Abstract
This study aimed to obtain and investigate ZnCr2Se4 single crystals doped with rhenium. The single crystals were obtained by applying chemical vapour transport. An X-ray study confirmed the cubic () structure of the tested crystals. Thermal, magnetic, electrical, and specific heat measurements accurately determined the physicochemical characteristics, which revealed that the obtained single crystals are p-type semiconductors with antiferromagnetic order below the Néel temperature TN = 21.7 K. The Debye temperature had a value of 295 K. The substitution of Re-paramagnetic ions, possessing a screened 5d-shell, in place of Zn-diamagnetic ions, caused an increase in the activation energy, Fermi energy, and Fermi temperature compared to the pure ZnCr2Se4. The boost of the dc magnetic field induced a shift of TN towards lower temperatures and a spin fluctuation peak visible at Hdc = 40 and 50 kOe. The obtained single crystals are thermally stable up to 1100 °C.
1. Introduction
In the modern world, the synthesis of single crystals is a vast field of activity, encompassing theoretical aspects and device fabrication. Single crystals create the foundations of modern technology. Many types of crystals are needed for lasers, optical components, light-emitting diodes, electron emitters for electron microscopes, and countless other applications.
Materials with a spinel structure based on chromium and selenium (ACr2Se4, where A=Cu, Cd, and Zn) and doped with various elements are attractive for their magnetic, electrical, and thermal properties. They can be insulators, semiconductors, conductors, superconductors, ferrimagnets, ferromagnets, antiferromagnets, and Pauli paramagnets. In addition, these compounds are stable at high temperatures, so they can be applied in machines and devices operating at high temperatures. Doped spinel compounds, in which thermal conductivity exists, are possible for commercial application because thermoelectricity is a phenomenon that allows energy to be converted inside a solid. Thermoelectric cooling is the only environmentally friendly cooling used in power generators, computers, infrared detectors, and electronics and optoelectronics [1]. Doped chromite selenides have catalytic properties and could replace expensive silver catalysts in many chemical reactions [2]. In laser technology, the same compounds are used as materials that enable the use of solar energy [3,4].
The parent compound ZnCr2Se4, possessing semiconducting and antiferromagnetic (AFM) properties, belongs to the group of compounds crystallising in the spinel structure [5,6,7]. Because of the significant lattice parameter of ZnCr2Se4 (10.484 Å), an antiferromagnetic order below the Néel temperature TN = 21 K, and the positive value of the paramagnetic Curie–Weiss temperature (θCW = 115 K), the compounds based on doped ZnCr2Se4 are promising new materials with desired properties [8,9,10].
Numerous experimental and theoretical studies have been devoted to ZnCr2Se4 doped with various elements. The matrix was doped with p-elements (Al, Ga, In, Sn, and Pb [11,12,13,14,15,16,17,18]), d-elements (Cu, Mn, Ni, and Ta [19,20,21,22,23,24,25,26]), and f-elements (Dy, Gd, Ho, and Nd [27,28,29,30,31]).
The presence of an additional cation in the crystal lattice of ZnCr2Se4 caused significant phenomena, e.g., the re-entrant spin-glass, geometric spin-glass, and metal-insulator transition and heavy fermion properties [16,27,30,31]. The current article continues our earlier research, focusing on introducing d-elements to the crystal lattice of ZnCr2Se4 to obtain new materials with desired properties. This work presents the synthesis and physicochemical characteristics of ZnCr2Se4 single crystals doped with rhenium ions instead of zinc ions. The thermodynamic model, describing the growth of single crystals, has been elaborated using a computer program.
The determination of the Fermi energy and Fermi temperature from the diffusion component of the thermoelectric power has demonstrated the importance of this work. These phenomena have not been reported earlier in the literature.
2. Materials and Methods
2.1. Materials
To synthesise the ZnCr2Se4 single crystals doped with rhenium, the elements Zn, Re, Cr, and Se, and anhydrous CrCl3 (5N, Sigma Aldrich, Poznań, Poland) were applied.
2.2. Methods
2.2.1. Chemical Vapour Transport (CVT)
The growth of ZnCr2Se4 single crystals doped with rhenium was carried out using chemical vapour transport (CVT). The CVT method is of great practical and cognitive importance. Crystallisation may occur at a temperature below the melting point. Thanks to this fact, it is possible to obtain structurally pure, low-temperature polymorphs.
Chemical vapour transport (CVT) is based on heterogeneous reversible reactions that can occur in the investigated system.
The order of magnitude of the equilibrium constants Kp (logKp ≈ 0) regulates the transport ability of the reactions. The change of free energy ΔG° (close to zero) guarantees the process’s reversibility and ensures that significant amounts of products and substrates are in the equilibrium state. The value of ΔG° is calculated based on the formula below:
where: R—gas constant, T—absolute temperature, Kp—equilibrium constant, ΔG°, ΔH°, and ΔS°—changes in the reaction’s free energy, enthalpy, and entropy. The set of transport reactions is chosen based on thermodynamic data. As a transport agent, volatile halides (e.g., NbCl5, AsCl3, CrCl3, and CdCl2) or chlorine are usually used [32,33,34]. The details describing the basis of the chemical vapour transport are presented in [35]. The HSC Chemistry v6 computer program was used for preparing the model of single-crystal growth [36].
2.2.2. Physicochemical Characteristic
The physicochemical characteristics were achieved using various methods: (1) magnetic and electrical measurements; (2) X-ray study; (3) specific heat measurements; and (4) thermal analysis. Electrical conductivity, σ(T), of single crystals under study was measured along the [001] direction by the DC method using a Keithley 6517B Electrometer/High Resistance Meter (Keithley Instruments, LLC, Solon, OH, USA) and within the temperature range of 77–400 K. The crystal, whose surfaces at the edges of the octahedron were polished into a rectangular parallelepiped, was placed between copper electrodes and pressed mechanically. The Seebeck coefficient, S(T), was measured within the temperature range of 100–400 K with the help of a Seebeck Effect Measurement System (MMR Technologies, Inc., San Jose, CA, USA). The electrical and thermal contact between the single crystal and electrodes was achieved by a silver lacquer mixture (Degussa Leitsilber 200) [9,20,21,22,23,24,25,26,27].
Dynamic magnetic susceptibility (ac) was measured at an internal oscillating magnetic field of Hac = 1 Oe with an internal frequency of f = 120 Hz. Magnetization isotherms were measured at 2, 4, 10, 20, 40, 60, and 300 K using a Quantum Design MPMS-XL-7AC SQUID magnetometer (Quantum Design, San Diego, CA, USA) in applied external fields up to 70 kOe. The details of research methods, conditions of measurements, and types of equipment used to prepare the physicochemical characteristic of Zn1−xRexCr2Se4 single crystals are described in Refs. [17,27,28,29,30,31,37]. The effective magnetic moment µeff was calculated using the equation presented in Refs. [38,39]. The effective number of Bohr magnetons peff was calculated from the equation:
where x is the content of rhenium ions in the sample, [40] for Cr3+ (S = 3/2; L = 3; J = 3/2, g = 2/5, basic term 4F3/2; for g = 2, peff = 3.873 [40]) and Re2+ (S = 5/2; J = 5/2 for L = 5; g = 2/7, basic term 6S5/2; for L = 0 and g = 2, peff = 5.916 [41]) ions with 3d3 and 5d5 electronic configuration, respectively. The equations for the magnetic superexchange integrals J1 and J2 are presented in Ref. [42]. Specific heat C(T) was measured in the 2–300 K temperature range and in the external magnetic field up to 4 T using Quantum Design PPMS (Physical Properties Measurement System) with heat-capacity and -resistivity options.
Thermal measurements were conducted using a Labsys Evo (Setaram Inc., Cranbury, NJ, USA) apparatus. The measurements were carried out in the flowing high-purity Ar-atmosphere with a heating rate of 10 °C/min.
The scanning electron microscope JEM 6480 (JEOL USA, INC., Peabody, MA, USA) was applied with an energy-dispersive X-ray spectrometer (SEM/EDS) to determine the chemical composition.
The X-ray diffraction was conducted at 293(1) K. The data were collected using a Super Nova X-ray diffractometer (Agilnt, Oxfordshire, UK) with a microfocus X-ray tube, optimised multi-layer optics for Mo-Kα (λ = 0.71073 Å) radiation, and an Atlas CCD detector. Accurate cell parameters were determined and refined with CrysAlisPro software (version 1.171.37.35, Agilent Technologies, Wrocław, 2014). Also, the CrysAlisPro program was used to integrate the collected data. The spinel structure () was refined using the SHELXL-2013 program [43,44]. All atoms were refined with anisotropic displacement parameters.
3. Results and Discussion
3.1. Thermodynamic Model of Chemical Vapour Transport in the ZnSe-Re-Se-CrCl3 System
The thermodynamic model is based on a set of hypothetical reactions that can occur in the ZnSe-Re-Se-CrCl3 system (a set of reactions is presented in Supplementary Materials). Anhydrous CrCl3, used as a transport agent in temperatures above 773 K, dissociates on CrCl2 and Cl2. The compound CrCl2 reacts with Cl2, and CrCl4 forms. The partial pressure of Cl2 and CrCl2 are very low compared to that of CrCl3 and CrCl4. The partial pressure coefficient Q (Q = p(CrCl3)/p(CrCl4) is 20 at 830 K because, during the sublimation of CrCl3, the gas phase contains 5% CrCl4. In the reaction system, three transport agents co-exist. For this reason, the consideration of hypothetical reactions which could appear in the reaction system should consider reactions with CrCl3, CrCl4, and Cl2 [45,46].
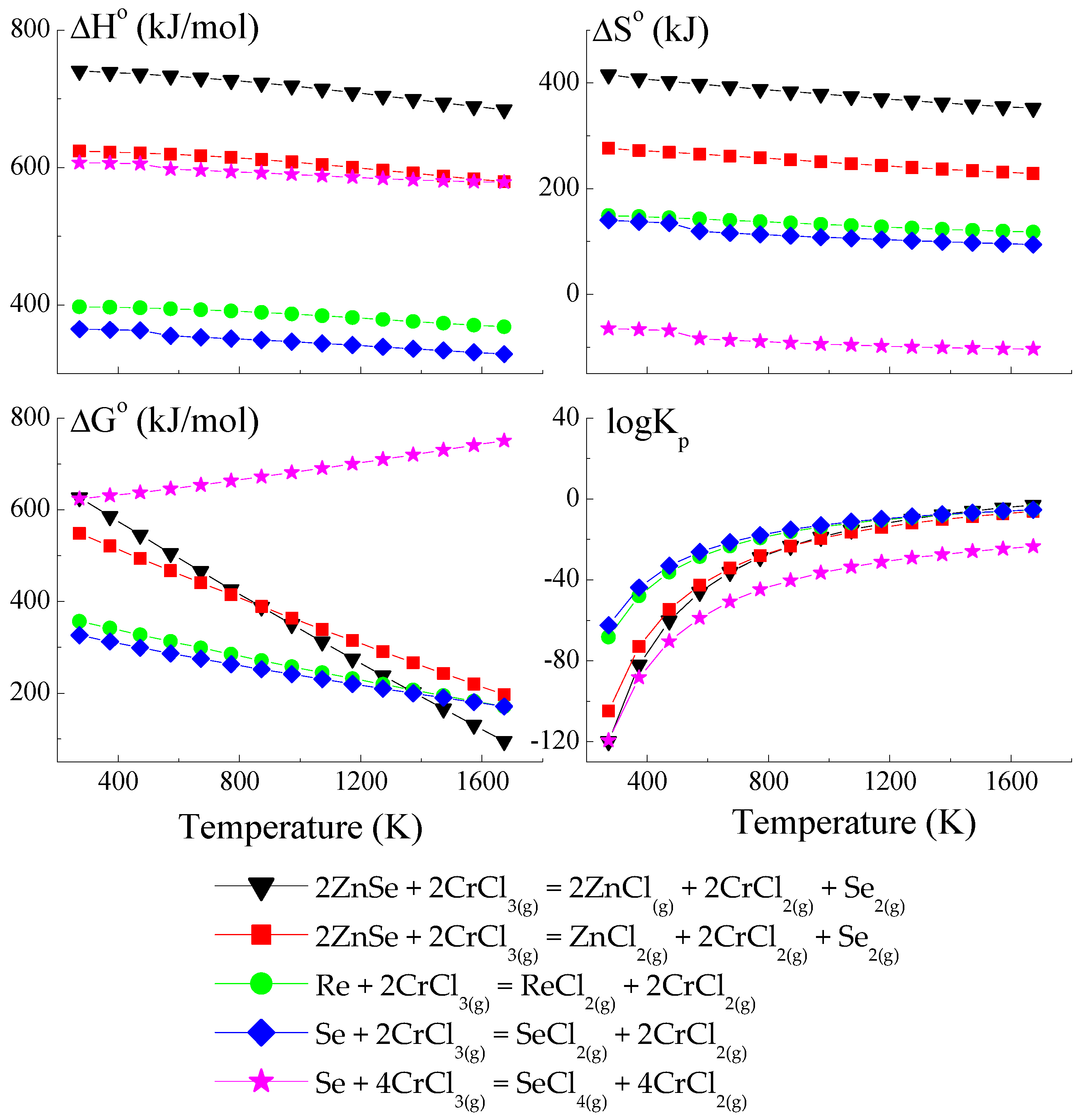
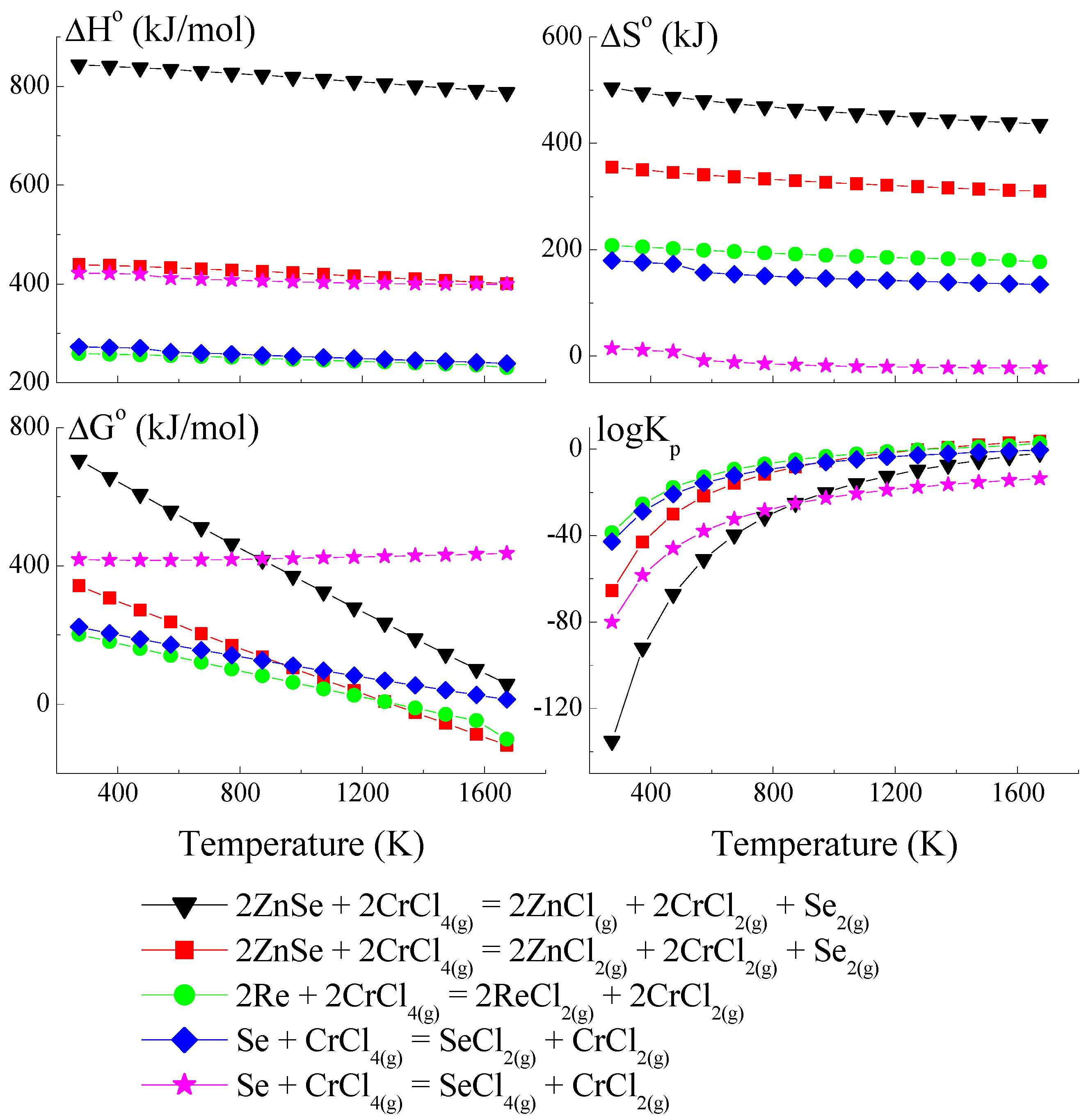
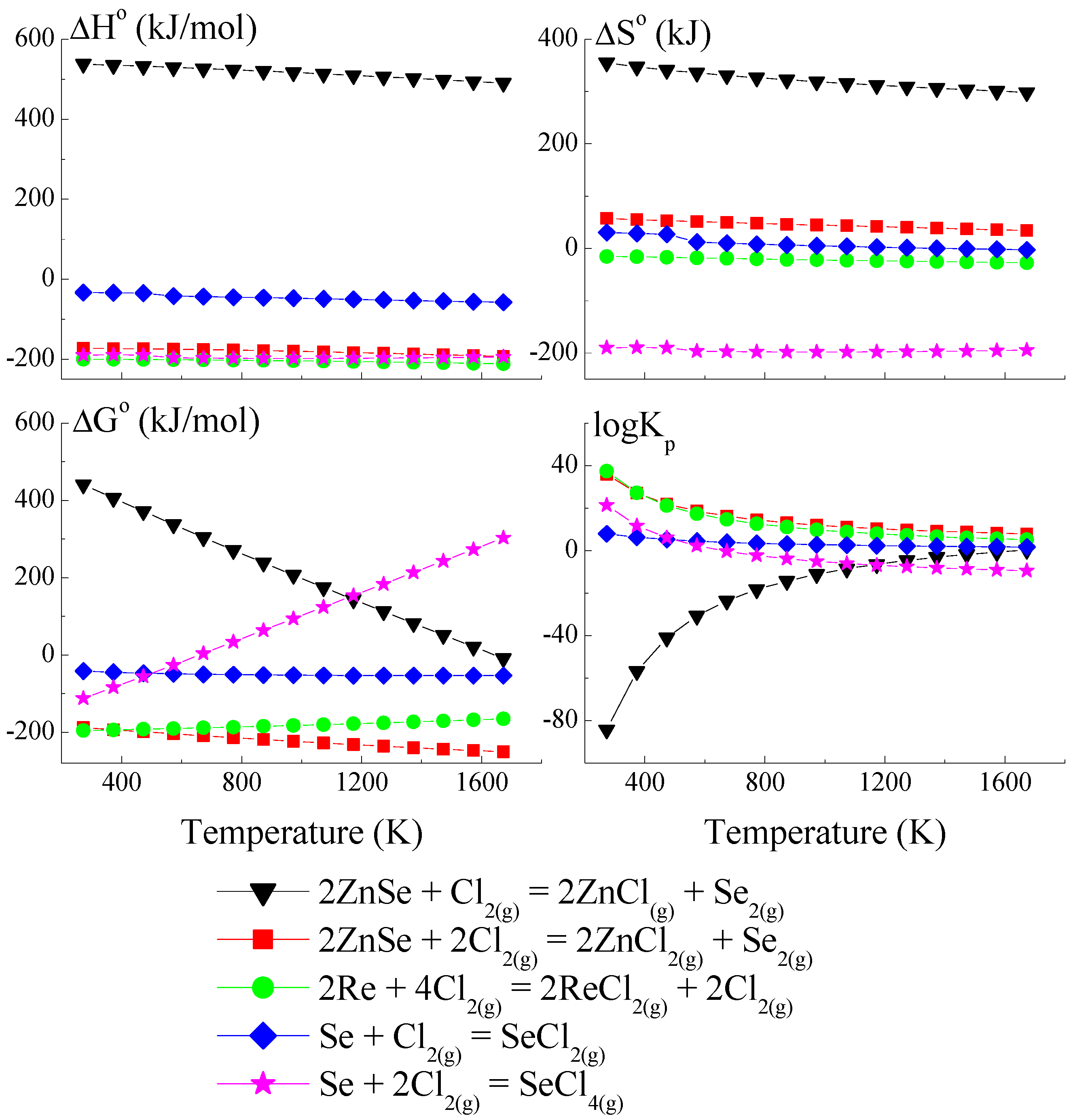
Because the compound ReSe does not exist, pure Re was utilised to calculate the crystal growth model for the ZnCr2Se4 single crystals doped with rhenium. The thermodynamic model of the chemical vapour transport in the ZnSe-Re-Se-CrCl3 system is presented in Figure 1, Figure 2 and Figure 3. The thermodynamic parameters (ΔH°, ΔG°, ΔS°, and logKp) were calculated using the HSC Chemistry 6 computer program [36].
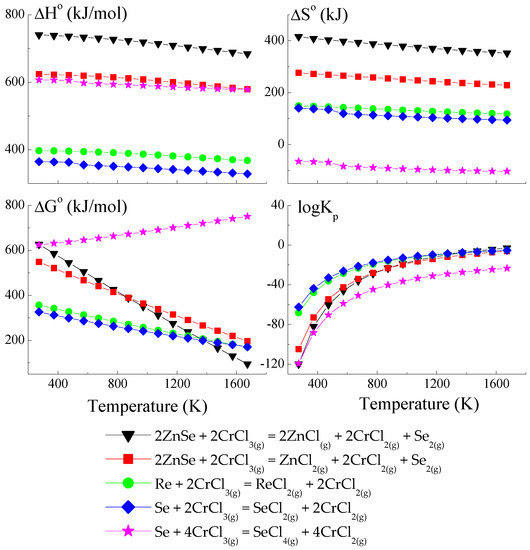
Figure 1.
The dependence of the thermodynamic parameters vs. temperature T for the reactions with CrCl3 as a transport agent (theoretical calculation using HSC Chemistry v6 computer program [36]).
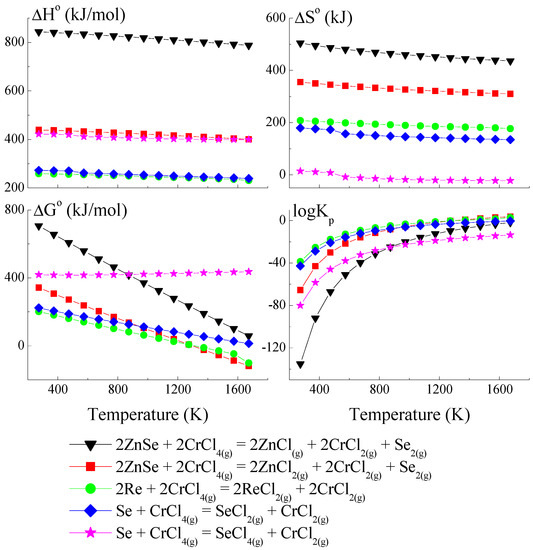
Figure 2.
The dependence of the thermodynamic parameters vs. temperature T for reactions with CrCl4 as a transport agent (theoretical calculation using HSC Chemistry v6 computer program [36]).
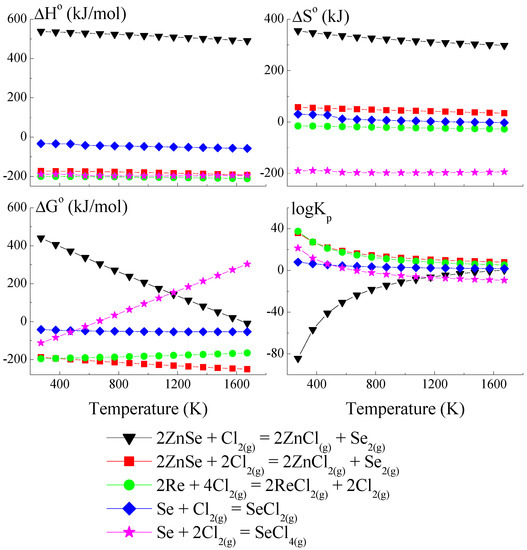
Figure 3.
The dependence of the thermodynamic parameters vs. temperature T for reactions with Cl2 as a transport agent (theoretical calculation using HSC Chemistry v6 computer program [36]).
The thermodynamic model of the chemical vapour transport in the ZnSe-Re-Se-CrCl3 system proved that the synchronous transport of ZnSe, Re, and Se would occur via gaseous CrCl3 and CrCl4 in the temperature range: 1000–1300 K. In this temperature range, logKp and ΔG° values are close to zero (Figure 1 and Figure 2), which indicates the proper conditions for chemical transport.
The prepared thermodynamic crystal growth model confirmed that for the ZnSe-Re-Se-CrCl3 system, the conditions for the simultaneous transport of ZnSe, Re, and Se are fulfilled.
3.2. Growth of ZnCr2Se4 Single Crystals Doped with Rhenium
The process of growth of the ZnCr2Se4 single crystals doped with rhenium was carried on in quartz-glass ampoules using a solid-state reaction in a high vacuum (10−5 mbarr). The experiments were carried out in ampoules with an outer diameter of 20 mm and a length of about 200 mm. The stoichiometric amounts of ZnSe, Re, and Se, weighed according to the reaction:
4(1 − x)ZnSe + 4xRe + 4xSe + 2CrCl3 = Zn1−xRexCrxSe4 + 3(1 − x)ZnCl2 + 3xReCl2
for x = 0.1–0.4.
for x = 0.1–0.4.
Were introduced to the quartz-glass ampoule, sealed, and put into the two-zone pipe furnace.
Based on the thermodynamic model data, reaction conditions were selected: the dissolution zone 1143–1203 K, crystallisation zone 1103–1173 K, and temperature gradient 30–40 K (Table 1), according to Refs. [24,25,26,27,28,29,30,31,32]. The stoichiometric amounts of ZnSe, Re, Se, and CrCl3 placed in quartz ampoules were heated for 336 h and then cooled at about 50 degrees per hour. Based on the ideal gas equation of state
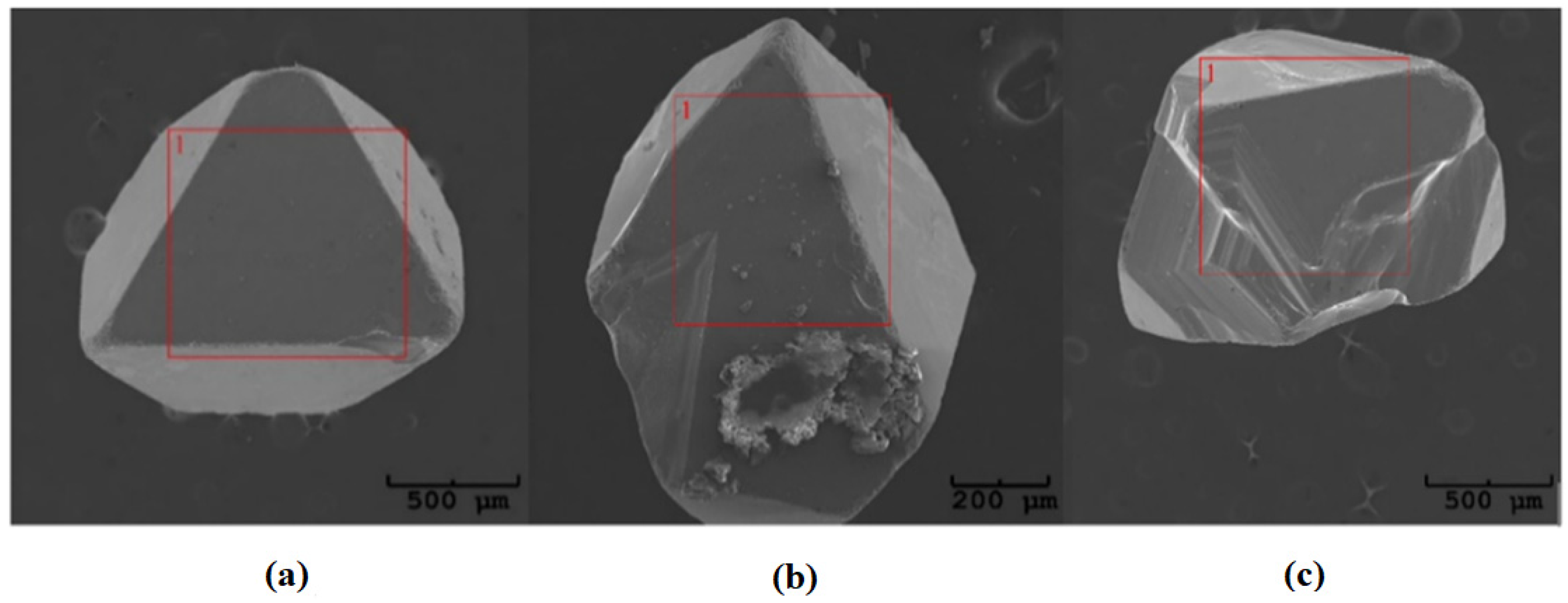
where p—pressure, V—the volume of glass ampoule, n—the number of moles of transport agent, R—the universal gas constant, and T—temperature, it can be estimated that the average pressure in the quartz ampoule during the crystal growth. According to our calculations, the pressure inside the ampoule is about 0.3 MPa, which indicates that the substrates were transferred into the gas phase by diffusion [32,33]. The obtained ZnCr2Se4:Re single crystals are shown in Figure 4.

Table 1.
Conditions of growth and chemical composition of ZnCr2Se4:Re single crystals.
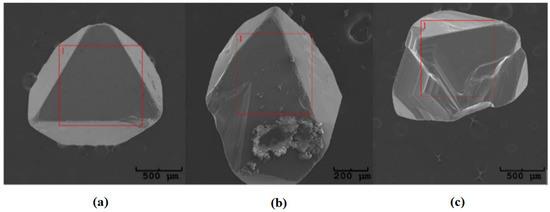
Figure 4.
Examples of single crystals obtained in the ZnCr2Se4:Re system: (a) Zn0.94Re0.06Cr2Se4, (b) Zn0.92Re0.08Cr2Se4, and (c) Zn0.88Re0.12Cr2Se4.
3.3. Chemical Composition
3.4. Structural Study
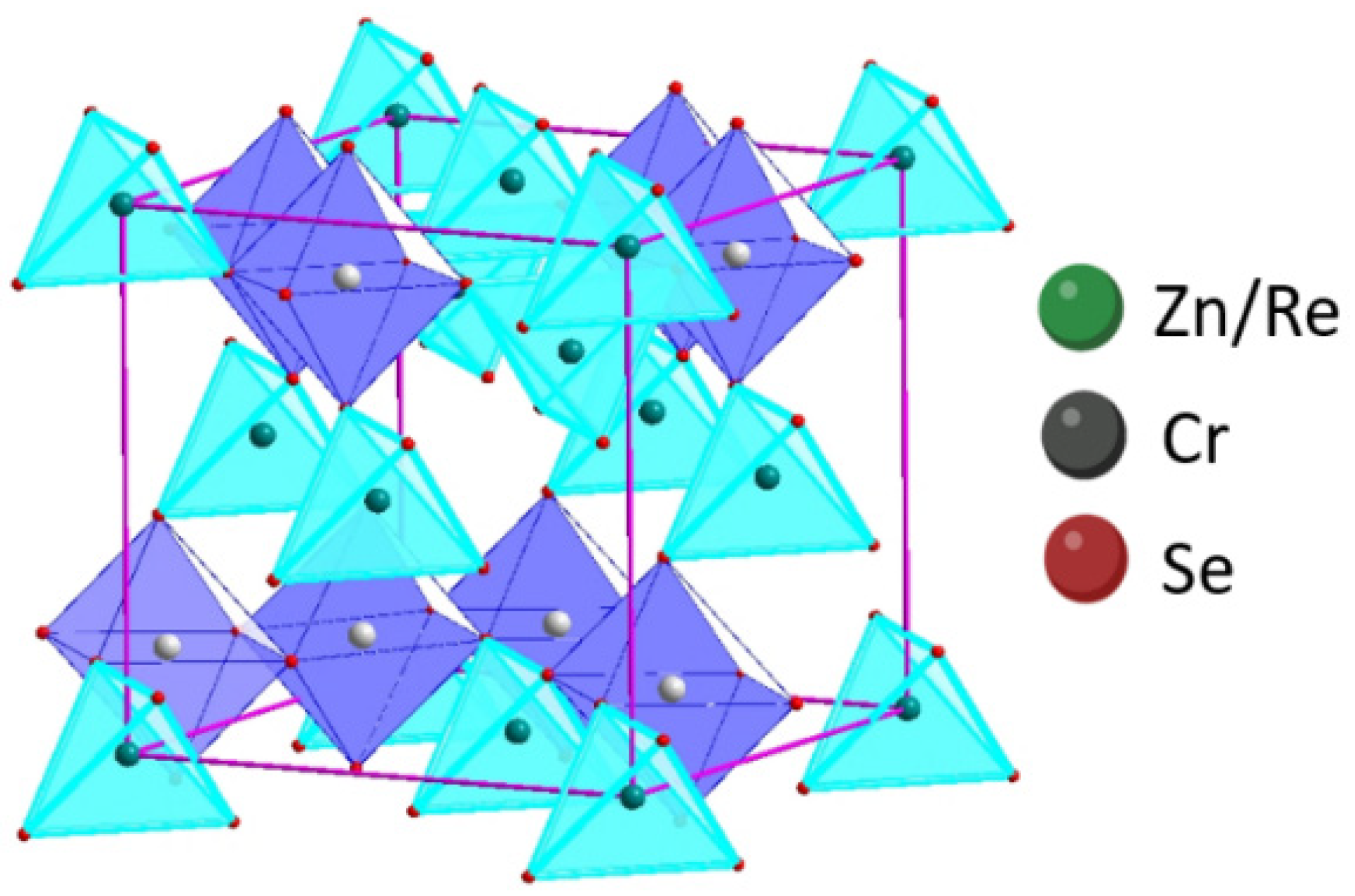
The structural parameters were calculated based on the procedure depicted in Refs. [18,31]. The results showed that the Re ions share the tetrahedral positions with Zn-cations in every fifth crystal. The structural study confirmed that the obtained samples crystallise in the cubic system (SG ). The formula describing cation distribution in the obtained ZnCr2Se4 single crystals doped with rhenium is Zn1−xRexCr2Se4 (Figure 5).
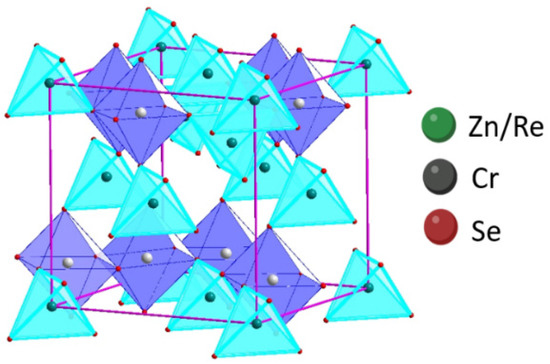
Figure 5.
Projection of cubic structure of Zn1−xRexCr2Se4 crystals.
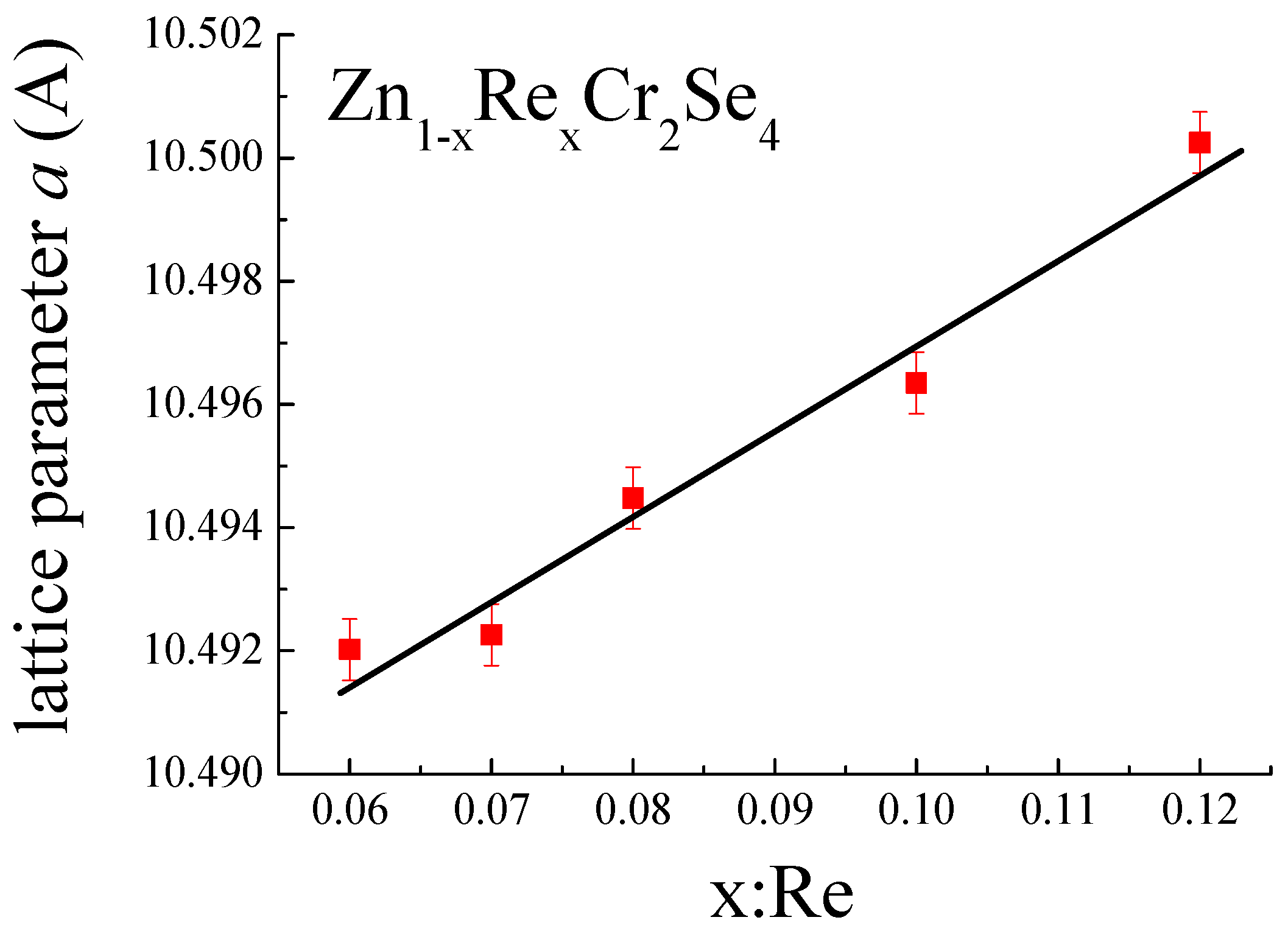
The presence of Re ions in ZnCr2Se4 is confirmed by an increase in lattice parameters of the unit cell (Table 2, Figure 6), by the difference of ionic and covalent radii (, where r indices ionic radius, and R indices covalent radius [47,48]). The linear dependence of lattice parameters indicates that Vegard’s rule is observed in Zn1−xRexCr2Se4.

Table 2.
Structural parameters of Zn1−xRexCr2Se4 single crystals.
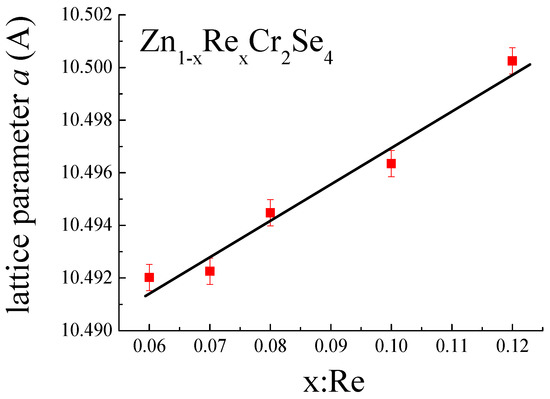
Figure 6.
Change in lattice parameter a as function of rhenium quantity.
The details about other structural parameters (CIF files, all measurement parameters, values of the parameter u, atomic coordinates, equivalent isotropic displacement parameters, interatomic distances, and bond angles) of the Zn1−xRexCr2Se4 single crystals are presented in the Supplementary Materials.
3.5. Electrical Studies
The activation energy Ea was calculated according to the formula:
where k is the Boltzmann constant and σ0 is the reference conductivity.
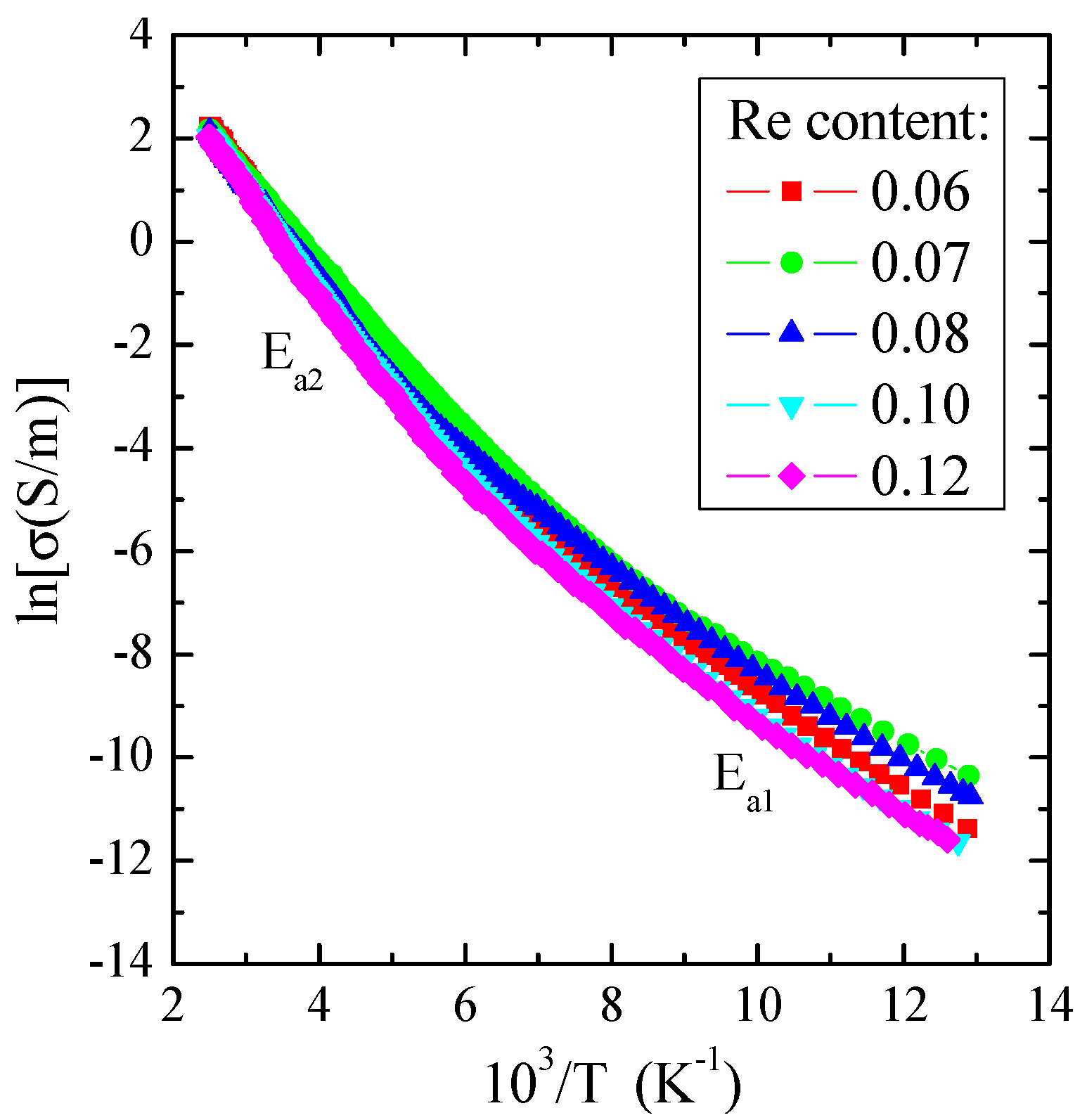
As illustrated in Figure 7, the electrical conductivity for measured crystals revealed two areas: the external area in the narrow temperature range of 77–130 K, in which the weak thermal activation of Ea1~0.08 eV is observed, and the internal area in the temperature range of 200–400 K with stronger thermal activation of Ea2~0.16 eV (Table 3). In the region of stronger activation, the value of electrical conductivity at 400 K is about 9 S/m, a typical value for a spinel matrix with an energy gap of 1.28 eV at room temperature [49]. The admixture of rhenium generally enhances the thermal activation of current carriers in the internal region compared to ZnCr2Se4, for which Ea is about 1.35 eV [13] (Table 3). Similar behaviour was found for the ZnCr2Se4 crystals doped with elements 3d [14], 5d [19,24], and 4f [29,31].
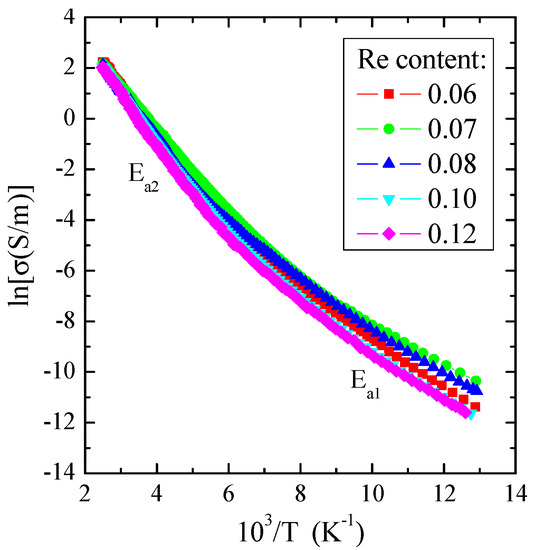
Figure 7.
Electrical conductivity (lnσ) vs. reciprocal temperature (103/T) for Zn1−xRexCr2Se4 single crystals.

Table 3.
Electrical parameters of Zn1−xRexCr2Se4 single crystals.
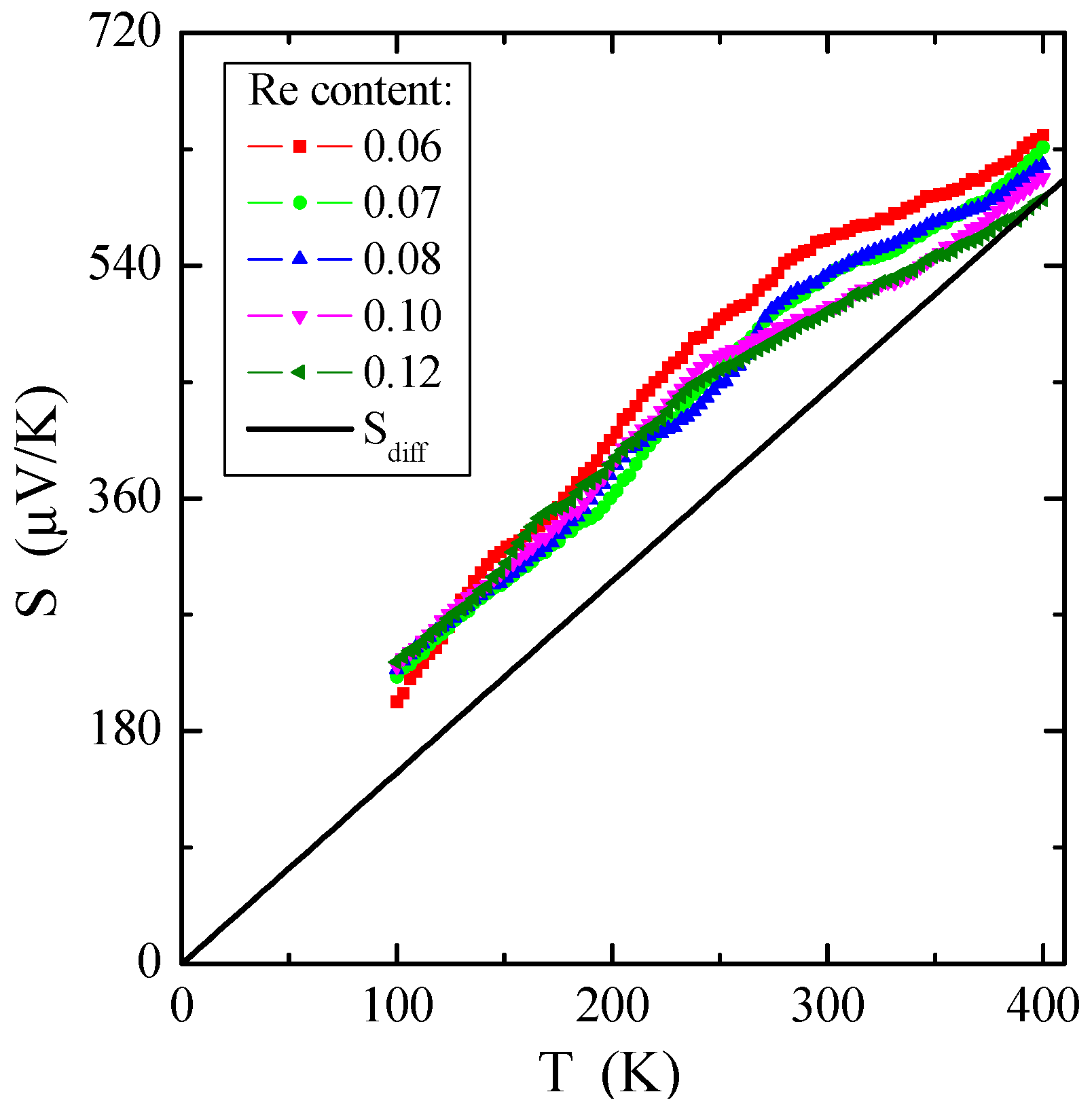
Figure 8 demonstrates the dependence of thermoelectric power on temperature S(T). On the whole, the thermopower in conventional metals is composed of two various components: (1) a diffusion component (Sdiff), which is proportional to temperature according to the Mott formula at higher temperatures [50], and a phonon drag component (Sph), which is more complex. The Sph contribution comes from transferring phonon momentum to the electron gas. Both components drop at low temperatures, such as T3 below θD/10, when phonons freeze out (θD is the Debye temperature), and at high temperatures, such as T−1 above approximately θD/2, when the phonon’s excess momentum is limited by anharmonic phonon–phonon scattering [51]. A Debye temperature of Zn1−xRexCr2Se4, obtained from specific heat measurement, has a value of 295 K. Therefore, the peak of the phonon drag component of thermoelectric power should be in the temperature range of 30–140 K and is not visible in Figure 8. The diffusion share Sdiff is a direct application of the Boltzmann transport equation [50], described by the formula:
where e is the elementary charge, EF is the Fermi energy, and a is an empirical slope. Using Equation (5), the Fermi energy, EF, can be determined by the formula:
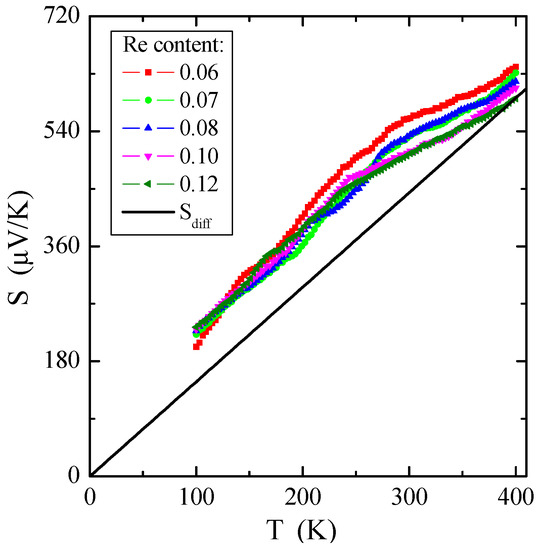
Figure 8.
Thermoelectric power S vs. temperature T of Zn1−xRexCr2Se4 single crystals. Sdiff is the diffusion component of thermopower (marked with a solid line).
The experimental dependence of Sdiff on temperature is evident in Figure 8 by solid lines. Based on Equation (6), it is possible to estimate the Fermi energy EF and the Fermi temperature TF (defined as EF/k), knowing the experimental value of the slope of thermopower for every single crystal. The values of EF and TF are given in Table 3. Comparing metals, e.g., for pure copper, EF = 7 eV and TF = 8.19 × 104 K [52], and non-metallic conductors, e.g., for Cu1−xGaxCr2Se4 single crystals, EF~0.3 eV and TF~3 × 103 K [53], it can be concluded that the Fermi energy EF has smaller values for the tested crystals. It bears out that the Fermi level is near the valence-band border, and the shallow acceptor level is just above the valence band. The source of the observed low p-type electrical conductivity, which is more thermally activated above room temperature, could be cationic vacancies in the spinel structure. Structural defects seem to always exist at thermal equilibrium in the crystal lattice, even in perfect samples.
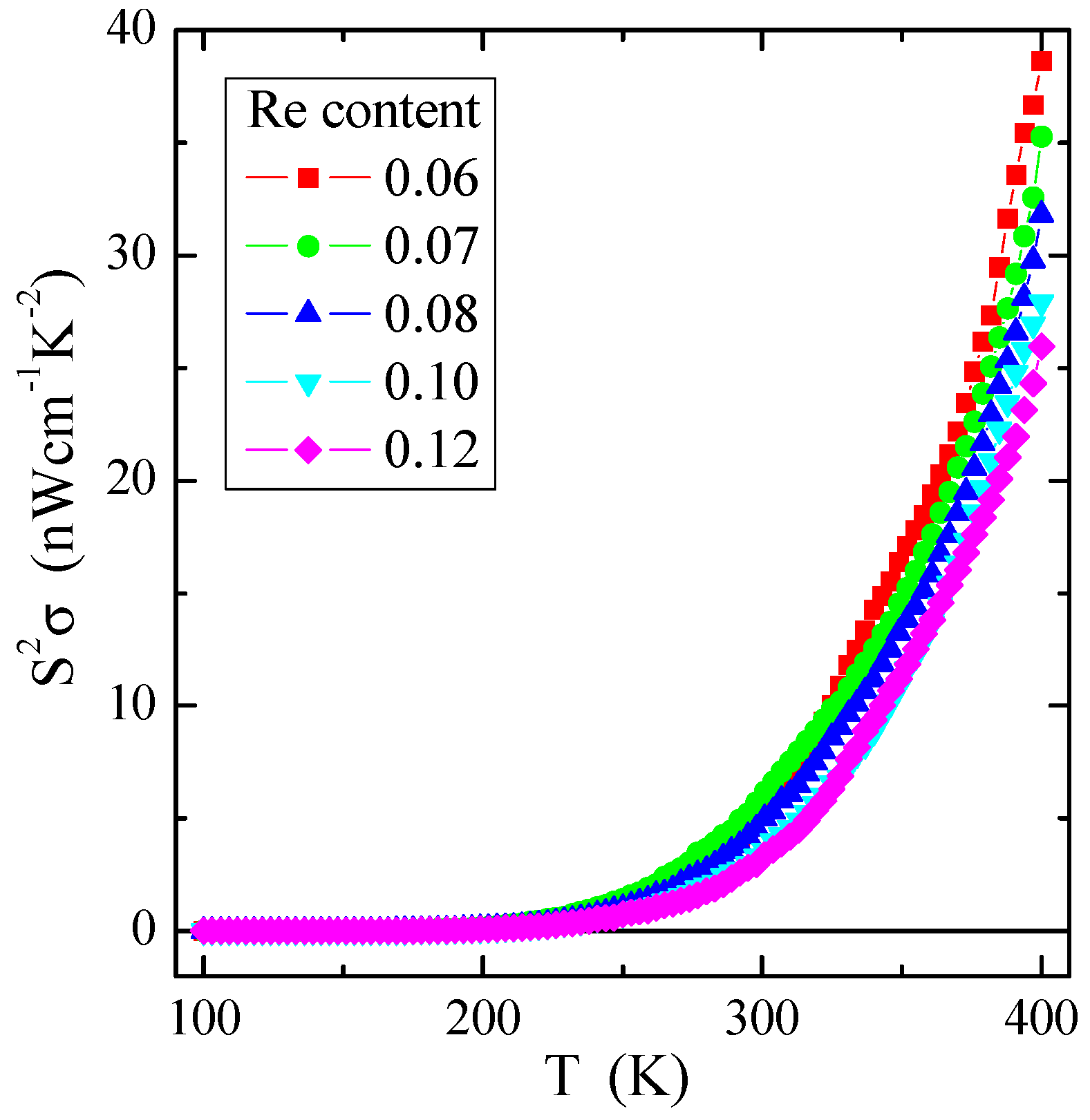
As evident from Figure 9, the power factor S2σ of the investigated spinel semiconductors has a small value of several tens of nW/(cmK2), e.g., compared to the value of 0.1 µW/(cmK2) for the non-metallic spinel conductor CuCr2Se4:Ga [1]. The value of S2σ substantially increases with increasing temperature, i.e., in the internal area already above 250 K for all investigated samples with rhenium. Similar behaviour of the power factor as a function of temperature, but, on a somewhat smaller scale, was observed in the somewhat conductive molybdate-tungstate ceramics doped with Ga3+ and Co2+ [54], as well as Nd3+ and Mn2+ [55]. The above studies indicate that, regardless of the chemical bond type, the appropriate admixture influences the power factor, increasing the thermal activation of electric current carriers.
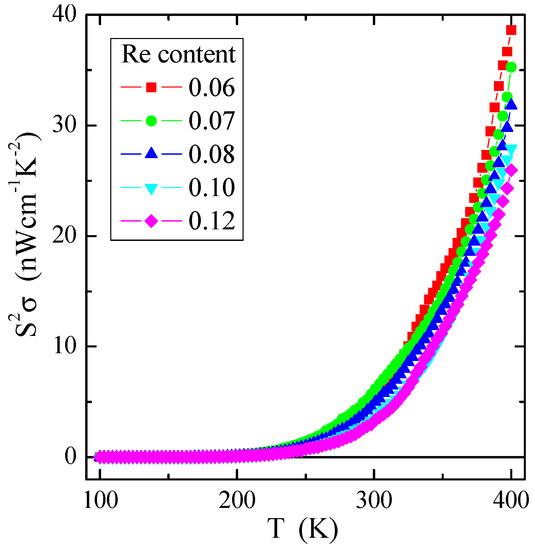
Figure 9.
Power factor S2σ vs. temperature T of Zn1−xRexCr2Se4 single crystals.
3.6. Magnetic Properties
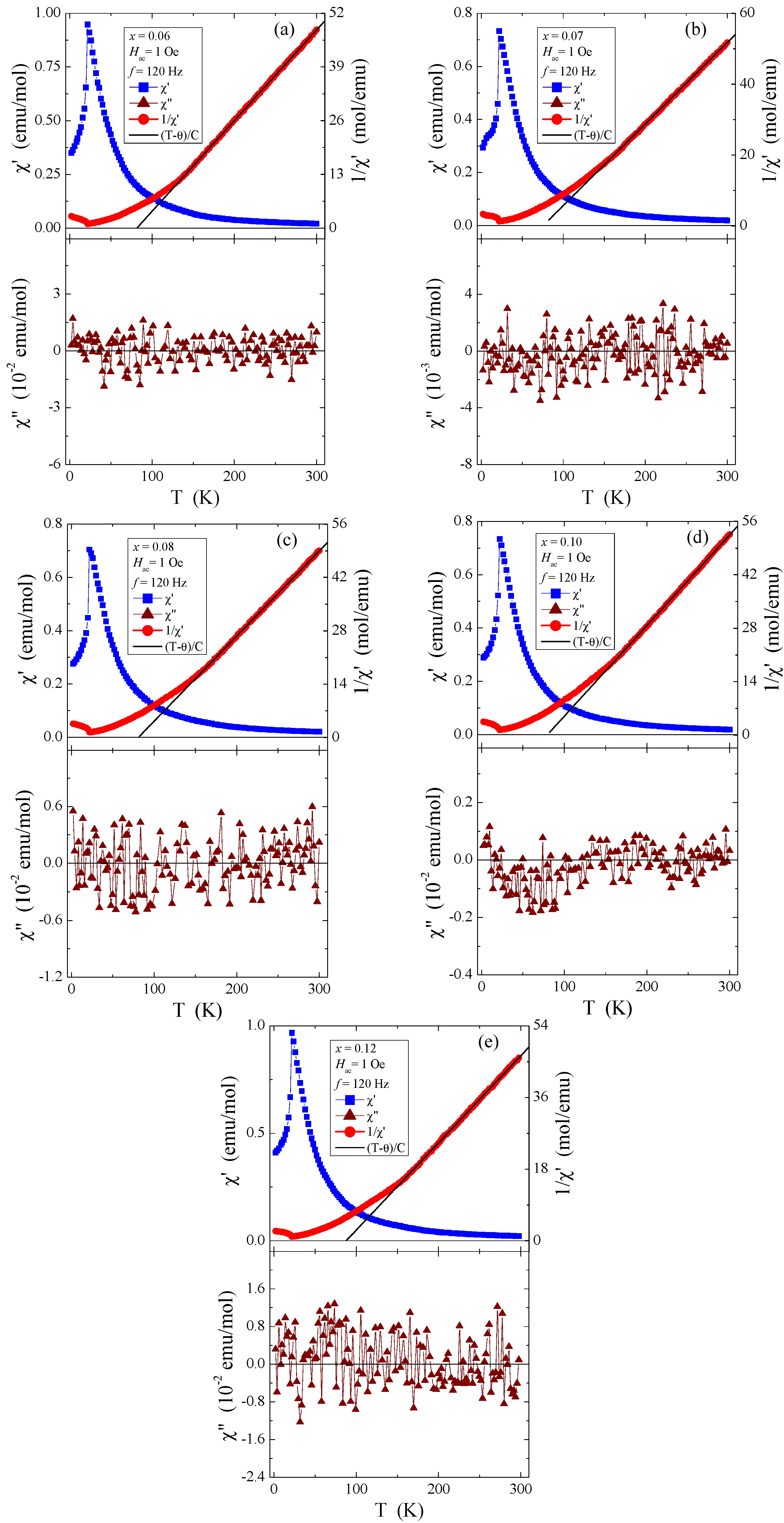
The measurement data in Figure 10a–e, recorded in the internal oscillating magnetic field Hac = 1 Oe with the internal frequency f = 120 Hz and with zero external static magnetic field, suggest that the dependence of both magnetic susceptibility components (real (χ′) and imaginary (χ″)) on temperature indicates the AFM behaviour below the Néel temperature TN = 21.7 K and at positive Curie–Weiss temperatures (θ) of about 80 K. These values are typical for short-range FM interactions, which are independent of the quantity of rhenium in the studied samples. On the other hand, the oscillations of the imaginary component of the ac magnetic susceptibility, χ″, visible around the value of zero (Figure 10), suggest the lack of energy losses caused, among others, by spin reorientation or rotation of the domain walls.
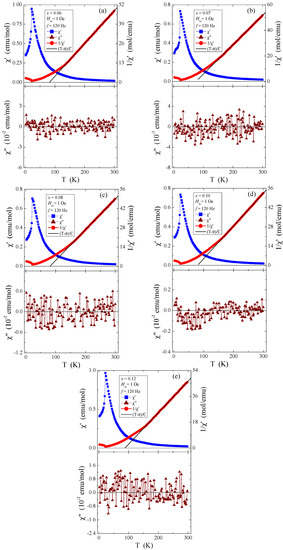
Figure 10.
The real (χ′) and imaginary (χ″) components of ac magnetic susceptibility and 1/χ′ vs. temperature T of Zn1−xRexCr2Se4 single crystals: (a) Zn0.94Re0.06Cr2.0Se4.0, (b) Zn0.93Re0.07Cr2.0Se4.0, (c) Zn0.92Re0.08Cr2.0Se4.0, (d) Zn0.90Re0.10Cr2.0Se4.0, (e) Zn0.88Re0.12Cr2.0Se4.0.
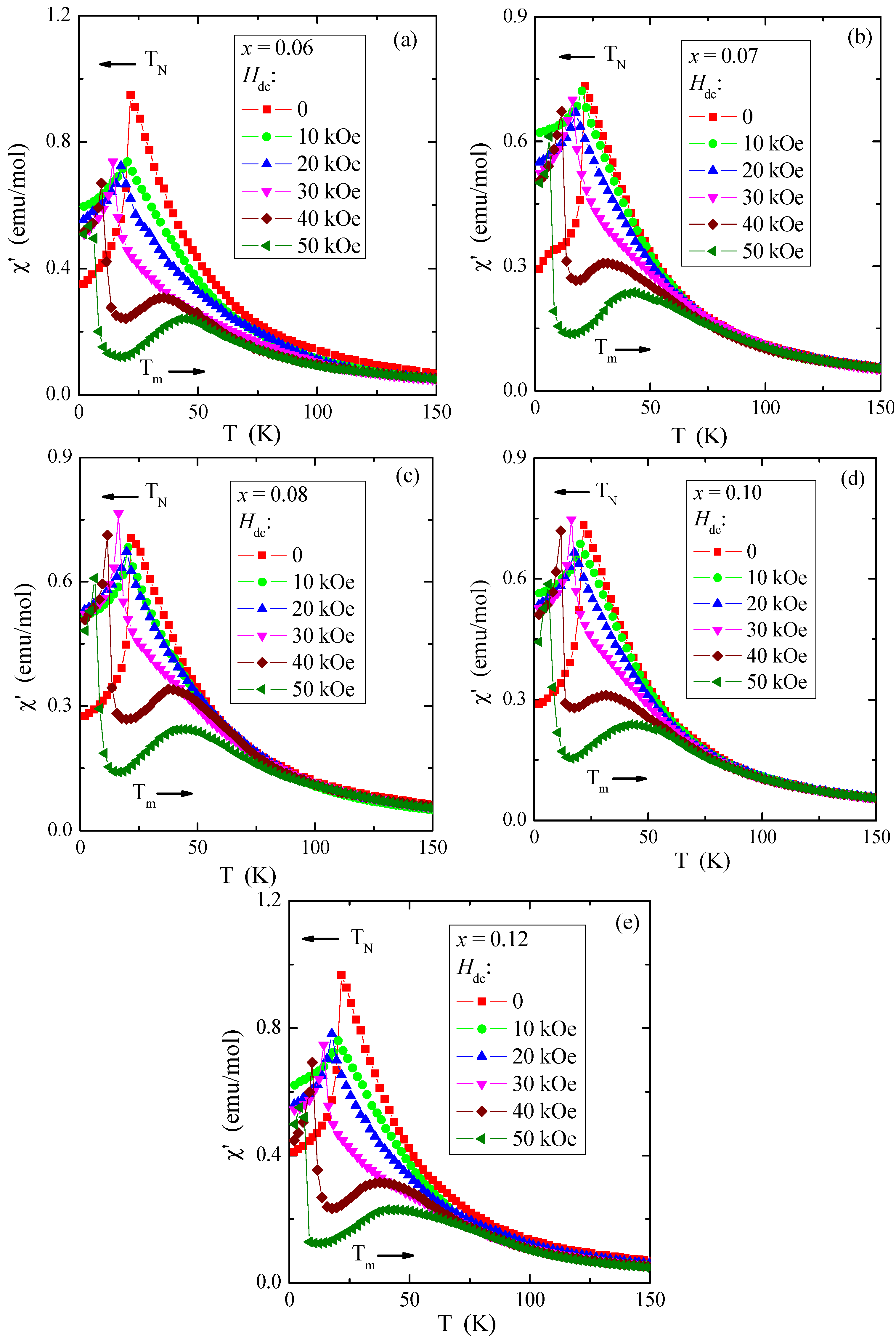
The determined values of θ, presented in Table 4, are much lower than the literature data shown in Refs. [5,6] and slightly lower than in [8], where θ is 115 K and 90 K, respectively. In contrast, the determined TN values for all tested crystals are the same and close to those of pure ZnCr2Se4 [5,6,9]. Long-range AFM interactions are less sensitive to doping with paramagnetic ions, whose 4f and 5d orbitals are intensely screened. On the other hand, FM short-range interactions are more sensitive to the local spin ordering visible in the oscillatory character of the values of both the paramagnetic Curie–Weiss temperature and the J1 and J2 superexchange integrals for the first two coordination spheres (Table 4). The values of the effective magnetic moment (μeff) are substantially similar to the values of the effective number of Bohr magnetons (peff) for the electron configuration of rhenium 5d5 and the base term 6S5/2. It may mean that the orbital magnetic contribution has been quenched and the contribution to the magnetic moment comes solely from the spin. The temperature dependences of ac magnetic susceptibility χ′, recorded in the internal oscillating magnetic field Hac = 1 Oe with the internal frequency f = 120 Hz and taken at external static magnetic fields Hdc = 0, 10, 20, 30, 40, and 50 kOe, are depicted in Figure 11a–e. This figure shows that TN is shifted toward lower temperatures and Tm is shifted toward higher ones. A strong magnetic field diminishes the AFM order and enlarges the FM order.

Table 4.
Magnetic parameters of Zn1−xRexCr2Se4 single crystals recorded in the internal oscillating magnetic field Hac = 1 Oe with the internal frequency f = 120 Hz and with zero external static magnetic field.
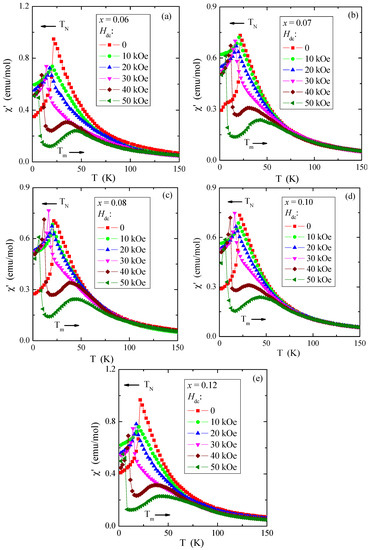
Figure 11.
Ac magnetic susceptibility χ′ vs. temperature T recorded at internal oscillating magnetic field Hac = 1 Oe with internal frequency f = 120 Hz for Zn1−xRexCr2Se4 single crystals. Horizontal arrows indicate the shift of TN and Tm with increasing magnetic field Hdc: (a) Zn0.94Re0.06Cr2.0Se4.0, (b) Zn0.93Re0.07Cr2.0Se4.0, (c) Zn0.92Re0.08Cr2.0Se4.0, (d) Zn0.90Re0.10Cr2.0Se4.0, (e) Zn0.88Re0.12Cr2.0Se4.0.
Additionally, the Curie constant C and the effective moment µeff are slightly higher than the chromium ion value per molecule. It is confirmed that the rhenium ions influence the magnetic moment. For Hdc = 50 kOe, the J1 superexchange integral for the first coordination sphere changes the sign from negative to positive, while the J2 integral remains positive (not shown here), as in Zn1−xPbxCr2Se4 [18].
It corroborates that the short-range FM interaction expands through the whole temperature range. The (χ′(T) curves above TN, in the paramagnetic region, illustrate character istic broad peaks at Tm = 31–35 and 42–45 K in the fields Hdc = 40 and 50 kOe, respectively. These broad peaks of ac magnetic susceptibility may be caused by the spin fluctuations that appear due to a static magnetic field strengthening short-range FM interactions. The thermal energy kT opposes this phenomenon. Similar peaks were observed in the ZnCr2Se4 crystals doped with Al [56], Ce, Ga, In [57], and Pb [18].
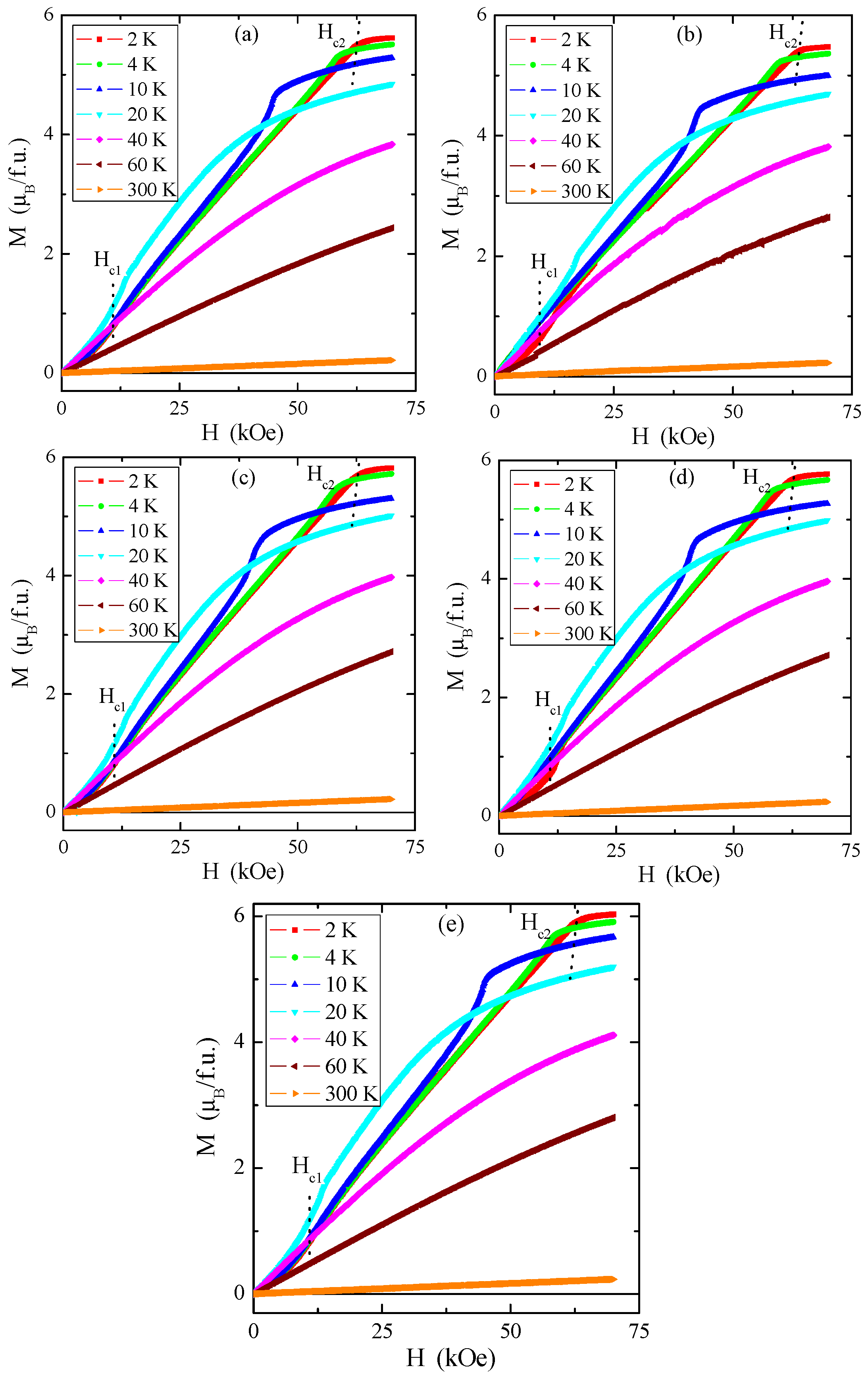
Magnetic isotherms in Figure 12a–e indicate that the magnetic saturation value of the tested single crystals is close to 6 μB/f.u., similar to pure ZnCr2Se4 [4].
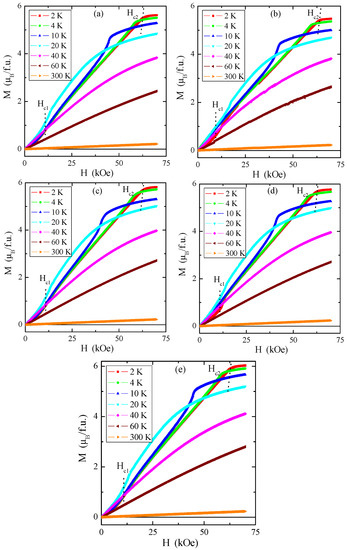
Figure 12.
Magnetization M vs. magnetic field H for Zn1−xRexCr2Se4 single crystals: (a) Zn0.94Re0.06Cr2.0Se4.0, (b) Zn0.93Re0.07Cr2.0Se4.0, (c) Zn0.92Re0.08Cr2.0Se4.0, (d) Zn0.90Re0.10Cr2.0Se4.0, (e) Zn0.88Re0.12Cr2.0Se4.0. The first (Hc1) and second (Hc2) critical magnetic fields registered at 2 K are marked with dotted lines.
The first critical field Hc1, i.e., metamagnetic transition, is connected with the transition from the helical to the conical phase, and the second critical field Hc2 is related to the change of the spiral spin order to the ferromagnetic phase. The critical fields Hc1 (a value of approx. 12 kOe) and Hc2 (a value of approx. 61 kOe) insignificantly depend on the rhenium quantity in the sample. Comparison with pure ZnCr2Se4 revealed that Hc1 has a somewhat higher value and Hc2 has a slightly lower value (Table 4) [8]. The hysteresis loops have zero-field coercivity and zero remanences.
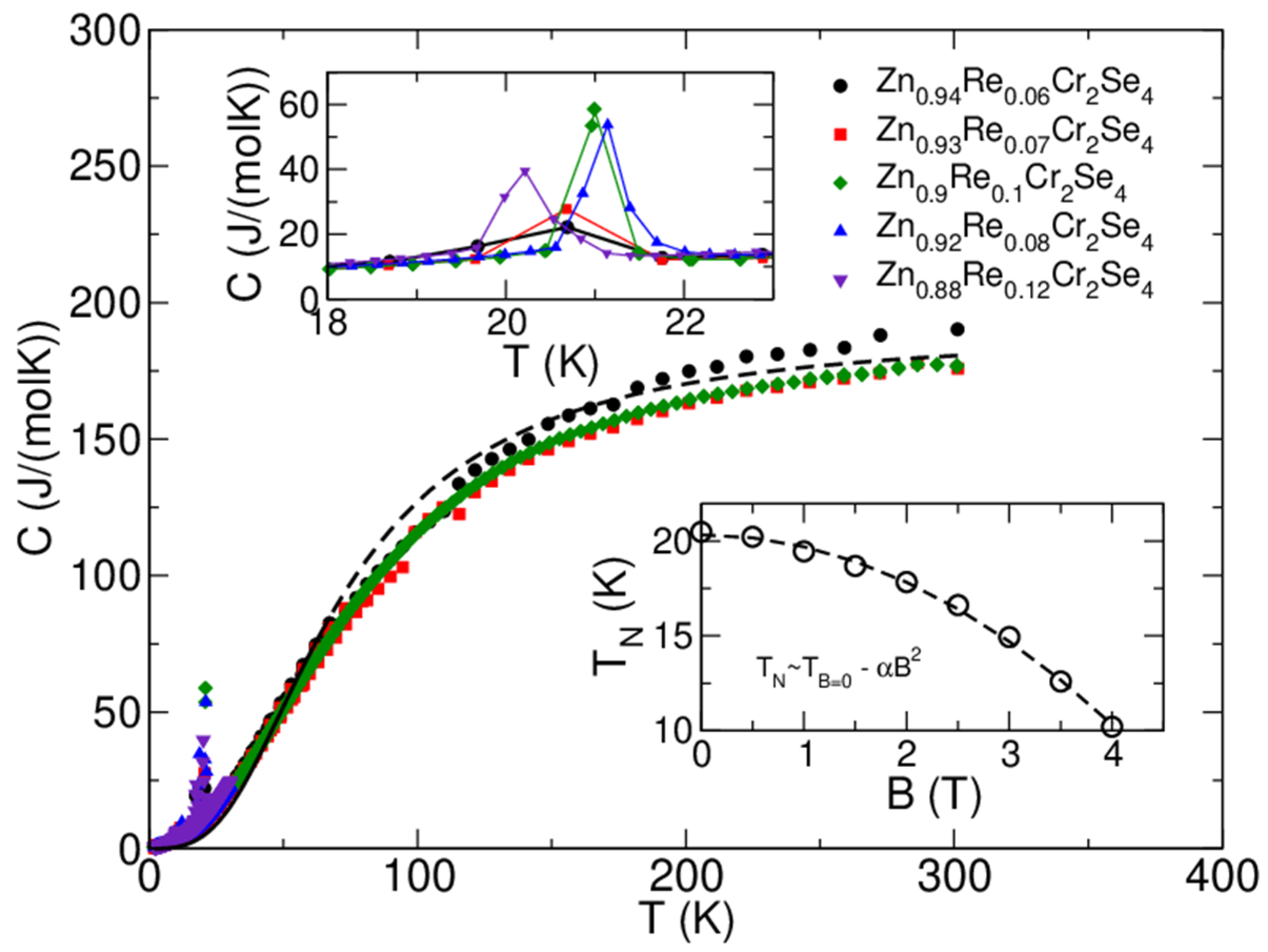
3.7. Specific Heat Measurements
The results of specific heat measurements are shown in Figure 13. Moreover, the dashed line indicates a Debye model fit for T > 35 K and sample composition Zn0.95Re0.06Cr2Se4 with the following fit parameters: number of atoms nD~7.59 and Debye temperature θD~295 K [30]. The number of atoms fits the stoichiometry quite reasonably, and the pattern of the heat-capacity curves is similar for all the samples (which is expected, due to their composition). Therefore, the Debye temperature is also similar. The upper inset shows the magnetic peak at TN~21 K for various stoichiometries. The position of the peak does not shift monotonously with Re concentration. The difference in ordering temperature, as seen from the heat-capacity measurement, is below~1 K. Taking into account limitations of the pulse-measurement technique employed in the PPMS instrument, we can say that Re substitution does not change the ordering temperature in an observable and consistent way. The lower inset shows the position of the magnetic peak for the Zn0.95Re0.06Cr2Se4 sample in magnetic fields up to 4 T. We see that the increasing field shifts the peak to lower temperatures, confirming the antiferromagnetic ordering. The dependence can be very well fitted with the quadratic formula TN = T(B = 0T) − α2 (α = 0.63 and T(B = 0) = 20.34 K).
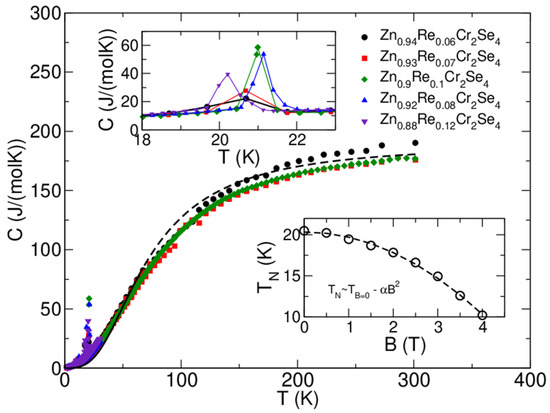
Figure 13.
Specific heat, C, as a function of temperature T, measured for Zn1−xRexCr2Se4 single crystals at zero magnetic field (central figure). The dashed lines indicate that the Debye model fit experimental data for T > 40 K and the Zn0.94Re0.06Cr2Se4 sample (black-filled circles). The upper inset shows how the magnetic peak is affected by Re substitution. The lower inset shows how the magnetic field affects the magnetic transition temperature for the Zn0.93Re0.07Cr2Se4 sample. Experimental data is presented here with open circles, and the quadratic fit is given with the dashed line. Note that the temperature scales on the lower and upper insets are different. Hence, the peaks in the upper inset appear much broader than in the lower inset or the central figure.
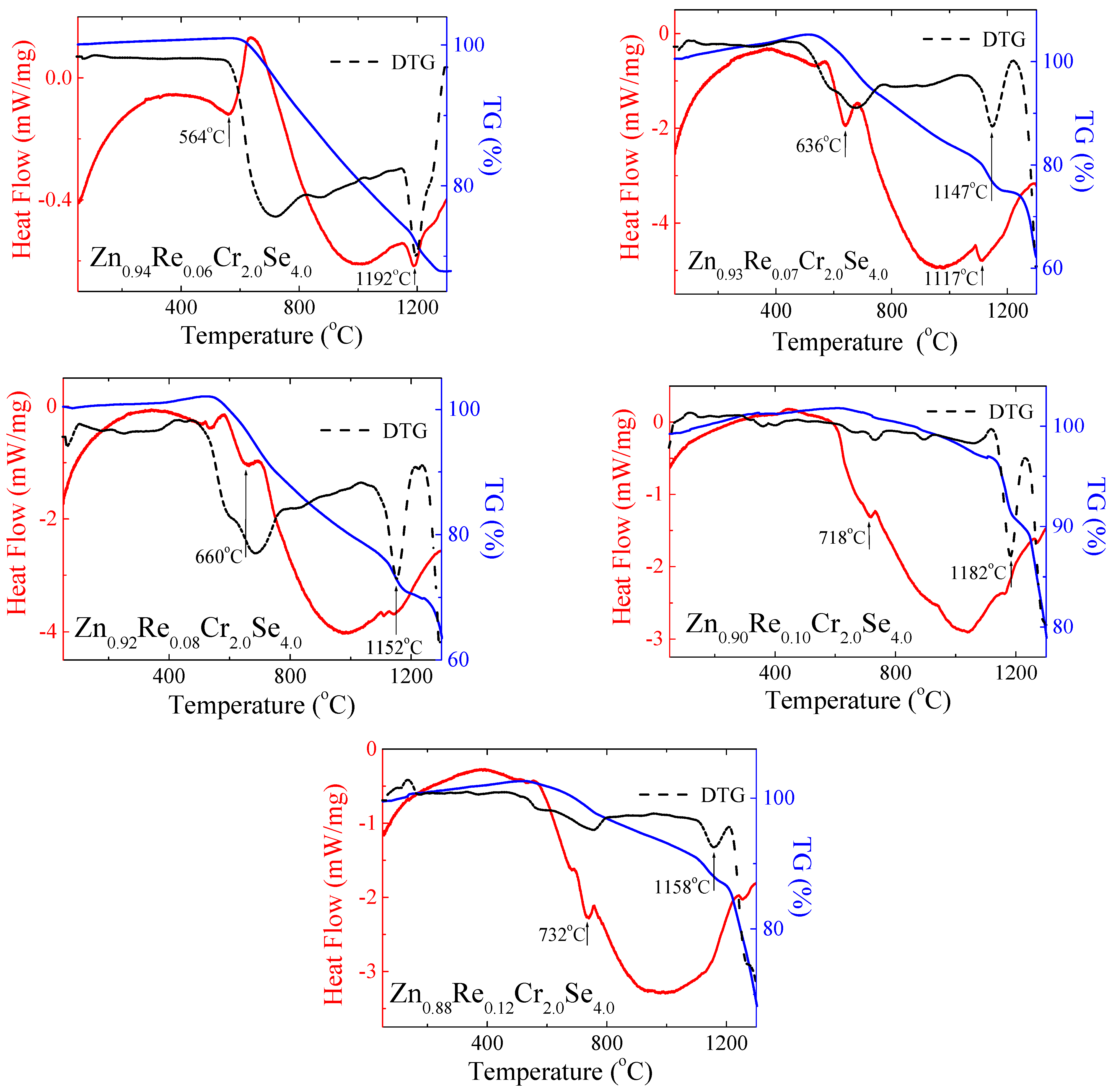
3.8. Thermal Study
The thermal study results are presented in Figure 14 and Table 5. The shape of DSC curves revealed that the obtained Zn1−xRexCr2Se4 single crystals are stable up to above 500 °C. For the smaller amount of rhenium (x = 0.06; 0.07), the first endothermic peaks appear at 564 °C and 636 °C, respectively. For both compounds, the second endothermic peak is visible above 1100 °C (Figure 12). With increasing rhenium, the endothermic peaks are shifted towards higher temperatures, and the second endothermic peak disappears. This phenomenon can be associated with rhenium ions in the crystal lattice of ZnCr2Se4.
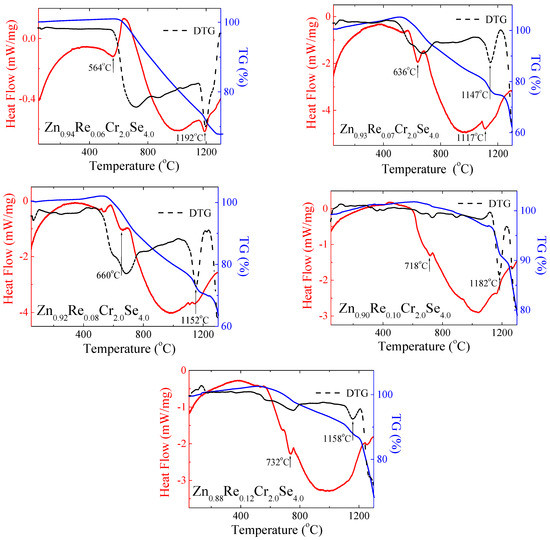
Figure 14.
DSC/TG curves for Zn1−xRexCr2Se4 single crystals (DTG—Derivative Thermogravimetry).

Table 5.
Parameters determined from DSC/TG analysis for Zn1−xRexCr2Se4 single crystals.
Re2+ ions have a bigger ionic radius (0.96 Å) than Zn2+ ions (0.60 Å), which can influence the stability of the crystal lattice. Small changes in the sample mass are observed with the endothermic peaks. It may indicate melting and evaporation processes, which can occur in the system during heating. However, it is worth highlighting that the observed changes are insignificant and suggest the thermal resistance of obtained crystals. The mass loss is observed with increasing temperature on the DTG curve. The extensive mass loss is observed above 1100 °C.
4. Conclusions
We have presented a new family of Zn1−xRexCr2Se4 single crystals. These single crystals have been obtained using the chemical vapour transport (CVT) technique. The conditions of the crystal growth process were refined using thermodynamic calculations. SEM and XRD studies indicated that the obtained single crystals are chemically pure and crystallised in the cubic system (SG: ), which aligns with the spinel structure. Thermal measurements confirmed the thermal stability of single crystals at temperatures up to 1100 °C. Increasing the external magnetic field shifts TN and the specific heat peak towards lower temperatures, while the values of Tm—towards higher temperatures. A significant weakness of long-range AFM interactions is evidenced in the reduction of the superexchange integral J1 for the first coordination sphere and spin fluctuations in the paramagnetic region. The dependence of magnetisation on the magnetic field showed two untypical phenomena below TN. These phenomena occurred at critical fields Hc1 = 12 and Hc2 = 57 kOe. It is correlated well with the change of the sign of the J1 integral from negative to positive at Hdc = 50 kOe, which is caused by the short-range FM interaction extending through the whole temperature range. Calculations of the Fermi energy (EF) and the Fermi temperature (TF) derived from the diffusive component of thermoelectric power revealed a slight increase in EF and TF with increasing rhenium content, indicating shallow acceptor levels above the valence band. A substantial increase in the thermoelectric power factor S2σ in the internal region above 250 K was observed for all samples containing rhenium.
Based on our investigations, we can conclude that obtained results provided compelling evidence that the materials found on the doped ZnCr2Se4 compound could be appropriately implemented in a broad spectrum of new technological areas, e.g., as thermomagnetic and thermoelectric materials in electronic devices. Our results encourage future studies and should be explored on this type of material.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma16134565/s1. Refs. [5,6] are cited in the supplementary materials.
Author Contributions
Conceptualisation, I.J. and T.G. (Tadeusz Groń).; methodology, I.J., T.G. (Tadeusz Groń), J.K., B.S., E.P., J.J., Z.S. and B.W.-K.; validation, I.J. and T.G. (Tadeusz Groń).; formal analysis, I.J., J.K. and T.G. (Tadeusz Groń); investigation, I.J., Z.S., J.K., E.P., T.G. (Tomasz Goryczka), B.S., J.J. and J.G.; resources, I.J.; writing—original draft preparation, I.J., T.G. (Tadeusz Groń) and J.G; writing—review and editing, I.J., T.G. (Tadeusz Groń) and J.G.; visualisation, I.J. and T.G. (Tadeusz Groń); supervision, I.J. and T.G. (Tadeusz Groń); project administration, I.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by the Slovak Research and Development Agency (project APVV-17-0318) and VEGA 1/0116/22.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Snyder, G.J.; Caillat, T.; Fleurial, J.P. Thermoelectric properties of Chalcogenides with Spinel Structure. Mat. Res. Innov. 2001, 5, 67. [Google Scholar] [CrossRef]
- Korenskii, V.I.; Ignateva, I.S.; Kolenko, I.P.; Potiev, A.A.; Surat, L.L. Osobennosti Elektronnogo Stroeniya i Svoistva Twerdofaznych Soedinenii Titana i Vanadya; UNTS AN SSSR: Sverdlovsk, Russia, 1982. [Google Scholar]
- Armand, N.B. Materials for Advances Batteries; Minphy, M.W., Broadhead, J., Steele, B.C.H., Eds.; New York Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Thakeray, M.M.; David, W.I.F.; Goodenough, J.B. High-temperature lithiation of α-Fe2O3: A mechanistic study. J. Solid State Chem. 1984, 55, 280–286. [Google Scholar] [CrossRef]
- Lotgering, F.K. Ferromagnetic interactions in sulphides, selenides and tellurides with spinel structure. In Proceedings of the International Conference on Magnetism, Nottingham, UK, 7–11 September 1964; Institute of Physics and the Physical Society: London, UK, 1965; pp. 533–538. [Google Scholar]
- Plumier, R. Étude par diffraction de neutrons de l’antiferromagnétisme hélicoïdal du spinelle ZnCr2Se4 en présence d’un champ magnétique. J. Phys. 1966, 27, 213–219. [Google Scholar] [CrossRef]
- Greenwood, N.N. Ionenkristalle, Gitterdefekte und Nichtstöchiometrische Verbindungen; Verlag Chemie: Weinheim, Germany, 1973. [Google Scholar]
- Park, S.; Kwon, S.; Lee, S.; Khim, S.; Bhoi, D.; Park, C.B.; Kim, K.H. Interactions in the bond-frustrated helimagnet ZnCr2Se4 investigated by NMR. Sci. Rep. 2019, 9, 16627. [Google Scholar] [CrossRef] [PubMed]
- Hemberger, J.; von Nidda, H.-A.K.; Tsurkan, V.; Loidl, A. Large Magnetostriction and Negative Thermal Expansion in the Frustrated Antiferromagnet ZnCr2Se4. Phys. Rev. Lett. 2007, 98, 147203. [Google Scholar] [CrossRef]
- Yaresko, A.N. Electronic band structure and exchange coupling constants in ACr2X4 spinels (A=Zn, Cd, Hg; X=O, S, Se). Phys. Rev. 2008, 77, 115106. [Google Scholar] [CrossRef]
- Malicka, E.; Groń, T.; Pacyna, A.W.; Maciążek, E.; Duda, H.; Pawełczyk, M.; Zawisza, B.; Sitko, R. Influence of temperature on the critical fields in ZnCr2−xAlxSe4 antiferromagnets. J. Alloys Compd. 2009, 480, 67–69. [Google Scholar] [CrossRef]
- Groń, T.; Malicka, E.; Duda, H.; Pacyna, A.W.; Mydlarz, T.; Sitko, R.; Pawełczyk, M. Spin-glass-like behavior in ZnxCryAlzSe4. J. Phys. Chem. Solids 2009, 70, 1175–1180. [Google Scholar] [CrossRef]
- Okońska-Kozłowska, I.; Lutz, H.D.; Groń, T.; Krok, J.; Mydlarz, T. Darstellung, elektrische und magnetische Eingenschaften von-Zn1−xGa0.667xCr2Se4-Spinell-Einkristallen. Mater. Res. Bull. 1984, 19, 1–5. [Google Scholar] [CrossRef]
- Groń, T.; Duda, H.; Warczewski, J. Transport phenomena in the antiferromagnetic spinels Zn1−xGa2x/3Cr2Se4 (where 0.0 < × < 0.5). J. Mag. Mag. Mater. 1990, 83, 487–489. [Google Scholar]
- Groń, T.; Kopyczok, J.; Okońska-Kozłowska, I.; Warczewski, J. Seebeck effect in the antiferromagnetic single crystals of ZnCr2−xInxSe4 (0.0 < × < 0.15). J. Mag. Mag. Mater. 1992, 111, 53–55. [Google Scholar]
- Jendrzejewska, I.; Groń, T.; Kusz, J.; Żelechower, M.; Maciążek, E.; Ślebarski, A.; Fijałkowski, M. Spin-glass-like behaviour in tin doped ZnCr2Se4 single crystals. J. Alloys Compd. 2015, 635, 238–244. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Mroziński, J.; Groń, T.; Duda, H.; Zajdel, P.; Pacyna, A.W.; Maciążek, E.; Hanc, A. Effect of cation substitution on Fermi level of n-type ZnxSnyCrzSe4 spinels. J. Alloys Compd. 2009, 480, 63–66. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Groń, T.; Kusz, J.; Stokłosa, Z.; Pietrasik, E.; Goryczka, T.; Sawicki, B.; Goraus, J.; Jampilek, J.; Duda, H.; et al. The Zn1−xPbxCr2Se4-single crystals obtained by chemical vapour transport—structure and magnetic, electrical, and thermal properties. Materials 2022, 15, 5289. [Google Scholar] [CrossRef] [PubMed]
- Groń, T.; Wolff, J.; Hehenkamp, T.; Bärner, K.; Okońska-Kozłowska, I.; Jendrzejewska, I.; Malicka, E. Positron annihilation studies in single and polycrystals of Zn1−xCuxCr2Se4 spinel series. Radiat. Eff. Defects Solids 1996, 139, 97–107. [Google Scholar] [CrossRef]
- Groń, T.; Jendrzejewska, I.; Gołąbek, S.; Duda, H.; Krajewski, A.; Bärner, K. The thermoelectric power of ferromagnetically ordered ZnxCuyCrzSe4 single crystals. Phys. B Condens. Matter. 2003, 327, 88–95. [Google Scholar] [CrossRef]
- Mazur, S.; Groń, T.; Jendrzejewska, I. Influence of spin arrangement on thermopower in ZnxCuyCrzSe4 spinels. J. Alloys Compd. 2009, 480, 19–22. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Waśkowska, A.; Mydlarz, T. Influence of nickel substitution on the cation distribution and magnetic properties of ZnCr2Se4. J. Alloys Compd. 2001, 327, 73–77. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Zajdel, P.; Goryczka, T.; Goraus, J.; Kita, A.; Mydlarz, T. Influence of covalency and anion polarisation on magnetic and electronic properties of ZnCr2−xNixSe4. J. Alloys Compd. 2012, 520, 153–157. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Groń, T.; Goraus, J.; Pilch, M.; Pietrasik, E.; Barsova, Z.; Czerniewski, J.; Goryczka, T.; Witkowska-Kita, B.; Bienko, A.; et al. Synthesis and structural, magnetic, thermal and electronic properties of Mn-doped ZnCr2Se4. Mater. Chem. Phys. 2019, 238, 121901. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Zajdel, P.; Heimann, J.; Krok-Kowalski, J.; Mydlarz, T.; Mrzigod, J. Influence of manganese on magnetic and electronic properties of ZnCr2Se4 single Crystals. Mater. Res. Bull. 2012, 47, 1881–1886. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Groń, T.; Kwapuliński, P.; Kusz, J.; Pietrasik, E.; Goryczka, T.; Sawicki, B.; Ślebarski, A.; Fijałkowski, M.; Jampilek, J.; et al. Study of the structure, magnetic, thermal and electrical characterisation of ZnCr2Se4:Ta single crystals obtained by chemical vapour transport. Materials 2021, 14, 2749. [Google Scholar] [CrossRef] [PubMed]
- Jendrzejewska, I.; Groń, T.; Knizek, K.; Pilch, M.; Ślebarski, A.; Goraus, J.; Zajdel, P.; Stokłosa, Z.; Pietrasik, E.; Goryczka, T.; et al. Preparation, structure and magnetic, electronic and thermal properties of Dy3+-doped ZnCr2Se4 with unique geometric type spin-glass. J. Solid State Chem. 2021, 298, 122114. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Groń, T.; Maciążek, E.; Duda, H.; Kubisztal, M.; Ślebarski, A.; Pietrasik, E.; Fijałkowski, M. Specific heat and magnetic properties of single-crystalline ZnxDyyCrzSe4 spinels. J. Magn. Magn. Mater. 2016, 407, 122–128. [Google Scholar] [CrossRef]
- Maciążek, E.; Karolus, M.; Kubisztal, M.; Jendrzejewska, I.; Sitko, R.; Groń, T.; Ślebarski, A.; Fijałkowski, M. Magnetic and specific heat properties of a new Gd-doped ZnCr2Se4. Mater. Chem. Phys. 2015, 168, 187–192. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Groń, T.; Kusz, J.; Goraus, J.; Barsova, Z.; Pietrasik, E.; Czerniewski, J.; Goryczka, T.; Kubisztal, M. Growth, structure and physico-chemical properties of monocrystalline ZnCr2Se4:Ho prepared by chemical vapour transport. J. Solid State Chem. 2020, 281, 121024. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Groń, T.; Kusz, J.; Barsova, Z.; Pietrasik, E.; Goryczka, T.; Sawicki, B.; Ślebarski, A.; Fijałkowski, M.; Jampilek, J.; et al. Synthesis, crystal structure and characterisation of monocrystalline ZnCr2Se4 doped with neodymium. J. Solid State Chem. 2020, 292, 121661. [Google Scholar] [CrossRef]
- Piekarczyk, J. Thermodynamic model of chemical vapour transport and its application to some ternary compounds: I. The model. J. Cryst. Growth 1987, 82, 367–376. [Google Scholar] [CrossRef]
- Piekarczyk, J. Thermodynamic model of chemical vapour transport and its application to some ternary compounds: II. Application of the model to the complex oxides: ZnCr2O4, Y3Fe5O12 and Fe2TiO5. J. Cryst. Growth 1988, 89, 267–286. [Google Scholar] [CrossRef]
- Schmidt, P.M.; Binnewies, M.; Glaum, R.M.; Schmidt, M. Chemical Vapor Transport Reactions—Methods, Materials, Modeling. In Advanced Topics on Crystal Growth; IntechOpen: London, UK, 2013; Chapter 9; pp. 227–305. [Google Scholar]
- Jendrzejewska, I.; Żelechower, M.; Szamocka, K.; Mydlarz, T.; Waśkowska, A.; Okońska-Kozłowska, I. Growth of the CdxNiyCrzSe4 single crystals and their magnetic properties. J. Cryst. Growth 2004, 270, 30–37. [Google Scholar] [CrossRef]
- HSC Chemistry, version 6.01; Metso Outotec Corporation: Helsinki, Finland, 2009.
- Jurczyk, J.; Sitko, R.; Jendrzejewska, I. Thin sample in the XRF analysis. A new method of preparing microsamples of mono- and polycrystals, and silicate rocks. Chem. Anal. 1999, 44, 167–185. [Google Scholar]
- Groń, T.; Krok-Kowalski, J.; Duda, H.; Mydlarz, T.; Gilewski, A.; Walczak, J.; Filipek, E.; Bärner, K. Metamagnetism in Cr2V4−xMoxO13+0.5x. Phys. Rev. B 1995, 51, 16021–16024. [Google Scholar] [CrossRef]
- Krok-Kowalski, J.; Groń, T.; Warczewski, J.; Mydlarz, T.; Okońska-Kozłowska, I. Ferrimagnetism and metamagnetism in Cd1−xCuxCr2S4, spinels. J. Magn. Magn. Mater. 1997, 168, 129–138. [Google Scholar] [CrossRef]
- Morrish, A.H. Physical Principles of Magnetism; John Wiley & Sons, Inc.: New York, NY, USA, 1965. [Google Scholar]
- Particle Data Group; Workman, R.L.; Burkert, V.D.; Crede, V.; Klempt, E.; Thoma, U.; Tiator, L.; Agashe, K.; Aielli, G.; Allanach, B.C.; et al. The Review of Particle Physics. Prog. Theor. Exp. Phys. 2022, 2022, 083C01. [Google Scholar]
- Holland, W.E.; Brown, H.A. Application of the Weiss molecular field theory to the B-site spinel. Phys. Status Solidi 1972, 10, 249–253. [Google Scholar] [CrossRef]
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr. 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Plies, V. Massenspektrometrische Untersuchungen der Gasphase über CrCl3 und CrCl3/Cl2. Z. Anorg. Allg. Chem. 1988, 556, 120–125. [Google Scholar] [CrossRef]
- Von Oppermann, H. Das Reaktionsgleichgewicht 2CrCl3,f, g + Cl2,g = 2 CrCl4,g. Z. Anorg. Allg. Chem. 1968, 359, 51–57. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Dzięgielewski, J. Inorganic Chemistry—Script for Students; University of Silesia: Katowice, Poland, 1987. [Google Scholar]
- Busch, G.; Magyar, B.; Wachter, P. Optical absorption of some ferro- and antiferromagnetic spinels, containing Cr3+-ions. Phys. Lett. 1966, 23, 438–440. [Google Scholar] [CrossRef]
- Barnard, R.D. Thermoelectricity in Metals and Alloys; Taylor & Francis: London, UK, 1972. [Google Scholar]
- Trodahl, H.J. Thermopower of the superconducting cuprates. Phys. Rev. B 1995, 51, 5178–6175. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C. Introduction to Solid State Physics; John Wiley & Sons, Inc.: New York, NY, USA, 1971; p. 641. [Google Scholar]
- Groń, T.; Bärner, K.; Kleeberg, C.; Okońska-Kozłowska, I. The thermoelectric power of ferromagnetically ordered Cu1−xGaxCr2Se4 spinels. Phys. B Condens. Matter 1996, 225, 191–196. [Google Scholar] [CrossRef]
- Sawicki, B.; Karolewicz, M.; Tomaszewicz, E.; Oboz, M.; Groń, T.; Kukuła, Z.; Pawlus, S.; Nowok, A.; Duda, H. Effect of Gd3+ substitution on thermoelectric power factor of paramagnetic Co2+-doped calcium molybdato-tungstates. Materials 2021, 14, 3692. [Google Scholar] [CrossRef]
- Sawicki, B.; Tomaszewicz, E.; Guzik, M.; Groń, T.; Oboz, M.; Duda, H.; Pawlus, S.; Urbanowicz, P. Effect of Ca2+ site substitution on structural, optical, electrical and magnetic properties in Nd3+ and Mn2+-co-doped calcium molybdato-tungstates. Ceram. Int. 2023, 49, 944–955. [Google Scholar] [CrossRef]
- Malicka, E.; Groń, T.; Ślebarski, A.; Pacyna, A.W.; Goraus, J.; Fijałkowski, M.; Heimann, J. Specific heat and magnetic susceptibility of single-crystalline ZnCr2−xAlxSe4 (x = 0.15, 0.23). J. Phys. Chem. Solids 2011, 72, 974–979. [Google Scholar] [CrossRef]
- Malicka, E.; Groń, T.; Ślebarski, A.; Gągor, A.; Pacyna, A.W.; Sitko, R.; Goraus, J.; Mydlarz, T.; Heimann, J. Specific heat and magnetic susceptibility of single-crystalline ZnCr2Se4 spinels doped with Ga, In and Ce. Mater. Chem. Phys. 2011, 131, 142–150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).