The Flow Stress Behavior and Physical-Based Constitutive Model for As-Quenched Al-Zn-Mg-Cu Alloy
Abstract
1. Introduction
2. Experiments
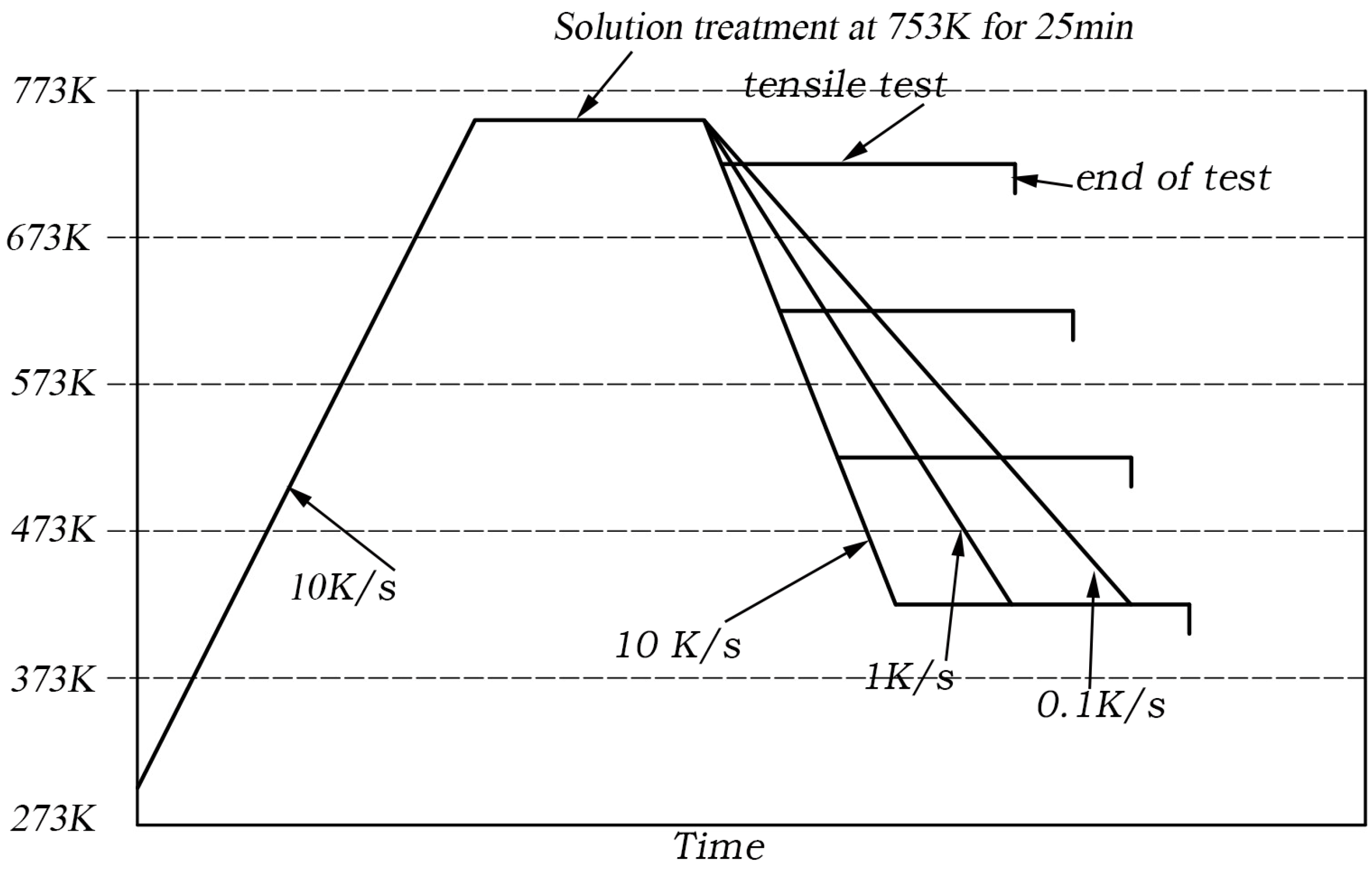
2.1. Material and Experimental Procedure
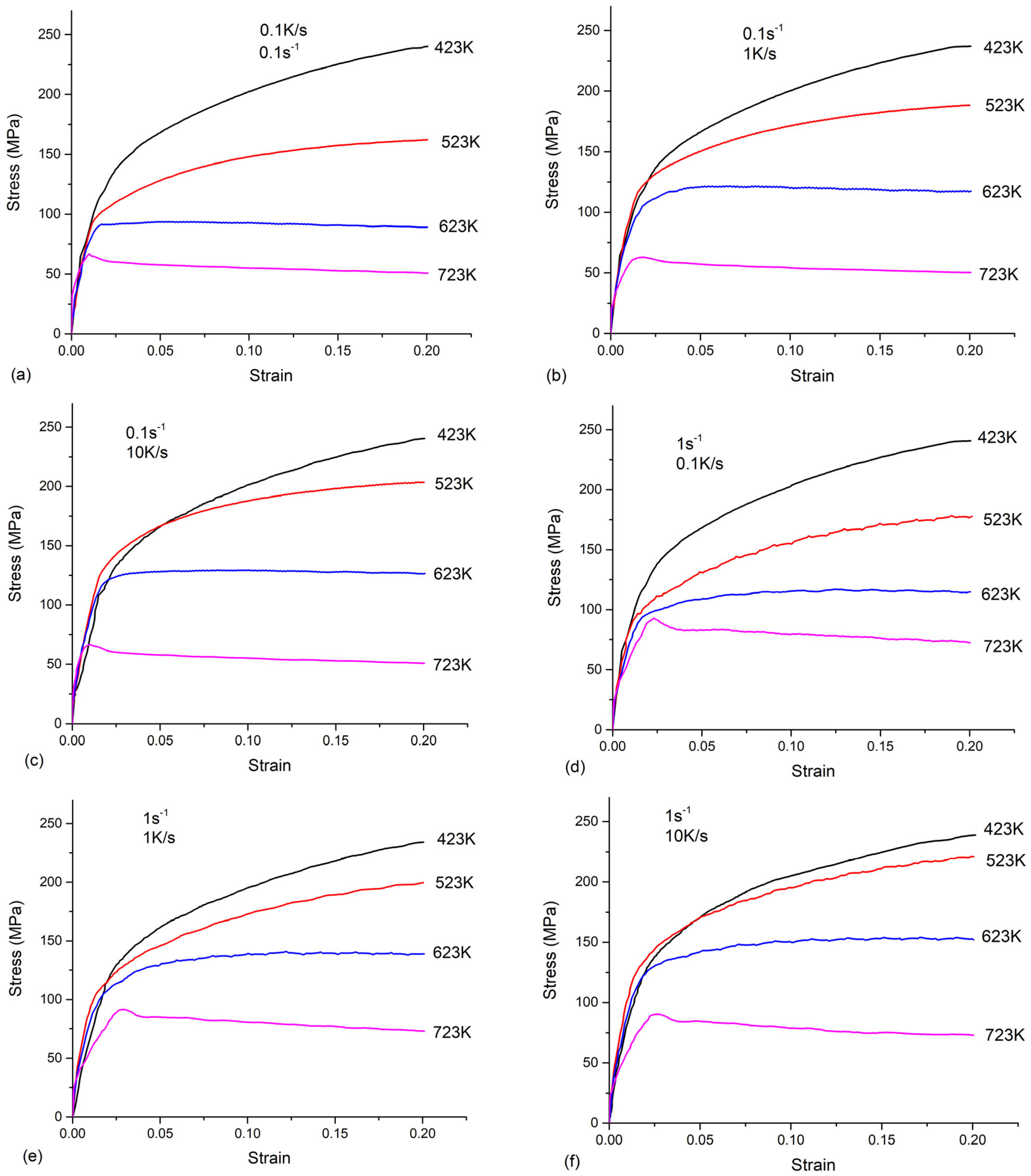
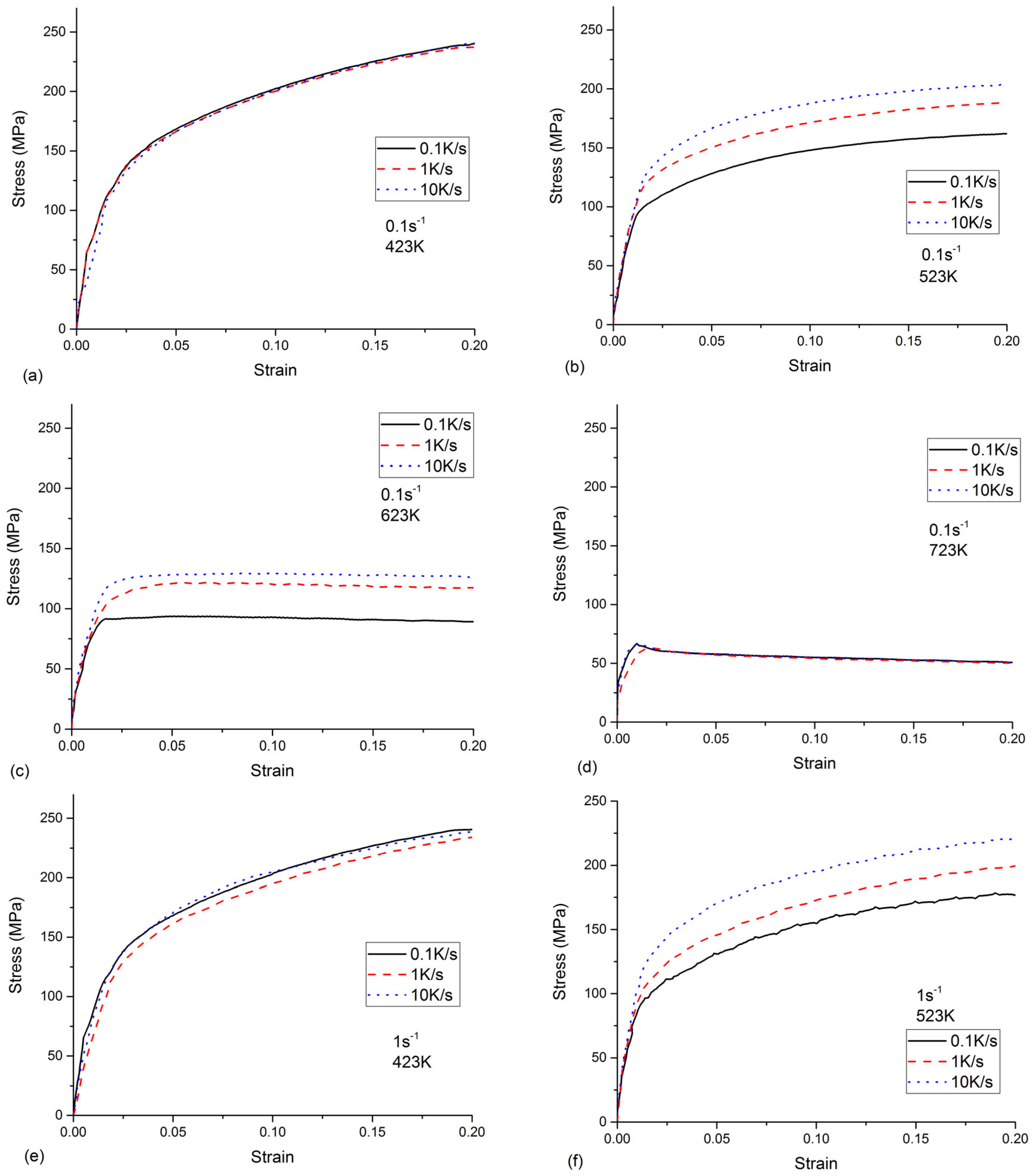
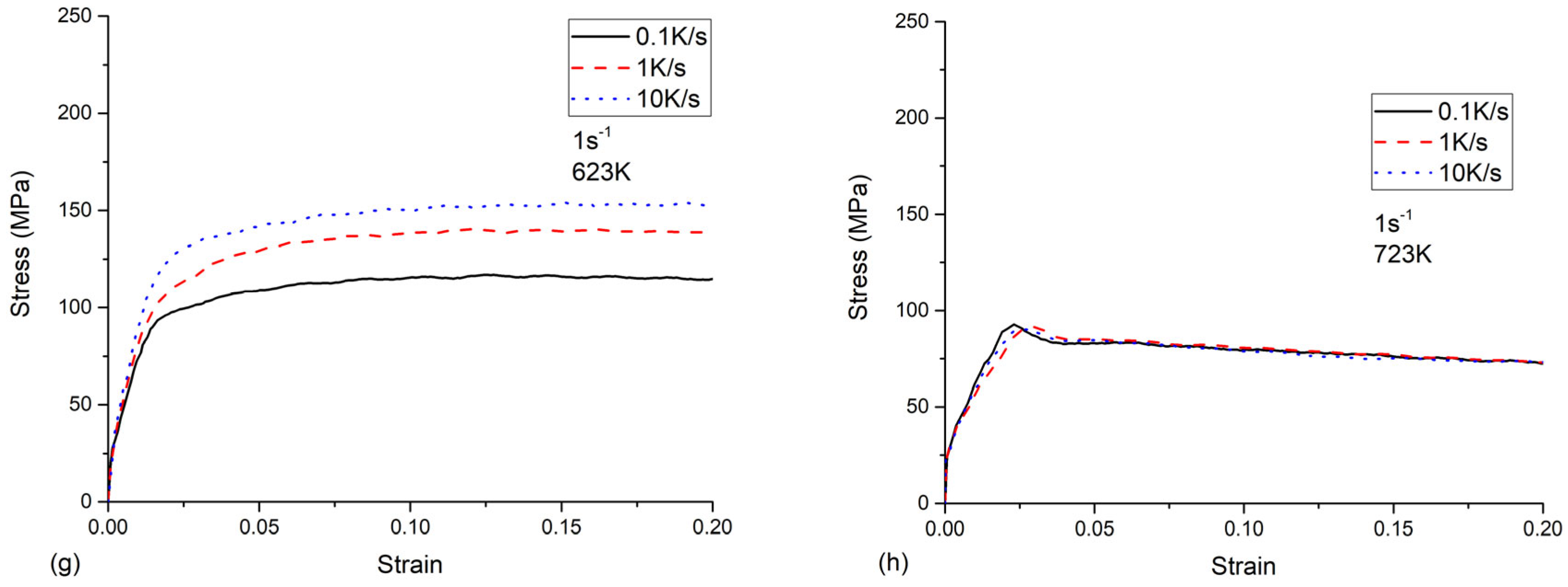
2.2. Experimental Results of Tensile Test
3. Physical-Based Constitutive Model
3.1. The Kinetics Model of Precipitation
3.2. Dislocation Density Flow Stress Model
3.3. Summary of the Precipitation Kinetics and Constitutive Models
4. Model Prediction Results and Discussions
5. Conclusions
- (1)
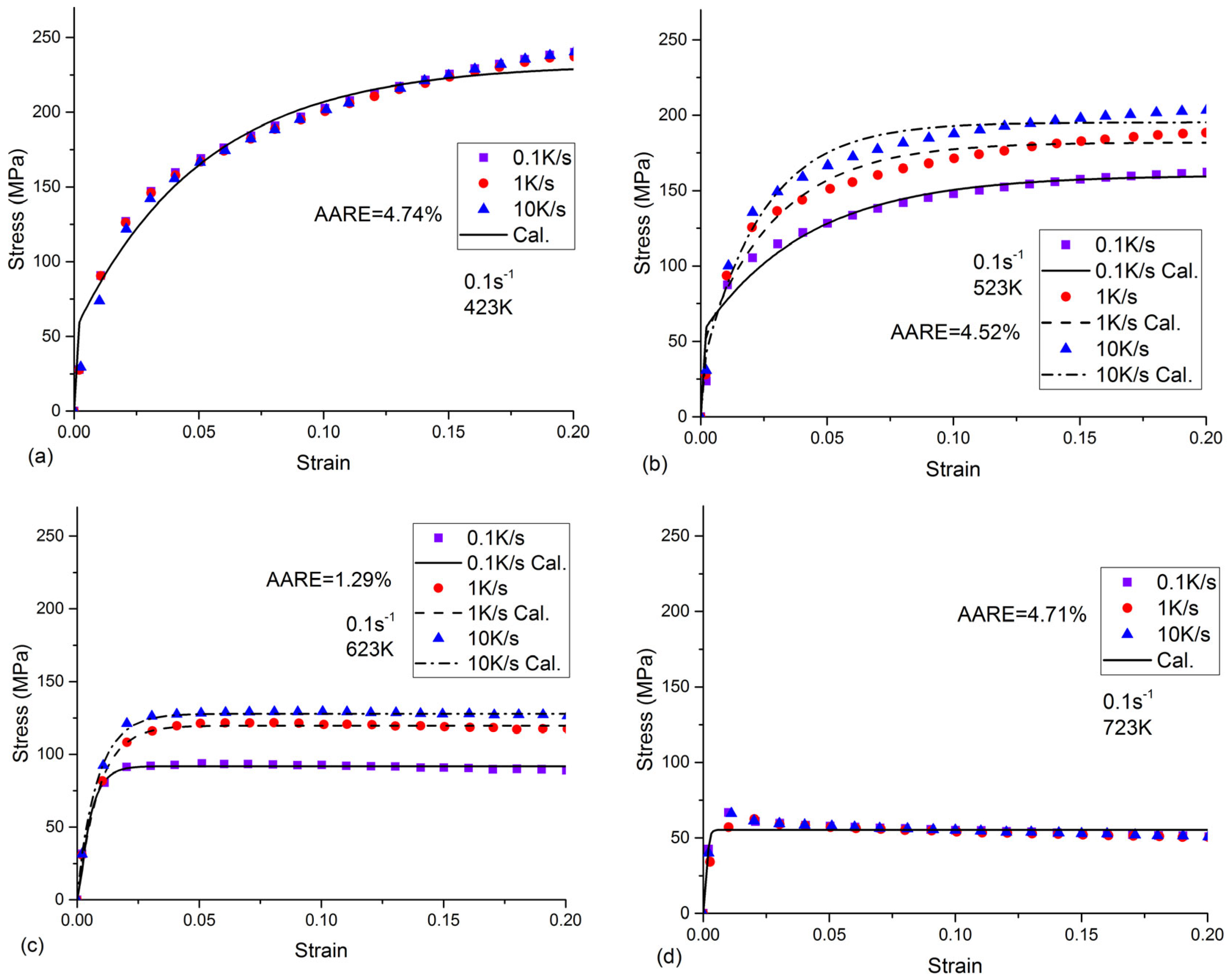
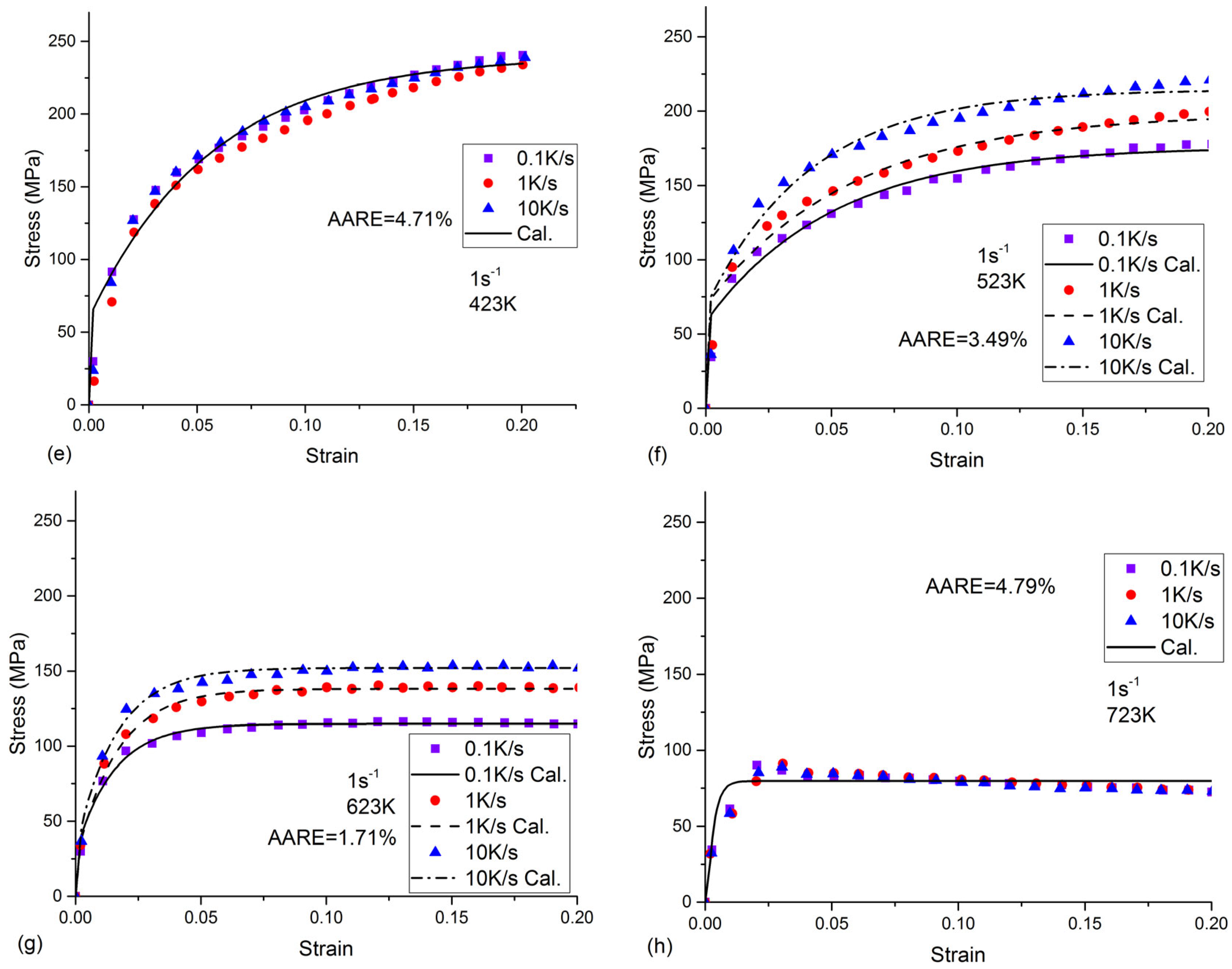
- All the tested parameters, i.e., cooling rates, strain rates, and temperatures, can have effects on the flow stress behavior of the as-quenched AA7050 alloy. The values of the flow stress increased with the increased strain rate and the decreased temperature. When the temperature was 523 K or 623 K, the flow stress values increased with the increasing cooling rate. The flow stress values increased with the increasing cooling rate at a temperature of 523 K or 623 K, compared to the negligible effects on flow stress behavior at a temperature of 423 K or 723 K.
- (2)
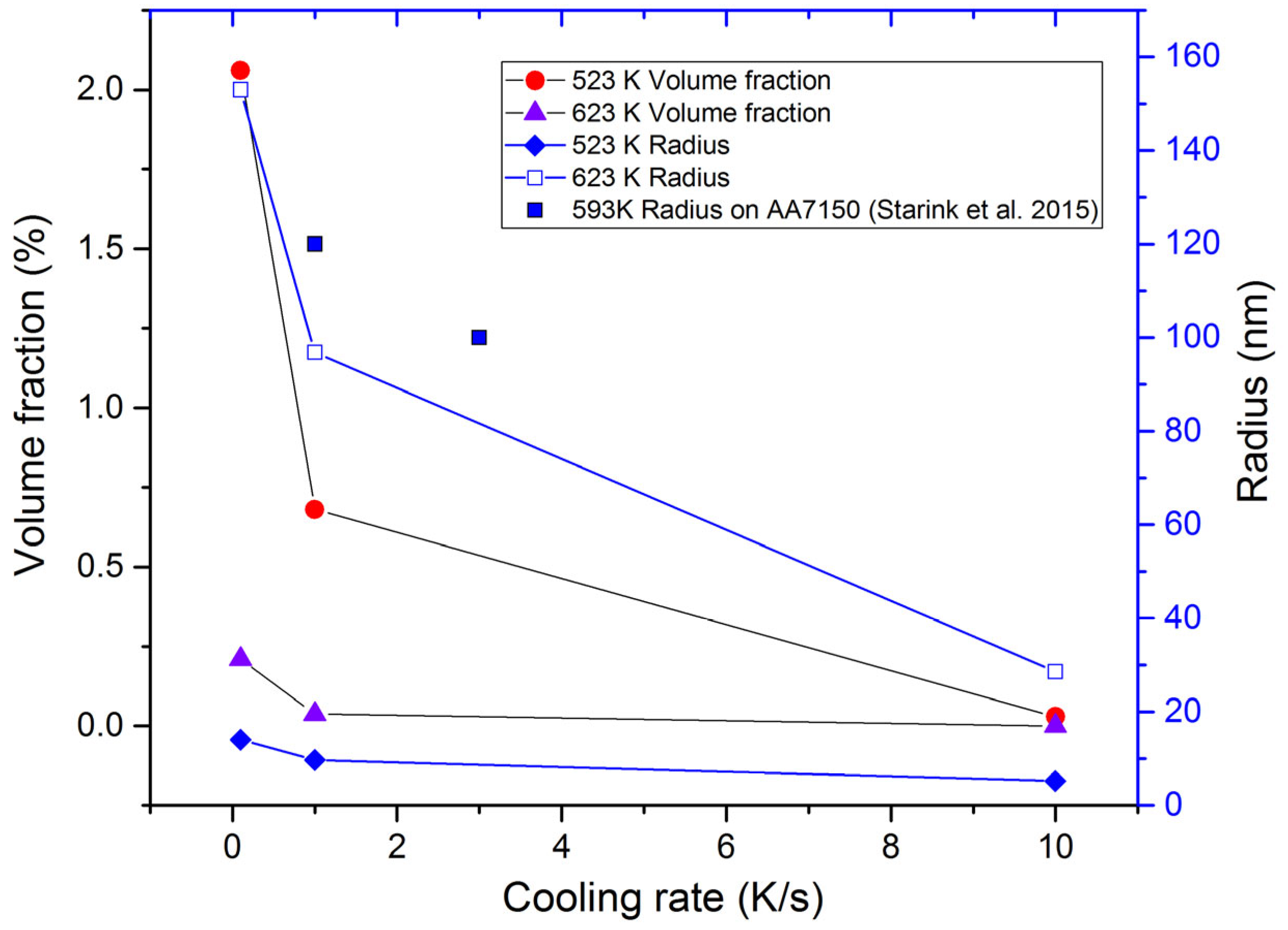
- In the present precipitation prediction, both the volume fraction and the radius of the precipitates decreased with the increasing cooling rate. The physical-based constitutive model was applied to predict the flow stress behavior in the isothermal tensile test of the as-quenched AA 7050 alloy. The AARE between the calculated and the experimental flow stress was about 4%. The present constitutive model, which considered the effects of the precipitates, can be used to describe the flow stress behavior of other series of as-quenched aluminum alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Chen, Y.; Hu, J. Recent advances in the development of aerospace materials. Prog. Aerosp. Sci. 2018, 97, 22–34. [Google Scholar] [CrossRef]
- Li, J.; Wang, S. Distortion caused by residual stresses in machining aeronautical aluminum alloy parts: Recent advances. Int. J. Adv. Manuf. Technol. 2017, 89, 997–1012. [Google Scholar] [CrossRef]
- Akhtar, W.; Lazoglu, I.; Liang, S.Y. Prediction and control of residual stress-based distortions in the machining of aerospace parts: A review. J. Manuf. Process. 2022, 76, 106–122. [Google Scholar] [CrossRef]
- Robinson, J.S.; Tanner, D.A.; Truman, C.E. 50th anniversary article: The origin and management of residual stress in heat-treatable aluminium alloys. Strain 2014, 50, 185–207. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, X.M. A critical review of experimental results and constitutive descriptions for metals and alloys in hot working. Mater. Des. 2011, 32, 1733–1759. [Google Scholar] [CrossRef]
- Ulysse, P.; Schultz, R.W. The effect of coatings on the thermo-mechanical response of cylindrical specimens during quenching. J. Mater. Process. Technol. 2008, 204, 39–47. [Google Scholar] [CrossRef]
- Ulysse, P. Thermo-mechanical characterization of forged coated products during water quench. J. Mater. Process. Technol. 2009, 209, 5584–5592. [Google Scholar] [CrossRef]
- Chobaut, N.; Carron, D.; Arsène, S.; Schloth, P.; Drezet, J.-M. Quench induced residual stress prediction in heat treatable 7xxx aluminium alloy thick plates using Gleeble interrupted quench tests. J. Mater. Process. Technol. 2015, 222, 373–380. [Google Scholar] [CrossRef]
- Chobaut, N.; Repper, J.; Pirling, T.; Carron, D.; Drezet, J.M. Residual stress analysis in AA7449 as-quenched thick plates using neutrons and FE modelling. In Proceedings of the 13th International Conference on Aluminum Alloys Pittsburgh, Pittsburgh, PA, USA, 3–7 June 2012; Springer: Cham, Switzerland, 2012; pp. 285–291. [Google Scholar]
- Reich, M.; Kessler, O. Mechanical properties of undercooled aluminium alloys and their implementation in quenching simulation. Mater. Sci. Technol. 2012, 28, 769–772. [Google Scholar] [CrossRef]
- Robinson, J.S.; Tanner, D.A.; Truman, C.E.; Paradowska, A.; Wimpory, R. The influence of quench sensitivity on residual stresses in the aluminium alloys 7010 and 7075. Mater. Charact. 2012, 65, 73–85. [Google Scholar] [CrossRef]
- Schumacher, P.; Pogatscher, S.; Starink, M.J.; Schick, C.; Mohles, V.; Milkereit, B. Quench-induced precipitates in Al–Si alloys: Calorimetric determination of solute content and characterisation of microstructure. Thermochim. Acta 2015, 602, 63–73. [Google Scholar] [CrossRef]
- Guo, R.; Wu, J. Dislocation density based model for Al-Cu-Mg alloy during quenching with considering the quench-induced precipitates. J. Alloys Compd. 2018, 741, 432–441. [Google Scholar] [CrossRef]
- Chobaut, N.; Carron, D.; Drezet, J.M. Characterisation of precipitation upon cooling of an AA2618 Al–Cu–Mg alloy. J. Alloys Compd. 2016, 654, 56–62. [Google Scholar] [CrossRef]
- Starink, M.J.; Milkereit, B.; Zhang, Y.; Rometsch, P.A. Predicting the quench sensitivity of Al–Zn–Mg–Cu alloys: A model for linear cooling and strengthening. Mater. Des. 2015, 88, 958–971. [Google Scholar] [CrossRef]
- Zhang, Y.; Weyland, M.; Milkereit, B.; Reich, M.; Rometsch, P.A. Precipitation of a new platelet phase during the quenching of an Al-Zn-Mg-Cu alloy. Sci. Rep. 2016, 6, 23109. [Google Scholar] [CrossRef]
- Yang, B.; Milkereit, B.; Zhang, Y.; Rometsch, P.; Kessler, O.; Schick, C. Continuous cooling precipitation diagram of aluminium alloy AA7150 based on a new fast scanning calorimetry and interrupted quenching method. Mater. Charact. 2016, 120, 30–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Milkereit, B.; Kessler, O.; Schick, C.; Rometsch, P. Development of continuous cooling precipitation diagrams for aluminium alloys AA7150 and AA7020. J. Alloys Compd. 2014, 584, 581–589. [Google Scholar] [CrossRef]
- GB/T 4338-2006; Metallic Materials-Tensile Testing at Elevated Temperature. People’s Republic of China State Administration of Quality Supervision, Inspection and Quarantine. China National Standardization Management Committee: Beijing, China, 2007; pp. 1–9. (In Chinese)
- Milkereit, B.; Starink, M.J.; Rometsch, P.A.; Schick, C.; Kessler, O. Review of the quench sensitivity of aluminium alloys: Analysis of the kinetics and nature of quench-induced precipitation. Materials 2019, 12, 4083. [Google Scholar] [CrossRef]
- Wang, S.; Hou, L.G.; Luo, J.R.; Zhang, J.S.; Zhuang, L.Z. Characterization of hot workability in AA 7050 aluminum alloy using activation energy and 3-D processing map. J. Mater. Process. Technol. 2015, 225, 110–121. [Google Scholar] [CrossRef]
- Wang, S.; Luo, J.R.; Hou, L.G.; Zhang, J.S.; Zhuang, L.Z. Physically based constitutive analysis and microstructural evolution of AA7050 aluminum alloy during hot compression. Mater. Des. 2016, 107, 277–289. [Google Scholar] [CrossRef]
- Myhr, O.R.; Grong, Ø. Modelling of non-isothermal transformations in alloys containing a particle distribution. Acta Mater. 2000, 48, 1605–1615. [Google Scholar] [CrossRef]
- Perez, M.; Dumont, M.; Acevedo-Reyes, D. Implementation of classical nucleation and growth theories for precipitation. Acta Mater. 2008, 56, 2119–2132. [Google Scholar] [CrossRef]
- Starink, M.J.; Cao, L.F.; Rometsch, P.A. A model for the thermodynamics of and strengthening due to co-clusters in Al–Mg–Si-based alloys. Acta Mater. 2012, 60, 4194–4207. [Google Scholar] [CrossRef]
- Nicolas, M.; Deschamps, A. Characterisation and modelling of precipitate evolution in an Al–Zn–Mg alloy during non-isothermal heat treatments. Acta Mater. 2003, 51, 6077–6094. [Google Scholar] [CrossRef]
- Perez, M. Gibbs–Thomson effects in phase transformations. Scr. Mater. 2005, 52, 709–712. [Google Scholar] [CrossRef]
- Charkhchian, J.; Zarei-Hanzaki, A.; Moshiri, A.; Abedi, H.R.; Schwarz, T.M.; Lawitzki, R.; Schmitz, G.; Chadha, K.; Aranas, C.; Shen, J., Jr.; et al. Spinodal decomposition of B2-phase and formation of Cr-rich nano-precipitates in AlCoCrFeNi2.1 eutectic high entropy alloy. Adv. Eng. Mater. 2023, 2300164. [Google Scholar] [CrossRef]
- Varvenne, C.; Leyson, G.; Ghazisaeidi, M.; Curtin, W. Solute strengthening in random alloys. Acta Mater. 2017, 124, 660–683. [Google Scholar] [CrossRef]
- Fribourg, G.; Bréchet, Y.; Deschamps, A.; Simar, A. Microstructure-based modelling of isotropic and kinematic strain hardening in a precipitation-hardened aluminium alloy. Acta Mater. 2011, 59, 3621–3635. [Google Scholar] [CrossRef]
- Deschamps, A.; Brechet, Y. Influence of predeformation and ageing of an Al–Zn–Mg alloy—II. Modeling of precipitation kinetics and yield stress. Acta Mater. 1998, 47, 293–305. [Google Scholar] [CrossRef]
- Stoller, R.E.; Zinkle, S.J. On the relationship between uniaxial yield strength and resolved shear stress in polycrystalline materials. J. Nucl. Mater. 2000, 283, 349–352. [Google Scholar] [CrossRef]
- Schloth, P.; Deschamps, A.; Gandin, C.-A.; Drezet, J.-M. Modeling of GP (I) zone formation during quench in an industrial AA7449 75 mm thick plate. Mater. Des. 2016, 112, 46–57. [Google Scholar] [CrossRef]
- Chobaut, N. Measurements and Modelling of Residual Stresses during Quenching of Thick Heat Treatable Aluminium Components in Relation to Their Precipitation State. Ph.D. Thesis, Ecole Polytechnique Federale de Lausanne, Lausanne, Switzerland, 2015. [Google Scholar]
- Liu, C.; Davis, A.; Fellowes, J.; Prangnell, P.B.; Raabe, D.; Shanthraj, P. CALPHAD-informed phase-field model for two-sublattice phases based on chemical potentials: η-phase precipitation in Al-Zn-Mg-Cu alloys. Acta Mater. 2022, 226, 117602. [Google Scholar] [CrossRef]
- Anjabin, N.; Taheri, A.K. Physically based material model for evolution of stress–strain behavior of heat treatable aluminum alloys during solution heat treatment. Mater. Des. 2010, 31, 433–437. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Q.; Guo, H.; Xia, Z.; Wu, Q.; Liu, B. Modeling the precipitation kinetics and tensile properties in Al-7Si-Mg cast aluminum alloys. Mater. Sci. Eng. A 2017, 685, 403–416. [Google Scholar] [CrossRef]
- Babu, B.; Lindgren, L.E. Dislocation density based model for plastic deformation and globularization of Ti-6Al-4V. Int. J. Plast. 2013, 50, 94–108. [Google Scholar] [CrossRef]
- Mohamadizadeh, A.; Zarei-Hanzaki, A.; Abedi, H.R. Modified constitutive analysis and activation energy evolution of a low-density steel considering the effects of deformation parameters. Mech. Mater. 2016, 95, 60–70. [Google Scholar] [CrossRef]

| Chemistry | Zn | Cu | Mg | Fe | Si | Mn | Ti | Others | Al |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 6.2 | 2.1 | 2.2 | 0.05 | 0.02 | 0.66 | 0.02 | 0.05 | Bal. |
| Aluminum Alloy | Temperature (K) | Cooling Rate () | Strain Rate () |
|---|---|---|---|
| 7050 | 723, 623, 523, 423 | 0.1, 1, 10 | 0.1, 1 |
| Parameters | Meaning | Value | Source |
|---|---|---|---|
| a | Lattice parameter | 4.04 × 10−10 m | [33] |
| Interfacial energy | 0.3 J/m2 | [26] | |
| k | Boltzmann’s constant | 1.38 × 10−23 J/K | - |
| R | Gas constant | 8.314 J/(mol·K) | - |
| Molar volume of MgZn2 | 3.025 × 10−5 m3/mol | [26] | |
| Nucleation site number per unit volume | 1 × 1028 m−3 | [33] | |
| Material coefficient of solute | 1.49 × 10−5 m2/s | [34] | |
| Activation energy for diffusion | 120.5 kJ/mol | [34] | |
| Zf | Zeldovich factor | 1/20 | [31] |
| Molar fraction of solute Mg in MgZn2 | 0.33 (at.%) | [35] | |
| Formation enthalpy | 75 kJ/mol | [26] | |
| Initial solute concentration of Mg in the matrix | 0.0376 (at.%) | - |
| Parameters | Meaning | Value or Domain | Source |
|---|---|---|---|
| M | Taylor factor | 3.06 | [32] |
| b | Burger’s vector | 2.84 × 10−10 m | - |
| Dislocation line tension parameter | 0.43 | [31] | |
| Vacancy migration activation energy | 68 kJ/mol | [33] | |
| Reference strain rate | [29] | ||
| Initial immobile dislocation density | [36] | ||
| Proportionality factor | [37] | ||
| Dimensionless factor | [38] | ||
| Shear stress at zero temperature | - | ||
| k1 | Material constant | [36] | |
| Material constant | [36] | ||
| C | Material constant | [36] | |
| q | Material constant | - | |
| p | Material constant | - | |
| Efficiency factor | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Liang, D.; Qin, G. The Flow Stress Behavior and Physical-Based Constitutive Model for As-Quenched Al-Zn-Mg-Cu Alloy. Materials 2023, 16, 4982. https://doi.org/10.3390/ma16144982
Guo R, Liang D, Qin G. The Flow Stress Behavior and Physical-Based Constitutive Model for As-Quenched Al-Zn-Mg-Cu Alloy. Materials. 2023; 16(14):4982. https://doi.org/10.3390/ma16144982
Chicago/Turabian StyleGuo, Ruichao, Dandan Liang, and Guohua Qin. 2023. "The Flow Stress Behavior and Physical-Based Constitutive Model for As-Quenched Al-Zn-Mg-Cu Alloy" Materials 16, no. 14: 4982. https://doi.org/10.3390/ma16144982
APA StyleGuo, R., Liang, D., & Qin, G. (2023). The Flow Stress Behavior and Physical-Based Constitutive Model for As-Quenched Al-Zn-Mg-Cu Alloy. Materials, 16(14), 4982. https://doi.org/10.3390/ma16144982





