Microstructural Characterization and Prior Particle Boundary (PPB) of PM Nickel-Based Superalloys by Spark Plasma Sintering (SPS)
Abstract
1. Introduction
2. Materials and Methods
3. Results
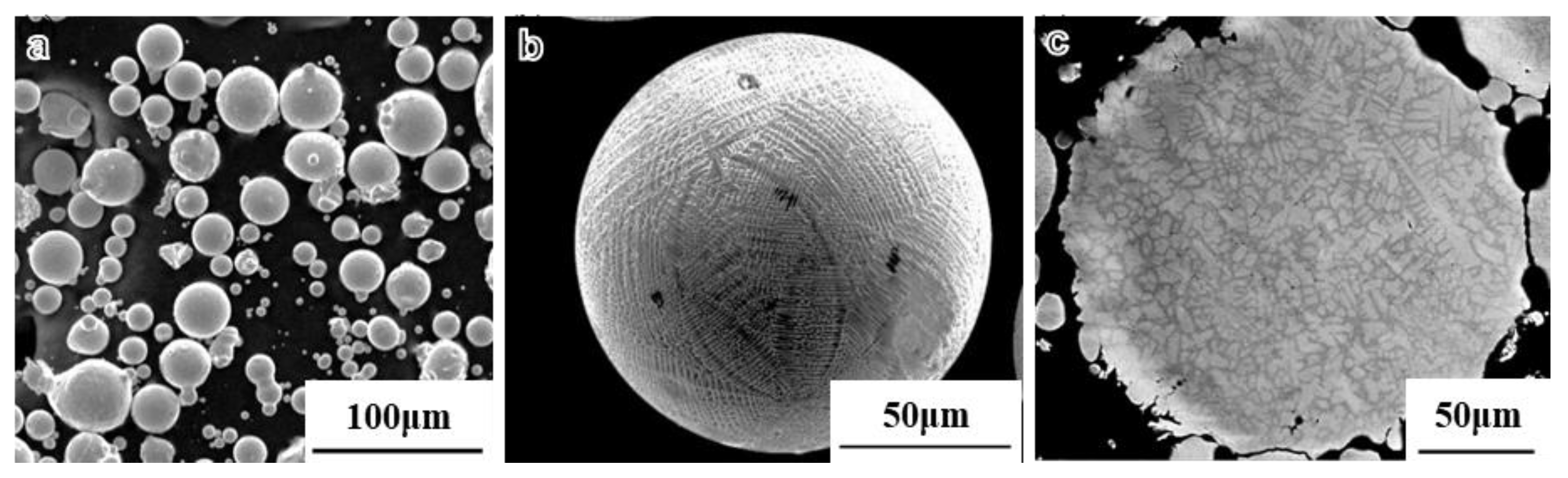
3.1. The Densification Process and Microstructure of SPS Samples
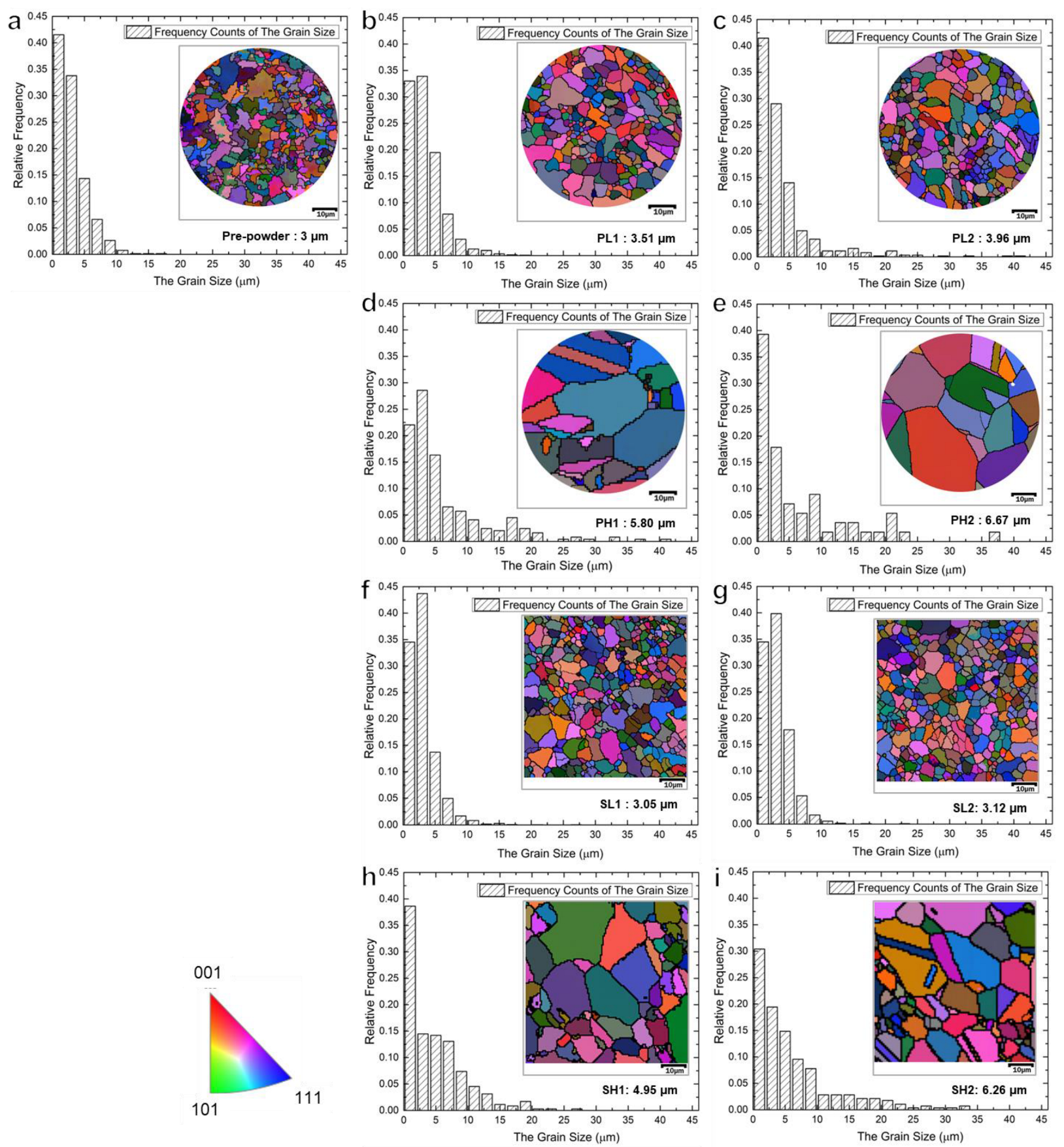
3.2. The Effect of Temperature and Technology on Grain Size
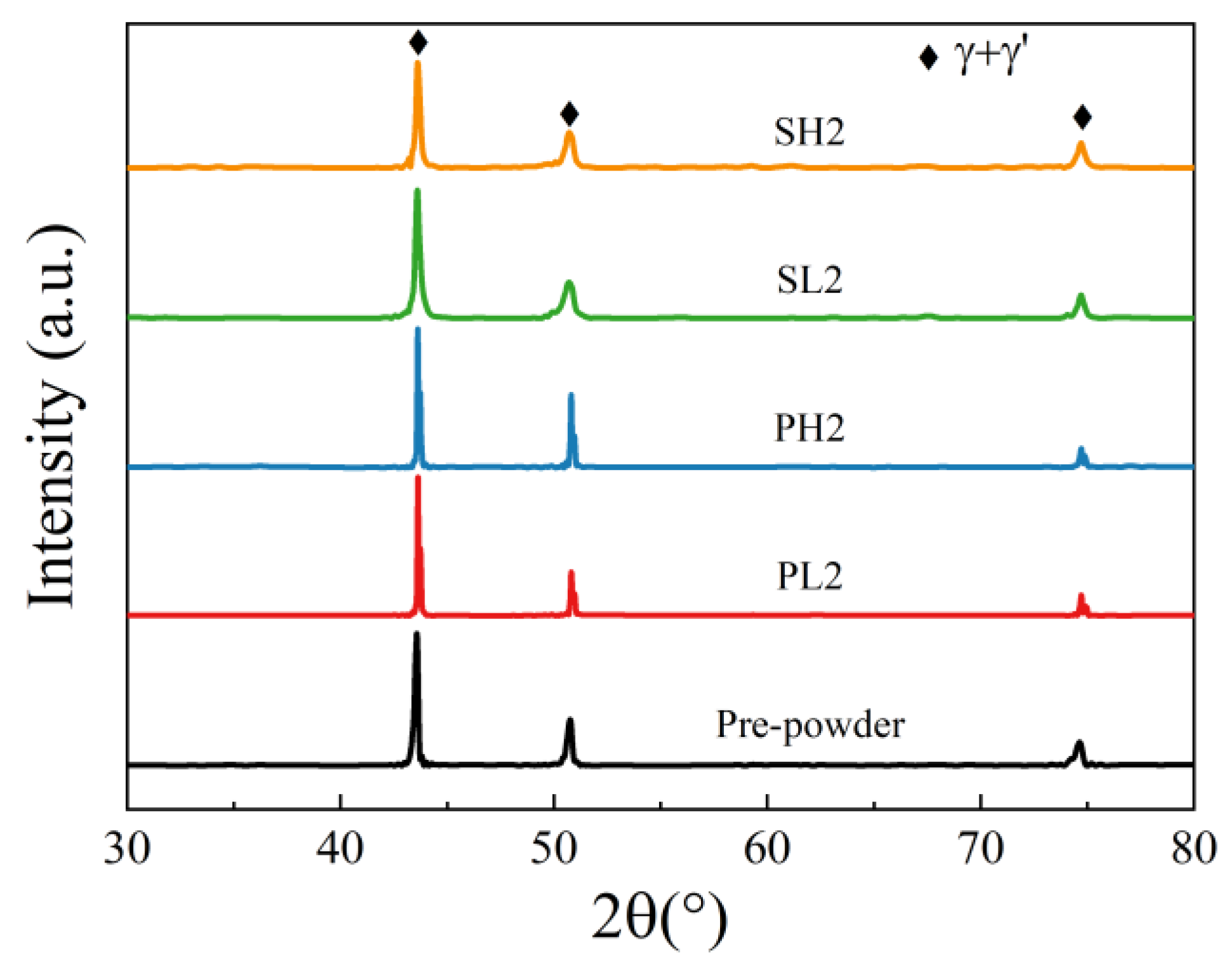
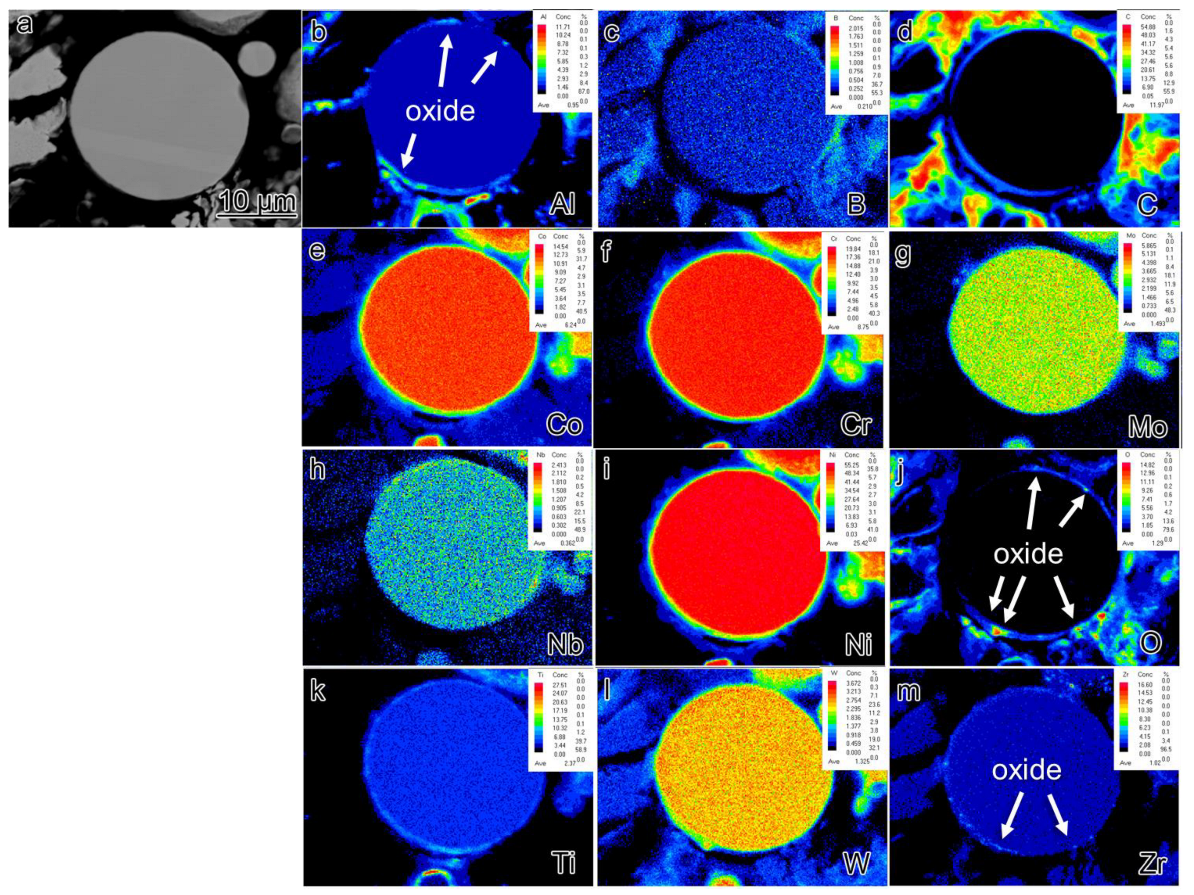
3.3. The Precipitations under Different Processing Conditions
4. Discussion
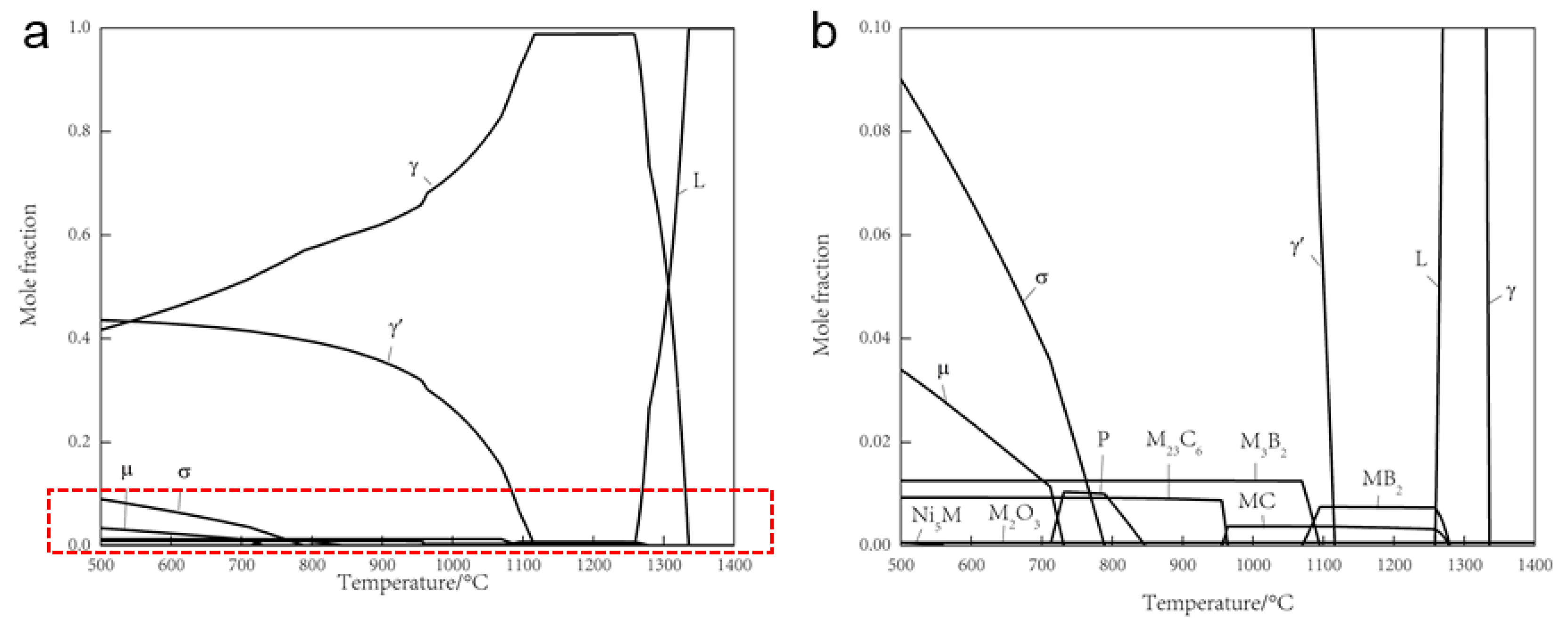
4.1. The Thermodynamic and Kinetic Calculation
4.2. The Evolution of PPB
5. Conclusions
- (1)
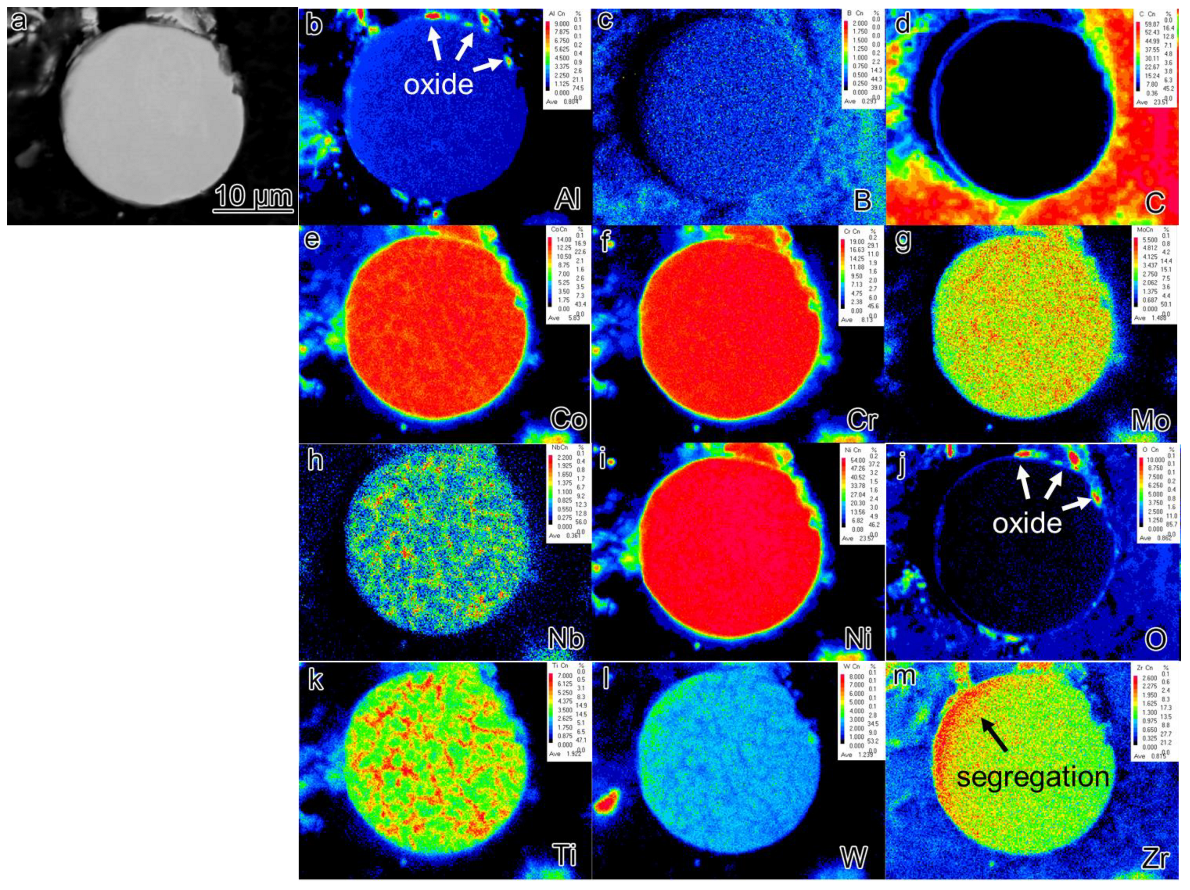
- During the rapid solidification process, there were very few Al2O3 precipitates on the surface of the pre-alloyed powders. Subsequently, after heat treatment, Nb and Ti-rich carbides are observed in the powders.
- (2)
- The SPS samples exhibited almost negligible PPB at a sintering temperature of 1070 °C. Conversely, the presence of prominent PPB was observed at a sintering temperature of 1170 °C. This indicates that the SPS method is effective in reducing PPB formation at low sintering temperatures while facilitating grain size control within a short duration.
- (3)
- The mechanism underlying PPB formation involves the segregation of certain elements already present on the surface of the pre-alloyed powder, with carbides and oxides preferentially precipitating at the sintering neck under the influence of thermodynamic and dynamic factors. The predominant precipitates within the PPB are ZrO2 and MC carbides enriched with Ti and Nb elements.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwary, C.S.; Kashiwar, A.; Bhowmick, S.; Kumar, K.H.; Chattopadhyay, K.; Banerjee, D. Engineering an ultrafine intermetallic eutectic ternary alloy for high strength and high temperature applications. Scr. Mater. 2018, 157, 67–71. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Z.; Cai, B.; Zhou, N.; Magdysyuk, O.; Gao, Y.; Srivatsa, S.; Tan, L.; Jiang, L. Formation mechanism of abnormally large grains in a polycrystalline nickel-based superalloy during heat treatment processing. Acta Mater. 2019, 168, 287–298. [Google Scholar] [CrossRef]
- Wilson, A.S. Formation and effect of topologically close-packed phases in nickel-base superalloys. Mater. Sci. Technol. 2017, 33, 1108–1118. [Google Scholar] [CrossRef]
- Belan, J. GCP and TCP phases presented in nickel-base superalloys. Mater. Today: Proc. 2016, 3, 936–941. [Google Scholar] [CrossRef]
- Hou, J.; Dong, J.; Yao, Z.; Jiang, H.; Zhang, M. Influences of PPB, PPB affect zone, grain boundary and phase boundary on crack propagation path for a P/M superalloy FGH4096. Mater. Sci. Eng. A 2018, 724, 17–28. [Google Scholar] [CrossRef]
- Chai, L.; Li, H.; Yang, Z.; Min, X.; Liao, Q.; Liu, Y.; Men, S.; Yan, Y.; Xu, J. Heavy metals and metalloids in the surface sediments of the Xiangjiang River, Hunan, China: Distribution, contamination, and ecological risk assessment. Environ. Sci. Pollut. Res. 2017, 24, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Liu, G.; Hu, B.; Jia, C. Formation of previous particle boundary of nickel base pm superalloy FGH96. Acta Met. Sin. 2013, 49, 1248–1254. [Google Scholar] [CrossRef]
- Semiatin, S.L.; Obstalecki, M.; Payton, E.J.; Pilchak, A.L.; Shade, P.A.; Levkulich, N.C.; Shank, J.M.; Pagan, D.C.; Zhang, F.; Tiley, J.S. Dissolution of the Alpha Phase in Ti-6Al-4V During Isothermal and Continuous Heat Treatment. Met. Mater. Trans. A 2019, 50, 2356–2370. [Google Scholar] [CrossRef]
- Garosshen, T.J. Influence of alloy chemistry on carbide precipitation in a nickel base superalloy. Met. Trans. A 1986, 17, 2075–2077. [Google Scholar] [CrossRef]
- Thamburaj, R.; Koul, A.; Wallace, W.; de Malherbe, M. Prior particle boundary precipitation in P/M superalloys. Mod. Dev. Powder Metall. 1984, 16, 635–673. [Google Scholar]
- Rao, G.A.; Srinivas, M.; Sarma, D. Effect of thermomechanical working on the microstructure and mechanical properties of hot isostatically pressed superalloy Inconel 718. Mater. Sci. Eng. A 2004, 383, 201–212. [Google Scholar] [CrossRef]
- Pierron, X.; Banik, A.; Maurer, G.; Lemsky, J.; Furrer, D.; Jain, S. Sub-solidus HIP process for P/M superalloy conventional billet conversion. In Proceedings of the 9th International Symposium on Superalloys, Warrendale, PA, USA, 17–21 September 2000; pp. 425–433. [Google Scholar]
- Ma, W.-B.; Liu, G.-Q.; Hu, B.-F.; Zhang, Y.-W.; Liu, J.-T. Effect of Hf on carbides of FGH4096 superalloy produced by hot isostatic pressing. Mater. Sci. Eng. A 2013, 587, 313–319. [Google Scholar] [CrossRef]
- Azarniya, A.; Azarniya, A.; Sovizi, S.; Hosseini, H.R.M.; Varol, T.; Kawasaki, A.; Ramakrishna, S. Physicomechanical properties of spark plasma sintered carbon nanotube-reinforced metal matrix nanocomposites. Prog. Mater. Sci. 2017, 90, 276–324. [Google Scholar] [CrossRef]
- Khouzani, M.K.; Bahrami, A.; Mehr, M.Y. Spark plasma sintering of Stellite®-6 superalloy. J. Alloys Compd. 2019, 782, 461–468. [Google Scholar] [CrossRef]
- Huang, Z.; Ren, X.; Liu, M.; Xu, C.; Zhang, X.; Guo, S.; Chen, H. Effect of Cu on the microstructures and properties of WC-6Co cemented carbides fabricated by SPS. Int. J. Refract. Met. Hard Mater. 2017, 62, 155–160. [Google Scholar] [CrossRef]
- Trzaska, Z.; Couret, A.; Monchoux, J.-P. Spark plasma sintering mechanisms at the necks between TiAl powder particles. Acta Mater. 2016, 118, 100–108. [Google Scholar] [CrossRef]
- Lagos, M.; Agote, I. SPS synthesis and consolidation of TiAl alloys from elemental powders: Microstructure evolution. Intermetallics 2013, 36, 51–56. [Google Scholar] [CrossRef]
- Ogunbiyi, O.; Jamiru, T.; Sadiku, R.; Beneke, L.; Adesina, O.; Obadele, B.A. Corrosion and wear behaviour of Spark plasma-sintered NiCrCoAlTiW-Ta superalloy. J. Bio-Tribo-Corros. 2020, 6, 1. [Google Scholar] [CrossRef]
- Meng, B.; Zhang, Z.; Ma, L.; Wan, M. Effect of sintering temperature on microstructure and mechanical properties of Inconel 718 superalloy prepared by micro-FAST. Mater. Sci. Eng. A 2022, 836, 142733. [Google Scholar] [CrossRef]
- Hua, X.-J.; Hu, P.; Xing, H.-R.; Han, J.-Y.; Ge, S.-W.; Li, S.-L.; He, C.-J.; Wang, K.-S.; Cui, C.-J. Development and property tuning of refractory high-entropy alloys: A review. Acta Met. Sin. (Engl. Lett.) 2022, 35, 1231–1265. [Google Scholar] [CrossRef]
- Onawale, O.T.; Cobbinah, P.V.; Nzeukou, R.A.; Matizamhuka, W.R. Synthesis route, microstructural evolution, and mechanical property relationship of high-entropy alloys (HEAs): A review. Materials 2021, 14, 3065. [Google Scholar] [CrossRef]
- Burachynsky, V.; Cahoon, J.R. A theory for solute impurity diffusion, which considers engel-brewer valences, balancing the fermi energy levels of solvent and solute, and differences in zero point energy. Met. Mater. Trans. A 1997, 28, 563–582. [Google Scholar] [CrossRef]
- Swalin, R.A.; Martin, A.; Olson, R. Diffusion of magnesium, silicon, and molybdenum in nickel. Jom J. Miner. Met. Mater. Soc. 1957, 9, 936–939. [Google Scholar] [CrossRef]
- Ye, D.; Hu, J. Data Manual for Practical Inorganic Thermodynamics; Metallurgical Industry Press: Beijing, China, 1981. [Google Scholar]
- Huo, C.; Zhou, L.; Guo, L.; Wang, J.; Li, Y.; Sun, J.; Liu, L. Effect of the Al2O3 additive on the high temperature ablation behavior of the ZrC–ZrO2 coating for SiC-coated carbon/carbon composites. Ceram. Int. 2019, 45, 23180–23195. [Google Scholar] [CrossRef]
- Luthra, K.L.; Briant, C.L. Comments on? rare earth-inert-dispersoid effect? Oxid. Met. 1988, 30, 257–258. [Google Scholar] [CrossRef]
- Ge, Z.; Chen, K.; Guo, J.; Zhou, H.; Ferreira, J.M.J.F. Combustion synthesis of ternary carbide Ti3AlC2 in Ti–Al–C system. J. Eur. Ceram. Soc. 2003, 23, 567–574. [Google Scholar] [CrossRef]
- German, R.M.; Pierre, G.R.S. The high temperature thermodynamic properties of Ni-Ti alloys. Met. Trans. 1972, 3, 2819–2823. [Google Scholar] [CrossRef]
- Shatynski, S.R. The thermochemistry of transition metal carbides. Oxid. Met. 1979, 13, 105–118. [Google Scholar] [CrossRef]
- Kajikawa, K.; Oikawa, K.; Takahashi, F.; Yamada, H.; Anzai, K. Reassessment of Liquid/Solid Equilibrium in Ni-Rich Side of Ni-Nb and Ni-Ti Systems. Mater. Trans. 2010, 51, 781–786. [Google Scholar] [CrossRef]
- Richardson, F.D. Physical Chemistry of Melts in Metallurgy; Academic Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Schlesinger, M.E.; Okamoto, H.; Gokhale, A.B.; Abbaschian, R. The Nb-Si (Niobium-Silicon) system. J. Phase Equilibria Diffus. 1993, 14, 502–509. [Google Scholar] [CrossRef]
- Ogawa, Y.; Noguchi, K.; Takakuwa, O. Criteria for hydrogen-assisted crack initiation in Ni-based superalloy 718. Acta Mater. 2022, 229, 117789. [Google Scholar] [CrossRef]






| Co | Cr | Mo | W | Al | Ti | Nb | B | Zr | C | Ni | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominal | 13 | 16 | 4 | 4 | 2.3 | 3.75 | 0.90 | 0.013 | 0.04 | 0.05 | Bal. |
| Actual | 12.36 | 16.68 | 3.75 | 3.89 | 2.18 | 3.95 | 1.05 | 0.094 | 0.036 | 0.0416 | Bal. |
| Parameters | SL1 | SL2 | SH1 | SH2 | PL1 | PL2 | PH1 | PH2 |
|---|---|---|---|---|---|---|---|---|
| Method | SPS | SPS | SPS | SPS | PHT | PHT | PHT | PHT |
| Temperature (°C) | 1070 | 1070 | 1170 | 1170 | 1070 | 1070 | 1170 | 1170 |
| Time (min) | 5 | 40 | 5 | 40 | 5 | 40 | 5 | 40 |
| Preparation Process | SL1 | SL2 | SH1 | SH2 | Master Alloy | After-HIPed |
|---|---|---|---|---|---|---|
| Density (g/cm3) | 8.1291 | 8.3104 | 8.2543 | 8.2608 | 8.2937 | 8.2931 |
| Temperature (°C) | Mole Fraction/% | |||||
|---|---|---|---|---|---|---|
| γ | MB2 | M2O3 | γ’ | M3B2 | MC | |
| 1070 | 83.26 | 0.02 | 0.06 | 15.07 | 1.22 | 0.37 |
| 1170 | 98.84 | 0.74 | 0.06 | - | - | 0.36 |
| Temperature (°C) | Phase | Composition Mole Fraction/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Ti | Zr | Nb | W | Mo | Cr | O | Al | Ti | ||
| 1070 | MC | 47.65 | 38.31 | 0.81 | 11.55 | 1.16 | 0.20 | 0.31 | - | - | - |
| M2O3 | - | - | - | - | - | - | - | 60.00 | 39.81 | 0.19 | |
| 1170 | MC | 47.48 | 40.37 | 0.48 | 9.84 | 1.23 | 0.25 | 0.34 | - | - | - |
| M2O3 | - | - | - | - | - | - | - | 60.00 | 39.62 | 0.38 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Z.; Li, Q.; Wang, G.; Liu, F. Microstructural Characterization and Prior Particle Boundary (PPB) of PM Nickel-Based Superalloys by Spark Plasma Sintering (SPS). Materials 2023, 16, 4664. https://doi.org/10.3390/ma16134664
Qin Z, Li Q, Wang G, Liu F. Microstructural Characterization and Prior Particle Boundary (PPB) of PM Nickel-Based Superalloys by Spark Plasma Sintering (SPS). Materials. 2023; 16(13):4664. https://doi.org/10.3390/ma16134664
Chicago/Turabian StyleQin, Zijun, Qianyi Li, Guowei Wang, and Feng Liu. 2023. "Microstructural Characterization and Prior Particle Boundary (PPB) of PM Nickel-Based Superalloys by Spark Plasma Sintering (SPS)" Materials 16, no. 13: 4664. https://doi.org/10.3390/ma16134664
APA StyleQin, Z., Li, Q., Wang, G., & Liu, F. (2023). Microstructural Characterization and Prior Particle Boundary (PPB) of PM Nickel-Based Superalloys by Spark Plasma Sintering (SPS). Materials, 16(13), 4664. https://doi.org/10.3390/ma16134664







