Innovative Metal-Organic Frameworks for Targeted Oral Cancer Therapy: A Review
Abstract
:1. Introduction
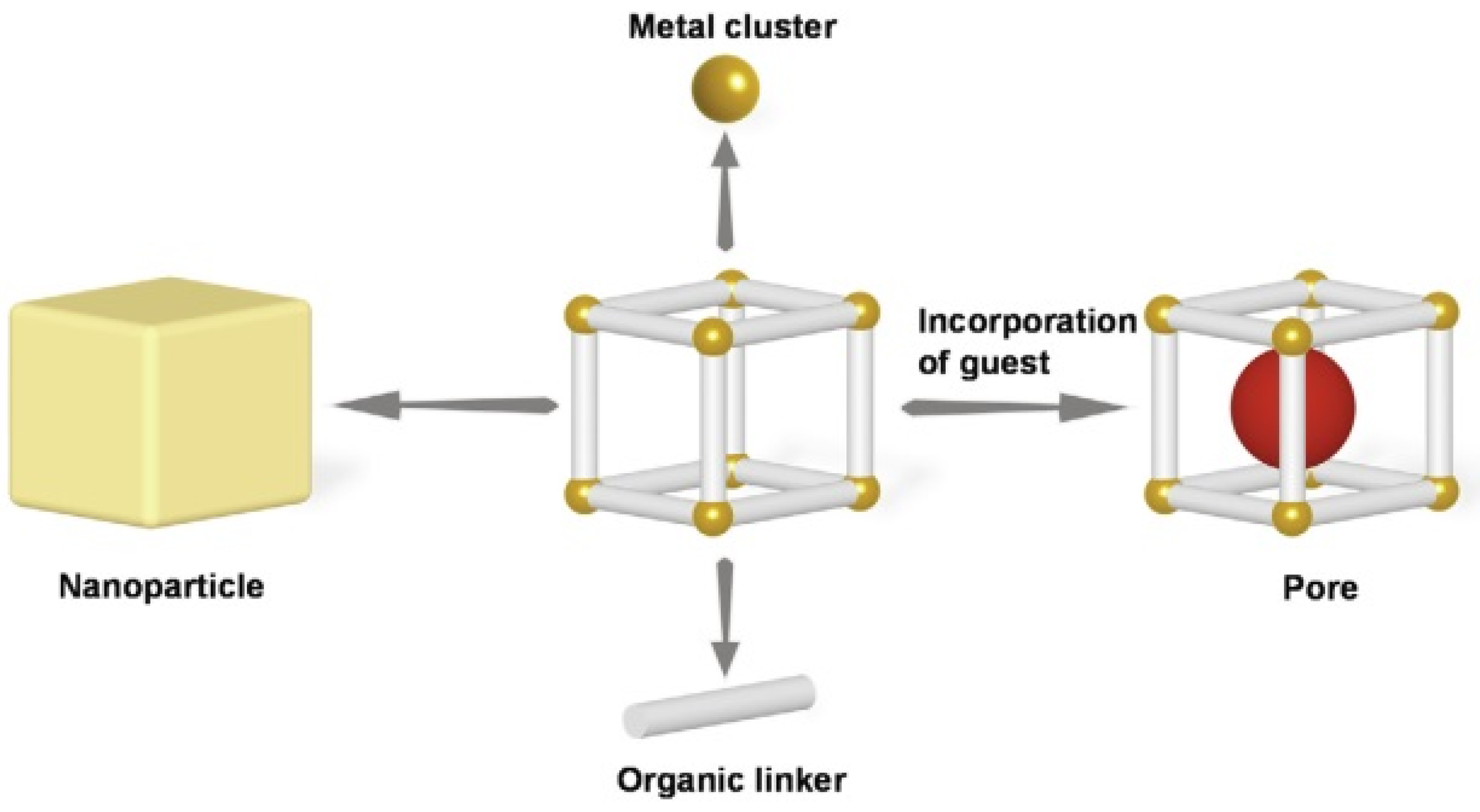
2. Metal-Organic Frameworks
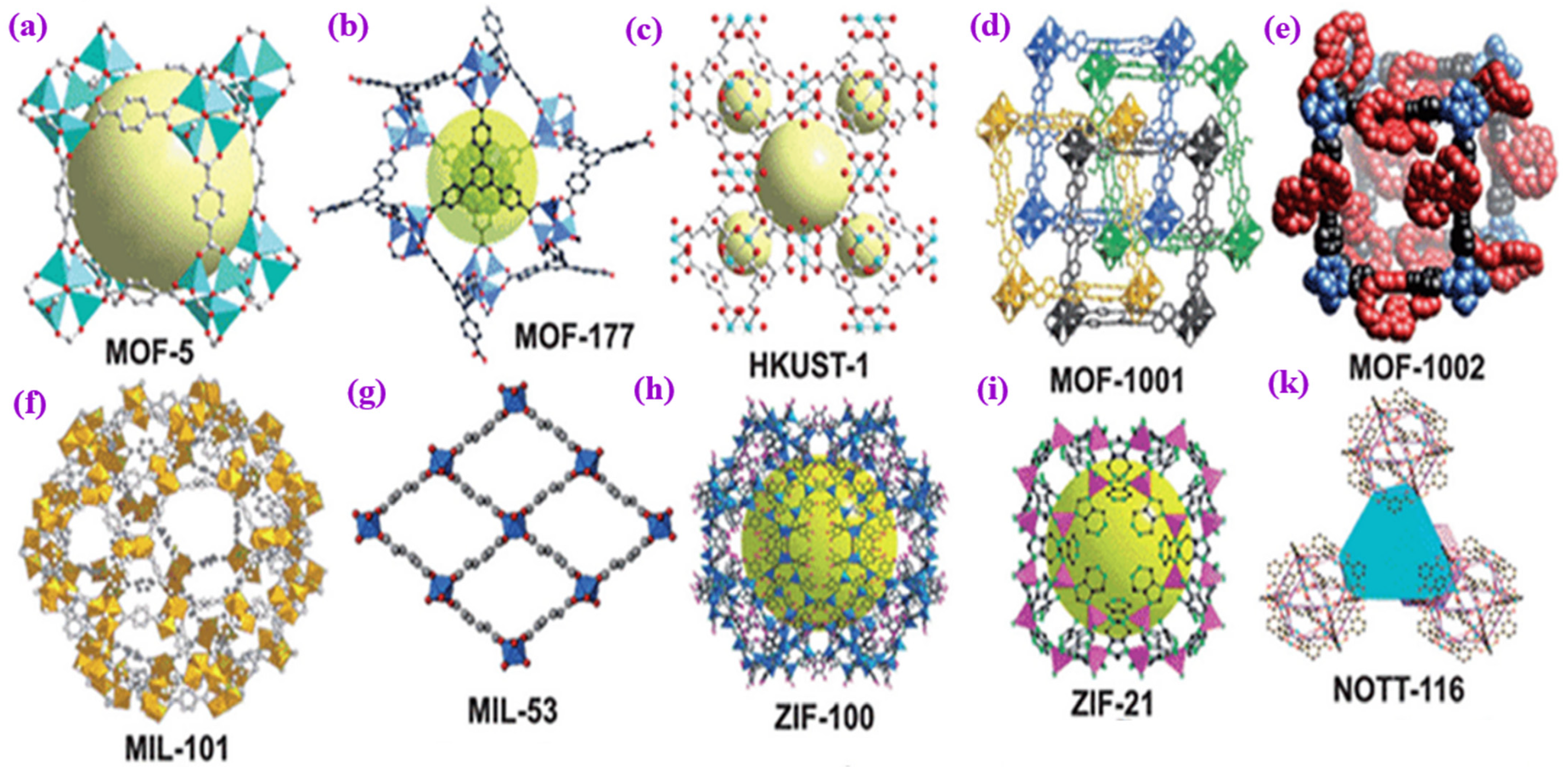

3. Biological Metal-Organic Frameworks (BioMOFs)
4. Metal-Organic Frameworks (MOFs) for Biomedical Applications
4.1. Bioimaging
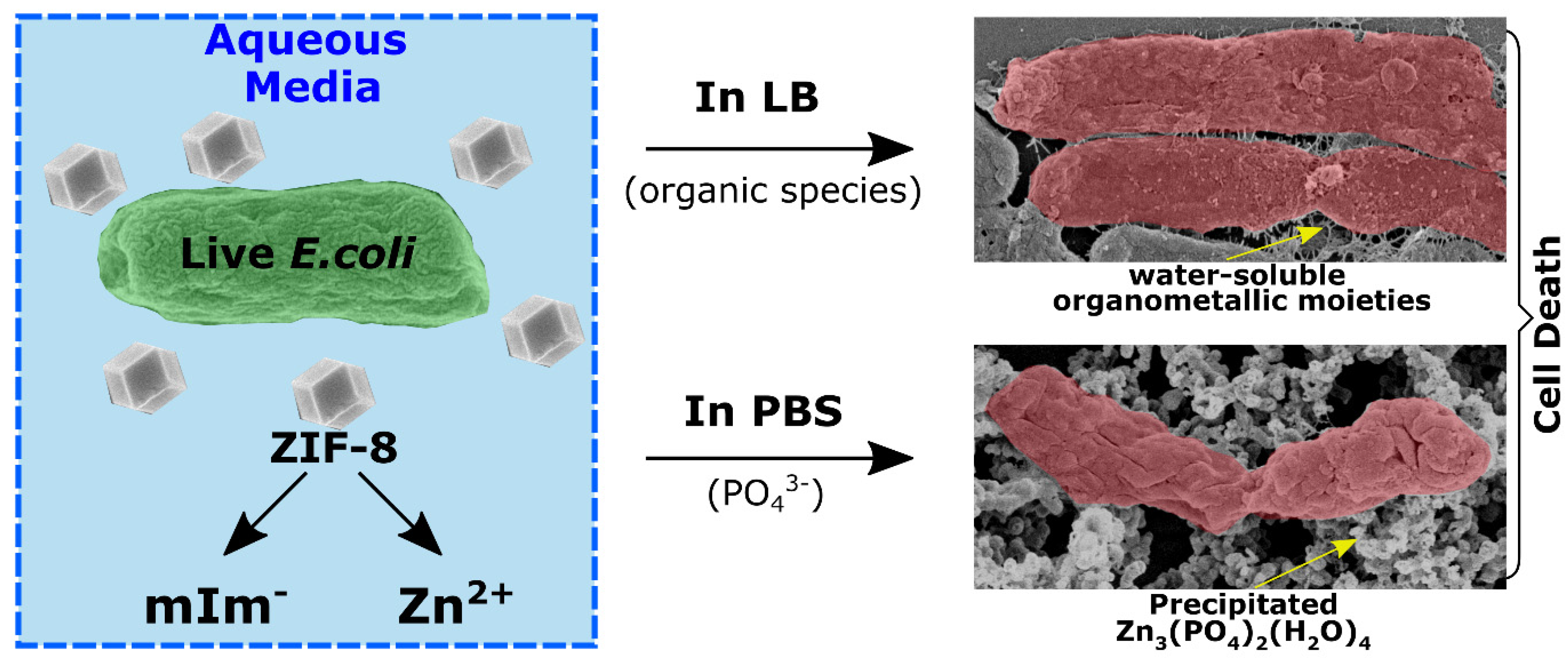
4.2. Antibacterial
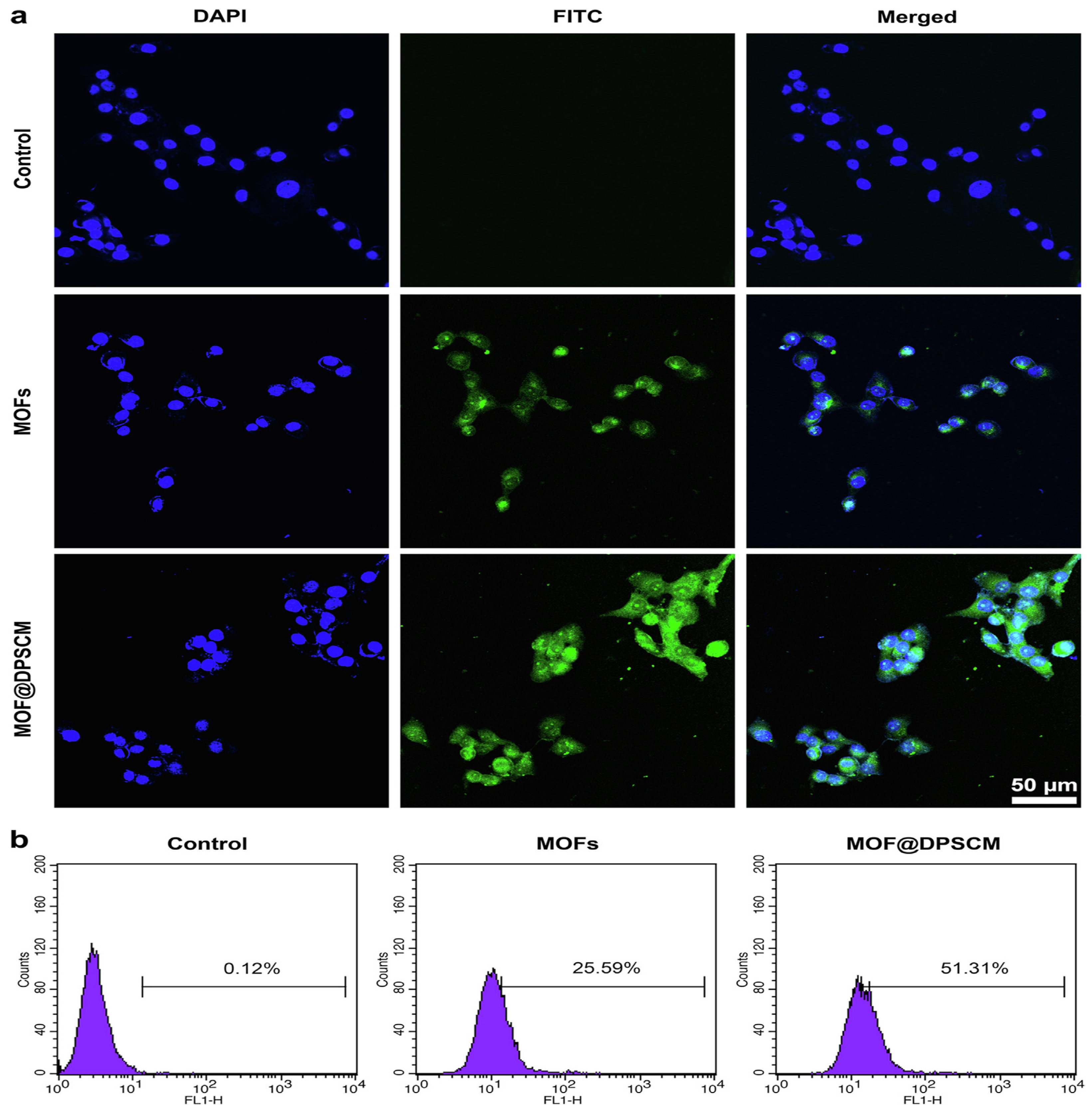
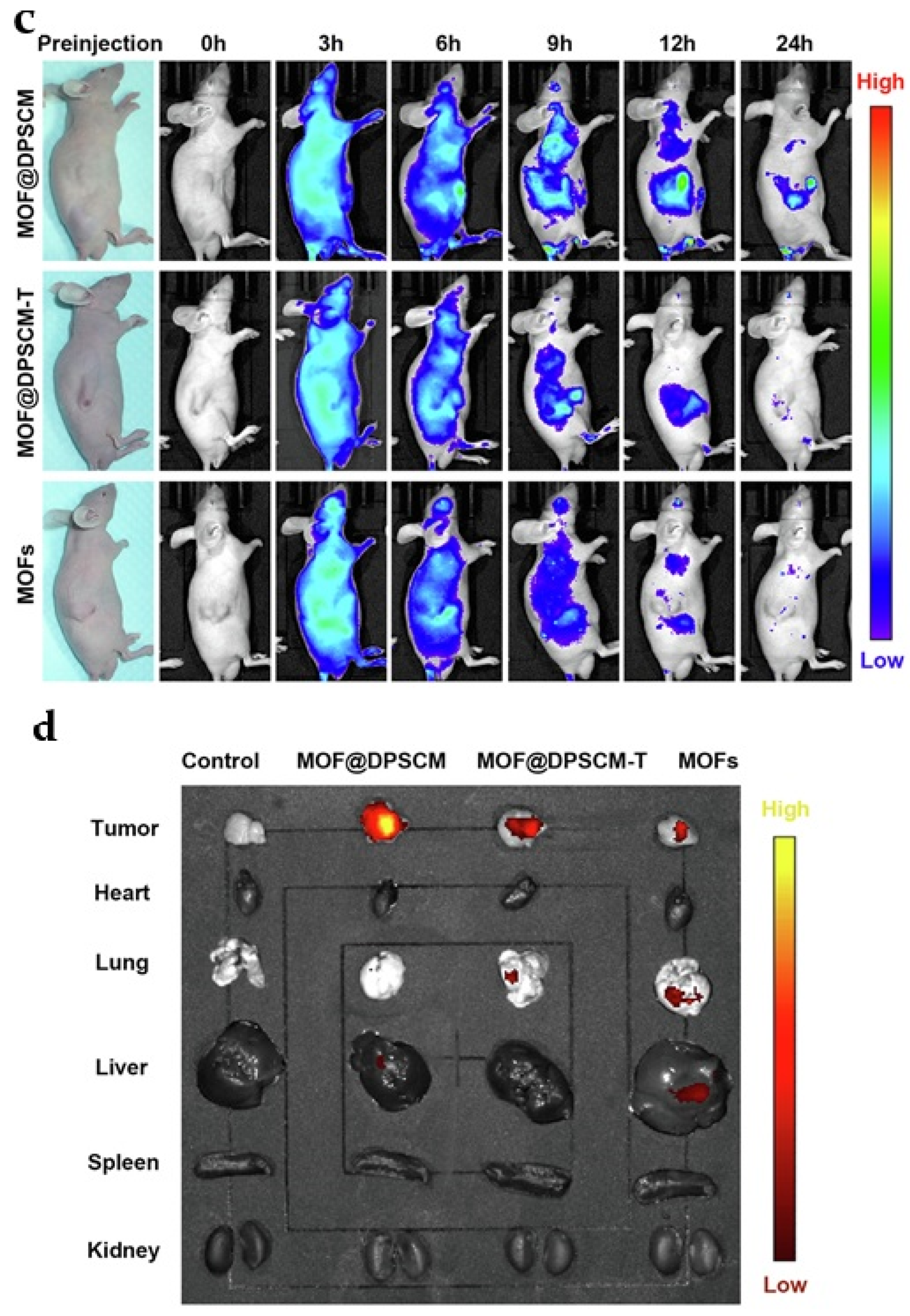
4.3. Drug Delivery System
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, M.X.; Yang, Y.W. Metal–organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Bahrani, S.; Hashemi, S.A.; Mousavi, S.M.; Azhdari, R. Zinc-based metal–organic frameworks as nontoxic and biodegradable platforms for biomedical applications: Review study. Drug Metab. Rev. 2019, 51, 356–377. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Su, Z.-M. Metal-organic frameworks as potential drug delivery systems. Expert Opin. Drug Deliv. 2013, 10, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y.W. Metal–organic frameworks for biomedical applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cui, Z.; Li, Y.-H.; Hsu, W.-H.; Lee, B.-H. In vitro and in vivo anticancer activity of pardaxin against proliferation and growth of oral squamous cell carcinoma. Mar. Drugs 2015, 14, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luque-Michel, E.; Imbuluzqueta, E.; Sebastián, V.; Blanco-Prieto, M.J. Clinical advances of nanocarrier-based cancer therapy and diagnostics. Expert Opin. Drug Deliv. 2017, 14, 75–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Liu, F.; Gupta, S.; Li, C. Interventional nanotheranostics of pancreatic ductal adenocarcinoma. Theranostics 2016, 6, 1393. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.-Y.; Su, Y.-L.; Cheng, W.; Hu, P.-F.; Chiang, C.-S.; Chen, W.-T.; Hu, S.-H. Graphene quantum dots-mediated theranostic penetrative delivery of drug and photolytics in deep tumors by targeted biomimetic nanosponges. Nano Lett. 2018, 19, 69–81. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Salahi, S.; Hosseini, M.; Amani, A.M.; Babapoor, A. Development of Clay Nanoparticles toward Bio and Medical Applications; IntechOpen: London, UK, 2018. [Google Scholar]
- Abootalebi, S.N.; Mousavi, S.M.; Hashemi, S.A.; Shorafa, E.; Omidifar, N.; Gholami, A. Antibacterial effects of green-synthesized silver nanoparticles using Ferula asafoetida against Acinetobacter baumannii isolated from the hospital environment and assessment of their cytotoxicity on the human cell lines. J. Nanomater. 2021, 2021, 6676555. [Google Scholar] [CrossRef]
- Ali, S.; Varghese, L.; Pereira, L.; Tulunay-Ugur, O.E.; Kucuk, O.; Carey, T.E.; Wolf, G.T.; Sarkar, F.H. RETRACTED: Sensitization of Squamous Cell Carcinoma to Cisplatin Induced Killing by Natural Agents; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Babapoor, A.; Amani, A.M. A conceptual review of rhodanine: Current applications of antiviral drugs, anticancer and antimicrobial activities. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1132–1148. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.-P.; Chou, T.-H.; Ding, H.-Y.; Chen, P.-R.; Chiang, F.-Y.; Kuo, P.-L.; Liang, C.-H. Apigenin induces apoptosis via tumor necrosis factor receptor-and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Minhas, S.; Kashif, M.; Altaf, W.; Afzal, N.; Nagi, A.H. Concomitant-chemoradiotherapy-associated oral lesions in patients with oral squamous-cell carcinoma. Cancer Biol. Med. 2017, 14, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjornmalm, M.; Thurecht, K.J.; Michael, M.; Scott, A.M.; Caruso, F. Bridging bio–nano science and cancer nanomedicine. ACS Nano 2017, 11, 9594–9613. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Bilal, M.; Jia, S.; Jiang, Y.; Cui, J. Design and bio-applications of biological metal-organic frameworks. Korean J. Chem. Eng. 2019, 36, 1949–1964. [Google Scholar] [CrossRef]
- Jones, C.W. Metal–Organic Frameworks and Covalent Organic Frameworks: Emerging Advances and Applications. JACS Au 2022, 2, 1504–1505. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Mosca, N.; Tosi, G.; Drozdov, A. Application of metal-organic frameworks. Polym. Int. 2017, 66, 731–744. [Google Scholar] [CrossRef]
- André, V.N.; da Silva, A.R.F.; Fernandes, A.; Frade, R.; Garcia, C.; Rijo, P.; Antunes, A.M.; Rocha, J.O.; Duarte, M.T. Mg-and Mn-MOFs boost the antibiotic activity of nalidixic acid. ACS Appl. Bio Mater. 2019, 2, 2347–2354. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, F.; Wang, D. MOFs and MOF-Derived Materials for Antibacterial Application. J. Funct. Biomater. 2022, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Xu, W.; Ren, X.; Wang, Y.; Dong, B.; Wang, L. Microporous frameworks as promising platforms for antibacterial strategies against oral diseases. Front. Bioeng. Biotechnol. 2020, 8, 628. [Google Scholar] [CrossRef]
- Karpiński, T.M. Role of oral microbiota in cancer development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noureen, C.; Rushikesh, D.; Pankaj, C. The role of bacteria in oral cancer. Indian J. Med. Paediatr. Oncol. 2010, 31, 126–131. [Google Scholar]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The oral microbiota may have influence on oral cancer. Front. Cell. Infect. Microbiol. 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusama, K.; Inoue, H.; Miyazaki, Y.; Kikuchi, K.; Sakashita, H.; Ochiai, K. Microorganisms and cancer of the oral cavity. Integr. Cancer Sci. 2016, 3, 510–515. [Google Scholar] [CrossRef] [Green Version]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of metal–organic frameworks with rod secondary building units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef]
- Fallahinezhad, F.; Afsa, M.; Ghahramani, Y. Graphene Quantum Dots and their applications in regenerative medicine: A mini-review. Adv. Appl. NanoBio-Technol. 2021, 4, 59–72. [Google Scholar]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, J.-D.; Mao, X.-D.; Song, L.-L.; Yang, X.-Y.; Liu, S.-J. Toughening mechanisms of epoxy resin using aminated metal-organic framework as additive. Mater. Lett. 2019, 240, 113–116. [Google Scholar] [CrossRef]
- Xing, X.-S.; Fu, Z.-H.; Zhang, N.-N.; Yu, X.-Q.; Wang, M.-S.; Guo, G.-C. High proton conduction in an excellent water-stable gadolinium metal–organic framework. Chem. Commun. 2019, 55, 1241–1244. [Google Scholar] [CrossRef]
- Mirkovic, I.; Lei, L.; Ljubic, D.; Zhu, S. Crystal growth of metal–organic framework-5 around cellulose-based fibers having a necklace morphology. ACS Omega 2019, 4, 169–175. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Ahmadi, S.; Fazilati, M.; Mousavi, S.M.; Nazem, H. Anti-bacterial/fungal and anti-cancer performance of green synthesized Ag nanoparticles using summer savory extract. J. Exp. Nanosci. 2020, 15, 363–380. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Yang, D.-A.; Kim, J.; Jeong, S.-Y.; Ahn, W.-S. CO2 adsorption and catalytic application of Co-MOF-74 synthesized by microwave heating. Catal. Today 2012, 185, 35–40. [Google Scholar] [CrossRef]
- Kim, J.; Yang, S.-T.; Choi, S.B.; Sim, J.; Kim, J.; Ahn, W.-S. Control of catenation in CuTATB-n metal–organic frameworks by sonochemical synthesis and its effect on CO2 adsorption. J. Mater. Chem. 2011, 21, 3070–3076. [Google Scholar] [CrossRef]
- Hartmann, M.; Kunz, S.; Himsl, D.; Tangermann, O.; Ernst, S.; Wagener, A. Adsorptive separation of isobutene and isobutane on Cu3 BTC2. Langmuir 2008, 24, 8634–8642. [Google Scholar] [CrossRef]
- Friščić, T.; Reid, D.G.; Halasz, I.; Stein, R.S.; Dinnebier, R.E.; Duer, M.J. Ion-and liquid-assisted grinding: Improved mechanochemical synthesis of metal–organic frameworks reveals salt inclusion and anion templating. Angew. Chem. Int. Ed. 2010, 49, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Black, C.A.; Hanton, L.R.; Spicer, M.D. A coordination polymer strategy for anion encapsulation: Anion–π interactions in (4, 4) nets formed from Ag (I) salts and a flexible pyrimidine ligand. Chem. Commun. 2007, 14, 3171–3173. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Bahrani, S.; Savardashtaki, A.; Esmaeili, H.; Lai, C.W.; Mazraedoost, S.; Abassi, M.; Ramavandi, B. Data on cytotoxic and antibacterial activity of synthesized Fe3O4 nanoparticles using Malva sylvestris. Data Brief 2020, 28, 104929. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Esmaeili, H.; Parvin, H.; Mojoudi, A.; Fateh, M.A.; Fateh, H.; Babapoor, A.; Mazraedoost, S.; Zarei, M. Investigating the activity of antioxidants activities content in Apiaceae and to study antimicrobial and insecticidal activity of antioxidant by using SPME Fiber assembly carboxen/polydimethylsiloxane (CAR/PDMS). J. Environ. Treat. Tech. 2020, 8, 214–224. [Google Scholar]
- Shen, K.; Zhang, M.; Zheng, H. Critical factors influencing the structures and properties of metal–organic frameworks. CrystEngComm 2015, 17, 981–991. [Google Scholar] [CrossRef]
- Paz, F.A.A.; Klinowski, J.; Vilela, S.M.; Tome, J.P.; Cavaleiro, J.A.; Rocha, J. Ligand design for functional metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 1088–1110. [Google Scholar]
- Keskin, S.; Kızılel, S. Biomedical applications of metal organic frameworks. Ind. Eng. Chem. Res. 2011, 50, 1799–1812. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Mousavi, S.M.; Arjmand, M.; Yan, N.; Sundararaj, U. Electrified single-walled carbon nanotube/epoxy nanocomposite via vacuum shock technique: Effect of alignment on electrical conductivity and electromagnetic interference shielding. Polym. Compos. 2018, 39, E1139–E1148. [Google Scholar] [CrossRef]
- Li, G.; Han, Y. Two-In-One Mof Structure with Tunable Porosity for Enhanced Separation; ACS Publications: Washington, DC, USA, 2022. [Google Scholar]
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-like metal–organic frameworks (ZMOFs): Design, synthesis, and properties. Chem. Soc. Rev. 2015, 44, 228–249. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M. Metal azolate frameworks: From crystal engineering to functional materials. Chem. Rev. 2012, 112, 1001–1033. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kravtsov, V.C.; Eddaoudi, M. Template-Directed Assembly of Zeolite-like Metal–Organic Frameworks (ZMOFs): A usf-ZMOF with an Unprecedented Zeolite Topology. Angew. Chem. 2008, 120, 8574–8577. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Alezi, D.; Peedikakkal, A.M.P.; Weseliński, Ł.J.; Guillerm, V.; Belmabkhout, Y.; Cairns, A.J.; Chen, Z.; Wojtas, Ł.; Eddaoudi, M. Quest for highly connected metal–organic framework platforms: Rare-earth polynuclear clusters versatility meets net topology needs. J. Am. Chem. Soc. 2015, 137, 5421–5430. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.T.; Furukawa, H.; Gándara, F.; Nguyen, H.T.; Cordova, K.E.; Yaghi, O.M. Selective capture of carbon dioxide under humid conditions by hydrophobic chabazite-type zeolitic imidazolate frameworks. Angew. Chem. Int. Ed. 2014, 53, 10645–10648. [Google Scholar] [CrossRef]
- Wu, H.; Yildirim, T.; Zhou, W. Exceptional mechanical stability of highly porous zirconium metal–organic framework UiO-66 and its important implications. J. Phys. Chem. Lett. 2013, 4, 925–930. [Google Scholar] [CrossRef]
- Valtchev, V.; Mintova, S. 1. Zeolites and MOFs? Dare to Know Them! Zeolites Met.-Org. Framew. 2018, 24, 13. [Google Scholar]
- Sene, S.; Marcos-Almaraz, M.T.; Menguy, N.; Scola, J.; Volatron, J.; Rouland, R.; Greneche, J.-M.; Miraux, S.; Menet, C.; Guillou, N. Maghemite-nanoMIL-100 (Fe) bimodal nanovector as a platform for image-guided therapy. Chem 2017, 3, 303–322. [Google Scholar] [CrossRef]
- Ahmadi, S.; Fazilati, M.; Nazem, H.; Mousavi, S.M. Green synthesis of magnetic nanoparticles using Satureja hortensis essential oil toward superior antibacterial/fungal and anticancer performance. BioMed Res. Int. 2021, 2021, 8822645. [Google Scholar] [CrossRef]
- Chen, G.; Luo, J.; Cai, M.; Qin, L.; Wang, Y.; Gao, L.; Huang, P.; Yu, Y.; Ding, Y.; Dong, X. Investigation of metal-organic framework-5 (MOF-5) as an antitumor drug oridonin sustained release carrier. Molecules 2019, 24, 3369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-B.; Furukawa, H.; Ko, N.; Nie, W.; Park, H.J.; Okajima, S.; Cordova, K.E.; Deng, H.; Kim, J.; Yaghi, O.M. Introduction of functionality, selection of topology, and enhancement of gas adsorption in multivariate metal–organic framework-177. J. Am. Chem. Soc. 2015, 137, 2641–2650. [Google Scholar] [CrossRef]
- Saifutdinov, B.R.; Isaeva, V.I.; Chernyshev, V.V.; Vergun, V.V.; Kapustin, G.I.; Ivanova, Y.P.; Ilyin, M.M.; Tkachenko, O.P.; Buryak, A.K.; Kustov, L.M. Understanding the Working Mechanism of the Novel HKUST-1@BPS Composite Materials as Stationary Phases for Liquid Chromatography. Polymers 2022, 14, 1373. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.; Liu, T.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T., III; et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev 2014, 43, 5561–5593. [Google Scholar] [CrossRef]
- Brown, K.S.; Marean, C.W.; Herries, A.I.; Jacobs, Z.; Tribolo, C.; Braun, D.; Roberts, D.L.; Meyer, M.C.; Bernatchez, J. Fire as an engineering tool of early modern humans. Science 2009, 325, 859–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharjee, S.; Chen, C.; Ahn, W.-S. Chromium terephthalate metal–organic framework MIL-101: Synthesis, functionalization, and applications for adsorption and catalysis. Rsc Adv. 2014, 4, 52500–52525. [Google Scholar] [CrossRef]
- Zou, M.; Dong, M.; Zhao, T. Advances in Metal-Organic Frameworks MIL-101 (Cr). Int. J. Mol. Sci. 2022, 23, 9396. [Google Scholar] [CrossRef] [PubMed]
- Ashling, C.W.; Johnstone, D.N.; Widmer, R.N.; Hou, J.; Collins, S.M.; Sapnik, A.F.; Bumstead, A.M.; Midgley, P.A.; Chater, P.A.; Keen, D.A. Synthesis and properties of a compositional series of MIL-53 (Al) metal–organic framework crystal-glass composites. J. Am. Chem. Soc. 2019, 141, 15641–15648. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Cooper, A. Functional Metal-Organic Frameworks: Gas Storage, Separation and Catalysis; Springer: Berlin/Heidelberg, Germany, 2010; Volume 293, pp. 1–33. [Google Scholar]
- Wang, N.; Liu, Y.; Qiao, Z.; Diestel, L.; Zhou, J.; Huang, A.; Caro, J. Polydopamine-based synthesis of a zeolite imidazolate framework ZIF-100 membrane with high H2/CO2 selectivity. J. Mater. Chem. A 2015, 3, 4722–4728. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Crawford, J.M.; Wolden, C.A.; Carreon, M.A. ZIF-21 Crystals: Its Morphology Control and Potential as an Adsorbent for Ammonia Capture. J. Phys. Chem. C 2022, 126, 12951–12957. [Google Scholar] [CrossRef]
- Yan, Y.; Telepeni, I.; Yang, S.; Lin, X.; Kockelmann, W.; Dailly, A.; Blake, A.J.; Lewis, W.; Walker, G.S.; Allan, D.R. Metal−organic polyhedral frameworks: High H2 adsorption capacities and neutron powder diffraction studies. J. Am. Chem. Soc. 2010, 132, 4092–4094. [Google Scholar] [CrossRef]
- Kampouraki, Z.-C.; Giannakoudakis, D.A.; Nair, V.; Hosseini-Bandegharaei, A.; Colmenares, J.C.; Deliyanni, E.A. Metal organic frameworks as desulfurization adsorbents of DBT and 4, 6-DMDBT from fuels. Molecules 2019, 24, 4525. [Google Scholar] [CrossRef] [Green Version]
- Mubarak, N.S.A.; Foo, K.; Schneider, R.; Abdelhameed, R.M.; Sabar, S. The chemistry of MIL-125 based materials: Structure, synthesis, modification strategies and photocatalytic applications. J. Environ. Chem. Eng. 2022, 10, 106883. [Google Scholar] [CrossRef]
- Bergaoui, M.; Khalfaoui, M.; Awadallah-F, A.; Al-Muhtaseb, S. A review of the features and applications of ZIF-8 and its derivatives for separating CO2 and isomers of C3-and C4-hydrocarbons. J. Nat. Gas Sci. Eng. 2021, 96, 104289. [Google Scholar] [CrossRef]
- Winarta, J.; Shan, B.; Mcintyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A decade of UiO-66 research: A historic review of dynamic structure, synthesis mechanisms, and characterization techniques of an archetypal metal–organic framework. Cryst. Growth Des. 2019, 20, 1347–1362. [Google Scholar] [CrossRef]
- Vahabi, A.H.; Norouzi, F.; Sheibani, E.; Rahimi-Nasrabadi, M. Functionalized Zr-UiO-67 metal-organic frameworks: Structural landscape and application. Coord. Chem. Rev. 2021, 445, 214050. [Google Scholar] [CrossRef]
- Zhang, Z.; Peh, S.B.; Kang, C.; Chai, K.; Zhao, D. Metal-organic frameworks for C6–C8 hydrocarbon separations. EnergyChem 2021, 3, 100057. [Google Scholar] [CrossRef]
- Kholdeeva, O.; Maksimchuk, N. Metal-organic frameworks in oxidation catalysis with hydrogen peroxide. Catalysts 2021, 11, 283. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.-L.; Li, D. Biological metal–organic frameworks: Structures, host–guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Mousavi, S.; Arjmand, O.; Talaghat, M.; Azizi, M.; Shooli, H. Modifying the properties of polypropylene-wood composite by natural polymers and eggshell Nano-particles. Polym. Renew. Resour. 2015, 6, 157–173. [Google Scholar] [CrossRef]
- Imaz, I.; Rubio-Martinez, M.; An, J.; Sole-Font, I.; Rosi, N.L.; Maspoch, D. Metal–biomolecule frameworks (MBioFs). Chem. Commun. 2011, 47, 7287–7302. [Google Scholar] [CrossRef]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal–organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Rojas, S.; Devic, T.; Horcajada, P. Metal organic frameworks based on bioactive components. J. Mater. Chem. B 2017, 5, 2560–2573. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Karimipourfard, M.; Mousavi, S.M.; Sina, S.; Bahrani, S.; Omidifar, N.; Ramakrishna, S.; Arjmand, M. Transparent sodium polytungstate polyoxometalate aquatic shields toward effective X-ray radiation protection: Alternative to lead glasses. Mater. Today Commun. 2022, 31, 103822. [Google Scholar] [CrossRef]
- André, V.; Quaresma, S.; da Silva, J.L.F.; Duarte, M.T. Exploring mechanochemistry to turn organic bio-relevant molecules into metal-organic frameworks: A short review. Beilstein J. Org. Chem. 2017, 13, 2416–2427. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.M.; Hashemi, S.A.; Mazraedoost, S.; Yousefi, K.; Gholami, A.; Behbudi, G.; Ramakrishna, S.; Omidifar, N.; Alizadeh, A.; Chiang, W.-H. Multifunctional gold nanorod for therapeutic applications and pharmaceutical delivery considering cellular metabolic responses, oxidative stress and cellular longevity. Nanomaterials 2021, 11, 1868. [Google Scholar] [CrossRef]
- Beldon, P.J.; Fábián, L.; Stein, R.S.; Thirumurugan, A.; Cheetham, A.K.; Friščić, T. Rapid room-temperature synthesis of zeolitic imidazolate frameworks by using mechanochemistry. Angew. Chem. 2010, 122, 9834–9837. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Amani, A.M.; Babapoor, A.; Arjmand, O. Applications of graphene oxide in case of nanomedicines and nanocarriers for biomolecules: Review study. Drug Metab. Rev. 2019, 51, 12–41. [Google Scholar] [CrossRef]
- Wang, H.-S.; Wang, Y.-H.; Ding, Y. Development of biological metal–organic frameworks designed for biomedical applications: From bio-sensing/bio-imaging to disease treatment. Nanoscale Adv. 2020, 2, 3788–3797. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Bahrani, S.; Mousavi, S.M.; Omidifar, N.; Behbahan, N.G.G.; Arjmand, M.; Ramakrishna, S.; Lankarani, K.B.; Moghadami, M.; Shokripour, M. Ultra-precise label-free nanosensor based on integrated graphene with Au nanostars toward direct detection of IgG antibodies of SARS-CoV-2 in blood. J. Electroanal. Chem. 2021, 894, 115341. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yu, Z.; Huang, X.; Wang, Z.; Niu, L.; Teng, M.; Li, J. Rational design of MOFs constructed from modified aromatic amino acids. Chem.–A Eur. J. 2007, 13, 9399–9405. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, A.C.; Morris, R.E.; Horcajada, P.; Férey, G.; Gref, R.; Couvreur, P.; Serre, C. BioMOFs: Metal–organic frameworks for biological and medical applications. Angew. Chem. Int. Ed. 2010, 49, 6260–6266. [Google Scholar] [CrossRef] [PubMed]
- André, V.; Quaresma, S. Bio-Inspired Metal-Organic Frameworks in the Pharmaceutical World: A Brief Review; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Della Rocca, J.; Liu, D.; Lin, W. Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–organic frameworks as efficient materials for drug delivery. Angew. Chem. 2006, 118, 6120–6124. [Google Scholar] [CrossRef]
- Ma, Y.; Qu, X.; Liu, C.; Xu, Q.; Tu, K. Metal-organic frameworks and their composites towards biomedical applications. Front. Mol. Biosci. 2021, 8, 805228. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Mousavi, S.M.; Faghihi, R.; Arjmand, M.; Rahsepar, M.; Bahrani, S.; Ramakrishna, S.; Lai, C.W. Superior X-ray radiation shielding effectiveness of biocompatible polyaniline reinforced with hybrid graphene oxide-iron tungsten nitride flakes. Polymers 2020, 12, 1407. [Google Scholar] [CrossRef]
- Singh, N.; Qutub, S.; Khashab, N.M. Biocompatibility and biodegradability of metal organic frameworks for biomedical applications. J. Mater. Chem. B 2021, 9, 5925–5934. [Google Scholar] [CrossRef]
- Alyami, M.Z.; Alsaiari, S.K.; Li, Y.; Qutub, S.S.; Aleisa, F.A.; Sougrat, R.; Merzaban, J.S.; Khashab, N.M. Cell-type-specific CRISPR/Cas9 delivery by biomimetic metal organic frameworks. J. Am. Chem. Soc. 2020, 142, 1715–1720. [Google Scholar] [CrossRef]
- Cai, M.; Qin, L.; Pang, L.; Ma, B.; Bai, J.; Liu, J.; Dong, X.; Yin, X.; Ni, J. Amino-functionalized Zn metal organic frameworks as antitumor drug curcumin carriers. New J. Chem. 2020, 44, 17693–17704. [Google Scholar] [CrossRef]
- Alsaiari, S.K.; Qutub, S.S.; Sun, S.; Baslyman, W.; Aldehaiman, M.; Alyami, M.; Almalik, A.; Halwani, R.; Merzaban, J.; Mao, Z. Sustained and targeted delivery of checkpoint inhibitors by metal-organic frameworks for cancer immunotherapy. Sci. Adv. 2021, 7, eabe7174. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, X.; Gao, H.; Chen, S.; Wang, J. Metal-organic framework-based nanomaterials for biomedical applications. Chin. Chem. Lett. 2020, 31, 1060–1070. [Google Scholar] [CrossRef]
- Wen, T.; Quan, G.; Niu, B.; Zhou, Y.; Zhao, Y.; Lu, C.; Pan, X.; Wu, C. Versatile nanoscale metal–organic frameworks (nMOFs): An emerging 3D nanoplatform for drug delivery and therapeutic applications. Small 2021, 17, 2005064. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Aung, T.; Guo, N.; Weichselbaum, R.; Lin, W. Nanoscale metal–organic frameworks for therapeutic, imaging, and sensing applications. Adv. Mater. 2018, 30, 1707634. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Lu, K.; Poon, C.; Lin, W. Metal–organic frameworks as sensory materials and imaging agents. Inorg. Chem. 2014, 53, 1916–1924. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Huxford-Phillips, R.C.; Lin, W. Silica-based nanoprobes for biomedical imaging and theranostic applications. Chem. Soc. Rev. 2012, 41, 2673–2685. [Google Scholar] [CrossRef] [Green Version]
- Maranescu, B.; Visa, A. Applications of metal-organic frameworks as drug delivery systems. Int. J. Mol. Sci. 2022, 23, 4458. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Fytory, M.; Arafa, K.K.; El Rouby, W.M.; Farghali, A.A.; Abdel-Hafiez, M.; El-Sherbiny, I.M. Dual-ligated metal organic framework as novel multifunctional nanovehicle for targeted drug delivery for hepatic cancer treatment. Sci. Rep. 2021, 11, 19808. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yu, T.; Shi, Z.; Wang, Z. The preparation of metal–organic frameworks and their biomedical application. Int. J. Nanomed. 2016, 11, 1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, Y.; Yang, Z.; Zhang, L.; Xiao, L.; Liu, P.; Wang, J.; Yi, C.; Xu, Z.; Ren, J. Albumin/sulfonamide stabilized iron porphyrin metal organic framework nanocomposites: Targeting tumor hypoxia by carbonic anhydrase IX inhibition and T 1–T 2 dual mode MRI guided photodynamic/photothermal therapy. J. Mater. Chem. B 2018, 6, 265–276. [Google Scholar] [CrossRef]
- Hosseini, H.; Mousavi, S.M. Bacterial cellulose/polyaniline nanocomposite aerogels as novel bioadsorbents for removal of hexavalent chromium: Experimental and simulation study. J. Clean. Prod. 2021, 278, 123817. [Google Scholar] [CrossRef]
- Mousavi, S.-M.; Nejad, Z.M.; Hashemi, S.A.; Salari, M.; Gholami, A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W. Bioactive agent-loaded electrospun nanofiber membranes for accelerating healing process: A review. Membranes 2021, 11, 702. [Google Scholar] [CrossRef]
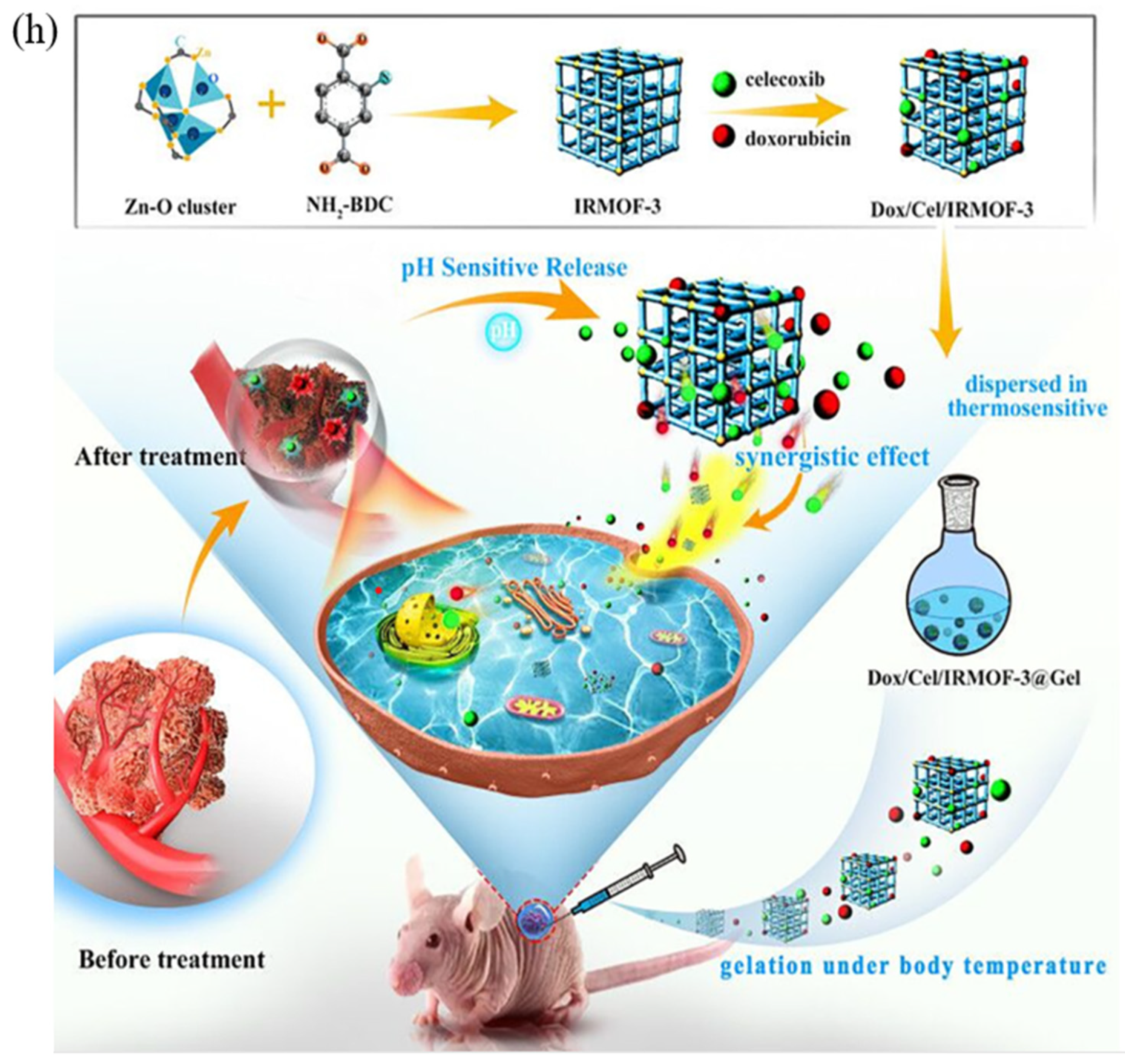
- Tan, G.; Zhong, Y.; Yang, L.; Jiang, Y.; Liu, J.; Ren, F. A multifunctional MOF-based nanohybrid as injectable implant platform for drug synergistic oral cancer therapy. Chem. Eng. J. 2020, 390, 124446. [Google Scholar] [CrossRef]
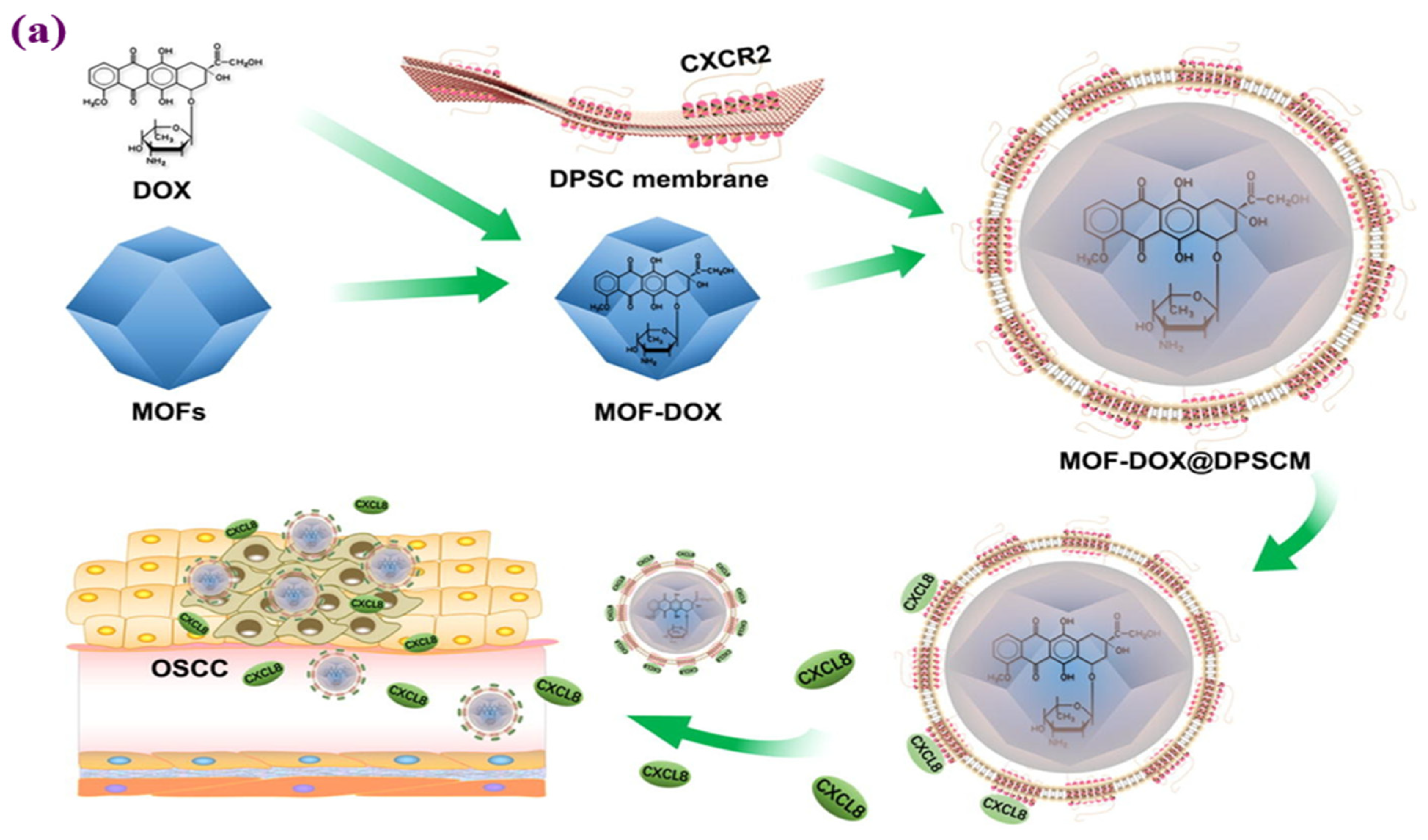
- Zhou, D.; Chen, Y.; Bu, W.; Meng, L.; Wang, C.; Jin, N.; Chen, Y.; Ren, C.; Zhang, K.; Sun, H. Modification of metal-organic framework nanoparticles using dental pulp mesenchymal stem cell membranes to target oral squamous cell carcinoma. J. Colloid Interface Sci. 2021, 601, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Midoux, P.; Pichon, C.; Ahmad, F.J.; Akhter, S. Nanoporous metal organic frameworks as hybrid polymer–metal composites for drug delivery and biomedical applications. Drug Discov. Today 2017, 22, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ye, J.; Zhang, D.; Xie, R.; Bogale, R.F.; Sun, Y.; Zhao, L.; Zhao, Q.; Ning, G. Silver carboxylate metal–organic frameworks with highly antibacterial activity and biocompatibility. J. Inorg. Biochem. 2014, 138, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, D.; Zhu, N.; Wu, Y.; Li, G. Antibacterial mechanisms and applications of metal-organic frameworks and their derived nanomaterials. Trends Food Sci. Technol. 2021, 109, 413–434. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Bahrani, S.; Yousefi, K.; Behbudi, G.; Babapoor, A.; Omidifar, N.; Lai, C.W.; Gholami, A.; Chiang, W.-H. Recent advancements in polythiophene-based materials and their biomedical, geno sensor and DNA detection. Int. J. Mol. Sci. 2021, 22, 6850. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Neu, H.C. The crisis in antibiotic resistance. Science 1992, 257, 1064–1073. [Google Scholar] [CrossRef] [Green Version]
- Nong, W.; Wu, J.; Ghiladi, R.A.; Guan, Y. The structural appeal of metal–organic frameworks in antimicrobial applications. Coord. Chem. Rev. 2021, 442, 214007. [Google Scholar] [CrossRef]
- Shen, M.; Forghani, F.; Kong, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Antibacterial applications of metal–organic frameworks and their composites. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1397–1419. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.M.; Hashemi, S.A.; Ghahramani, Y.; Azhdari, R.; Yousefi, K.; Gholami, A.; Fallahi Nezhad, F.; Vijayakameswara Rao, N.; Omidifar, N.; Chiang, W.-H. Antiproliferative and apoptotic effects of graphene oxide@AlFu MOF based saponin natural product on OSCC line. Pharmaceuticals 2022, 15, 1137. [Google Scholar] [CrossRef]
- Berchel, M.; Le Gall, T.; Denis, C.; Le Hir, S.; Quentel, F.; Elleouet, C.; Montier, T.; Rueff, J.-M.; Salaün, J.-Y.; Haelters, J.-P. A silver-based metal–organic framework material as a ‘reservoir’of bactericidal metal ions. New J. Chem. 2011, 35, 1000–1003. [Google Scholar] [CrossRef]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorożyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Sheta, S.M.; El-Sheikh, S.M.; Abd-Elzaher, M.M. Simple synthesis of novel copper metal–organic framework nanoparticles: Biosensing and biological applications. Dalton Trans. 2018, 47, 4847–4855. [Google Scholar] [CrossRef]
- Taheri, M.; Ashok, D.; Sen, T.; Enge, T.G.; Verma, N.K.; Tricoli, A.; Lowe, A.; Nisbet, D.R.; Tsuzuki, T. Stability of ZIF-8 nanopowders in bacterial culture media and its implication for antibacterial properties. Chem. Eng. J. 2021, 413, 127511. [Google Scholar] [CrossRef]
- Aguado, S.; Quirós, J.; Canivet, J.; Farrusseng, D.; Boltes, K.; Rosal, R. Antimicrobial activity of cobalt imidazolate metal–organic frameworks. Chemosphere 2014, 113, 188–192. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Allan, P.K.; Renouf, C.L.; Duncan, M.J.; Wheatley, P.S.; Warrender, S.J.; Dawson, D.; Ashbrook, S.E.; Gil, B.; Marszalek, B. Multirate delivery of multiple therapeutic agents from metal-organic frameworks. APL Mater. 2014, 2, 124108. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.M.; Hashemi, S.A.; Kalashgrani, M.Y.; Omidifar, N.; Bahrani, S.; Vijayakameswara Rao, N.; Babapoor, A.; Gholami, A.; Chiang, W.-H. Bioactive graphene quantum dots based polymer composite for biomedical applications. Polymers 2022, 14, 617. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Geng, Z.; Yin, Y.; Ma, X.; Wang, Z. Morphology effect on the luminescent property and antibacterial activity of coordination polymer particles with identical crystal structures. CrystEngComm 2011, 13, 5100–5104. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y. Enhanced biomimic bactericidal surfaces by coating with positively-charged ZIF nano-dagger arrays. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2199–2207. [Google Scholar] [CrossRef]
- Hasan, M.N.; Bera, A.; Maji, T.K.; Pal, S.K. Sensitization of nontoxic MOF for their potential drug delivery application against microbial infection. Inorg. Chim. Acta 2021, 523, 120381. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Bagchi, D.; Katouah, H.A.; Hasan, M.N.; Altass, H.M.; Pal, S.K. Enhanced water stability and photoresponsivity in metal-organic framework (MOF): A potential tool to combat drug-resistant bacteria. Sci. Rep. 2019, 9, 19372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sava Gallis, D.F.; Butler, K.S.; Agola, J.O.; Pearce, C.J.; McBride, A.A. Antibacterial countermeasures via metal–organic framework-supported sustained therapeutic release. ACS Appl. Mater. Interfaces 2019, 11, 7782–7791. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.M.; Mathlouthi, E.; Cahu, M.; Sene, S.; Daurat, M.; Long, J.; Guari, Y.; Salles, F.; Chopineau, J.; Devoisselle, J.-M. Synergic effect of doxorubicin release and two-photon irradiation of Mn2+-doped Prussian blue nanoparticles on cancer therapy. RSC Adv. 2020, 10, 2646–2649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Wang, L.; Zheng, X.; Xie, Z. Zirconium-based nanoscale metal–organic framework/poly (ε-caprolactone) mixed-matrix membranes as effective antimicrobials. ACS Appl. Mater. Interfaces 2017, 9, 41512–41520. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, P.K.; Baloda, S.; Madras, G.; Bose, S. A designer membrane tool-box with a mixed metal organic framework and RAFT-synthesized antibacterial polymer perform in tandem towards desalination, antifouling and heavy metal exclusion. J. Mater. Chem. A 2018, 6, 16664–16679. [Google Scholar] [CrossRef]
- Qian, L.; Lei, D.; Duan, X.; Zhang, S.; Song, W.; Hou, C.; Tang, R. Design and preparation of metal-organic framework papers with enhanced mechanical properties and good antibacterial capacity. Carbohydr. Polym. 2018, 192, 44–51. [Google Scholar] [CrossRef]
- Seyedpour, S.F.; Rahimpour, A.; Najafpour, G. Facile in-situ assembly of silver-based MOFs to surface functionalization of TFC membrane: A novel approach toward long-lasting biofouling mitigation. J. Membr. Sci. 2019, 573, 257–269. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Akhbari, K.; Morsali, A. Dense coating of surface mounted CuBTC metal–organic framework nanostructures on silk fibers, prepared by layer-by-layer method under ultrasound irradiation with antibacterial activity. Ultrason. Sonochemistry 2012, 19, 846–852. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks; ACS Publications: Washington, DA, USA, 2012; Volume 112, pp. 673–674. [Google Scholar]
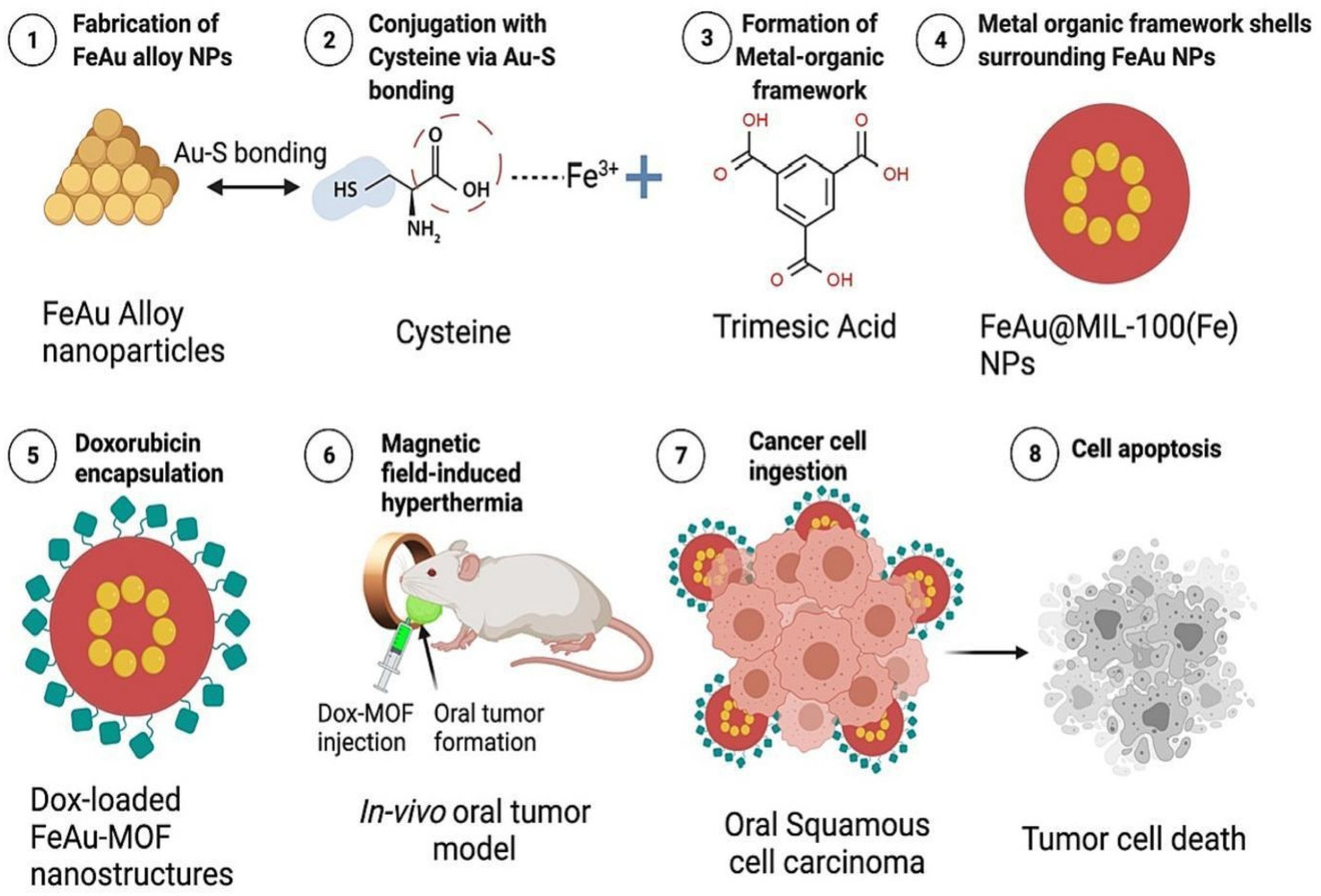
- Dhawan, U.; Tseng, C.-L.; Wu, P.-H.; Liao, M.-Y.; Wang, H.-Y.; Wu, K.C.-W.; Chung, R.-J. Theranostic doxorubicin encapsulated FeAu alloy@metal-organic framework nanostructures enable magnetic hyperthermia and medical imaging in oral carcinoma. Nanomed. Nanotechnol. Biol. Med. 2023, 52, 102652. [Google Scholar] [CrossRef]
- Cui, J.; Li, W.; Bu, W.; Liu, J.; Chen, X.; Li, X.; Liu, C.; Meng, L.; Chen, M.; Sun, H. Folic acid-modified disulfiram/Zn-IRMOF3 nanoparticles for oral cancer therapy by inhibiting ALDH1A1+ cancer stem cells. Biomater. Adv. 2022, 139, 213038. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Parvin, N.; Gholami, A.; Ramakrishna, S.; Omidifar, N.; Moghadami, M.; Chiang, W.-H.; Mazraedoost, S. Recent biotechnological approaches for treatment of novel COVID-19: From bench to clinical trial. Drug Metab. Rev. 2021, 53, 141–170. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Qin, C.; Wang, C.G.; Su, Z.M.; Wang, S.; Wang, X.L.; Yang, G.S.; Shao, K.Z.; Lan, Y.Q.; Wang, E.B. Chiral nanoporous metal-organic frameworks with high porosity as materials for drug delivery. Adv. Mater. 2011, 23, 5629–5632. [Google Scholar] [CrossRef]
- Oh, H.; Li, T.; An, J. Drug Release Properties of a Series of Adenine-Based Metal–Organic Frameworks. Chem.–A Eur. J. 2015, 21, 17010–17015. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.; Jin, E.; Palanikumar, L.; Lee, J.H.; Kim, J.C.; Nam, J.S.; Jana, B.; Kwon, T.-H.; Kwak, S.K. MOF × biopolymer: Collaborative combination of metal–organic framework and biopolymer for advanced anticancer therapy. ACS Appl. Mater. Interfaces 2019, 11, 27512–27520. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Low, F.W.; Hashemi, S.A.; Lai, C.W.; Ghasemi, Y.; Soroshnia, S.; Savardashtaki, A.; Babapoor, A.; Pynadathu Rumjit, N.; Goh, S.M. Development of graphene based nanocomposites towards medical and biological applications. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1189–1205. [Google Scholar] [CrossRef]
- Xiang, Z.; Qi, Y.; Lu, Y.; Hu, Z.; Wang, X.; Jia, W.; Hu, J.; Ji, J.; Lu, W. MOF-derived novel porous Fe3O4@C nanocomposites as smart nanomedical platforms for combined cancer therapy: Magnetic-triggered synergistic hyperthermia and chemotherapy. J. Mater. Chem. B 2020, 8, 8671–8683. [Google Scholar] [CrossRef]
- Awasthi, G.; Shivgotra, S.; Nikhar, S.; Sundarrajan, S.; Ramakrishna, S.; Kumar, P. Progressive Trends on the Biomedical Applications of Metal Organic Frameworks. Polymers 2022, 14, 4710. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot synthesis of metal–organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Cai, W.; Gao, H.; Chu, C.; Wang, X.; Wang, J.; Zhang, P.; Lin, G.; Li, W.; Liu, G.; Chen, X. Engineering phototheranostic nanoscale metal–organic frameworks for multimodal imaging-guided cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 2040–2051. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Li, S.; Li, L.; Zhang, L.; Wang, T.; Yu, M.; Mou, Z.; Wang, C. Facile synthesis of polypyrrole@metal–organic framework core–shell nanocomposites for dual-mode imaging and synergistic chemo-photothermal therapy of cancer cells. J. Mater. Chem. B 2017, 5, 1772–1778. [Google Scholar] [CrossRef]
- Nicum, S.J.; O’Brien, M.E. Topotecan for the treatment of small-cell lung cancer. Expert Rev. Anticancer Ther. 2007, 7, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Pashow, K.M.; Della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic modifications of iron-carboxylate nanoscale metal−organic frameworks for imaging and drug delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-G.; Dong, Z.-Y.; Cheng, H.; Wan, S.-S.; Chen, W.-H.; Zou, M.-Z.; Huo, J.-W.; Deng, H.-X.; Zhang, X.-Z. A multifunctional metal–organic framework based tumor targeting drug delivery system for cancer therapy. Nanoscale 2015, 7, 16061–16070. [Google Scholar] [CrossRef]
- Hu, Q.; Yu, J.; Liu, M.; Liu, A.; Dou, Z.; Yang, Y. A low cytotoxic cationic metal–organic framework carrier for controllable drug release. J. Med. Chem. 2014, 57, 5679–5685. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Q.-W.; Zhuang, C.; Tang, P.-P.; Lin, N.; Wei, L.-Q. Controlled release of drug molecules in metal–organic framework material HKUST-1. Inorg. Chem. Commun. 2017, 79, 78–81. [Google Scholar] [CrossRef]
- Anand, R.; Borghi, F.; Manoli, F.; Manet, I.; Agostoni, V.; Reschiglian, P.; Gref, R.; Monti, S. Host–guest interactions in Fe(III)-trimesate MOF nanoparticles loaded with doxorubicin. J. Phys. Chem. B 2014, 118, 8532–8539. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Wang, S.; Li, P.; Mirkin, C.A.; Farha, O.K. DNA-functionalized metal–organic framework nanoparticles for intracellular delivery of proteins. J. Am. Chem. Soc. 2019, 141, 2215–2219. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, P.; Modica, J.A.; Drout, R.J.; Farha, O.K. Acid-resistant mesoporous metal–organic framework toward oral insulin delivery: Protein encapsulation, protection, and release. J. Am. Chem. Soc. 2018, 140, 5678–5681. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Zhang, Y.; Li, Z.-Y. A low cytotoxic metal–organic framework carrier: pH-responsive 5-fluorouracil delivery and anti-cervical cancer activity evaluation. J. Clust. Sci. 2018, 29, 1285–1290. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Zou, Q.; Sun, S.-K.; Yu, C.; Zhang, X.; Li, R.-J.; Fu, Y.-Y. Theranostic metal–organic framework core–shell composites for magnetic resonance imaging and drug delivery. Chem. Sci. 2016, 7, 5294–5301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippousi, M.; Turner, S.; Leus, K.; Siafaka, P.I.; Tseligka, E.D.; Vandichel, M.; Nanaki, S.G.; Vizirianakis, I.S.; Bikiaris, D.N.; Van Der Voort, P. Biocompatible Zr-based nanoscale MOFs coated with modified poly (ε-caprolactone) as anticancer drug carriers. Int. J. Pharm. 2016, 509, 208–218. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lu, K.; Liu, D.; Lin, W. Nanoscale metal–organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J. Am. Chem. Soc. 2014, 136, 5181–5184. [Google Scholar] [CrossRef] [PubMed]


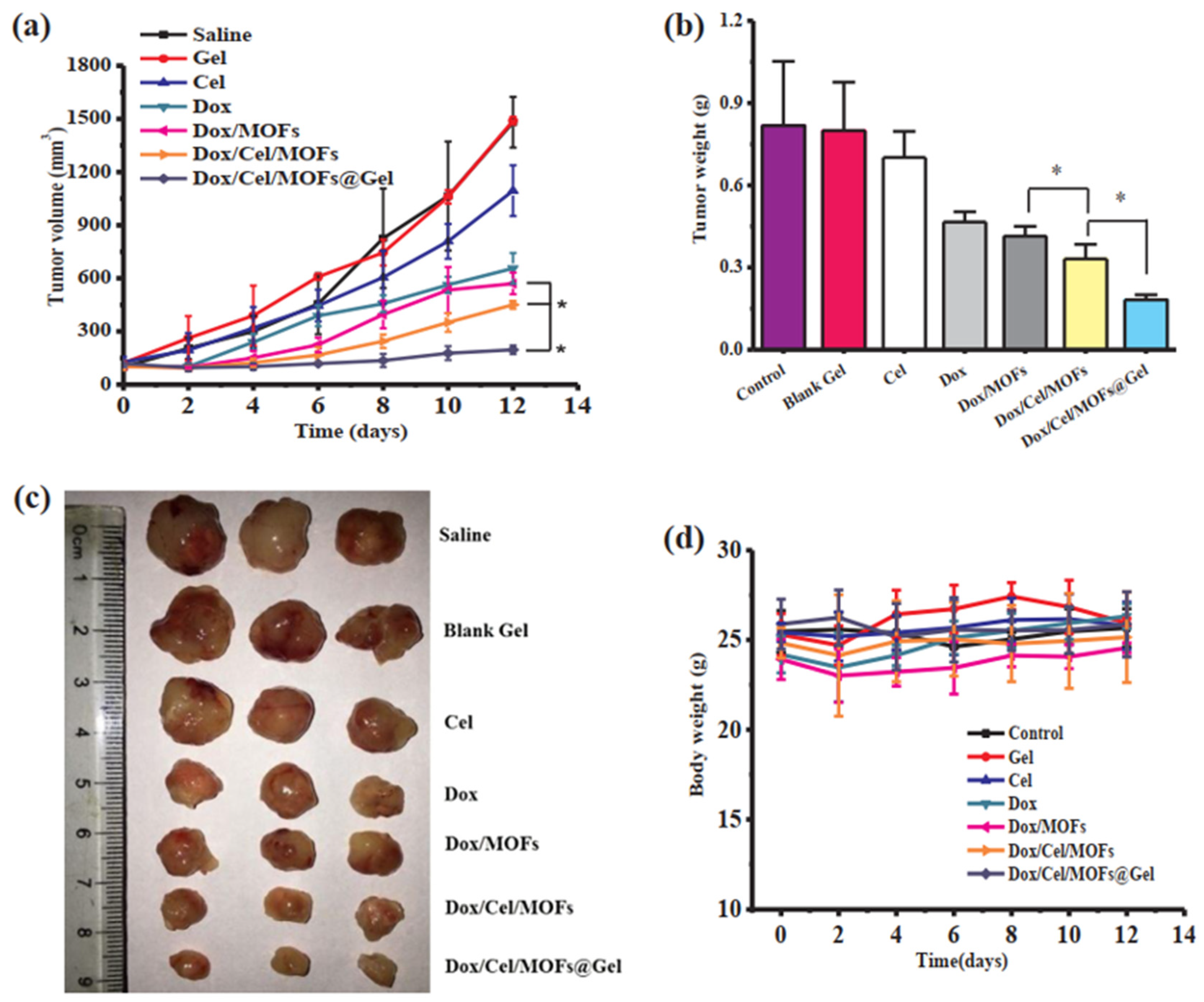
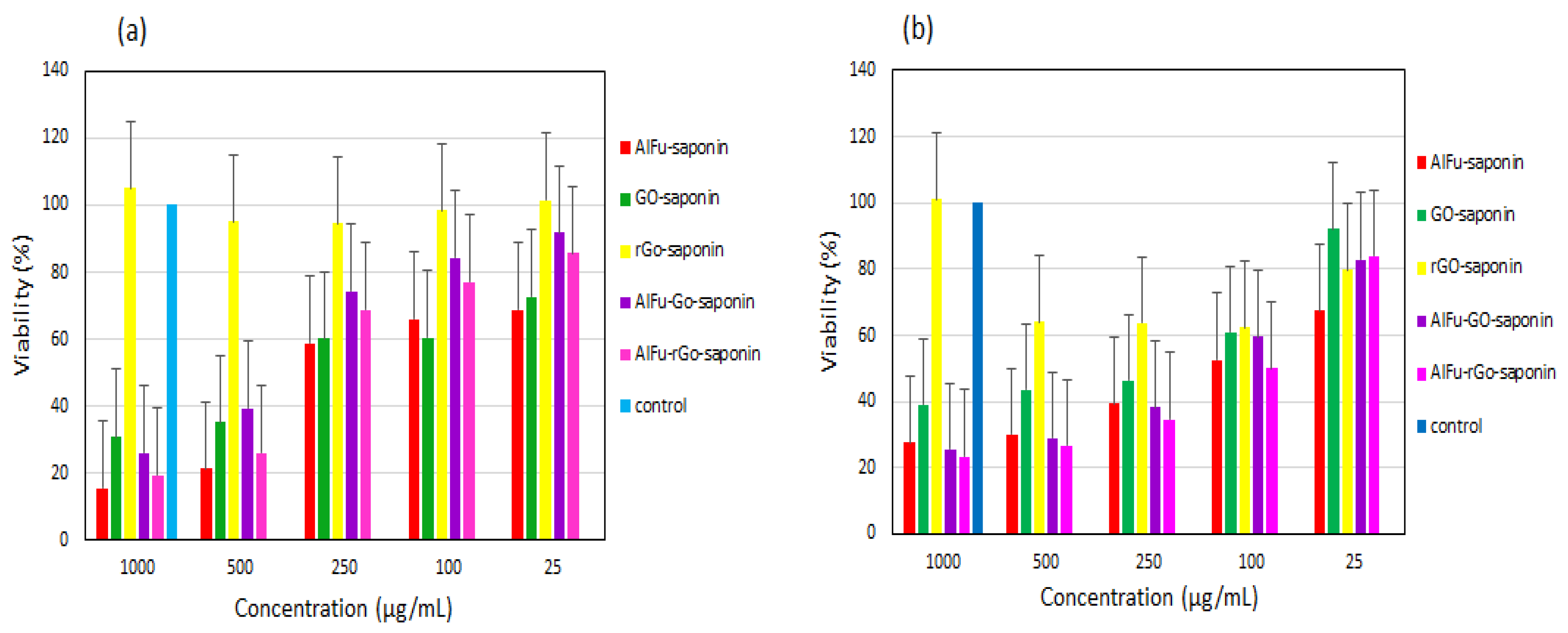
| S. No. | MOFs Composites | MOF’s Diameter | Antibacterial Compound | Kind of Bacteria | Combination Type | Antibacterial Activity | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Zn50Co50-ZIF | 100 nm | Zn50Co50-ZIF | S. aureus | one-pot method | Concentration = 1 mg/mL | [136] |
| 2 | Ceftazidime@ZIF-8 | 400 nm | Ceftazidime | E. coli | Mixture procedure | [137] | |
| 3 | MIL-100 (Fe)@gentamicin | 145–200 nm | gentamicin | S. epidermidis and S. aureus | simple impregnation procedure | S. epidermidis: MIC = 0.125 mg/L S. aureus: MIC = 0.5–1 mg/L | [138] |
| 4 | UiO-66/Poly(ε-caprolactone) MMMs | Poly(ε-caprolactone)/Mixed-matrix membranes | E. coli | Drawdown coating method | Standard plate count: Effectively inhibited within 30 min irradiation | [139] | |
| 5 | Tool-box with a mixed MOF | <50 nm | Commercially plover membrane | S. aureus and E. coli | Sandwich multilayered membrane by casting | Standard plate count: Exhibited 5-log fold reduction within 15 min of incubation | [140] |
| 6 | CP/CNF/ZIF-67 | 10 nm | Carboxylated cellulose nanofiber | E. coli | In Situ on the fiber | Standard plate count: Antibacterial rate reached 80% ZOI = 12 mm | [141] |
| 7 | Ag-MOF functionalized TFC membrane | Polyamide membrane | E. coli | In Situ functionalization | Fluorescence microscopy: Bacterial mortality of approximately 100% was attained | [142] | |
| 8 | Cu-BTC@silk fibers | Silk fibers | S.aureus and E. coli | layer-by-layer method | S. aureus: ZOI = 6.5 to 7.5 mm E. coli: ZOI = 7.7 to 8.0 mm | [143] |
| Compound | Late Apoptosis (%) | Early Apoptosis (%) | Cumulative Apoptosis (%) | |||
|---|---|---|---|---|---|---|
| PDL Cell Line | PDL Cell Line | OSCC Cell Line | OSCC Cell Line | PDL Cell Line | OSCC Cell Line | |
| AlFu-rGO | 11.25 ± 3.12 | 5.35 ± 0.58 | 7.66 ± 0.96 | 9.51 ± 0.51 | 16.60 ± 2.54 | 17.17 ± 2.80 |
| AlFu-GO | 8.79 ± 0.74 | 6.9 ± 3.39 | 5.69 ± 1.85 | 11.5 ± 1.33 | 15.69 ± 3.93 | 17.19 ± 2.26 |
| AlFu-GO-saponin | 7.13 ± 1.57 | 3.85 ± 0.07 | 11.30 ± 0.07 | 15.60 ± 0.16 | 10.98 ± 2.36 | 26.90 ± 3.24 |
| AlFu-rGO-saponin | 11.2 ± 1.43 | 4.7 ± 1.90 | 16.29 ± 3.48 | 13.59 ± 2.11 | 15.9 ± 4.08 | 29.88 ± 0.41 |
| saponin | 3.45 ± 0.41 | 11.51 ± 0.14 | 9.95 ± 1.05 | 12.80 ± 2.57 | 14.96 ± 3.04 | 22.75 ± 1.28 |
| control | 1.32 ± 0.08 | 1.20 ± 0.45 | 0.35 ± 0.81 | 0195 ± 0.31 | 2.52 ± 0.78 | 1.31 ± 0.62 |
| S. No. | MOFs | Drugs | Mechanism | Biological Test System | Reference |
|---|---|---|---|---|---|
| 1 | ZIF-8 | Doxorubicin | Encapsulation | Breast cancer cell lines | [155] |
| 2 | ZIF-8 | Ceftazidime | NA | Escherichia coli | [137] |
| 3 | MIL-100 (Fe) | Indocyanine green | π–π interaction | xenograft tumors/MCF-7 cells | [156] |
| 4 | MIL-100 (Fe) | Doxorubicin | NA | HepG-2 cells | [157] |
| 5 | MIL-100 (Fe) | Metformin hydrochloride | pH-cleavable bonds | PBS Buffer | [158] |
| 6 | MIL-101 (Fe) | BODIPY | NA | HT-29 human colon adenocarcinoma cells | [159] |
| 7 | MIL-101 (Fe) | Doxorubicin | NA | H-22 tumor-bearing mice | [160] |
| 8 | MOF-74 (Fe) | Ibuprofen | Ion exchange | PC12 cells | [161] |
| 9 | HKUST-1 | Ibuprofen and guaiacol anethole | NA | PBS buffer | [162] |
| 10 | MIL-100 (Fe) | Doxorubicin | Host-Guest Interactions | Tris Buffer | [163] |
| 11 | NU-1000 | Insulin | NA | Nucleic acids | [164] |
| 12 | NU-1000 | Insulin | NA | PBS Buffer | [165] |
| 13 | Zn-MOF | 5-Fluorouracil | pH-controlled | PBS Buffer | [166] |
| 14 | UiO-66@ Fe3O4 | Doxorubicin | π–π interaction | HeLa, 3T3 | [167] |
| 15 | UiO-66 | Cisplatin | Encapsulation | U-87 MG cancer cell and HSC-3 | [168] |
| 16 | UiO-68 | Cisplatin | Encapsulation | SKOV-3 cells | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, S.M.; Hashemi, S.A.; Fallahi Nezhad, F.; Binazadeh, M.; Dehdashtijahromi, M.; Omidifar, N.; Ghahramani, Y.; Lai, C.W.; Chiang, W.-H.; Gholami, A. Innovative Metal-Organic Frameworks for Targeted Oral Cancer Therapy: A Review. Materials 2023, 16, 4685. https://doi.org/10.3390/ma16134685
Mousavi SM, Hashemi SA, Fallahi Nezhad F, Binazadeh M, Dehdashtijahromi M, Omidifar N, Ghahramani Y, Lai CW, Chiang W-H, Gholami A. Innovative Metal-Organic Frameworks for Targeted Oral Cancer Therapy: A Review. Materials. 2023; 16(13):4685. https://doi.org/10.3390/ma16134685
Chicago/Turabian StyleMousavi, Seyyed Mojtaba, Seyyed Alireza Hashemi, Fatemeh Fallahi Nezhad, Mojtaba Binazadeh, Milad Dehdashtijahromi, Navid Omidifar, Yasamin Ghahramani, Chin Wei Lai, Wei-Hung Chiang, and Ahmad Gholami. 2023. "Innovative Metal-Organic Frameworks for Targeted Oral Cancer Therapy: A Review" Materials 16, no. 13: 4685. https://doi.org/10.3390/ma16134685
APA StyleMousavi, S. M., Hashemi, S. A., Fallahi Nezhad, F., Binazadeh, M., Dehdashtijahromi, M., Omidifar, N., Ghahramani, Y., Lai, C. W., Chiang, W.-H., & Gholami, A. (2023). Innovative Metal-Organic Frameworks for Targeted Oral Cancer Therapy: A Review. Materials, 16(13), 4685. https://doi.org/10.3390/ma16134685










