Properties of Red Mud Neutralized with Sulfuric Acid and Effects on Cement Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Testing Methods
3. Results and Discussion
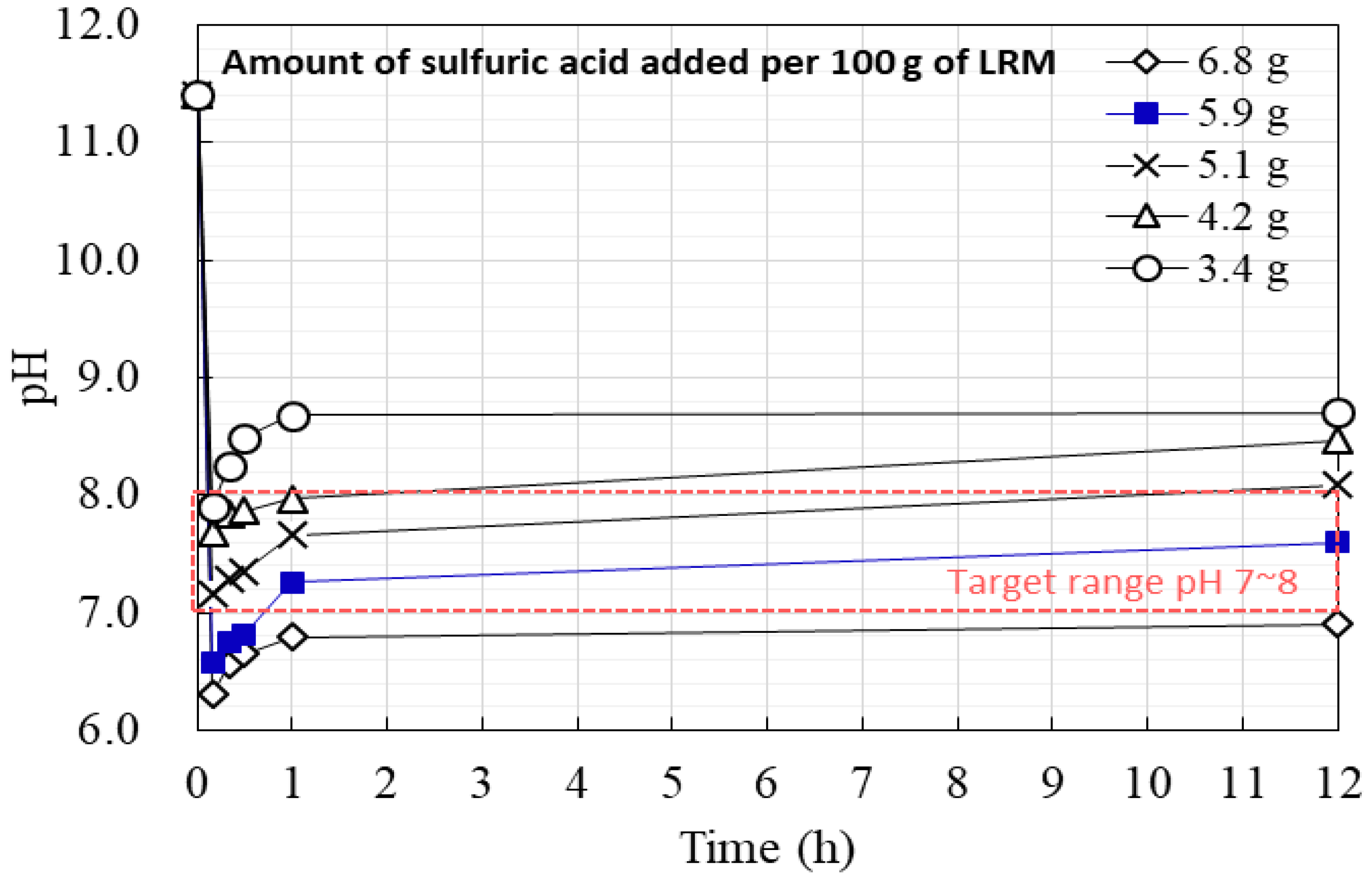
3.1. Properties of LRM + S
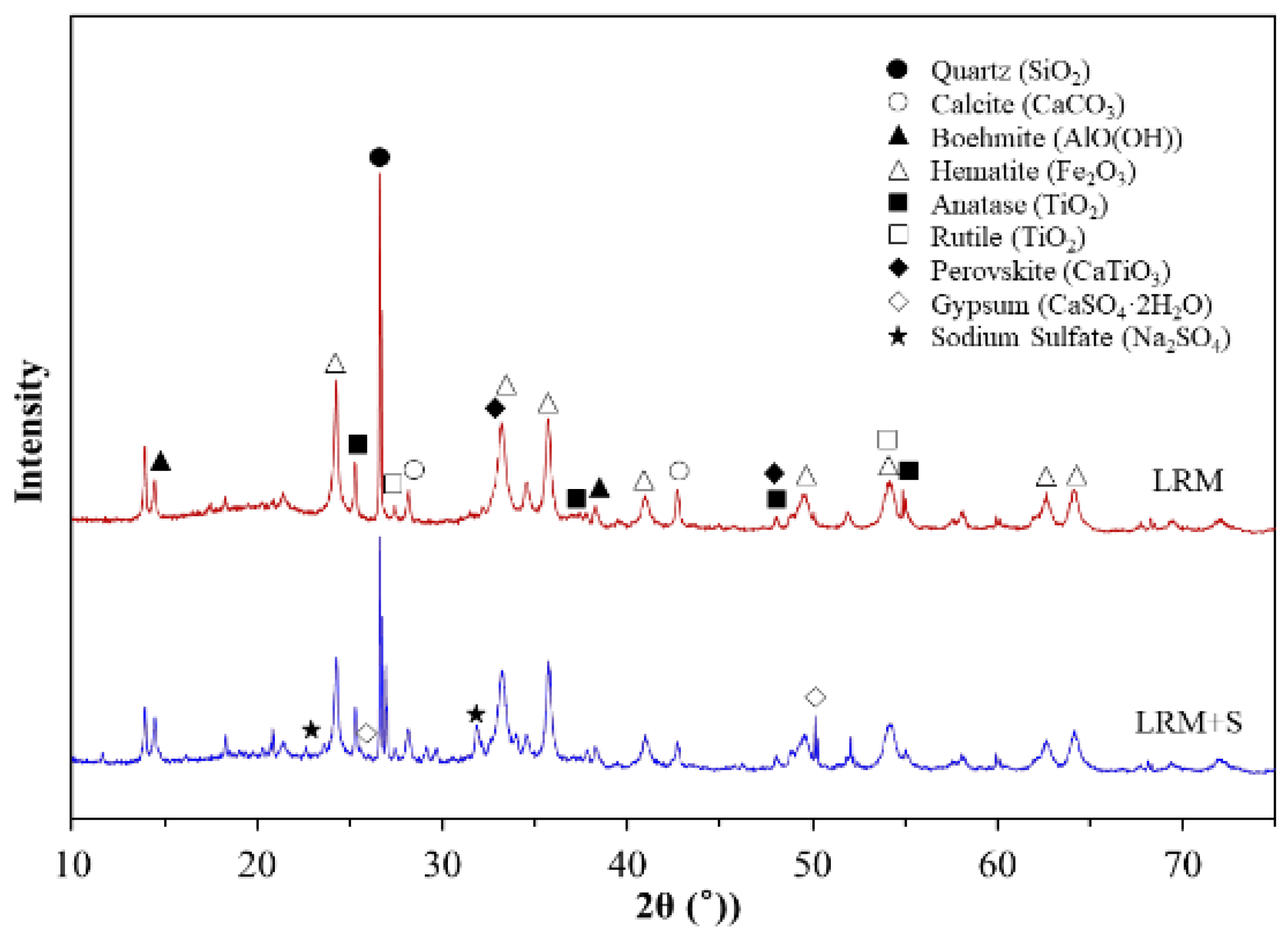
3.1.2. XRD
3.1.3. Physical Properties
3.2. Flow
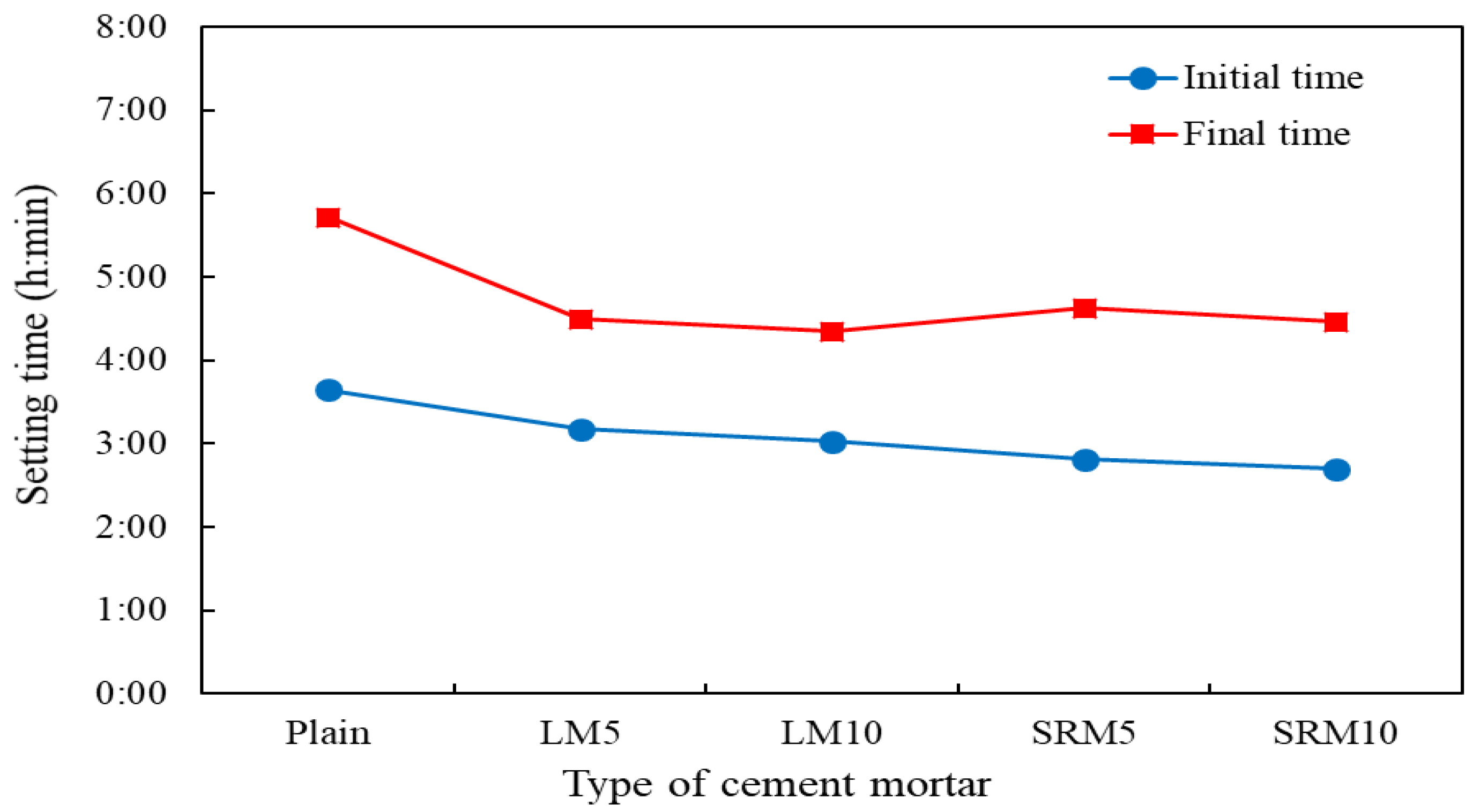
3.3. Setting Time
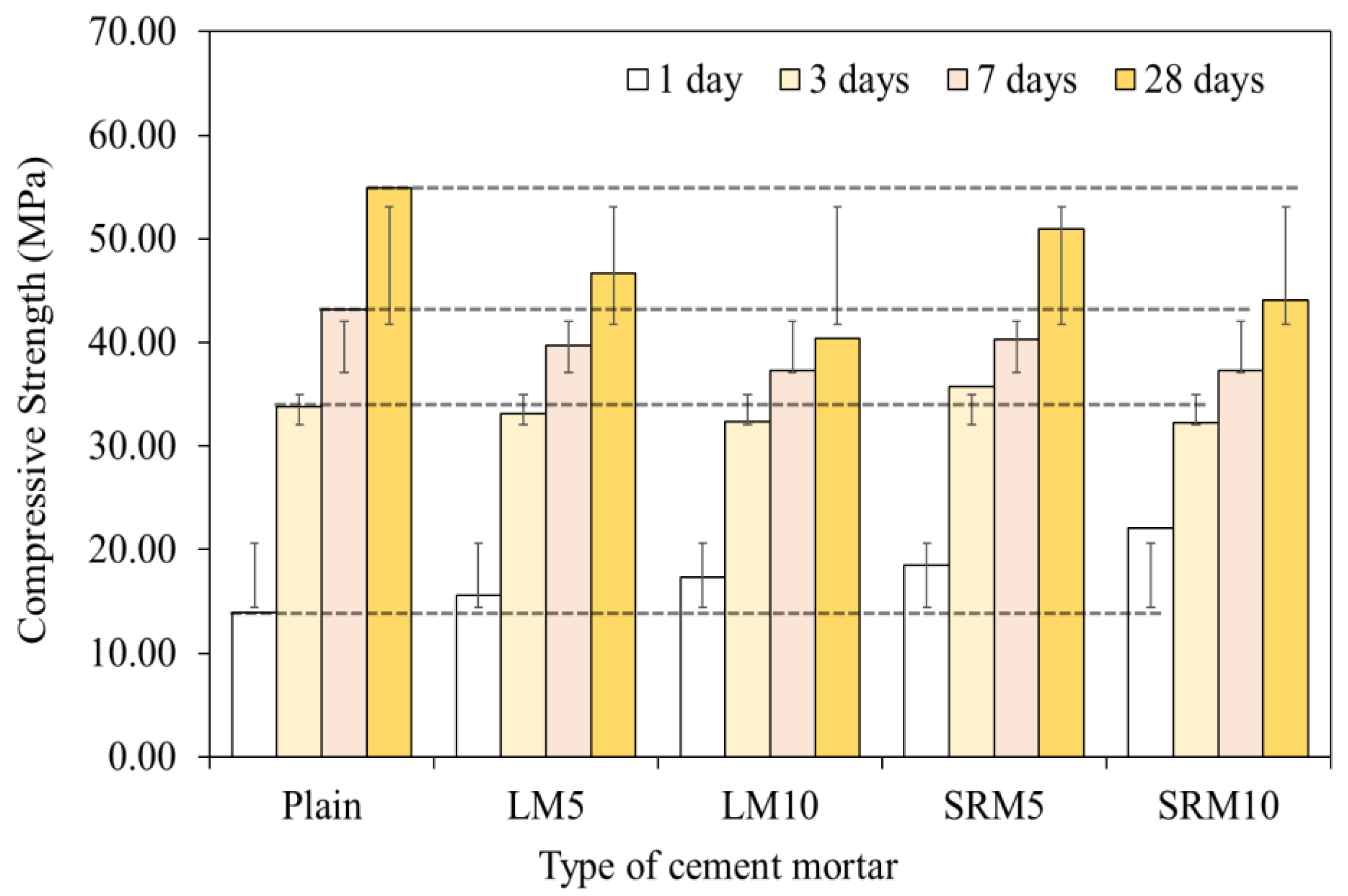
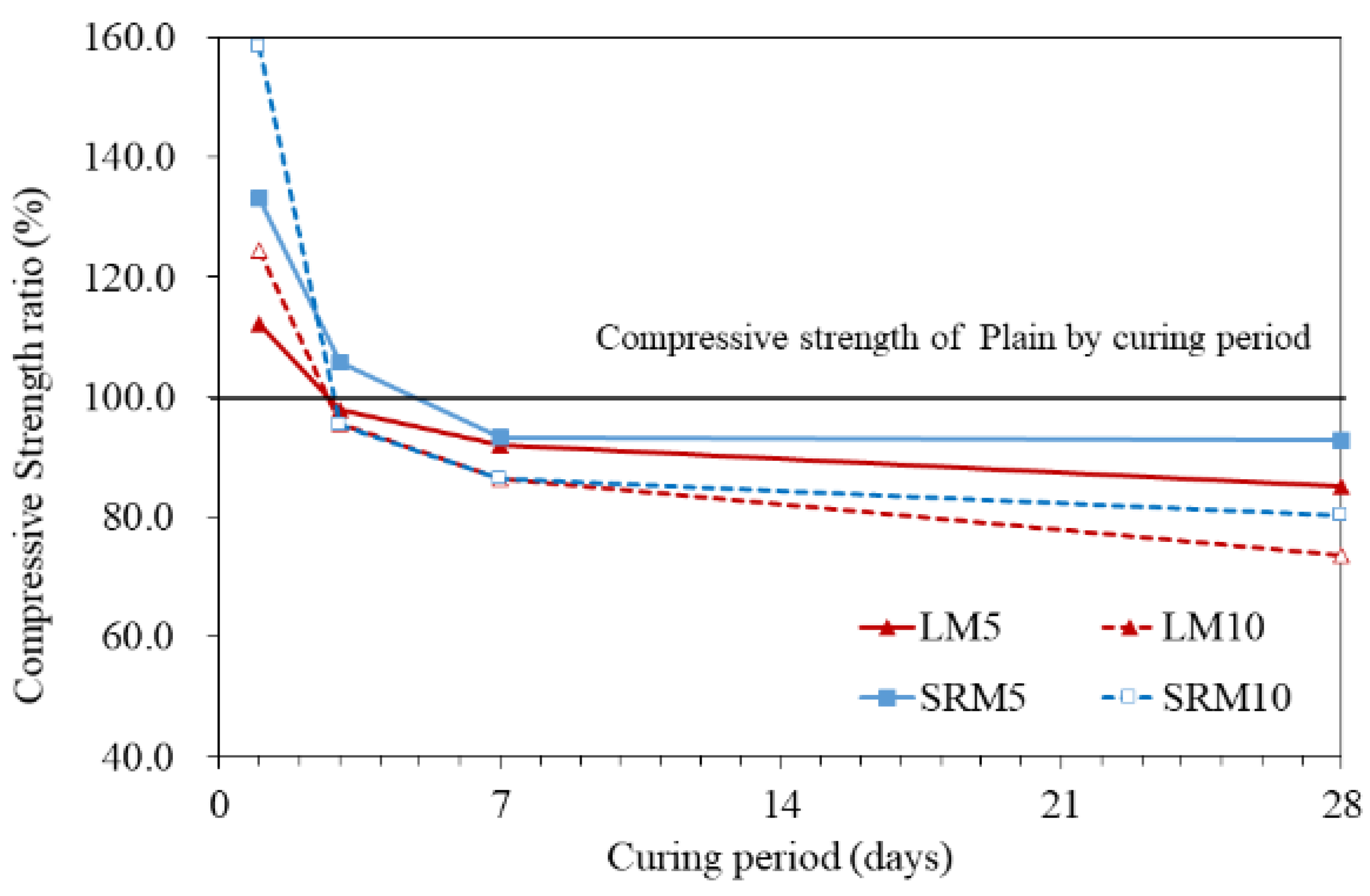
3.4. Compressive Strength
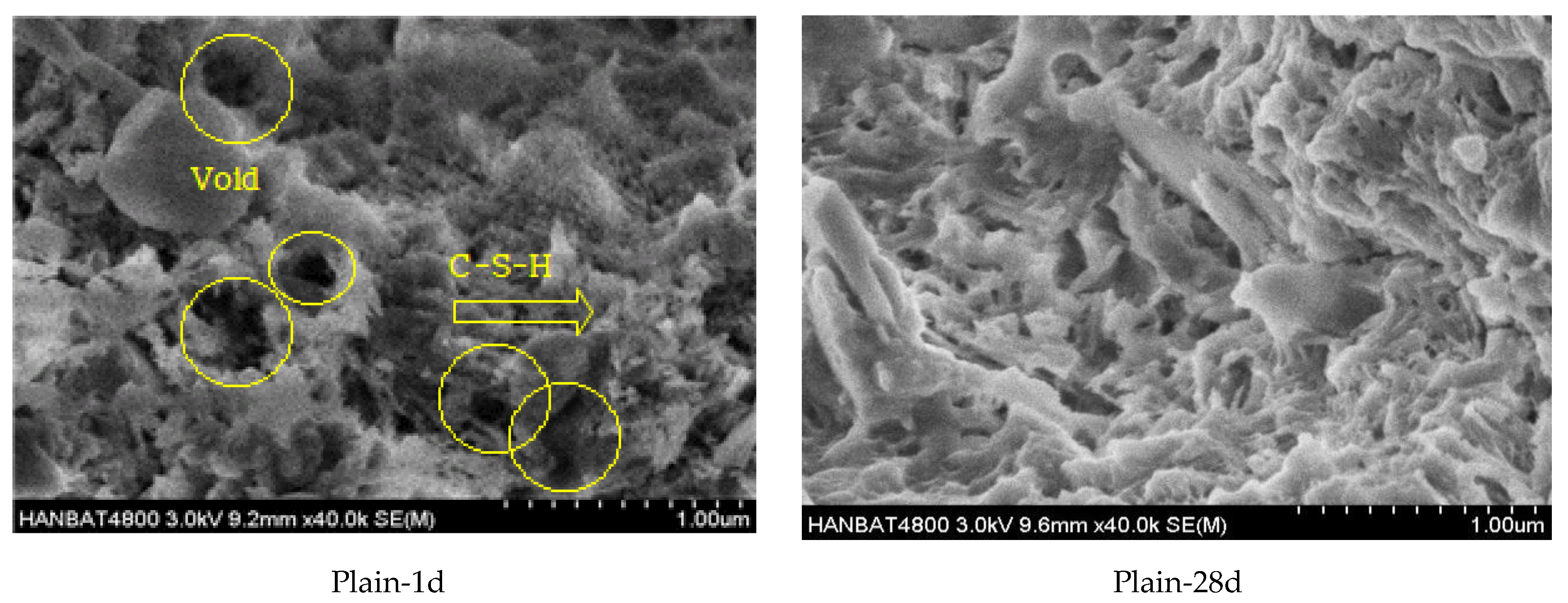
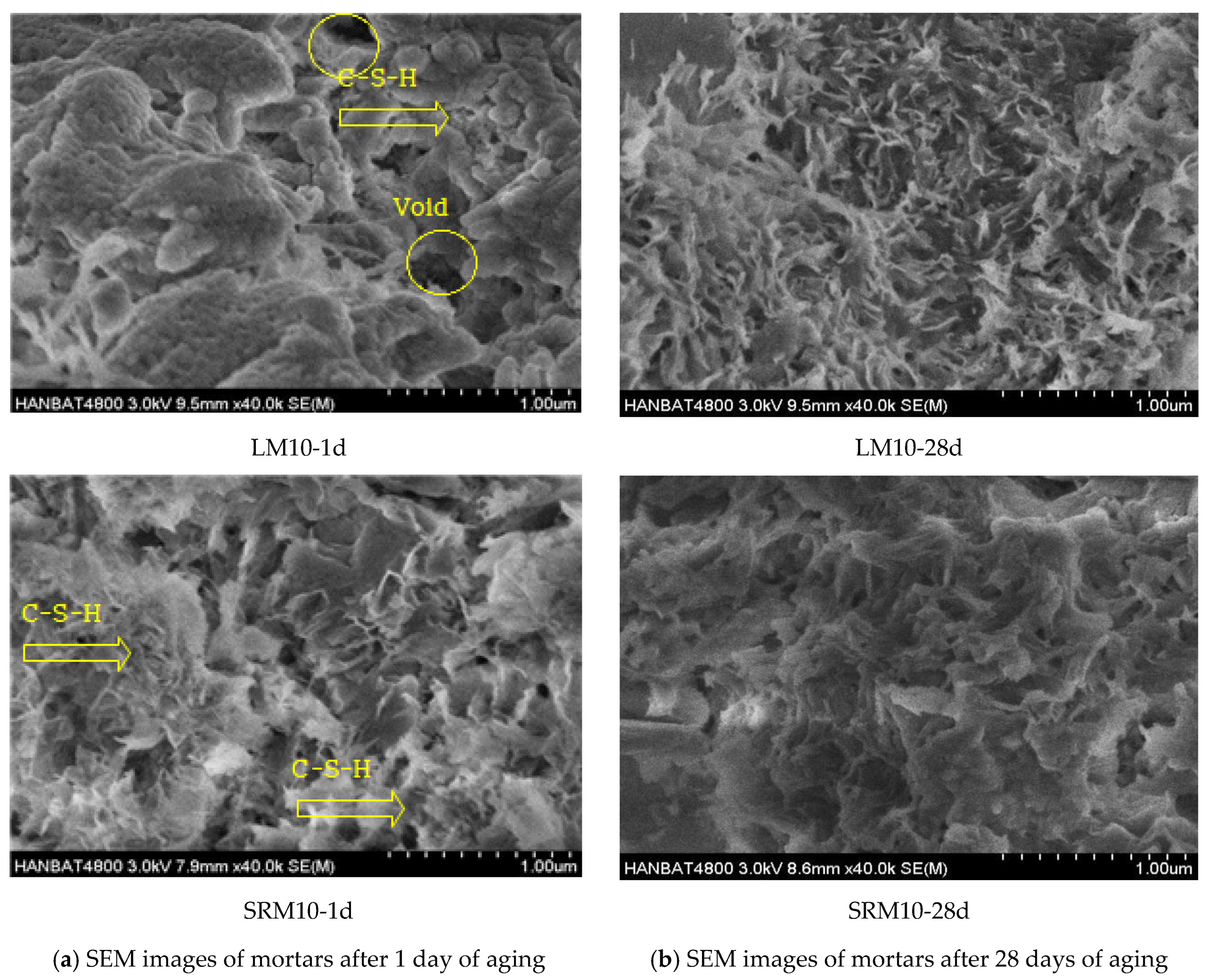
3.5. SEM Observations Instead
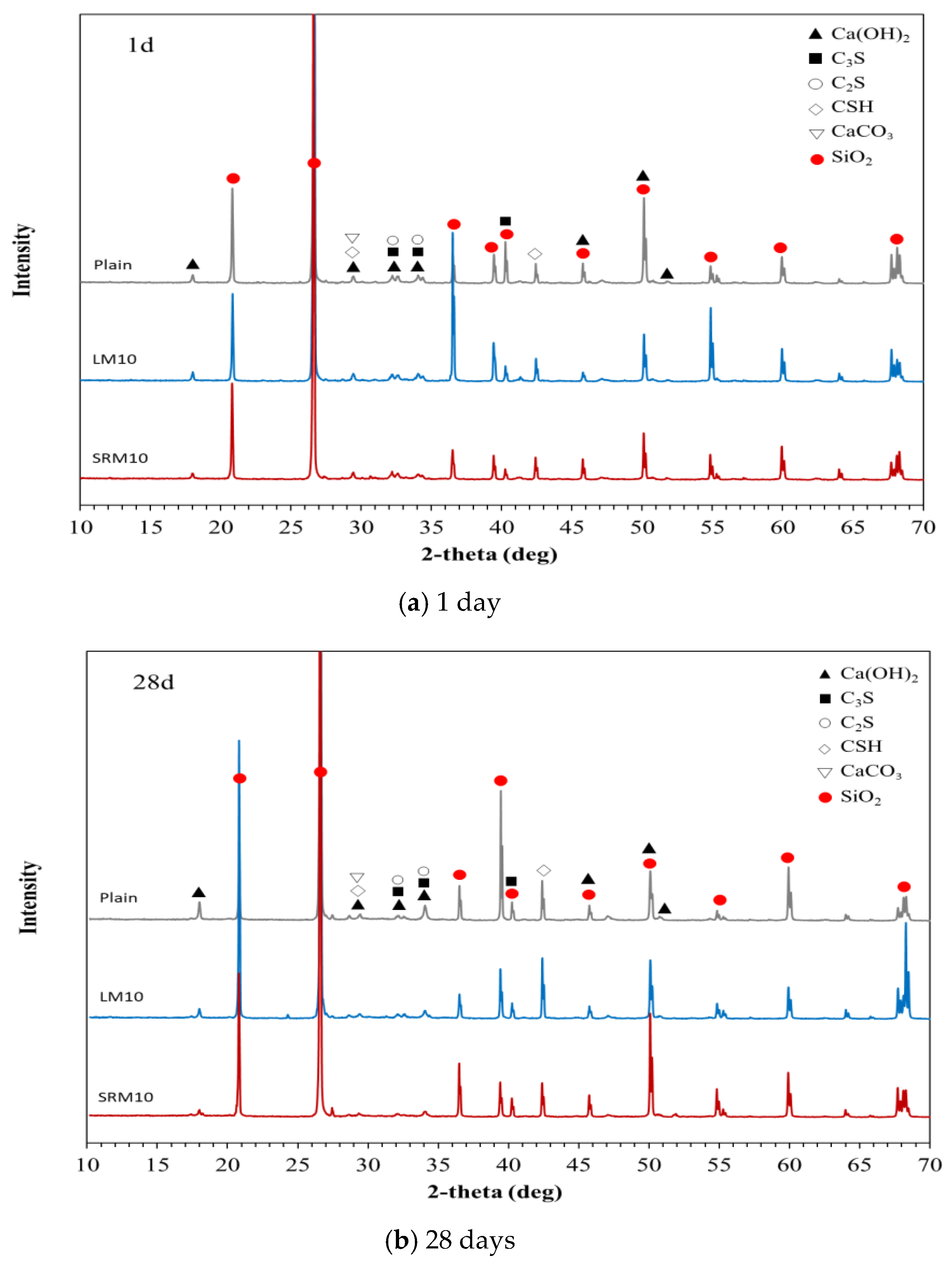
3.6. XRD
4. Conclusions
- When LRM was neutralized with sulfuric acid and stabilized to a pH of 7 to 8, gypsum and sodium sulfate were generated, and the physical properties of the LRM, including particle size, specific surface area, and viscosity, were changed. In particular, the viscosity increased by 1.6 times.
- Substituting LRM and LRM + S for cement in cement mortar decreased the flow. The flow value decreased as the amount of red mud increased. The measurement results showed that the setting times of LM and SRM, which contained LRM and LRM + S, were shorter than those of plain mortar. In particular, the final setting times were significantly shortened.
- The compressive strengths of LM and SRM were initially higher than that of plain mortar but decreased with aging. The compressive strengths of the LM10 and SRM10 were approximately 24% and 58% higher than that of plain mortar, respectively, after 1 day of aging. After approximately 3 days of aging, the compressive strengths of the LM10 and SRM10 were comparable to that of plain mortar. After 28 days of aging, it was lower than plain mortar by approximately 26 and 19%, respectively. The compressive strength decreased as the amounts of LRM and LRM + S in the mortar increased. The compressive strength of SRM at 28 days was higher than that of LM, confirming the compressive strength improvement effect of sulfuric acid neutralization.
- More pores were observed in the microstructure of the plain mortar compared to those of the LM10 and SRM10 mortars at 1 day of aging. This can explain the higher initial compressive strengths of LM and SRM compared to that of plain mortar. At 28 days of aging, the plain and SRM10 mortars exhibited similar microstructures, unlike the LM10 mortar, which exhibited a fine fibrous structure. This difference in hydration products can explain the slightly lower compressive strength of LM10 after 28 days of aging. The XRD analysis showed that the hydration products of the plain, LM10, and SRM10 mortars were identical at 1 day of aging. XRD indicated the formation of xonotlite in the LM10 after 28 days of aging.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, T.; Cui, K.; Chang, J. Development of low-carbon cement: Carbonation of compounded C2S by β-C2S and γ-C2S. Cem. Concr. Compos. 2023, 139, 105071. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Provis, J.L.; Tsang, D.C.W.; Poon, C.S. Accelerated carbonation of reactive MgO and Portland cement blends under flowing CO2 Gas. Cem. Concr. Compos. 2019, 106, 103489. [Google Scholar] [CrossRef]
- Cui, K.; Liang, K.; Jiang, T.; Zhang, J.; Lau, D.; Chang, J. Understanding the role of carbon nanotubes in low-carbon concrete: From experiment to molecular dynamics. Cem. Concr. Compos. 2023, 105189. [Google Scholar] [CrossRef]
- Cui, K.; Lu, D.; Jiang, T.; Zhang, J.; Jiang, Z.; Zhang, G.; Lau, D. Understanding the role of carbon nanotubes in low carbon sulfoaluminate cement-based composite. J. Clean. Prod. 2023, 137843. [Google Scholar] [CrossRef]
- Lima, M.S.S.; Thives, L.P. Evaluation of red mud as filler in Brazilian dense graded asphalt mixtures. Constr. Build. Mater. 2020, 260, 119894. [Google Scholar] [CrossRef]
- Menzie, W.D.; Barry, J.J.; Bleiwas, D.I.; Bray, E.L.; Goonan, T.G.; Matos, G. The Global Flow of Aluminum from 2006 through 2025; United States Department of the Interior, United States Geological Survey: Reston, VA, USA, 2010. [Google Scholar]
- Liu, X.; Zhang, N.; Yao, Y.; Sun, H.; Feng, H. Micro-structural characterization of the hydration products of bauxite-calcination-method red mud-coal gangue based cementitious materials. J. Hazard. Mater. 2013, 262, 428–438. [Google Scholar] [CrossRef]
- Hildebrando, E.A.; Souza, J.A.d.S.; Angélica, R.S.; Neves, R.F. Application of bauxite waste from Amazon region in the heavy clay industry. Mater. Res. 2013, 16, 1418–1422. [Google Scholar] [CrossRef]
- Nath, H.; Sahoo, A. A study on the characterization of red mud. Int. J. Appl. Bio-Eng. 2014, 8, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recy. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J. Hydration, reinforcing mechanism, and macro performance of multi-layer graphene-modified cement composites. J. Build. Eng. 2022, 57, 104880. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tadé, M.O. Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 2008, 72, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ma, S.; Chen, Y.; Ning, S.; Wei, Y.; Fujita, T. Recovery of scandium from red mud by leaching with titanium white waste acid and solvent extraction with P204. Hydrometallurgy 2021, 204, 105724. [Google Scholar] [CrossRef]
- Bonomi, C.; Alexandri, A.; Vind, J.; Panagiotopoulou, A.; Tsakiridis, P.; Panias, D. Scandium and titanium recovery from bauxite residue by direct leaching with a Brønsted acidic ionic liquid. Metals 2018, 8, 834. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Afshinnia, K.; Rangaraju, P.R. Effect of alkali content of cement on properties of high performance cementitious mortar. Constr. Build. Mater. 2016, 102, 631–639. [Google Scholar] [CrossRef]
- Muraleedharan, M.; Nadir, Y. Factors affecting the mechanical properties and microstructure of geopolymers from red mud and granite waste powder: A review. Ceram. Int. 2021, 47, 13257–13279. [Google Scholar] [CrossRef]
- Kang, S.; Kang, H.; Lee, B. Hydration properties of cement with liquefied red mud neutralized by nitric acid. Materials 2021, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kang, H.; Lee, B. Effects of adding neutralized red mud on the hydration properties of cement paste. Materials 2020, 13, 4107. [Google Scholar] [CrossRef]
- Kang, S.P.; Kwon, S.J. Effects of red mud and alkali-activated slag cement on efflorescence in cement mortar. Constr. Build. Mater. 2017, 133, 459–467. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N.; Sun, H.; Zhang, J.; Li, L. Structural investigation relating to the cementitious activity of bauxite residue—Red Mud. Cem. Concr. Res. 2011, 41, 847–853. [Google Scholar] [CrossRef]
- Choe, G.; Kang, S.; Kang, H. Mechanical properties of concrete containing liquefied red mud subjected to uniaxial compression loads. Materials 2020, 13, 854. [Google Scholar] [CrossRef] [Green Version]
- ASTM C1437-20; Standard Test Method for the Flow of Hydraulic Cement Mortar. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- ASTM C191-21; Standard Test Method for Time of Setting of Hydraulic Cement by Vicat Needle. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- ASTM C349-18; Test Method (Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. American Society for Testing and Materials: West Conshohocken, PA, USA, 2018.
- Choe, G.; Kang, S.; Kang, H. Characterization of slag cement mortar containing nonthermally treated dried Red Mud. Appl. Sci. 2019, 9, 2510. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, D.V.; Labrincha, J.A.; Morelli, M.R. Potential use of natural red mud as pozzolan for Portland cement. Mater. Res. 2011, 14, 60–66. [Google Scholar] [CrossRef]
- Niu, M.; Li, G.; Zhang, J.; Cao, L. Preparation of alkali-free liquid accelerator based on aluminum sulfate and its accelerating mechanism on the hydration of cement pastes. Constr. Build. Mater. 2020, 253, 119246. [Google Scholar] [CrossRef]
- Kunther, W.; Lothenbach, B.; Scrivener, K. Influence of bicarbonate ions on the deterioration of mortar bars in sulfate solutions. Cem. Concr. Res. 2013, 44, 77–86. [Google Scholar] [CrossRef]
- Ortega, J.; Cabeza, M.; Tenza-Abril, A.J.; Real-Herraiz, T.; Climent, M.Á.; Sánchez, I. Effects of red mud addition in the microstructure, durability and mechanical performance of cement mortars. Appl. Sci. 2019, 9, 984. [Google Scholar] [CrossRef] [Green Version]
- Senff, L.; Modolo, R.C.E.; Silva, A.S.; Ferreira, V.M.; Hotza, D.; Labrincha, J.A. Influence of red mud addition on rheological behavior and hardened properties of mortars. Constr. Build. Mater. 2014, 65, 84–91. [Google Scholar] [CrossRef]
- Jawad, Z.F.; Ghayyib, R.J.; Salman, A.J. Microstructural Analysis for Cement Mortar with Different Nano Materials. Mater. Sci. Forum 2020, 1002, 615–626. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Liu, S.; Hooton, R.D.; Ji, T.; Kong, Y. Impacts of sulphates on rheological property and hydration performance of cement paste in the function of polycarboxylate superplasticizer. Constr. Build. Mater. 2020, 256, 119428. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Gao, F.; Zhang, G.; Cai, J.; Cheng, X. Resource utilization of red mud from the Solid Waste of aluminum Industry Used in Geothermal Wells. Materials 2022, 15, 8446. [Google Scholar] [CrossRef]
- Van, N.D.; Imasawa, K.; Hama, Y. Influence of hydrothermal synthesis conditions and carbonation on physical properties of xonotlite-based lightweight material. Constr. Build. Mater. 2022, 321, 126328. [Google Scholar] [CrossRef]

| Type | Chemical Composition (wt.%) | Moisture Content Ratio (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | ||
| Red mud sludge | 38.8 | 16.1 | 22.8 | 3.4 | 0.21 | 0.29 | 10.0 | 0.4 | 36 |
| Type | Blaine (cm2/g) | Setting Time | Density (g/cm3) | Chemical Composition (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial (min) | Final (h) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Lg. Loss | |||
| OPC * | 3300 | 200 | 5.5 | 3.15 | 21.7 | 5.7 | 3.2 | 63.1 | 2.8 | 2.2 | 1.3 |
| Type | Color | Specific Gravity | pH | Residue Content (%) | Viscosity (cP) | NaCl (wt.%) | Moisture (wt.%) |
|---|---|---|---|---|---|---|---|
| Polycarboxylic acid series | Light brown | 1.136 | 6.72 | 40.7 | 180 | - | - |
| Methyl cellulose | White | - | - | - | 32,900 | 1.36 | 1.40 |
| Mix ID | Mix Design (g) | ||||
|---|---|---|---|---|---|
| Cement | Sand | Water | LRM | LRM + S | |
| Plain | 100 | 300 | 50 | - | - |
| LM5 * | 95 | 45.27 | 9.72 | - | |
| LM10 | 90 | 40.54 | 19.46 | - | |
| SRM5 ** | 95 | 46.06 | - | 8.94 | |
| SRM10 | 90 | 42.11 | - | 17.89 | |
| Type of Red Mud | Moisture Content (%) | pH | Density (g/cm3) | Viscosity (cP) | Average Particle Size (µm) | Specific Surface Area (m²/kg) |
|---|---|---|---|---|---|---|
| LRM | 48.6 | 11.5 | 1.50 | 36,670 | 2.50 | 2871 |
| LRM + S | 44.1 | 7.6 | 1.54 | 60,670 | 3.02 | 2441 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-P.; Kim, S.-J.; Hong, S.-U.; Lee, B.-K. Properties of Red Mud Neutralized with Sulfuric Acid and Effects on Cement Mortar. Materials 2023, 16, 4730. https://doi.org/10.3390/ma16134730
Kang S-P, Kim S-J, Hong S-U, Lee B-K. Properties of Red Mud Neutralized with Sulfuric Acid and Effects on Cement Mortar. Materials. 2023; 16(13):4730. https://doi.org/10.3390/ma16134730
Chicago/Turabian StyleKang, Suk-Pyo, Sang-Jin Kim, Seong-Uk Hong, and Byoung-Ky Lee. 2023. "Properties of Red Mud Neutralized with Sulfuric Acid and Effects on Cement Mortar" Materials 16, no. 13: 4730. https://doi.org/10.3390/ma16134730
APA StyleKang, S.-P., Kim, S.-J., Hong, S.-U., & Lee, B.-K. (2023). Properties of Red Mud Neutralized with Sulfuric Acid and Effects on Cement Mortar. Materials, 16(13), 4730. https://doi.org/10.3390/ma16134730






