N, S, O Self-Doped Carbon Derived from Grapefruit Peel for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of NSOC-800 Electrode
2.3. Characterization
2.4. Computational Process
3. Results and Discussion
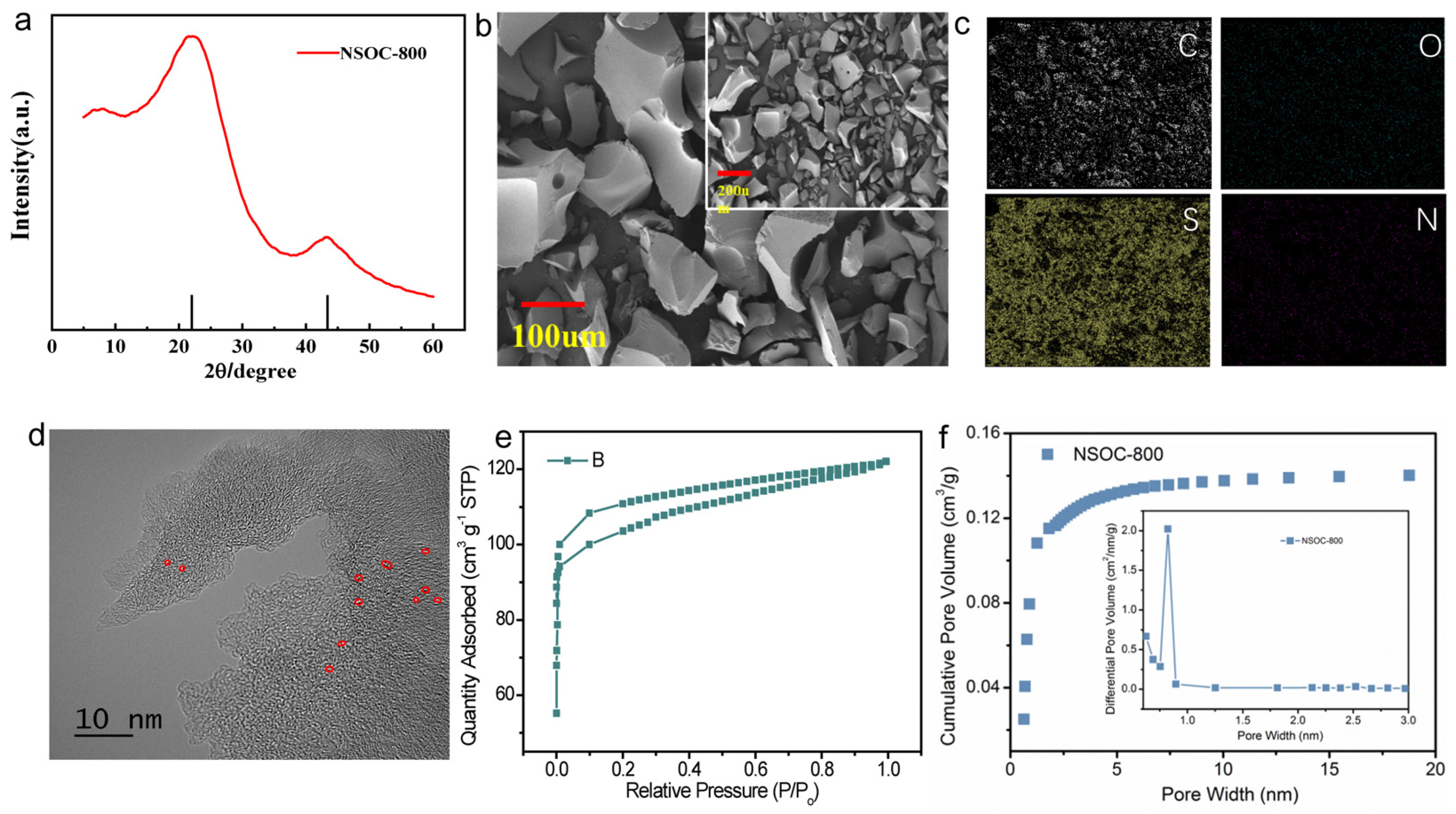
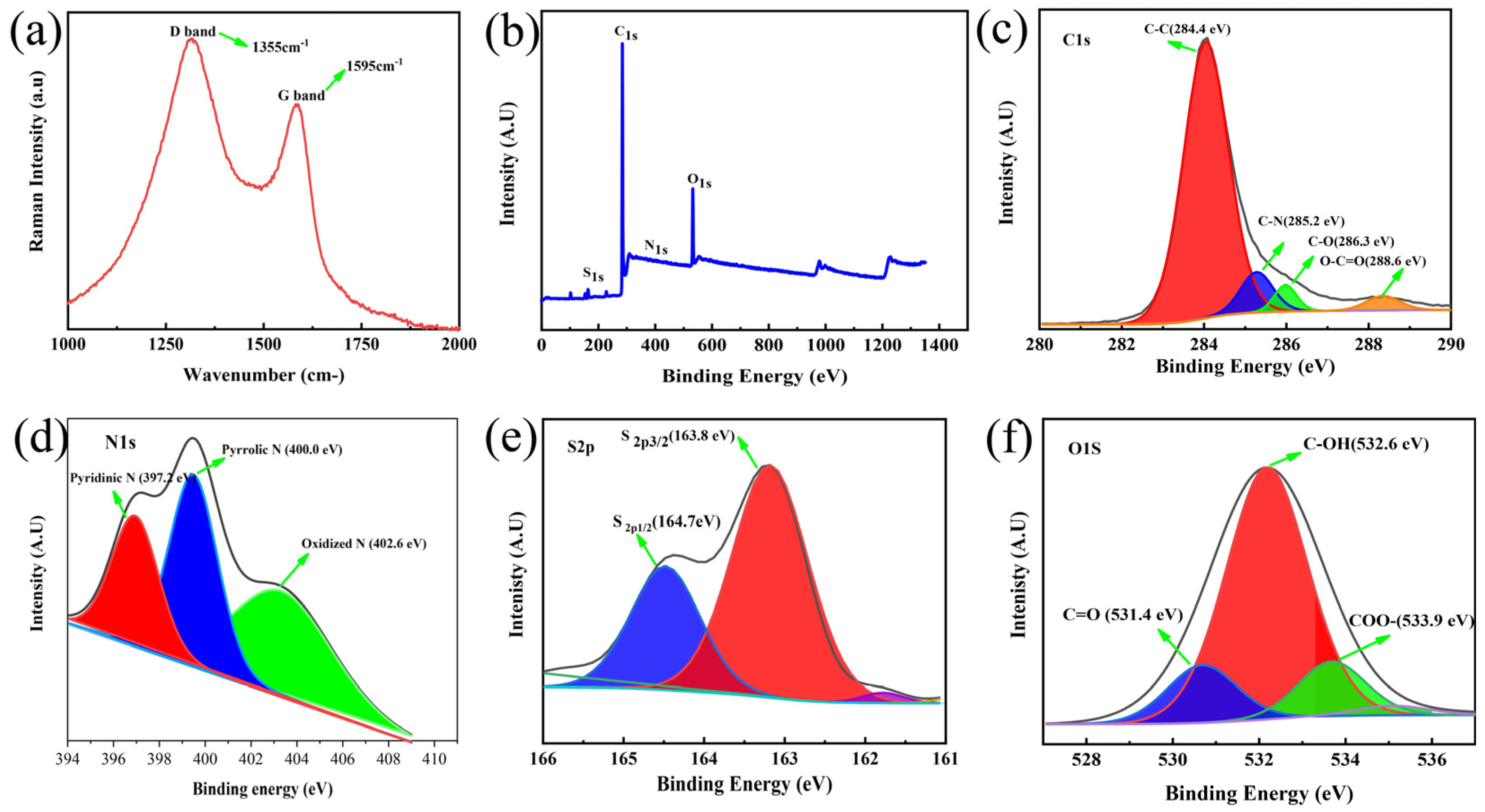
3.1. Material Characterization
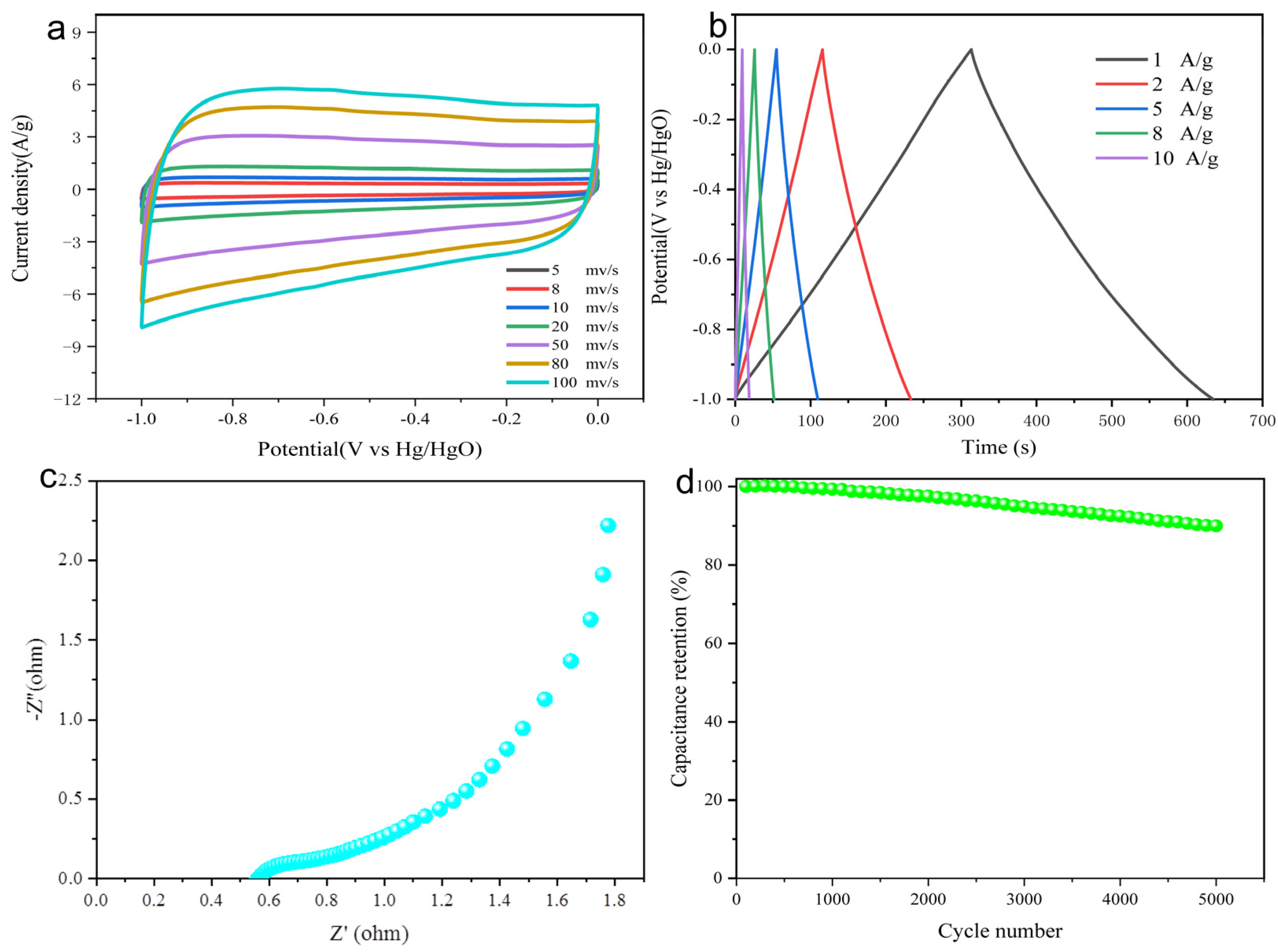
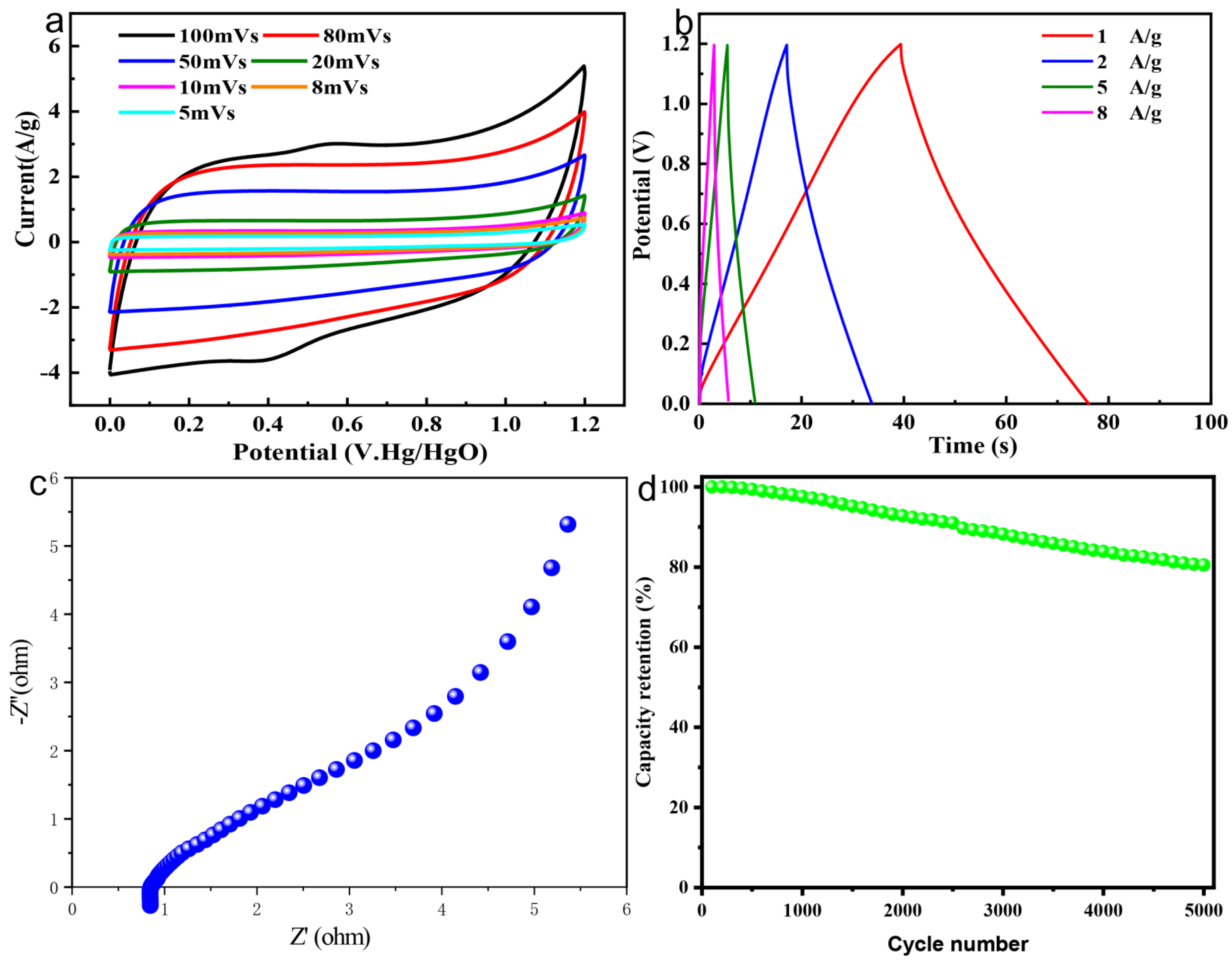
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, Y.; Zhang, M.; Xie, S.; Tong, Y.; Cheng, F.; Lu, X. Binder-free WS2 nanosheets with enhanced crystallinity as a stable negative electrode for flexible asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 21460–21466. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xiao, P.; Xue, L.; Li, H.; Zhai, T. The Rising Zinc Anodes for High-Energy Aqueous Batteries. Energy Chem. 2021, 3, 100052. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Hou, C.; Wang, Z.; Liang, C.; Zhao, C.; Tong, Y.; Lu, X.; Yang, S. Nitrogen-Doped Co3O4 Mesoporous Nanowire Arrays as an Additive-Free Air-Cathode for Flexible Solid-State Zinc–Air Batteries. Adv. Mater. 2017, 29, 1602868. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, Y.; Yang, F.; Liu, X.; Lu, X. Aromatic organic molecular crystal with enhanced π–π stacking interaction for ultrafast Zn-ion storage. Energy Environ. Sci. 2020, 13, 2515–2523. [Google Scholar] [CrossRef]
- Zhao, L.-F.; Hu, Z.; Lai, W.-H.; Tao, Y.; Peng, J.; Miao, Z.-C.; Wang, Y.-X.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X. Hard Carbon Anodes: Fundamental Understanding and Commercial Perspectives for Na-Ion Batteries beyond Li-Ion and K-Ion Counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Grey, C.P.; Hall, D.S. Prospects for lithium-ion batteries and beyond—A 2030 vision. Nat. Commun. 2020, 11, 6279. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, O.; Hawkes, A.; Gambhir, A.; Staffell, I. The future cost of electrical energy storage based on experience rates. Nat. Energy 2017, 2, 17110. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Kim, J.; Yun, S.; Choi, W.; Kim, H.; Yoon, W.-S. Multiscale factors in designing alkali-ion (Li, Na, and K) transition metal inorganic compounds for next-generation rechargeable batteries. Energy Environ. Sci. 2020, 13, 4406–4449. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, F.; Ding, H.; He, P.; Zhou, H. Lithium Metal Extraction from Seawater. Joule 2018, 2, 1648–1651. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Niu, C.; Duan, M.; Wang, X.; Huang, L.; Wang, J.; Pu, L.; Ren, W.; Shi, C.; Meng, J.; et al. Alkaline earth metal vanadates as sodium-ion battery anodes. Nat. Commun. 2017, 8, 460. [Google Scholar] [CrossRef]
- Zheng, S.; Wu, Z.-S.; Wang, S.; Xiao, H.; Zhou, F.; Sun, C.; Bao, X.; Cheng, H.-M. Graphene-based materials for high-voltage and high-energy asymmetric supercapacitors. Energy Storage Mater. 2017, 6, 70–97. [Google Scholar] [CrossRef]
- You, B.; Kang, F.; Yin, P.; Zhang, Q. Hydrogel-derived heteroatom-doped porous carbon networks for supercapacitor and electrocatalytic oxygen reduction. Carbon 2016, 103, 9–15. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chang, K.-H.; Hu, C.-C. Differentiate the pseudocapacitance and double-layer capacitance contributions for nitrogen-doped reduced graphene oxide in acidic and alkaline electrolytes. J. Power Sources 2013, 227, 300–308. [Google Scholar] [CrossRef]
- Jiao, X.; Xia, X.; Liu, P.; Lei, W.; Ouyang, Y.; Hao, Q. Nickel cobaltite nanosheets strongly anchored on boron and nitrogen co-doped graphene for high-performance asymmetric supercapacitors. Nanotechnology 2017, 28, 315403. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kim, H.-J. Effect of Time on a Hierarchical Corn Skeleton-Like Composite of CoO@ZnO as Capacitive Electrode Material for High Specific Performance Supercapacitors. Energies 2018, 11, 3285. [Google Scholar] [CrossRef] [Green Version]
- Moniruzzaman, M.; Anil Kumar, Y.; Pallavolu, M.R.; Arbi, H.M.; Alzahmi, S.; Obaidat, I.M. Two-Dimensional Core-Shell Structure of Cobalt-Doped@MnO2 Nanosheets Grown on Nickel Foam as a Binder-Free Battery-Type Electrode for Supercapacitor Application. Nanomaterials 2022, 12, 3187. [Google Scholar] [CrossRef]
- Karnan, M.; Raj, A.G.K.; Subramani, K.; Santhoshkumar, S.; Sathish, M. The fascinating supercapacitive performance of activated carbon electrodes with enhanced energy density in multifarious electrolytes. Sustain. Energy Fuels 2020, 4, 3029–3041. [Google Scholar] [CrossRef]
- Saini, S.; Chand, P.; Joshi, A. Biomass derived carbon for supercapacitor applications: Review. J. Energy Storage 2021, 39, 102646. [Google Scholar] [CrossRef]
- Chen, X.; Paul, R.; Dai, L. Carbon-based supercapacitors for efficient energy storage. Natl. Sci. Rev. 2017, 4, 453–489. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, P.; Zhou, Y.; Zhu, Y. High-performance symmetric supercapacitors based on carbon nanotube/graphite nanofiber nanocomposites. Sci. Rep. 2018, 8, 9005. [Google Scholar] [CrossRef] [Green Version]
- Dhavale, S.B.; Patil, V.L.; Patil, S.S.; Patil, D.S.; Dhavale, R.P.; Park, H.-H.; Patil, P.S. Porous carbon derived from Terminalia catappa leaves for energy storage application. Sustain. Energy Fuels 2023, 7, 1284–1301. [Google Scholar] [CrossRef]
- Chen, H.; Hu, H.; Han, F.; Liu, J.; Zhang, Y.; Zheng, Y. CoMoO4/bamboo charcoal hybrid material for high-energy-density and high cycling stability supercapacitors. Dalton Trans. 2020, 49, 10799–10807. [Google Scholar] [CrossRef]
- Zallouz, S.; Le Meins, J.-M.; Matei Ghimbeu, C. Alkaline hydrogel electrolyte from biosourced chitosan to enhance the rate capability and energy density of carbon-based supercapacitors. Energy Adv. 2022, 1, 1051–1064. [Google Scholar] [CrossRef]
- Liao, Y.; Huang, Y.; Shu, D.; Zhong, Y.; Hao, J.; He, C.; Zhong, J.; Song, X. Three-dimensional nitrogen-doped graphene hydrogels prepared via hydrothermal synthesis as high-performance supercapacitor materials. Electrochim. Acta 2016, 194, 136–142. [Google Scholar] [CrossRef]
- Arbi, H.M.; Yadav, A.A.; Anil Kumar, Y.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I.M. Polypyrrole-Assisted Ag Doping Strategy to Boost Co(OH)2 Nanosheets on Ni Foam as a Novel Electrode for High-Performance Hybrid Supercapacitors. Nanomaterials 2022, 12, 3982. [Google Scholar] [CrossRef]
- Thirumal, V.; Sreekanth, T.V.M.; Yoo, K.; Kim, J. Biomass-Derived Hard Carbon and Nitrogen-Sulfur Co-Doped Graphene for High-Performance Symmetric Sodium Ion Capacitor Devices. Energies 2023, 16, 802. [Google Scholar] [CrossRef]
- Thangavel, R.; Kannan, A.G.; Ponraj, R.; Kaliyappan, K.; Yoon, W.-S.; Kim, D.-W.; Lee, Y.-S. Cinnamon-Derived Hierarchically Porous Carbon as an Effective Lithium Polysulfide Reservoir in Lithium–Sulfur Batteries. Nanomaterials 2020, 10, 1220. [Google Scholar] [CrossRef]
- Thangavel, R.; Kannan, A.G.; Ponraj, R.; Thangavel, V.; Kim, D.-W.; Lee, Y.-S. Nitrogen- and sulfur-enriched porous carbon from waste watermelon seeds for high-energy, high-temperature green ultracapacitors. J. Mater. Chem. A 2018, 6, 17751–17762. [Google Scholar] [CrossRef]
- Zhou, J.; Shen, H.; Li, Z.; Zhang, S.; Zhao, Y.; Bi, X.; Wang, Y.; Cui, H.; Zhuo, S. Porous carbon materials with dual N, S-doping and uniform ultra-microporosity for high performance supercapacitors. Electrochim. Acta 2016, 209, 557–564. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Xie, L.; Su, F.; Xie, L.; Guo, X.; Wang, Z.; Kong, Q.; Sun, G.; Ahmad, A.; Li, X.; Yi, Z.; et al. Effect of pore structure and doping species on charge storage mechanisms in porous carbon-based supercapacitors. Mater. Chem. Front. 2020, 4, 2610–2634. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Alhebshi, N.A.; Salah, N.; Alshareef, H.N. Synthesis Strategies of Porous Carbon for Supercapacitor Applications. Small Methods 2020, 4, 1900853. [Google Scholar] [CrossRef]
- Chen, R.; Tang, H.; He, P.; Zhang, W.; Dai, Y.; Zong, W.; Guo, F.; He, G.; Wang, X. Interface Engineering of Biomass-Derived Carbon used as Ultrahigh-Energy-Density and Practical Mass-Loading Supercapacitor Electrodes. Adv. Funct. Mater. 2023, 33, 2212078. [Google Scholar] [CrossRef]
- Shaheen Shah, S.; Abu Nayem, S.M.; Sultana, N.; Saleh Ahammad, A.J.; Abdul Aziz, M. Preparation of Sulfur-doped Carbon for Supercapacitor Applications: A Review. ChemSusChem 2022, 15, e202101282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, L.; Shu, C.; Kong, L.; Ahmad, I.; Zhou, Y.-N.; Tang, W.; Sun, X.; Wu, Y. A Universal Strategy For N-Doped 2D Carbon Nanosheets with Sub-Nanometer Micropore for High-Performance Supercapacitor. Energy Environ. Mater. 2021, 4, 569–576. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S.; Xu, C.; Ma, X.; Huang, G.; Lu, C.; Li, Z. Mechanisms of Porous Carbon-based Supercapacitors. ChemNanoMat 2021, 7, 1273–1290. [Google Scholar] [CrossRef]
- Yuan, C.Z.; Zhou, L.; Hou, L.R. Facile fabrication of self-supported three-dimensional porous reduced graphene oxide film for electrochemical capacitors. Mater. Lett. 2014, 124, 253–255. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Guo, X.; Jin, K.; Wang, Y.; Li, L.; Zhang, Y.; Wang, Z.; Sun, L.; Zhang, T. N/S co-doped three-dimensional graphene hydrogel for high performance supercapacitor. Electrochim. Acta 2018, 278, 51–60. [Google Scholar] [CrossRef]
- Akhter, T.; Islam, M.M.; Faisal, S.N.; Haque, E.; Minett, A.I.; Liu, H.K.; Konstantinov, K.; Dou, S.X. Self-Assembled N/S Codoped Flexible Graphene Paper for High Performance Energy Storage and Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, S.; Penchev, M.; Ruiz, I.; Bozhilov, K.N.; Yan, D.; Ozkan, M.; Ozkan, C.S. Three dimensional few layer graphene and carbon nanotube foam architectures for high fidelity supercapacitors. Nano Energy 2013, 2, 294–303. [Google Scholar] [CrossRef]
- Zhang, S.; Ikoma, A.; Ueno, K.; Chen, Z.; Dokko, K.; Watanabe, M. Protic-Salt-Derived Nitrogen/Sulfur-Codoped Mesoporous Carbon for the Oxygen Reduction Reaction and Supercapacitors. ChemSusChem 2015, 8, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, Z.; Ba, Z.; Dong, J.; Zhang, Q.; Zhao, X. Three-dimensional N/S Co-doped holey graphene oxide based hydrogel electrodes for high performance supercapacitors. J. Energy Storage 2021, 39, 102658. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Xie, J.; Lv, M.; Luo, J.; Yang, F.; Yu, Y.; Lu, X. Iron-induced electron modulation of nickel hydroxide/carbon nanotubes composite to effectively boost the oxygen evolution activity. Chem. Eng. J. 2023, 452, 139369. [Google Scholar] [CrossRef]
- Karamanova, B.; Stoyanova, A.; Shipochka, M.; Veleva, S.; Stoyanova, R. Effect of Alkaline-Basic Electrolytes on the Capacitance Performance of Biomass-Derived Carbonaceous Materials. Materials 2020, 13, 2941. [Google Scholar] [CrossRef]
- Fic, K.; Meller, M.; Menzel, J.; Frackowiak, E. Around the thermodynamic limitations of supercapacitors operating in aqueous electrolytes. Electrochim. Acta 2016, 206, 496–503. [Google Scholar] [CrossRef]
- Chaudoy, V.; Tran Van, F.; Deschamps, M.; Ghamouss, F. Ionic liquids in a poly ethylene oxide cross-linked gel polymer as an electrolyte for electrical double layer capacitor. J. Power Sources 2017, 342, 872–878. [Google Scholar] [CrossRef]
| Supercapacitors | Current Densities (A g−1) | Cycles | Capacity Retention (%) |
|---|---|---|---|
| YP-50F//YP-50F (KOH) [48] | 0.06 | 6000 | ~76.9 |
| N/S-3DGH//N/S-3DGH [42] | 2 | 6000 | ~76 |
| 5 mol L−1 LiNO3||6 mol L−1 [49] | 1 | 5000 | ~79.5 |
| CLPE based supercapacitors [50] | 1 | 2000 | ~100 |
| CSC/S//CSC/S [30] | 0.335 | 150 | ~85 |
| NSOC-800//NSOC-800 (Our work) | 1 | 5000 | ~81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, L.; Lu, X. N, S, O Self-Doped Carbon Derived from Grapefruit Peel for High-Performance Supercapacitors. Materials 2023, 16, 4577. https://doi.org/10.3390/ma16134577
Wang Y, Wang L, Lu X. N, S, O Self-Doped Carbon Derived from Grapefruit Peel for High-Performance Supercapacitors. Materials. 2023; 16(13):4577. https://doi.org/10.3390/ma16134577
Chicago/Turabian StyleWang, Yi, Liangqun Wang, and Xihong Lu. 2023. "N, S, O Self-Doped Carbon Derived from Grapefruit Peel for High-Performance Supercapacitors" Materials 16, no. 13: 4577. https://doi.org/10.3390/ma16134577




