A Comparison of Capacitive Deionization and Membrane Capacitive Deionization Using Novel Fabricated Ion Exchange Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrode Fabrication Method
2.3. Ion Exchange Membranes Fabrication
2.4. The Characterisation of IEMs
2.4.1. The Ion-Exchange Capacity (IEC)
2.4.2. The Percentage of Swelling
2.4.3. Determination of Perm-Selectivity for Membranes
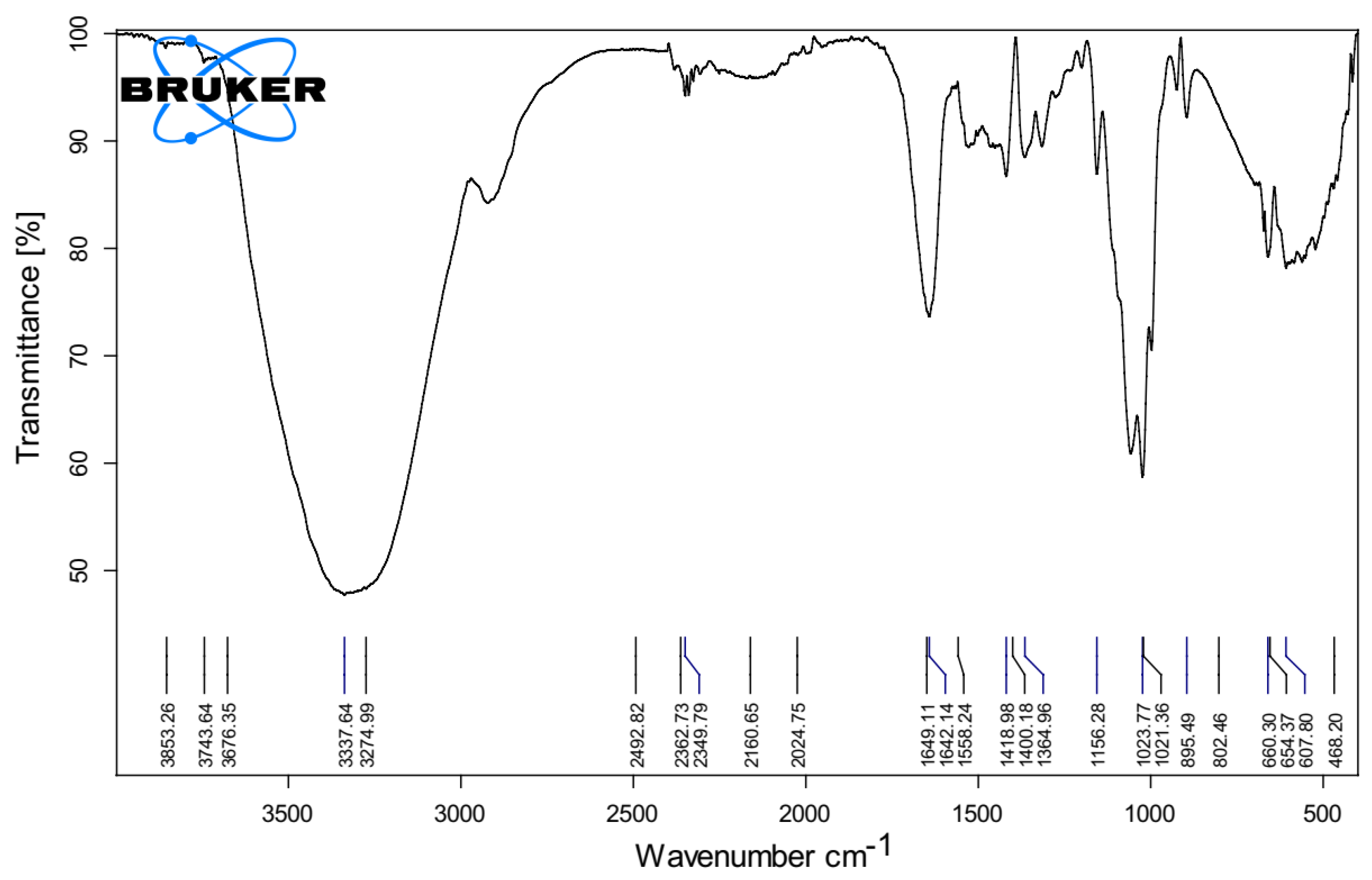
2.4.4. The Fourier Transform Infrared (FTIR)
2.4.5. Water Contact Angle
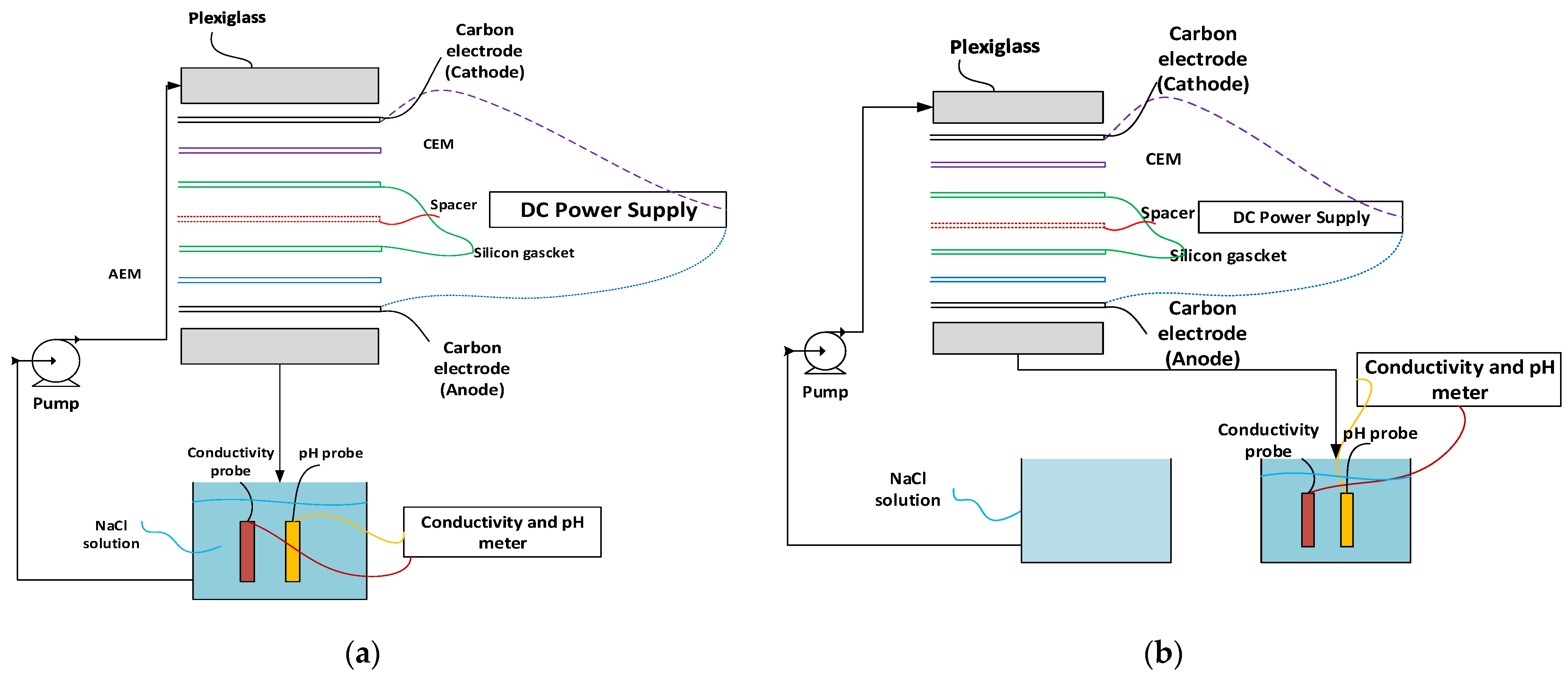
2.5. Capacitive Deionization Set-Up
2.6. Experimental Procedure
2.7. Evaluation of the Efficiency and Performance of the Capacitive Deionization
3. Results and Discussions
3.1. Ion-Exchange Membranes Characterisations
3.1.1. Characterization of Fabricated Cellulose Acetate IEMs vs. Other Membranes Used in MCDI
3.1.2. Fourier Transform Infrared (FTIR)
3.1.3. Contact Angles
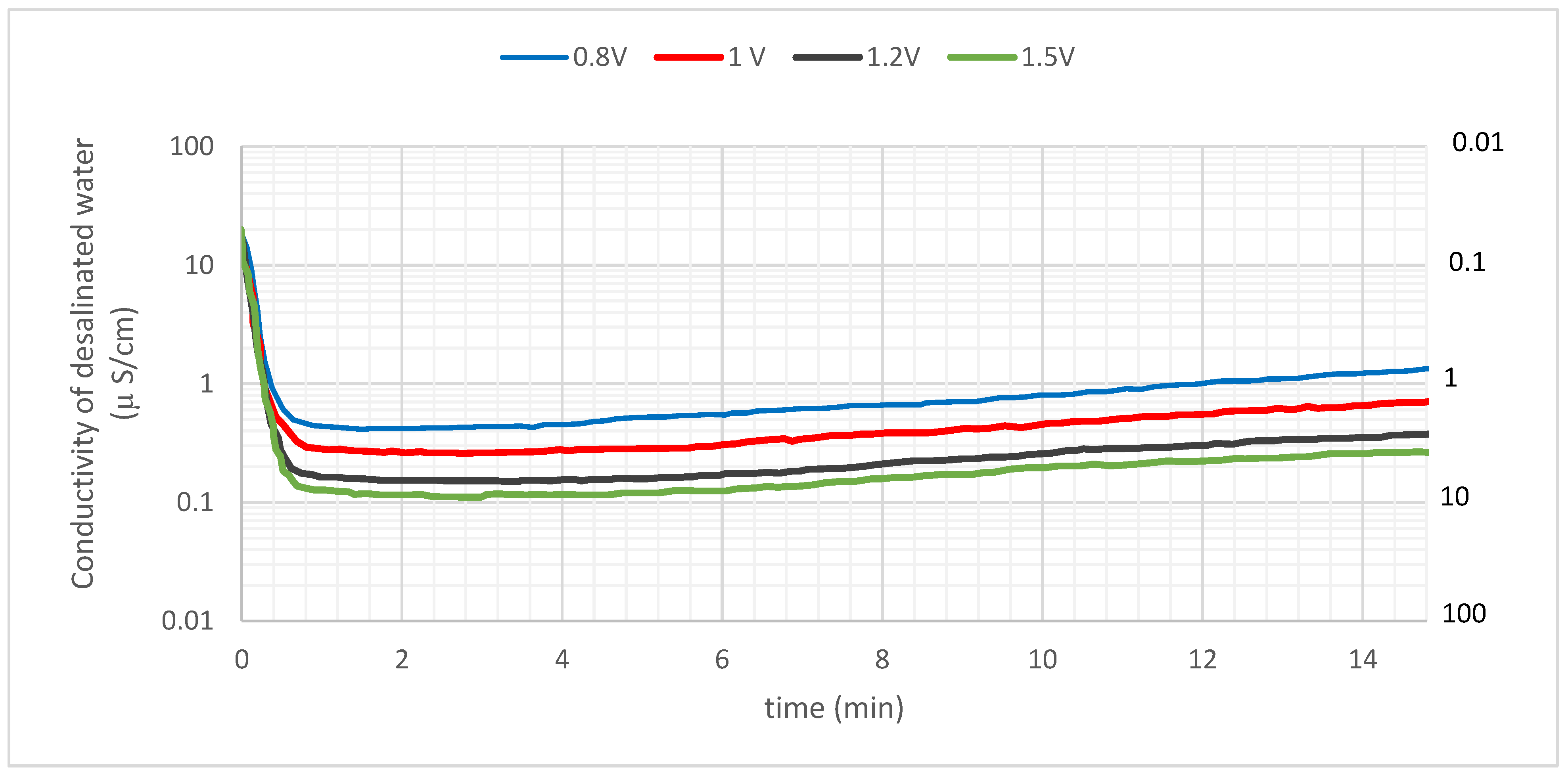
3.2. Changes in Treated Water Conductivity with Cell Potential (Single Pass MCDI)
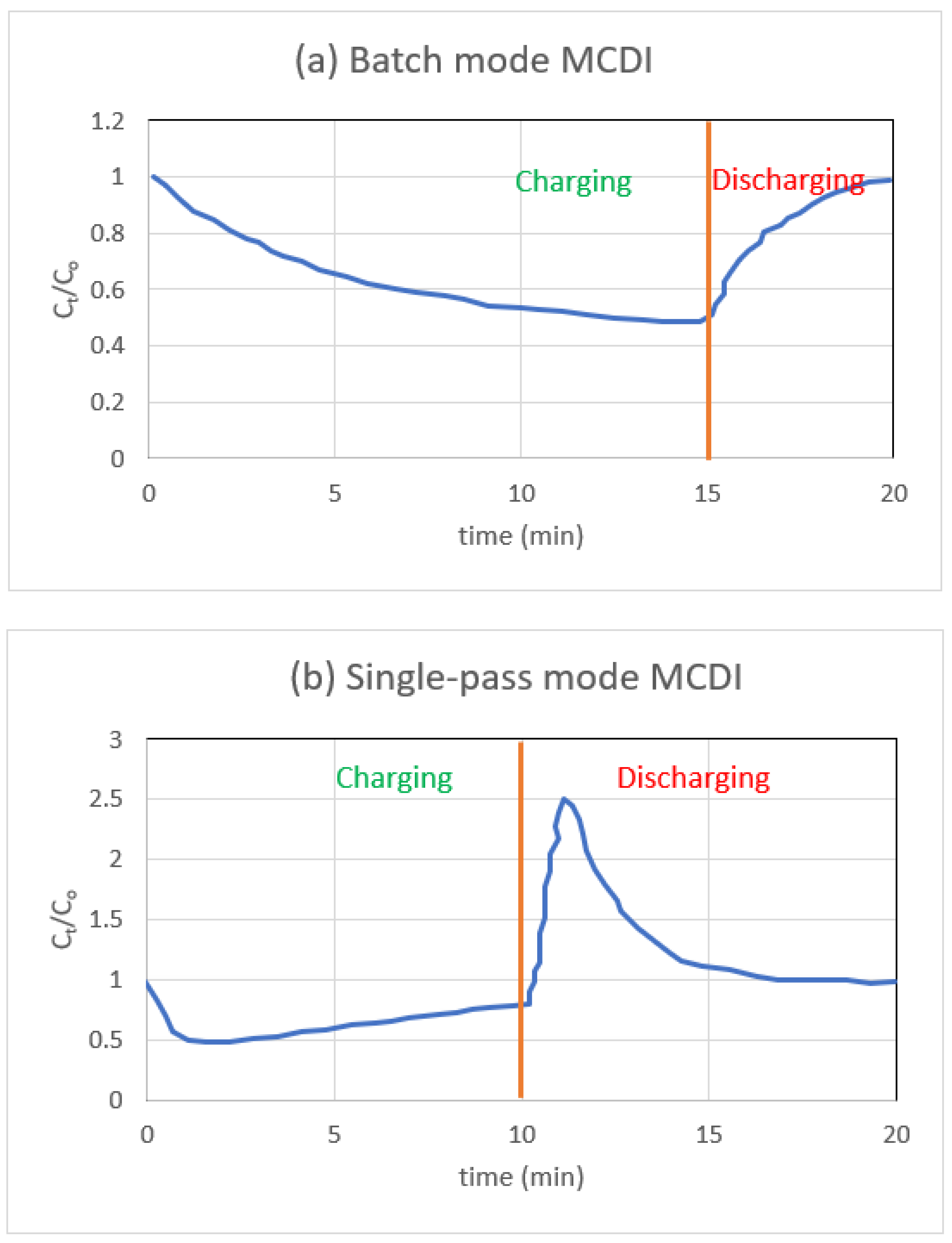
3.3. Batch vs. Single-Pass Mode of Operation
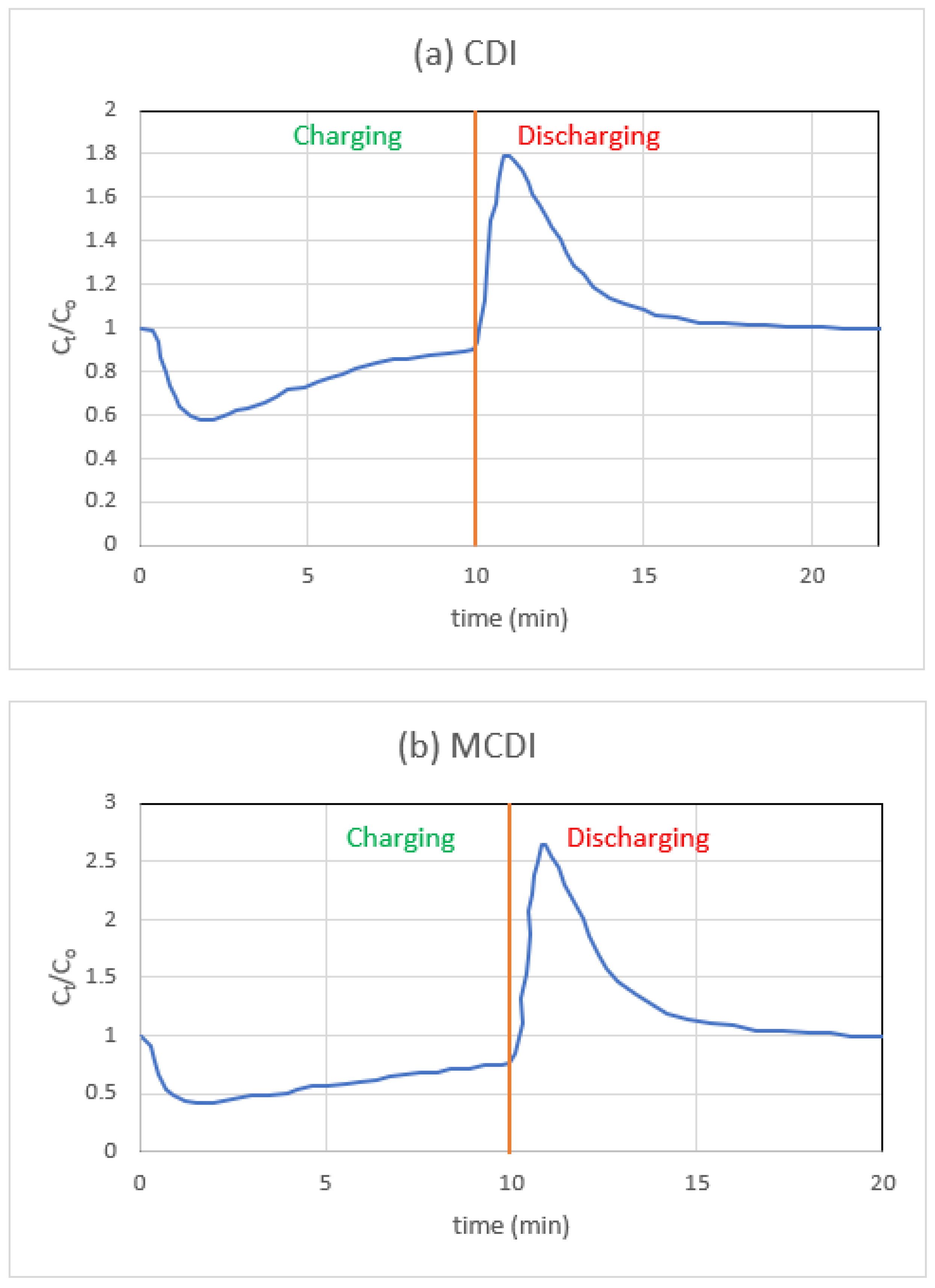
3.4. CDI vs. MCDI (Single-Pass Mode)
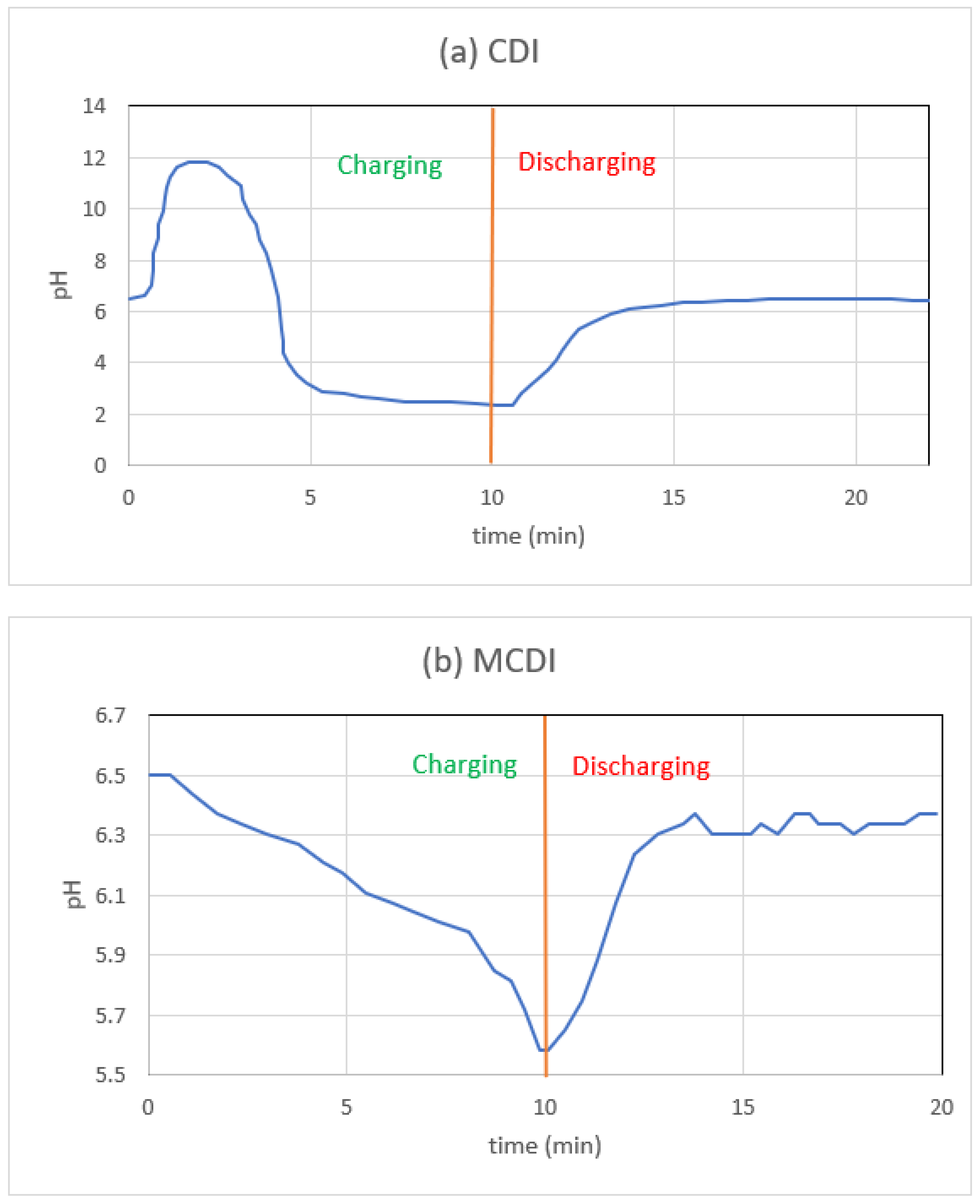
3.5. Effect of pH
4. Conclusions
- This study demonstrates that the deacetylated heterogeneous IEMs may be simply manufactured using the phase inversion approach.
- When IEMs were placed in front of the electrodes, there was a discernible increase in both the charge efficiency and the salt adsorption rates.
- Since a sudden increase or reduction in pH is not desirable for the generation of fresh water, MCDI, as opposed to CDI, only slightly alters the pH of the water. This is an additional advantage of MCDI over CDI.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obaid, M.; Abdelkareem, M.A.; Kook, S.; Kim, H.Y.; Hilal, N.; Ghaffour, N.; Kim, I.S. Breakthroughs in the Fabrication of Electrospun-Nanofiber-Supported Thin Film Composite/Nanocomposite Membranes for the Forward Osmosis Process: A Review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1727–1795. [Google Scholar] [CrossRef]
- Elsheniti, M.B.; Elbessomy, M.O.; Wagdy, K.; Elsamni, O.A.; Elewa, M.M. Augmenting the Distillate Water Flux of Sweeping Gas Membrane Distillation Using Turbulators: A Numerical Investigation. Case Stud. Therm. Eng. 2021, 26, 101180. [Google Scholar] [CrossRef]
- Elsheniti, M.B.; Ibrahim, A.; Elsamni, O.; Elewa, M. Experimental and Economic Investigation of Sweeping Gas Membrane Distillation/Pervaporation Modules Using Novel Pilot Scale Device. Sep. Purif. Technol. 2023, 310, 123165. [Google Scholar] [CrossRef]
- Naim, M.; Elewa, M.; El-Shafei, A.; Moneer, A. Desalination of Simulated Seawater by Purge-Air Pervaporation Using an Innovative Fabricated Membrane. Water Sci. Technol. 2015, 72, 785–793. [Google Scholar] [CrossRef]
- Elewa, M.M.; El-Shafei, A.A.; Moneer, A.A.; Naim, M.M. Effect of Cell Hydrodynamics in Desalination of Saline Water by Sweeping Air Pervaporation Technique Using Innovated Membrane. Desalination Water Treat. 2016, 57, 23293–23307. [Google Scholar] [CrossRef]
- Sayed, E.T.; Al Radi, M.; Ahmad, A.; Abdelkareem, M.A.; Alawadhi, H.; Atieh, M.A.; Olabi, A.G. Faradic Capacitive Deionization (FCDI) for Desalination and Ion Removal from Wastewater. Chemosphere 2021, 275, 130001. [Google Scholar] [CrossRef]
- Al Radi, M.; Sayed, E.T.; Alawadhi, H.; Abdelkareem, M.A. Progress in Energy Recovery and Graphene Usage in Capacitive Deionization. Crit. Rev. Environ. Sci. Technol. 2021, 52, 3080–3136. [Google Scholar] [CrossRef]
- Elsaid, K.; Elkamel, A.; Sayed, E.T.; Wilberforce, T.; Abdelkareem, M.A.; Olabi, A.G. Carbon-Based Nanomaterial for Emerging Desalination Technologies: Electrodialysis and Capacitive Deionization. In Encyclopedia of Smart Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 411–420. ISBN 9780128157336. [Google Scholar]
- Xu, H.; Ji, X.; Wang, L.; Huang, J.; Han, J.; Wang, Y. Performance Study on a Small-Scale Photovoltaic Electrodialysis System for Desalination. Renew. Energy 2020, 154, 1008–1013. [Google Scholar] [CrossRef]
- AlMadani, H.M.N. Water Desalination by Solar Powered Electrodialysis Process. Renew. Energy 2003, 28, 1915–1924. [Google Scholar] [CrossRef]
- Hansima, M.A.C.K.; Makehelwala, M.; Jinadasa, K.B.S.N.; Wei, Y.; Nanayakkara, K.G.N.; Herath, A.C.; Weerasooriya, R. Fouling of Ion Exchange Membranes Used in the Electrodialysis Reversal Advanced Water Treatment: A Review. Chemosphere 2021, 263, 127951. [Google Scholar] [CrossRef]
- Sayed, E.T.; Shehata, N.; Abdelkareem, M.A.; Atieh, M.A. Recent Progress in Environmentally Friendly Bio-Electrochemical Devices for Simultaneous Water Desalination and Wastewater Treatment. Sci. Total Environ. 2020, 748, 141046. [Google Scholar] [CrossRef] [PubMed]
- Kokabian, B.; Ghimire, U.; Gude, V.G. Water Deionization with Renewable Energy Production in Microalgae—Microbial Desalination Process. Renew. Energy 2018, 122, 354–361. [Google Scholar] [CrossRef]
- Elsaid, K.; Sayed, E.T.; Abdelkareem, M.A.; Mahmoud, M.S.; Ramadan, M.; Olabi, A.G. Environmental Impact of Emerging Desalination Technologies: A Preliminary Evaluation. J. Environ. Chem. Eng. 2020, 8, 104099. [Google Scholar] [CrossRef]
- Ali, A.; Quist-Jensen, C.A.; Jørgensen, M.K.; Siekierka, A.; Christensen, M.L.; Bryjak, M.; Hélix-Nielsen, C.; Drioli, E. A Review of Membrane Crystallization, Forward Osmosis and Membrane Capacitive Deionization for Liquid Mining. Resour. Conserv. Recycl. 2021, 168, 105273. [Google Scholar] [CrossRef]
- Cheng, Y.; Hao, Z.; Hao, C.; Deng, Y.; Li, X.; Li, K.; Zhao, Y. A Review of Modification of Carbon Electrode Material in Capacitive Deionization. RSC Adv. 2019, 9, 24401–24419. [Google Scholar] [CrossRef]
- Wang, L.; Dykstra, J.E.; Lin, S. Energy Efficiency of Capacitive Deionization. Environ. Sci. Technol. 2019, 53, 3366–3378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, H.; Zhao, H.; Wang, Y.; Tang, N. Electrode Materials for Capacitive Deionization: A Review. J. Electroanal. Chem. 2020, 873, 1327–1356. [Google Scholar] [CrossRef]
- Lei, J.; Yu, F.; Xie, H.; Ma, J. Ti3C2T x MXene/Carbon Nanofiber Multifunctional Electrode for Electrode Ionization with Antifouling Activity. Chem. Sci. 2023, 14, 3610–3621. [Google Scholar] [CrossRef]
- Rabaia, M.K.H.; Abdelkareem, M.A.; Sayed, E.T.; Elsaid, K.; Chae, K.J.; Wilberforce, T.; Olabi, A.G. Environmental Impacts of Solar Energy Systems: A Review. Sci. Total Environ. 2021, 754, 141989. [Google Scholar] [CrossRef]
- Anderson, M.A.; Cudero, A.L.; Palma, J. Capacitive Deionization as an Electrochemical Means of Saving Energy and Delivering Clean Water. Comparison to Present Desalination Practices: Will It Compete? Electrochim. Acta 2010, 55, 3845–3856. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; Van Der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the Science and Technology of Water Desalination by Capacitive Deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water Desalination via Capacitive Deionization: What Is It and What Can We Expect from It? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef] [Green Version]
- Hawks, S.A.; Knipe, J.M.; Campbell, P.G.; Loeb, C.K.; Hubert, M.A.; Santiago, J.G.; Stadermann, M. Quantifying the Flow Efficiency in Constant-Current Capacitive Deionization. Water Res. 2018, 129, 327–336. [Google Scholar] [CrossRef]
- Hemmatifar, A.; Palko, J.W.; Stadermann, M.; Santiago, J.G. Energy Breakdown in Capacitive Deionization. Water Res. 2016, 104, 303–311. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Xu, J.; Wang, C.; Wang, Y.; Yuan, D.; Chew, J.W. Electrosorption Performance on Graphene-Based Materials: A Review. RSC Adv. 2023, 13, 6518–6529. [Google Scholar] [CrossRef]
- Dykstra, J.E. Desalination with Porous Electrodes: Mechanisms of Ion Transport and Adsorption. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2018. [Google Scholar]
- Yasin, A.S.; Obaid, M.; Mohamed, I.M.A.; Yousef, A.; Barakat, N.A.M. ZrO2 Nanofibers/Activated Carbon Composite as a Novel and Effective Electrode Material for the Enhancement of Capacitive Deionization Performance. RSC Adv. 2017, 7, 4616–4626. [Google Scholar] [CrossRef] [Green Version]
- Hawks, S.A.; Ramachandran, A.; Porada, S.; Campbell, P.G.; Suss, M.E.; Biesheuvel, P.M.; Santiago, J.G.; Stadermann, M. Performance Metrics for the Objective Assessment of Capacitive Deionization Systems. Water Res. 2019, 152, 126–137. [Google Scholar] [CrossRef]
- Agartan, L.; Hayes-Oberst, B.; Byles, B.W.; Akuzum, B.; Pomerantseva, E.; Caglan Kumbur, E. Influence of Operating Conditions and Cathode Parameters on Desalination Performance of Hybrid CDI Systems. Desalination 2019, 452, 1–8. [Google Scholar] [CrossRef]
- Srimuk, P.; Zeiger, M.; Jäckel, N.; Tolosa, A.; Krüner, B.; Fleischmann, S.; Grobelsek, I.; Aslan, M.; Shvartsev, B.; Suss, M.E.; et al. Enhanced Performance Stability of Carbon/Titania Hybrid Electrodes during Capacitive Deionization of Oxygen Saturated Saline Water. Electrochim. Acta 2017, 224, 314–328. [Google Scholar] [CrossRef]
- Sayed, E.T.; Olabi, A.G.; Shehata, N.; Al Radi, M.; Majdy Muhaisen, O.; Rodriguez, C.; Ali Atieh, M.; Abdelkareem, M.A. Application of Bio-Based Electrodes in Emerging Capacitive Deionization Technology for Desalination and Wastewater Treatment. Ain Shams Eng. J. 2022, 14, 102030. [Google Scholar] [CrossRef]
- Rommerskirchen, A.; Kalde, A.; Linnartz, C.J.; Bongers, L.; Linz, G.; Wessling, M. Unraveling Charge Transport in Carbon Flow-Electrodes: Performance Prediction for Desalination Applications. Carbon 2019, 145, 507–520. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Ma, J.; Zhang, C.; Song, J.; Waite, T.D. Short-Circuited Closed-Cycle Operation of Flow-Electrode CDI for Brackish Water Softening. Environ. Sci. Technol. 2018, 52, 9350–9360. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; Wu, L.; Sun, J.; Wang, L.; Li, T.; Waite, T.D. Flow Electrode Capacitive Deionization (FCDI): Recent Developments, Environmental Applications, and Future Perspectives. Environ. Sci. Technol. 2021, 55, 4243–4267. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion Exchange Membranes for Alkaline Fuel Cells: A Review. J. Memb. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- McNair, R.; Szekely, G.; Dryfe, R.A.W. Ion-Exchange Materials for Membrane Capacitive Deionization. ACS EST Water 2021, 1, 217–239. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, J.H. Enhanced Desalination Efficiency in Capacitive Deionization with an Ion-Selective Membrane. Sep. Purif. Technol. 2010, 71, 70–75. [Google Scholar] [CrossRef]
- Długołecki, P.; Van Der Wal, A. Energy Recovery in Membrane Capacitive Deionization. Environ. Sci. Technol. 2013, 47, 4904–4910. [Google Scholar] [CrossRef]
- Han, L.; Karthikeyan, K.G.; Gregory, K.B. Energy Consumption and Recovery in Capacitive Deionization Using Nanoporous Activated Carbon Electrodes. J. Electrochem. Soc. 2015, 162, E282–E288. [Google Scholar] [CrossRef]
- Kang, J.; Kim, T.; Jo, K.; Yoon, J. Comparison of Salt Adsorption Capacity and Energy Consumption between Constant Current and Constant Voltage Operation in Capacitive Deionization. Desalination 2014, 352, 52–57. [Google Scholar] [CrossRef]
- Kim, T.; Yoon, J. CDI Ragone Plot as a Functional Tool to Evaluate Desalination Performance in Capacitive Deionization. RSC Adv. 2015, 5, 1456–1461. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, J.H. Improvement of Desalination Efficiency in Capacitive Deionization Using a Carbon Electrode Coated with an Ion-Exchange Polymer. Water Res. 2010, 44, 990–996. [Google Scholar] [CrossRef]
- Li, H.; Liang, S.; Li, J.; He, L. The Capacitive Deionization Behaviour of a Carbon Nanotube and Reduced Graphene Oxide Composite. J. Mater. Chem. A Mater. 2013, 1, 6335–6341. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, L.; Xu, X.; Lu, T.; Sun, Z.; Chua, D.H.C. Enhanced Desalination Efficiency in Modified Membrane Capacitive Deionization by Introducing Ion-Exchange Polymers in Carbon Nanotubes Electrodes. Electrochim. Acta 2014, 130, 619–624. [Google Scholar] [CrossRef]
- Zhao, R.; Satpradit, O.; Rijnaarts, H.H.M.; Biesheuvel, P.M.; van der Wal, A. Optimization of Salt Adsorption Rate in Membrane Capacitive Deionization. Water Res. 2013, 47, 1941–1952. [Google Scholar] [CrossRef]
- Zhao, R.; Biesheuvel, P.M.; Van Der Wal, A. Energy Consumption and Constant Current Operation in Membrane Capacitive Deionization. Energy Environ. Sci. 2012, 5, 9520–9527. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, Y.; Wang, R.; Wu, Y.; Xu, S.; Wang, J. Performance Comparison and Energy Consumption Analysis of Capacitive Deionization and Membrane Capacitive Deionization Processes. Desalination 2013, 324, 127–133. [Google Scholar] [CrossRef]
- Dykstra, J.E.; Keesman, K.J.; Biesheuvel, P.M.; van der Wal, A. Theory of PH Changes in Water Desalination by Capacitive Deionization. Water Res. 2017, 119, 178–186. [Google Scholar] [CrossRef]
- He, D.; Wong, C.E.; Tang, W.; Kovalsky, P.; David Waite, T. Faradaic Reactions in Water Desalination by Batch-Mode Capacitive Deionization. Environ. Sci. Technol. Lett. 2016, 3, 222–226. [Google Scholar] [CrossRef]
- Tang, W.; He, D.; Zhang, C.; Kovalsky, P.; Waite, T.D. Comparison of Faradaic Reactions in Capacitive Deionization (CDI) and Membrane Capacitive Deionization (MCDI) Water Treatment Processes. Water Res. 2017, 120, 229–237. [Google Scholar] [CrossRef]
- Jain, A.; Kim, J.; Owoseni, O.M.; Weathers, C.; Caña, D.; Zuo, K.; Walker, W.S.; Li, Q.; Verduzco, R. Aqueous-Processed, High-Capacity Electrodes for Membrane Capacitive Deionization. Environ. Sci. Technol. 2018, 52, 5859–5867. [Google Scholar] [CrossRef]
- Ahualli, S.; Iglesias, G.R.; Fernández, M.M.; Jiménez, M.L.; Delgado, Á.V. Use of Soft Electrodes in Capacitive Deionization of Solutions. Environ. Sci. Technol. 2017, 51, 5326–5333. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Hamelers, H.V.M.; Suss, M.E. Theory of Water Desalination by Porous Electrodes with Immobile Chemical Charge. Colloids Interface Sci. Commun. 2015, 9, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Porada, S.; Omosebi, A.; Liu, K.L.; Biesheuvel, P.M.; Landon, J. Complementary Surface Charge for Enhanced Capacitive Deionization. Water Res. 2016, 92, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Omosebi, A.; Landon, J.; Liu, K. Surface Charge Enhanced Carbon Electrodes for Stable and Efficient Capacitive Deionization Using Inverted Adsorption-Desorption Behavior. Energy Environ. Sci. 2015, 8, 897–909. [Google Scholar] [CrossRef]
- Yu, T.H.; Shiu, H.Y.; Lee, M.; Chiueh, P.T.; Hou, C.H. Life Cycle Assessment of Environmental Impacts and Energy Demand for Capacitive Deionization Technology. Desalination 2016, 399, 53–60. [Google Scholar] [CrossRef]
- Kim, S.J.; Ko, S.H.; Kang, K.H.; Han, J. Direct Seawater Desalination by Ion Concentration Polarization. Nat. Nanotechnol. 2010, 5, 297–301. [Google Scholar] [CrossRef]
- Togashi, Y.; Shirakawa, J.; Okuyama, T.; Yamazaki, S.; Kyohara, M.; Miyazawa, A.; Suzuki, T.; Hamada, M.; Terauchi, Y. Evaluation of the Appropriateness of Using Glucometers for Measuring the Blood Glucose Levels in Mice. Sci. Rep. 2016, 6, 25465. [Google Scholar] [CrossRef]
- Liu, M.; Xue, Z.; Zhang, H.; Li, Y. Dual-Channel Membrane Capacitive Deionization Based on Asymmetric Ion Adsorption for Continuous Water Desalination. Electrochem. Commun. 2021, 125, 106974. [Google Scholar] [CrossRef]
- Yasin, A.S.; Mohamed, A.Y.; Mohamed, I.M.A.; Cho, D.Y.; Park, C.H.; Kim, C.S. Theoretical Insight into the Structure-Property Relationship of Mixed Transition Metal Oxides Nanofibers Doped in Activated Carbon and 3D Graphene for Capacitive Deionization. Chem. Eng. J. 2019, 371, 166–181. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Sufiani, O.; Sahini, M.G.; Elisadiki, J. Towards Attaining SDG 6: The Opportunities Available for Capacitive Deionization Technology to Provide Clean Water to the African Population. Environ. Res. 2023, 216, 114671. [Google Scholar] [CrossRef]
- Jung, Y.J.; Baek, K.W.; Oh, B.S.; Kang, J.W. An Investigation of the Formation of Chlorate and Perchlorate during Electrolysis Using Pt/Ti Electrodes: The Effects of PH and Reactive Oxygen Species and the Results of Kinetic Studies. Water Res. 2010, 44, 5345–5355. [Google Scholar] [CrossRef] [PubMed]
- Likhachev, D.S.; Li, F.C. Large-Scale Water Desalination Methods: A Review and New Perspectives. Desalination Water Treat. 2013, 51, 2836–2849. [Google Scholar] [CrossRef]
- Amy, G.; Ghaffour, N.; Li, Z.; Francis, L.; Linares, R.V.; Missimer, T.; Lattemann, S. Membrane-Based Seawater Desalination: Present and Future Prospects. Desalination 2017, 401, 16–21. [Google Scholar] [CrossRef]
- Qin, M.; Deshmukh, A.; Epsztein, R.; Patel, S.K.; Owoseni, O.M.; Walker, W.S.; Elimelech, M. Comparison of Energy Consumption in Desalination by Capacitive Deionization and Reverse Osmosis. Desalination 2019, 455, 100–114. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Elimelech, M. Energy Requirements of Ammonia-Carbon Dioxide Forward Osmosis Desalination. Desalination 2007, 207, 370–382. [Google Scholar] [CrossRef]
- He, T.X.; Yan, L.-J. Application of Alternative Energy Integration Technology in Seawater Desalination. Desalination 2009, 249, 104–108. [Google Scholar] [CrossRef]
- Abdel-Jawad, M. Energy Sources for Coupling with Desalination Plants in the GCC Countries; United Nations Economic and Social Commission for Western Asia: Beirut, Lebanon, 2001; pp. 1–6. [Google Scholar]
- Wittholz, M.K.; O’Neill, B.K.; Colby, C.B.; Lewis, D. Estimating the Cost of Desalination Plants Using a Cost Database. Desalination 2008, 229, 10–20. [Google Scholar] [CrossRef]
- Cay-Durgun, P.; McCloskey, C.; Konecny, J.; Khosravi, A.; Lind, M.L. Evaluation of Thin Film Nanocomposite Reverse Osmosis Membranes for Long-Term Brackish Water Desalination Performance. Desalination 2017, 404, 304–312. [Google Scholar] [CrossRef]
- Pan, S.Y.; Haddad, A.Z.; Kumar, A.; Wang, S.W. Brackish Water Desalination Using Reverse Osmosis and Capacitive Deionization at the Water-Energy Nexus. Water Res. 2020, 183, 116064. [Google Scholar] [CrossRef]
- El Batouti, M.; Al-Harby, N.F.; Elewa, M.M. A Review on Promising Membrane Technology Approaches for Heavy Metal Removal from Water and Wastewater to Solve Water Crisis. Water 2021, 13, 3241. [Google Scholar] [CrossRef]
- El Batouti, M.; Alharby, N.F.; Elewa, M.M. Review of New Approaches for Fouling Mitigation in Membrane Separation Processes in Water Treatment Applications. Separations 2021, 9, 1. [Google Scholar] [CrossRef]
- Scott, K. (Ed.) Membranes for Electrochemical Cells. In Handbook of Industrial Membranes; Elsevier: Amsterdam, The Netherlands, 1995; pp. 773–790. ISBN 978-1-85617-233-2. [Google Scholar]
- Naim, M.M.; Batouti, M.E.; Elewa, M.M. Novel Heterogeneous Cellulose-Based Ion-Exchange Membranes for Electrodialysis. Polym. Bull. 2022, 79, 9753–9777. [Google Scholar] [CrossRef]
- Shin, Y.U.; Lim, J.; Boo, C.; Hong, S. Improving the Feasibility and Applicability of Flow-Electrode Capacitive Deionization (FCDI): Review of Process Optimization and Energy Efficiency. Desalination 2021, 502, 114930. [Google Scholar] [CrossRef]
- Ha, Y.; Lee, H.; Yoon, H.; Shin, D.; Ahn, W.; Cho, N.; Han, U.; Hong, J.; Anh Thu Tran, N.; Yoo, C.Y.; et al. Enhanced Salt Removal Performance of Flow Electrode Capacitive Deionization with High Cell Operational Potential. Sep. Purif. Technol. 2021, 254, 117500. [Google Scholar] [CrossRef]
- Kim, N.; Lee, J.; Kim, S.; Hong, S.P.; Lee, C.; Yoon, J.; Kim, C. Short Review of Multichannel Membrane Capacitive Deionization: Principle, Current Status, and Future Prospect. Appl. Sci. 2020, 10, 683. [Google Scholar] [CrossRef] [Green Version]
- Rommerskirchen, A.; Linnartz, C.J.; Egidi, F.; Kendir, S.; Wessling, M. Flow-Electrode Capacitive Deionization Enables Continuous and Energy-Efficient Brine Concentration. Desalination 2020, 490, 114453. [Google Scholar] [CrossRef]
- Benvenuti, T.; Giacobbo, A.; Santana Barros, K.; Scarazzato, T.; da Trindade, C.D.M. Electrodialysis, Electrodialysis Reversal and Capacitive Deionization Technologies. In Advancement in Polymer-Based Membranes for Water Remediation; Nayak, S.K., Dutta, K., Gohil, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 505–539. ISBN 9780323885140. [Google Scholar]
- Beke, M.; Velempini, T.; Pillay, K. Synthesis and Application of NiO-ZrO2@g-C3N4 Nanocomposite for High-Performance Hybrid Capacitive Deionisation. Results Chem. 2023, 5, 100799. [Google Scholar] [CrossRef]
- Datar, S.D.; Mohanapriya, K.; Ahirrao, D.J.; Jha, N. Comparative Study of Electrosorption Performance of Solar Reduced Graphene Oxide in Flow-between and Flow-through Capacitive Deionization Architectures. Sep. Purif. Technol. 2021, 257, 117972. [Google Scholar] [CrossRef]
- Sun, K.; Tebyetekerwa, M.; Wang, C.; Wang, X.; Zhang, X.; Zhao, X.S. Electrocapacitive Deionization: Mechanisms, Electrodes, and Cell Designs. Adv. Funct. Mater. 2023, 33, 2213578. [Google Scholar] [CrossRef]
- Fang, K.; Gong, H.; He, W.; Peng, F.; He, C.; Wang, K. Recovering Ammonia from Municipal Wastewater by Flow-Electrode Capacitive Deionization. Chem. Eng. J. 2018, 348, 301–309. [Google Scholar] [CrossRef]
- Agartan, L.; Akuzum, B.; Caglan Kumbur, E.; Agar, E. Impact of Flow Configuration on Electrosorption Performance and Energy Consumption of CDI Systems. J. Water Supply Res. Technol. AQUA 2020, 69, 134–144. [Google Scholar] [CrossRef]
- Koo, J.S.; Kwak, N.-S.; Hwang, T.S. Synthesis and Properties of Nonfluoro Aminated Poly (Vinylbenzyl Chloride-Co-Ethyl Methacrylate-Co-Styrene) Anion Exchange Membranes for MCDI Process. Polymer 2012, 36, 564–572. [Google Scholar]
- Tian, G.; Liu, L.; Meng, Q.; Cao, B. Preparation and Characterization of Cross-Linked Quaternised Polyvinyl Alcohol Membrane/Activated Carbon Composite Electrode for Membrane Capacitive Deionization. Desalination 2014, 354, 107–115. [Google Scholar] [CrossRef]
- Jeong, K.S.; Hwang, W.C.; Hwang, T.S. Synthesis of an Aminated Poly (Vinylidene Fluride-g-4-Vinyl Benzyl Chloride) Anion Exchange Membrane for Membrane Capacitive Deionization (MCDI). J. Memb. Sci. 2015, 495, 316–321. [Google Scholar] [CrossRef]
- Ul Haq, O.; Choi, J.-H.; Lee, Y.-S. Anion-Exchange Membrane for Membrane Capacitive Deionization Prepared via Pore-Filling Polymerization in a Porous Polyethylene Supporting Membrane. React. Funct. Polym. 2018, 132, 36–42. [Google Scholar] [CrossRef]
- Chang, J.; Tang, K.; Cao, H.; Zhao, Z.; Su, C.; Li, Y.; Duan, F.; Sheng, Y. Application of Anion Exchange Membrane and the Effect of Its Properties on Asymmetric Membrane Capacitive Deionization. Sep. Purif. Technol. 2018, 207, 387–395. [Google Scholar] [CrossRef]
- Qiu, Q.; Cha, J.-H.; Choi, Y.-W.; Choi, J.-H.; Shin, J.; Lee, Y.-S. Preparation of Polyethylene Membranes Filled with Crosslinked Sulfonated Polystyrene for Cation Exchange and Transport in Membrane Capacitive Deionization Process. Desalination 2017, 417, 87–93. [Google Scholar] [CrossRef]
- Kang, K.W.; Hwang, C.W.; Hwang, T.S. Synthesis and Properties of Sodium Vinylbenzene Sulfonate-Grafted Poly (Vinylidene Fluoride) Cation Exchange Membranes for Membrane Capacitive Deionization Process. Macromol. Res. 2015, 23, 1126–1133. [Google Scholar] [CrossRef]
- Cha, J.-H.; ul Haq, O.; Choi, J.-H.; Lee, Y.-S. Sulfonated Poly (Ether Ether Ketone) Ion-Exchange Membrane for Membrane Capacitive Deionization Applications. Macromol. Res. 2018, 26, 1273–1275. [Google Scholar] [CrossRef]
- Jain, A.; Weathers, C.; Kim, J.; Meyer, M.D.; Walker, W.S.; Li, Q.; Verduzco, R. Self Assembled, Sulfonated Pentablock Copolymer Cation Exchange Coatings for Membrane Capacitive Deionization. Mol. Syst. Des. Eng. 2019, 4, 348–356. [Google Scholar] [CrossRef]
- Oren, Y. Capacitive Deionization (CDI) for Desalination and Water Treatment—Past, Present and Future (a Review). Desalination 2008, 228, 10–29. [Google Scholar] [CrossRef]
- Welgemoed, T.J.; Schutte, C.F. Capacitive Deionization TechnologyTM: An Alternative Desalination Solution. Desalination 2005, 183, 327–340. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, W.S.; Choi, J.H. Electrode Reactions and Adsorption/Desorption Performance Related to the Applied Potential in a Capacitive Deionization Process. Desalination 2010, 258, 159–163. [Google Scholar] [CrossRef]
- Hassanvand, A.; Chen, G.Q.; Webley, P.A.; Kentish, S.E. A Comparison of Multicomponent Electrosorption in Capacitive Deionization and Membrane Capacitive Deionization. Water Res. 2018, 131, 100–109. [Google Scholar] [CrossRef] [Green Version]

| Desalination Technology | Driving Force | Energy Consumption (kWh/m3) | Cost of Water USD/m3 | Refs. |
|---|---|---|---|---|
| MSF | Thermally driven | 5–6 | 0.56–1.75 | [69] |
| MED | 3–4 | 0.52–8 | [70] | |
| ED | Potential difference | 2.6–5.5 | 0.6–1.05 | [71] |
| Seawater RO | Pressure | 5.1–7.45 | 0.45–1.72 | [72] |
| Brackish Water RO | 1 | 0.26–1.3 | [73] | |
| CDI | Potential difference | 0.1–1.5 | 0.11 | [68,74] |
| Membrane Type | Characterizations | Ref. | ||||
|---|---|---|---|---|---|---|
| Thickness (μm) | % Swelling | % Perm-Selectivity | IEC, mmol eq/g | Electrical Resistance (Ω cm2) | ||
| VBC-EMA-St | – | 23–68 | 0.9–1.7 | 1.6–8.8 | [89] | |
| QPVA | – | 7–31 | 1.2–2.8 | – | [90] | |
| PVDF-g-VBC | – | 12–56 | 0.4–1.3 | 2.0–15 | [91] | |
| PE-CMS | 29 | 5 | 3.0 | 0.3 | [92] | |
| QPPO | 62–70 | 10–90 | 1.3–2.6 | 1.0–9.0 | [93] | |
| CA-AEM | 0.581 | 70.87 | 37 | 1.34 | 5 | This study |
| Membrane Type | Characterizations | Ref. | ||||
|---|---|---|---|---|---|---|
| Thickness (μm) | % Swelling | % Perm-Selectivity | IEC, mmol eq/g | Electrical Resistance (Ω cm2) | ||
| VBC-EMA-St | – | 76–121 | 0.5–1.0 | 0.6–2.8 | [89] | |
| PE-CSPS | 25 | 26–36 | 0.7–1.0 | 0.3–0.6 | [94] | |
| PVDF-g-PSVBS | – | 7–61 | 0.1–1.1 | 2–60 | [95] | |
| sPEEK | 100 | 28–47 | 2.0–2.3 | 0.5–0.9 | [96] | |
| sPBC | – | 40–200 | 1.0–2.0 | – | [97] | |
| CA-CEM | 0.526 | 74.28 | 44.88 | 1.47 | 5 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elewa, M.M.; El Batouti, M.; Al-Harby, N.F. A Comparison of Capacitive Deionization and Membrane Capacitive Deionization Using Novel Fabricated Ion Exchange Membranes. Materials 2023, 16, 4872. https://doi.org/10.3390/ma16134872
Elewa MM, El Batouti M, Al-Harby NF. A Comparison of Capacitive Deionization and Membrane Capacitive Deionization Using Novel Fabricated Ion Exchange Membranes. Materials. 2023; 16(13):4872. https://doi.org/10.3390/ma16134872
Chicago/Turabian StyleElewa, Mahmoud M., Mervette El Batouti, and Nouf F. Al-Harby. 2023. "A Comparison of Capacitive Deionization and Membrane Capacitive Deionization Using Novel Fabricated Ion Exchange Membranes" Materials 16, no. 13: 4872. https://doi.org/10.3390/ma16134872
APA StyleElewa, M. M., El Batouti, M., & Al-Harby, N. F. (2023). A Comparison of Capacitive Deionization and Membrane Capacitive Deionization Using Novel Fabricated Ion Exchange Membranes. Materials, 16(13), 4872. https://doi.org/10.3390/ma16134872






