Organic—Inorganic Hybrid Interfaces Enable the Preparation of Nitrogen-Doped Hollow Carbon Nanospheres as High-Performance Anodes for Lithium and Potassium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
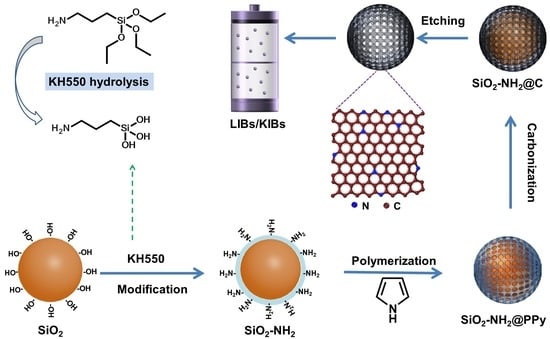
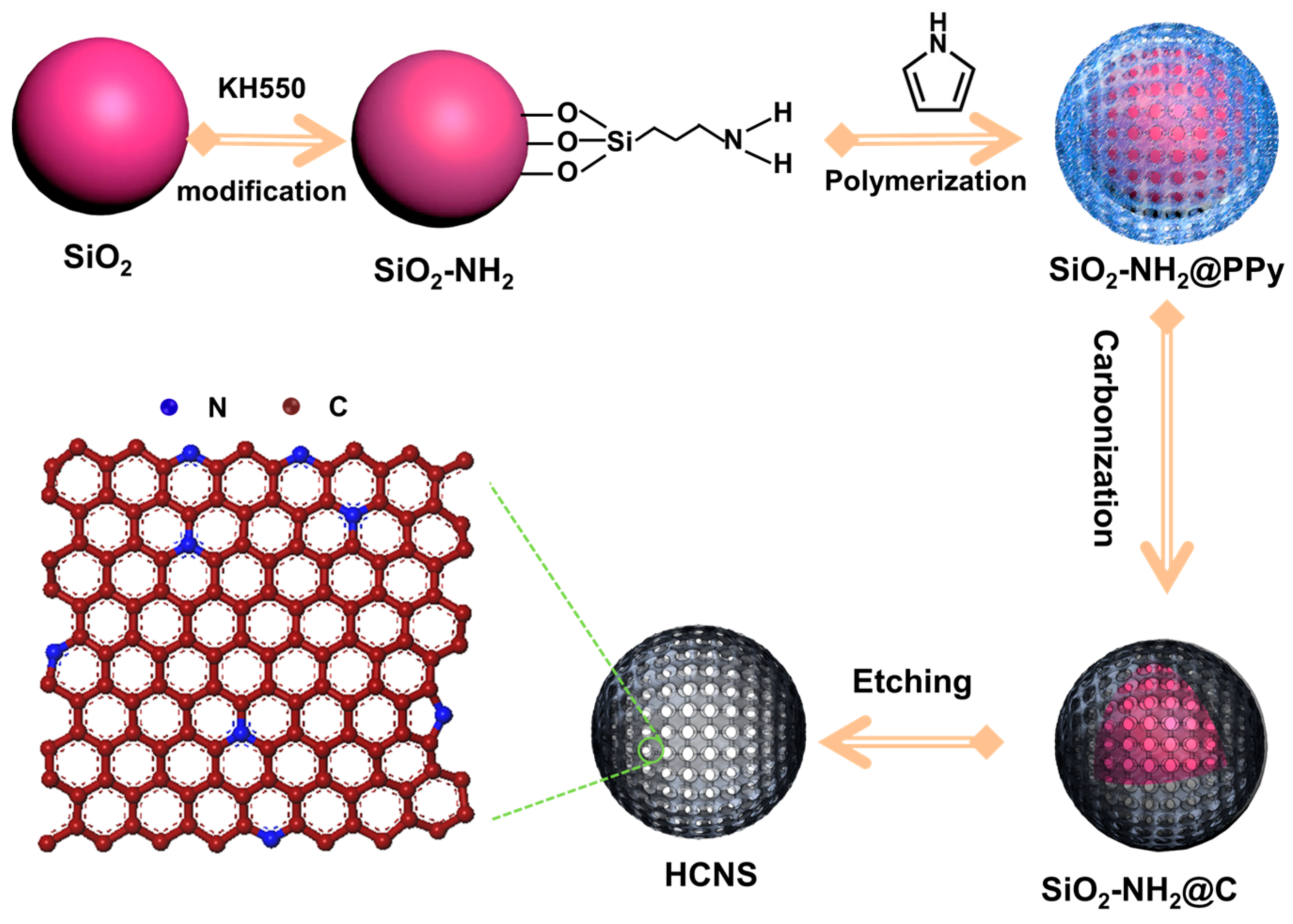
2.1. Synthesis of HCNS
2.2. Characterization
2.3. Electrochemical Measurements
2.4. Molecular Dynamics Computational Methods
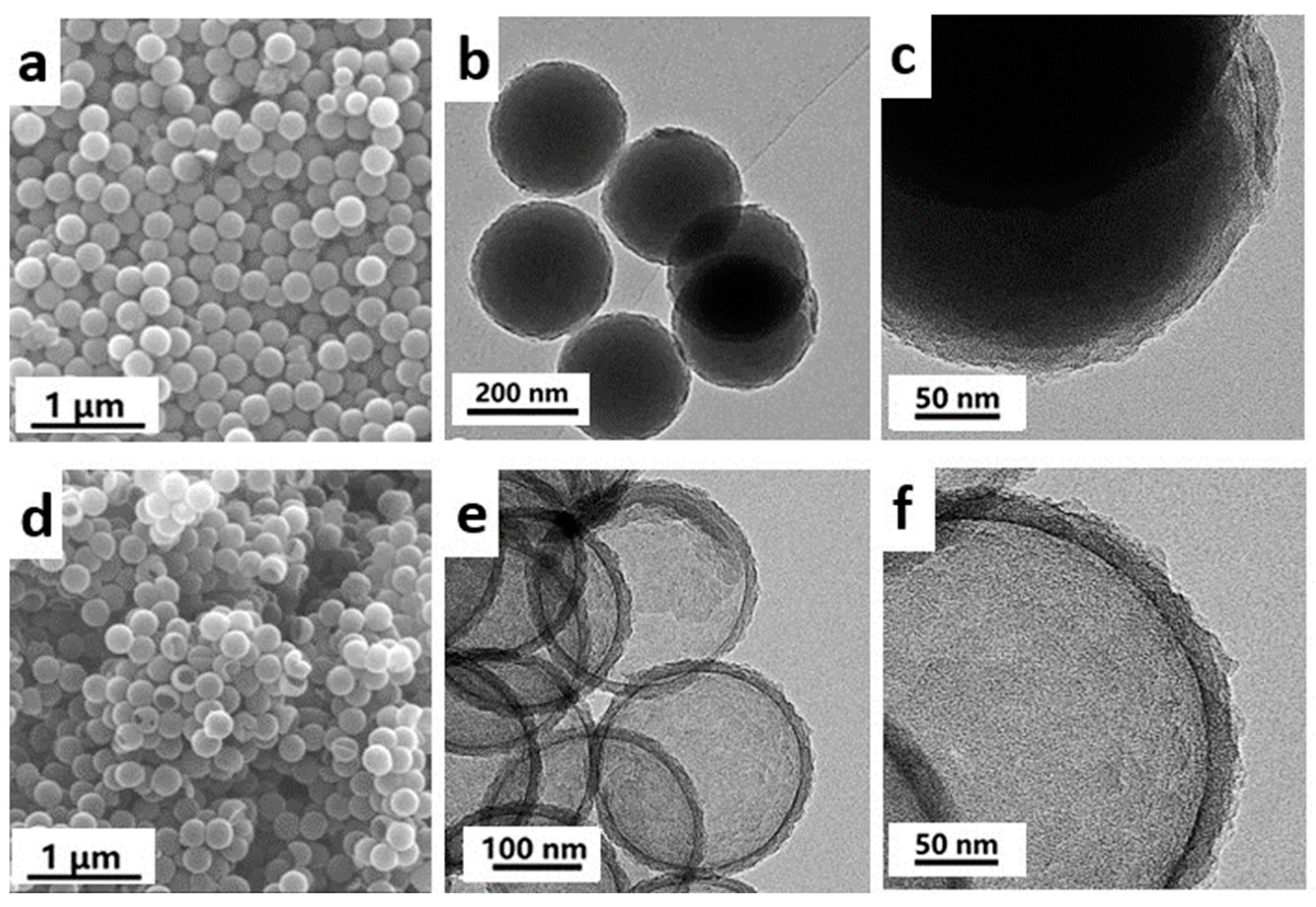
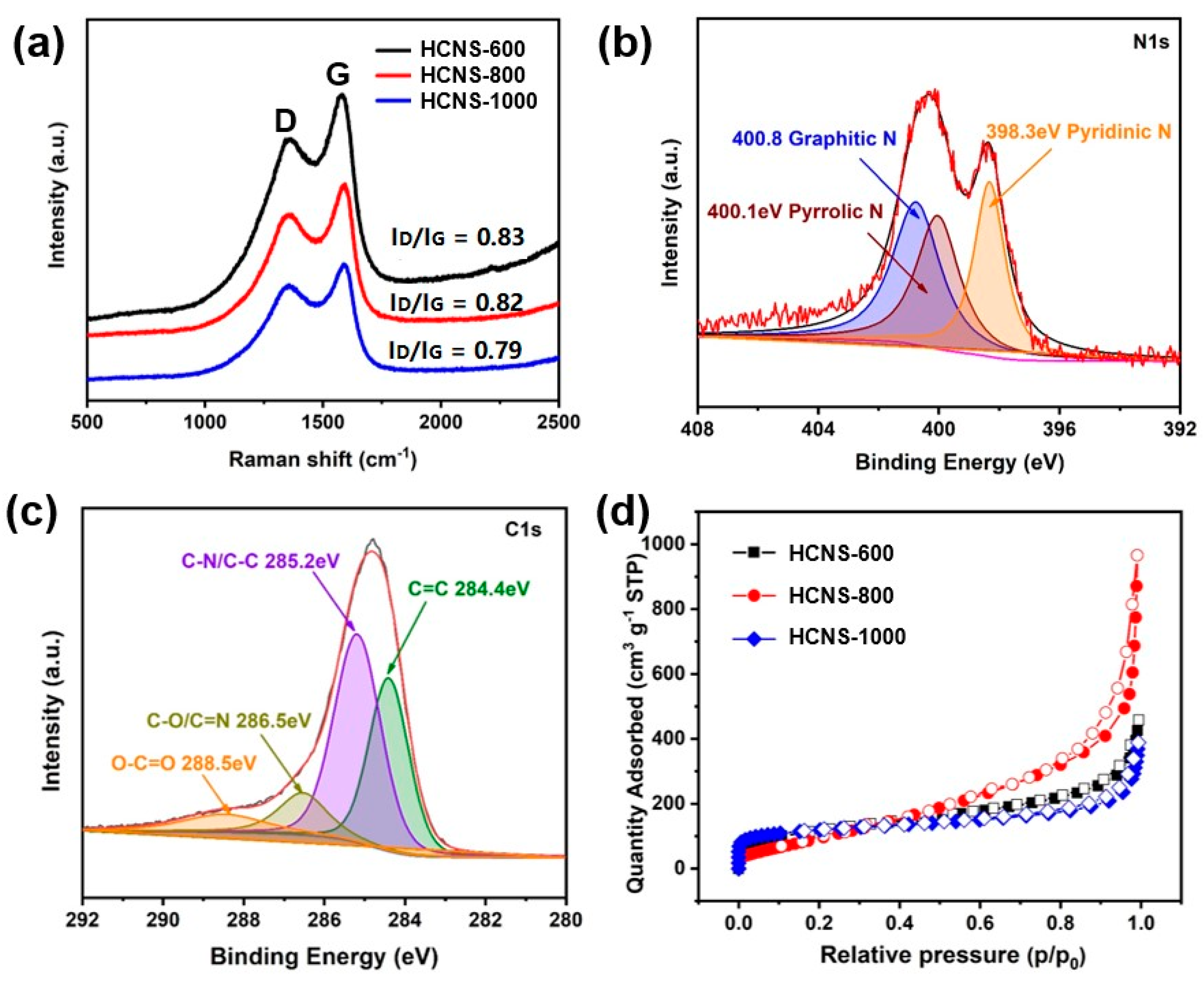
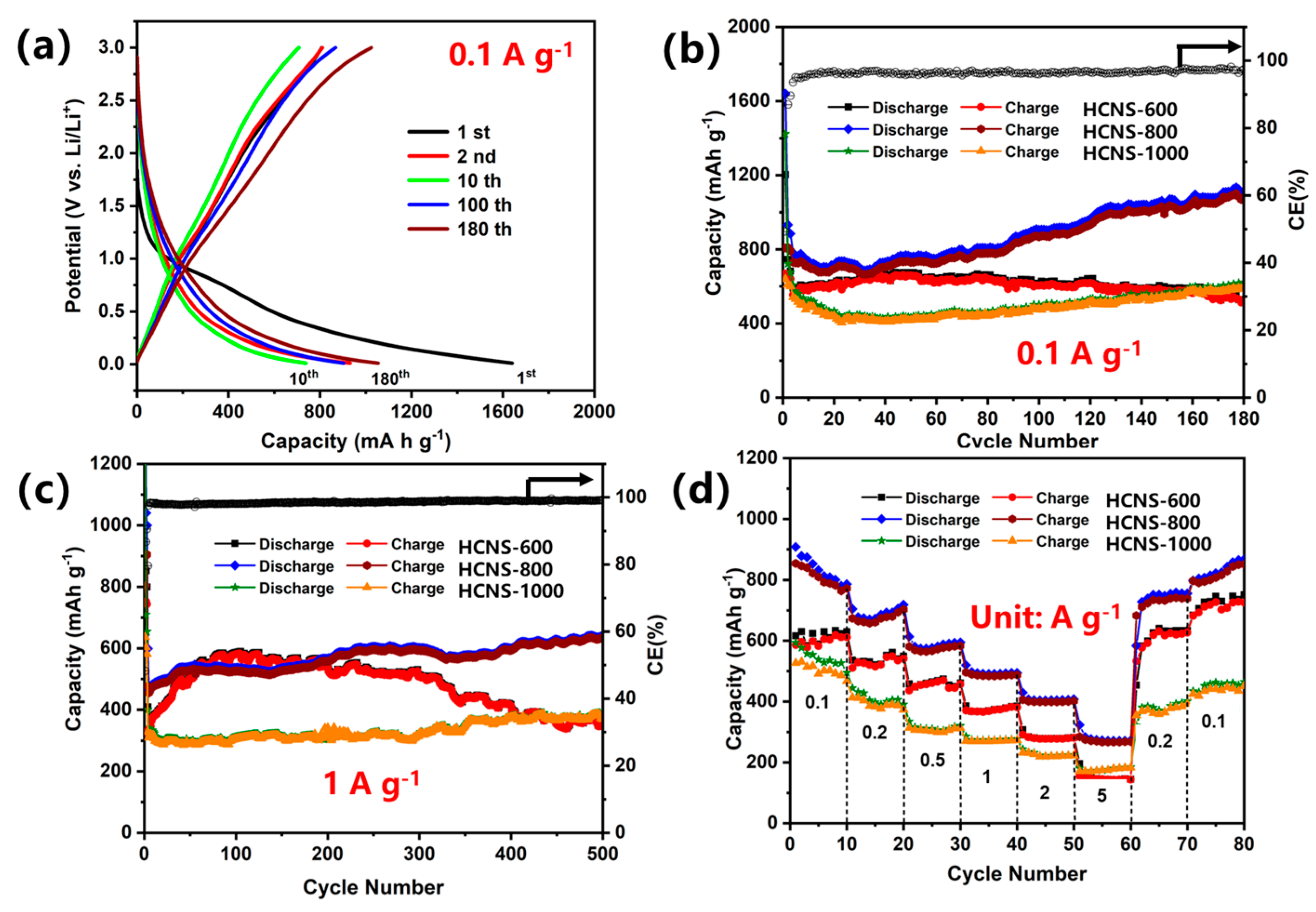
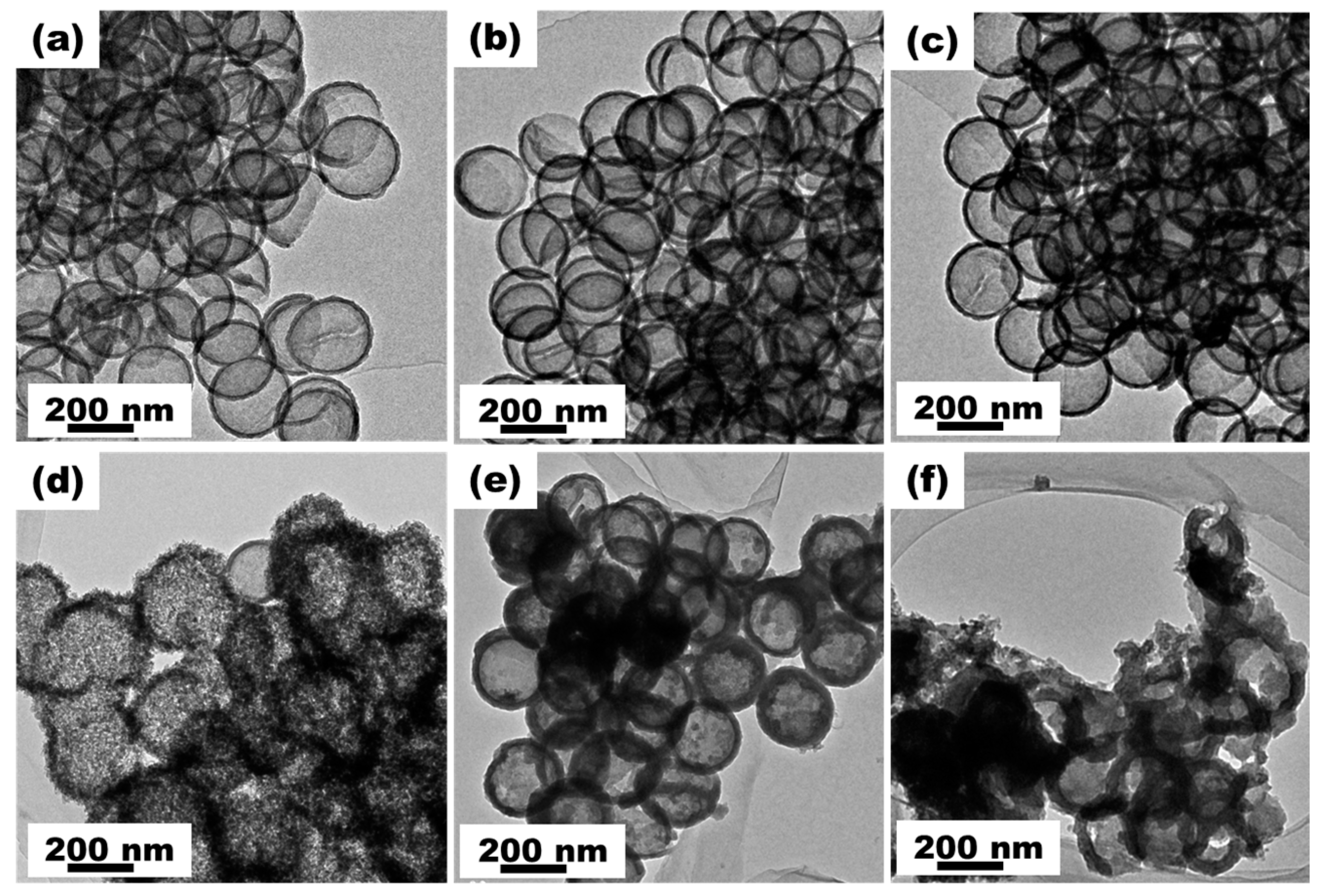
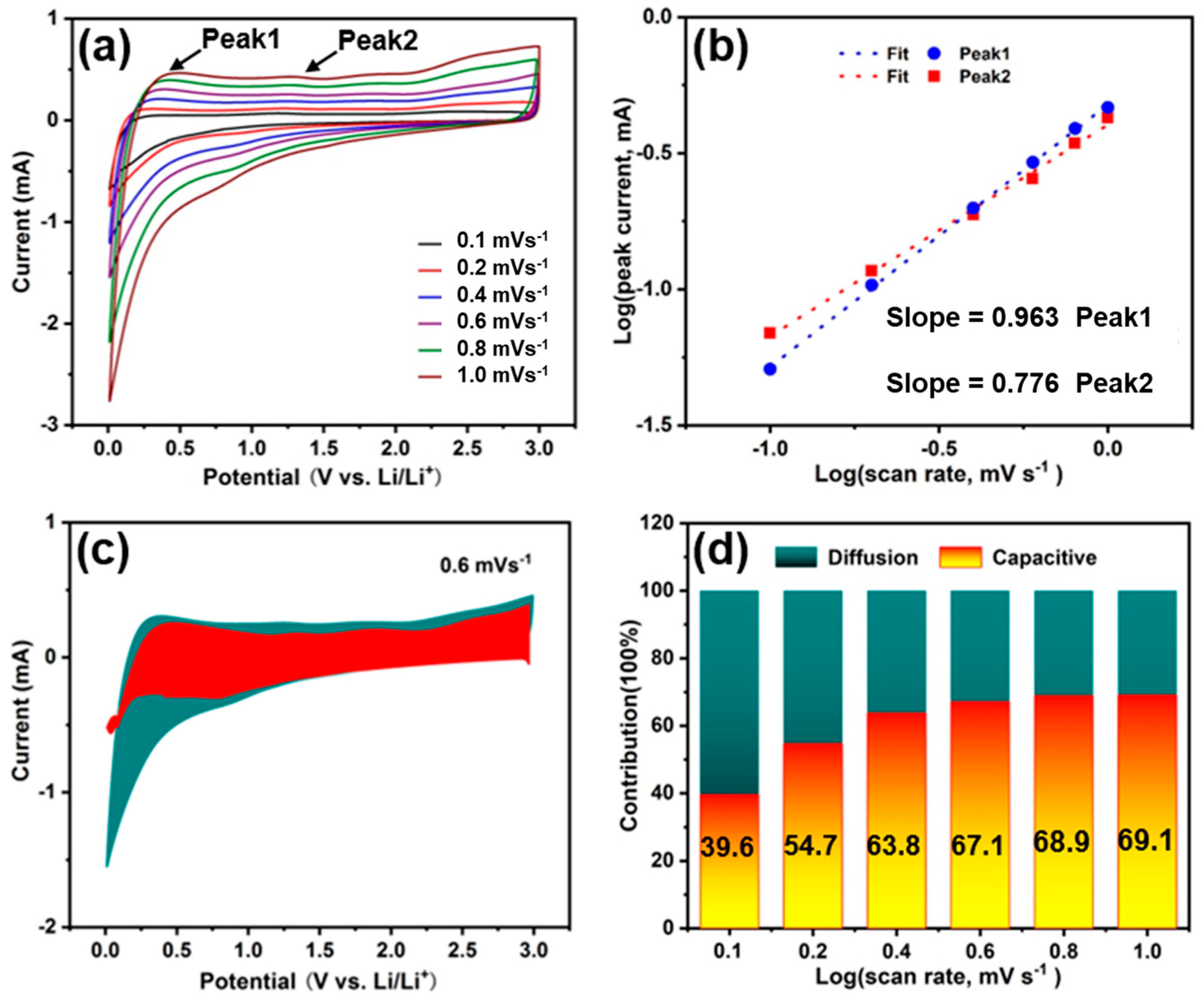
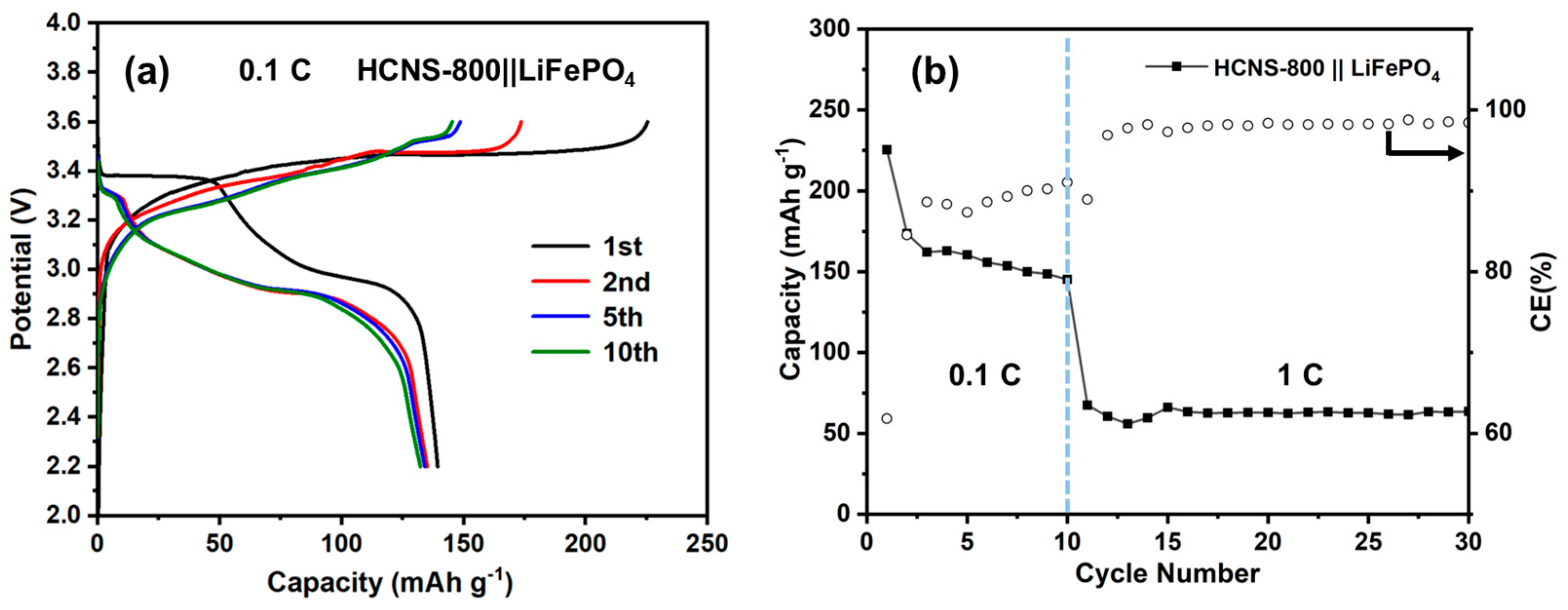
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energ. Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Kim, M.G.; Cho, J. Reversible and High-Capacity Nanostructured Electrode Materials for Li-Ion Batteries. Adv. Funct. Mater. 2009, 19, 1497–1514. [Google Scholar] [CrossRef]
- Amin, K.; Mao, L.; Wei, Z. Recent Progress in Polymeric Carbonyl-Based Electrode Materials for Lithium and Sodium Ion Batteries. Macromol. Rapid Comm. 2019, 40, 1800565. [Google Scholar] [CrossRef] [PubMed]
- Kam, K.C.; Doeff, M.M. Aliovalent titanium substitution in layered mixed Li-Ni-Mn-Co oxides for lithium battery applications. J. Mater. Chem. 2011, 21, 9991–9993. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Ding, D.; Bu, Y.; Wang, Z.; Huang, B.; Guo, H.; Li, X. Enhanced electrochemical properties of LiNiO2-based cathode materials by nanoscale manganese carbonate treatment. Appl. Surf. Sci. 2017, 403, 426–434. [Google Scholar]
- Nazer, N.S.; Yartys, V.A.; Azib, T.; Latroche, M.; Cuevas, F.; Forseth, S.; Vie, P.J.S.; Henry, P.F.; Arnberg, L.; Henry, P.F. In operando neutron diffraction study of a commercial graphite/(Ni, Mn, Co) oxide-based multi-component lithium ion battery. J. Power Sources 2016, 326, 93–103. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.S. Atomistic Simulation Study of Mixed-Metal Oxide (LiNi1/3Co1/3Mn1/3O2) Cathode Material for Lithium Ion Battery. J. Phys. Chem. C. 2012, 116, 6484–6489. [Google Scholar] [CrossRef]
- Sultana, I.; Rahman, M.M.; Chen, Y.; Glushenkov, A.M. Potassium-Ion Battery Anode Materials Operating through the Alloying-Dealloying Reaction Mechanism. Adv. Funct. Mater. 2018, 28, 1703857. [Google Scholar] [CrossRef]
- Han, S.A.; Qutaish, H.; Lee, J.; Park, M.; Kim, J.H. Metal-Organic Frame Work Derived Porous Structures towards Lithium Recharge able Batteries. EcoMat 2023, 5, e12283. [Google Scholar] [CrossRef]
- Diez, N.; Sevilla, M.; Fuertes, A.B. Dense (Non-Hollow) Carbon Nanospheres: Synthesis and Electrochemical Energy Applications. Mater. Today Nano 2021, 16, 100147. [Google Scholar] [CrossRef]
- Natarajan, S.; Lee, Y.S.; Aravindan, V. Biomass-Derived Carbon Materials as Prospective Electrodes for High-Energy Lithium- and Sodium-Ion Capacitors. Chem.-Asian J. 2019, 14, 936–951. [Google Scholar] [CrossRef]
- Hussain, I.; Sahoo, S.; Sayed, M.S.; Ahmad, M.; Javed, M.S.; Lamiel, C.; Li, Y.; Shim, J.; Ma, X.; Zhang, K. Hollow Nano- and Microstructures: Mechanism, Composition, Applications, and Factors affecting Morphology and Performance. Coord. Chem. Rev. 2022, 458, 214429. [Google Scholar] [CrossRef]
- Jang, Y.J.; Oh, H.G.; Park, S. Rational Design of Ultrafine FeSe2 Nanocrystals Embedded within Hollow Mesoporous Carbon Bowls for Potassium-Ion Batteries with Long-term Cycling Stability and High Volumetric Capacity. J. Mater. Sci. Technol. 2023, 143, 129–139. [Google Scholar] [CrossRef]
- Kang, B.K.; Choi, Y.J.; Choi, H.W.; Kwon, S.B.; Kim, S.; Kim, Y.J.; Park, J.S.; Yang, W.S.; Yoon, D.H.; Ryu, W. Rational Design and in-Situ Formation of Nickel-Cobalt Nitride Multi-Core/Hollow N-Doped Carbon Shell Anode for Li-Ion Batteries. Chem. Eng. J. 2021, 420, 129630. [Google Scholar] [CrossRef]
- Kim, G.H.; Choi, W.H.; Choi, J.W.; Kim, K.; Park, D.G.; Park, M.G.; Kim, M.G.; Jang, H.; Kim, U.; Kang, J.K. Coiled Conformation Hollow Carbon Nanosphere Cathode and Anode for High Energy Density and Ultrafast Chargeable Hybrid Energy Storage. ACS Nano 2022, 16, 6552–6564. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Martin, A.; Martinez-Fernandez, J.; Ruttert, M.; Heckmann, A.; Winter, M.; Placke, T.; Ramirez-Rico, J. Iron-Catalyzed Graphitic Carbon Materials from Biomass Resources as Anodes for Lithium-Ion Batteries. ChemSusChem 2018, 11, 2776–2787. [Google Scholar] [CrossRef]
- Mahmood, A.; Li, S.; Ali, Z.; Tabassum, H.; Zhu, B.; Liang, Z.; Meng, W.; Aftab, W.; Guo, W.; Zhao, Y. Ultrafast Sodium/Potassium-Ion Intercalation into Hierarchically Porous Thin Carbon Shells. Adv. Mater. 2019, 31, 1805430. [Google Scholar] [CrossRef]
- Cui, R.C.; Xu, B.; Dong, H.J.; Yang, C.C.; Jiang, Q. N/O Dual-Doped Environment-Friendly Hard Carbon as Advanced Anode for Potassium-Ion Batteries. Adv. Sci. 2020, 7, 1902547. [Google Scholar] [CrossRef]
- Kim, J.M.; Cho, Y.; Koo, C.; Lee, C.; Ramirez, P.D.; Ko, D.; Oh, J.; Park, S.; Kofinas, P.; Begin-Colin, S.; et al. Microwave-Assisted Preparation of Carbon Coating Layer on Raspberry-Shaped Iron Oxide Particles for Lithium-Ion Battery Anodes. J. Electroanal. Chem. 2021, 895, 115520. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Q.; Xie, W.; Yuan, T.; Zang, Q.; Wang, N.; Fan, H.; Xie, S.; Ouyang, X. B, F Co-doping Flexible Carbon Nanofibers as a Fast and Stable Anode for Potassium-Ion Hybrid Capacitor. J. Alloys Compd. 2022, 914, 165285. [Google Scholar] [CrossRef]
- Wang, J.; Yan, X.; Zhang, Z.; Ying, H.; Guo, R.; Yang, W.; Han, W.Q. Facile Preparation of High-Content N-Doped CNT Microspheres for High-Performance Lithium Storage. Adv. Funct. Mater. 2019, 29, 1904819. [Google Scholar] [CrossRef]
- Wang, J.; Yan, X.; Zhang, Z.; Ying, H.; Guo, R.; Yang, W.; Han, W.Q. Insights into the Na+ Storage Mechanism of Phosphorus-Functionalized Hard Carbon as Ultrahigh Capacity Anodes. Adv. Energy Mater. 2018, 8, 1702781. [Google Scholar]
- Yang, S.H.; Park, S.; Kang, Y.C. MOF-Derived CoSe2@N-Doped Carbon Matrix Confined in Hollow Mesoporous Carbon Nanospheres as High-Performance Anodes for Potassium-Ion Batteries. Nano-Micro Lett. 2021, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Alkarmo, W.; Ouhib, F.; Aqil, A.; Thomassin, J.M.; Yuan, J.; Gong, J.; Vertruyen, B.; Detrembleur, C.; Jérôme, C. Poly(ionic liquid)-Derived N-Doped Carbons with Hierarchical Porosity for Lithium- and Sodium-Ion Batteries. Macromol. Rapid Comm. 2019, 40, 1800545. [Google Scholar] [CrossRef] [Green Version]
- Ni, D.; Sun, W.; Wang, Z.; Bai, Y.; Lei, H.; Lai, X.; Sun, K. Heteroatom-Doped Mesoporous Hollow Carbon Spheres for Fast Sodium Storage with an Ultralong Cycle Life. Adv. Energy Mater. 2019, 9, 1900036. [Google Scholar] [CrossRef]
- Yin, L.; Park, M.; Jeon, I.; Hwang, J.H.; Kim, J.P.; Lee, H.W.; Park, M.; Jeong, S.Y.; Cho, C. Silicon Nanoparticle Self-Incorporated in Hollow Nitrogen-Doped Carbon Microspheres for Lithium-Ion Battery Anodes. Electrochim. Acta 2021, 368, 137630. [Google Scholar] [CrossRef]
- Nam, K.C.; Seon, Y.H.; Bandyopadhyay, P.; Cho, J.S.; Jeong, S.M. Porous Nanofibers Comprising Hollow Co3O4/Fe3O4 Nanospheres and Nitrogen-Doped Carbon Derived by Fe@ZIF-67 as Anode Materials for Lithium-Ion Batteries. Int. J. Energy Res. 2022, 46, 8934–8948. [Google Scholar] [CrossRef]
- Chen, J.M.; Cheng, Y.; Zhang, Q.B.; Luo, C.; Li, H.Y.; Wu, Y.; Zhang, H.H.; Wang, X.; Liu, H.D.; He, X.; et al. Designing and Understanding the Superior Potassium Storage Performance of Nitrogen/Phosphorus Co-Doped Hollow Porous Bowl-Like Carbon Anodes. Adv. Funct. Mater. 2021, 31, 2007158. [Google Scholar] [CrossRef]
- Liang, J.; Kou, H.R.; Ding, S. Complex Hollow Bowl-Like Nanostructures: Synthesis, Application, and Perspective. Adv. Funct. Mater. 2021, 31, 2007801. [Google Scholar] [CrossRef]
- Kim, Y.B.; Seo, H.Y.; Kim, S.; Kim, T.H.; Choi, J.H.; Cho, J.S.; Kang, Y.C.; Park, G.D. Controllable Synthesis of Carbon Yolk-Shell Microsphere and Application of Metal Compound-Carbon Yolk-Shell as Effective Anode Material for Alkali-Ion Batteries. Small Methods 2023, 7, 2201370. [Google Scholar] [CrossRef]
- Trukawka, M.; Wenelska, K.; Singer, L.; Klingeler, R.; Chen, X.; Mijowska, E. Hollow Carbon Spheres Loaded with Uniform Dispersion of Copper Oxide Nanoparticles for Anode in Lithium-Ion Batteries. J. Alloys Compd. 2021, 853, 156700. [Google Scholar] [CrossRef]
- Abbas, S.A.; Forghani, M.; Anh, S.; Donne, S.W.; Jung, K.D. Carbon Hollow Spheres as Electrochemical Capacitors: Mechanistic Insights. Energy Storage Mater. 2020, 24, 550–556. [Google Scholar] [CrossRef]
- Fu, Y.X.; Sun, Y.H. Comparative study of synthesis and characterization of monodispersed SiO2@Y2O3:Eu3+ and SiO2@Y2O3:Eu3+@SiO2 core-shell structure phosphor particles. J. Alloys Compd. 2009, 471, 190–196. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, Z.; Wang, S.; Cheng, Z.; Li, P.; Wang, R. Engineered Interfusion of Hollow Nitrogen-Doped Carbon Nanospheres for Improving Electrochemical Behavior and Energy Density of Lithium-Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1902322. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium Ion Insertion in Hollow Carbon Nanowires for Battery Applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef]
- Sui, D.; Xu, L.; Zhang, H.; Sun, Z.; Kan, B.; Ma, Y.; Chen, Y. 3D Cross-Linked Graphene-Based Honeycomb Carbon Composite with Excellent Confinement Effect of Organic Cathode Material for Lithium-Ion Batteries. Carbon 2020, 157, 656–662. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Z.; Eloi, J.; Titirici, M.; Eichhorn, S.J. Ice-Templated, Sustainable Carbon Aerogels with Hierarchically Tailored Channels for Sodium- and Potassium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2110862. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.; Xiong, X.; Hu, C.; Zou, F.; Hu, P.; Huang, Y. Sulfur-Doped Carbon with Enlarged Interlayer Distance as a High-Performance Anode Material for Sodium-Ion Batteries. Adv. Sci. 2015, 2, 1500195. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yuan, F.; Li, Z.; Zhang, D.; Yu, Q.; Wang, B. Heteroatom-Doped Carbon Anode Materials for Potassium-Ion Batteries: From Mechanism, Synthesis to Electrochemical Performance. Apl. Mater. 2022, 10, 030902. [Google Scholar] [CrossRef]
- Ma, L.; Lyu, S.S.; Dai, Y.; Pei, X.Y.; Mo, D.C.; Fu, Y.X. Lithium storage properties of NiO/reduced graphene oxide composites derived from different oxidation degrees of graphite oxide. J. Alloys Compd. 2019, 810, 151954. [Google Scholar] [CrossRef]
- Thauer, E.; Shi, X.; Zhang, S.; Chen, X.; Deeg, L.; Klingeler, R.; Wenelska, K.; Mijowska, E. Mn3O4 Encapsulated in Hollow Carbon Spheres Coated by Graphene Layer for Enhanced Magnetization and Lithium-Ion Batteries Performance. Energy 2021, 217, 119399. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Qin, S.; Liu, D.; Zhang, P.; Hegh, D.; Zhang, J.; Naebe, M.; Lei, W.; Razal, J.M. Pore-Assisted Lithium Deposition in Hierarchically Porous and Hollow Carbon Textile for Highly Stable Lithium Anode. J. Power Sources 2021, 489, 229464. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Wang, J.; Li, X. N-Doped Hollow Carbon Nanofibers Anchored Hierarchical FeP Nanosheets as High-Performance Anode for Potassium-Ion Batteries. J. Alloys Compd. 2020, 821, 153268. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO2 (anatase) Nanoparticles. J. Phys. Chem. C. 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive Oxide Materials for High-Rate Electrochemical Energy Storage. Energ. Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Wenelska, K.; Trukawka, M.; Kukulka, W.; Chen, X.; Mijowska, E. Co-Existence of Iron Oxide Nanoparticles and Manganese Oxide Nanorods as Decoration of Hollow Carbon Spheres for Boosting Electrochemical Performance of Li-Ion Battery. Materials 2021, 14, 6902. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Zhao, X.; Yin, K.; Li, Y.; Chen, L.; Yang, X.; Zhang, W.; Su, B.-L.; Cao, G. Superior Pseudocapacitive Lithium-Ion Storage in Porous Vanadium Oxides@C Heterostructure Composite. ACS Appl. Mater. Inter. 2017, 9, 43665–43673. [Google Scholar] [CrossRef]
- Chao, D.; Zhu, C.; Yang, P.; Xia, X.; Liu, J.; Wang, J.; Fan, X.; Savilov, S.; Lin, J.; Shen, Z.X. Array of Nanosheets Render Ultrafast and High-Capacity Na-Ion Storage by Tunable Pseudocapacitance. Nat. Commun. 2016, 7, 12122. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhuang, N.; Xie, J.; Zhu, Y.; Lai, H.; Qin, W.; Mai, W. Carboxymethyl Cellulose Binder Greatly Stabilizes Porous Hollow Carbon Submicrospheres in Capacitive K-Ion Storage. Acs Appl. Mater. Inter. 2019, 11, 15581–15590. [Google Scholar] [CrossRef]
- Qiu, J.X.; Li, S.; Gray, E.; Liu, H.W.; Gu, Q.F.; Sun, C.H.; Lai, C.; Zhao, H.J.; Zhang, S.Q. Hydrogenation Synthesis of Blue TiO2 for High-Performance Lithium-Ion Batteries. J. Phys. Chem. C 2014, 118, 8824–8830. [Google Scholar] [CrossRef]
- Zhong, S.; Liu, H.; Wei, D.; Hu, J.; Zhang, H.; Hou, H.; Peng, M.; Zhang, G.; Duan, H. Long-aspect-ratio N-rich carbon nanotubes as anode material for sodium and lithium ion batteries. Chem. Eng. J. 2020, 395, 125054. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, Q.-W.; Wang, Q.; Ma, J. Nitrogen doped porous carbon as excellent dual anodes for Li- and Na-ion batteries. Chin. Chem. Lett. 2019, 31, 583–588. [Google Scholar] [CrossRef]
- Kim, J.-G.; Kim, H.-C.; Kim, N.D.; Khil, M.-S. N-doped hierarchical porous hollow carbon nanofibers based on PAN/PVP@SAN structure for high performance supercapacitor. Compos. Part B Eng. 2020, 186, 107825. [Google Scholar] [CrossRef]
- Ding, Y.; Hu, L.; He, D.; Peng, Y.; Niu, Y.; Li, Z.; Zhang, X.; Chen, S. Design of multishell microsphere of transition metal oxides/carbon composites for lithium ion battery. Chem. Eng. J. 2019, 380, 122489. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, X.; Li, N.; Yang, C.; Ji, T.; Yan, K.; Chi, H.; Zhang, X.; Sun, F.; Sun, D.; et al. Three-dimensional porous graphene microsphere for high-performance anode of lithium ion batteries. Surf. Coat. Technol. 2019, 360, 232–237. [Google Scholar] [CrossRef]
- Liang, T.; Wang, H.; Fei, R.; Wang, R.; He, B.; Gong, Y.; Yan, C. A high-power lithium-ion hybrid capacitor based on a hollow N-doped carbon nanobox anode and its porous analogue cathode. Nanoscale 2019, 11, 20715–20724. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, S.; Zhang, Y.; Yu, B.; Hou, L.; Su, G.; Ma, S.; Zou, J.; Huang, H. Hollow Carbon Nanospheres with Extremely Small Size as Anode Material in Lithium-Ion Batteries with Outstanding Cycling Stability. J. Phys. Chem. C 2016, 120, 3139–3144. [Google Scholar] [CrossRef]
- Xu, C.; Niu, D.; Zheng, N.; Yu, H.; He, J.; Li, Y. Facile Synthesis of Nitrogen-Doped Double-Shelled Hollow Mesoporous Carbon Nanospheres as High-Performance Anode Materials for Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 5999–6007. [Google Scholar] [CrossRef]
- Wang, F.; Song, R.; Song, H.; Chen, X.; Zhou, J.; Ma, Z.; Li, M.; Lei, Q. Simple synthesis of novel hierarchical porous carbon microspheres and their application to rechargeable lithium-ion batteries. Carbon 2015, 81, 314–321. [Google Scholar] [CrossRef]
- Wang, H.-G.; Yuan, C.; Zhou, R.; Duan, Q.; Li, Y. Self-sacrifice template formation of nitrogen-doped porous carbon microtubes towards high performance anode materials in lithium ion batteries. Chem. Eng. J. 2017, 316, 1004–1010. [Google Scholar] [CrossRef]
- Zhou, S.; Li, J.; Fu, L.; Zhu, J.; Yang, W.; Li, D.; Zhou, L. Black Phosphorus/Hollow Porous Carbon for High Rate Performance Lithium-Ion Battery. Chemelectrochem 2020, 7, 2184–2189. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y.; Liu, B.; Wang, S.; Cao, M. Porous carbon nanofiber webs derived from bacterial cellulose as an anode for high performance lithium ion batteries. Carbon 2015, 91, 56–65. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Mo, D.-C.; Qu, Z.-T.; Wang, W.-K.; Lyu, S.-S. Organic—Inorganic Hybrid Interfaces Enable the Preparation of Nitrogen-Doped Hollow Carbon Nanospheres as High-Performance Anodes for Lithium and Potassium-Ion Batteries. Materials 2023, 16, 4936. https://doi.org/10.3390/ma16144936
Dai Y, Mo D-C, Qu Z-T, Wang W-K, Lyu S-S. Organic—Inorganic Hybrid Interfaces Enable the Preparation of Nitrogen-Doped Hollow Carbon Nanospheres as High-Performance Anodes for Lithium and Potassium-Ion Batteries. Materials. 2023; 16(14):4936. https://doi.org/10.3390/ma16144936
Chicago/Turabian StyleDai, Yao, Dong-Chuan Mo, Zong-Tao Qu, Wen-Kang Wang, and Shu-Shen Lyu. 2023. "Organic—Inorganic Hybrid Interfaces Enable the Preparation of Nitrogen-Doped Hollow Carbon Nanospheres as High-Performance Anodes for Lithium and Potassium-Ion Batteries" Materials 16, no. 14: 4936. https://doi.org/10.3390/ma16144936