Effects of Mo Particles Addition on the Microstructure and Properties of 316 L Stainless Steels Fabricated by Laser Powder Bed Fusion
Abstract
:1. Introduction
2. Material and Methods
2.1. LPBF Facility and Printing Procedure
2.2. Microstructural Observation
2.3. Mechanical Properties Testing
2.4. Electrochemical Testing
3. Results and Discussion
3.1. Phase Analysis
3.2. General Microstructure
3.3. Tensile Properties
3.4. Fracture Surface
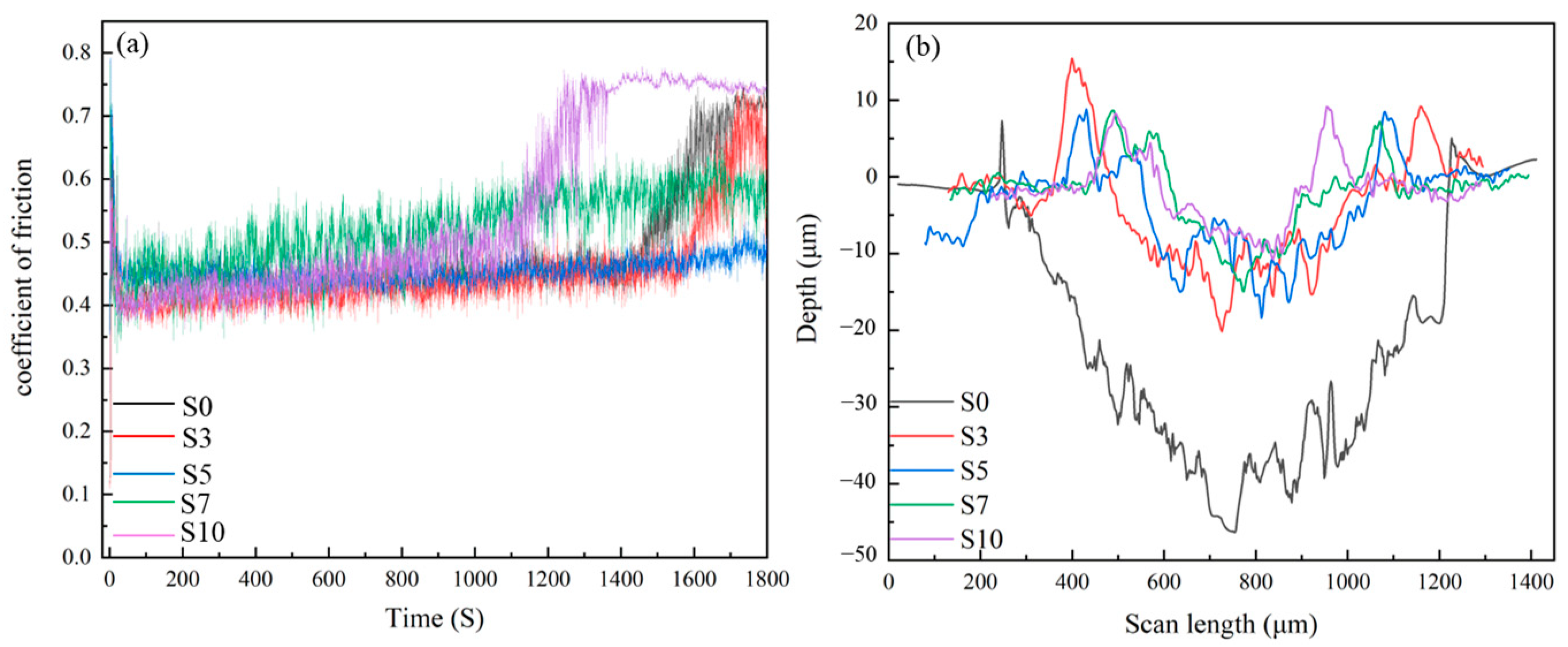
3.5. Tribological Performance
3.6. Worn Morphology
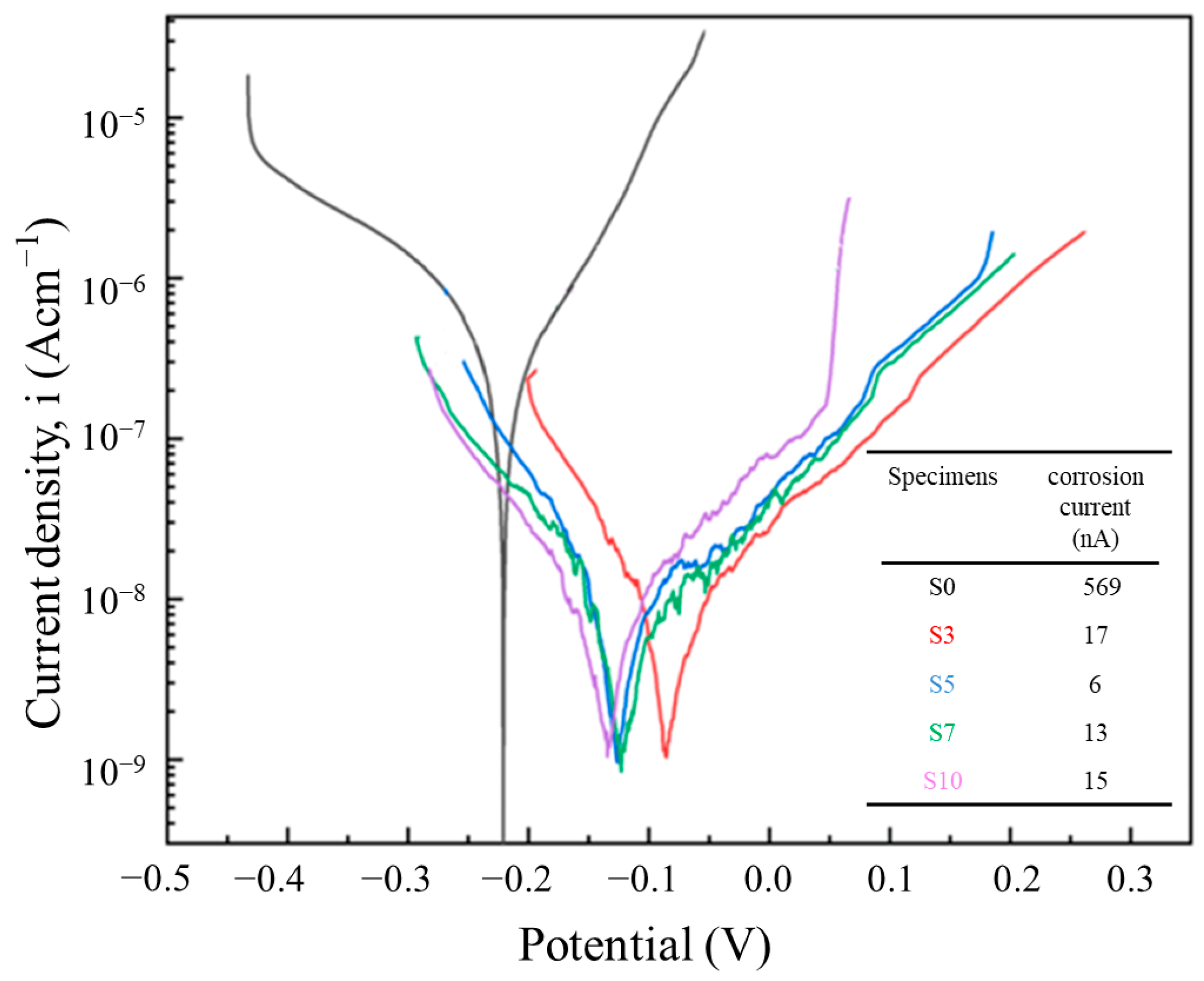
3.7. Electrochemical Results
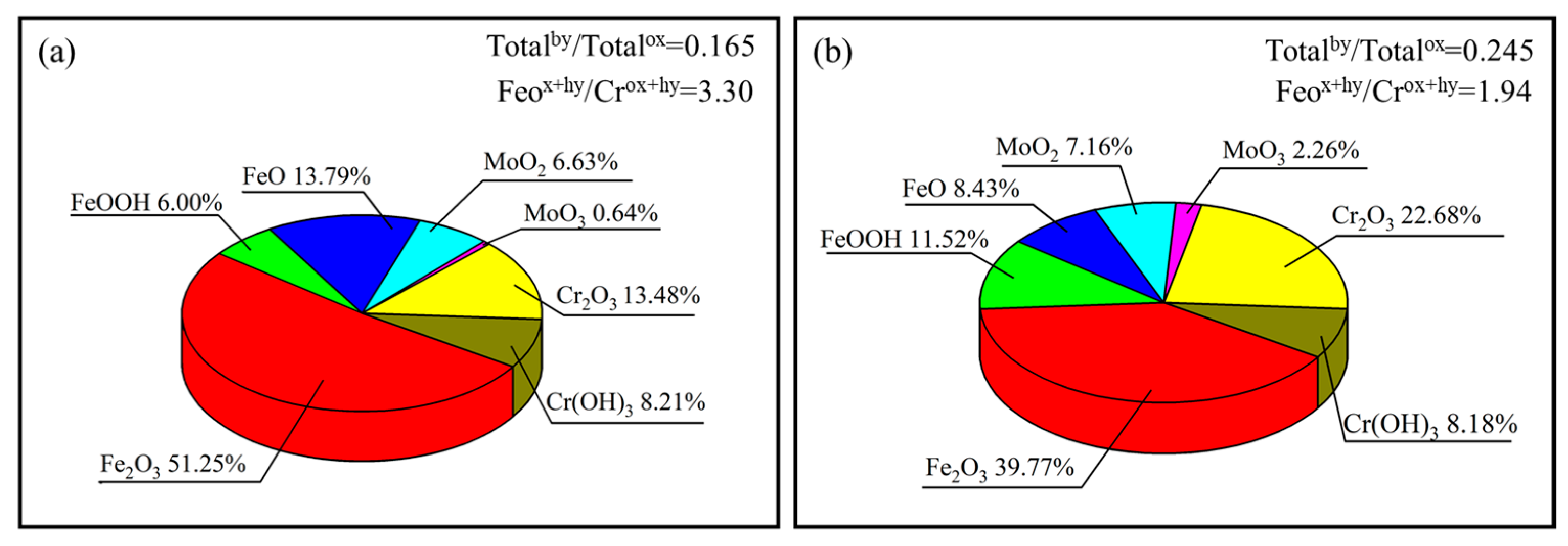
3.8. Passive Film Characterization
4. Conclusions
- With the increasing Mo particles addition, phase transformation occurs from γ-Fe for S0 to α-Fe for S10. Especially, both the phases are contained in S5. Moreover, the phase transformation can be attributed to the formation of MoCx and the increasing cooling rate as well as the increasing internal stress caused by Mo addition.
- With the increasing Mo particles addition, the ultimate tensile strength of the LPBF 316 L SS increases monotonically, while the friction coefficient decreases firstly till 5 wt.% Mo addition and then increases. Moreover, S5 shows the best comprehensive mechanical performance in this study, which can be attributed to the synergistic impact of solid solution strengthening, fine grain strengthening, and dislocation strengthening, as well as the relatively low content of defects caused by Mo addition.
- The addition of Mo can improve the corrosion resistance by increasing the content of Cr2O3 in the passivated film. Moreover, a low corrosion current up to 6 nA is achieved for S5, which has two orders of magnitude lower than that of S0. However, too much Mo particles (e.g., >5 wt.%) leads to the increase in defects. Therefore, S5 shows the best corrosion resistance in this study.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Yang, S.; Gu, J.; Xiong, Q.; Duan, C.; Meng, X.; Fang, Y. Microstructure and Wear Properties of Selective Laser Melting 316L. Mater. Chem. Phys. 2020, 254, 123487. [Google Scholar] [CrossRef]
- Al-Mamun, N.S.; Mairaj Deen, K.; Haider, W.; Asselin, E.; Shabib, I. Corrosion Behavior and Biocompatibility of Additively Manufactured 316L Stainless Steel in a Physiological Environment: The Effect of Citrate Ions. Addit. Manuf. 2020, 34, 101237. [Google Scholar] [CrossRef]
- Baker, M.A.; Castle, J.E. The Initiation of Pitting Corrosion at MnS Inclusions. Corros. Sci. 1993, 34, 667–682. [Google Scholar] [CrossRef]
- Zhu, Y.; Zou, J.; Chen, X.; Yang, H. Tribology of Selective Laser Melting Processed Parts: Stainless Steel 316L Under Lubricated Conditions. Wear 2016, 350–351, 46–55. [Google Scholar] [CrossRef]
- Laleh, M.; Hughes, A.E.; Xu, W.; Gibson, I.; Tan, M.Y. Unexpected Erosion-Corrosion Behaviour of 316 L Stainless Steel Produced by Laser powder bed fusion. Corros. Sci. 2019, 155, 67–74. [Google Scholar] [CrossRef]
- Bidulsky, R.; Gobber, F.S.; Bidulska, J.; Ceroni, M.; Kvackaj, T.; Grande, M.A. Coated Metal Powders for Laser Powder Bed Fusion (L-PBF) Processing: A Review. Metals 2021, 11, 1831. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, X.; Yang, J.; Teng, W.; Wang, Y. Densification Behavior and Mechanical Properties of Nanocrystalline TiC Reinforced 316L Stainless Steel Composite Parts Fabricated by Laser powder bed fusion. Opt. Laser Technol. 2018, 103, 239–250. [Google Scholar] [CrossRef]
- Yazıcı, M.; Kovacı, H.; Yetim, A.F.; Çelik, A. Structural, Mechanical and Tribological Properties of Ti and TiN Coatings On 316L Stainless Steel. Ceram. Int. 2018, 44, 14195–14201. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Y.; Jing, H.; Lin, D.; Zhao, L.; Xu, L.; Xin, P. Selective Laser Melting of Low-Content Graphene Nanoplatelets Reinforced 316L Austenitic Stainless Steel Matrix: Strength Enhancement without Affecting Ductility. Addit. Manuf. 2020, 34, 101381. [Google Scholar] [CrossRef]
- Tanprayoon, D.; Srisawadi, S.; Sato, Y.; Tsukamoto, M.; Suga, T. Microstructure and Hardness Response of Novel 316 L Stainless Steel Composite with TiN Addition Fabricated by LPBF. Opt. Laser Technol. 2020, 129, 106238. [Google Scholar] [CrossRef]
- AlMangour, B.; Baek, M.; Grzesiak, D.; Lee, K. Strengthening of Stainless Steel by Titanium Carbide Addition and Grain Refinement During Laser powder bed fusion. Mater. Sci. Eng. A 2018, 712, 812–818. [Google Scholar] [CrossRef]
- Zou, Y.; Tan, C.; Qiu, Z.; Ma, W.; Kuang, M.; Zeng, D. Additively Manufactured SiC-reinforced Stainless Steel with Excellent Strength and Wear Resistance. Addit. Manuf 2021, 41, 101971. [Google Scholar] [CrossRef]
- Song, B.; Wang, Z.; Yan, Q.; Zhang, Y.; Zhang, J.; Cai, C.; Wei, Q.; Shi, Y. Integral Method of Preparation and Fabrication of Metal Matrix Composite: Selective Laser Melting of In-Situ Nano/Submicro-Sized Carbides Reinforced Iron Matrix Composites. Mater. Sci. Eng. A 2017, 707, 478–487. [Google Scholar] [CrossRef]
- Duan, Z.; Man, C.; Dong, C.; Cui, Z.; Kong, D.; Wang, L.; Wang, X. Pitting Behavior of LPBF 316 L Stainless Steel Exposed to Chloride Environments with Different Aggressiveness: Pitting Mechanism Induced by Gas Pores. Corros. Sci. 2020, 167, 108520. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Y.; Kim, M.; Kim, H.; No, J.; Duan, Z.; Yuan, Q.; Suhr, J. An Austenite-Rich Composite of Stainless Steels with High Strength and Favorable Ductility Via Selective Laser Melting of a Powder Mixture. Mater. Sci. Eng. A 2022, 855, 143891. [Google Scholar] [CrossRef]
- Ghayoor, M.; Lee, K.; He, Y.; Chang, C.; Paul, B.K.; Pasebani, S. Selective Laser Melting of Austenitic Oxide Dispersion Strengthened Steel: Processing, Microstructural Evolution and Strengthening Mechanisms. Mater. Sci. Eng. A 2020, 788, 139532. [Google Scholar] [CrossRef]
- Sun, P.; Qiu, C. Influence of Addition of TiAl Particles on Microstructural and Mechanical Property Development in a Selectively Laser Melted Stainless Steel. Mater. Sci. Eng. A 2021, 826, 141925. [Google Scholar] [CrossRef]
- Quan, J.; Lin, K.; Gu, D. Laser powder bed fusion of Silver Submicron Powder Modified 316 L Stainless Steel: Influence of Silver Addition on Microstructures and Performances. Powder Technol. 2020, 364, 478–483. [Google Scholar] [CrossRef]
- Yin, X.; Zhai, Q.; Zhang, Q.; Wang, K.; Meng, L.; Ma, Z.; Chen, G.; Wang, S.; Wang, L. Effect of Tungsten Particles on Microstructure and Properties of 316 L Stainless Steel Manufactured by selective laser melting. J. Manuf. Process. 2021, 68, 210–221. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Yan, Q.; Zhang, L.; Wang, M.; Song, B.; Shi, Y. Amorphous Alloy Strengthened Stainless Steel Manufactured by Selective Laser Melting: Enhanced Strength and Improved Corrosion Resistance. Scr. Mater. 2018, 148, 20–23. [Google Scholar] [CrossRef]
- Cheng, P.M.; Zhang, G.J.; Zhang, J.Y.; Liu, G.; Sun, J. Coupling Effect of Intergranular and Intragranular Particles on Ductile Fracture of Mo-La2O3 Alloys. Mater. Sci. Eng. A 2015, 640, 320–329. [Google Scholar] [CrossRef]
- Zhang, T.; Deng, H.W.; Xie, Z.M.; Liu, R.; Yang, J.F.; Liu, C.S.; Wang, X.P.; Fang, Q.F.; Xiong, Y. Recent Progresses on Designing and Manufacturing of Bulk Refractory Alloys with High Performances Based on Controlling Interfaces. J. Mater. Sci. Technol. 2020, 52, 29–62. [Google Scholar] [CrossRef]
- Lutton Cwalina, K.; Demarest, C.R.; Gerard, A.Y.; Scully, J.R. Revisiting the Effects of Molybdenum and Tungsten Alloying on Corrosion Behavior of Nickel-Chromium Alloys in Aqueous Corrosion. Curr. Opin. Solid State Mater. Sci. 2019, 23, 129–141. [Google Scholar] [CrossRef]
- Giganto, S.; Martínez-Pellitero, S.; Barreiro, J.; Zapico, P. Influence of 17-4 PH Stainless Steel Powder Recycling on Properties of LPBF Additive Manufactured Parts. J. Mater. Res. Technol. 2022, 16, 1647–1658. [Google Scholar] [CrossRef]
- Guo, M.X.; Zhu, J.; Yi, L.; Wang, F.; Li, G.J.; Lei, R.S. Effects of Precipitation and Strain-Induced Martensitic Transformation of Fe-C Phases on the Mechanical Properties of Cu-Fe-C Alloy. Mater. Sci. Eng. A 2017, 697, 119–125. [Google Scholar] [CrossRef]
- Sun, W.W.; Zurob, H.S.; Hutchinson, C.R. Coupled Solute Drag and Transformation Stasis During Ferrite Formation in Fe-C-Mn-Mo. Acta Mater. 2017, 139, 62–74. [Google Scholar] [CrossRef]
- Mirzababaei, S.; Doddapaneni, V.V.K.; Lee, K.; Paul, G.E.; Pirgazi, H.; Tan, K.; Ertorer, O.; Chang, C.; Paul, B.K.; Pasebani, S. Remarkable Enhancement in Thermal Conductivity of Stainless-Steel Leveraging Metal Composite via Laser Powder Bed Fusion: 316 L-Cu Composite. Addit. Manuf 2023, 70, 103576. [Google Scholar] [CrossRef]
- Mistarihi, Q.M.; Raj, V.; Kim, J.H.; Ryu, H.J. Thermal Conductivity of Mo-reinforced ZrO2 Composites Fabricated by Spark Plasma Sintering for Inert Matrix Fuels. Mater. Des 2017, 134, 476–485. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Yang, J. Selective laser melting of TiC reinforced 316L stainless steel matrix nanocomposites: Influence of starting TiC particle size and volume content. Mater. Des. 2016, 104, 141–151. [Google Scholar] [CrossRef]
- Axen, N.; Zumgahr, K.H. Abrasive Wear of Tic-Steel Composite Clad Layers on Tool Steel. Wear 1992, 157, 189–201. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Liu, H.; Wang, M.; Xing, S.; Zhao, Y. Achievement of High Strength-Ductility Combination in Austenitic and Ferritic Duplex Stainless Steel by Heterogeneous Deformation. J. Mater. Res. Technol 2022, 21, 943–950. [Google Scholar] [CrossRef]
- Liao, J.; Liu, Z.; Ling, Y.; Zhang, Q.; Qiu, S.; Gu, J.; Li, J.; Dong, H.; Song, J.; Wang, T. Electronic and Surface Engineering of Mo Doped Ni@C Nanocomposite Boosting Catalytic Upgrading of Aqueous Bio-Ethanol to Bio-Jet Fuel Precursors. Chem. Eng. J. 2023, 461, 141888. [Google Scholar] [CrossRef]
- Zhai, W.; Zhu, Z.; Zhou, W.; Nai, S.M.L.; Wei, J. Selective laser melting of dispersed TiC Particles Strengthened 316 L Stainless Steel. Compos. Part B Eng. 2020, 199, 108291. [Google Scholar] [CrossRef]
- Bidulský, R.; Bidulská, J.; Gobber, F.S.; Kvačkaj, T.; Petroušek, P.; Actis-Grande, M.; Weiss, K.; Manfredi, D. Case Study of the Tensile Fracture Investigation of Additive Manufactured Austenitic Stainless Steels Treated at Cryogenic Conditions. Materials 2020, 13, 3328. [Google Scholar] [CrossRef]
- Casati, R.; Lemke, J.; Vedani, M. Microstructure and Fracture Behavior of 316L Austenitic Stainless Steel Produced by Selective Laser Melting. J. Mater. Sci. Technol. 2016, 32, 738–744. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, H.; Nie, X.; Zhang, C.; Zhang, H. On the role of atmospheric oxygen into mechanical properties and fracture behavior of selective laser melted AlCu5MnCdVA. Mater. Des 2018, 150, 18–27. [Google Scholar] [CrossRef]
- Li, H.; Ramezani, M.; Li, M.; Ma, C.; Wang, J. Tribological Performance of Selective Laser Melted 316 L Stainless Steel. Tribol. Int. 2018, 128, 121–129. [Google Scholar] [CrossRef]
- Toor, I. Mott-Schottky Analysis of Passive Films on Si Containing Stainless Steel Alloys. J. Electrochem. Soc. 2011, 158, C391. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Passive Films on Stainless Steels—Chemistry, Structure and Growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Schultze, J.W.; Lohrengel, M.M. Stability, Reactivity and Breakdown of Passive Films. Problems of Recent and Future Research. Electrochim. Acta 2000, 45, 2499–2513. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, D.; Zhou, J.; Qiu, Z.; Yang, X. Enhanced Corrosion Resistance of Laser Additive Manufactured 316L Stainless Steel by Ultrasonic Surface Rolling Process. Surf. Coat. Technol. 2023, 454, 129187. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, Z.J.; Gao, Y. The Bi-Layer Structure and the Higher Compactness of a Passive Film on Nanocrystalline 304 Stainless Steel. Thin Solid Films 2016, 599, 64–71. [Google Scholar] [CrossRef]
- Li, D.G.; Wang, J.D.; Chen, D.R.; Liang, P. Molybdenum Addition Enhancing the Corrosion Behaviors of 316 L Stainless Steel in the Simulated Cathodic Environment of Proton Exchange Membrane Fuel Cell. Int. J. Hydrog. Energ. 2015, 40, 5947–5957. [Google Scholar] [CrossRef]
- Jinlong, L.; Tongxiang, L.; Chen, W. Surface Enriched Molybdenum Enhancing the Corrosion Resistance of 316 L Stainless Steel. Mater. Lett. 2016, 171, 38–41. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Cui, Z.; Xiao, K.; Yu, Q.; Li, X. A Comparative Study of Primary and Secondary Passive Films Formed on AM355 Stainless Steel in 0.1 M NaOH. Appl. Surf. Sci. 2018, 427, 763–773. [Google Scholar] [CrossRef]

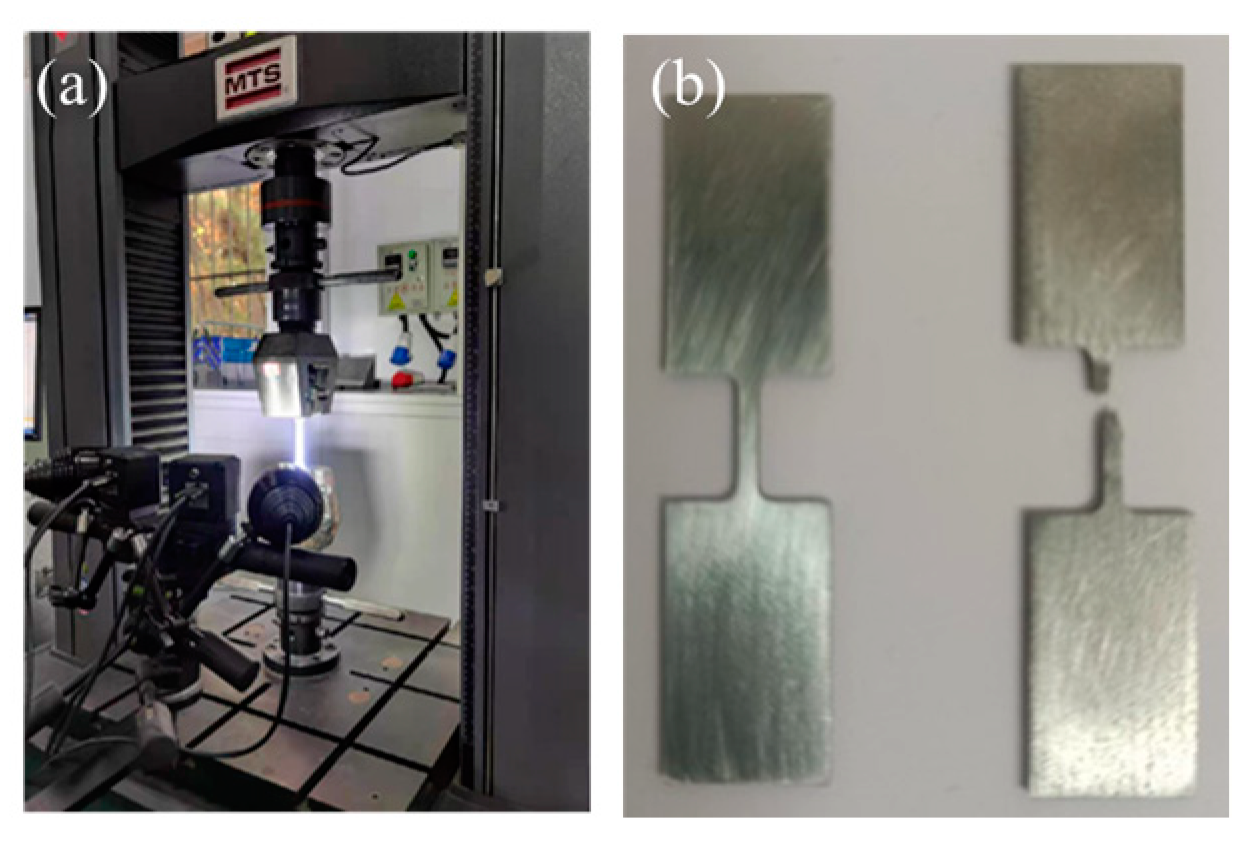
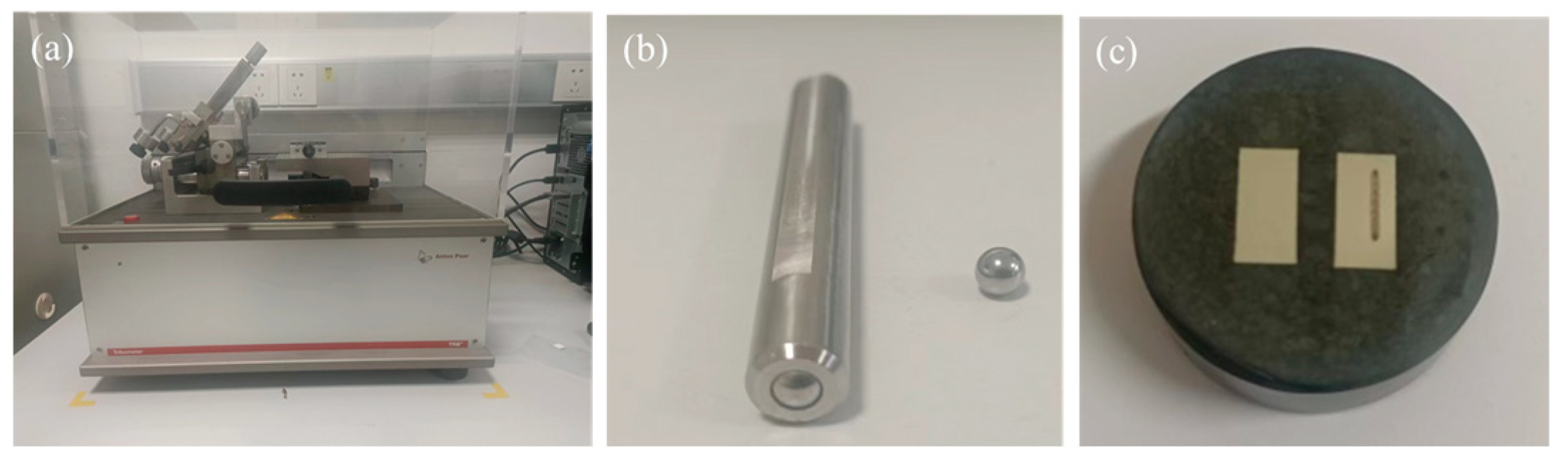


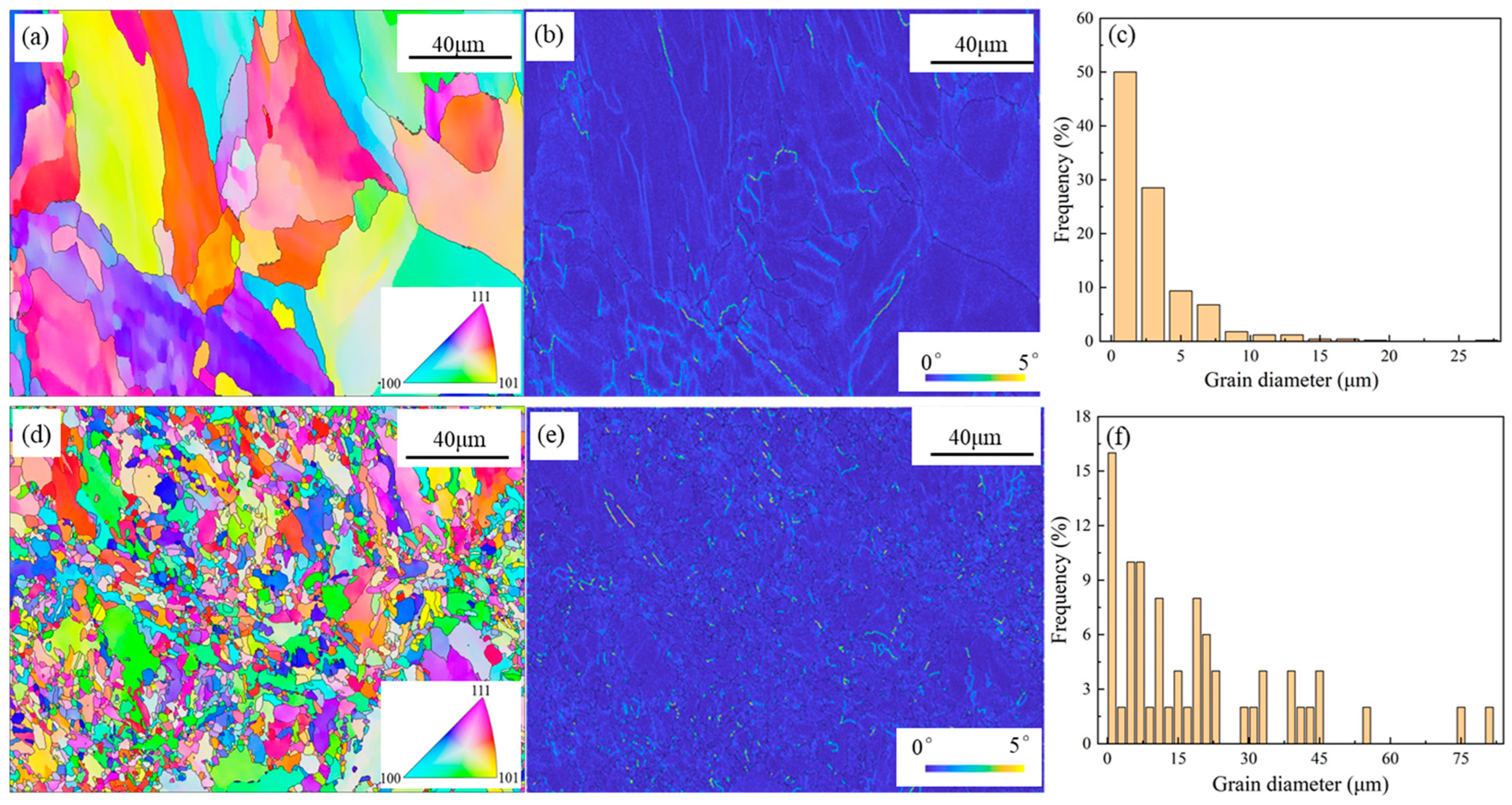

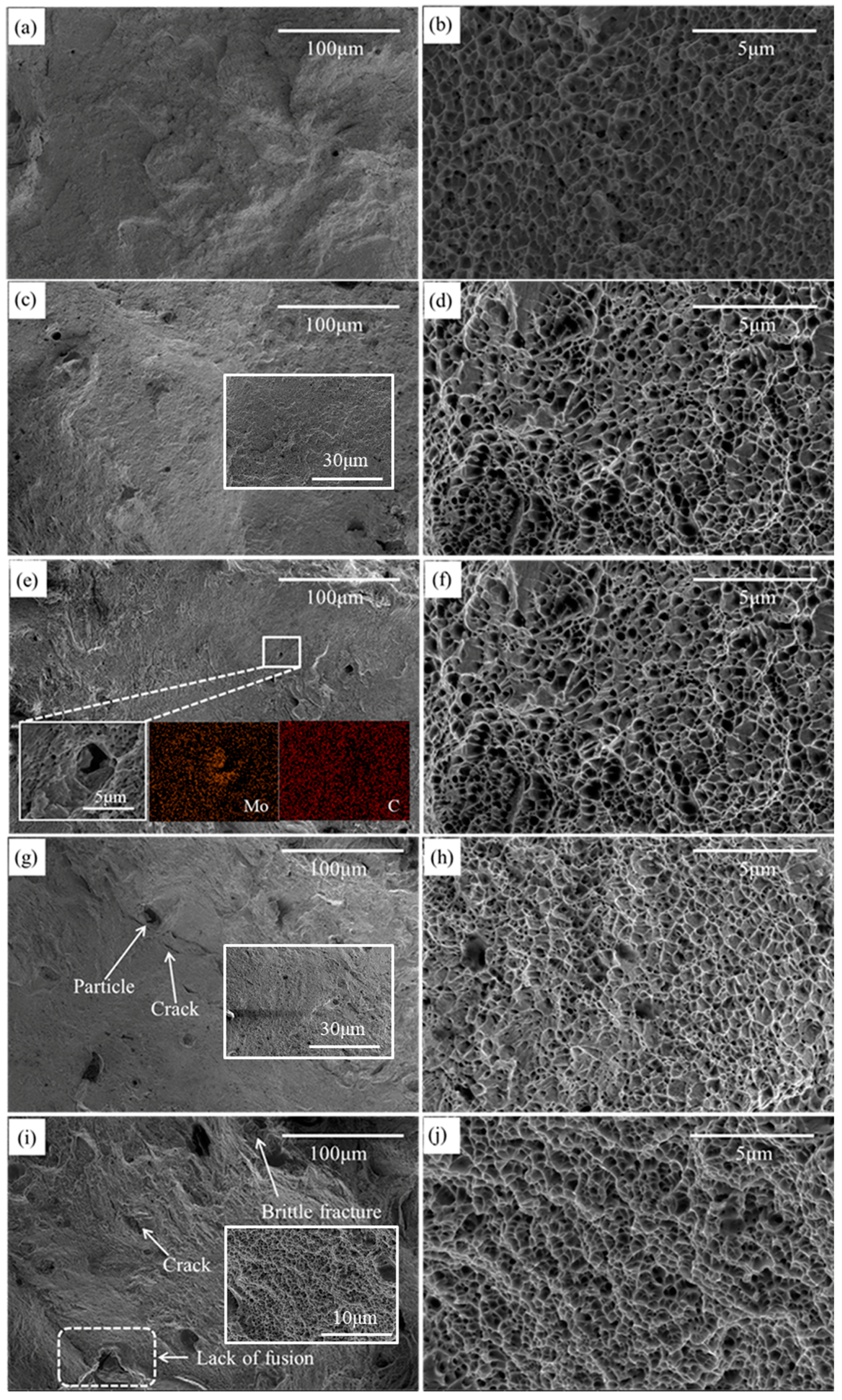


| Samples | Power (W) | Scanning Speed (mm/s) | Layer Thickness (mm) | Laser Energy (J/mm3) |
|---|---|---|---|---|
| S0 | 200 | 800 | 0.03 | 69 |
| S3 | 215 | 800 | 0.03 | 75 |
| S5 | 225 | 800 | 0.03 | 78 |
| S7 | 235 | 800 | 0.03 | 82 |
| S10 | 250 | 800 | 0.03 | 87 |
| Specimens | Frictions Coefficient (μ) | Wear Depth (μm) | Wear Width (μm) | Wear Track Area (μm2) |
|---|---|---|---|---|
| S0 | 0.473 | 47 ± 2.4 | 1018 ± 42 | 33,839 ± 342 |
| S3 | 0.432 | 21 ± 1.7 | 820 ± 29 | 10,915 ± 247 |
| S5 | 0.421 | 18 ± 1.6 | 650 ± 33 | 9592 ± 153 |
| S7 | 0.479 | 14 ± 2.1 | 585 ± 27 | 6738 ± 324 |
| S10 | 0.493 | 9 ± 1.4 | 473 ± 35 | 5382 ± 173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Zhang, S.; Wang, S.; Wang, L.; He, Y.; Cui, Y.; Liu, D.; Wang, M. Effects of Mo Particles Addition on the Microstructure and Properties of 316 L Stainless Steels Fabricated by Laser Powder Bed Fusion. Materials 2023, 16, 4827. https://doi.org/10.3390/ma16134827
Li B, Zhang S, Wang S, Wang L, He Y, Cui Y, Liu D, Wang M. Effects of Mo Particles Addition on the Microstructure and Properties of 316 L Stainless Steels Fabricated by Laser Powder Bed Fusion. Materials. 2023; 16(13):4827. https://doi.org/10.3390/ma16134827
Chicago/Turabian StyleLi, Bolin, Shuai Zhang, Shenghai Wang, Li Wang, Yinchuan He, Yaning Cui, Dan Liu, and Mingxu Wang. 2023. "Effects of Mo Particles Addition on the Microstructure and Properties of 316 L Stainless Steels Fabricated by Laser Powder Bed Fusion" Materials 16, no. 13: 4827. https://doi.org/10.3390/ma16134827




