Effects of Bi–Sn–Pb Alloy and Ball-Milling Duration on the Reactivity of Magnesium–Aluminum Waste-Based Materials for Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Properties of the Starting Materials and Ball-Milled Samples
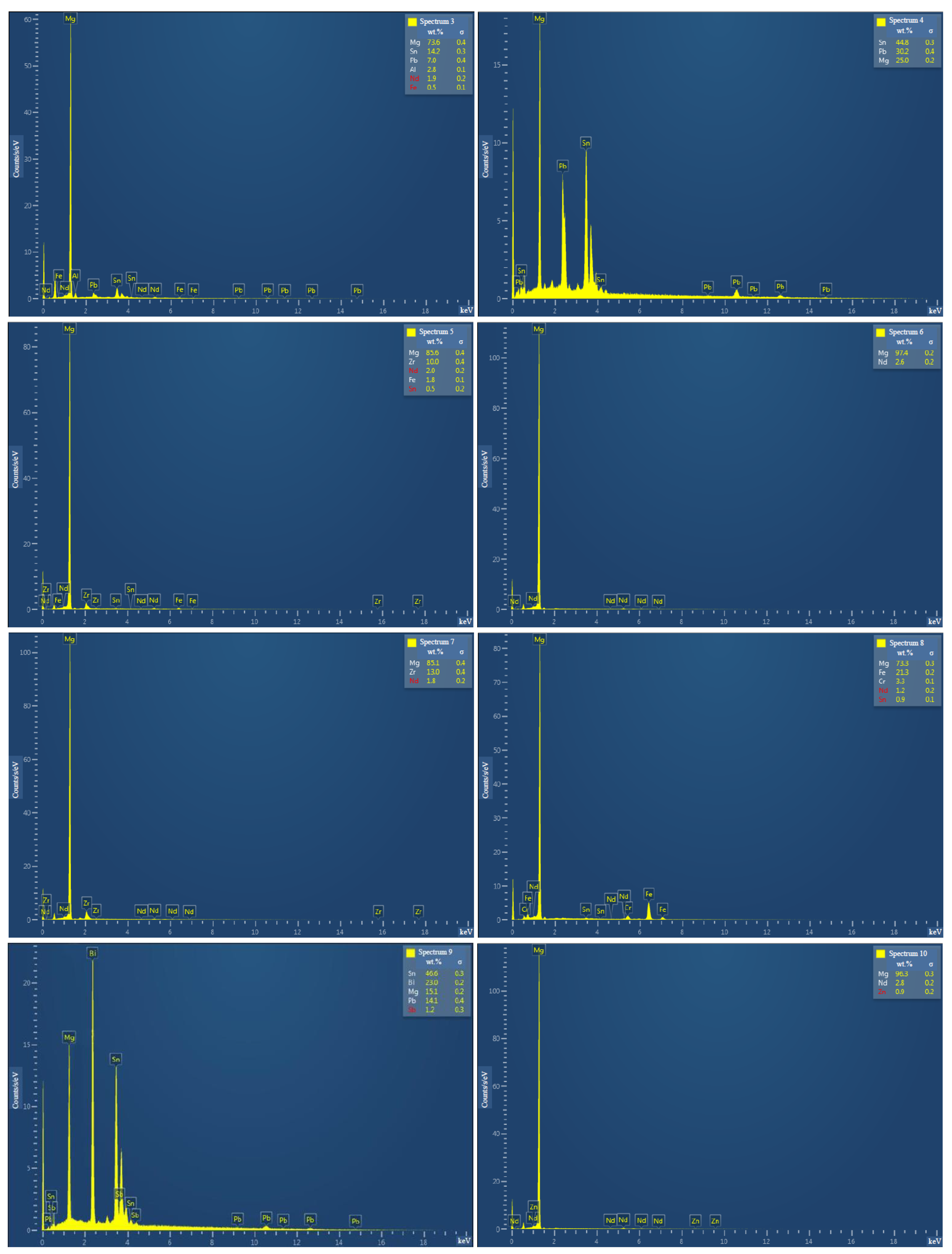
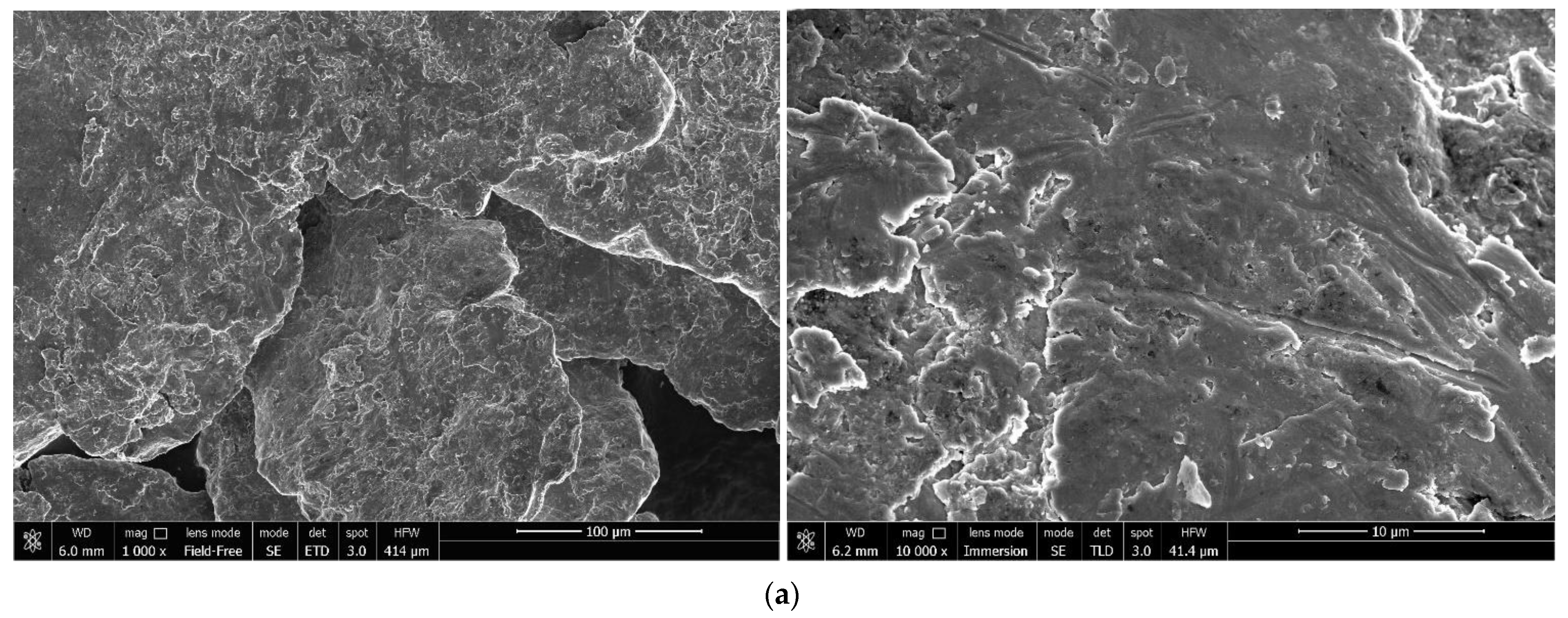
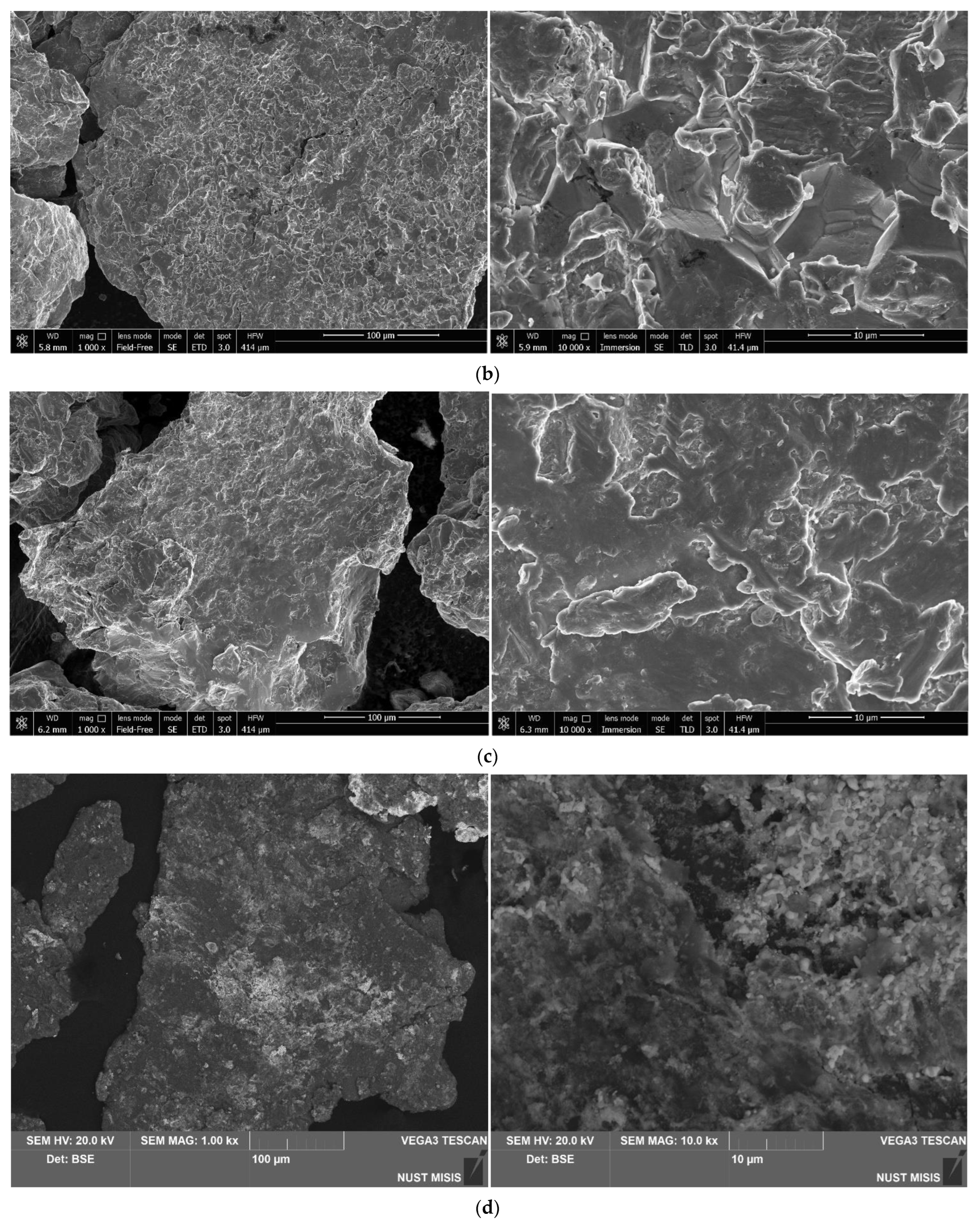
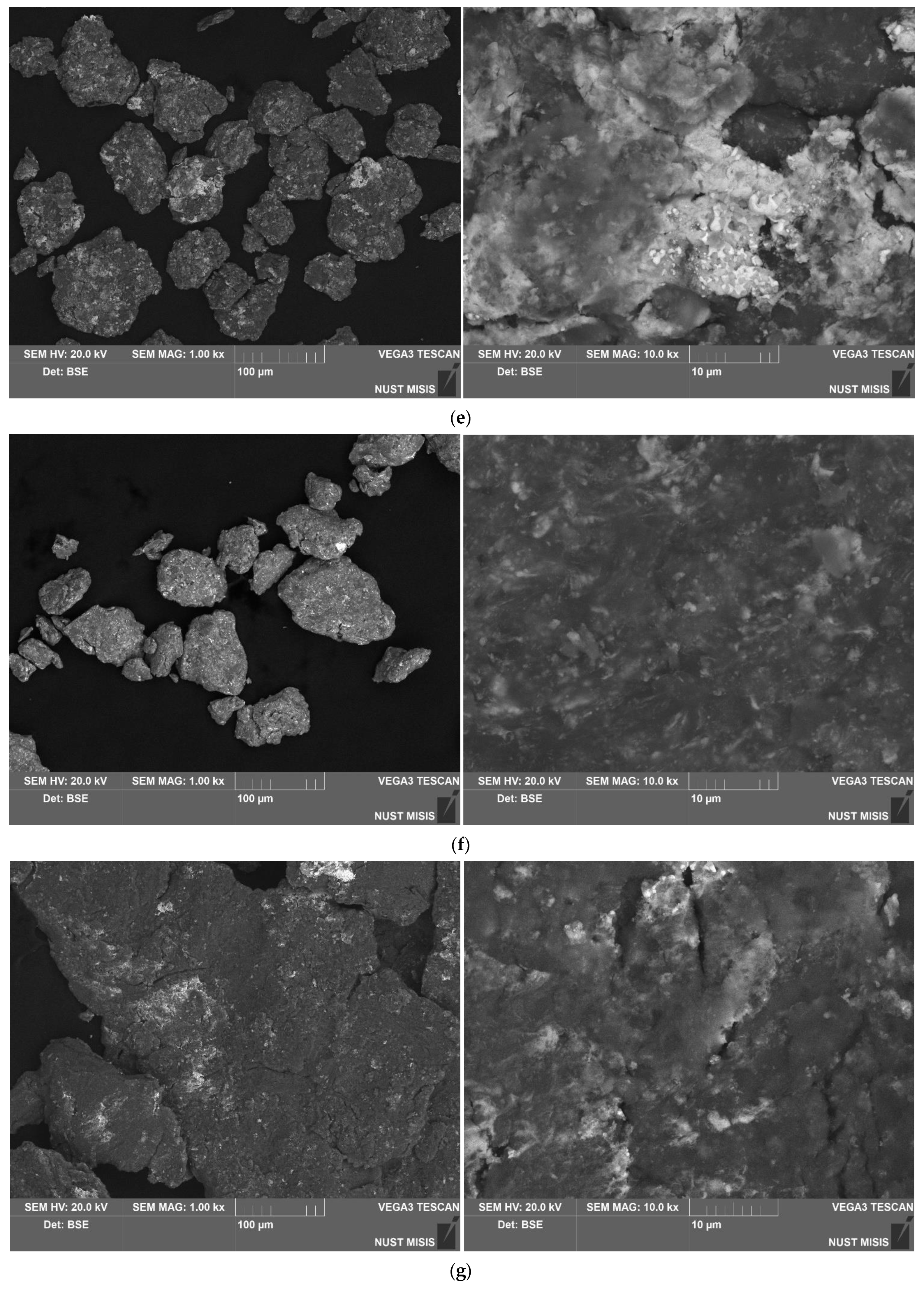
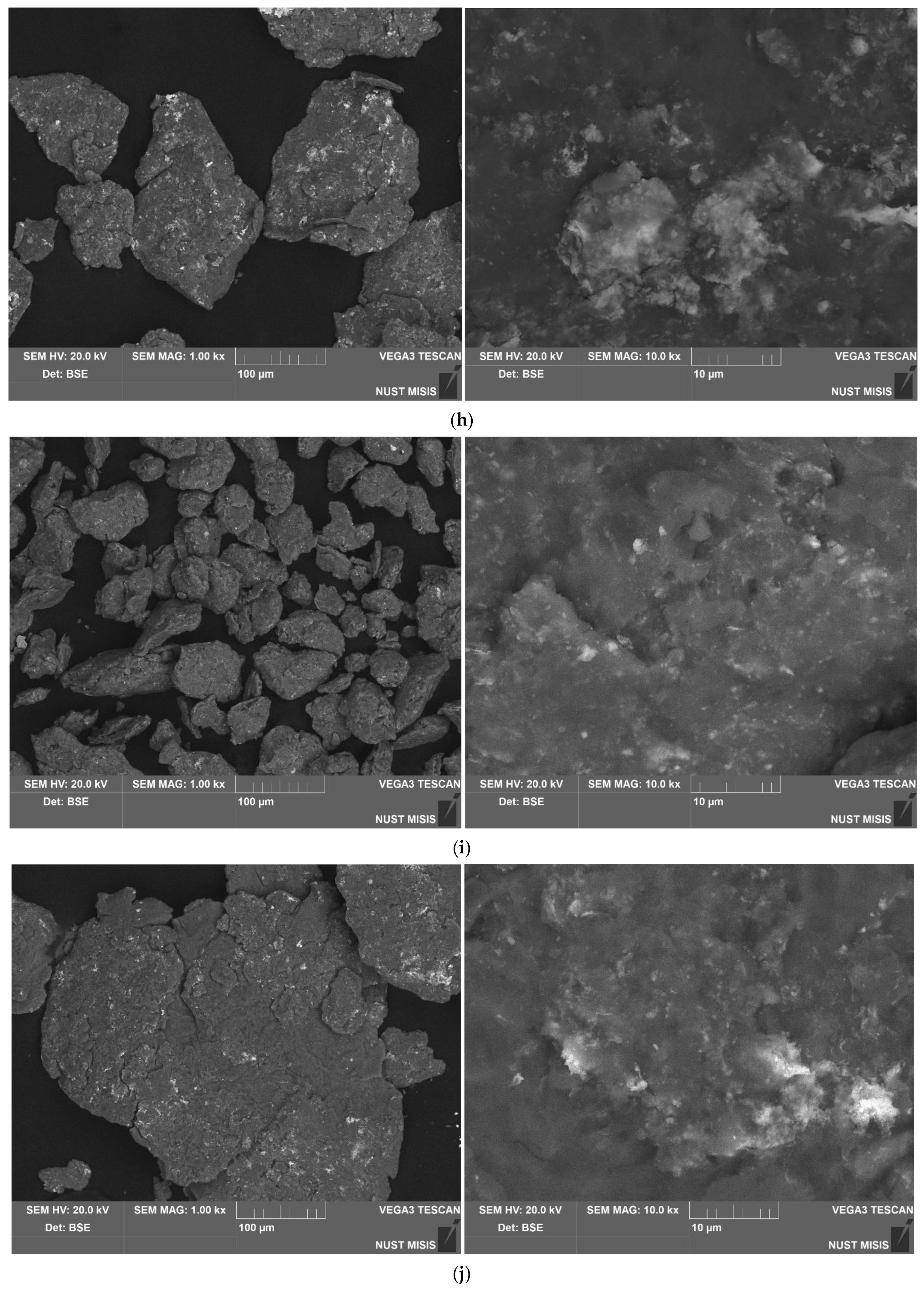
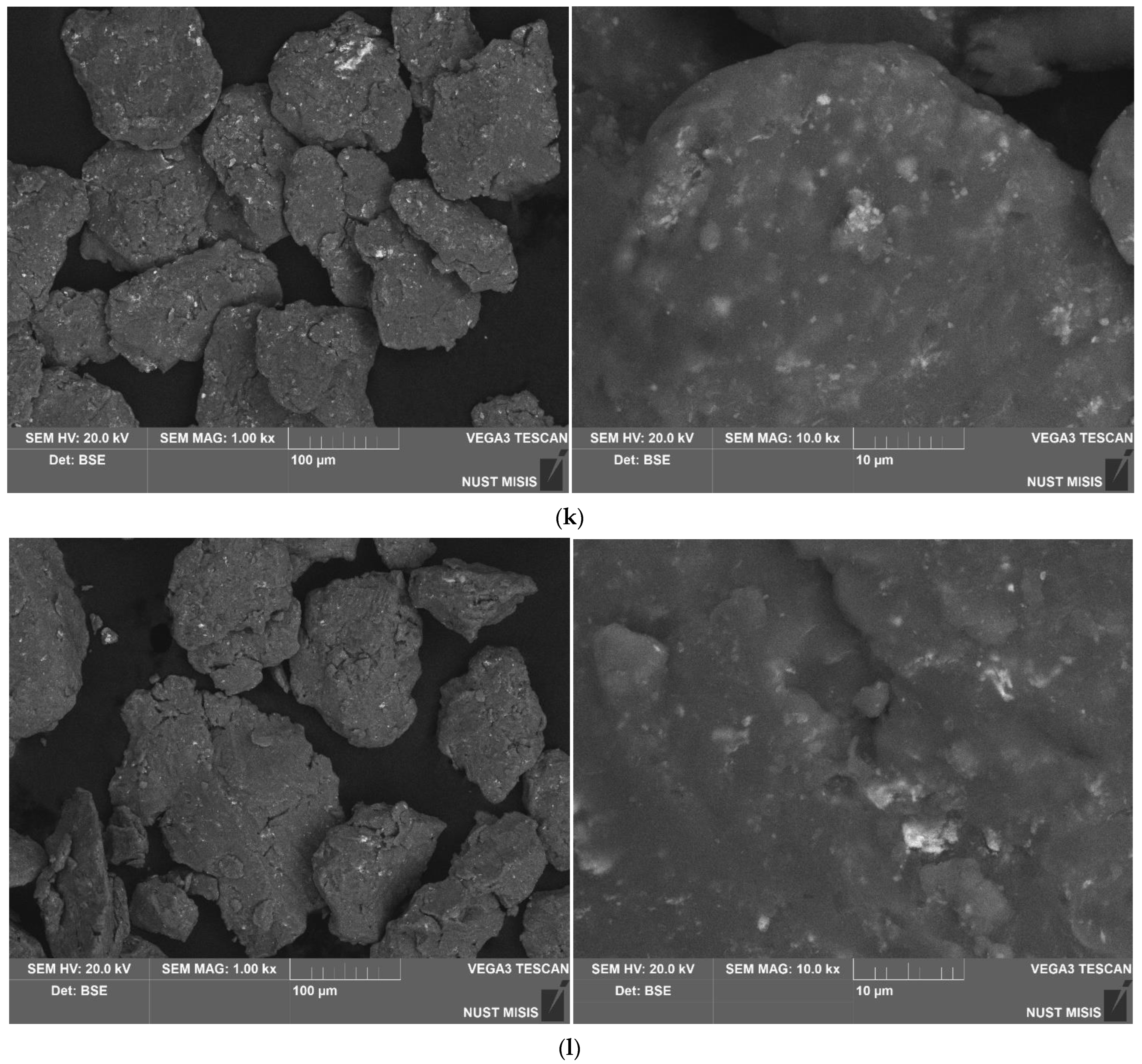
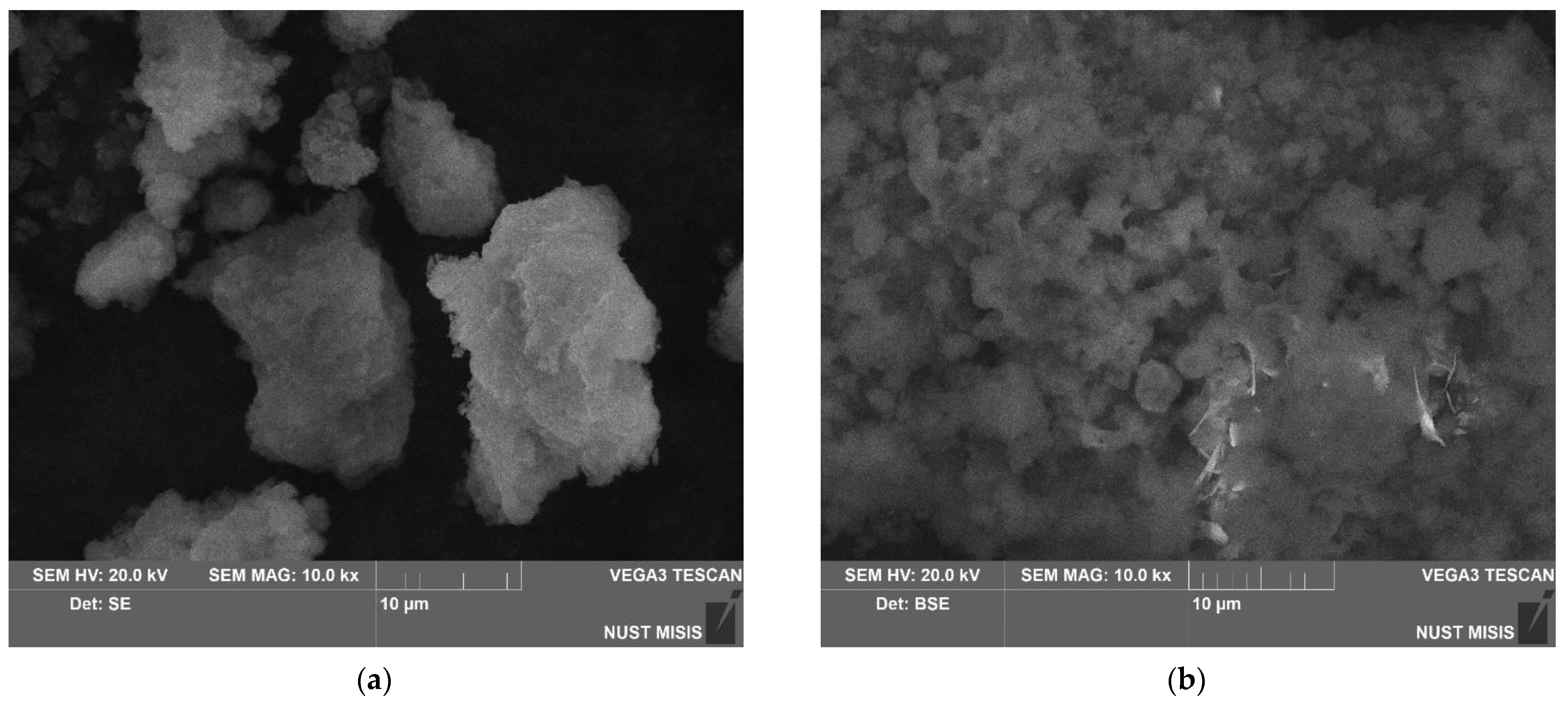
3.1.1. Investigation of Microstructure and Elemental Composition
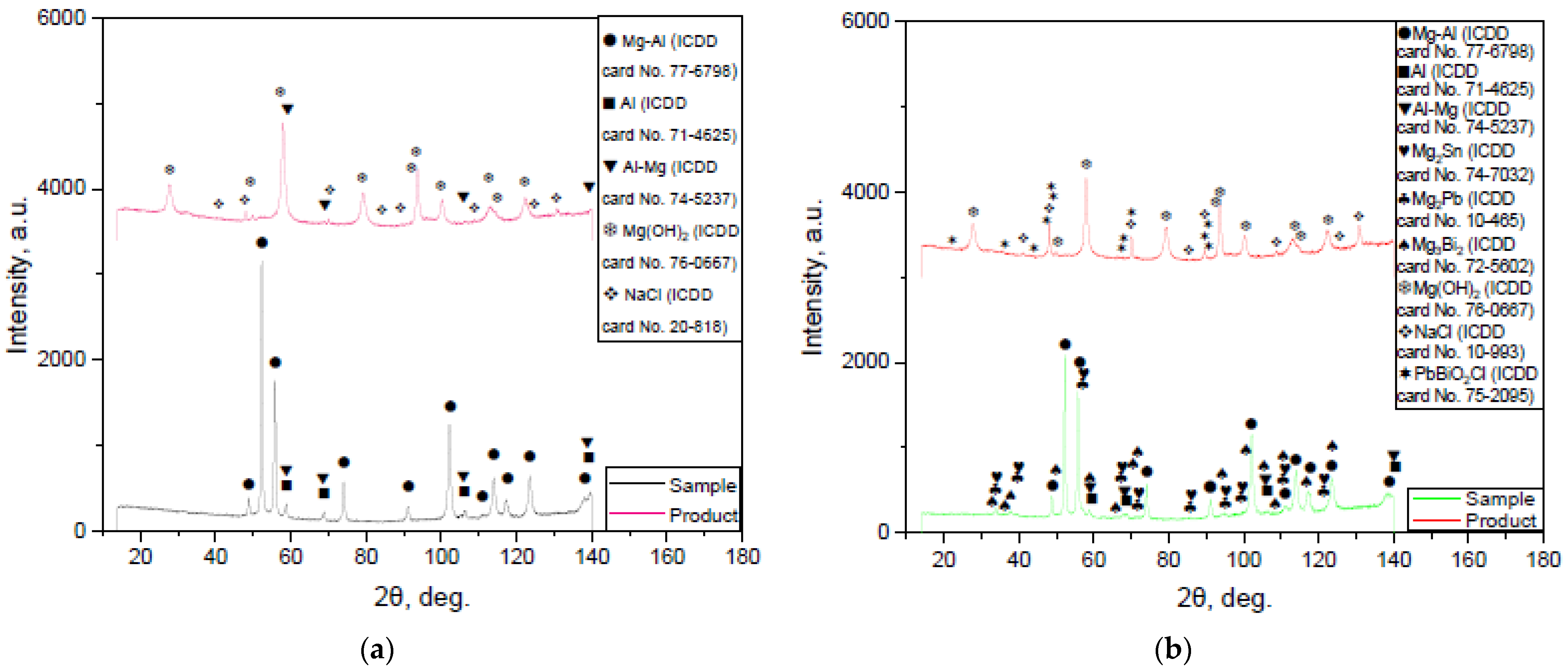
3.1.2. Results of XRD Analysis
3.2. Tests for Hydrogen Generation Performance
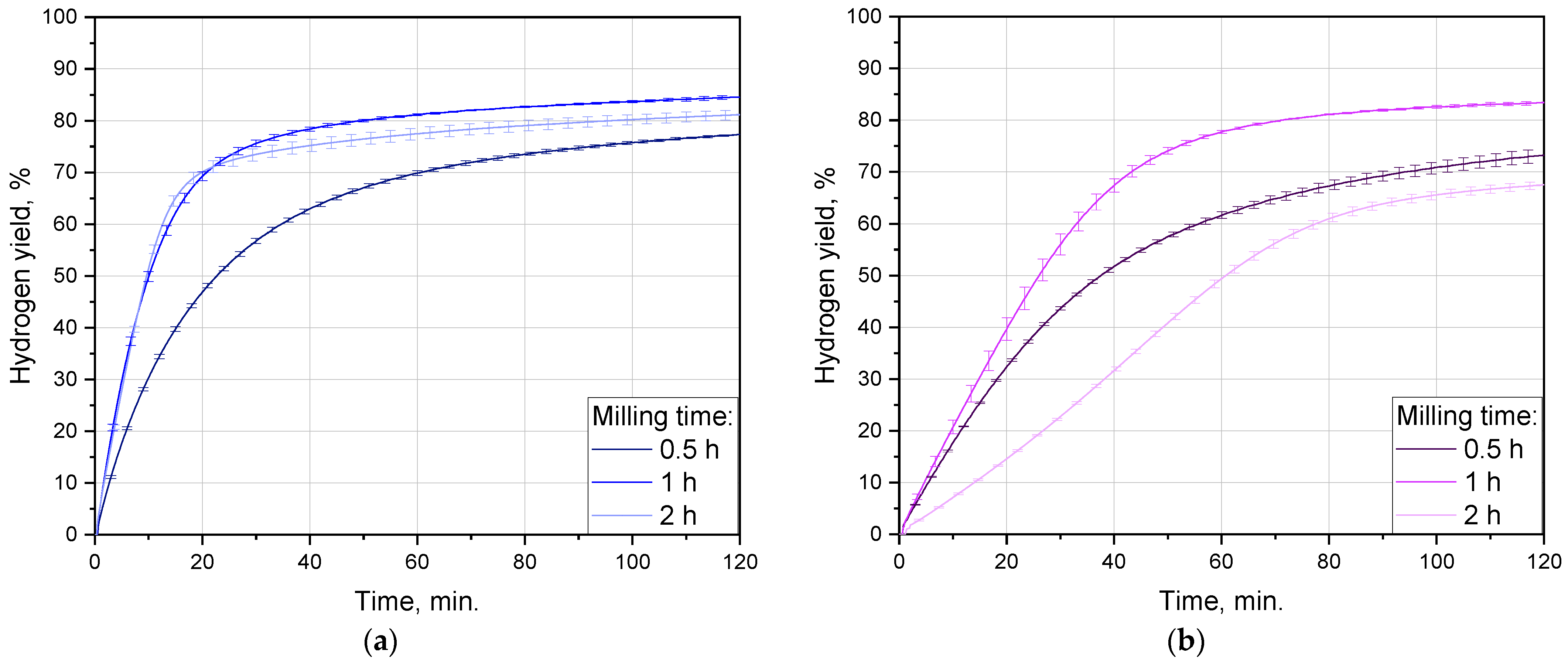
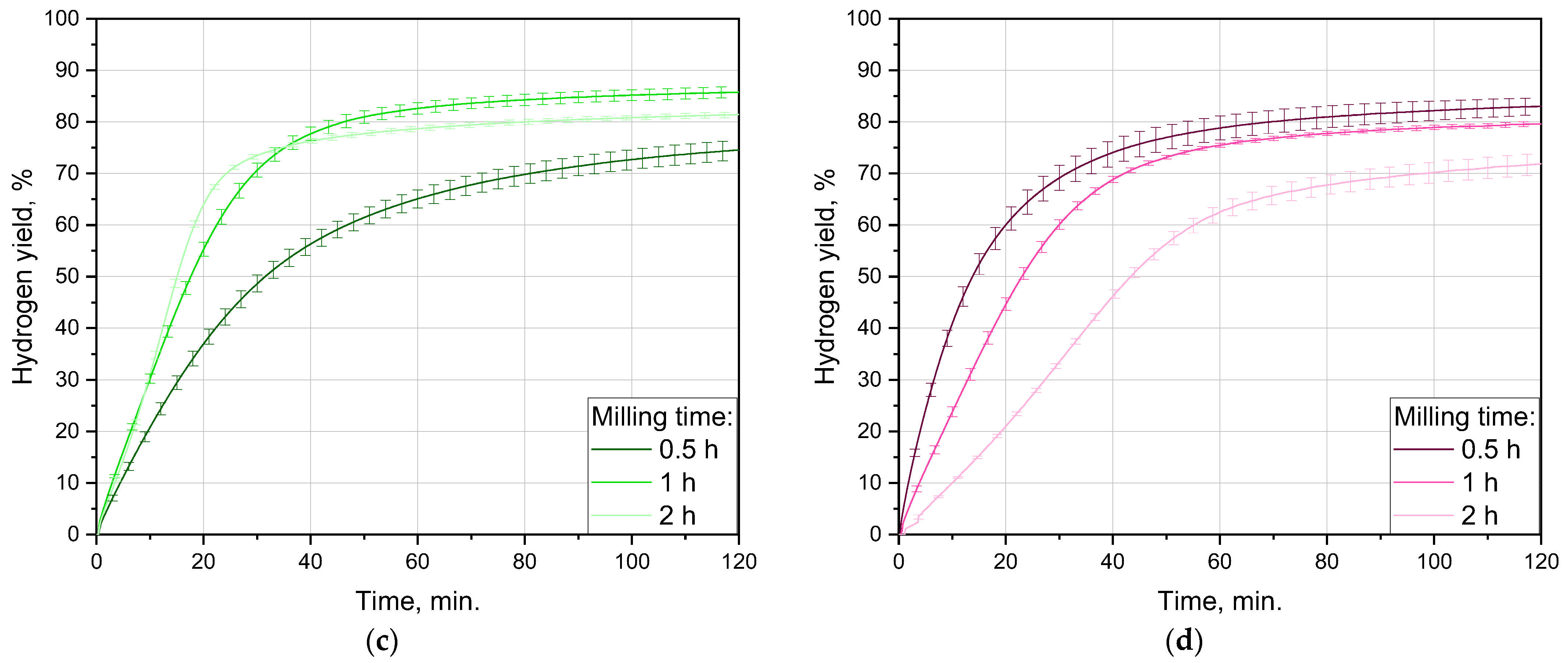
3.2.1. Impact of Ball-Milling Duration
3.2.2. Influence of Additive Content
3.3. Characteristics of the Reaction Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Ritchie, H.; Roser, M.; Rosado, P. CO2 and Greenhouse Gas Emissions. Our World Data 2020. Available online: https://ourworldindata.org/co2-and-greenhouse-gas-emissions (accessed on 27 May 2023).
- Elveny, M.S.; Jalil, A.T.; Davarpanah, A.; Alfakeer, M.; Bahajjaj, A.A.A.; Ouladsmane, M. CFD-based simulation to reduce greenhouse gas emissions from industrial plants. Int. J. Chem. React. Eng. 2021, 19, 1179–1186. [Google Scholar] [CrossRef]
- Samelak, I.; Balaban, M.; Antić, M.; Šolević Knudsen, T.; Jovančićević, B. Geochromatographic migration of oil pollution from a heating plant to river sediments. Environ. Chem. Lett. 2020, 18, 459–466. [Google Scholar] [CrossRef]
- Zhang, B.; Matchinski, E.J.; Chen, B.; Ye, X.; Jing, L.; Lee, K. Chapter 21—Marine Oil Spills—Oil Pollution, Sources and Effects. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 391–406. [Google Scholar]
- Dastan, S.A. The predicaments of the Turkish natural gas market between supply shortages and price hikes. Resour. Policy 2023, 82, 103566. [Google Scholar] [CrossRef]
- Xue, M.-M.; Wu, G.; Wang, Q.; Yao, Y.-F.; Liang, Q.-M. Socioeconomic impacts of a shortage in imported oil supply: Case of China. Nat. Hazards 2019, 99, 1415–1430. [Google Scholar] [CrossRef]
- Panchenko, V.A.; Daus, Y.V.; Kovalev, A.A.; Yudaev, I.V.; Litti, Y.V. Prospects for the production of green hydrogen: Review of countries with high potential. Int. J. Hydrogen Energy 2023, 48, 4551–4571. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Dudoladov, A.O.; Buryakovskaya, O.A.; Ambaryan, G.N. Modelling of aluminum-fuelled power plant with steam-hydrogen enthalpy utilization. Int. J. Hydrogen Energy 2018, 43, 4623–4631. [Google Scholar] [CrossRef]
- Yu, M.; Wang, K.; Vredenburg, H. Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen. Int. J. Hydrogen Energy 2021, 46, 21261–21273. [Google Scholar] [CrossRef]
- Alhuyi Nazari, M.; Fahim Alavi, M.; Salem, M.; Assad, M.E.H. Utilization of hydrogen in gas turbines: A comprehensive review. Int. J. Low-Carbon Technol. 2022, 17, 513–519. [Google Scholar] [CrossRef]
- Ha, C.; Jiao, Y.; Wang, C.; Qin, J.; Wang, S.; Liu, H.; Liu, Z.; Guo, F. Experimental study of hydrogen catalytic combustion wall temperature distribution characteristics and its effect on the coupling performance of autothermal reformers. Energy 2023, 271, 127052. [Google Scholar] [CrossRef]
- Hassan, Q.; Abdulateef, A.M.; Hafedh, S.A.; Al-samari, A.; Abdulateef, J.; Sameen, A.Z.; Salman, H.M.; Al-Jiboory, A.K.; Wieteska, S.; Jaszczur, M. Renewable energy-to-green hydrogen: A review of main resources routes, processes and evaluation. Int. J. Hydrogen Energy 2023, 48, 17383–17408. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, D.; Wang, J.; Tu, W.; Wu, D.; Koh, S.W.; Gao, P.; Xu, Z.J.; Deng, S.; Zhou, Y.; et al. Raw biomass electroreforming coupled to green hydrogen generation. Nat. Commun. 2021, 12, 2008. [Google Scholar] [CrossRef]
- Jovan, D.J.; Dolanc, G. Can Green Hydrogen Production Be Economically Viable under Current Market Conditions. Energies 2020, 13, 6599. [Google Scholar] [CrossRef]
- Armijo, J.; Philibert, C. Flexible production of green hydrogen and ammonia from variable solar and wind energy: Case study of Chile and Argentina. Int. J. Hydrogen Energy 2020, 45, 1541–1558. [Google Scholar] [CrossRef]
- Kakoulaki, G.; Kougias, I.; Taylor, N.; Dolci, F.; Moya, J.; Jäger-Waldau, A. Green hydrogen in Europe—A regional assessment: Substituting existing production with electrolysis powered by renewables. Energy Convers. Manag. 2021, 228, 113649. [Google Scholar] [CrossRef]
- Cho, S.M.; Kim, C.; Kim, K.S.; Kim, D.K. Lightweight hydrogen storage cylinder for fuel cell propulsion systems to be applied in drones. Int. J. Press. Vessel. Pip. 2021, 194, 104428. [Google Scholar] [CrossRef]
- Li, M.; Bai, Y.; Zhang, C.; Song, Y.; Jiang, S.; Grouset, D.; Zhang, M. Review on the research of hydrogen storage system fast refueling in fuel cell vehicle. Int. J. Hydrogen Energy 2019, 44, 10677–10693. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Uratani, J.; Huang, Y.; Xu, L.; Griffiths, S.; Ding, Y. Hydrogen liquefaction and storage: Recent progress and perspectives. Renew. Sustain. Energy Rev. 2023, 176, 113204. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, J.-H.; Kim, Y.-H.; Kim, J.-W.; Lee, K.-J.; Park, S.-J. Recent Progress Using Solid-State Materials for Hydrogen Storage: A Short Review. Processes 2022, 10, 304. [Google Scholar] [CrossRef]
- Tang, D.; Tan, G.-L.; Li, G.-W.; Liang, J.-G.; Ahmad, S.M.; Bahadur, A.; Humayun, M.; Ullah, H.; Khan, A.; Bououdina, M. State-of-the-art hydrogen generation techniques and storage methods: A critical review. J. Energy Storage 2023, 64, 107196. [Google Scholar] [CrossRef]
- Dvoynikov, M.; Buslaev, G.; Kunshin, A.; Sidorov, D.; Kraslawski, A.; Budovskaya, M. New Concepts of Hydrogen Production and Storage in Arctic Region. Resources 2021, 10, 3. [Google Scholar] [CrossRef]
- Zou, Q.; Tian, Y.; Han, F. Prediction of state property during hydrogen leaks from high-pressure hydrogen storage systems. Int. J. Hydrogen Energy 2019, 44, 22394–22404. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, H.; Kang, H.; Zhou, W.; Zhang, C. A literature review of failure prediction and analysis methods for composite high-pressure hydrogen storage tanks. Int. J. Hydrogen Energy 2019, 44, 25777–25799. [Google Scholar] [CrossRef]
- Faye, O.; Szpunar, J.; Eduok, U. A critical review on the current technologies for the generation, storage, and transportation of hydrogen. Int. J. Hydrogen Energy 2022, 47, 13771–13802. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Dewangan, S.K.; Mohan, M.; Kumar, V.; Sharma, A.; Ahn, B. A comprehensive review of the prospects for future hydrogen storage in materials-application and outstanding issues. Int. J. Energy Res. 2022, 46, 16150–16177. [Google Scholar] [CrossRef]
- Usman, M.R. Hydrogen storage methods: Review and current status. Renew. Sustain. Energy Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Uda, M.; Okuyama, H.; Suzuki, T.S.; Sakka, Y. Hydrogen generation from water using Mg nanopowder produced by arc plasma method. Sci. Technol. Adv. Mater. 2012, 13, 025009. [Google Scholar] [CrossRef]
- Ilyin, A.P.; Root, L.O.; Mostovshchikov, A.V. Application of Aluminum Nanopowder for Pure Hydrogen Production. Key Eng. Mater. 2016, 712, 261–266. [Google Scholar] [CrossRef]
- Uehara, K.; Takeshita, H.; Kotaka, H. Hydrogen gas generation in the wet cutting of aluminum and its alloys. J. Mater. Process. Technol. 2002, 127, 174–177. [Google Scholar] [CrossRef]
- Ambaryan, G.N.; Vlaskin, M.S.; Dudoladov, A.O.; Meshkov, E.A.; Zhuk, A.Z.; Shkolnikov, E.I. Hydrogen generation by oxidation of coarse aluminum in low content alkali aqueous solution under intensive mixing. Int. J. Hydrogen Energy 2016, 41, 17216–17224. [Google Scholar] [CrossRef]
- Yavor, Y.; Goroshin, S.; Bergthorson, J.M.; Frost, D.L.; Stowe, R.; Ringuette, S. Enhanced hydrogen generation from aluminum–water reactions. Int. J. Hydrogen Energy 2013, 38, 14992–15002. [Google Scholar] [CrossRef]
- Escobar-Alarcón, L.; Iturbe-García, J.L.; González-Zavala, F.; Solis-Casados, D.A.; Pérez-Hernández, R.; Haro-Poniatowski, E. Hydrogen production by laser irradiation of metals in water under an ultrasonic field: A novel approach. Int. J. Hydrogen Energy 2019, 44, 1579–1585. [Google Scholar] [CrossRef]
- Aduev, B.P.; Kraft, Y.V.; Nurmukhametov, D.R.; Belokurov, G.M.; Nelubina, N.V.; Ismagilov, Z.R. Hydrogen production by oxidation of aluminum nanopowder in water under the action of laser pulses. Int. J. Hydrogen Energy 2023, 48, 22484–22494. [Google Scholar] [CrossRef]
- Trowell, K.; Goroshin, S.; Frost, D.; Bergthorson, J. Hydrogen production rates of aluminum reacting with varying densities of supercritical water. RSC Adv. 2022, 12, 12335–12343. [Google Scholar] [CrossRef]
- Etminanbakhsh, M.; Reza Allahkaram, S. Reaction of aluminum particles with superheated steam to generate hydrogen gas as a readily usable clean fuel. Fuel 2023, 332, 126011. [Google Scholar] [CrossRef]
- Yavor, Y.; Goroshin, S.; Bergthorson, J.M.; Frost, D.L. Comparative reactivity of industrial metal powders with water for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 1026–1036. [Google Scholar] [CrossRef]
- Kravchenko, O.V.; Sevastyanova, L.G.; Genchel, V.K.; Bulychev, B.M. Hydrogen generation from magnesium oxidation by water in presence of halides of transition and non-transition metals. Int. J. Hydrogen Energy 2015, 40, 12072–12077. [Google Scholar] [CrossRef]
- Kantürk Figen, A.; Coşkuner, B.; Pişkin, S. Hydrogen generation from waste Mg based material in various saline solutions (NiCl2, CoCl2, CuCl2, FeCl3, MnCl2). Int. J. Hydrogen Energy 2015, 40, 7483–7489. [Google Scholar] [CrossRef]
- Dudoladov, A.O.; Buryakovskaya, O.A.; Vlaskin, M.S.; Zhuk, A.Z.; Shkolnikov, E.I. Generation of hydrogen by aluminium oxidation in aquaeous solutions at low temperatures. Int. J. Hydrogen Energy 2016, 41, 2230–2237. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Vlaskin, M.S. Hydrogen Recovery from Waste Aluminum–Plastic Composites Treated with Alkaline Solution. Materials 2022, 15, 8699. [Google Scholar] [CrossRef] [PubMed]
- Soberanis, M.A.E.; Vales-Pinzón, C.; Hernández-Núñez, E.; Flota-Bañuelos, M.; Medina, J.; Quintal-Palomo, R.; San-Pedro, L.; Ruiz-Gómez, M. Temperature dependence on hydrogen production from hydrolysis reaction of recycled aluminum. Clean Technol. Environ. Policy 2023, 25, 35–49. [Google Scholar] [CrossRef]
- Alviani, V.N.; Hirano, N.; Watanabe, N.; Oba, M.; Uno, M.; Tsuchiya, N. Local initiative hydrogen production by utilization of aluminum waste materials and natural acidic hot-spring water. Appl. Energy 2021, 293, 116909. [Google Scholar] [CrossRef]
- Saishu, H.; Kosaka, T.; Tsuchiya, N.; Watanabe, N. Empirical studies of the hydrogen generation system by using hot springs water and waste aluminum. J. Geotherm. Res. Soc. Jpn. 2017, 39, 15–24. [Google Scholar]
- Buryakovskaya, O.A.; Suleimanov, M.Z.; Vlaskin, M.S.; Kumar, V.; Ambaryan, G.N. Aluminum Scrap to Hydrogen: Complex Effects of Oxidation Medium, Ball Milling Parameters, and Copper Additive Dispersity. Metals 2023, 13, 185. [Google Scholar] [CrossRef]
- Al Bacha, S.; Thienpont, A.; Zakhour, M.; Nakhl, M.; Bobet, J.L. Clean hydrogen production by the hydrolysis of magnesium-based material: Effect of the hydrolysis solution. J. Clean. Prod. 2021, 282, 124498. [Google Scholar] [CrossRef]
- Kravchenko, O.V.; Sevastyanova, L.G.; Urvanov, S.A.; Bulychev, B.M. Formation of hydrogen from oxidation of Mg, Mg alloys and mixture with Ni, Co, Cu and Fe in aqueous salt solutions. Int. J. Hydrogen Energy 2014, 39, 5522–5527. [Google Scholar] [CrossRef]
- Rodríguez, M.; Urretavizcaya, G.; Bobet, J.-L.; Castro, F.J. Effective hydrogen production by hydrolysis of Mg wastes reprocessed by mechanical milling with iron and graphite. J. Alloys Compd. 2023, 946, 169352. [Google Scholar] [CrossRef]
- Li, S.-L.; Song, J.-M.; Uan, J.-Y. Mg-Mg2X (X = Cu, Sn) eutectic alloy for the Mg2X nano-lamellar compounds to catalyze hydrolysis reaction for H2 generation and the recycling of pure X metals from the reaction wastes. J. Alloys Compd. 2019, 772, 489–498. [Google Scholar] [CrossRef]
- Li, S.-L.; Lin, H.-M.; Uan, J.-Y. Production of an Mg/Mg2Ni lamellar composite for generating H2 and the recycling of the post-H2 generation residue to nickel powder. Int. J. Hydrogen Energy 2013, 38, 13520–13528. [Google Scholar] [CrossRef]
- Davies, J.; du Preez, S.P.; Bessarabov, D.G. On-Demand Hydrogen Generation by the Hydrolysis of Ball-Milled Aluminum–Bismuth–Zinc Composites. Materials 2022, 15, 1197. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Du Preez, S.P.; Bessarabov, D.G. The Hydrolysis of Ball-Milled Aluminum–Bismuth–Nickel Composites for On-Demand Hydrogen Generation. Energies 2022, 15, 2356. [Google Scholar] [CrossRef]
- Kolupula, A.P.; Pant, H.; Varam, S.; Srikanth, V.V.S.S. Novel nanocomposite constituted by Al-Bi alloy and MoS2 for hydrogen generation through hydrolysis. Mater. Lett. 2023, 337, 133946. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, H.; Shi, J.; Wang, H.; Gao, X.; Gao, Q.; Sun, X. Unveiling the role of indium and tin in Al–Ga based alloys for on-demand hydrogen supply from simulation to validation. J. Power Sources 2023, 554, 232268. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X.M.; Chen, D.M.; Yang, K. Insight into the reactivity of Al–Ga–In–Sn alloy with water. Int. J. Hydrogen Energy 2012, 37, 2187–2194. [Google Scholar] [CrossRef]
- Huang, T.; Gao, Q.; Liu, D.; Xu, S.; Guo, C.; Zou, J.; Wei, C. Preparation of Al-Ga-In-Sn-Bi quinary alloy and its hydrogen production via water splitting. Int. J. Hydrogen Energy 2015, 40, 2354–2362. [Google Scholar] [CrossRef]
- Wei, C.; Liu, D.; Xu, S.; Cui, T.; An, Q.; Liu, Z.; Gao, Q. Effects of Cu additives on the hydrogen generation performance of Al-rich alloys. J. Alloys Compd. 2018, 738, 105–110. [Google Scholar] [CrossRef]
- An, Q.; Hu, H.; Li, N.; Liu, D.; Xu, S.; Liu, Z.; Wei, C.; Luo, F.; Xia, M.; Gao, Q. Effects of Bi composition on microstructure and Al-water reactivity of Al-rich alloys with low-In. Int. J. Hydrogen Energy 2018, 43, 10887–10895. [Google Scholar] [CrossRef]
- Su, M.; Wang, H.; Xu, H.; Chen, F.; Hu, H.; Gan, J. Enhanced hydrogen production properties of a novel aluminum-based composite for instant on-site hydrogen supply at low temperature. Int. J. Hydrogen Energy 2022, 47, 9969–9985. [Google Scholar] [CrossRef]
- Ma, M.; Yang, L.; Ouyang, L.; Shao, H.; Zhu, M. Promoting hydrogen generation from the hydrolysis of Mg-Graphite composites by plasma-assisted milling. Energy 2019, 167, 1205–1211. [Google Scholar] [CrossRef]
- Wang, S.; Sun, L.-X.; Xu, F.; Jiao, C.-L.; Zhang, J.; Zhou, H.-Y.; Huang, F.-L. Hydrolysis reaction of ball-milled Mg-metal chlorides composite for hydrogen generation for fuel cells. Int. J. Hydrogen Energy 2012, 37, 6771–6775. [Google Scholar] [CrossRef]
- Czech, E.; Troczynski, T. Hydrogen generation through massive corrosion of deformed aluminum in water. Int. J. Hydrogen Energy 2010, 35, 1029–1037. [Google Scholar] [CrossRef]
- Alinejad, B.; Mahmoodi, K. A novel method for generating hydrogen by hydrolysis of highly activated aluminum nanoparticles in pure water. Int. J. Hydrogen Energy 2009, 34, 7934–7938. [Google Scholar] [CrossRef]
- Huang, M.; Ouyang, L.; Liu, J.; Wang, H.; Shao, H.; Zhu, M. Enhanced hydrogen generation by hydrolysis of Mg doped with flower-like MoS2 for fuel cell applications. J. Power Sources 2017, 365, 273–281. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Kurbatova, A.I.; Vlaskin, M.S.; Valyano, G.E.; Grigorenko, A.V.; Ambaryan, G.N.; Dudoladov, A.O. Waste to Hydrogen: Elaboration of Hydroreactive Materials from Magnesium-Aluminum Scrap. Sustainability 2022, 14, 4496. [Google Scholar] [CrossRef]
- Sun, Q.; Zou, M.; Guo, X.; Yang, R.; Huang, H.; Huang, P.; He, X. A study of hydrogen generation by reaction of an activated Mg–CoCl2 (magnesium–cobalt chloride) composite with pure water for portable applications. Energy 2015, 79, 310–314. [Google Scholar] [CrossRef]
- Huang, M.; Ouyang, L.; Ye, J.; Liu, J.; Yao, X.; Wang, H.; Shao, H.; Zhu, M. Hydrogen generation via hydrolysis of magnesium with seawater using Mo, MoO2, MoO3 and MoS2 as catalysts. J. Mater. Chem. A 2017, 5, 8566–8575. [Google Scholar] [CrossRef]
- Ostovan, F.; Hasanzadeh, E.; Toozandehjani, M.; Shafiei, E.; Jamaludin, K.R.; Amrin, A.B. A combined friction stir processing and ball milling route for fabrication Al5083-Al2O3 nanocomposite. Mater. Res. Express 2019, 6, 065012. [Google Scholar] [CrossRef]
- David, E.; Kopac, J. Hydrolysis of aluminum dross material to achieve zero hazardous waste. J. Hazard. Mater. 2012, 209–210, 501–509. [Google Scholar] [CrossRef]
- Kale, M.; Yılmaz, İ.H.; Kaya, A.; Çetin, A.E.; Söylemez, M.S. Pilot-scale hydrogen generation from the hydrolysis of black aluminum dross without any catalyst. J. Energy Inst. 2022, 100, 99–108. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. Hazardous aluminum dross characterization and recycling strategies: A critical review. J. Environ. Manag. 2018, 223, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Mary Joseph, A.; Snellings, R.; Nielsen, P.; Matthys, S.; De Belie, N. Pre-treatment and utilisation of municipal solid waste incineration bottom ashes towards a circular economy. Constr. Build. Mater. 2020, 260, 120485. [Google Scholar] [CrossRef]
- Moreno-Flores, R.; Loyola-Morales, F.; Valenzuela, E.; Sebastian, P.J. Design and performance evaluation of a prototype hydrogen generator employing hydrolysis of aluminum waste. Clean Technol. Environ. Policy 2023, 25, 747–753. [Google Scholar] [CrossRef]
- Salueña-Berna, X.; Marín-Genescà, M.; Massagués Vidal, L.; Dagà-Monmany, J.M. Waste Aluminum Application as Energy Valorization for Hydrogen Fuel Cells for Mobile Low Power Machines Applications. Materials 2021, 14, 7323. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Chen, S.; Xu, K. A new idea for industrial safety in magnesium grinding: Suppression of hydrogen generation in wet dust collectors. Int. J. Hydrogen Energy 2022, 47, 20333–20346. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, K.; Ge, J.; Liu, B. Study on hydrogen evolution risk and suppression methods of Mg–Al/Mg–Zn alloy waste dust in wet dust collector. Process Saf. Environ. Prot. 2022, 163, 321–329. [Google Scholar] [CrossRef]
- Al Bacha, S.; Pighin, S.A.; Urretavizcaya, G.; Zakhour, M.; Nakhl, M.; Castro, F.J.; Bobet, J.L. Effect of ball milling strategy (milling device for scaling-up) on the hydrolysis performance of Mg alloy waste. Int. J. Hydrogen Energy 2020, 45, 20883–20893. [Google Scholar] [CrossRef]
- Cinkilic, E.; Ridgeway, C.D.; Yan, X.; Luo, A.A. A Formation Map of Iron-Containing Intermetallic Phases in Recycled Cast Aluminum Alloys. Metall. Mater. Trans. A 2019, 50, 5945–5956. [Google Scholar] [CrossRef]
- Capuzzi, S.; Timelli, G. Preparation and Melting of Scrap in Aluminum Recycling: A Review. Metals 2018, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Itoh, S.; Tsujita, K.; Morishige, T.; Takenaka, T. The Effect of Vibration Condition for Magnesium Dry Gravity Sorting by Vibrating Particulate Bed. Mater. Sci. Forum 2019, 941, 1535–1539. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Effect of ball size on steady state of aluminum powder and efficiency of impacts during milling. Powder Technol. 2015, 284, 149–158. [Google Scholar] [CrossRef]
- Shelekhov, E.V.; Tcherdyntsev, V.V.; Pustov, L.Y.; Kaloshkin, S.D.; Tomilin, I.A. Computer Simulation of Mechanoactivation Process in the Planetary Ball Mill: Determination of the Energy Parameters of Milling. J. Metastable Nanocrystalline Mater. 2000, 8, 603–608. [Google Scholar] [CrossRef]
- Sato, A.; Kano, J.; Saito, F. Analysis of abrasion mechanism of grinding media in a planetary mill with DEM simulation. Adv. Powder Technol. 2010, 21, 212–216. [Google Scholar] [CrossRef]
- Vummidi Lakshman, S.; Gibbins, J.D.; Wainwright, E.R.; Weihs, T.P. The effect of chemical composition and milling conditions on composite microstructure and ignition thresholds of AlZr ball milled powders. Powder Technol. 2019, 343, 87–94. [Google Scholar] [CrossRef]
- Salili, S.M.; Ataie, A.; Sadighi, Z. Effect of Ball Size and Ball to Powder Ratio Variation on Crystallite Size and Formation of Nanocrystalline Materials in Planetary Ball Mill. AIP Conf. Proc. 2011, 1400, 127–130. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Effect of structural evolution of aluminum powder during ball milling on hydrogen generation in aluminum–water reaction. Int. J. Hydrogen Energy 2013, 38, 795–806. [Google Scholar] [CrossRef]
- Ostovan, F.; Matori, K.A.; Toozandehjani, M.; Oskoueian, A.; Yusoff, H.M.; Yunus, R.; Ariff, A.H.M. Microstructural evaluation of ball-milled nano Al2O3 particulate-reinforced aluminum matrix composite powders. Int. J. Mater. Res. 2015, 106, 636–640. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Luo, A.A.; Peng, L.; Zhang, P. Fatigue behavior and life prediction of cast magnesium alloys. Mater. Sci. Eng. A 2015, 647, 113–126. [Google Scholar] [CrossRef]
- Viswanadhapalli, B.; Bupesh Raja, V.K. Application of Magnesium Alloys in Automotive Industry-A Review. In Proceedings of the Emerging Trends in Computing and Expert Technology, Chennai, India, 22–23 March 2019; Springer: Cham, Switzerland, 2020; pp. 519–531. [Google Scholar]
- Oslanec, P.; Iždinský, K.; Simančík, F. Possibilities of magnesium recycling. Mater. Sci. Technol. 2008, 4, 83–88. [Google Scholar]
- Gouty, Q.; Castro, F.J.; Urretavizcaya, G.; Sabatier, J.; Bobet, J.L. Experimental and theoretical approach of the hydrolysis of pelleted magnesium alloys scraps. J. Alloys Compd. 2022, 919, 165784. [Google Scholar] [CrossRef]
- Leite, R.; Amorim, M.; Rodrigues, M.; Oliveira Neto, G. Overcoming barriers for adopting cleaner production: A case study in Brazilian small metal-mechanic companies. Sustainability 2019, 11, 4808. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Sun, Y.; He, J.; Guo, Z.; Fang, D. Corrosion behavior of as-cast Pb-Mg-Al alloys in 3.5% NaCl solution. Corrosion 2012, 68, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Gabalcová, Z.; Gogola, P.; Gerhátová, Ž.; Palcut, M. Corrosion Behavior of an Mg2Sn Alloy. Materials 2022, 15, 2025. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-L.; Ma, S.-C.; Bai, Y.; Cui, Z.-Q.; Wang, H.-X. Corrosion behavior of Mg-6Bi-2Sn alloy in the simulated body fluid solution: The influence of microstructural characteristics. J. Alloys Compd. 2018, 731, 945–954. [Google Scholar] [CrossRef]
- Deady, E.; Moon, C.; Moore, K.; Goodenough, K.M.; Shail, R.K. Bismuth: Economic geology and value chains. Ore Geol. Rev. 2022, 143, 104722. [Google Scholar] [CrossRef]
- Periyapperuma, K.; Tran, T.T.; Purcell, M.I.; Obrovac, M.N. The Reversible Magnesiation of Pb. Electrochim. Acta 2015, 165, 162–165. [Google Scholar] [CrossRef]
- Babayigit, A.; Duy Thanh, D.; Ethirajan, A.; Manca, J.; Muller, M.; Boyen, H.-G.; Conings, B. Assessing the toxicity of Pb- and Sn-based perovskite solar cells in model organism Danio rerio. Sci. Rep. 2016, 6, 18721. [Google Scholar] [CrossRef]
- Kim, W.J.; Seo, S.; Lee, S.I.; Kim, D.W.; Kim, M.J. A study on pyro-hydrometallurgical process for selective recovery of Pb, Sn and Sb from lead dross. J. Hazard. Mater. 2021, 417, 126071. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, Y.; Yang, R.; Li, J. Hydrogen generation from hydrolysis of activated magnesium/low-melting-point metals alloys. Int. J. Hydrogen Energy 2019, 44, 1366–1373. [Google Scholar] [CrossRef]
- Pilidi, A.; Tzanis, A.; Helm, T.; Arfanis, M.; Falaras, P.; Speliotis, T. Nanometer-Thick Bismuth Nanocrystal Films for Sensoric Applications. ACS Appl. Nano Mater. 2020, 3, 9669–9678. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, K.N. Low melting point solders based on Sn, Bi, and In elements. Mater. Today Adv. 2020, 8, 100115. [Google Scholar] [CrossRef]
- Radetić, T.; Popović, M.; Alil, A.; Markoli, B.; Naglič, I.; Romhanji, E. Effect of homogenization temperature on microstructure and mechanical properties of Al-Mg-Si alloy containing low-melting point elements. J. Alloys Compd. 2022, 902, 163719. [Google Scholar] [CrossRef]
- GOST 2856-79; Casting Magnesium Alloys. Grades. IPK Standards Publishing House: Moscow, Russia, 1981.
- GOST 4233-77; Reagents. Sodium Chloride. Specifications. IPK Standards Publishing House: Moscow, Russia, 1978.
- GOST 10157-79; Gaseous and Liquid Argon. Specifications. IPK Standards Publishing House: Moscow, Russia, 1980.
- Buryakovskaya, O.A.; Vlaskin, M.S. Microstructural Transformation and Hydrogen Generation Performance of Magnesium Scrap Ball Milled with Devarda’s Alloy. Materials 2022, 15, 8058. [Google Scholar] [CrossRef] [PubMed]
- Bobrynina, E.; Koltsova, T.; Larionova, T. Investigation of Copper–Carbon Composite Microstructure and Properties. Metals 2023, 13, 1052. [Google Scholar] [CrossRef]
- Tang, L.; Peng, X.; Huang, J.; Ma, A.; Deng, Y.; Xu, G. Microstructure and mechanical properties of severely deformed Al-Mg-Sc-Zr alloy and their evolution during annealing. Mater. Sci. Eng. A 2019, 754, 295–308. [Google Scholar] [CrossRef]
- Calka, A.; Wexler, D. A Study of the Evolution of Particle Size and Geometry during Ball Milling. Mater. Sci. Forum 2001, 360–362, 301–310. [Google Scholar] [CrossRef]
- Czerwinski, F. The reactive element effect on high-temperature oxidation of magnesium. Int. Mater. Rev. 2015, 60, 264–296. [Google Scholar] [CrossRef]
- Feng, H.; Liu, S.; Du, Y.; Lei, T.; Zeng, R.; Yuan, T. Effect of the second phases on corrosion behavior of the Mg-Al-Zn alloys. J. Alloys Compd. 2017, 695, 2330–2338. [Google Scholar] [CrossRef]
- Candan, S.; Candan, E. Comparative study on corrosion behaviors of Mg-Al-Zn alloys. Trans. Nonferrous Met. Soc. China 2018, 28, 642–650. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Wei, Q.; Wang, N.; Hou, B. Electrochemical behavior of Mg-Al-Zn-In alloy as anode materials in 3.5wt.% NaCl solution. Electrochim. Acta 2017, 238, 156–167. [Google Scholar] [CrossRef]
- Ren, T.; Yang, S.; Wu, S.; Wang, M.; Xue, Y. High-energy ball milling enhancing the reactivity of microscale zero-valent aluminum toward the activation of persulfate and the degradation of trichloroethylene. Chem. Eng. J. 2019, 374, 100–111. [Google Scholar] [CrossRef]
- Wang, C.; Yang, T.; Liu, Y.; Ruan, J.; Yang, S.; Liu, X. Hydrogen generation by the hydrolysis of magnesium–aluminum–iron material in aqueous solutions. Int. J. Hydrogen Energy 2014, 39, 10843–10852. [Google Scholar] [CrossRef]
- Zhao, W. The Corrosion Behavior and Mechanical Properties of AZ31 Magnesium Alloy. Master’s Thesis, Washington State University, Pullman, WA, USA, 2017. [Google Scholar]
- Ruggiero, M.A.; Rutter, J.W. Formation of microstructure during solidification of Bi–Pb–Sn ternary eutectic. Mater. Sci. Technol. 1999, 15, 1110–1114. [Google Scholar] [CrossRef]
- Gierlotka, W.; Tung, Y.-C. A New Thermodynamic Description of the Bi-Pb-Sn System. J. Phase Equilibria Diffus. 2017, 38, 814–828. [Google Scholar] [CrossRef]
- Kamal, M.; Moharram, B.M.; Farag, H.; El-Bediwi, A.; Abosheiasha, H.F. Structure, attenuation coefficients and physical properties of Bi–Pb–Sn fusible alloys. Radiat. Eff. Defects Solids 2006, 161, 137–142. [Google Scholar] [CrossRef]
- Guo, T.; Lu, X.; Varma, R.K.; Zhao, C.; Wang, J.; You, J.; Chen, J. Influence of Coarse Mg3Bi2 Particles on Deformation Behaviors of Mg-Bi Alloys. Front. Mater. 2021, 8, 633789. [Google Scholar] [CrossRef]
- Wan, D.-Q.; Hu, Y.-L.; Ye, S.-T.; Wang, H.-B.; Wei, S.-M. Effects of Pb on microstructure, mechanical properties and corrosion resistance of as-cast Mg97Zn1Y2 alloys. China Foundry 2018, 15, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Sun, W.; Yang, Y.; Zhan, H.; Yan, K.; Zeng, G. Effects of Mg2Sn precipitation on the age-hardening and deformation behaviour of a Mg-Sn-Al-Zn alloy. Mater. Sci. Eng. A 2023, 867, 144714. [Google Scholar] [CrossRef]
- Michael, E.B.; Brown, E. Handbook of Thermal Analysis and Calorimetry: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Birchal, V.; Rocha, S.; Mansur, M.; Ciminelli, V. A simplified mechanistic analysis of magnesia hydration. Can. J. Chem. Eng. 2001, 79, 507–511. [Google Scholar] [CrossRef]
- Al Bacha, S.; Aubert, I.; Devos, O.; Zakhour, M.; Nakhl, M.; Bobet, J.L. Corrosion of pure and milled Mg17Al12 in “model” seawater solution. Int. J. Hydrogen Energy 2020, 45, 15805–15813. [Google Scholar] [CrossRef]
- Rodríguez, M.; Niro, F.; Urretavizcaya, G.; Bobet, J.-L.; Castro, F.J. Hydrogen production from hydrolysis of magnesium wastes reprocessed by mechanical milling under air. Int. J. Hydrogen Energy 2022, 47, 5074–5084. [Google Scholar] [CrossRef]
- Grosjean, M.H.; Zidoune, M.; Roué, L.; Huot, J.Y. Hydrogen production via hydrolysis reaction from ball-milled Mg-based materials. Int. J. Hydrogen Energy 2006, 31, 109–119. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, R.; Gao, W.; Hu, J.; Li, J. Effect of carbon materials and bismuth particle size on hydrogen generation using aluminum-based composites. J. Alloys Compd. 2020, 817, 152800. [Google Scholar] [CrossRef]
- Zou, M.-S.; Yang, R.-J.; Guo, X.-Y.; Huang, H.-T.; He, J.-Y.; Zhang, P. The preparation of Mg-based hydro-reactive materials and their reactive properties in seawater. Int. J. Hydrogen Energy 2011, 36, 6478–6483. [Google Scholar] [CrossRef]
- Wang, C.-H.; Guo, X.-Y.; Zou, M.-S.; Yang, R.-J. Preparation and hydro-reactivity of ball-milled aluminum-based hydro-reactive metal materials. Acta Armamentarii 2016, 37, 817–822. [Google Scholar] [CrossRef]
- Südholz, A.D.; Birbilis, N.; Bettles, C.J.; Gibson, M.A. Corrosion behaviour of Mg-alloy AZ91E with atypical alloying additions. J. Alloys Compd. 2009, 471, 109–115. [Google Scholar] [CrossRef]
- Çiçek, B.; Sun, Y. A study on the mechanical and corrosion properties of lead added magnesium alloys. Mater. Des. 2012, 37, 369–372. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Feng, Y.; Deng, M.; Wang, N. Effect of Al and Pb Contents on the Corrosion Electrochemical Properties and Activation of Mg-Al-Pb Alloy Anode. J. Electrochem. Soc. 2017, 164, A438. [Google Scholar] [CrossRef]
- Wang, N.-G.; Wang, R.-C.; Peng, C.-Q.; Yan, F.; Zhang, X.-Y. Influence of aluminium and lead on activation of magnesium as anode. Trans. Nonferrous Met. Soc. China 2010, 20, 1403–1411. [Google Scholar] [CrossRef]
- Wang, N.-G.; Wang, R.-C.; Peng, C.-Q.; FENG, Y.; Zhang, X.-Y. Corrosion behavior of Mg-Al-Pb and Mg-Al-Pb-Zn-Mn alloys in 3.5% NaCl solution. Trans. Nonferrous Met. Soc. China 2010, 20, 1936–1943. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.; Peng, C.; Feng, Y.; Chen, B. Effect of hot rolling and subsequent annealing on electrochemical discharge behavior of AP65 magnesium alloy as anode for seawater activated battery. Corros. Sci. 2012, 64, 17–27. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.; Peng, C.; Peng, B.; Feng, Y.; Hu, C. Discharge behaviour of Mg-Al-Pb and Mg-Al-Pb-In alloys as anodes for Mg-air battery. Electrochim. Acta 2014, 149, 193–205. [Google Scholar] [CrossRef]
- Li, B.; Duan, Y.; Zheng, S.; Li, M.; Peng, M.; Qi, H. Microstructure evolution and corrosion properties of ECAPed Mg–Pb-9.2Al-0.8B alloys. J. Mater. Res. Technol. 2023, 24, 6048–6064. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.P.; Guo, Y.C.; Yang, Z.; Xia, F. Effect of Tin on Microstructure and Electrochemistrical Properties of Mg-Al-Sn-Zn Magnesium Alloys Anodic Materials. Adv. Mater. Res. 2011, 197–198, 1129–1134. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.; Guo, Y.; Yang, Z.; Xia, F.; Wang, J. Effect of tin addition on microstructure and electrochemical properties of rolled AZ61-Sn magnesium anodic materials. Rare Met. 2011, 30, 639. [Google Scholar] [CrossRef]
- Ping, W.; Jianping, L.; Yongchun, G.; Zhong, Y.; Feng, X.; Jianli, W. Effect of Sn on microstructure and electrochemical properties of Mg alloy anode materials. Rare Met. Mater. Eng. 2012, 41, 2095–2099. [Google Scholar] [CrossRef]
- Song, G.-L. Effect of tin modification on corrosion of AM70 magnesium alloy. Corros. Sci. 2009, 51, 2063–2070. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Huang, S.; Zhou, X. Study of the corrosion behavior and the corrosion films formed on the surfaces of Mg–xSn alloys in 3.5wt.% NaCl solution. Appl. Surf. Sci. 2014, 317, 1143–1150. [Google Scholar] [CrossRef]
- Jihua, C.; Zhenhua, C.; Hongge, Y.; Fuquan, Z.; Yingliang, C. Effects of Sn and Ca additions on microstructure, mechanical properties, and corrosion resistance of the as-cast Mg-Zn-Al-based alloy. Mater. Corros. 2008, 59, 934–941. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kang, J.-Y.; Yang, J.; Yim, C.D.; You, B.S. Role of Sn in corrosion and passive behavior of extruded Mg-5 wt% Sn alloy. Corros. Sci. 2016, 102, 355–362. [Google Scholar] [CrossRef]
- Tong, F.; Chen, X.; Wei, S.; Malmström, J.; Vella, J.; Gao, W. Microstructure and battery performance of Mg-Zn-Sn alloys as anodes for magnesium-air battery. J. Magnes. Alloy. 2021, 9, 1967–1976. [Google Scholar] [CrossRef]
- Cain, T.W.; Glover, C.F.; Scully, J.R. The corrosion of solid solution Mg-Sn binary alloys in NaCl solutions. Electrochim. Acta 2019, 297, 564–575. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kang, J.-Y.; Kim, S.G.; Kim, B.; Park, S.S.; Yim, C.D.; You, B.S. Influences of metallurgical factors on the corrosion behaviour of extruded binary Mg–Sn alloys. Corros. Sci. 2014, 82, 369–379. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Qi, W.-C.; Cui, H.-Z.; Zhang, F.; Li, S.-Q.; Han, E.-H. In vitro corrosion of as-extruded Mg–Ca alloys—The influence of Ca concentration. Corros. Sci. 2015, 96, 23–31. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Mohedano, M.; Mendis, C.L.; del Olmo, R.; Matykina, E.; Hort, N.; Merino, M.C.; Pardo, A. Corrosion of Mg-9Al alloy with minor alloying elements (Mn, Nd, Ca, Y and Sn). Mater. Des. 2017, 130, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Aung, N.N.; Sun, Y. Effect of antimony, bismuth and calcium addition on corrosion and electrochemical behaviour of AZ91 magnesium alloy. Corros. Sci. 2009, 51, 403–408. [Google Scholar] [CrossRef]
- Tok, H.Y.; Hamzah, E.; Bakhsheshi-Rad, H.R. The role of bismuth on the microstructure and corrosion behavior of ternary Mg–1.2Ca–xBi alloys for biomedical applications. J. Alloys Compd. 2015, 640, 335–346. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Jiang, B.; Yu, W.; Kang, S.; Cheng, W.; Park, S.; Chen, D.; Yin, F.; Shin, K.; et al. A Comprehensive Study of Dynamic Recrystallization Behavior of Mg Alloy with 3 wt.% Bi Addition. Metals 2021, 11, 838. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Cheng, W.-L.; Gu, X.-J.; Yu, H.; Wang, H.-X.; Niu, X.-F.; Wang, L.-F.; Li, H. Discharge performance of extruded Mg-Bi binary alloys as anodes for primary Mg-air batteries. J. Alloys Compd. 2021, 886, 161271. [Google Scholar] [CrossRef]
- Wu, C.; Meng, X.; Wang, W. High-Performance Mg–Al–Bi Alloy Anode for Seawater Batteries and Related Mechanisms. Processes 2020, 8, 1362. [Google Scholar] [CrossRef]
- Xu, W.-T.; Cheng, W.; Guo, Z.-Y.; Yu, H.; Wang, L.-F.; Li, H.; Wang, H.-X.; Wang, J.-H.; Meng, S.-J. Development of Dilute Mg-Bi Alloy Sheet with Good Synergy of Corrosion-Resistance and Tensile Properties. SSRN 2023. [Google Scholar] [CrossRef]
- Go, J.; Lee, J.U.; Yu, H.; Park, S.H. Influence of Bi addition on dynamic recrystallization and precipitation behaviors during hot extrusion of pure Mg. J. Mater. Sci. Technol. 2020, 44, 62–75. [Google Scholar] [CrossRef]
- Yu, H.; Fan, S.; Meng, S.; Choi, J.O.; Li, Z.; Go, Y.; Kim, Y.M.; Zhao, W.; You, B.S.; Shin, K.S. Microstructural evolution and mechanical properties of binary Mg–xBi (x = 2, 5, and 8 wt%) alloys. J. Magnes. Alloy. 2021, 9, 983–994. [Google Scholar] [CrossRef]
- Liu, R.L.; Scully, J.R.; Williams, G.; Birbilis, N. Reducing the corrosion rate of magnesium via microalloying additions of group 14 and 15 elements. Electrochim. Acta 2018, 260, 184–195. [Google Scholar] [CrossRef] [Green Version]
- Nayeb-Hashemi, A.A.; Clark, J.B. The Mg–Pb (Magnesium–Lead) system. Bull. Alloy Phase Diagr. 1985, 6, 56–66. [Google Scholar] [CrossRef]
- Elsayed, F.R.; Sasaki, T.T.; Mendis, C.L.; Ohkubo, T.; Hono, K. Compositional optimization of Mg–Sn–Al alloys for higher age hardening response. Mater. Sci. Eng. A 2013, 566, 22–29. [Google Scholar] [CrossRef]
- Guangyin, Y.; Yangshan, S.; Wenjiang, D. Effects of bismuth and antimony additions on the microstructure and mechanical properties of AZ91 magnesium alloy. Mater. Sci. Eng. A 2001, 308, 38–44. [Google Scholar] [CrossRef]


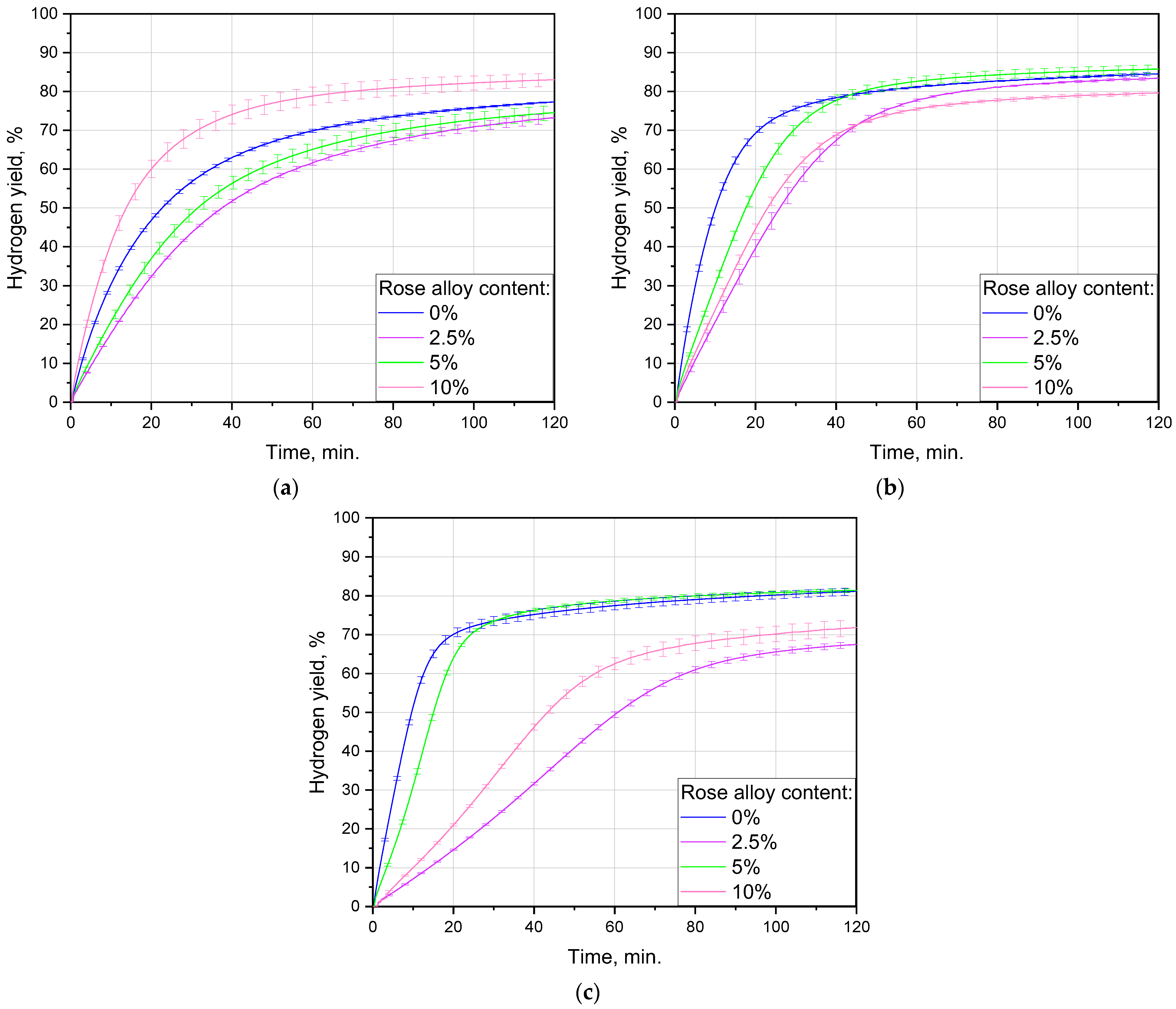
| Milling Speed, rpm | Balls Diameter, mm | Balls-to-Powder Mass ratio | Powder Portion Mass, g | Milling Time, h | Rose Alloy Content, wt.% | Experiment Temperature, °C | Aqueous Solution | Sample Mass, g |
|---|---|---|---|---|---|---|---|---|
| 580 | 10 | 24:1 | 4 | 0.5; 1; 2 | 0; 2.5; 5; 10 | 25 | 3.5 wt.% NaCl | 0.75 |
| Composition | Ball-Milling Time, h | Hydrogen Yield, % | Maximum Reaction Rate, mL/g/min. |
|---|---|---|---|
| Scrap without additives | 0.5 | 77.3 ± 0.2 | 56 |
| 1 | 84.6 ± 0.4 | 86 | |
| 2 | 81.2 ± 0.9 | 77 | |
| Scrap + 2.5 wt.% Rose alloy | 0.5 | 73.2 ± 1.3 | 31 |
| 1 | 83.4 ± 0.3 | 35 | |
| 2 | 67.5 ± 0.7 | 19 | |
| Scrap + 5 wt.% Rose alloy | 0.5 | 74.5 ± 1.9 | 35 |
| 1 | 85.8 ± 1.1 | 49 | |
| 2 | 81.4 ± 0.5 | 43 | |
| Scrap + 10 wt.% Rose alloy | 0.5 | 83.0 ± 1.6 | 66 |
| 1 | 79.6 ± 0.5 | 39 | |
| 2 | 71.9 ± 2.1 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buryakovskaya, O.A.; Ambaryan, G.N.; Tarasenko, A.B.; Suleimanov, M.Z.; Vlaskin, M.S. Effects of Bi–Sn–Pb Alloy and Ball-Milling Duration on the Reactivity of Magnesium–Aluminum Waste-Based Materials for Hydrogen Production. Materials 2023, 16, 4745. https://doi.org/10.3390/ma16134745
Buryakovskaya OA, Ambaryan GN, Tarasenko AB, Suleimanov MZ, Vlaskin MS. Effects of Bi–Sn–Pb Alloy and Ball-Milling Duration on the Reactivity of Magnesium–Aluminum Waste-Based Materials for Hydrogen Production. Materials. 2023; 16(13):4745. https://doi.org/10.3390/ma16134745
Chicago/Turabian StyleBuryakovskaya, Olesya A., Grayr N. Ambaryan, Alexey B. Tarasenko, Musi Zh. Suleimanov, and Mikhail S. Vlaskin. 2023. "Effects of Bi–Sn–Pb Alloy and Ball-Milling Duration on the Reactivity of Magnesium–Aluminum Waste-Based Materials for Hydrogen Production" Materials 16, no. 13: 4745. https://doi.org/10.3390/ma16134745






