right below the reactor core) where it rapidly solidified without interaction with silicate construction material (serpentine and concrete). According to a very cautious estimate, room 305/2 contains about 60 tons of the fuel [5].
It was found (for the first time in 1990) that matrices of Chernobyl “lava” interact with the environment. This process is accompanied with the formation of uranyl-phases such as UO4·4H2O; UO3·2H2O; UO2·CO3; Na4 (UO2) (CO3)3, etc. [6,7]. Moreover, the formation of uranyl phases, as assumed, could happen on the surface of some “lava” samples stored under laboratory conditions without humidity control [3,8].
The experimental study of the chemical alteration of Chernobyl corium and “lava” is very important in order to model behavior of these highly radioactive materials over long periods of time [9,10,11]. The information obtained can be applied to predict properties of molten fuel materials contacting water since 2011 at Units#1, 2 and 3 of the Fukushima Daiichi Nuclear Power Plant (F-1 NPP).
Herein, we report the results of precise phase identifications of two uranyl compounds, which were formed on the surface of the Chernobyl sample collected in room 305/2 of the Chernobyl “Shelter” [4] and used in previous experiments on hydrochemical alteration [10]. New-formed phases were characterized using several experimental techniques including Single-Crystal X-ray Diffraction Analysis (SCXRD) as well as optical and scanning electron microscopy. Features of the structural architecture of novel phases, which come from the specific chemical composition of the initial fragment of the Chernobyl sample, are reported and discussed.
2. Materials and Methods
2.1. Chernobyl Corium-Containing Sample
The Chernobyl corium-containing sample (Figure 1) consisted of mainly Fe3O4 and inclusions of solid solutions “UO2-ZrO2” (i.e., corium solidification products) with a broad range of U/Zr ratio and was used for chemical alteration experiment in distilled water at 150 °C for one year. Details about chemical and phase composition of this sample have been reported before [4]. The main interest to study this particular type of Chernobyl highly radioactive sample is related to evaluating of physico-chemical durability of corium–steel interaction products over a long time in water under increased temperature. It is assumed that similar materials can be discovered in the near future at Units #1, 2 and 3 of F-1 NPP.
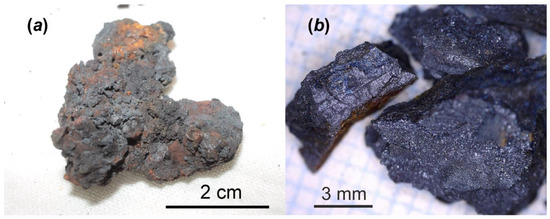
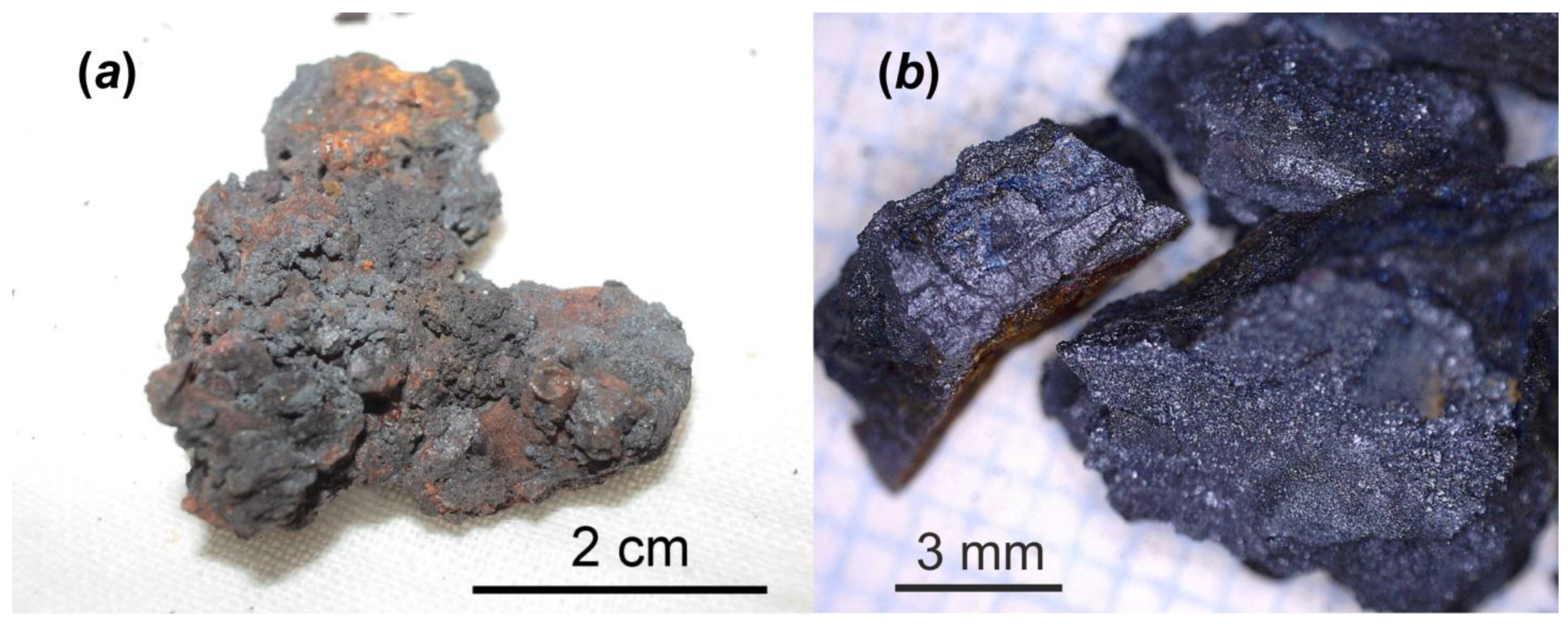
Figure 1.
One of the highly radioactive samples consisted of molten and oxidized steel and corium. It was collected by V.A. Zirlin and L.D. Nikolaeva in room 305/2 (right below former reactor core) of the Chernobyl “Shelter” in 1990: general view (a); and small broken fragments prepared for alteration test (b).
2.2. Hydrothermal Alteration Experiment
The 0.15-g fragment of the Chernobyl corium-containing sample and 10 mL of distilled water were placed in a steel autoclave equipped with a 25-mL Teflon liner. The experiment was carried out at a temperature of 150 °C and lasted about a year.
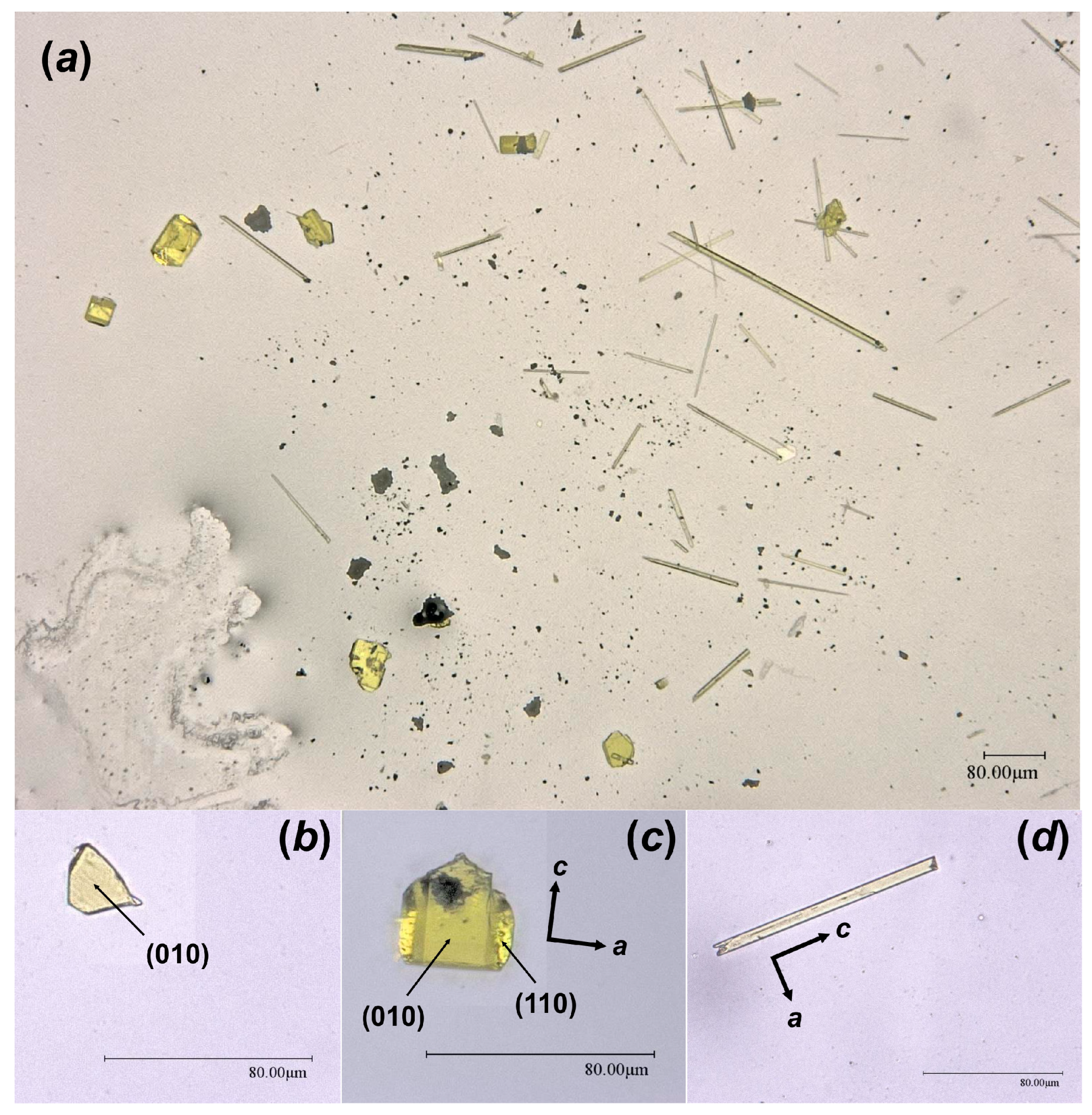
As the result of this hydrothermal experiment, a highly altered sample of corium-containing material was obtained, the surface of which was covered with yellowish crystals of various sizes and shapes (Figure 2). According to the visual observation of secondary phases using an optical microscope under polarized and cross polarized light, three types of morphologies were found: prismatic, lamellar and flattened needle-like crystals (Figure 3). Pictures of the secondary phases were collected using a digital microscope, Keyence VHX-1000. Further SCXRD studies showed that lamellar (Bqr_1) and prismatic (Bqr_2) crystals belong to the same structural type, an analog of the uranyl-oxide hydroxy-hydrate mineral becquerelite (Ca[(UO2)6O4 (OH)6]·8H2O) [12] (Figure 3b,c). Despite morphological differences, both types of crystals are flattened on {010}. Needle-like crystals (Phu) appeared to be analogs to another secondary U-bearing mineral, phurcalite (Ca2[(UO2)3O2 (PO4)2]·7H2O [13,14]) (Figure 3d).
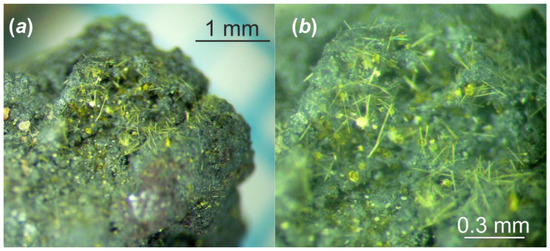
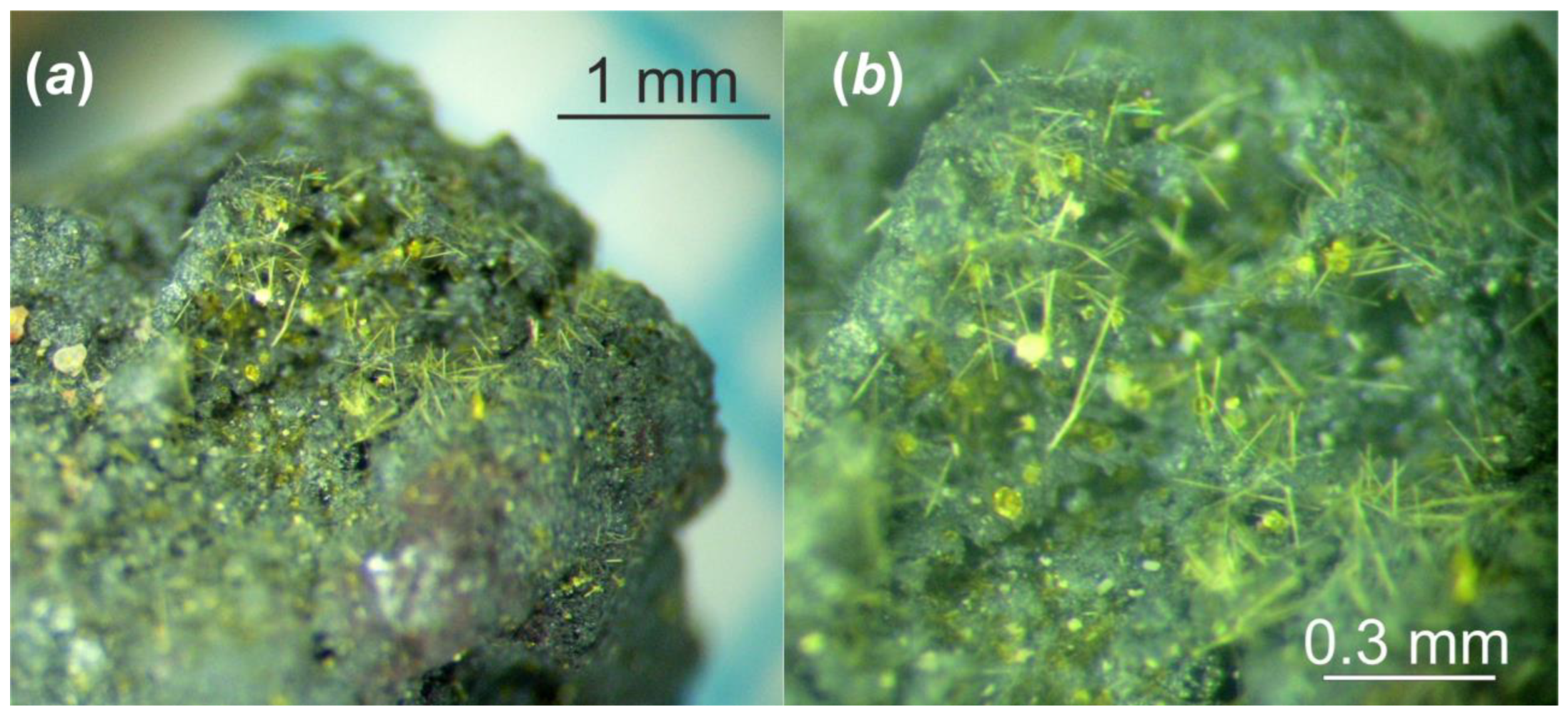
Figure 2.
The fragment of the Chernobyl corium-containing sample after hydrothermal alteration at (150 °C in distilled water for 1 year): general view (a); and its magnified image (b).
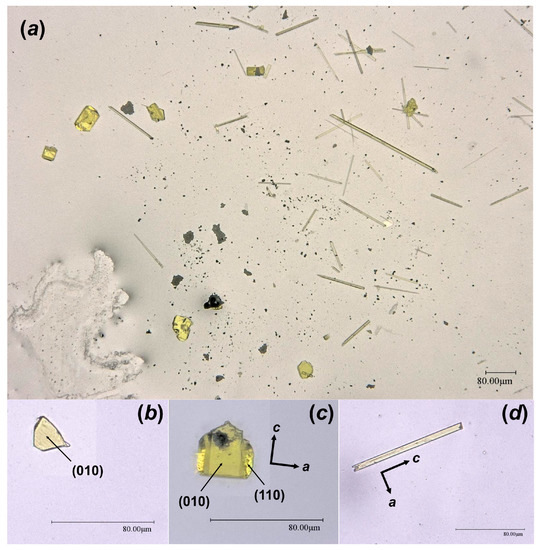
Figure 3.
Crystalline phases collected from the surface of the Chernobyl corium-containing sample after the hydrothermal alteration experiment (a); examined single crystals of Bqr_1 (b), Bqr_2 (c), and Phu (d) with shown indexed faces and crystallographic axes orientation.
2.3. Chemical Composition
The chemical analyses were carried out with a Hitachi FlexSEM 1000 scanning electron microscope equipped with EDS Xplore Contact 30 detector and Oxford AZtecLive STD system of analysis. Analytical conditions were: accelerating voltage 20 kV and beam current 5 nA. Only Ca, Mn, P, Si, U and O were recorded in Phu; Ca, U and O–in Bqr. Contents of other elements with atomic numbers higher than that of beryllium were below the detection limits. The following standards and X-ray lines were used: Ca–CaF2, Kα; Mn–Mn2SiO4, Kα; Si–SiO2, Kα; P–NdP5O14, Kα; U–UO2, Mβ.
The chemical composition of Bqr is (wt.%, mean of five spots, H2O content calculated based on structure): CaO 2.77, UO3 87.32, H2O 10.07, total 100.16. The empirical formula based on 30 O apfu is Ca0.97U6+6.01H22O30, or, taking into consideration the structural data, Ca0.97U6+6.01O16 (OH)6 ·8H2O.
The chemical composition of Phu is (wt.%, mean of seven spots, H2O content calculated based on structure): CaO 8.69, MnO 0.21, SiO2 0.43, P2O5 10.89, UO3 69.87, H2O 8.71, total 98.80. The empirical formula based on 23 O apfu is Ca1.92Mn0.04P1.91Si0.09U6+3.03H14O23, or, taking in consideration the structural data, (Ca1.92Mn0.04)Σ1.96U6+3.03 (P1.91Si0.09)Σ2.00O16 · 7H2O.
2.4. Single-Crystal X-ray Diffraction Studies
Single crystals of Bqr_1, Bqr_2 and Phu were selected under an optical microscope in polarized light, coated in oil-based cryoprotectant and mounted on a cryoloops. The diffraction data were collected using a Rigaku XtaLAB Synergy S X-ray diffractometer operated with a monochromated microfocus MoKα tube PhotonJet-S (λ = 0.71073 Å) at 50 kV and 1.0 mA and equipped with a CCD HyPix 6000HE hybrid photon-counting detector [15]. The frame width was 1.0o in ω, and exposures ranged from 12 to 110 s for each frame. CrysAlisPro software [16] was used for the integration and correction of diffraction data for polarization, background and Lorentz effects, as well as for absorption correction. An empirical absorption correction based on spherical harmonics implemented in the SCALE3 ABSPACK algorithm was applied. The unit-cell parameters (Table 1) were refined using the least-squares techniques. The structures were solved by a dual-space algorithm and refined using SHELX programs [17,18], incorporated in the OLEX2 program package [19]. The final model included coordinates and anisotropic displacement parameters for all non-H atoms. H atoms were localized from different Fourier maps and were included in the refinement with bond lengths and isotropic displacement parameters restraints. The crystal structures of Bqr_1 and Bqr_2 were refined as two-component inversion twins with statistically equal contribution of components (0.54 (3)/0.46 (3) and 0.56 (3)/0.44 (3), respectively). Supplementary crystallographic data were deposited in the Inorganic Crystal Structure Database (ICSD) and can be obtained by quoting the CSD 2256603-2256605 via www.ccdc.cam.ac.uk/structures/ (see Supplementary Materials).

Table 1.
Crystallographic data for lamellar (Bqr_1) and prismatic (Bqr_2) crystal analogs of becquerelite, and for needle-like (Phu) crystal analog of phurcalite.
3. Results
The mineral becquerelite was discovered a century ago [20], and its chemical composition and lattice parameters were then additionally reported [21,22]. The crystal structure of becquerelite was first reported by Piret-Meunier and Piret [12]. Later, the structural model of becquerelite was refined to better values of convergence factors [23,24] and spectroscopic studies have been performed [25,26,27]. Our SCXRD investigations confirm known structural models, and atom arrangements; naming from the latest model reported by Burns and Li [24] was taken as a starting set in the current work. It should be noted that all previous studies described a becquerelite unit cell in a non-conventional Pn21a setting (Table 2). Structural models of Bqr_1 and Bqr_2 are reported in a standard setting, which corresponds to the mm2 point group.

Table 2.
Comparison of the becquerelite and phurcalite unit cell parameters reported previously and in the current work.
The crystal structure of Bqr contains of six crystallographically independent U6+ cations. Each U6+ cation is strongly bonded to two O2- atoms, forming almost linearly within 7° O2-≡U6+≡O2- uranyl cations (Ur) with U–OUr bond lengths ranging from 1.724 (16) to 1.854 (19) Å (Table 3 and Table 4). All six Ur ions are equatorially coordinated by five O atoms, which results in the formation of pentagonal bipyramids (U–Oeq = 2.16 (2)–2.78 (3) Å). Besides, three out of five equatorial bonds are accounted for by O atoms of the hydroxyl groups. There is also one crystallographically unique Ca2+ cation in the structure of Bqr, which is coordinated by four OUr atoms and another four O atoms of H2O molecules with Ca–O = 2.36 (2)–3.049 (18) Å to form square antiprism coordination polyhedron.

Table 3.
Selected geometrical parameters in the structures of Bqr_1 and Bqr_2: bond lengths, Å; and bond-valence sums (BVS *, values are given in valence units).

Table 4.
Selected geometrical parameters in the structures of Bqr_1 and Bqr_2: bond lengths, Å; angles, °; and BVS * (v. u.).
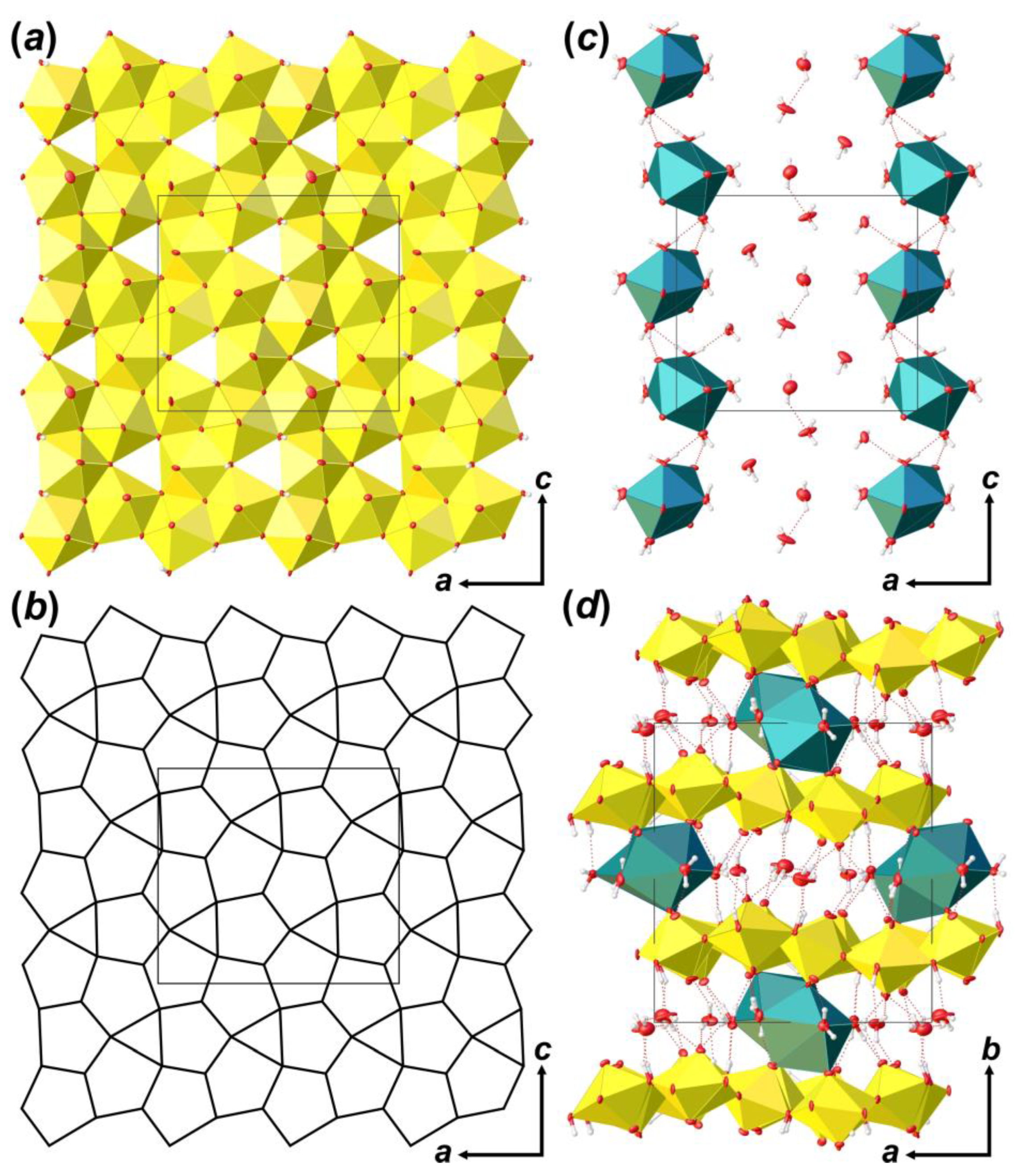
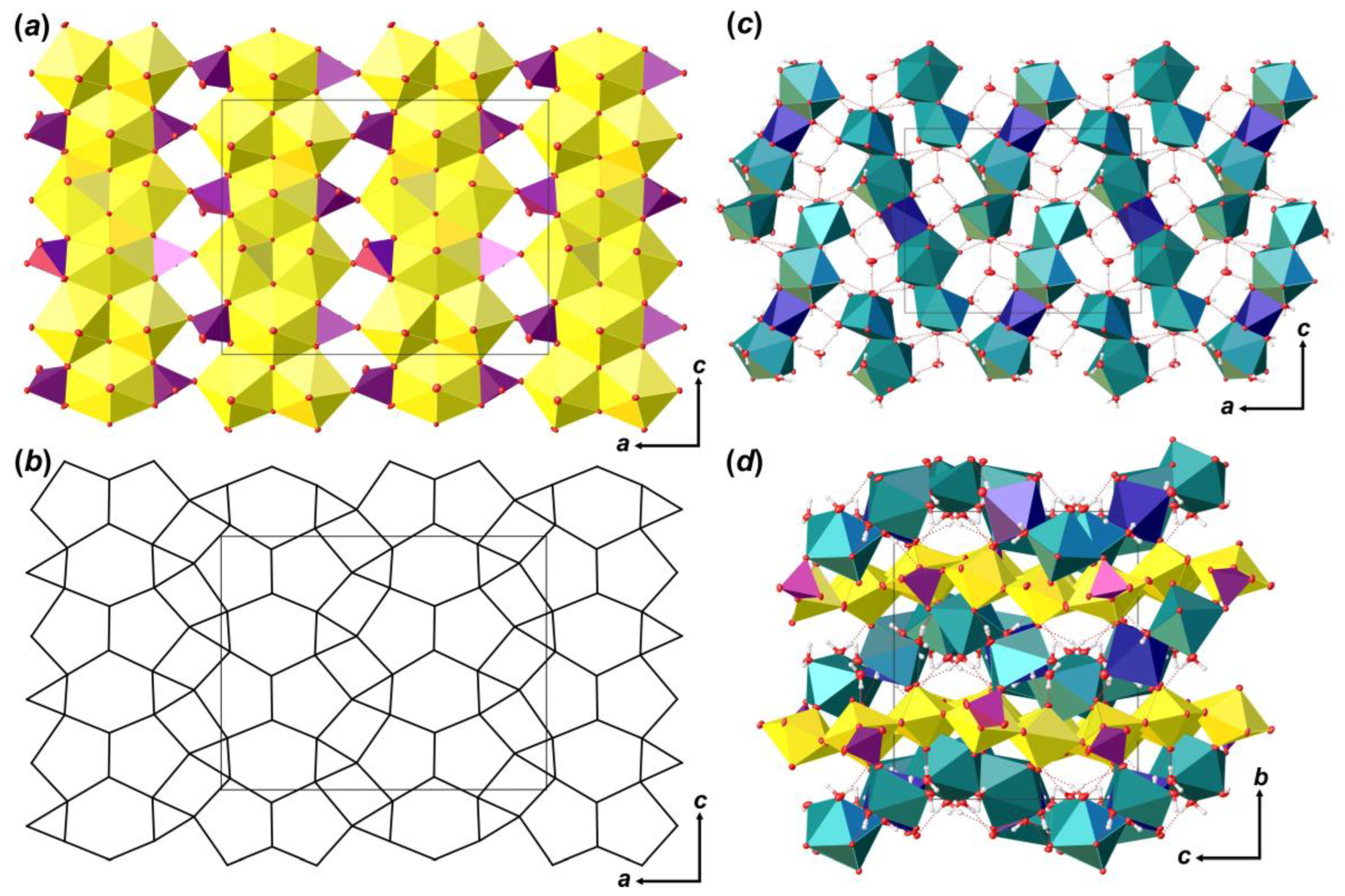
Coordination polyhedra of U atoms share equatorial edges and vertices to form layers of [(UO2)6O4 (OH)6]2– composition that are arranged parallel to (010) (Figure 4a). The layer of uranyl pentagonal bipyramids can be described in terms of anion-topology as formed by triangles and pentagons [34] with a …PDPD… stacking sequence of polygonal chains [35,36,37] and 5431 cyclic symbol [38,39] (Figure 4b). All pentagons are occupied by Ur, while all triangles are empty. This type of polygon arrangement is attributed to the so-called protasite or α-U3O8 anion-topology, which was also found in the structures of a number of minerals and synthetic compounds like protasite [23], billietite [23], compreignacite [40], masuyite [41], agrinierite [42], α-U3O8 [43], Na2[(UO2)3O3 (OH)2] [44], etc. In between the U-bearing layers, one crystallographically non-equivalent Ca2+ cation and eight H2O molecules are arranged (Figure 4c). Ca-centered polyhedra are organized in 1D units that are stretched along the [001]. Four out of eight H2O molecules are arranged in the coordination sphere of Ca2+ cations, and four molecules fill the gap between the chains of Ca-polyhedra and link with U-layers and Ca-chains only through the system of H-bonds (Figure 4d; Table 5). It should be noted that the system of H-bonds in the structure of Bqr, which was revealed after the assignment of H atoms sites, in general, corresponds to that proposed by Burns and Li [24]. However, several discrepancies can be found; for instance, OW24⋯O8 instead of OW24⋯OW27, or OW30⋯O10 instead of OW30⋯OW24 in Bqr and [24], respectively.
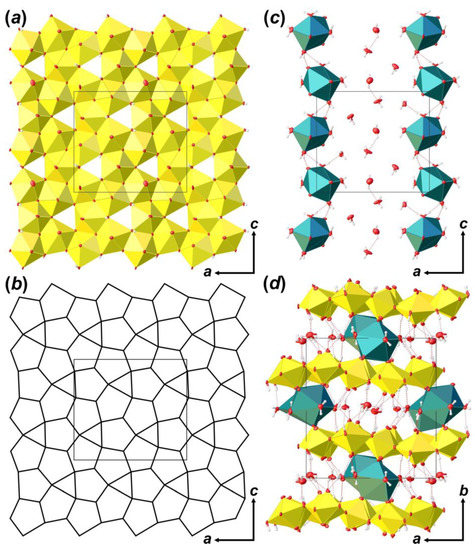
Figure 4.
The crystal structure of Bqr: polyhedral representation of the uranyl-hydroxy-oxide layer (a); its anion-topology (b); fragment of the interlayer space (c); and the structure of Bqr projected along the [001] (d). Legend: U polyhedra = yellow; Ca polyhedra = cyan; O atoms are red; hydrogen atoms are small white circles; H-bonds = dashed red lines; thermal ellipsoids are shown at the 50% probability level.

Table 5.
H-bonding system in the structure of Bqr_1. The most likely contacts are marked bold.
The mineral phurcalite was discovered by Deliens and Piret [13], who have reported on its orthorhombic symmetry, chemical composition and its lattice parameters. The structural model of phurcalite was reported the same year [14]. Later, the structure of phurcalite was refined several times for different specimens from various localities (Table 2) [28,29,30]. The most recent study reports on the H-bonding system, which was determined by a combination of SCXRD and modern computational methods [31]. The structural model of phurcalite reported in [31] was taken as a starting set of atoms in the current work.
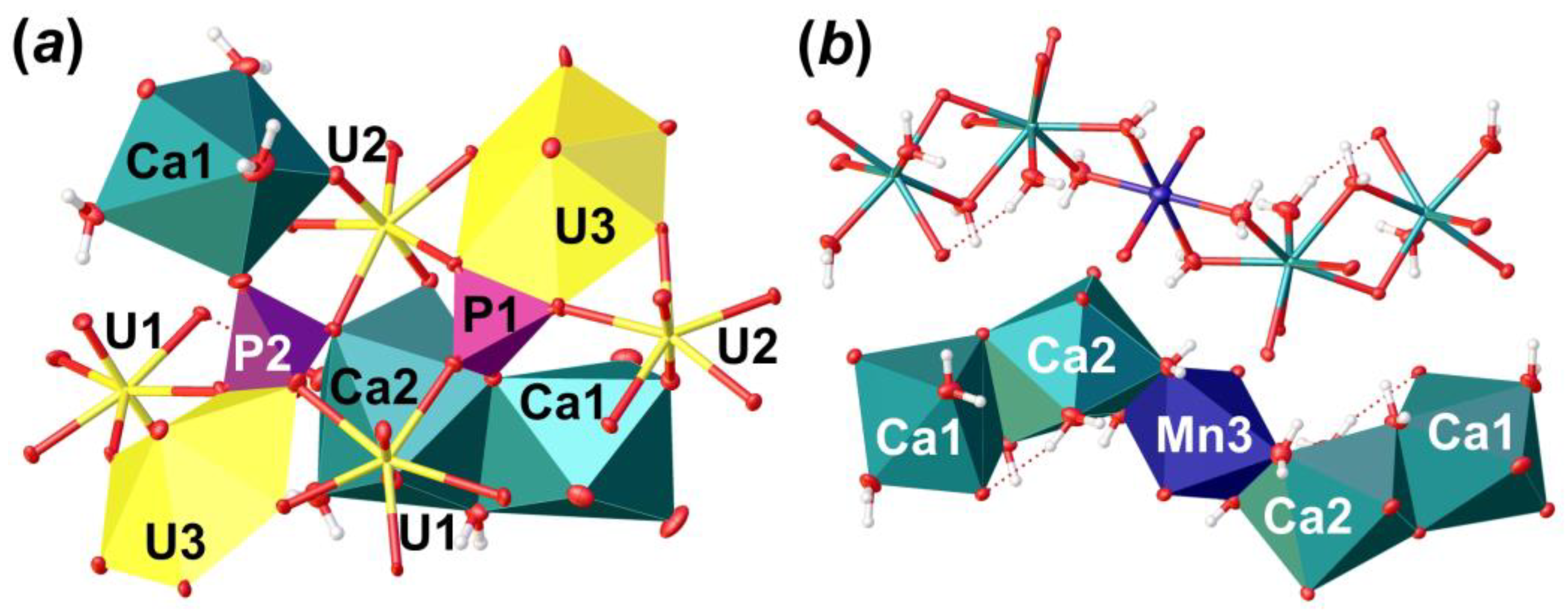
The crystal structure of Phu (Figure 5) contains three crystallographically independent U6+ cations. The U–OUr bond lengths range from 1.798 (3) to 1.822 (3) Å (Table 6). Ur1 and Ur2 ions are equatorially coordinated by five O atoms, which results in the formation of pentagonal bipyramids (U–Oeq = 2.252 (3)–2.512 (3) Å). The Ur3 ion is equatorially coordinated by six O atoms to form hexagonal bipyramid (U–Oeq = 2.221 (3)–2.790 (3) Å). There are two crystallographically non-equivalent P5+ cations in the structure of Phu, tetrahedrally coordinated by four O atoms each with <P–O> = 1.535 and 1.546 Å for P1 and P2, respectively. It is of interest that P-centered tetrahedra has slightly different coordination environment (Figure 6a). [P1O4]3– oxyanion shares an equatorial O2···O6 edge with Ur3 hexagonal bipyramid, an equatorial O11 vertex with Ur3 cation, and a bridged O13 atom, which is a part of a common O13···H2O20 edge between Ca1 and Ca2 polyhedra. The [P2O4]3– oxyanion also shares an equatorial O8···O15 edge with Ur3 hexagonal bipyramid, O18 atom with Ca1 coordination polyhedron, and O9 atom, which is a part of O5···O9 edge common between Ca2 and U2 coordination polyhedra. Slight deficiency of bond valence sums (BVS) for the P2 site, along with a slight elongation of the <P2–O> bond length (compared to that for P1; Table 6), and the results of chemical analysis, all indicate the presence of less than 0.1 Si atoms per formula unit (p.f.u.) in the structure of Phu; this allows considering P2 site as (P0.91Si0.09). Such a distribution most likely comes from the fact that the P1 site is more tightly bonded than the P2 site, which prevents a larger Si cation from occupying it. Similar crystal chemical restrictions for the larger Se6+ cations incorporation in tighter S6+ sites were observed in a course of phase formation studies in the mixed actinyl sulfate–selenate aqueous systems [45,46,47,48,49,50].
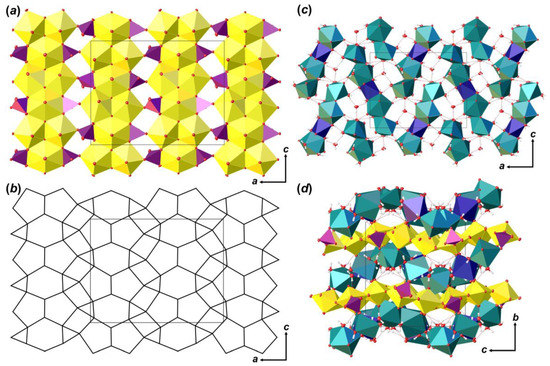
Figure 5.
The crystal structure of Phu: polyhedral representation of the uranyl phosphate layer (a); its anion-topology (b); fragment of the interlayer space (c); and the structure of Phu projected along the [100] (d). Legend: see Figure 4; Mn octahedra = dark blue; P tetrahedra = violet.

Table 6.
Selected geometrical parameters in the structure of Phu: bond lengths, Å; angles, °; and BVS* (v. u.).
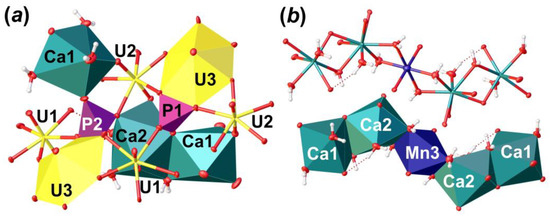
Figure 6.
The crystal structure of Phu: an arrangement of phosphate tetrahedra (a); and an organization of Ca-Mn pentamers from the interlayer space in polyhedral and ellipsoidal representation (b). Legend: see Figure 5.
The crystal structure of Phu is based on the uranyl phosphate layers of [(UO2)3O2 (PO4) (P0.91Si0.09O4)] compositions (Figure 5a), which are arranged parallel to (010). Anion-topology of the layer corresponds to the phosphuranylite type with 61524232 cyclic symbol [38,39], and can be described as formed by triangles, squares, pentagons and hexagons [34], where all hexagons and pentagons are occupied by Ur, all triangles are occupied by phosphate oxyanions (Figure 5b), and all squares stay vacant. This is one of the most common topological types of U-bearing 2D units. About 50 compounds of both natural and synthetic origin and various chemical compositions are known nowadays (e.g. [34,51,52,53,54,55,56,57]). Layers are formed by the large number of chains of dimers of edge-shared uranyl pentagonal bipyramids that are further connected by edge-shared U-centered hexagonal bipyramids. Neighbor chains are shifted by the half period as they lengthen, so that hexagonal bipyramids are arranged in front of dimers of pentagonal bipyramids. In these places, the chains are linked into a layer through the phosphate tetrahedra, which share an edge with hexagonal bipyramid from one chain, and a vertex with pentagonal bipyramid from a neighbor chain.
There are two non-equivalent Ca2+ sites, one Mn2+ site and six H2O molecules arranged in between the uranyl phosphate layers (Figure 5c). Ca1 site is surrounded by three H2O molecules and two OUr atoms, and two O atoms are shared with two distinct phosphate groups with <Ca1–O> = 2.424 Å. Ca2 site is surrounded by four H2O molecules, two OUr atoms, and two O atoms are shared with two distinct phosphate groups with <Ca1–O> = 2.498 Å. Ca1 and Ca2 coordination polyhedra share common O13···H2O20 edge to form dimer. The Mn3 site occupies an inversion center, which is arranged between two neighbor dimers of Ca-centered polyhedra. This site represents a rather classical octahedron surrounded by four H2O molecules (Mn3–H2O = 2.207 (3)–2.266 (4) Å) in the equatorial plane and another two apical OUr atoms with slightly elongated bonds (Mn3–OUr12 = 2.387 (3) Å), which can fit any of divalent cations. In the case of Phu crystal, an electron density peak of c.a. 1.1 e/Å3 was arranged in this site. Chemical analyses showed the presence of Mn in the examined samples, the amount of which corresponds to the site occupancy revealed in a course of SCXRD studies. Moreover, the presence of a cation at the Mn3 site results in a formation of the Ca1-Ca2-Mn3-Ca2-Ca1 pentamers via sharing common edges between Ca- and Mn-centered coordination polyhedra (Figure 6b). Pentamers are stretched along c.a. [102] and [-102] and separated by an additional H2O23 molecule, which links uranyl phosphate layers and pentamers only through H-bonds.
4. Discussion
Analogues of becquerelite discussed within this paper do not significantly differ in chemical composition and crystal structure from the previously studied natural samples. However, we report the crystal structure of becquerelite in the standard Pna21 setting for the first time, along with all H atom site assignments, which allow us to demonstrate the branchy H-bonding system. Investigation of phurcalite analogs have demonstrated differences in the structural architecture of known natural and obtained synthetic phases. Thus, the new octahedral site between the uranyl phosphate layers occupied by Mn atoms was found. It can be assumed that incorporation of a cation into the Mn3 site and the formation of pentamers result in a stronger linkage of uranyl phosphate layers into 3D structure. Compensation of an additional positive charge that comes with the incorporation of Mn2+ cations occurs due to the heterovalent isomorphism of Si4+ cations in the P5+ sites. Additional compensation, if needed, may come from the replacement of H2O16 and H2O19 molecules, which form an equatorial plane of Mn-centered octahedron and are included in the coordination sphere of Ca2 cations, by O2– anions or OH– groups. Thus, the formula of the studied Phu crystal according to the SCXRD and SEM data could be given as Ca2Mn0.03[(UO2)3O2 (PO4) (P0.94Si0.06O4)]·7H2O. It is of interest that, in previous studies of natural phurcalite crystals, no additional cation sites except for Ca1 and Ca2 have been found within the interlayer space. This example shows that a possible re-investigation of phurcalite mineral samples is needed to check if any additional cations that may occupy the Mn3 octahedral site.
The Chernobyl corium-containing sample used in this research is a product of high temperature co-melting of U-oxide fuel, zircaloy cladding, steel, serpentine and concrete [4]. As a result, it has a unique and complex chemical and phase compositions. It can explain the composition of uranyl phases formed during the alteration experiment. Uranium comes from the relicts of overheated nuclear fuel (UOx) and corium inclusions (U-Zr-O with high U/Zr ratio), which is easy to oxidize to the 6+ state in aqueous solutions. Calcium and Si come from the concrete. Phosphorus and Mn, most likely, come from construction steel of 10HSND grade (10XCHД in Russian), used in the low basic reactor plate “OR” (“OP” in Russian). This steel contains 0.5–0.8 wt.% Mn and up to 0.035 wt.% P [58].
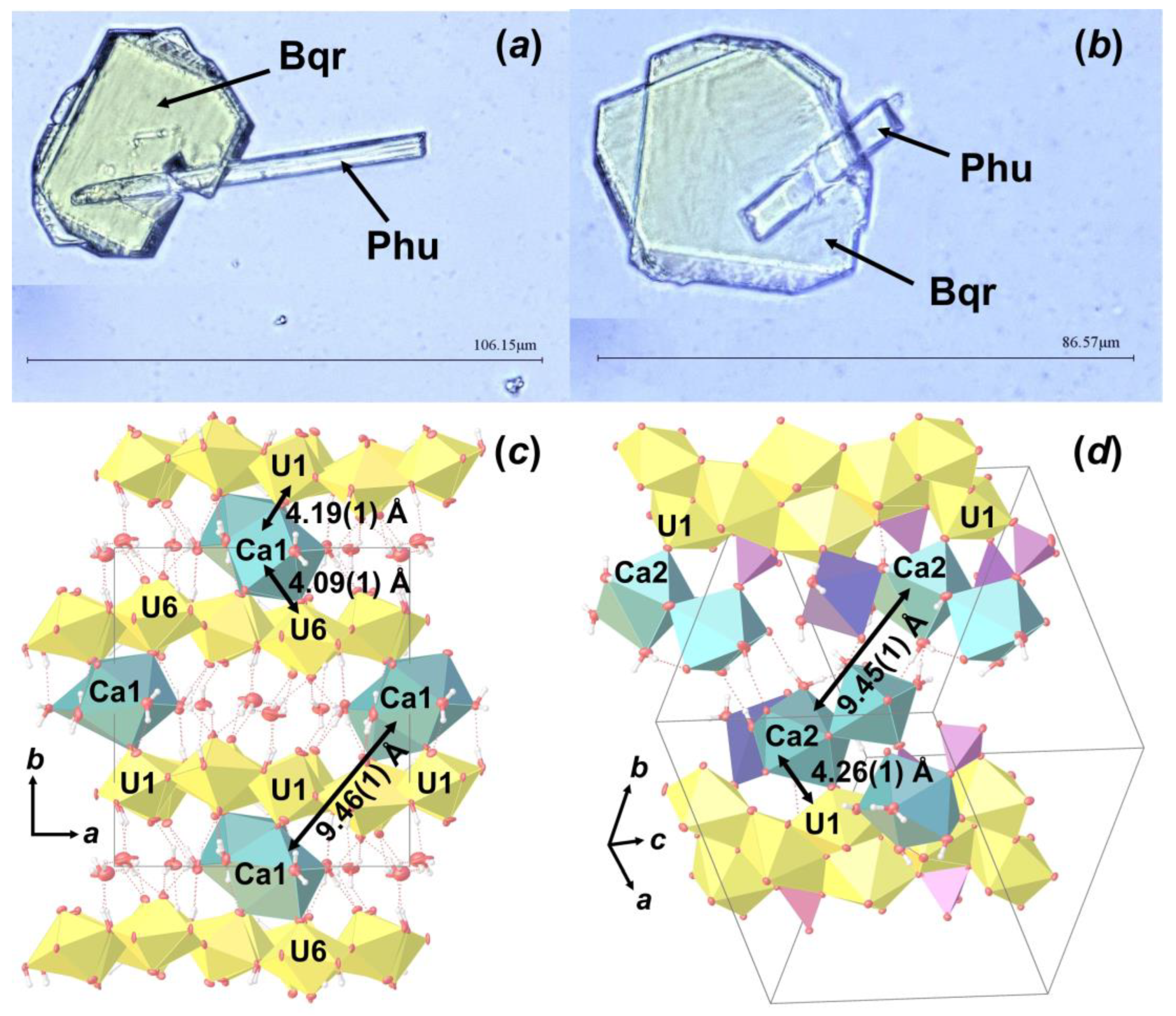
During optical microscopy studies of the alteration products, several intergrowth of lamina and needle crystals were found (Figure 7a,b). SCXRD experiments showed that these are intergrowths of Bqr and Phu, which can be described as follows: rotation of Phu unit cell relative to the Bqr by 142.83 ° around the c.a. [−0.25 0 1] axis, which corresponds to the approximate coincidence of the [001] direction in the structure of Bqr with the [−1−11] direction in the structure of Phu (Figure 7b,c). In these directions both structures have similar arrangement of Ca polyhedra and U bipyramids neighbor to them. Hence, one can assume that intergrowing relates exactly to these structural fragments. To our knowledge, this is the first reported evidence of becquerelite and phurcalite intergrowth.
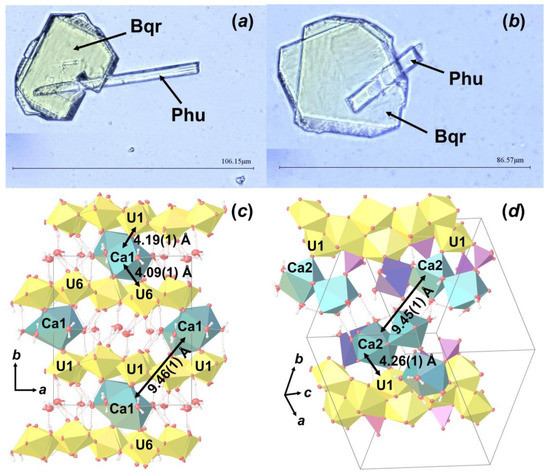
Figure 7.
Intergrowth of lamina Bqr crystals with needle-like Phu (a,b); and the arrangement of Bqr (c) and Phu (d) crystal structures, in which projections intergrowing occurs. Legend: see Figure 5.
5. Conclusions
Two analogues of common secondary uranyl minerals, becquerelite and phurcalite, formed on the surface of a Chernobyl corium-containing sample affected by hydrothermal alteration were identified and studied in detail. The results obtained are proposed to be included into a database for modelling of long-term behavior of corium–steel interaction products forming as a consequence of severe nuclear accidents.
The fact that, during hydrothermal experiment, only crystals with dense polymerization of uranyl polyhedra (i.e., that share common edges) were obtained, confirms our recently made assumption [56,57,59,60] that such structures should not crystalize at ambient temperature and an additional energy source is needed to obtain phases with dense architecture, while uranyl minerals and compounds with sparse structural units (i.e., that share only common vertices) can crystallize from aqueous solutions at ambient conditions.
The results of reported studies are important not only for predicting corium aging in anticipation of decommissioning, but also for evaluating the stability of corium, spent fuel, and cemented U-bearing wastes under temporary storage and final repository conditions [61,62,63].
The chemical stability of the corium should be modelled taking into account potential formation of secondary uranyl phases and their further chemical and physical alteration. Short-term leach tests do not provide enough time for the growth of secondary mineral-like phases. Therefore, such an important process is usually not taken into account in the models [64,65,66,67,68,69], although uranyl phases are obviously less stable than U oxide.
It is known from the model experiments that analogues of becquerelite are formed during the aging of spent fuel [70]. Thus, one can assume that the initial chemical forms of uranium are less important in most cases for the formation of these phases than particular oxidizing conditions and properties of the environment [71,72,73,74,75,76,77]. Corium, which possibly formed at F-1 NPP may differ chemically from Chernobyl corium [4,10,78], but the products of its alteration in water would be similar.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma16134533/s1, CSD 2256603-2256605 for Bqr_1, Bqr_2, and Phu, respectively: contain the supplementary crystallographic data for this paper.
Author Contributions
Conceptualization, methodology, investigation, writing—original draft preparation; writing-review & editing, visualization, V.V.G., B.E.B., B.Y.Z., A.V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (grant No. 23-17-00080).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Vladimir Zirlin and Larisa Nikolaeva (of the V.G. Khlopin Radium Institute, Russia), who have collected manually the highly radioactive corium-containing samples under extremely dangerous conditions inside Chernobyl “Shelter” in 1990. The XRD and optical microscopy studies have been performed at the X-ray Diffraction Centre of St. Petersburg State University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burakov, B.E.; Anderson, E.B.; Shabalev, S.I.; Strykanova, E.E.; Ushakov, S.V.; Trotabas, M.; Blanc, J.-Y.; Winter, P.; Duco, J. The Behaviour of Nuclear Fuel in First Days of the Chernobyl Accident. MRS Online Proc. Libr. 1997, 465, 1297–1308. [Google Scholar] [CrossRef]
- Burakov, B.E.; Shabalev, S.I.; Anderson, E.B. Principal Features of Chernobyl Hot Particles: Phase, Chemical and Radionuclide Compositions. In Role of Interfaces in Environmental Protection; NATO Science Series; Barany, S., Ed.; Springer: Dordrecht, The Netherlands, 2003; Volume 24, pp. 145–151. [Google Scholar]
- Burakov, B.E. Lava-like materials formed and solidified during Chernobyl accident. In Comprehensive Nuclear Materials, 2nd ed.; Konings, R.J.M., Stoller, R.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, pp. 525–540. [Google Scholar]
- Shiryaev, A.A.; Burakov, B.E.; Yapaskurt, V.O.; Zubekhina, B.Y.; Averin, A.A.; Petrov, Y.; Orlova, V.; Silantyeva, E.; Nickolsky, M.S.; Zirlin, V.A.; et al. Products of molten corium-metal interaction in Chernobyl accident: Composition and leaching of radionuclides. Progr. Nucl. Energy 2022, 152, 104373. [Google Scholar] [CrossRef]
- Borovoi, A.A.; Lagunenko, A.S.; Pazukhin, E.M. Estimating the Amount of Fuel in Cellar Building 305/2 at Chernobyl Unit 4. At. Energy 1998, 84, 295–299. [Google Scholar] [CrossRef]
- Burakov, B.E.; Anderson, E.B.; Strykanova, E.E. Secondary uranium minerals on the surface of Chernobyl “lava”. MRS Online Proc. Libr. 1997, 465, 1309–1311. [Google Scholar] [CrossRef]
- Teterin, Y.A.; Baev, A.S.; Bogatov, S.A. X-Ray photoelectron study of samples containing reactor fuel from “lava” and products growing on it which formed at Chernobyl NPP due to the accident. J. Electron Spectrosc. Relat. Phenom. 1994, 68, 685–694. [Google Scholar] [CrossRef]
- Zubekhina, B.Y.; Burakov, B.E. Leaching of actinides and other radionuclides from matrices of Chernobyl “lava” as analogues of vitrified HLW. J. Chem. Thermodyn. 2017, 114, 25–29. [Google Scholar] [CrossRef]
- Zubekhina, B.Y.; Burakov, B.E.; Bogdanova, O.G.; Petrov, Y.Y. Leaching of 137Cs from Chernobyl fuel debris: Corium and “lava”. Radiochim. Acta 2019, 107, 1155–1160. [Google Scholar] [CrossRef]
- Zubekhina, B.; Burakov, B.; Silanteva, E.; Petrov, Y.; Yapaskurt, V.; Danilovich, D. Long-Term Aging of Chernobyl Fuel Debris: Corium and “Lava”. Sustainability 2021, 13, 1073. [Google Scholar] [CrossRef]
- Olkhovyk, Y.A.; Ojovan, M.I. Corrosion Resistance of Chernobyl NPP Lava Fuel-Containing Masses. Innov. Corros. Mater. Sci. 2015, 5, 36–42. [Google Scholar] [CrossRef]
- Piret-Meunier, J.; Piret, P. Nouvelle détermination de la structure cristalline de la bequerelite. Bull. Minéral. 1982, 105, 606–610. [Google Scholar] [CrossRef]
- Deliens, M.; Piret, P. La phurcalite, Ca2(UO2)3(PO4)2(OH)4·4H2O, nouveau minéral. Bull. Minéral. 1978, 101, 356–358. [Google Scholar] [CrossRef]
- Piret, P.; Declercq, J.-P. Phurcalite. Acta Cryst. 1978, B34, 1677–1679. [Google Scholar] [CrossRef]
- Fraser, W. Diffractometers for modern X-ray crystallography: The XtaLAB Synergy X-ray diffractometer platform. Rigaku J. 2020, 36, 37–47. [Google Scholar]
- CrysAlisPro Software System; Version 1.171.41.94a; Rigaku Oxford Diffraction: Oxford, UK, 2021.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Schoep, A. Sur la becquerélite, nouveau minéral radioactif. C.R. Hebd. Séances Acad. Sci. 1922, 174, 1240–1242. [Google Scholar]
- Frondel, J.W.; Cuttitta, F. Studies of uranium minerals (XII): The status of billietite and becquerelite. Amer. Mineral. 1953, 30, 1019–1024. [Google Scholar]
- Christ, C.L.; Clark, J.R. Crystal chemical studies of uranyl oxide hydrates. Amer. Mineral. 1960, 45, 1026–1061. [Google Scholar]
- Pagoaga, M.K.; Appleman, D.E.; Stewart, J.M. Crystal structures and crystal chemistry of the uranyl oxide hydrates becquerelite, billietite, and protasite. Amer. Mineral. 1987, 72, 1230–1238. [Google Scholar]
- Burns, P.C.; Li, Y. The structures of becquerelite and Sr-exchanged becquerelite. Amer. Mineral. 2002, 87, 550–557. [Google Scholar] [CrossRef]
- Amayri, S.; Arnold, T.; Foerstendorf, H.; Geipel, G.; Bernhard, G. Spectroscopic characterization of synthetic becquerelite, Ca[UO2)6O4(OH)6]·8H2O, and swartzite, CaMg[UO2(CO3)3]·12H2O. Can. Mineral. 2004, 42, 953–962. [Google Scholar] [CrossRef]
- Schindler, M.; Hawthorne, F.C.; Burns, P.C.; Maurice, P.A. Dissolution of uranyl-oxide-hydroxy-hydrate minerals. II. Becquerelite. Can. Mineral. 2006, 44, 1207–1225. [Google Scholar] [CrossRef]
- Frost, R.L.; Čejka, J.; Weier, M.L. Raman spectroscopic study of the uranyl oxyhydroxide hydrates: Becquerelite, billietite, curite, schoepite and vandendriesscheite. J. Raman Spec. 2007, 38, 460–466. [Google Scholar] [CrossRef]
- Braithwaite, R.S.W.; Paar, W.H.; Chisholm, J.E. Phurcalite from Dartmoor, Southwest England, and its identity with ‘nisaite’ from Portugal. Mineral. Mag. 1989, 53, 583–589. [Google Scholar] [CrossRef]
- Atencio, D.; Neumann, R.; Silva, A.J.G.C.; Mascarenhas, Y.P. Phurcalite from Perus, São Paulo, Brazil, and redetermination of its crystal structure. Can. Mineral. 1991, 29, 95–105. [Google Scholar]
- Plášil, J. Crystal structure of phurcalite, Ca2[(UO2)3O2(PO4)2]·7H2O, from Jáchymov. Bull. Mineral. Petrolog. 2020, 28, 276–280. [Google Scholar] [CrossRef]
- Plášil, J.; Kiefer, B.; Ghazisaeed, S.; Philippo, S. Hydrogen bonding in the crystal structure of phurcalite, Ca2[(UO2)3O2(PO4)2]·7H2O: Single-crystal X-ray study and TORQUE calculations. Acta Cryst. 2020, B76, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.C.; Ewing, R.C.; Hawthorne, F.C. The crystal chemistry of hexavalent uranium: Polyhedron geometries, bond-valence parameters, and polymerization of polyhedra. Can. Mineral. 1997, 35, 1551–1570. [Google Scholar]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Cryst. 2015, B71, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Lussier, A.J.; Lopez, R.A.K.; Burns, P.C. A revised and expanded structure hierarchy of natural and synthetic hexavalent uranium compounds. Can. Mineral. 2016, 54, 177–283. [Google Scholar] [CrossRef]
- Miller, M.L.; Finch, R.J.; Burns, P.C.; Ewing, R.C. Description and classification of uranium oxide hydrate sheet anion topologies. J. Mater. Res. 1996, 11, 3048–3056. [Google Scholar] [CrossRef]
- Burns, P.C. The Crystal Chemistry of Uranium. In Uranium: Mineralogy, Geochemistry, and the Environment; Burns, P.C., Finch, R., Eds.; Walter de Gruyter GmbH & Co KG: Munich, Germany, 1999; Volume 38, pp. 23–90. [Google Scholar]
- Krivovichev, S.V.; Plášil, J. Mineralogy and Crystallography of Uranium. In Uranium: From Cradle to Grave; Burns, P.C., Sigmon, G.E., Eds.; Mineralogical Association of Canada Short Courses: Quebec, QC, Canada, 2013; Volume 43, pp. 15–119. [Google Scholar]
- Krivovichev, S.V.; Burns, P.C. Actinide compounds containing hexavalent cations of the VI group elements (S, Se, Mo, Cr, W). In Structural Chemistry of Inorganic Actinide Compounds; Krivovichev, S.V., Burns, P.C., Tananaev, I.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 95–182. [Google Scholar]
- Krivovichev, S.V. Structural Crystallography of Inorganic Oxysalts; Oxford University Press: Oxford, UK, 2008; 303p. [Google Scholar]
- Burns, P.C. The structure of compreignacite, K2[(UO2)3O2(OH)3]2(H2O)7. Can. Mineral. 1998, 36, 1061–1067. [Google Scholar]
- Burns, P.C.; Hanchar, J.M. The structure of masuyite, Pb[(UO2)3O3(OH)2](H2O)3, and its relationship to protasite. Can. Mineral. 1999, 37, 1483–1491. [Google Scholar]
- Cahill, C.L.; Burns, P.C. The structure of agrinierite: A Sr-containing uranyl oxide hydrate mineral. Amer. Mineral. 2000, 85, 1294–1297. [Google Scholar] [CrossRef]
- Loopstra, B.O. Crystal-structure of α-U3O8. J. Inorg. Nucl. Chem. 1977, 39, 713–1714. [Google Scholar] [CrossRef]
- Li, Y.P.; Burns, P.C. The structures of two sodium uranyl compounds relevant to nuclear waste disposal. J. Nucl. Mater. 2001, 299, 219–226. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Krivovichev, V.G.; Tananaev, I.G. Mixed uranyl sulfate-selenates: Variable composition and crystal structures. Cryst. Growth Des. 2016, 16, 4482–4492. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Tananaev, I.G. Selective Se-for-S substitution in Cs-bearing uranyl compounds. J. Solid State Chem. 2017, 248, 126–133. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Izatulina, A.R.; Krivovichev, S.V.; Tananaev, I.G. Chemically Induced Polytypic Phase Transitions in the Mg[(UO2)(TO4)2(H2O)](H2O)4 (T = S, Se) System. Inorg. Chem. 2019, 58, 14760–14768. [Google Scholar] [CrossRef]
- Kornyakov, I.V.; Gurzhiy, V.V.; Szymanowski, J.E.S.; Zhang, L.; Perry, S.N.; Krivovichev, S.V.; Burns, P.C. A Novel family of Np(VI) oxysalts: Crystal structures, calorimetry, thermal behavior and comparison with U(VI) compounds. Cryst. Growth Des. 2019, 19, 2811–2819. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Kornyakov, I.V.; Szymanowski, J.E.S.; Felton, D.; Tyumentseva, O.S.; Krzhizhanovskaya, M.G.; Krivovichev, S.V.; Burns, P.C. Chemically-induced structural variations of a family of Cs2[(AnO2)2(TO4)3] (An = U, Np; T = S, Se, Cr, Mo) compounds: Thermal behavior, calorimetry studies and spectroscopy characterization of Cs uranyl sulfate and selenate. J. Solid State Chem. 2020, 282, 121077. [Google Scholar] [CrossRef]
- Kornyakov, I.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Tananaev, I.G.; Gurzhiy, V.V. Crystal chemistry of the M2+[(UO2)(T6+O4)2(H2O)](H2O)4 (M2+ = Mg, Mn, Fe, Co, Ni and Zn; T6+ = S, Se) compounds: The interplay between chemical composition, pH and structural architecture. CrystEngComm 2021, 23, 1140–1148. [Google Scholar] [CrossRef]
- Demartin, F.; Diella, V.; Donzelli, S.; Gramaccioli, C.M.; Pilati, T. The importance of accurate crystal structure determination of uranium minerals. I. Phosphuranylite KCa(H3O)3(UO2)7(PO4)4O4·8H2O. Acta Crystallogr. 1991, B47, 439–446. [Google Scholar] [CrossRef]
- Locock, A.; Burns, P.C. The crystal structure of bergenite, a new geometrical isomer of the phosphuranilite group. Can. Mineral. 2003, 41, 91–101. [Google Scholar] [CrossRef]
- Serezhkina, L.B.; Peresypkina, E.V.; Virovets, A.V.; Pushkin, D.V.; Verevkin, A.G. Synthesis and structure of Cs[UO2(SeO4)(OH)]·nH2O (n = 1.5 or 1). Crystallogr. Rep. 2010, 55, 381–385. [Google Scholar] [CrossRef]
- Juillerat, C.A.; Moore, E.E.; Kocevski, V.; Besmann, T.; Zur Loye, H.-C. A Family of Layered Phosphates Crystallizing in a Rare Geometrical Isomer of the Phosphuranylite Topology: Synthesis, Characterization, and Computational Modeling of A4[(UO2)3O2(PO4)2] (A = Alkali Metal) Exhibiting Intralayer Ion Exchange. Inorg. Chem. 2018, 57, 4726–4738. [Google Scholar] [CrossRef] [PubMed]
- Gurzhiy, V.V.; Plášil, J. Structural complexity of natural uranyl sulfates. Acta Crystallogr. 2019, B75, 39–48. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Kuporev, I.V.; Kovrugin, V.M.; Murashko, M.N.; Kasatkin, A.V.; Plášil, J. Crystal Chemistry and Structural Complexity of Natural and Synthetic Uranyl Selenites. Crystals 2019, 9, 639. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Kalashnikova, S.A.; Kuporev, I.V.; Plášil, J. Crystal Chemistry and Structural Complexity of the Uranyl Carbonate Minerals and Synthetic Compounds. Crystals 2021, 11, 704. [Google Scholar] [CrossRef]
- GOST 6713-91; Low-Alloyed Structural Rolled Stock for Bridge Building. GostPerevod: Moscow, Russia, Interstate Standard. 1991.
- Tyumentseva, O.S.; Kornyakov, I.V.; Britvin, S.N.; Zolotarev, A.A.; Gurzhiy, V.V. Crystallographic Insights into Uranyl Sulfate Minerals Formation: Synthesis and Crystal Structures of Three Novel Cesium Uranyl Sulfates. Crystals 2019, 9, 660. [Google Scholar] [CrossRef]
- Kornyakov, I.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Gurzhiy, V.V. Dimensional evolution in hydrated K+-bearing uranyl sulfates: From 2D-sheets to 3D-frameworks. CrystEngComm 2020, 22, 4621–4629. [Google Scholar] [CrossRef]
- Gelfort, E. Nutzung der Spaltprodtikte nach Aufarbeitung Ausgedienter Brennelemente. Atomwirtsch. Atomtech. 1985, 30, 32–36. [Google Scholar]
- Finch, R.J.; Ewing, R.C. The corrosion of uraninite under oxidizing conditions. J. Nucl. Mater. 1992, 190, 133–156. [Google Scholar] [CrossRef]
- Finch, R.; Murakami, T. Systematics and paragenesis of Uranium minerals. Rev. Mineral. 1999, 38, 91–179. [Google Scholar]
- Hofmann, P.; Politis, C. The kinetic of the uranium dioxide-zircaloy reactions at high temperatures. J. Nucl. Mater. 1979, 87, 375–397. [Google Scholar] [CrossRef]
- Skokan, A.; Holleck, H. The significance of chemical reactions between reactor materials under core melting conditions. Nucl. Eng. Des. 1987, 103, 107–113. [Google Scholar] [CrossRef]
- Shiozawa, S.; Ichikawa, M.; Fujishiro, T. Studies of the UO2-zircaloy chemical interaction and fuel rod relocation modes in a severe fuel damage accident. J. Nucl. Mater. 1988, 154, 116–122. [Google Scholar] [CrossRef]
- Kim, K.T.; Olander, D.R. Dissolution of uranium dioxide by molten zircaloy. II. Convection-controlled reaction. J. Nucl. Mater. 1988, 154, 102–115. [Google Scholar] [CrossRef]
- Hayward, P.J.; George, I.M. Dissolution of UO2 in molten zircaloy-4 part 2: Phase evolution during dissolution and cooling. J. Nucl. Mater. 1994, 208, 43–52. [Google Scholar] [CrossRef]
- Hayward, P.J.; Hofmann, P.; Stuckert, J.; Berdyshev, A.V.; Veshchunov, M.S. UO2 Dissolution by Molten Zircaloy: New Experimental Results and Modeling; Forschungszentrum Karlsruhe GmbH: Karlsruhe, Germany, 1999; 81p. [Google Scholar]
- Wronkiewicz, D.J.; Bates, J.K.; Gerding, T.J.; Veleckis, E.; Tani, B.S. Uranium release and secondary phase formation during unsaturated testing of UO2 at 90 °C. J. Nucl. Mater. 1992, 190, 107–127. [Google Scholar] [CrossRef]
- Alwan, A.K.; Williams, P.A. The aqueous chemistry of uranium minerals. Part 2. Minerals of the liebigite group. Mineral. Mag. 1980, 43, 665–667. [Google Scholar] [CrossRef]
- Clark, D.L.; Hobart, D.E.; Neu, M.P. Actinide carbonate complexes and their importance in actinide environmental chemistry. Chem. Rev. 1995, 95, 25–48. [Google Scholar] [CrossRef]
- Stefaniak, E.A.; Alsecz, A.; Frost, R.; Mathe, Z.; Sajo, I.E.; Torok, S.; Worobiec, A.; Van Grieken, R. Combined SEM/EDX and micro-Raman spectroscopy analysis of uranium minerals from a former uranium mine. J. Hazard Mater. 2009, 168, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Plášil, J. Oxidation–hydration weathering of uraninite: The current state-of-knowledge. J. Geosci. 2014, 59, 99–114. [Google Scholar] [CrossRef]
- Driscoll, R.J.P.; Wolverson, D.; Mitchels, J.M.; Skelton, J.M.; Parker, S.C. A Raman spectroscopic study of uranyl minerals from Cornwall, UK. RSC Adv. 2014, 4, 59137–59149. [Google Scholar] [CrossRef]
- Pauliš, P.; Babka, K.; Sejkora, J.; Škácha, P. Uranové Minerály České Republiky a Jejich Nejvýznamnější Naleziště; Kuttna: Kutná Hora, Czech Republic, 2016; 570p. (In Czech) [Google Scholar]
- Plášil, J. Uranyl-oxide hydroxy-hydrate minerals: Their structural complexity and evolution trends. Eur. J. Mineral. 2018, 30, 237–251. [Google Scholar] [CrossRef]
- Grambow, B.; Nitta, A.; Shibata, A.; Koma, Y.; Utsunomiya, S.; Takami, R.; Fueda, K.; Ohnuki, T.; Jegou, C.; Laffolley, H.; et al. Ten years after the NPP accident at Fukushima: Review on fuel debris behavior in contact with water. J. Nucl. Sci. Technol. 2022, 59, 1–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).