Microscopic Analysis of the Wetting Morphology and Interfacial Bonding Mechanism of Preoxidised Kovar Alloys with Borosilicate Glass
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Materials
2.2. Oxidation Treatment of the Kovar Alloys
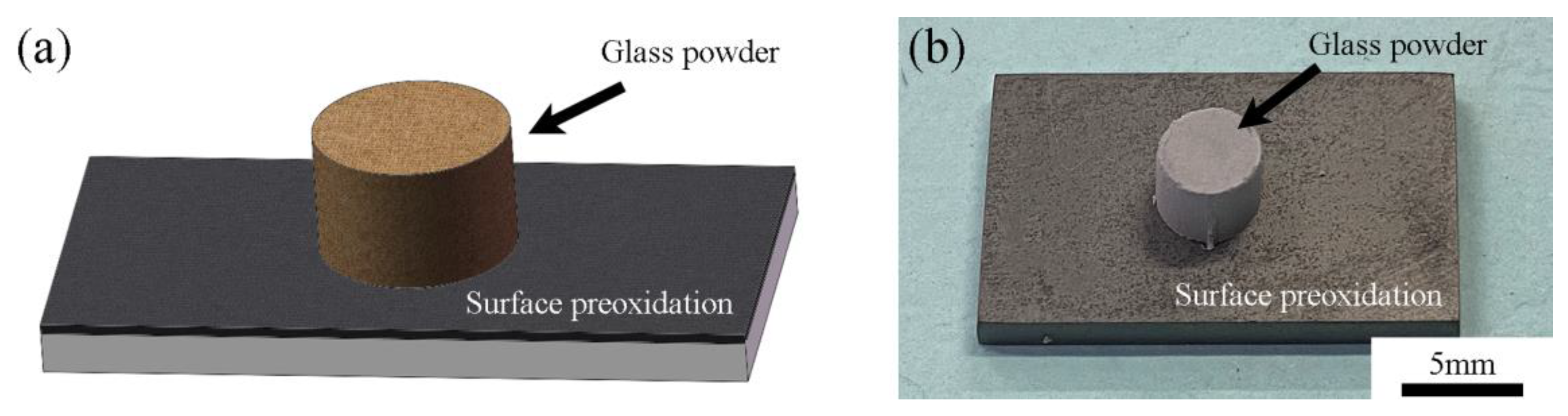
2.3. Wetting Experiments
2.4. Microscopic Observation and Elemental Analysis
3. Experimental Results
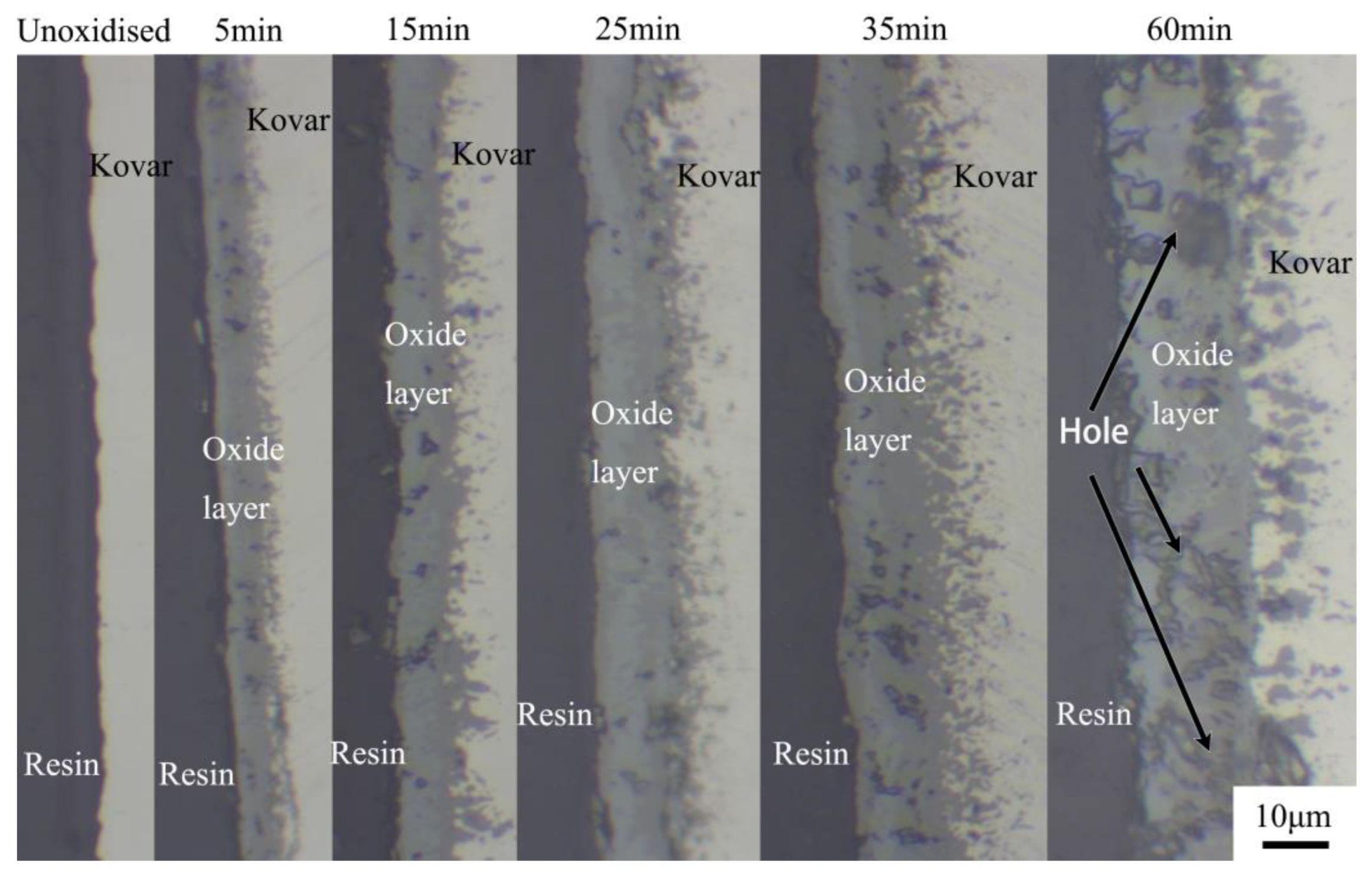
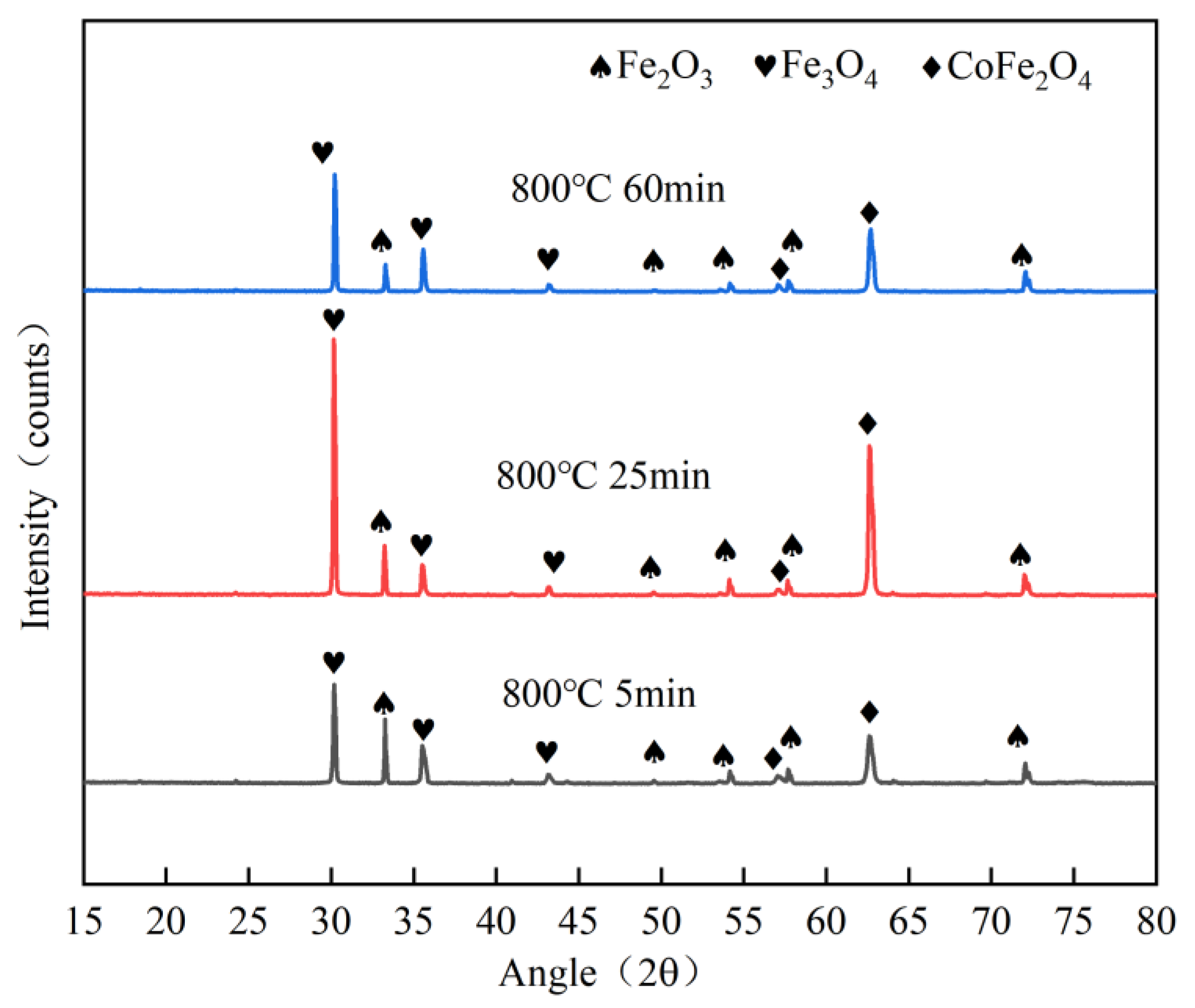
3.1. Oxidation Experiment of the Kovar Alloy
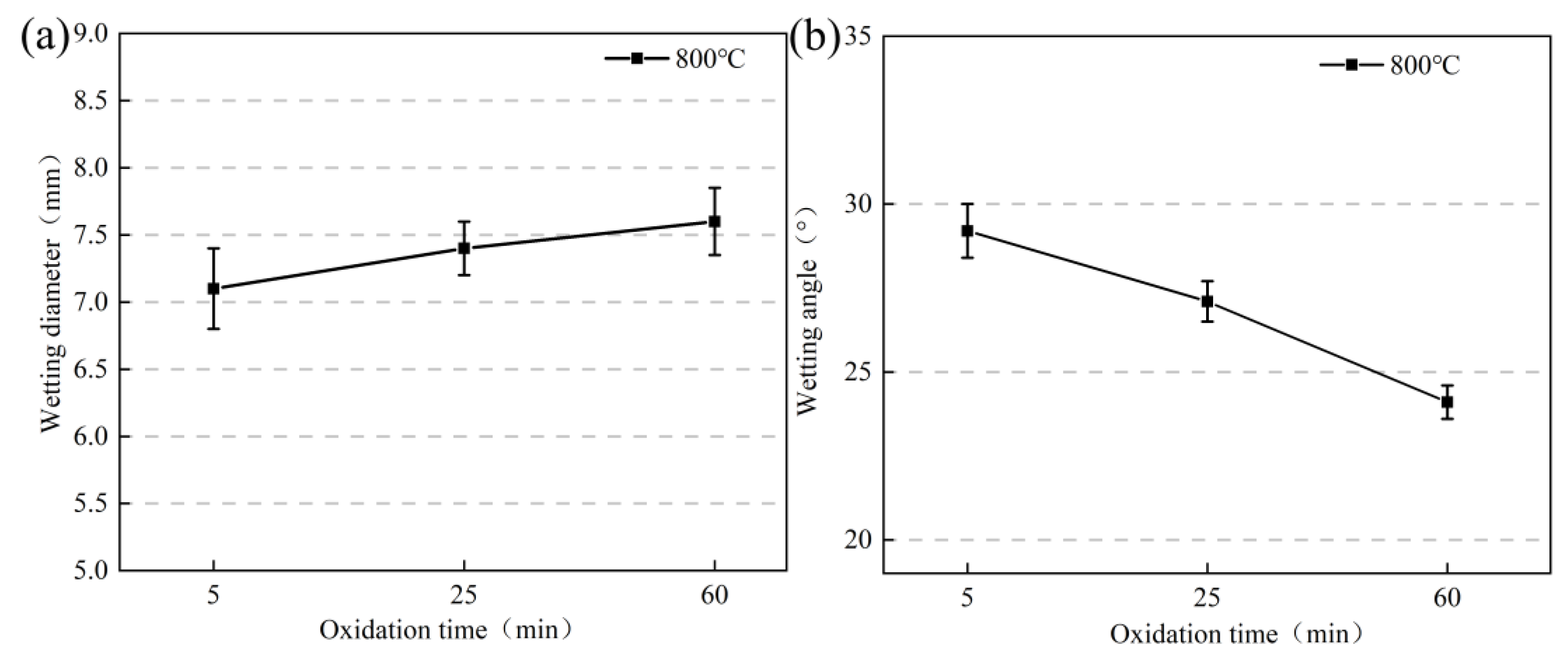
3.2. Wetting Experiments with Borosilicate Glass and Kovar
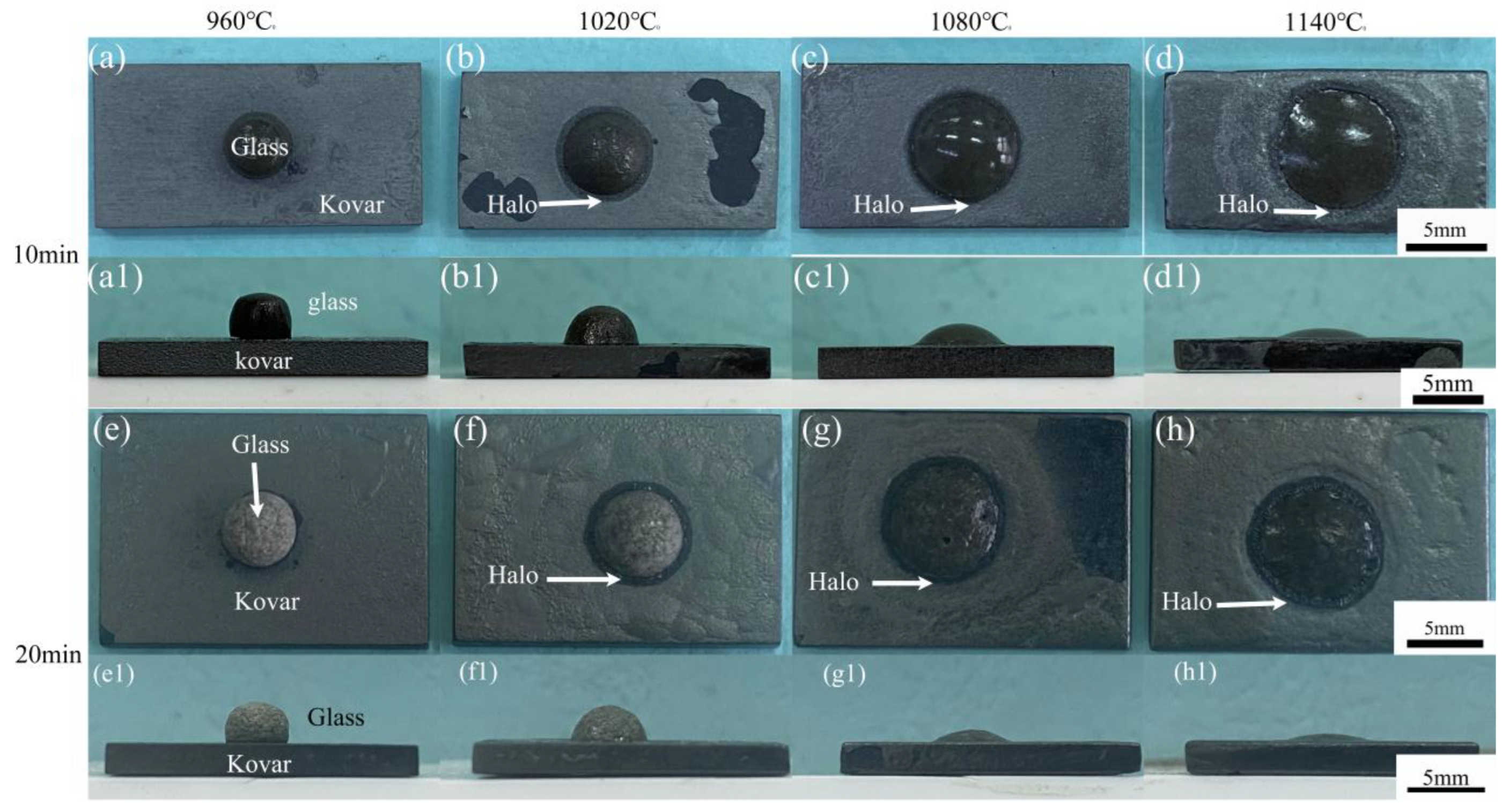
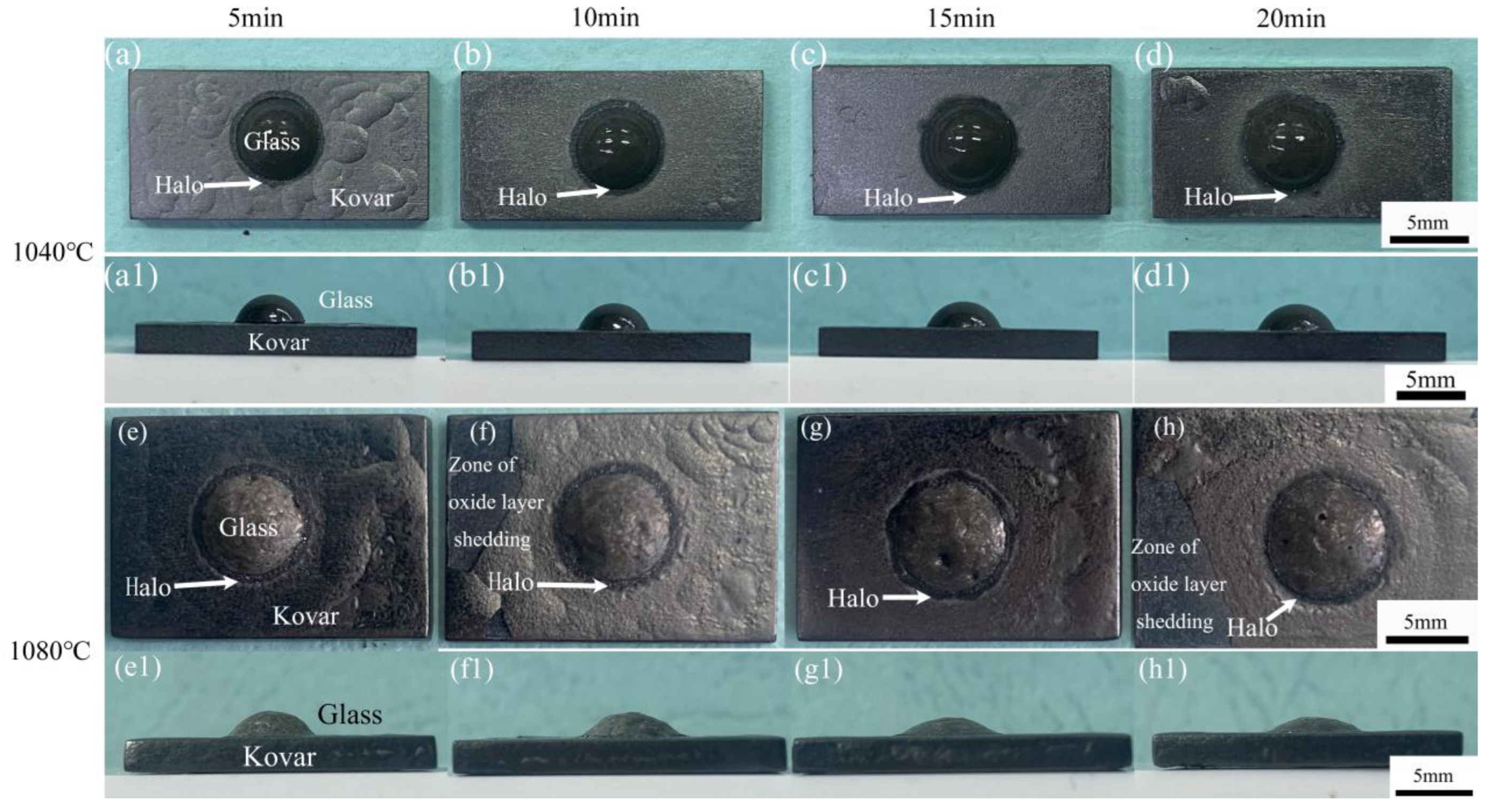
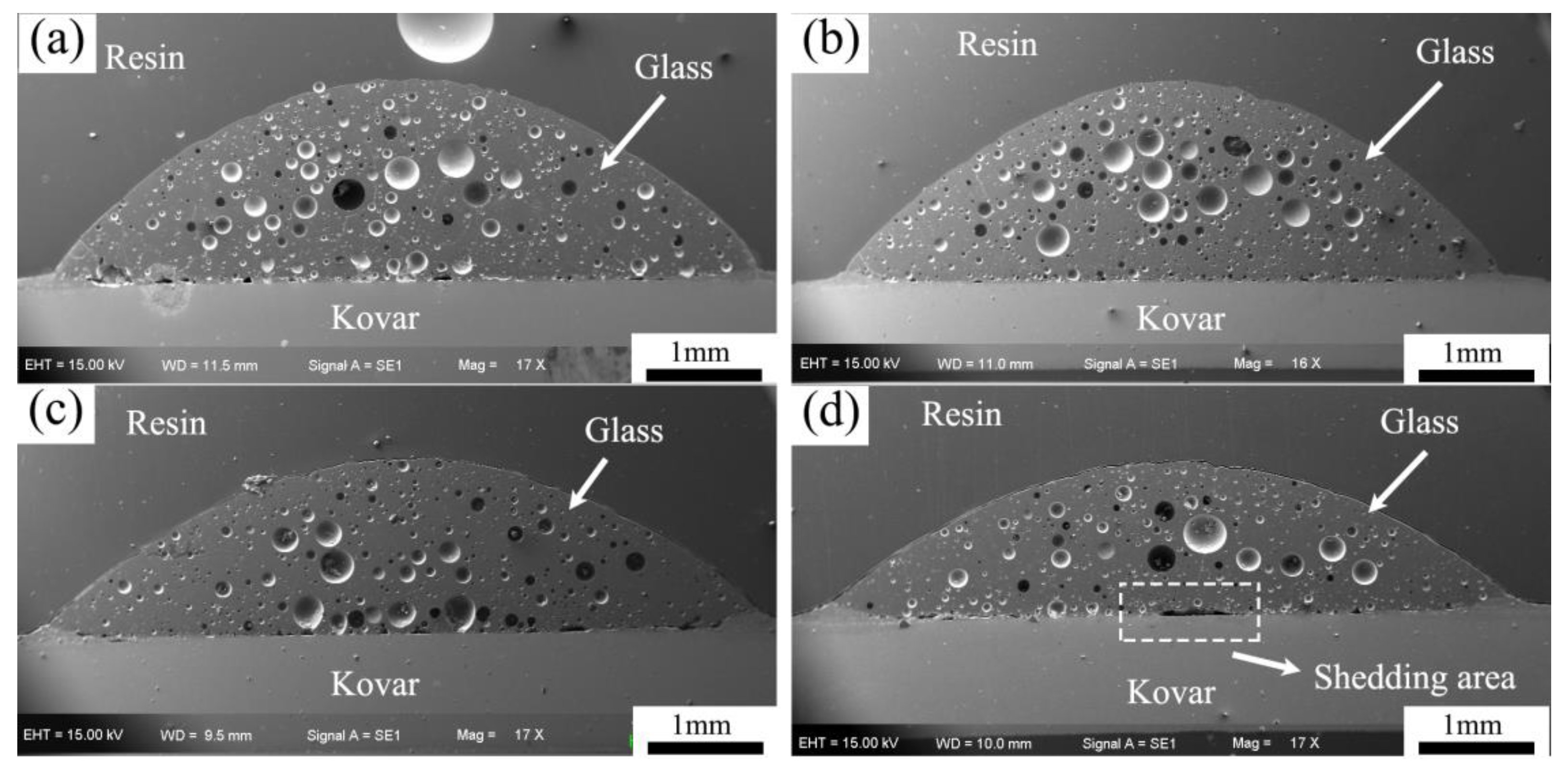
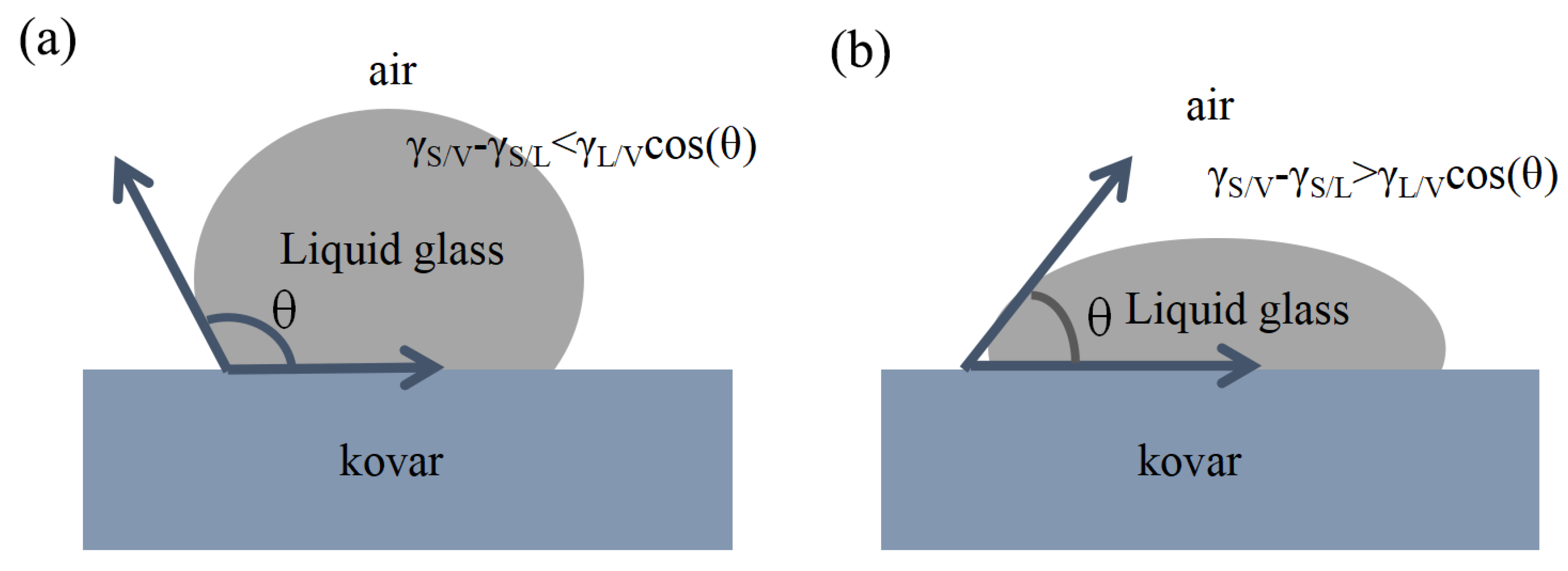
3.2.1. Analysis of Borosilicate Glass with Kovar Wetted Morphology
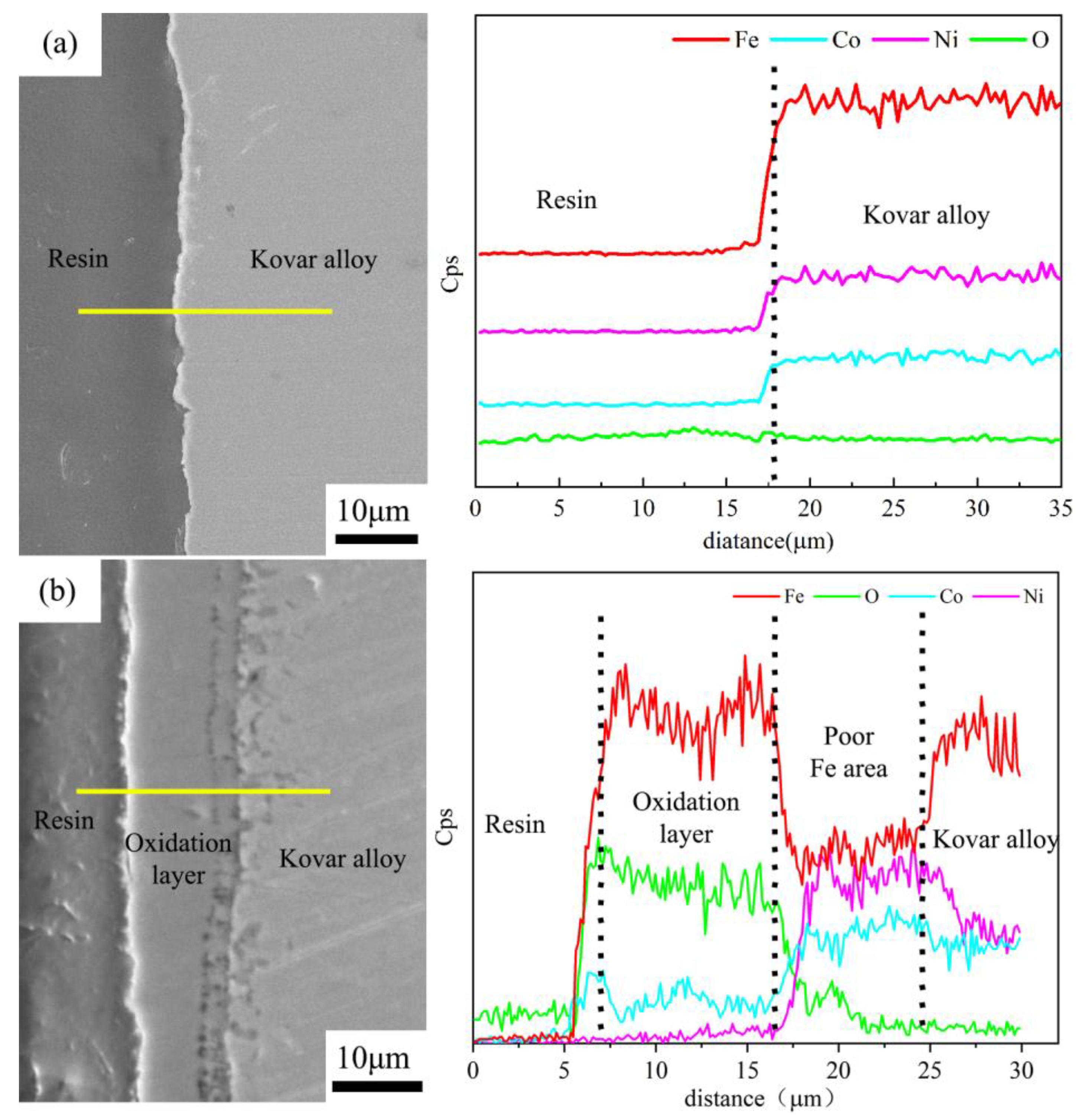
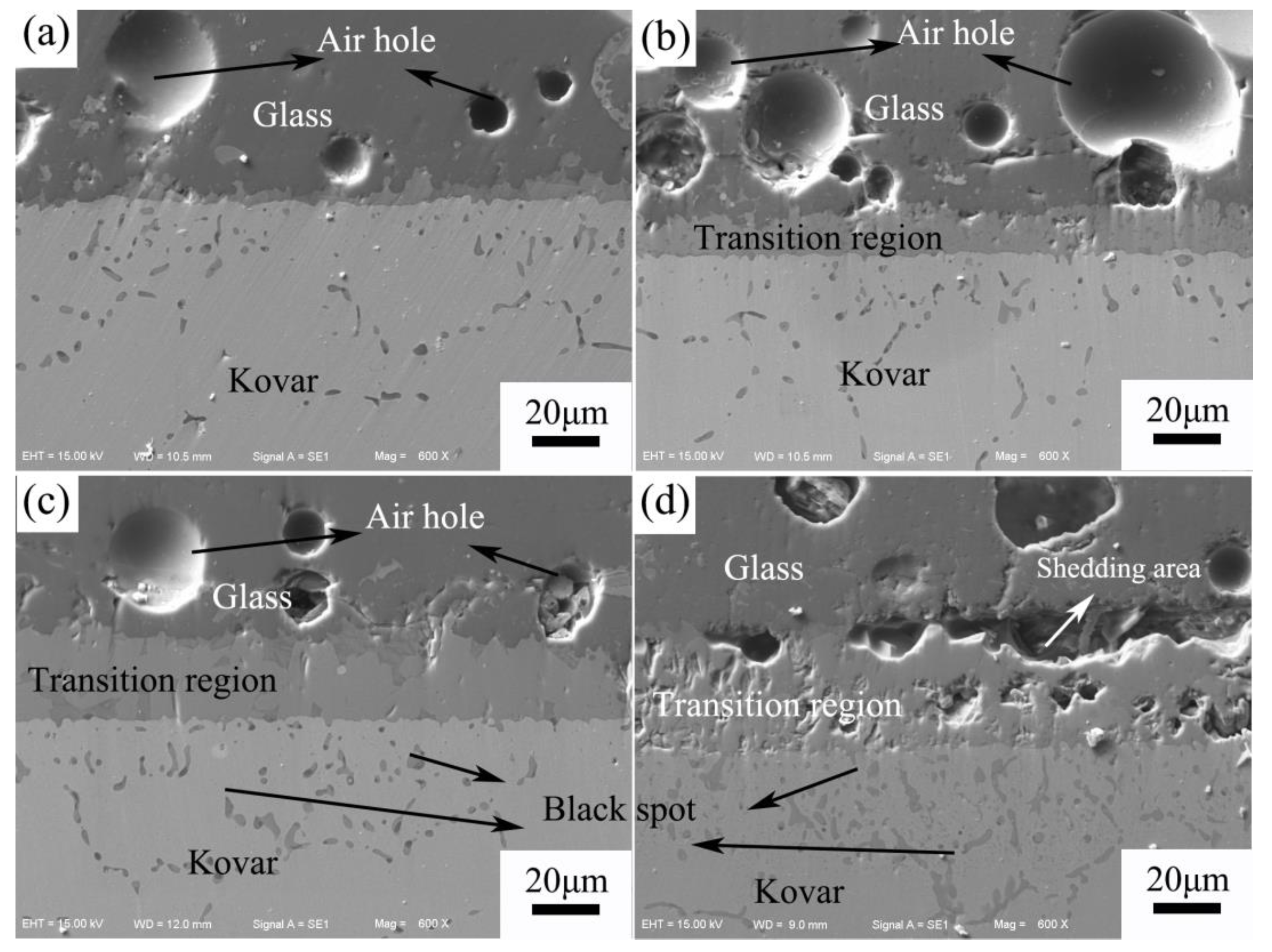
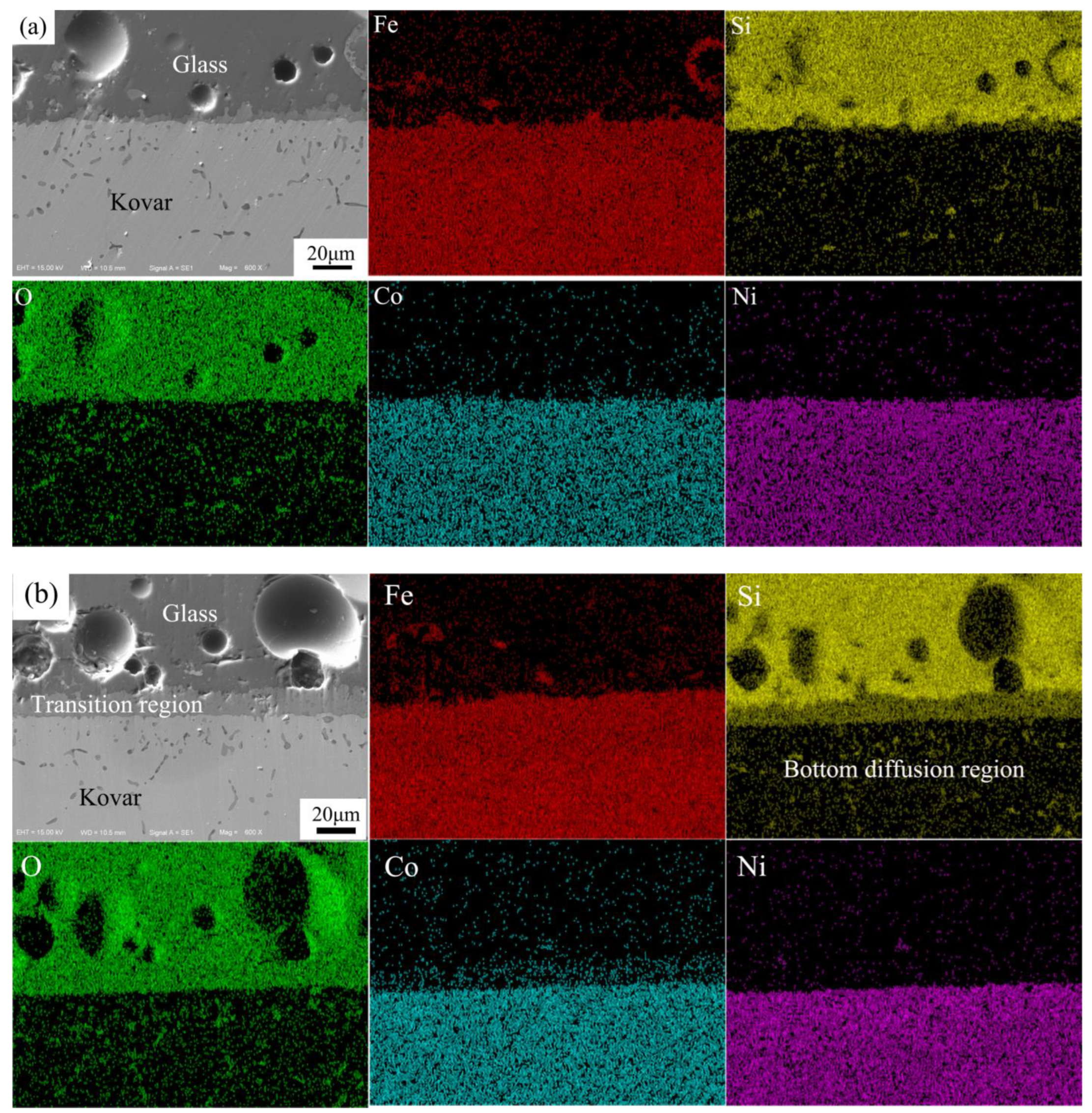
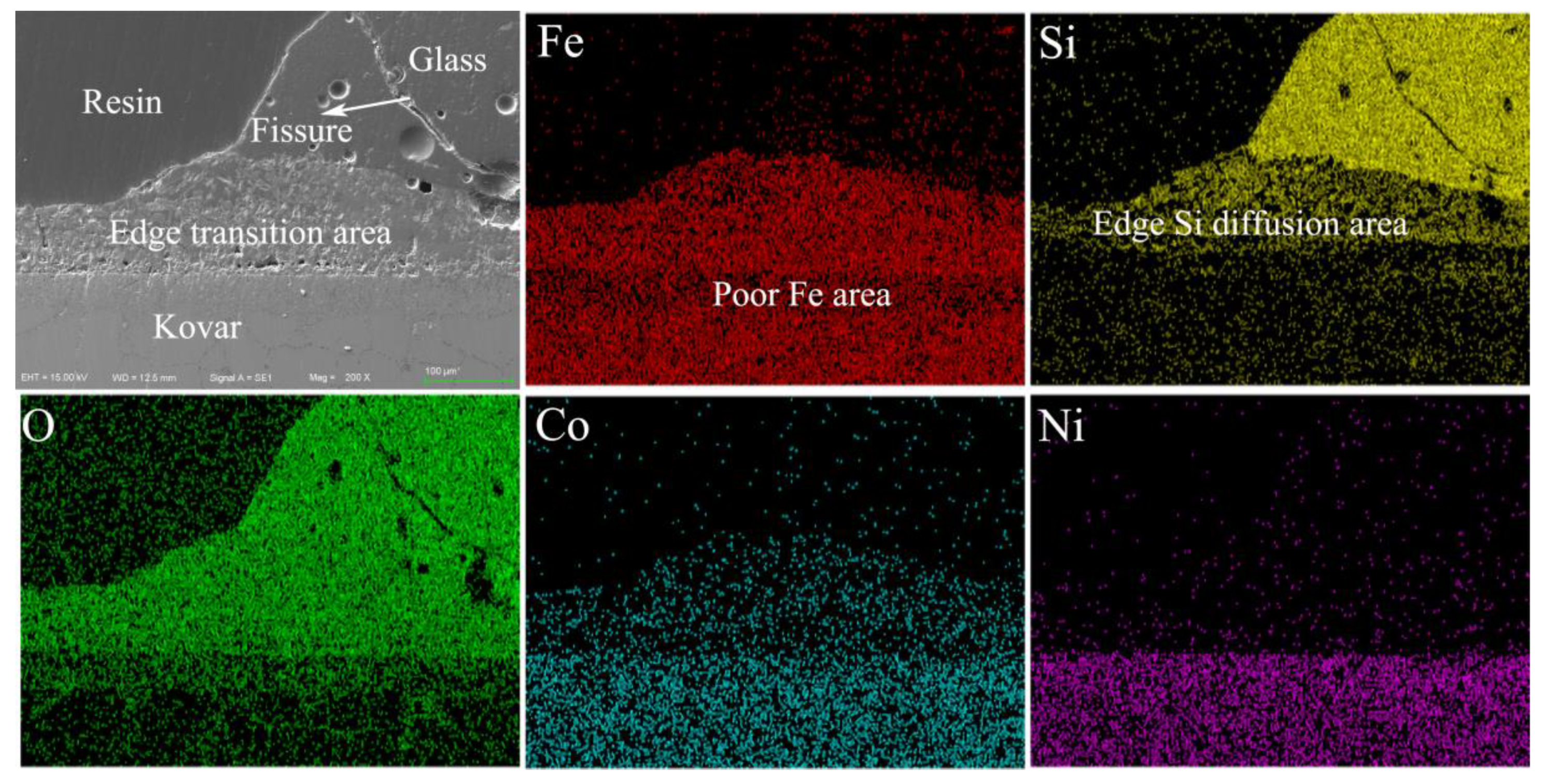
3.2.2. Analysis of the Interface between Borosilicate Glass and Kovar Wetting
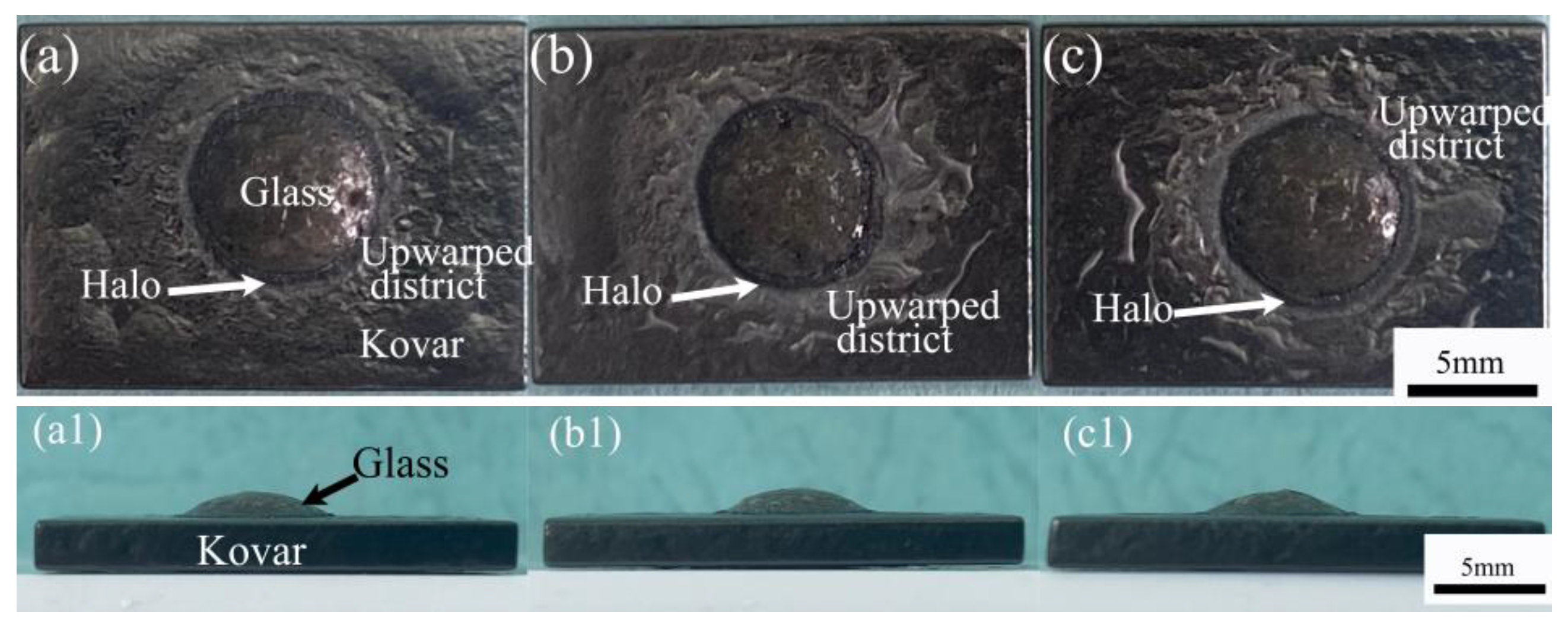
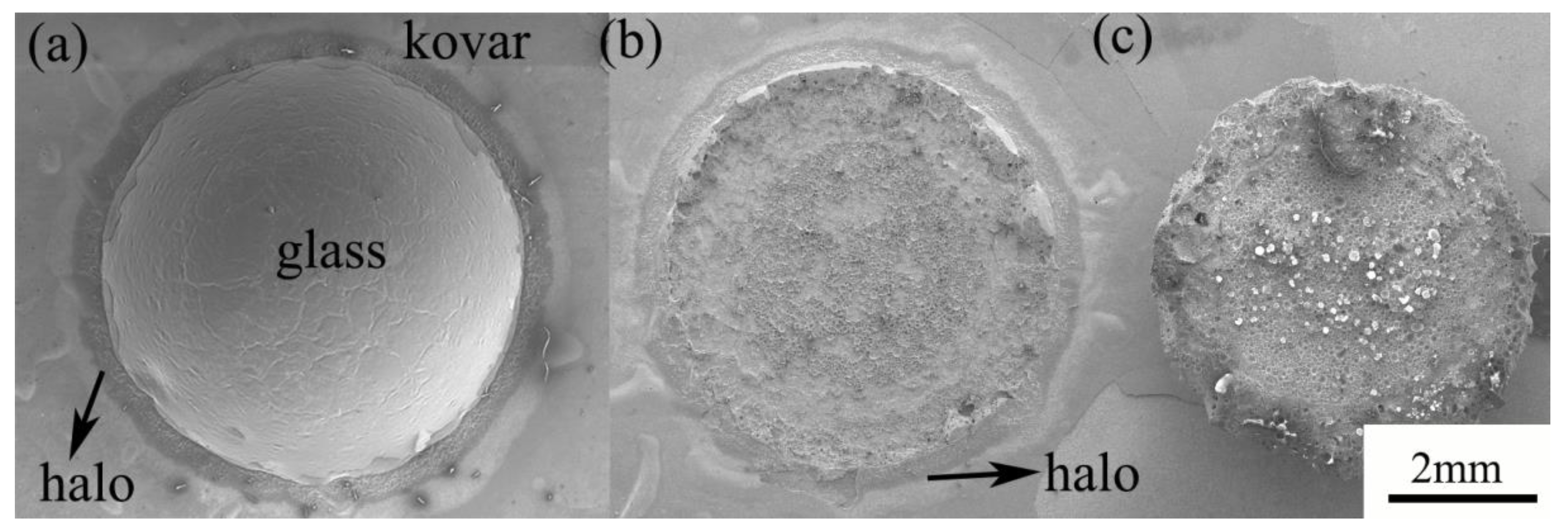
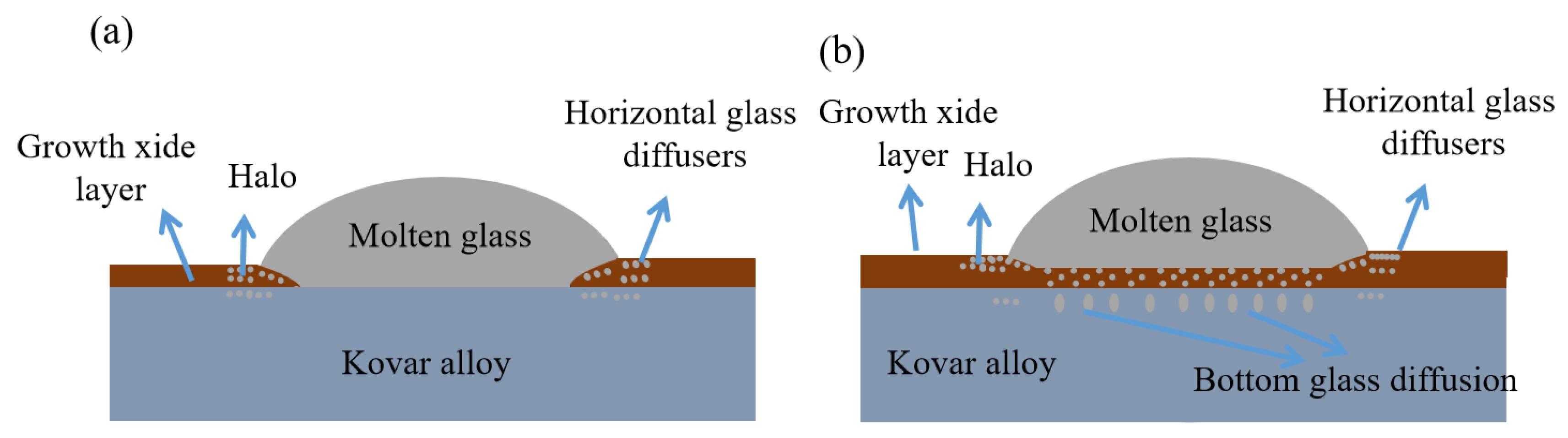
3.2.3. Study of the Wetting Boundary and Black Halo Formation between High-Borosilicate Glass and Kovar Alloys
3.2.4. Study of the Fracture and Composition of High-Borosilicate Glass with Frangible Alloys
3.3. Discussion
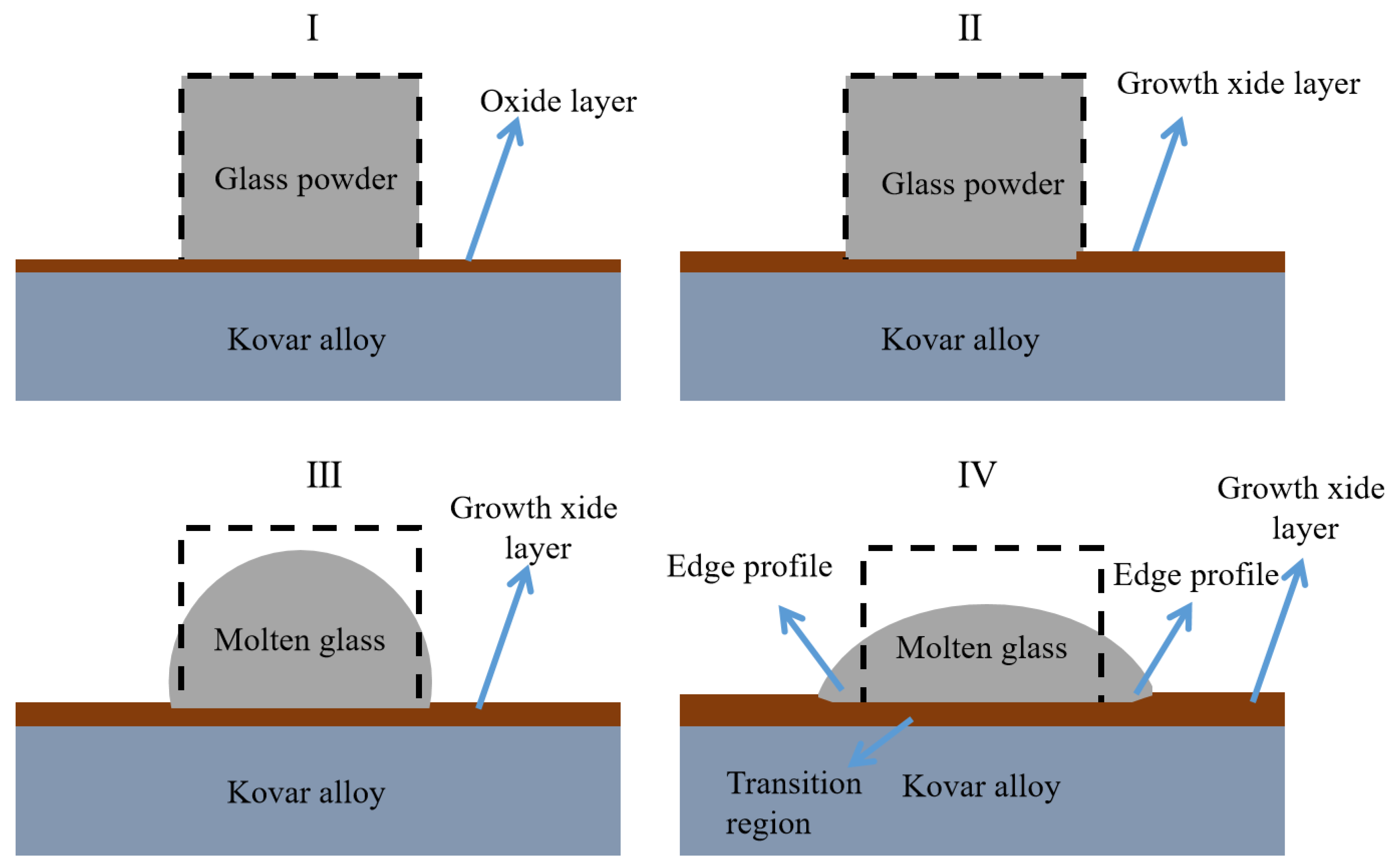
3.3.1. Mechanism for the Formation of High-Borosilicate Glass in the Wetted Form of Kovar Alloys
3.3.2. Wetting Microdiffusion of High-Borosilicate Glass with Kovar and Composition Formation Mechanisms
4. Conclusions
- (1)
- The oxide film generation thickness was 0–15 μm at 800 °C oxidation in the range of 0 to 60 min, and the thickness due to oxidation was denser in the range of 0 to 25 min; however, with the extension of time to 60 min, the oxide layer exhibited a loose structure and large pores. The oxide layer was divided into three regions according to the Fe element distribution (the Fe-rich matrix of Kovar alloy, Fe-poor porous region and Fe-rich oxidation region), and the main components of the oxide layer were Fe3O4 and Fe2O3.
- (2)
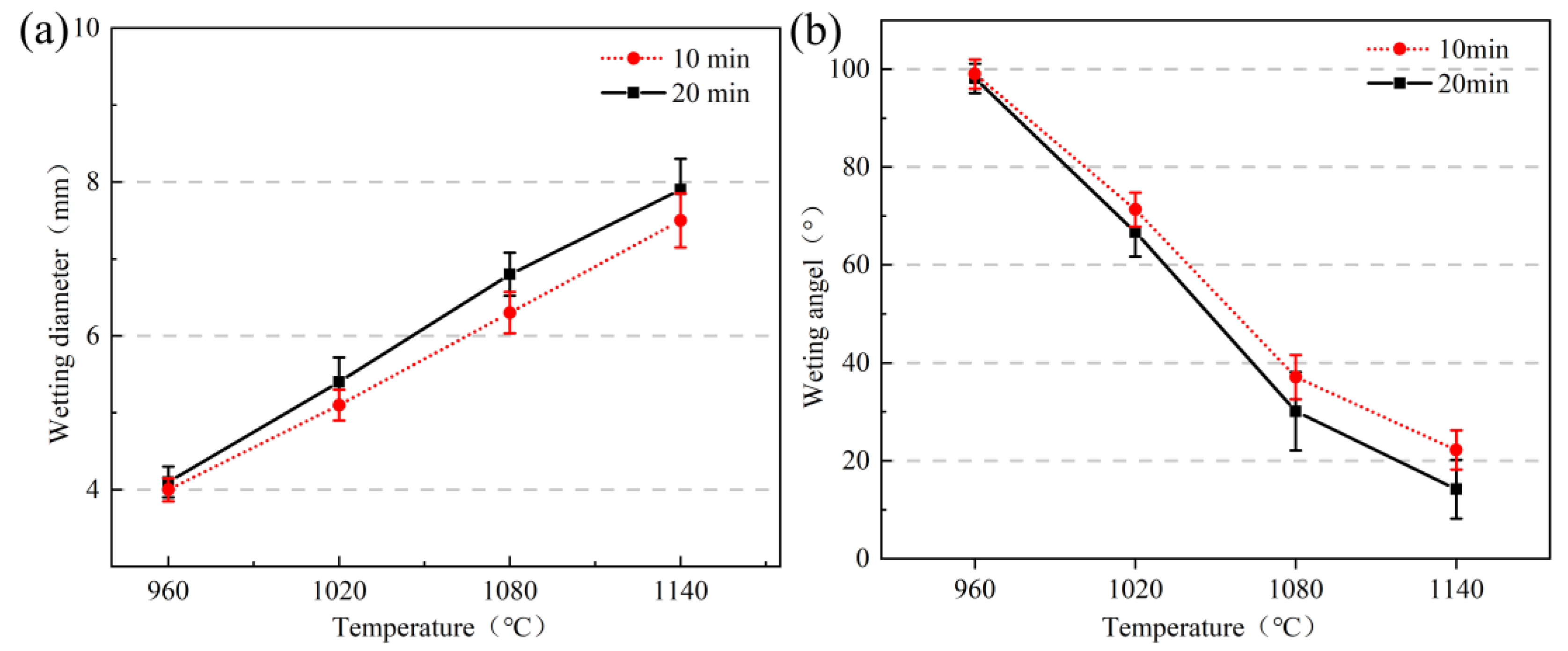
- The wettability of the Kovar alloy and high-borosilicate glass depended on temperature and time. As the temperature and holding time increased, the area of borosilicate glass spread on the surface of the Kovar alloy increased, and the wettability angle between the two decreased; however, a high temperature and time increase tended to damage the surface layer of the Kovar alloy.
- (3)
- The wetting mechanism of high-borosilicate glass on the surface of the Kovar alloy was mainly attributed to mechanical bonding. The entire wetting process of liquid glass filling the gap between the glass and the metal was accompanied by the reoxidation process on the surface of the Kovar alloy and the flow of liquid glass driven by thermodynamics to form the final macroscopic wetting profile.
- (4)
- The glass-to-metal wetting mechanism was divided into two aspects: longitudinal and transverse diffusion. The transverse diffusion of glass in the oxide layer of the Kovar alloy caused the formation of the black halo. During the wetting process, the high-borosilicate glass diffused longitudinally at the Kovar oxide interface under the action of capillary driving forces to form new chemical bonds, resulting in a new substance, Fe2SiO4, and the glass diffused further along the intergranular oxide towards the interior of the Kovar alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smeacetto, F.; Salvo, M.; Ferraris, M.; Cho, J.; Boccaccini, A.R. Glass–ceramic seal to join Crofer 22 APU alloy to YSZ ceramic in planar SOFCs. J. Eur. Ceram. Soc. 2007, 28, 61–68. [Google Scholar] [CrossRef]
- Guedes, A.; Pinto, A.; Vieira, M.; Viana, F. The effect of brazing temperature on the titanium/glass-ceramic bonding. J. Mater. Process. Technol. 1999, 92, 102–106. [Google Scholar] [CrossRef]
- Zhang, L.X.; Wu, L.Z.; Liu, D.; Feng, J.C.; Liu, H.B. Interface microstructure and mechanical properties of the brazed SiO2 glass ceramic and 30Cr3 high-tensile steel joint. Mater. Sci. Eng. A 2008, 496, 393–398. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Han, X.; Cui, M.; Fan, S. Investigation of the height-reducing method for clinched joint with AL5052 and AL6061. Int. J. Adv. Manuf. Technol. 2017, 89, 2269–2276. [Google Scholar] [CrossRef]
- Donald, I.W.; Mallinson, P.M.; Metcalfe, B.L.; Gerrard, L.A.; Fernie, J.A. Recent developments in the preparation, characterization and applications of glass- and glass–ceramic-to-metal seals and coatings. J. Mater. Sci. 2011, 46, 1975–2000. [Google Scholar] [CrossRef] [Green Version]
- Chou, Y.-S.; Stevenson, J.W.; Singh, P. Effect of pre-oxidation and environmental aging on the seal strength of a novel high-temperature solid oxide fuel cell (SOFC) sealing glass with metallic interconnect. J. Power Sources 2008, 184, 238–244. [Google Scholar] [CrossRef]
- Lei, D.; Wang, Z.; Li, J.; Li, J.B.; Wang, Z. Experimental study of glass to metal seals for parabolic trough receivers. Renew. Energy 2012, 48, 85–91. [Google Scholar] [CrossRef]
- Briand, D.; Weber, P.; Rooij, N. Metal to glass anodic bonding for microsystems packaging. In Proceedings of the International Conference on Transducers, Solid-State Sensors, Actuators & Microsystems, Boston, MA, USA, 8–12 June 2003. [Google Scholar]
- Li, Z.; Feng, G.; Wang, S.; Feng, S. High-efficiency Joining of C_f/Al Composites and TiAl Alloys under the Heat Effect of Laser-ignited Self-propagating High-temperature Synthesis. J. Mater. Sci. Technol. 2016, 32, 1111–1116. [Google Scholar] [CrossRef]
- Chern, T.S.; Tsai, H.L. Wetting and sealing of interface between 7056 Glass and Kovar alloy. Mater. Chem. Phys. 2007, 104, 472–478. [Google Scholar] [CrossRef]
- Zanchetta, A.; Lefort, P.; Gabbay, E. Thermal expansion and adhesion of ceramic to metal sealings: Case of porcelain-kovar junctions. J. Eur. Ceram. Soc. 1995, 15, 233–238. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Cheng, P.-Y.; Chou, C.-P. Matched glass-to-Kovar seals in N_2 and Ar atmospheres. Int. J. Miner. Metall. Mater. 2013, 20, 874–882. [Google Scholar] [CrossRef]
- Zanchetta, A.; Lortholary, P.; Lefort, P. Ceramic to metal sealings: Interfacial reactions mechanism in a porcelain-kovar junction. J. Alloys Compd. 1995, 228, 86–95. [Google Scholar] [CrossRef]
- Ardestani, S.; Dashtizad, V.; Kaflou, A. Effects of temperature, time, atmosphere and sealing geometry on defects occurred in borosilicate glass-kovar alloy seal. Ceram. Int. 2020, 47, 2008–2015. [Google Scholar] [CrossRef]
- Chanmuang, C.; Naksata, M.; Chairuangsri, T.; Jain, H.; Lyman, C.E. Microscopy and strength of borosilicate glass-to-Kovar alloy joints. Mater. Sci. Eng. A 2008, 474, 218–224. [Google Scholar] [CrossRef]
- Luo, D.; Shen, Z. Wetting and spreading behavior of borosilicate glass on Kovar. J. Alloys Compd. 2008, 477, 407–413. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, C.; Li, C. Wetting and sealing of the interface between silicate glass and copper. Int. J. Mater. Res. 2019, 110, 163–173. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Kathy, L. Effect of Atmosphere on Interconnect-Seal Glass Interaction for Solid Oxide Fuel/Electrolyzer Cells. J. Am. Ceram. Soc. 2011, 94, 875–885. [Google Scholar] [CrossRef]
- Legtenberg, R.; Bouwstra, S.; Elwenspoek, M. Low-temperature glass bonding for sensor application using boron oxide thin films. J. Micromech. Microeng. 1991, 1, 157. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, M.K.; Lu, K. Interfacial study of Crofer 22 APU interconnect-SABS-0 seal glass for solid oxide fuel/electrolyzer cells. J. Mater. Sci. 2009, 44, 5569–5578. [Google Scholar] [CrossRef]
- Hu, K.; Li, S.; Zhao, Z.; Liang, X.; Cai, Y.; Zhang, Y. Zinc diffusion affects the chemical stability of the borosilicate glass and AISI 304 interface. Mater. Charact. 2021, 171, 110792. [Google Scholar] [CrossRef]
- Magdefrau, N.J.; Lei, C.; Sun, E.Y.; Yamanis, J.; Aindow, M. Formation of spinel reaction layers in manganese cobaltite-coated Crofer22 APU for solid oxide fuel cell interconnects. J. Power Sources 2013, 227, 318–326. [Google Scholar] [CrossRef]
- Zhang, M.; Lawrence Yao, Y.; Jun Chen, C.; Kongsuwan, P.; Brandal, G.; Bian, D. Effects of Laser Radiation on the Wetting and Diffusion Characteristics of Kovar Alloy on Borosilicate Glass. J. Manuf. Sci. Eng. 2017, 140, 011012. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Kim, Y.G.; Lee, H.W.; Chung, W.S. Kinetics of Fe-30% Ni-12.5% Co invar alloy during high temperature oxidation. Met. Mater. Int. 2002, 8, 367–373. [Google Scholar] [CrossRef]
- Yates, P.M.; Mallinson, C.F.; Mallinson, P.M.; Whiting, M.J. An Investigation into the Nature of the Oxide Layer Formed on Kovar (Fe-29Ni-17Co) Wires Following Oxidation in Air at 700 and 800 °C. Oxid. Met. 2017, 88, 733–747. [Google Scholar] [CrossRef] [Green Version]
- Ghaedi, H.; Ayoub, M.; Sufian, S.; Shariff, A.M.; Lal, B. The study on temperature dependence of viscosity and surface tension of several Phosphonium-based deep eutectic solvents. J. Mol. Liq. 2017, 241, 500–510. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Q.; Zhang, K.; Ji, B. Effect of triple-phase contact line on contact angle hysteresis. Sci. China Phys. Mech. Astron. 2012, 55, 1045–1050. [Google Scholar] [CrossRef]
- Loudjani, M.K.; Cortès, R. Study of the local environment around zirconium ions in polycrystalline α-alumina in relation with kinetics of grain growth and solute drag. J. Eur. Ceram. Soc. 2000, 20, 1483–1491. [Google Scholar] [CrossRef]





| Element | Fe | Co | Ni | C, Mn, Si, Cu, Cr, Mo |
|---|---|---|---|---|
| Composition in wt.% | 52.5 | 17.27 | 28.78 | 1.45 |
| Element | SiO2 | B2O3 | (Na, K)2O | Al2O3 |
|---|---|---|---|---|
| Composition in wt.% | 81 | 13 | 4 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Chen, C.; Zhang, M. Microscopic Analysis of the Wetting Morphology and Interfacial Bonding Mechanism of Preoxidised Kovar Alloys with Borosilicate Glass. Materials 2023, 16, 4628. https://doi.org/10.3390/ma16134628
Shen J, Chen C, Zhang M. Microscopic Analysis of the Wetting Morphology and Interfacial Bonding Mechanism of Preoxidised Kovar Alloys with Borosilicate Glass. Materials. 2023; 16(13):4628. https://doi.org/10.3390/ma16134628
Chicago/Turabian StyleShen, Jiajia, Changjun Chen, and Min Zhang. 2023. "Microscopic Analysis of the Wetting Morphology and Interfacial Bonding Mechanism of Preoxidised Kovar Alloys with Borosilicate Glass" Materials 16, no. 13: 4628. https://doi.org/10.3390/ma16134628
APA StyleShen, J., Chen, C., & Zhang, M. (2023). Microscopic Analysis of the Wetting Morphology and Interfacial Bonding Mechanism of Preoxidised Kovar Alloys with Borosilicate Glass. Materials, 16(13), 4628. https://doi.org/10.3390/ma16134628






