Studying on Alloying Elements, Phases, Microstructure and Texture in FH36 Ship Plate Steel
Abstract
:1. Introduction
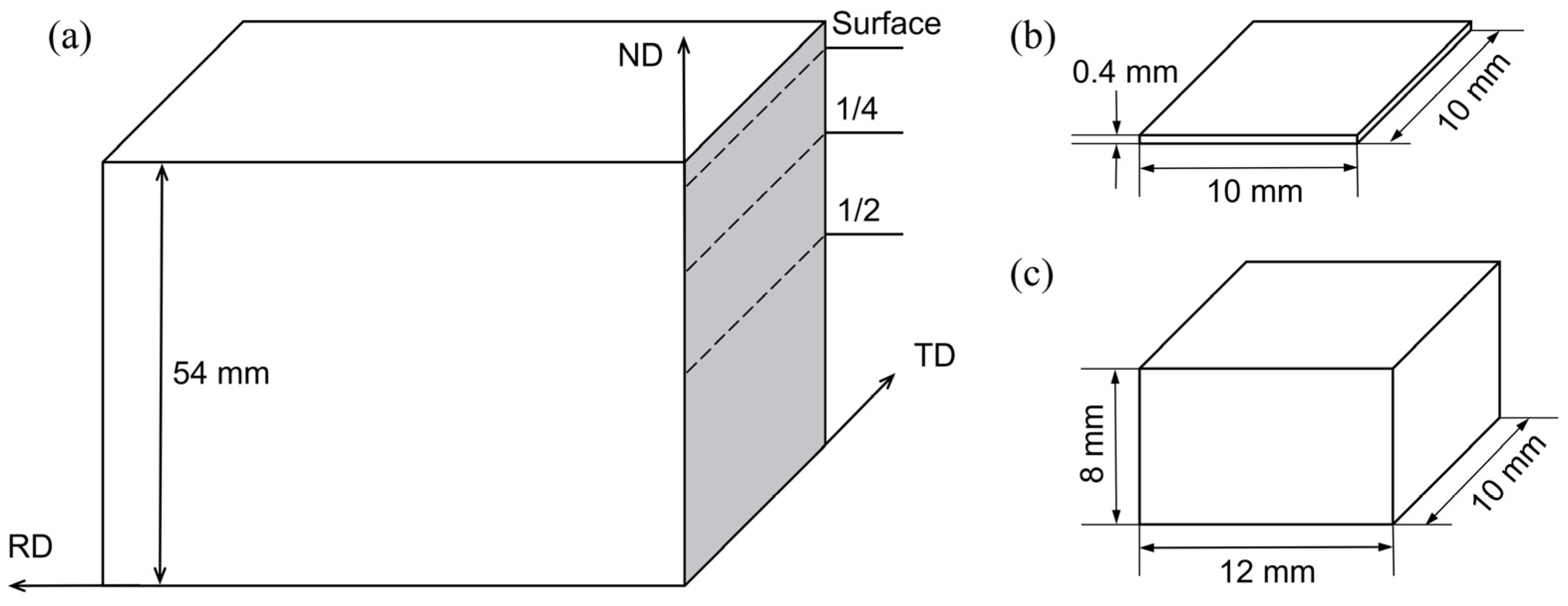
2. Material and Methods
2.1. Material and Process
2.2. Methods
3. Results and Discussion
3.1. Variation of Microstructure, Phases and Element Content by Temperature
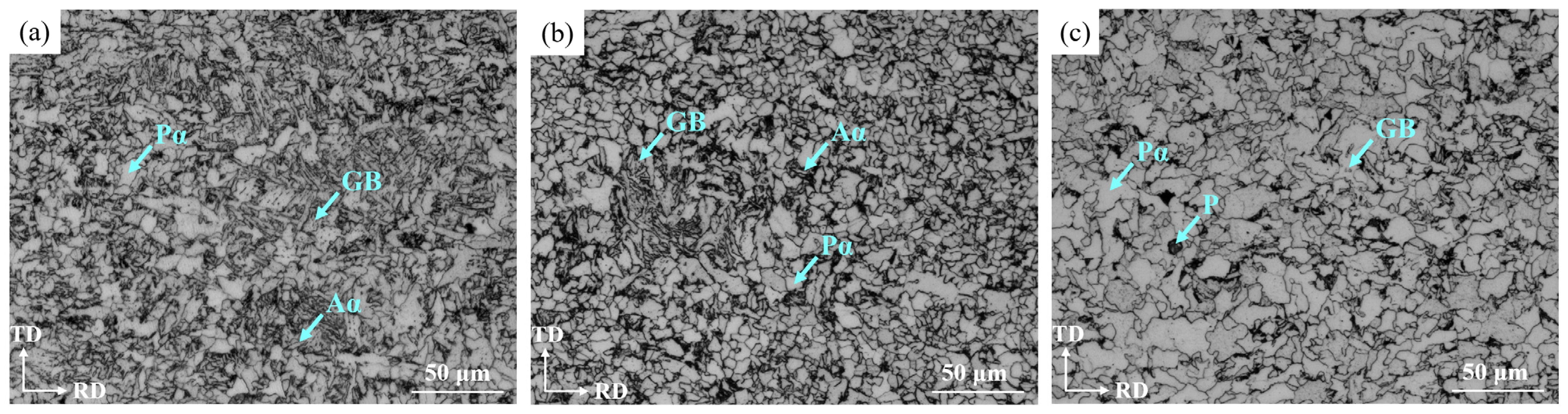
3.1.1. Microstructure Variation
3.1.2. Phases Content Variation
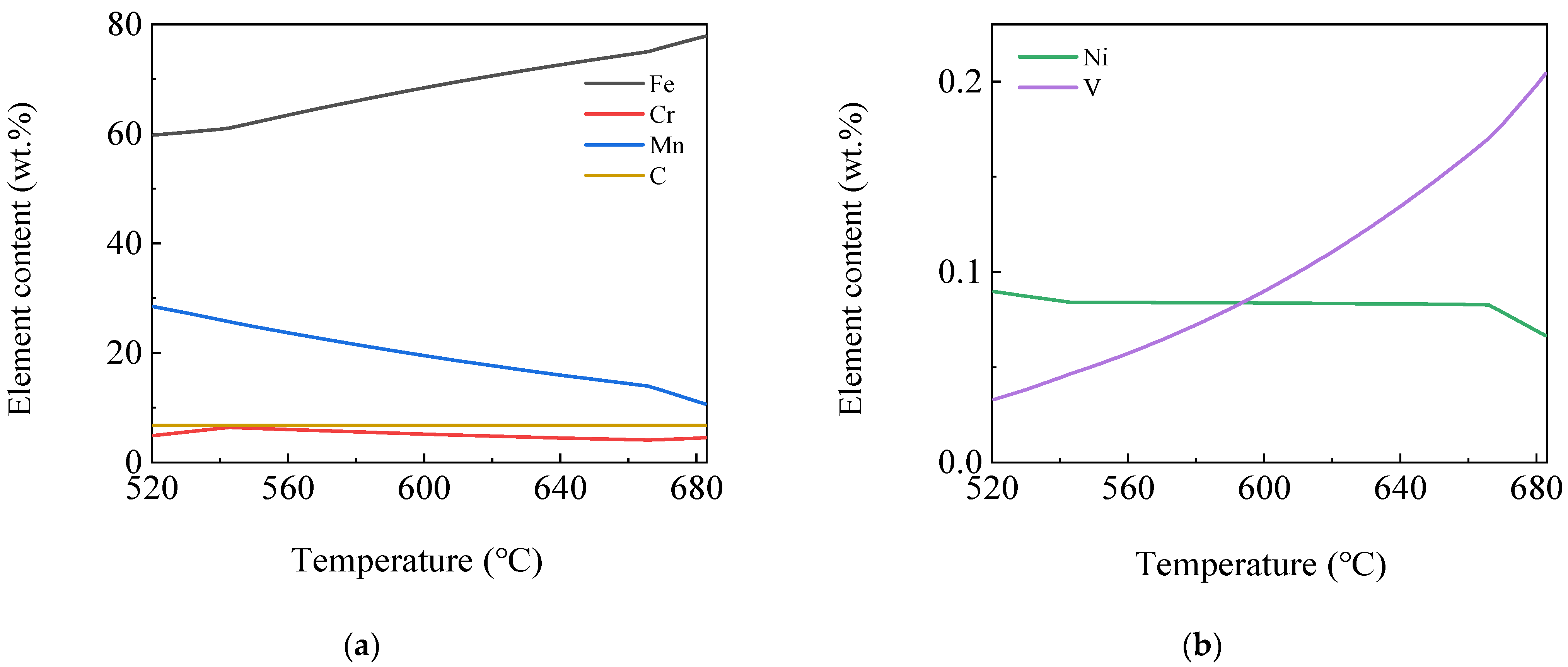
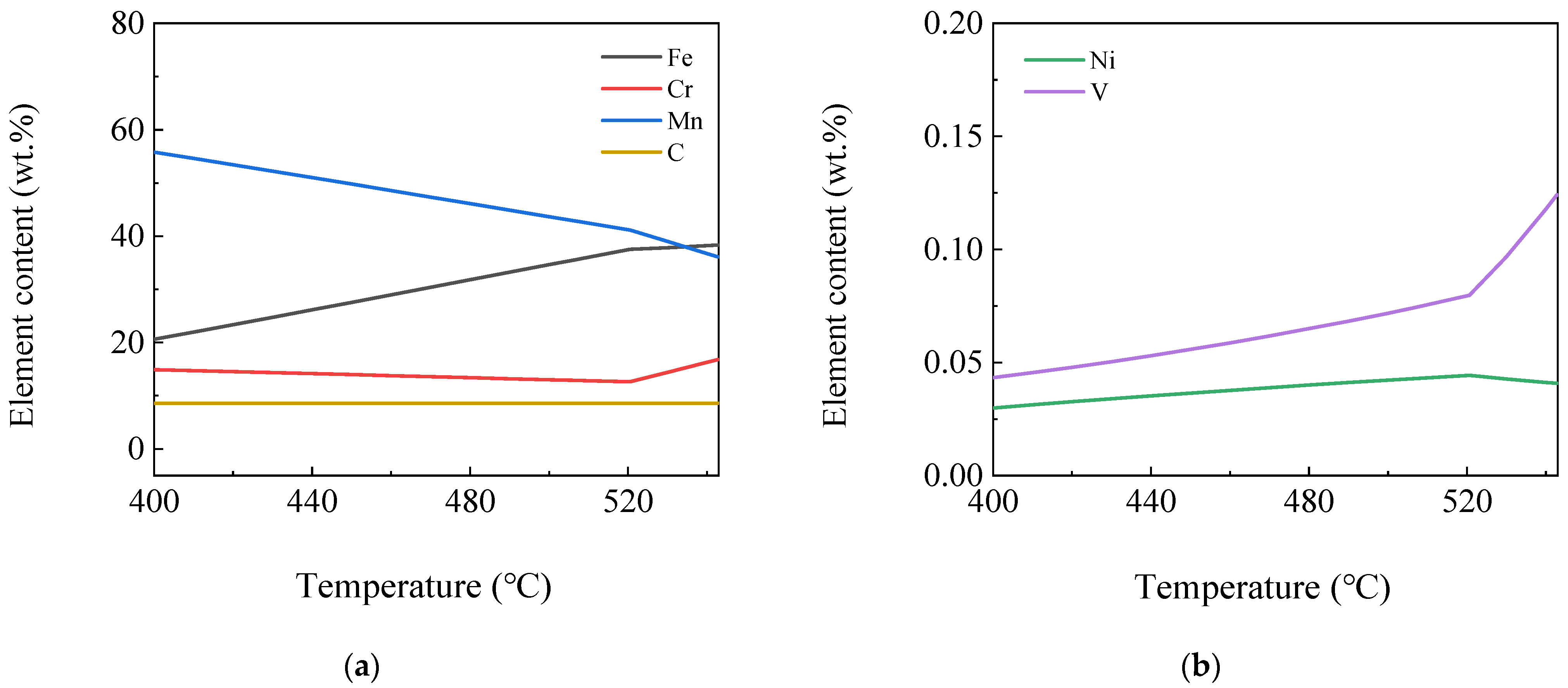
3.1.3. Element Content Variation in the Same Phase
3.1.4. Element Content Variation in Different Phases
3.2. Observation of Microstructure and Phases and Element Analysis
3.2.1. Observation of Microstructure
3.2.2. Observation of Precipitated Phases
3.2.3. Analysis of Element Content in Microstructure
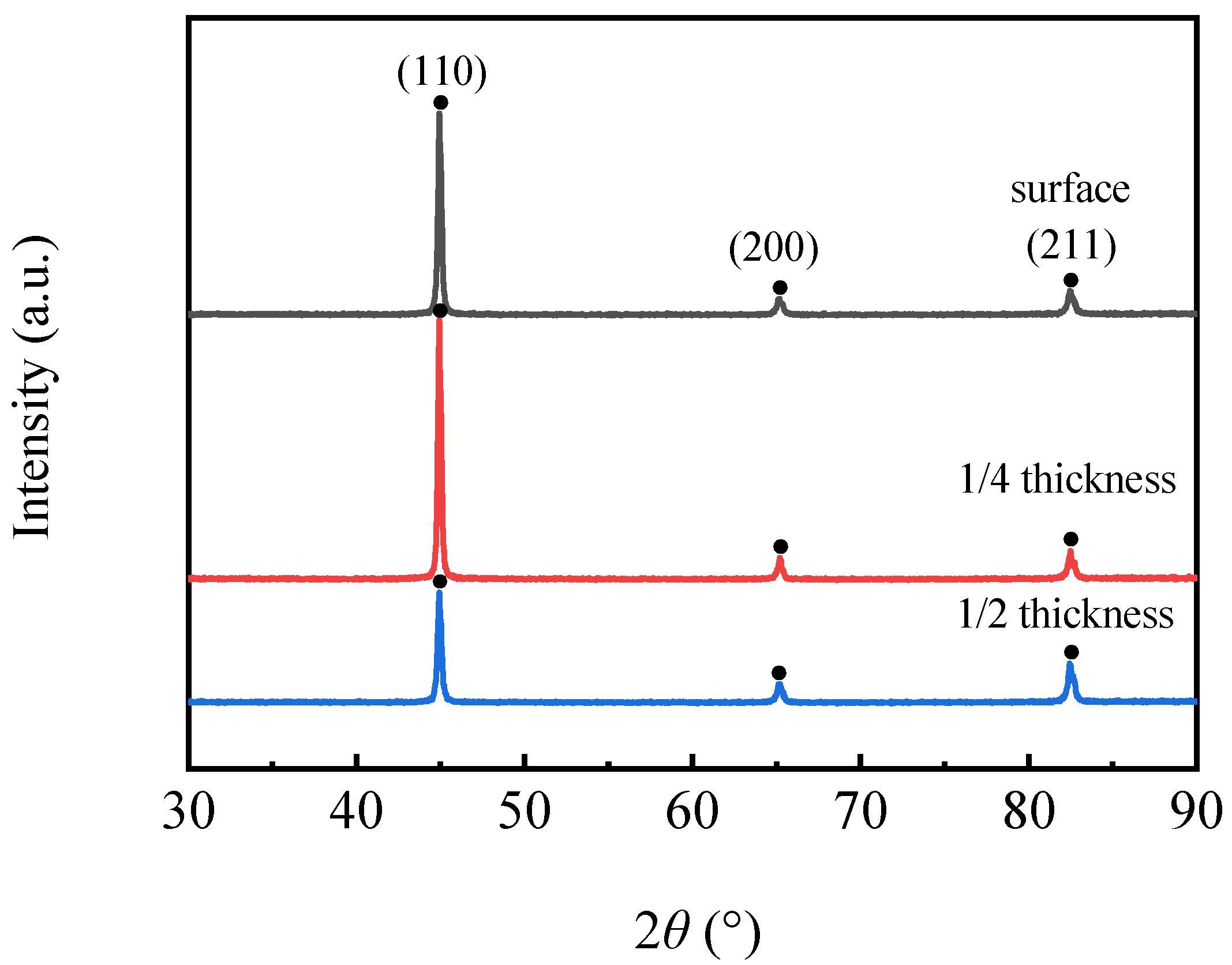
3.3. Orientation and Macro-Texture Analysis
3.3.1. Orientation Analysis
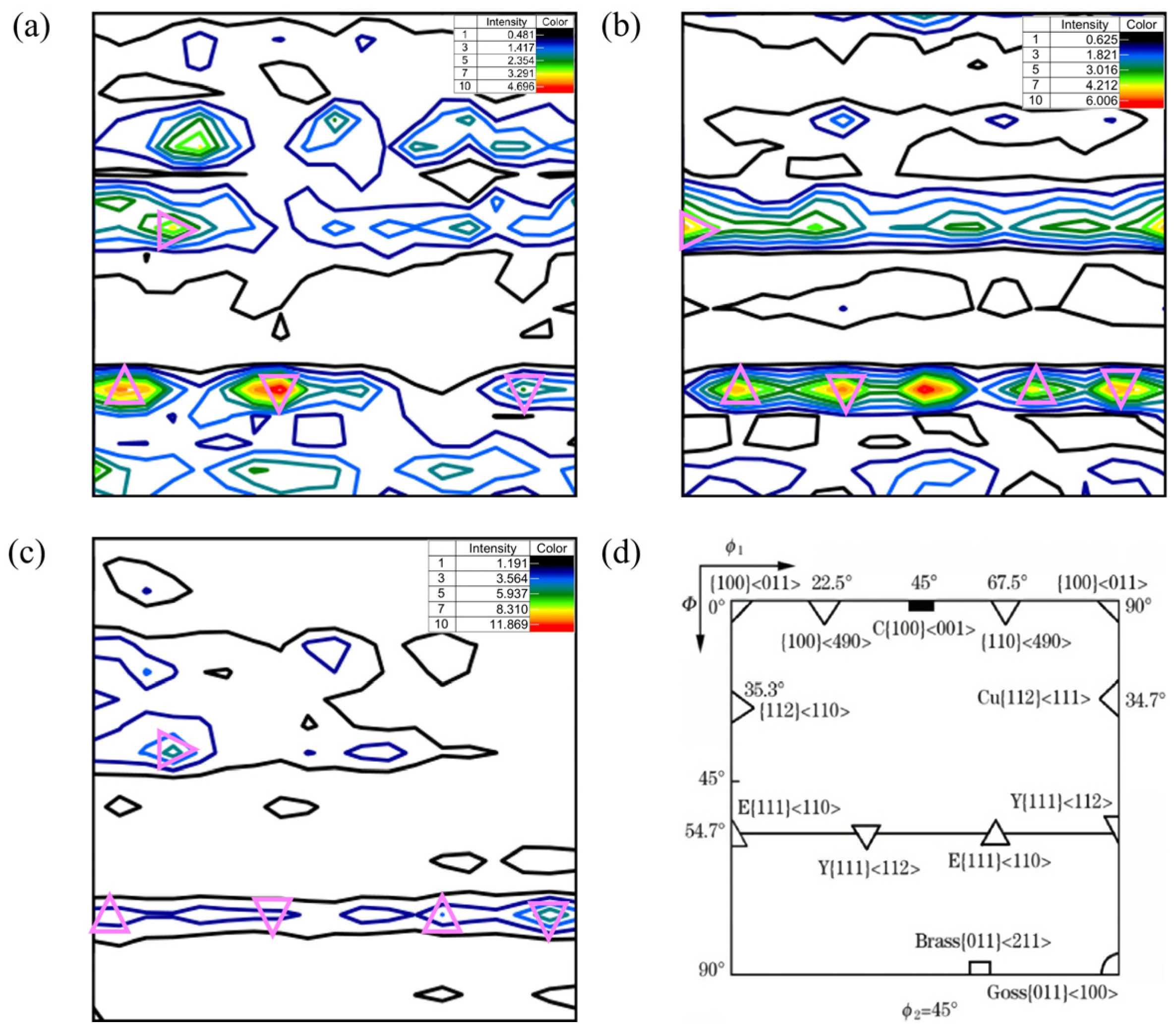
3.3.2. ODF Analysis
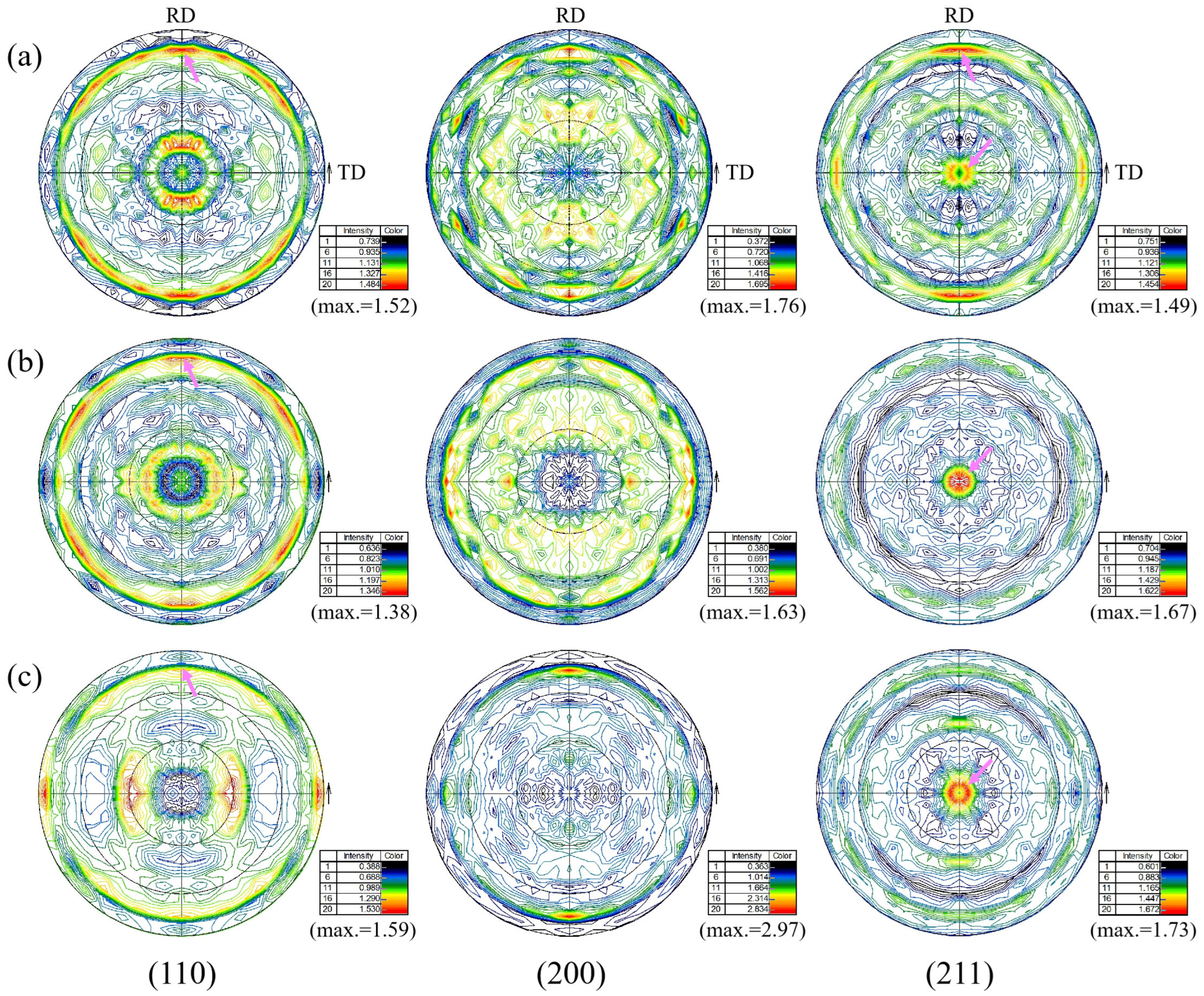
3.3.3. Pole Figure Analysis
3.4. Grain Boundary, Microstructure and Subgrain Analysis
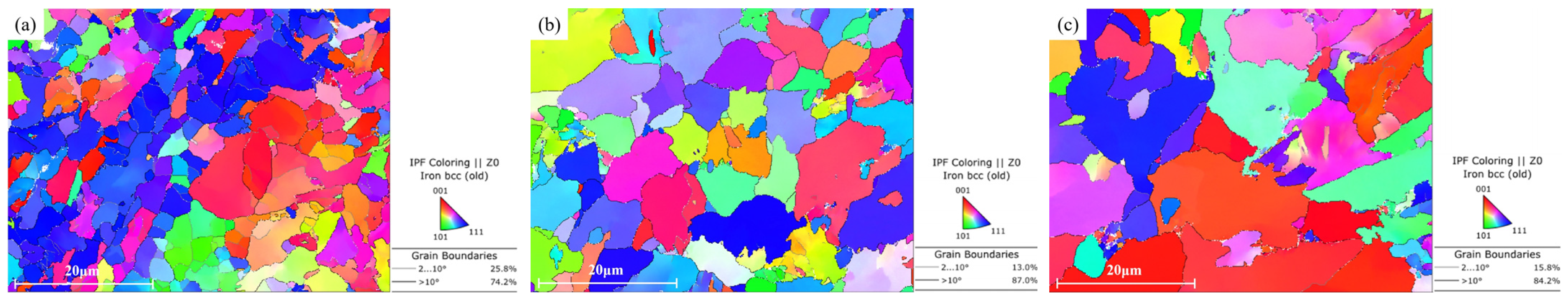
3.4.1. Grain Boundary Analysis
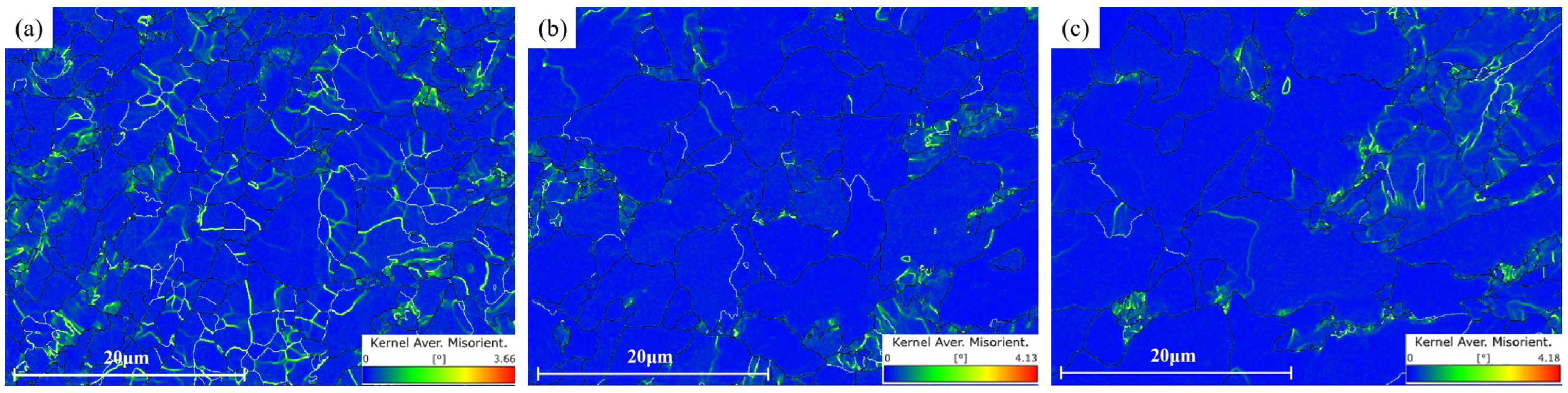
3.4.2. Subgrain Analysis
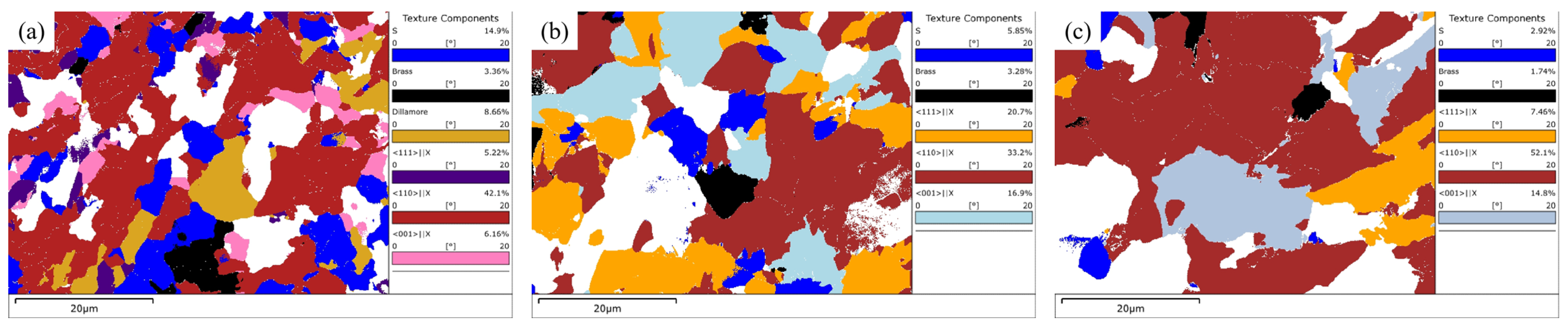
3.4.3. Texture Components Analysis
4. Conclusions
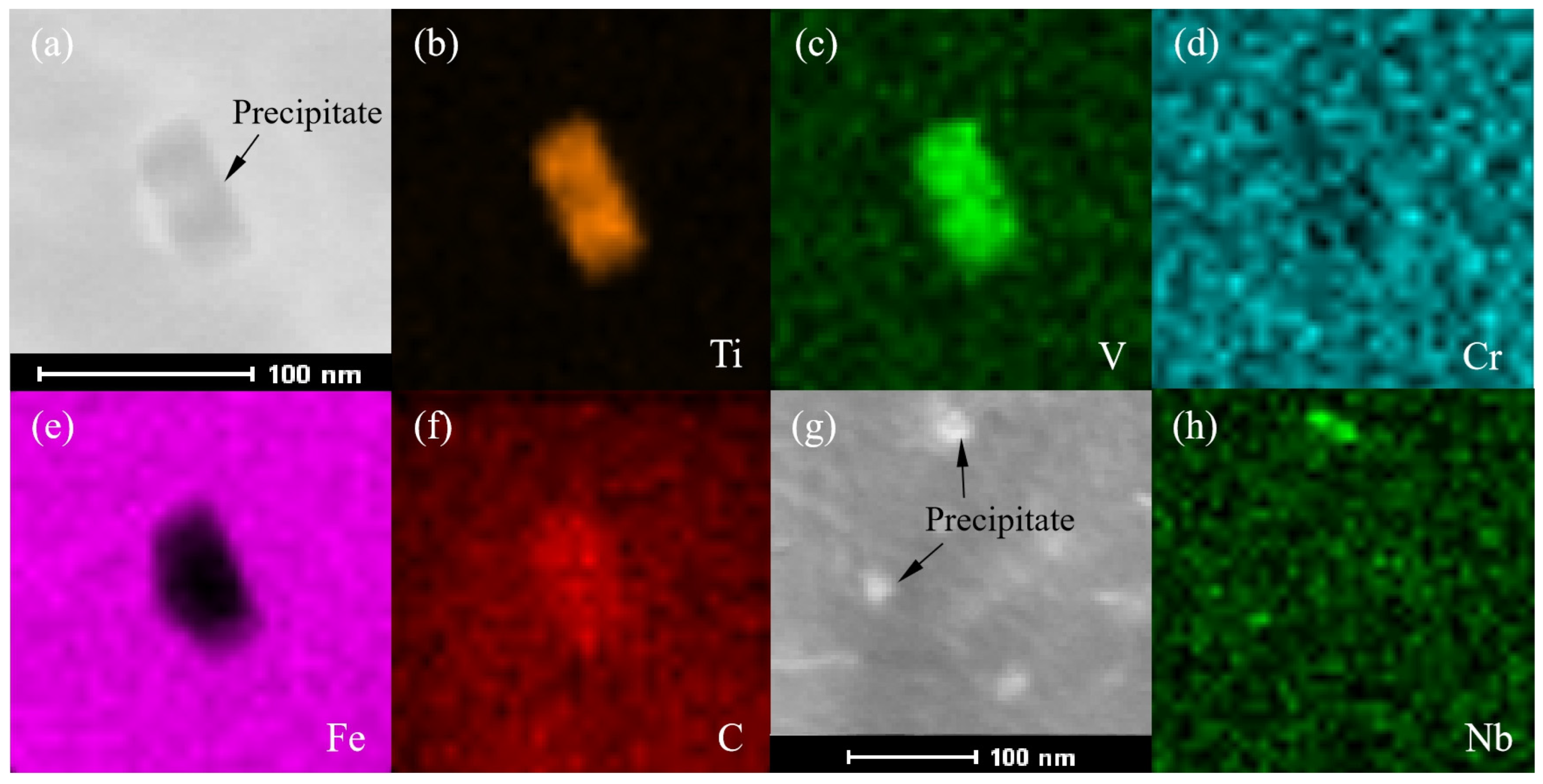
- The software calculation results showed that the γ phase started to transform into the α phase at 830 °C, and the transformation ended at 666 °C. The MC phase started to precipitate from the γ phase at 1150 °C and remained almost stable at 750 °C. The alloyed Fe3C phase started to precipitate at 683 °C, dissolved and disappeared at 521 °C. The M7C3 phase began to precipitate at 543 °C and subsequently remained almost unchanged. At 400 °C, the steel plate consisted of 99.17% α phase, 0.117% MC (TiC, VC, NbC and a small amount of CrC) phase and 0.713% M7C3 (Cr7C3, Mn7C3, a small amount of V7C3 and trace Ni7C3) phase. Among the phases, the Ni element was almost completely dissolved in the α phase. Most of the Mn element was dissolved in the α phase, and the rest was distributed in the Mn7C3 phase. Most of the Cr elements were distributed in the Cr7C3 phase and dissolved in the α phase. The Ti, Nb and V elements were almost or predominantly distributed in the TiC, NbC and VC phases. The TiC and VC phases observed by TEM existed in the form of a composite phase with a rectangular shape, while the Nb element existed as an independent phase of NbC, whose size was much lower than that of the composite phase of TiC and VC.
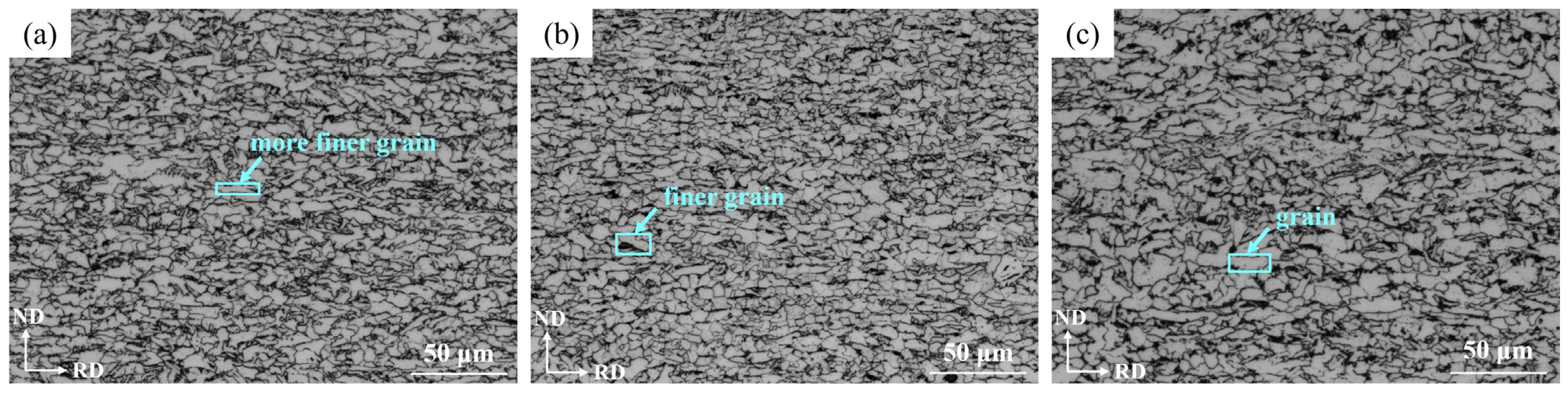
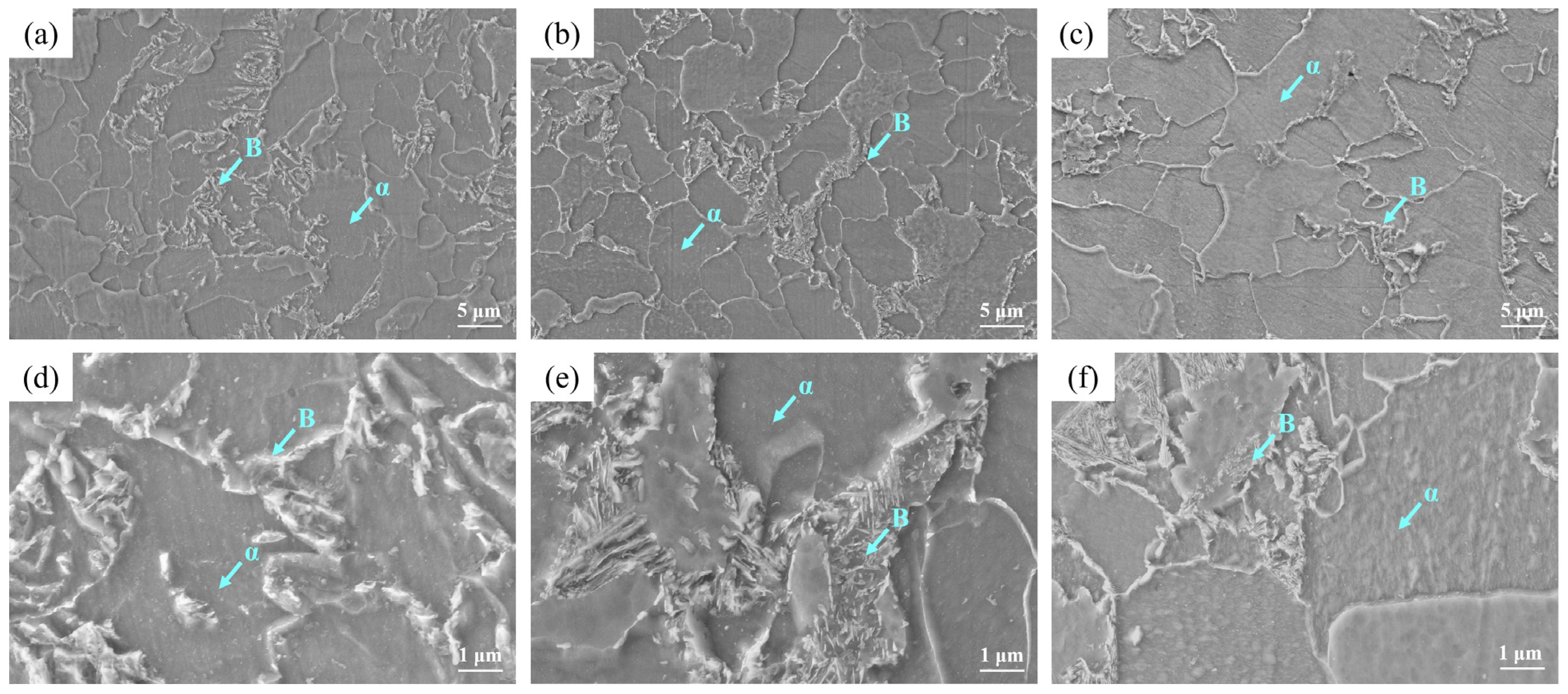
- OM observation showed that the microstructure at the surface was mainly composed of the Pα and Aα phases and GB, in which the bainite content was 12.87%, which was consistent with the calculation result. The microstructure at 1/4 thickness was mainly composed of Pα, a small amount of Aα phase and GB, and at 1/2 thickness was composed of the Pα phase and a small amount of pearlite and GB due to the slow cooling rate. Due to the higher cooling rate and large amount of deformation, the Pα phase at the surface was more uniform and finer than that at 1/4 and 1/2 thickness and there was more Aα phase and bainite than at 1/4 thickness.
- ODF analysis showed that there were strong {111}<110> and {111} <112> and weak {112}<110> texture components at the surface and 1/4 and 1/2 thickness of the steel plate. The formation of the {111}<uvw> texture component may be related to the rolling deformation mechanism in the γ phase region and the orientation relationship during the transformation from the γ phase to the α phase during the final rolling process. The formation of a weak {112}<110> texture component may be related to rolling in the α phase region. In addition, there was also a strong {001}<110> texture component at 1/2 thickness, which was related to a slower cooling rate. Compared with the other two locations, the weaker {111}<110> texture component at a 1/2 thickness of the steel plate was related to the lower final rolling temperature.
- EBSD measurements showed that the grain sizes at the surface and 1/4 and 1/2 thicknesses of the steel plate were 2.47 μm, 3.28 μm and 3.72 μm, respectively, and the grain sizes decreased gradually from 1/2 thickness to the surface, related to the gradual increase in the cooling rate after rolling. The proportions of high-angle grain boundaries at the surface and 1/4 and 1/2 thicknesses of the steel plate were 74.2%, 87% and 84.2%, respectively, and the proportion at the surface was obviously lower than that at 1/4 and 1/2 thickness, related to the larger number of subgrains that had not completed recrystallization at the surface because of the slower cooling rate.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Zhang, P.; Peng, X.D.; Yan, L.; Li, G. Comparison of microstructure and mechanical properties of high strength and toughness ship plate steel. Materials 2021, 14, 5886. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, P.; Hu, K.; Yan, L.; Li, G. Simultaneous improvement in mechanical properties and fatigue crack propagation resistance of low carbon offshore structural steel EH36 by Cu–Cr microalloying. Metals 2021, 11, 1880. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Q.; Ao, L.; Yan, L.; Zhou, C. Influence of control rolling and cooling on microstructure and mechanical properties of AH32 ship-plate steel. Heat Treat. Met. 2011, 36, 16–19. [Google Scholar]
- Zhou, C.; Ye, Q.; Ling, Y. Effect of ultra-fast cooling on microstructure and properties of high strength steel for shipbuilding. Trans. Mater. Heat Treat. 2014, 35, 161–166. [Google Scholar]
- Li, F.; Wu, H.; Tang, D.; Cheng, L. Microstructure characteristics of FH690 ultra-strength ship structure steel and its effects on low temperature toughness. Mater. Heat Treat. 2010, 39, 67–69. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, X.; Che, M.; Cui, Q.; Wu, K. Effect of center microstructure on mechanical properties of extra-thickness ultra-high strength marine steel. Heat Treat. Met. 2018, 43, 100–105. [Google Scholar] [CrossRef]
- Chen, A.; Ma, G.; Zhou, P.; Ren, J.; Shi, D. Trial-production of FH36 low-temperature high-strength hull plate. Shandong Metall. 2011, 33, 36–37. [Google Scholar] [CrossRef]
- Di, X.; Wang, Z.; Cui, C.; Qin, G.; Teng, Y.; Su, H.; Cui, H. Development and main technical issues discussion of FH36 steel plate with special requirements. Wide Heavy Plate 2022, 28, 38–42. [Google Scholar]
- Wu, J.; Shi, X.; Guo, H.; Guo, J. A mathematical model of mechanical properties influenced by manganese in DH36 high-strength ship plate steel. Chin. J. Chem. Eng. 2016, 38, 101–108. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, H.; Wu, J.; Shao, G.; Xu, L. Effect of heat treatment on the microstructure and mechanical property of high strength marine steel. Heat Treat. 2008, 2, 23–26. [Google Scholar] [CrossRef]
- Li, X. Research of the Microstructure and Properties Based on Mg or Zr Treated Ship-Building Steel Plates. Master’s Thesis, Northeastern University, Shenyang, China, 2016; p. 123. [Google Scholar]
- Zhou, D.; Chai, Y.; Liang, R.; Yang, Y.; Tong, S. Influence of V, V-N and V-Nb micro-alloying on microstructure and properties of high strength steel for shipbuilding. Heat Treat. Met. 2019, 44, 60–64. [Google Scholar] [CrossRef]
- Masahito, K. Characteristics of brittle crack arrest steel plate for large heat input welding for large container ships. KOBELCO Technol. Rev. 2011, 30, 66–69. [Google Scholar]
- Ansteel Iron and Steel Information Research Institute. Controlled Rolling Translation Collection; Ansteel Iron and Steel Information Research Institute: Beijing, China, 1980; p. 1. [Google Scholar]
- Han, S.; Choi, S.; Choi, J.; Seong, H.; Kim, I. Effect of hot-rolling processing on texture and r -value of annealed dual-phase steels. Mater. Sci. Eng. 2009, 527, 1686–1694. [Google Scholar] [CrossRef]
- Su, Y.; Li, L.; Fu, R. Through-thickness texture gradient in Hot-Rolled 25Mn-2.5Si-2Al TWIP Steel. J. Iron Steel Res. Int. 2013, 20, 46–53. [Google Scholar] [CrossRef]
- Inagaki, H. Fundamental aspect of texture formation in low carbon steel. ISIJ Int. 1994, 34, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Cheng, B.; Luo, M.; Qu, J. Microstructure and anti-cleavage fracture properties of ultra-thick crack arrest steel plate prepared by TMCP. Trans. Mater. Heat Treat. 2020, 41, 118–128. [Google Scholar] [CrossRef]
- Zhou, C.; Yan, L.; Zhang, P.; Zhu, L. Microstructure and mechanical properties of EH47 high strength brittle crack arrest steel for container ship. Trans. Mater. Heat Treat. 2017, 38, 83–87. [Google Scholar] [CrossRef]
- Li, P. Texture Formation and Its Effect on the Mechanical Properties of High Strength Low Alloy Steel. Master’s Thesis, Harbin Engineering University, Harbin, China, 2019; p. 66. [Google Scholar]
- Guan, X. Super-Low Carbon High-Strength BH Steel Plate; Shandong Science and Technology Press: Jinan, China, 2000; p. 97. [Google Scholar]
- Ray, R.; Jonas, J.; Butron-Guillen, M. Transformation texture in steels (review). ISIJ Inter. 1994, 34, 927–942. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Y.; Zhao, Z.; Sun, D. Developing an ultra-low carbon deep-drawing sheet steel by ferritic hot rolling process. J Northeast Univ: Nat Sci. 2005, 8, 747–750. [Google Scholar] [CrossRef]
- Liu, Z.; He, X.; Yang, Z. SA508Gr.4N Steel for a New Generation of Nuclear Pressure Vessels; Metallurgical Industry Press: Beijing, China, 2018; p. 169. [Google Scholar]
- Lambert-Perlade, A.; Sturel, T.; Gourgues, A.F. Mechanisms and modeling of cleavage fracture in simulated heat-affected zone microstructures of a high-strength low alloy steel. Metall. Mater. Trans. 2004, 35, 1039–1053. [Google Scholar] [CrossRef]
- Gourgues, A. Electron backscatter diffraction and cracking. J. Mater. Sci. Technol. 2002, 18, 119–133. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Knott, J.; Davis, C. Charpy-impact-toughness prediction using an “effective” grain size for thermomechanically controlled rolled microalloyed steels. Metall. Mater. Trans. 2004, 35, 121–130. [Google Scholar] [CrossRef]
- Dáz-Fuentes, M.; Iza-Mendia, A.; Gutiérrez, I. Analysis of different acicular ferrite microstructures in low-carbon steels by electron backscattered diffraction. Study of their toughness behavior. Metall. Mater. Trans. 2003, 34, 2505–2516. [Google Scholar] [CrossRef]
- Fan, C.; Shen, T.; Fan, Y.; Yang, J.; Ou, L.; Chen, X. Effects of rolling methods on microstructure and anisotropy of aluminium alloy sheet for industrial packing. Packag. J. 2018, 10, 52–58. [Google Scholar]
- Yu, Y. Principles of Metallography; Metallurgical Industry Press: Beijing, China, 2013; p. 966. [Google Scholar]









| Grade | Chemical Composition | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Nb | V | Ti | Als | Cu | Cr | Ni | Fe | |
| FH36 | 0.08 | 0.17 | 1.42 | 0.002 | 0.012 | 0.03 | 0.040 | 0.013 | 0.030 | 0.10 | 0.16 | 0.35 | Bal. |
| Grade | The First-Stage Rolling Temperature | The Second-Stage Rolling Temperature | Final Rolling Temperature | Cooling Rate/°C·s−1 | Final Cooling Temperature | Reddening Temperature |
|---|---|---|---|---|---|---|
| FH36 | 1050 | 830 | 790 | 11 | 450 | 500 |
| Phase | Alloying Elements | |||||
|---|---|---|---|---|---|---|
| Ni | Mn | Cr | V | Ti | Nb | |
| α | 99.9 | 71 | 30.8 | 0.6 | − | − |
| MC | − | − | 0.2 | 98.6 | 100 | 100 |
| M7C3 | 0.1 | 29 | 69 | 0.8 | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Li, G.; Yin, W.; Yan, L.; Wang, Z.; Zhang, P.; Hu, X.; Li, B.; Zhang, W. Studying on Alloying Elements, Phases, Microstructure and Texture in FH36 Ship Plate Steel. Materials 2023, 16, 4762. https://doi.org/10.3390/ma16134762
Wang D, Li G, Yin W, Yan L, Wang Z, Zhang P, Hu X, Li B, Zhang W. Studying on Alloying Elements, Phases, Microstructure and Texture in FH36 Ship Plate Steel. Materials. 2023; 16(13):4762. https://doi.org/10.3390/ma16134762
Chicago/Turabian StyleWang, Dong, Guanglong Li, Wei Yin, Ling Yan, Zhenmin Wang, Peng Zhang, Xiaodong Hu, Boyong Li, and Wanshun Zhang. 2023. "Studying on Alloying Elements, Phases, Microstructure and Texture in FH36 Ship Plate Steel" Materials 16, no. 13: 4762. https://doi.org/10.3390/ma16134762




