Two New Red/Near-Infrared Ir(Ⅲ) Complexes with Reversible and Force-Induced Enhanced Mechanoluminescence
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
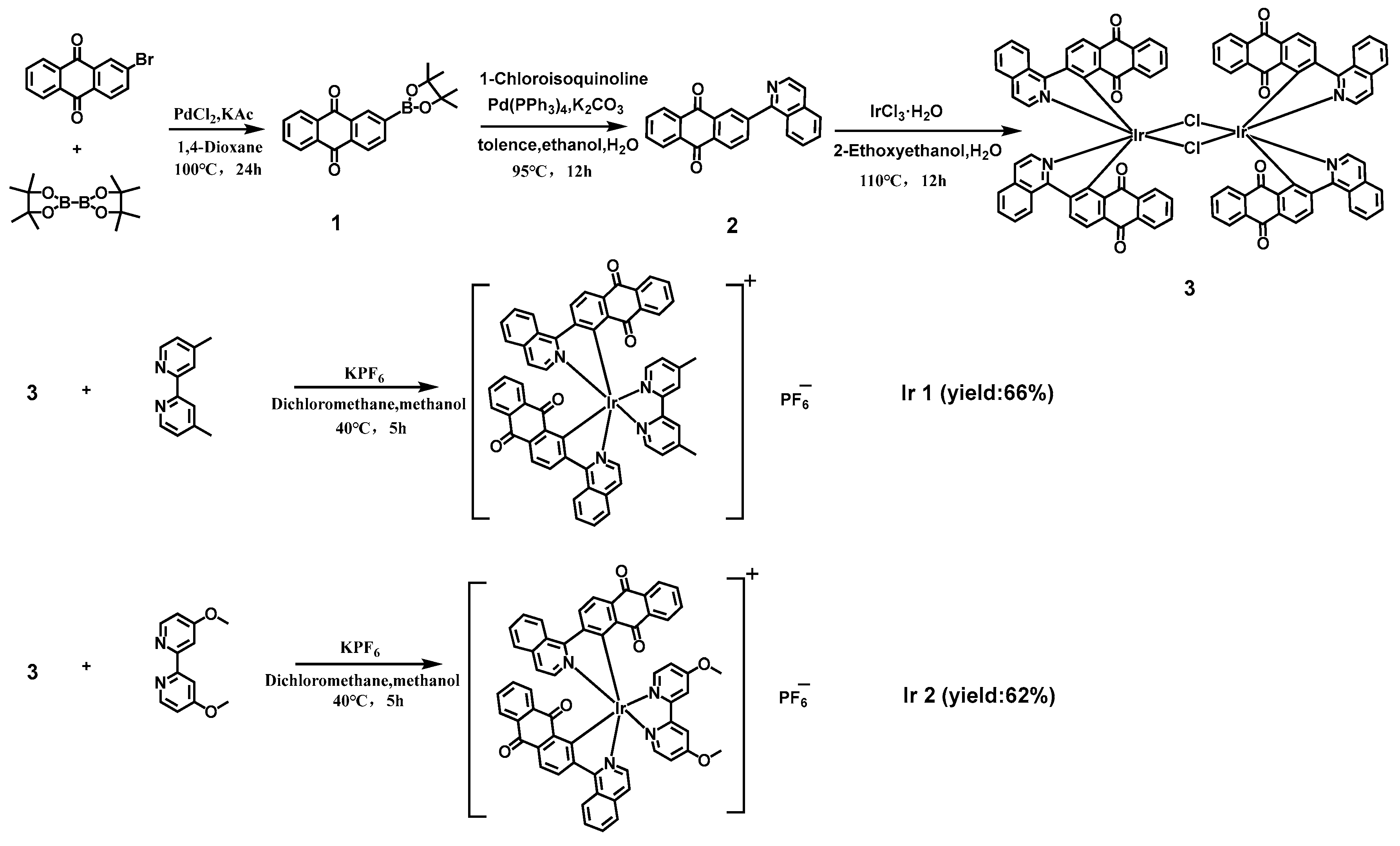
3.1. Syntheses and Characterization
3.2. Photophysical Properties
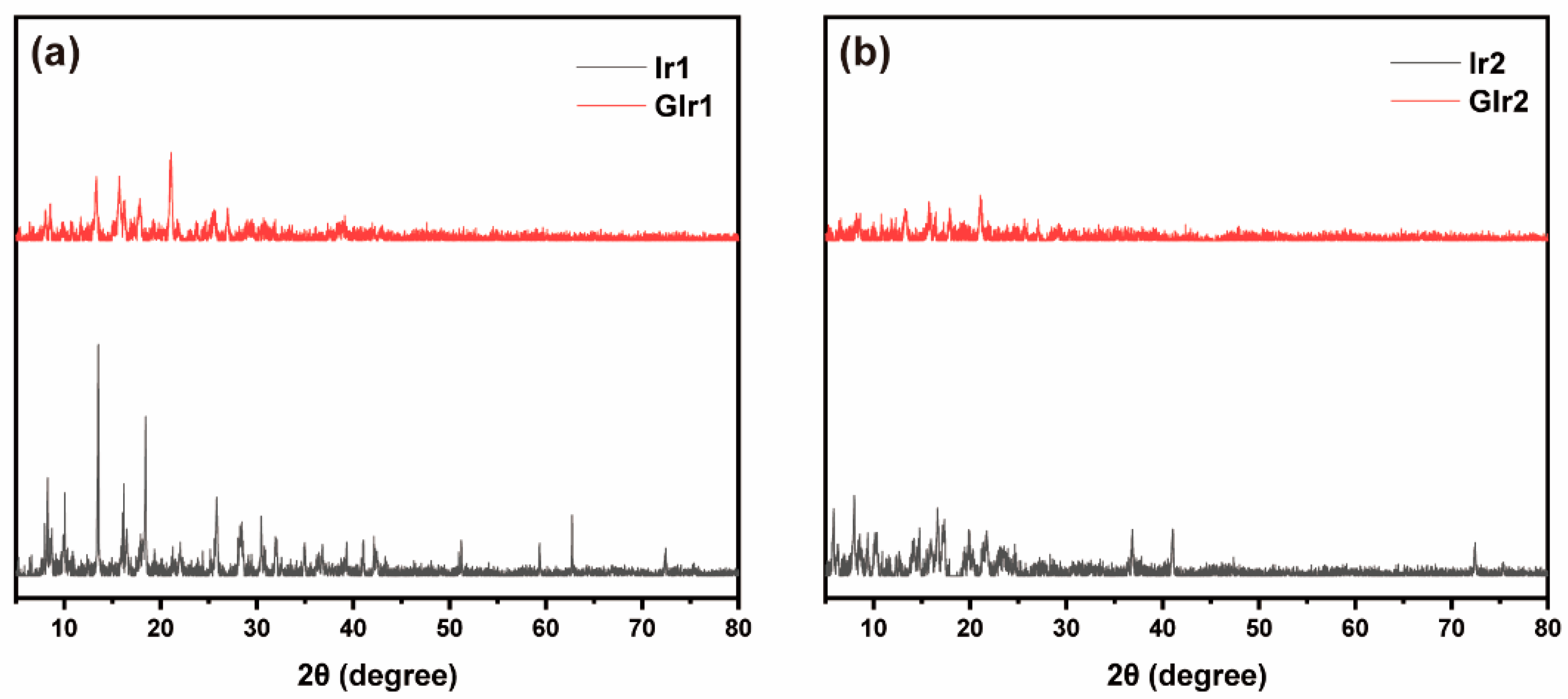
3.3. Mechanochromic Luminescent Mechanism Investigations
3.4. Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, K.; Wang, Y.; Cai, C.; Lin, H. Conversion of carbon dots from fluorescence to ultralong room-temperature phosphorescence by heating for security applications. Adv. Mater. 2018, 30, 1800783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, J.; Wang, J.; Shi, R.; Chen, L.; Zhang, A.; Yang, P. A vanadate-based white light emitting luminescent material for temperature sensing. RSC Adv. 2019, 9, 30045–30051. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xia, Q.; Huang, W.; Tian, J.; He, Z.; Li, B.S.; Tang, B.Z. Multiple anti-counterfeiting guarantees from a simple tetraphenylethylene derivative—high-contrasted and multi-state mechanochromism and photochromism. Angew. Chem. Int. Ed. 2019, 58, 17814–17819. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Li, C.; Liu, X.; Jiang, S.; Xu, Z.; Lee, R.; Zhu, M.; Xu, B.; Tian, W. Solid-state photoinduced luminescence switch for advanced anticounterfeiting and super-resolution imaging applications. J. Am. Chem. Soc. 2017, 139, 16036–16039. [Google Scholar] [CrossRef]
- Yang, W.; Yang, Y.; Qiu, Y.; Cao, X.; Huang, Z.; Gong, S.; Yang, C. AIE-active multicolor tunable luminogens: Simultaneous mechanochromism and acidochromism with high contrast beyond 100 nm. Mater. Chem. Front. 2020, 4, 2047–2053. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Lin, W.; Zhang, K.Y.; Lv, W.; Huang, X.; Huo, F.; Yang, H.; Jenkins, G.; Zhao, Q.; et al. Smart responsive phosphorescent materials for data recording and security protection. Nat. Commun. 2014, 5, 3601. [Google Scholar] [CrossRef]
- Takeda, Y.; Data, P.; Minakata, S. Alchemy of donor–acceptor–donor multi-photofunctional organic materials: From construction of electron-deficient azaaromatics to exploration of functions. Chem. Commun. 2020, 56, 8884–8894. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, B.; Liu, C.; Ji, X.; Huang, Y.; Sui, J.; Liu, D.; Wang, N.; Hao, H. Mechanistic study on the effect of magnetic field on the crystallization of organic small molecules. Ind. Eng. Chem. Res. 2021, 60, 15741–15751. [Google Scholar] [CrossRef]
- Cui, J.; Kim, G.; Kim, S.; Kwon, J.E.; Park, S.Y. Ultra-pH-sensitive small molecule probe showing a ratiometric fluorescence color change. ChemPhotoChem 2020, 4, 393–397. [Google Scholar] [CrossRef]
- Huang, W.; Xu, M.; Liu, J.; Wang, J.; Zhu, Y.; Liu, J.; Rong, H.; Zhang, J. Hydrophilic doped quantum dots “ink” and their inkjet-printed patterns for dual mode anticounterfeiting by reversible cation exchange mechanism. Adv. Funct. Mater. 2019, 29, 1808762. [Google Scholar] [CrossRef]
- Long, P.; Feng, Y.; Cao, C.; Li, Y.; Han, J.; Li, S.; Peng, C.; Li, Z.; Feng, W. Self-protective room-temperature phosphorescence of fluorine and nitrogen codoped carbon dots. Adv. Funct. Mater. 2018, 28, 1800791. [Google Scholar] [CrossRef]
- Sagara, Y.; Mutai, T.; Yoshikawa, I.; Araki, K. Material design for piezochromic luminescence: Hydrogen-bond-directed assemblies of a pyrene derivative. J. Am. Chem. Soc. 2007, 129, 1520–1521. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-J.; Chung, J.W.; Gierschner, J.; Kim, K.S.; Choi, M.-G.; Kim, D.; Park, S.Y. Multistimuli two-color luminescence switching via different slip-stacking of highly fluorescent molecular sheets. J. Am. Chem. Soc. 2010, 132, 13675–13683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yue, S.; Sun, H.; Wang, X.; Hao, X.; An, S. Nondestructive up-conversion readout in Er/Yb co-doped Na0.5Bi2.5Nb2O9-based optical storage materials for optical data storage device applications. J. Mater. Chem. C 2017, 5, 3838–3847. [Google Scholar] [CrossRef]
- Luo, J.; Li, L.-Y.; Song, Y.; Pei, J. A piezochromic luminescent complex: Mechanical force induced patterning with a high contrast ratio. Chem.—Eur. J. 2011, 17, 10515–10519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Y.; Gao, K.; Li, Z.; Liu, Y.; Liao, Y.; Duan, Y.; Han, T. A turn-on mechanochromic luminescent material serving as pressure sensor and rewritable optical data storage. Dye. Pigment. 2020, 173, 107928. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Qi, F.; Yan, H.; Jiang, Z.; Chen, Y. Flexible π-conjugated 2,5-diarylamino-terephthalates: A new class of mechanochromic luminophores with tunable aggregation states. Chem. A Eur. J. 2020, 26, 14963–14968. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, J.; Tan, X.; Wang, L.; Chen, J.; Li, B.; Ye, L.; Xu, B.; Zou, B.; Tian, W. Multi-stimuli responsive fluorescence switching: The reversible piezochromism and protonation effect of a divinylanthracene derivative. J. Mater. Chem. C 2013, 1, 7554–7559. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, J.; Zhai, S.; Wang, Y.; Xu, Z.; Liu, R.; Zhu, H. AIPE-active Pt(II) complexes with a tunable triplet excited state: Design, mechanochromism and application in anti-counterfeiting. Inorg. Chem. Front. 2020, 7, 4677–4686. [Google Scholar] [CrossRef]
- Han, Y.; Cao, H.-T.; Sun, H.-Z.; Wu, Y.; Shan, G.-G.; Su, Z.-M.; Hou, X.-G.; Liao, Y. Effect of alkyl chain length on piezochromic luminescence of iridium(III)-based phosphors adopting 2-phenyl-1H-benzoimidazole type ligands. J. Mater. Chem. C 2014, 2, 7648–7655. [Google Scholar] [CrossRef]
- Manbeck, G.F.; Brennessel, W.W.; Stockland, R.A., Jr.; Eisenberg, R. Luminescent Au(I)/Cu(I) alkynyl clusters with an ethynyl steroid and related aliphatic ligands: An octanuclear Au4Cu4 cluster and luminescence polymorphism in Au3Cu2 clusters. J. Am. Chem. Soc. 2010, 132, 12307–12318. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tong, J.; Ding, G.; Sun, C.; Wang, X.; Su, Z.; Sun, J.; Wen, L.-L.; Shan, G.-G. Highly emissive coordination polymer derived from tetraphenylethylene-tetrazole chromophore: Synthesis, characterization and piezochromic luminescent behavior. Chin. Chem. Lett. 2023, 34, 107255. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Yang, J.; Li, X.; Wang, X.; Ma, L. The abnormal solvatochromism, high-contrast mechanochromism and internal mechanism of two AIEE-active β-diketones. Dye. Pigment. 2020, 175, 108149. [Google Scholar] [CrossRef]
- Costa, R.D.; Ortí, E.; Bolink, H.J.; Monti, F.; Accorsi, G.; Armaroli, N. Luminescent ionic transition-metal complexes for light-emitting electrochemical cells. Angew. Chem. Int. Ed. 2012, 51, 8178–8211. [Google Scholar] [CrossRef]
- Ulbricht, C.; Beyer, B.; Friebe, C.; Winter, A.; Schubert, U.S. Recent developments in the application of phosphorescent iridium(III) complex systems. Adv. Mater. 2009, 21, 4418–4441. [Google Scholar] [CrossRef]
- Chi, Y.; Chou, P.-T. Transition-metal phosphors with cyclometalating ligands: Fundamentals and applications. Chem. Soc. Rev. 2010, 39, 638–655. [Google Scholar] [CrossRef]
- Tao, P.; Lü, X.; Zhou, G.; Wong, W.-Y. Asymmetric tris-heteroleptic cyclometalated phosphorescent iridium(III) complexes: An emerging class of metallophosphors. Acc. Mater. Res. 2022, 3, 830–842. [Google Scholar] [CrossRef]
- Su, N.; Li, S.; Yang, K.; Zhou, F.; Song, J.; Zhou, L.; Qu, J. High efficiency electroluminescence of orange-red iridium(III) complexes for OLEDs with an EQE over 30%. Dye. Pigment. 2021, 195, 109733. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; Liu, J.; Liu, Y.; Wu, D. Highly efficient dual-modal phosphorescence/computed tomography bioprobes based on an iridium complex and AuNP polyiohexol composite nanoparticles. Nanoscale 2017, 9, 9447–9456. [Google Scholar] [CrossRef]
- Tao, P.; Lv, Z.; Zheng, X.-K.; Jiang, H.; Liu, S.; Wang, H.; Wong, W.-Y.; Zhao, Q. Isomer engineering of lepidine-based iridophosphors for far-red hypoxia imaging and photodynamic therapy. Inorg. Chem. 2022, 61, 17703–17712. [Google Scholar] [CrossRef]
- Song, Z.; Liu, R.; Zhu, H.; Lu, Y.; Li, X.; Zhu, H. Smart inks based on AIPE-active heteroleptic Ir(III) complexes. Sens. Actuators B Chem. 2019, 279, 385–392. [Google Scholar] [CrossRef]
- Orselli, E.; Kottas, G.S.; Konradsson, A.E.; Coppo, P.; Fröhlich, R.; De Cola, L.; van Dijken, A.; Büchel, M.; Börner, H. Blue-emitting iridium complexes with substituted 1,2,4-triazole ligands: Synthesis, photophysics, and devices. Inorg. Chem. 2007, 46, 11082–11093. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Tao, P.; Ma, C.; Tang, R.; Gong, T.; Liu, S.; Zhao, Q. Iridium(iii) complex-containing non-conjugated polymers for non-volatile memory induced by switchable through-space charge transfer. J. Mater. Chem. C 2020, 8, 5449–5455. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.; Liu, X.; Li, G.; Che, W.; Zhu, D.; Su, Z.; Bryce, M.R. Reversible tricolour luminescence switching based on a piezochromic iridium(III) complex. Chem. Commun. 2019, 55, 14582–14585. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, T.; Shao, K.; Gao, Y.; Shan, G.; Su, Z.; Wang, X.; Zhu, D. Understanding mechanochromic luminescence on account of molecular level based on phosphorescent iridium(III) complex isomers. Inorg. Chem. 2021, 60, 3741–3748. [Google Scholar] [CrossRef] [PubMed]
- Tsukuda, T.; Kawase, M.; Dairiki, A.; Matsumoto, K.; Tsubomura, T. Brilliant reversible luminescent mechanochromism of silver(I) complexes containing o-bis(diphenylphosphino)benzene and phosphinesulfide. Chem. Commun. 2010, 46, 1905–1907. [Google Scholar] [CrossRef]
- Balch, A.L. Dynamic crystals: Visually detected mechanochemical changes in the luminescence of gold and other transition-metal complexes. Angew. Chem. Int. Ed. 2009, 48, 2641–2644. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Zhang, Y.; Yang, J.; Zhu, S.; Sheng, L.; Wang, X.; Yang, B.; Zhang, S.X.-A. Stress acidulated amphoteric molecules and mechanochromism via reversible intermolecular proton transfer. Chem. Commun. 2013, 49, 6587–6589. [Google Scholar] [CrossRef]
- Nonoyama, M. Benzo[h]quinolin-10-yl-n iridium(III) complexes. Bull. Chem. Soc. Jpn. 1974, 47, 767–768. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, F.; Zheng, Y.; Zuo, J. Efficient deep red electroluminescence of iridium(III) complexes with 2,3-diphenylquinoxaline derivatives and tetraphenylimidodiphosphinate. J. Mater. Chem. C 2017, 5, 3714–3724. [Google Scholar] [CrossRef]
- Guo, C.; Guo, S.; Lu, Q.; Jiang, Z.; Yang, Y.; Zhou, W.; Zeng, Q.; Liang, J.; Miao, Y.; Liu, Y. Solution-processed yellow organic light-emitting diodes based on two new ionic Ir (III) complexes. Molecules 2022, 27, 2840. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Lu, Q.; Yang, Y.; Jiang, Z.; Zeng, Q.; Zhou, W.; Jun, L.; Gong, Y.; Liu, Y.; Miao, Y.; et al. Solution-processed high-performance orange-red organic light-emitting diode (OLED) based on ionic phosphorescent iridium(III) complex. J. Organomet. Chem. 2022, 967, 122333. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16 Revision c. 01; Gaussian Inc.: Wallingford, CT, USA, 2016; 421. [Google Scholar]
- Tao, P.; Li, W.L.; Zhang, J.; Guo, S.; Zhao, Q.; Wang, H.; Wei, B.; Liu, S.J.; Zhou, X.H.; Yu, Q.; et al. Facile synthesis of highly efficient lepidine-based phosphorescent iridium(III) complexes for yellow and white organic light-emitting diodes. Adv. Funct. Mater. 2016, 26, 881. [Google Scholar] [CrossRef]



| Complexes | Emission in Degassed CH2Cl2 | Emission in Solid States | Eg [eV] a | Eonsetox [eV] | T1 [eV] b | ||
|---|---|---|---|---|---|---|---|
| λem [nm] | τ [μs] | λem [nm] | τ [μs] | ||||
| Ir1 | 616 | 4.58 | 677 | 2.21 | 2.01 | 0.72 | 1.97 |
| GIr1 | - | - | 641 | 0.94 | - | - | - |
| Ir2 | 620 | 3.55 | 677 | 1.86 | 2.00 | 0.69 | 1.97 |
| GIr2 | - | - | 661 | 0.84 | - | - | - |
| Complexes | State | HOMO | LUMO | Configuration | Character |
|---|---|---|---|---|---|
| (eV) | (eV) | ||||
| Ir1 | T1 | −5.97 | −2.98 | HOMO−1→LUMO+1, 6.48% | LLCT |
| HOMO−1→LUMO+3, 3.38% | MLCT/LLCT | ||||
| HOMO→LUMO, 84.5% | MLCT/LLCT | ||||
| Ir2 | T1 | −5.89 | −2.98 | HOMO−1→LUMO+1, 5.78% | LLCT |
| HOMO−1→LUMO+3, 2.88% | MLCT/LLCT | ||||
| HOMO→LUMO, 79.4% | MLCT/LLCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zeng, Q.; Zhou, W.; Jiang, J.; Zhang, Z.; Guo, S.; Liu, Y. Two New Red/Near-Infrared Ir(Ⅲ) Complexes with Reversible and Force-Induced Enhanced Mechanoluminescence. Materials 2023, 16, 4702. https://doi.org/10.3390/ma16134702
Yang Y, Zeng Q, Zhou W, Jiang J, Zhang Z, Guo S, Liu Y. Two New Red/Near-Infrared Ir(Ⅲ) Complexes with Reversible and Force-Induced Enhanced Mechanoluminescence. Materials. 2023; 16(13):4702. https://doi.org/10.3390/ma16134702
Chicago/Turabian StyleYang, Yuzhen, Qin Zeng, Weiqiao Zhou, Junjie Jiang, Zihao Zhang, Song Guo, and Yuanli Liu. 2023. "Two New Red/Near-Infrared Ir(Ⅲ) Complexes with Reversible and Force-Induced Enhanced Mechanoluminescence" Materials 16, no. 13: 4702. https://doi.org/10.3390/ma16134702
APA StyleYang, Y., Zeng, Q., Zhou, W., Jiang, J., Zhang, Z., Guo, S., & Liu, Y. (2023). Two New Red/Near-Infrared Ir(Ⅲ) Complexes with Reversible and Force-Induced Enhanced Mechanoluminescence. Materials, 16(13), 4702. https://doi.org/10.3390/ma16134702






