Thermophysical Properties of Laser Powder Bed Fused Ti-6Al-4V and AlSi10Mg Alloys Made with Varying Laser Parameters
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- (1)
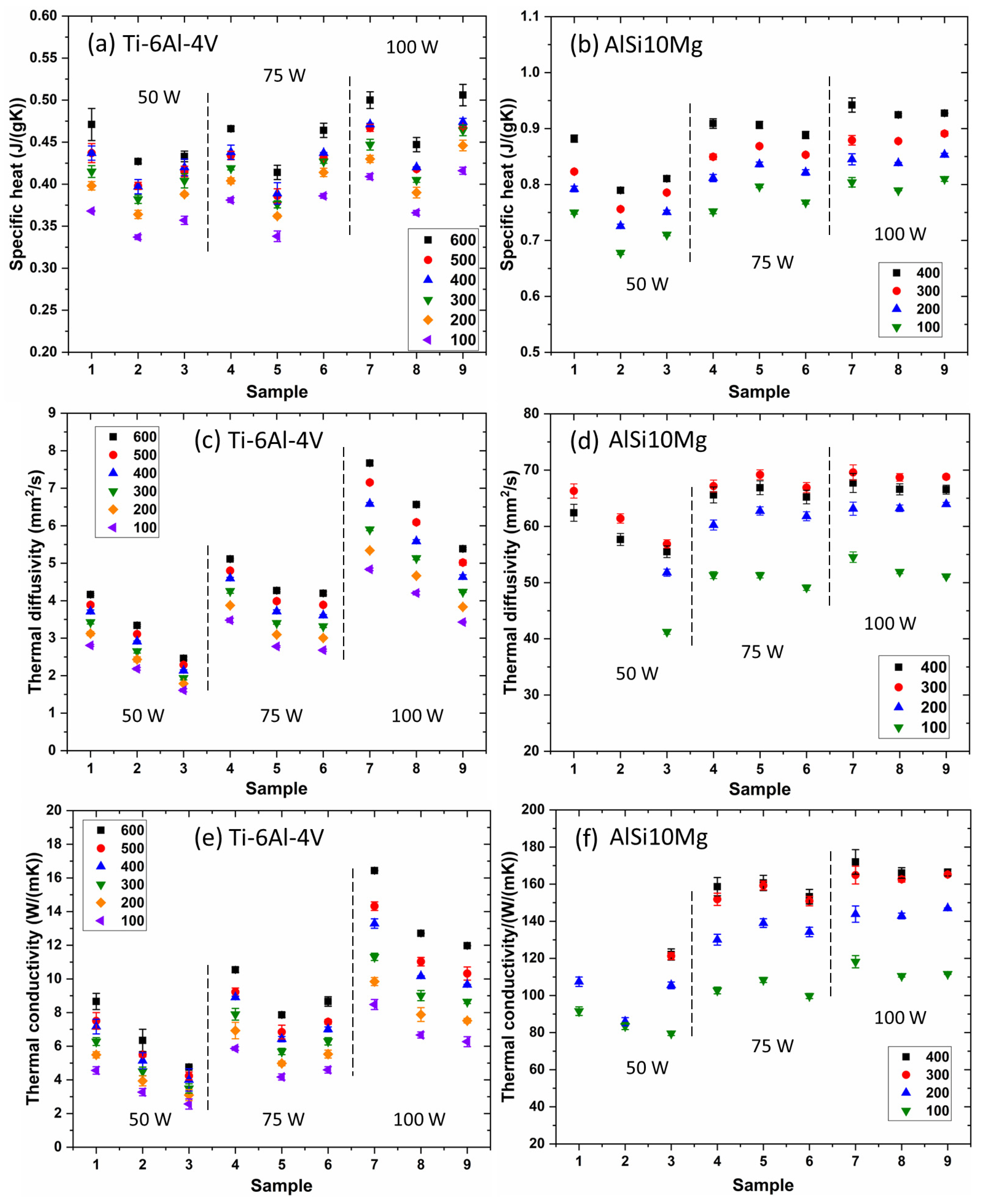
- As the test temperature rises, the specific heat values increase due to several factors, including enhanced atomic vibrations, excitation of more electrons to higher energy states, thermal expansion, and stronger phonon interactions at elevated temperatures.
- (2)
- The Ti-6Al-4V alloy exhibits low thermal conductivity, while the AlSi10Mg alloy demonstrates high thermal conductivity. The response of these alloys to changes in laser processing parameters varies. Thermophysical properties of L-PBF Ti-6Al-4V parts are significantly influenced by laser processing parameters. In contrast, the L-PBF AlSi10Mg alloy in this study does not demonstrate a clear sensitivity to variations in laser processing parameters. Specifically, at 100 °C, with the variation of laser processing parameters used in this study, the thermal conductivity of L-PBF Ti-6Al-4V alloy ranges from 2.6 to 8.5 W/(mK). This phenomenon is due to the fact that a higher cooling rate leads to an increase in the quantity of solute atoms within the L-PBF parts, as confirmed by Thermo-Calc simulation, which results in a reduction of thermal diffusivity and conductivity.
- (3)
- Furthermore, for both L-PBF Ti-6Al-4V and AlSi10Mg alloys, thermal conductivity increases with test temperature; for example, it rises from 8.5 to 16.4 W/(mK) and from 118.2 to 172.0 W/(mK) for L-PBF Ti-6Al-4V and AlSi10Mg alloys fabricated with a laser power of 100 W, and a laser scan speed of 200 mm/s, respectively. This trend can be attributed to the competition between impurity scattering and phonon-phonon scattering.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- du Plessis, A.; Razavi, N.; Benedetti, M.; Murchio, S.; Leary, M.; Watson, M.; Bhate, D.; Berto, F. Properties and applications of additively manufactured metallic cellular materials: A review. Prog. Mater. Sci. 2022, 125, 100918. [Google Scholar] [CrossRef]
- Zeng, C.; Guo, S.; Gradl, P.R.; Belcher, T. Thermophysical Properties of Select AM Alloys. In Metal Additive Manufacturing for Propulsion Applications; American Institute of Aeronautics and Astronautics, Inc.: Reston, VA, USA, 2022; pp. 775–824. [Google Scholar]
- Blakey-Milner, B.; Gradl, P.; Snedden, G.; Brooks, M.; Pitot, J.; Lopez, E.; Leary, M.; Berto, F.; du Plessis, A. Metal additive manufacturing in aerospace: A review. Mater. Des. 2021, 209, 110008. [Google Scholar] [CrossRef]
- Gradl, P.R.; Mireles, O.R.; Protz, C.S.; Garcia, C.P. Metal Additive Manufacturing for Propulsion Applications; American Institute of Aeronautics and Astronautics, Inc.: Reston, VA, USA, 2022. [Google Scholar]
- Manfredi, D.; Bidulský, R. Laser powder bed fusion of aluminum alloys. Acta Metall. Slovaca 2017, 23, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Wen, H.; Bernard, B.C.; Raush, J.R.; Gradl, P.R.; Khonsari, M.; Guo, S. Effect of temperature history on thermal properties of additively manufactured C-18150 alloy samples. Manuf. Lett. 2021, 28, 25–29. [Google Scholar] [CrossRef]
- Ettefagh, A.H.; Zeng, C.; Guo, S.; Raush, J. Corrosion behavior of additively manufactured Ti-6Al-4V parts and the effect of post annealing. Addit. Manuf. 2019, 28, 252–258. [Google Scholar] [CrossRef]
- Abedi, H.R.; Hanzaki, A.Z.; Azami, M.; Kahnooji, M.; Rahmatabadi, D. The high temperature flow behavior of additively manufactured Inconel 625 superalloy. Mater. Res. Express 2019, 6, 116514. [Google Scholar] [CrossRef]
- Ettefagh, A.H.; Guo, S.; Raush, J. Corrosion performance of additively manufactured stainless steel parts: A review. Addit. Manuf. 2021, 37, 101689. [Google Scholar] [CrossRef]
- Smith, T.M.; Kantzos, C.A.; Zarkevich, N.A.; Harder, B.J.; Heczko, M.; Gradl, P.R.; Thompson, A.C.; Mills, M.J.; Gabb, T.P.; Lawson, J.W. A 3D printable alloy designed for extreme environments. Nature 2023, 617, 513–518. [Google Scholar] [CrossRef]
- Yadav, P.; Rigo, O.; Arvieu, C.; Lacoste, E. Microstructural and mechanical aspects of AlSi7Mg0.6 alloy related to scanning strategies in L-PBF. Int. J. Adv. Manuf. Technol. 2022, 120, 6205–6223. [Google Scholar] [CrossRef]
- Sausto, F.; Carrion, P.; Shamsaei, N.; Beretta, S. Fatigue failure mechanisms for AlSi10Mg manufactured by L-PBF under axial and torsional loads: The role of defects and residual stresses. Int. J. Fatigue 2022, 162, 106903. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, B.; Ettefagh, A.H.; Wen, H.; Yao, H.; Meng, W.; Guo, S. Mechanical, thermal, and corrosion properties of Cu-10Sn alloy prepared by laser-powder-bed-fusion additive manufacturing. Addit. Manuf. 2020, 35, 101411. [Google Scholar] [CrossRef]
- Wang, Y.M.; Voisin, T.; McKeown, J.T.; Ye, J.; Calta, N.P.; Li, Z.; Zeng, Z.; Zhang, Y.; Chen, W.; Roehling, T.T.; et al. Additively manufactured hierarchical stainless steels with high strength and ductility. Nat. Mater. 2018, 17, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.; Tang, X.; Chen, X.; Sun, F.; Dong, L.; Tan, Y.; Chu, H.; Zhou, H.; Liu, P.; Fu, S. The effect of build orientations on mechanical and thermal properties on CuCrZr alloys fabricated by laser powder bed fusion. J. Mater. Res. Technol. 2023, 23, 3322–3336. [Google Scholar] [CrossRef]
- Yang, X.; Qi, Y.; Zhang, W.; Wang, Y.; Zhu, H. Laser powder bed fusion of C18150 copper alloy with excellent comprehensive properties. Mater. Sci. Eng. A 2023, 862, 144512. [Google Scholar] [CrossRef]
- Qi, X.; Takata, N.; Suzuki, A.; Kobashi, M.; Kato, M. Managing both high strength and thermal conductivity of a laser powder bed fused Al–2.5 Fe binary alloy: Effect of annealing on microstructure. Mater. Sci. Eng. A 2021, 805, 140591. [Google Scholar] [CrossRef]
- Azizi, A.; Hejripour, F.; Goodman, J.A.; Kulkarni, P.A.; Chen, X.; Zhou, G.; Schiffres, S.N. Process-dependent anisotropic thermal conductivity of laser powder bed fusion AlSi10Mg: Impact of microstructure and aluminum-silicon interfaces. Rapid Prototyp. J. 2023, 29, 1109–1120. [Google Scholar] [CrossRef]
- Saunders, N.; Miodownik, A.P. CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide; Elsevier: Oxford, UK, 1998. [Google Scholar]
- Huang, Q.; Hu, N.; Yang, X.; Zhang, R.; Feng, Q. Microstructure and inclusion of Ti–6Al–4V fabricated by selective laser melting. Front. Mater. Sci. 2016, 10, 428–431. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, Y.; Zhao, T.; Wang, P.; Wang, Y.; Chen, Z.; Li, Y.; Xiao, D.; Fang, D. Compression experiment and numerical evaluation on mechanical responses of the lattice structures with stochastic geometric defects originated from additive-manufacturing. Compos. Part B Eng. 2020, 194, 108030. [Google Scholar] [CrossRef]
- Eschner, N.; Weiser, L.; Häfner, B.; Lanza, G. Classification of specimen density in Laser Powder Bed Fusion (L-PBF) using in-process structure-borne acoustic process emissions. Addit. Manuf. 2020, 34, 101324. [Google Scholar] [CrossRef]
- Salloom, R.; Joshi, S.; Dahotre, N.; Srinivasan, S. Laser surface engineering of B4C/Fe nano composite coating on low carbon steel: Experimental coupled with computational approach. Mater. Des. 2020, 190, 108576. [Google Scholar] [CrossRef]
- Alhammad, M.; Esmaeili, S.; Toyserkani, E. Surface modification of Ti–6Al–4V alloy using laser-assisted deposition of a Ti–Si compound. Surf. Coat. Technol. 2008, 203, 1–8. [Google Scholar] [CrossRef]
- Chowdhury, S.; Yadaiah, N.; Prakash, C.; Ramakrishna, S.; Dixit, S.; Gupta, L.R.; Buddhi, D. Laser powder bed fusion: A state-of-the-art review of the technology, materials, properties & defects, and numerical modelling. J. Mater. Res. Technol. 2022, 20, 2109–2172. [Google Scholar] [CrossRef]
- Smith, D.S.; Puech, F.; Nait-Ali, B.; Alzina, A.; Honda, S. Grain boundary thermal resistance and finite grain size effects for heat conduction through porous polycrystalline alumina. Int. J. Heat Mass Transf. 2018, 121, 1273–1280. [Google Scholar] [CrossRef]
- Zhu, Y.; Lian, J.; Jiang, Q. Modeling of the melting point, Debye temperature, thermal expansion coefficient, and the specific heat of nanostructured materials. J. Phys. Chem. C 2009, 113, 16896–16900. [Google Scholar] [CrossRef]
- Matzke, H.; Lucuta, P.; Verrall, R.; Henderson, J. Specific heat of UO2-based SIMFUEL. J. Nucl. Mater. 1997, 247, 121–126. [Google Scholar] [CrossRef]
- Saha, R.; Biswas, P. Current status and development of external energy-assisted friction stir welding processes: A review. Weld. World 2022, 66, 577–609. [Google Scholar] [CrossRef]
- Mott, S.N. Metal–insulator transitions. Phys. Today 1978, 31, 42–47. [Google Scholar] [CrossRef]
- Meingast, C.; Junod, A.; Walker, E. Superconducting fluctuations and uniaxial-pressure dependence of Tc of a Bi2Sr2CaCu2O8+x single crystal from high-resolution thermal expansion. Phys. C Supercond. 1996, 272, 106–114. [Google Scholar] [CrossRef]
- Lv, W.; Henry, A. Non-negligible Contributions to Thermal Conductivity from Localized Modes in Amorphous Silicon Dioxide. Sci. Rep. 2016, 6, 35720. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Yuan, W.; Yue, H.; Zhu, Y. Study on microstructure and properties of Fe-based amorphous composite coating by high-speed laser cladding. Opt. Laser Technol. 2022, 146, 107574. [Google Scholar] [CrossRef]
- Zeng, C.; Ding, H.; Bhandari, U.; Guo, S.M. Design of crack-free laser additive manufactured Inconel 939 alloy driven by computational thermodynamics method. MRS Commun. 2022, 12, 844–849. [Google Scholar] [CrossRef]
- Li, J.; Huang, G.; Mi, X.; Peng, L.; Xie, H.; Kang, Y. Influence of the Ni/Co Mass Ratio on the Microstructure and Properties of Quaternary Cu-Ni-Co-Si Alloys. Materials 2019, 12, 2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rycerz, A. Wiedemann–Franz Law for Massless Dirac Fermions with Implications for Graphene. Materials 2021, 14, 2704. [Google Scholar] [CrossRef]
- Jeon, J.; Bae, D. Effect of cooling rate on the thermal and electrical conductivities of an A356 sand cast alloy. J. Alloy. Compd. 2019, 808, 151756. [Google Scholar] [CrossRef]
- Zhang, D.; Prasad, A.; Bermingham, M.J.; Todaro, C.J.; Benoit, M.J.; Patel, M.N.; Qiu, D.; StJohn, D.H.; Qian, M.; Easton, M.A. Grain Refinement of Alloys in Fusion-Based Additive Manufacturing Processes. Met. Mater. Trans. A 2020, 51, 4341–4359. [Google Scholar] [CrossRef]
- Chen, W.; Voisin, T.; Zhang, Y.; Florien, J.-B.; Spadaccini, C.M.; McDowell, D.L.; Zhu, T.; Wang, Y.M. Microscale residual stresses in additively manufactured stainless steel. Nat. Commun. 2019, 10, 4338. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, T.; Dutta, M.; Sarkar, D.; Biswas, K. Insights into Low Thermal Conductivity in Inorganic Materials for Thermoelectrics. J. Am. Chem. Soc. 2022, 144, 10099–10118. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, X.; Xu, J.; Zhang, P.; Lou, Z.; Reece, M.J.; Gao, F. Ultra-low thermal conductivity and enhanced mechanical properties of high-entropy rare earth niobates (RE3NbO7, RE = Dy, Y, Ho, Er, Yb). J. Eur. Ceram. Soc. 2021, 41, 1052–1057. [Google Scholar] [CrossRef]
- Hadadzadeh, A.; Asadi, E.; Shakil, S.I.; Amirkhiz, B.S.; Mohammadi, M.; Haghshenas, M. Indentation-derived mechanical properties of Ti-6Al-4V: Laser-powder bed fusion versus electron beam melting. Mater. Lett. 2021, 301, 130273. [Google Scholar] [CrossRef]
- Heidarzadeh, A.; Javidani, M.; Mofarrehi, M.; Motalleb-Nejad, P.; Mohammadzadeh, R.; Jafarian, H.; Chen, X.-G. Grain Structure Formation and Texture Modification through Multi-Pass Friction Stir Processing in AlSi10Mg Alloy Produced by Laser Powder Bed Fusion. Materials 2023, 16, 944. [Google Scholar] [CrossRef]
- Ślęzak, T.; Zmywaczyk, J.; Koniorczyk, P. Thermal diffusivity investigations of the Titanium Grade 1 in wide temperature range. AIP Conf. Proc. 2019, 2170, 020019. [Google Scholar] [CrossRef]
- Hrbek, J. Induction heating of thin nonmagnetic sheets in transverse time-variable magnetic field. Acta Tech. 2015, 60, 15–29. [Google Scholar]
- Tong, Z.; Li, S.; Ruan, X.; Bao, H. Comprehensive first-principles analysis of phonon thermal conductivity and electron-phonon coupling in different metals. Phys. Rev. B 2019, 100, 144306. [Google Scholar] [CrossRef] [Green Version]
- Tritt, T.M. Thermal Conductivity: Theory, Properties, and Applications; Springer Science & Business Media: New York, NY, USA, 2005. [Google Scholar]
- Galsin, J.S. Impurity Scattering in Metallic Alloys; Springer Science & Business Media: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Qiu, Q.; Liu, Y.; Xia, K.; Fang, T.; Yu, J.; Zhao, X.; Zhu, T. Grain Boundary Scattering of Charge Transport in n-Type (Hf,Zr)CoSb Half-Heusler Thermoelectric Materials. Adv. Energy Mater. 2019, 9, 1803447. [Google Scholar] [CrossRef]

| Alloy | Element Content (wt.%) | |||||
|---|---|---|---|---|---|---|
| Ti | Al | V | Si | Mg | Fe | |
| Ti-6Al-4V | Bal. | 6.02 | 3.98 | / | / | / |
| AlSi10Mg | / | Bal. | / | 9.92 | 0.291 | 0.137 |
| Samples | Laser Power (W) | Scanning Speed (m/s) | Sample Denotation for Ti-6Al-4V Alloy | Sample Denotation for AlSi10Mg Alloy |
|---|---|---|---|---|
| 1 | 50 | 0.2 | Ti-50-0.2 | Al-50-0.2 |
| 2 | 50 | 0.4 | Ti-50-0.4 | Al-50-0.4 |
| 3 | 50 | 0.6 | Ti-50-0.6 | Al-50-0.6 |
| 4 | 75 | 0.2 | Ti-75-0.2 | Al-75-0.2 |
| 5 | 75 | 0.4 | Ti-75-0.4 | Al-75-0.4 |
| 6 | 75 | 0.6 | Ti-75-0.6 | Al-75-0.6 |
| 7 | 100 | 0.2 | Ti-100-0.2 | Al-100-0.2 |
| 8 | 100 | 0.4 | Ti-100-0.4 | Al-100-0.4 |
| 9 | 100 | 0.6 | Ti-100-0.6 | Al-100-0.6 |
| Sample | Ti-6Al-4V | AlSi10Mg | ||
|---|---|---|---|---|
| Density (g/cm3) | Relative Density * (%) | Density (g/cm3) | Relative Density * (%) | |
| 50-0.2 | 4.417 ± 0.006 | 99.8 | 2.620 ± 0.003 | 97.0 |
| 50-0.4 | 4.448 ± 0.016 | 100.5 | 2.681 ± 0.007 | 99.3 |
| 50-0.6 | 4.465 ± 0.008 | 100.9 | 2.715 ± 0.005 | 100.4 |
| 75-0.2 | 4.425 ± 0.012 | 99.8 | 2.661 ± 0.008 | 98.5 |
| 75-0.4 | 4.444 ± 0.002 | 100.2 | 2.651 ± 0.004 | 98.1 |
| 75-0.6 | 4.444 ± 0.017 | 100.2 | 2.644 ± 0.006 | 97.8 |
| 100-0.2 | 4.286 ± 0.016 | 96.8 | 2.696 ± 0.005 | 100.0 |
| 100-0.4 | 4.331 ± 0.007 | 97.7 | 2.697 ± 0.003 | 100.0 |
| 100-0.6 | 4.394 ± 0.006 | 99.1 | 2.695 ± 0.005 | 99.6 |
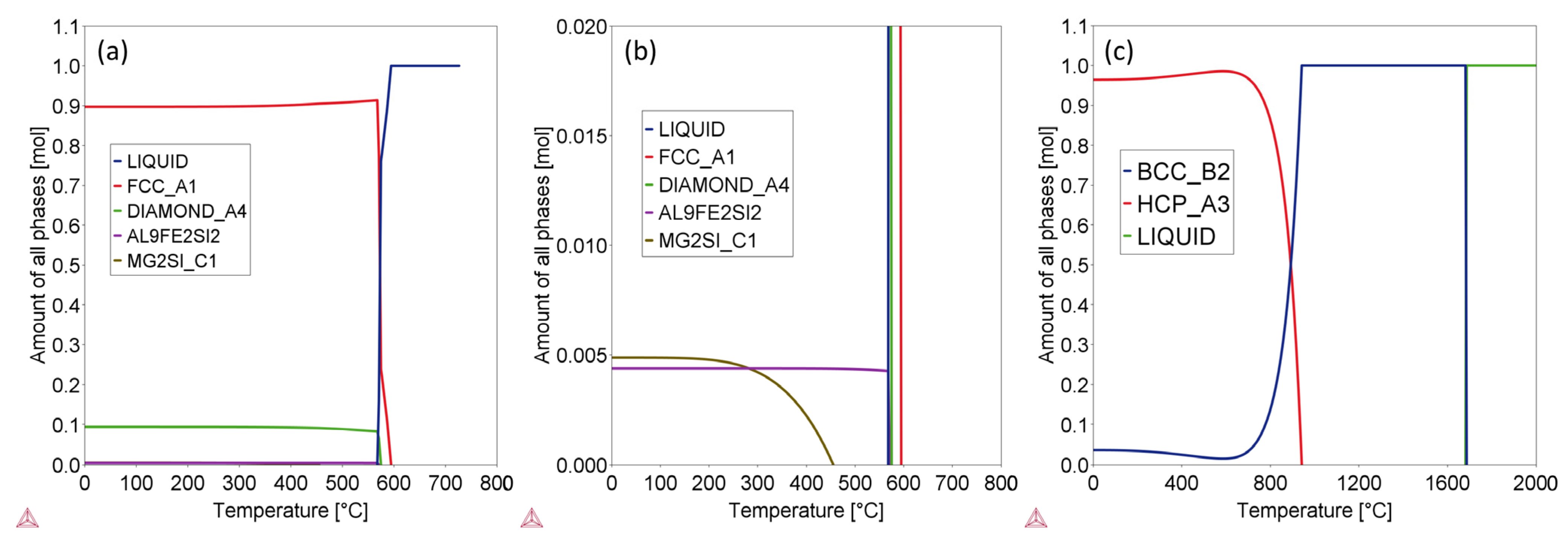
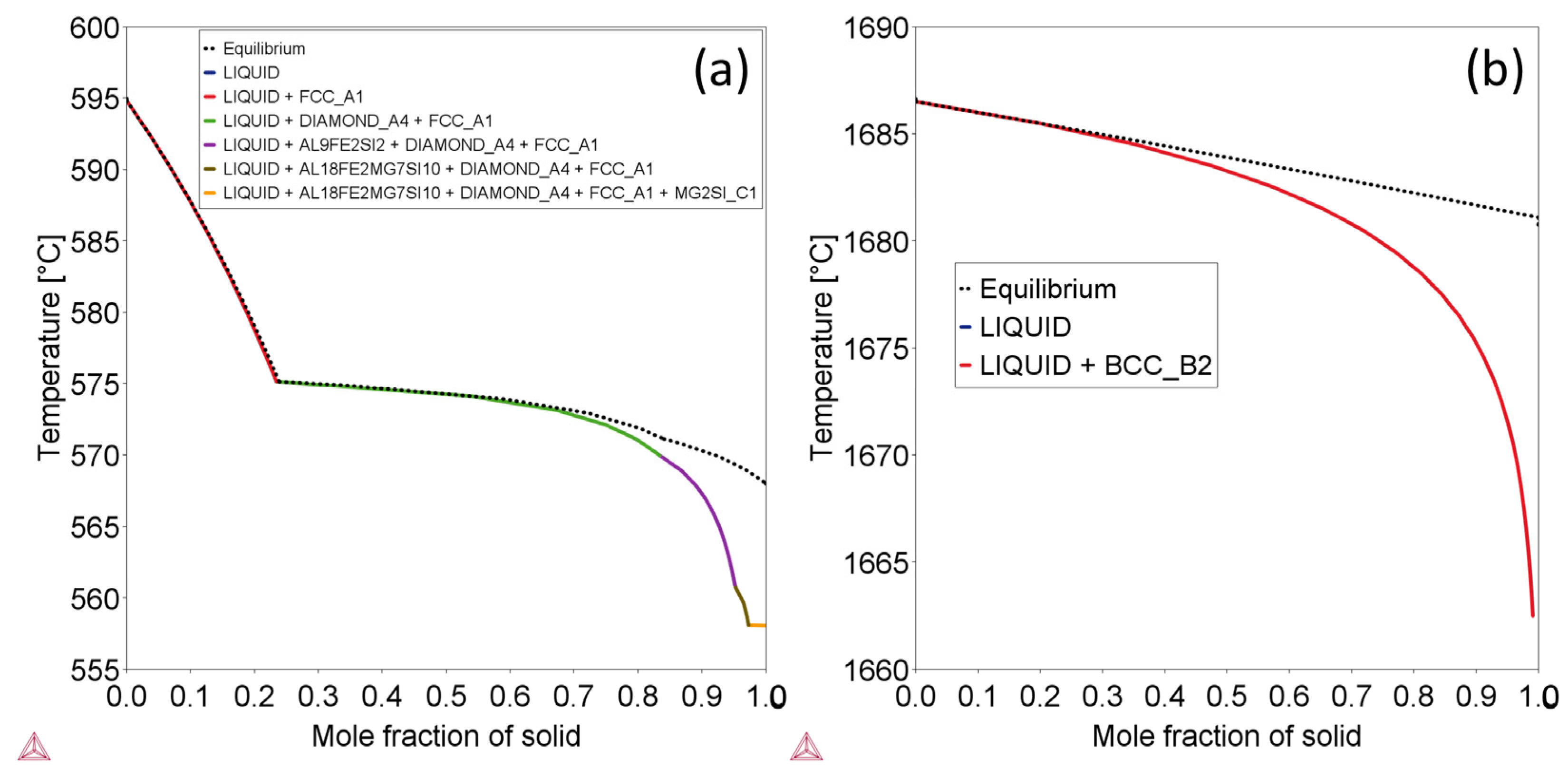
| Alloy | Stable Phases at RT | Phase Content (wt.%) | Phase Composition | |||||
|---|---|---|---|---|---|---|---|---|
| Ti | Al | V | Si | Mg | Fe | |||
| Ti-6Al-4V | HCP_A3 | 96.01 | 0.9373 | 0.0625 | 0.0002 | / | / | / |
| BCC_B2 | 3.99 | 0.0028 | 0.0002 | 0.9971 | / | / | / | |
| AlSi10Mg | AL9FE2SI2 | 0.51 | / | 0.6411 | / | 0.0892 | / | 0.2697 |
| Diamond_A4 | 9.71 | / | / | / | 1.0000 | / | / | |
| FCC_A1 | 89.33 | / | 1.0000 | / | / | / | / | |
| MG2SI_C1 | 0.46 | / | / | / | 0.3662 | 0.6338 | / | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akwaboa, S.; Zeng, C.; Amoafo-Yeboah, N.; Ibekwe, S.; Mensah, P. Thermophysical Properties of Laser Powder Bed Fused Ti-6Al-4V and AlSi10Mg Alloys Made with Varying Laser Parameters. Materials 2023, 16, 4920. https://doi.org/10.3390/ma16144920
Akwaboa S, Zeng C, Amoafo-Yeboah N, Ibekwe S, Mensah P. Thermophysical Properties of Laser Powder Bed Fused Ti-6Al-4V and AlSi10Mg Alloys Made with Varying Laser Parameters. Materials. 2023; 16(14):4920. https://doi.org/10.3390/ma16144920
Chicago/Turabian StyleAkwaboa, Stephen, Congyuan Zeng, Nigel Amoafo-Yeboah, Samuel Ibekwe, and Patrick Mensah. 2023. "Thermophysical Properties of Laser Powder Bed Fused Ti-6Al-4V and AlSi10Mg Alloys Made with Varying Laser Parameters" Materials 16, no. 14: 4920. https://doi.org/10.3390/ma16144920





