Antimicrobial Solutions for Endotracheal Tubes in Prevention of Ventilator-Associated Pneumonia
Abstract
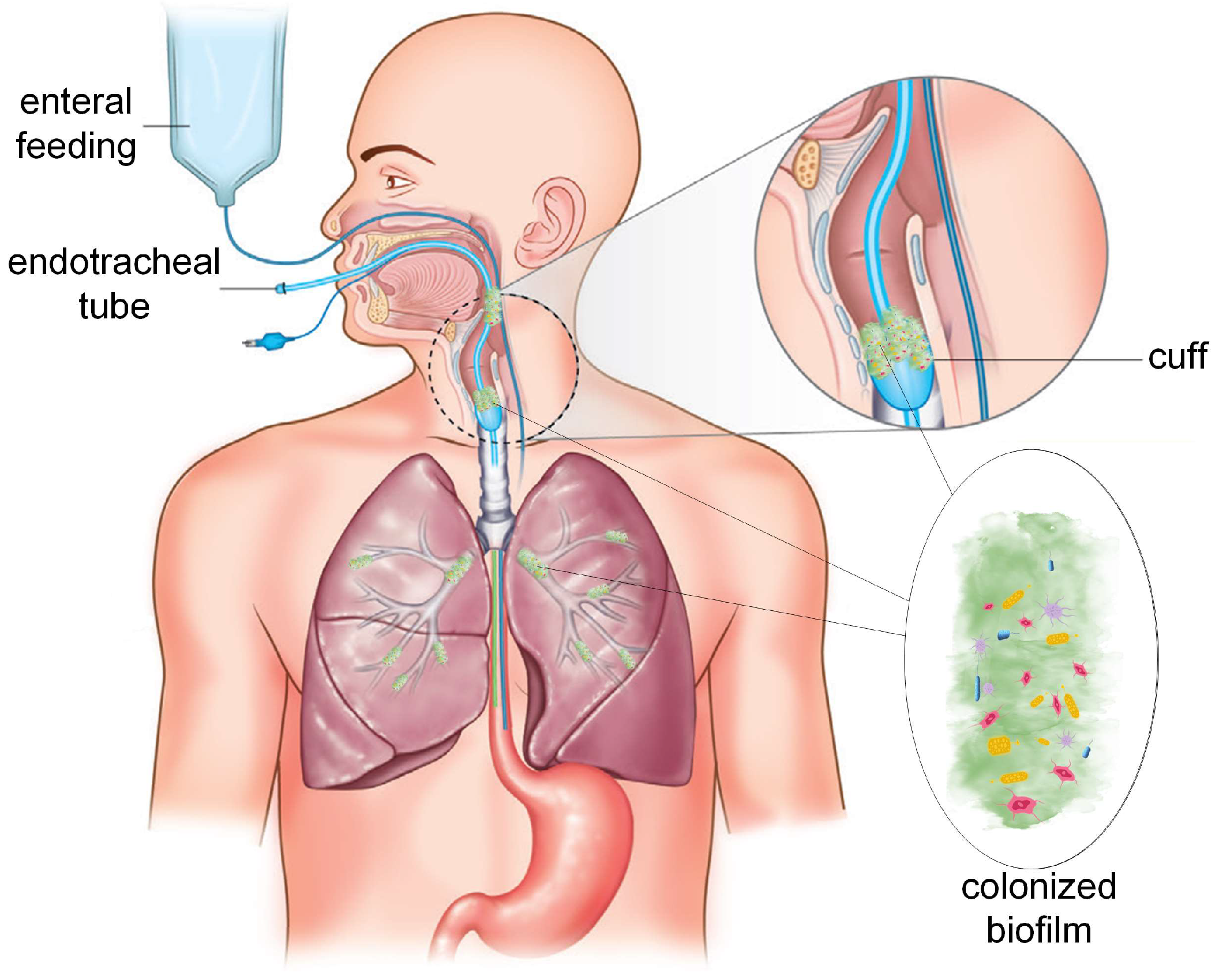
:1. Introduction
1.1. General Considerations
1.2. Endotracheal Tube a Life-Saving Medical Device with Negative Impact on Patient Health
2. Modern Technological Advances in Antimicrobial Coatings for ETTs
2.1. Active Antimicrobial Coatings
2.1.1. Antimicrobial Metal Coatings
2.1.2. Antimicrobial Coatings Based on Biocide Impregnation
2.1.3. Bio-Inspired Antimicrobial Coatings
2.2. Passive Coatings
2.2.1. Nanomodified Surfaces
2.2.2. Hydrophilic/Hydrophobic Surface Characteristics
2.2.3. Micropatterned Surface Modifications
2.3. Combinatorial Materials
2.4. Potential Side Effects and Disadvantages of Antibacterial Coatings
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antoniac, I.; Laptoiu, D.; Tecu, C.; Milea, C.; Gradinaru, S. Synthetic Materials for Osteochondral Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1058, 31–52. [Google Scholar] [CrossRef]
- Biel, M.A.; Sievert, C.; Usacheva, M.; Teichert, M.; Wedell, E.; Loebel, N.; Rose, A.; Zimmermann, R. Reduction of Endotracheal Tube Biofilms Using Antimicrobial Photodynamic Therapy. Lasers Surg. Med. 2011, 43, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.; Feit, C.; Grant, T.-A.; Brisbois, E.J. Antimicrobial Polymer Modifications to Reduce Microbial Bioburden on Endotracheal Tubes and Ventilator Associated Pneumonia. Acta Biomater. 2019, 91, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Antoniac, I.V.; Mohan, A.; Bodog, F.; Doicin, C.; Mates, I.; Ulmeanu, M.; Murzac, R.; Semenescu, A. Nanoparticles and Nanostructured Surface Fabrication for Innovative Cranial and Maxillofacial Surgery. Materials 2020, 13, 5391. [Google Scholar] [CrossRef]
- Kang, H.; Park, J.-K.; An, J.; Yi, J.-H.; Kim, H.-S. Determining Obstruction in Endotracheal Tubes Using Physical Respiratory Signals. Appl. Sci. 2023, 13, 4183. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef] [Green Version]
- Earar, K.; Antoniac, V.I.; Baciu, S.; Bran, S.; Onisor, F.; Milea, C.; Mohan, A.; Grigoroiu, R.; Saceleanu, A.; Manole, M. Etching Treatment Effect on Surface Morphology of Dental Structures. Rev. Chim. 2017, 68, 2700–2703. [Google Scholar] [CrossRef]
- Corobea, M.S.; Albu, M.G.; Ion, R.; Cimpean, A.; Miculescu, F.; Antoniac, I.V.; Raditoiu, V.; Sirbu, I.; Stoenescu, M.; Voicu, S.I.; et al. Modification of Titanium Surface with Collagen and Doxycycline as a New Approach in Dental Implants. J. Adhes. Sci. Technol. 2015, 29, 2537–2550. [Google Scholar] [CrossRef]
- Cavalu, S.; Kamel, E.; Laslo, V.; Fritea, L.; Costea, T.; Antoniac, I.V.; Vasile, E.; Antoniac, A.; Semenescu, A.; Mohan, A.; et al. Eco-Friendly, Facile and Rapid Way for Synthesis of Selenium Nanoparticles Production, Structural and Morphological Characterisation. Rev. Chim. 2018, 68, 2963–2966. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Burcea, M.; Ionescu, R.D.; Balta, F. IOL’s Opacification: A Complex Analysis Based on the Clinical Aspects, Biomaterials Used and Surface Characterization of Explanted IOL’s. Mater. Plast. 2015, 52, 109–112. [Google Scholar]
- Cavalu, S.; Antoniac, I.V.; Fritea, L.; Mates, I.M.; Milea, C.; Laslo, V.; Vicas, S.; Mohan, A. Surface Modifications of the Titanium Mesh for Cranioplasty Using Selenium Nanoparticles Coating. J. Adhes. Sci. Technol. 2018, 32, 2509–2522. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults With Hospital-Acquired and Ventilator-Associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [Green Version]
- Coelho, L.; Moniz, P.; Guerreiro, G.; Póvoa, P. Airway and Respiratory Devices in the Prevention of Ventilator-Associated Pneumonia. Medicina 2023, 59, 199. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Grainha, T.; Pereira, M.O.; Lopes, S.P. Antimicrobial Materials for Endotracheal Tubes: A Review on the Last Two Decades of Technological Progress. Acta Biomater. 2023, 158, 32–55. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. Summary of the International Clinical Guidelines for the Management of Hospital-Acquired and Ventilator-Acquired Pneumonia. ERJ. Open Res. 2018, 4, 00028–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arayasukawat, P.; So-ngern, A.; Reechaipichitkul, W.; Chumpangern, W.; Arunsurat, I.; Ratanawatkul, P.; Chuennok, W. Microorganisms and Clinical Outcomes of Early- and Late-Onset Ventilator-Associated Pneumonia at Srinagarind Hospital, a Tertiary Center in Northeastern Thailand. BMC Pulm. Med. 2021, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.; Marçal, P.; Amaro, P. Ventilator-Associated Pneumonia (VAP)—Early and Late-Onset Differences. Eur. Respir. J. 2013, 42. [Google Scholar]
- Mohan, A.G.; Ciurea, A.V.; Antoniac, I.; Manescu (Paltanea), V.; Bodog, A.; Maghiar, O.; Marcut, L.; Ghiurau, A.; Bodog, F. Cranioplasty after Two Giant Intraosseous Angiolipomas of the Cranium: Case Report and Literature Review. Healthcare 2022, 10, 655. [Google Scholar] [CrossRef]
- Monteiro-Neto, V.; Lima-Neto, L.G.; Abreu, A.G.; Monteiro, C.R.A.V. Microbiology of Ventilator-Associated Pneumonia. In Contemporary Topics of Pneumonia; Chroneos, Z.C., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3707-8. [Google Scholar]
- Khan, R.; Al-Dorzi, H.M.; Tamim, H.M.; Rishu, A.H.; Balkhy, H.; El-Saed, A.; Arabi, Y.M. The Impact of Onset Time on the Isolated Pathogens and Outcomes in Ventilator Associated Pneumonia. J. Infect. Public Health 2016, 9, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Hedrick, T.L.; Smith, R.L.; McElearney, S.T.; Evans, H.L.; Smith, P.W.; Pruett, T.L.; Young, J.S.; Sawyer, R.G. Differences in Early- and Late-Onset Ventilator-Associated Pneumonia between Surgical and Trauma Patients in a Combined Surgical or Trauma Intensive Care Unit. J. Trauma 2008, 64, 714–720. [Google Scholar] [CrossRef]
- Dudeck, M.A.; Horan, T.C.; Peterson, K.D.; Allen-Bridson, K.; Morrell, G.; Anttila, A.; Pollock, D.A.; Edwards, J.R. National Healthcare Safety Network Report, Data Summary for 2011, Device-Associated Module. Am. J. Infect. Control 2013, 41, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Koulenti, D.; Tsigou, E.; Rello, J. Nosocomial Pneumonia in 27 ICUs in Europe: Perspectives from the EU-VAP/CAP Study. Eur J. Clin. Microbiol. Infect. Dis. 2017, 36, 1999–2006. [Google Scholar] [CrossRef]
- Mehta, Y.; Jaggi, N.; Rosenthal, V.D.; Rodrigues, C.; Todi, S.K.; Saini, N.; Udwadia, F.E.; Karlekar, A.; Kothari, V.; Myatra, S.N.; et al. Effectiveness of a Multidimensional Approach for Prevention of Ventilator-Associated Pneumonia in 21 Adult Intensive-Care Units from 10 Cities in India: Findings of the International Nosocomial Infection Control Consortium (INICC). Epidemiol. Infect. 2013, 141, 2483–2491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Tawfiq, J.A.; Tambyah, P.A. Healthcare Associated Infections (HAI) Perspectives. J. Infect. Public Health 2014, 7, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health Care–Associated Infections: A Meta-Analysis of Costs and Financial Impact on the US Health Care System. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef]
- Wyncoll, D.; Camporota, L. Number Needed to Treat and Cost-Effectiveness in the Prevention of Ventilator-Associated Pneumonia. Crit. Care 2012, 16, 430. [Google Scholar] [CrossRef] [Green Version]
- Arefian, H.; Hagel, S.; Heublein, S.; Rissner, F.; Scherag, A.; Brunkhorst, F.M.; Baldessarini, R.J.; Hartmann, M. Extra Length of Stay and Costs Because of Health Care–Associated Infections at a German University Hospital. Am. J. Infect. Control 2016, 44, 160–166. [Google Scholar] [CrossRef]
- Arefian, H.; Hagel, S.; Fischer, D.; Scherag, A.; Brunkhorst, F.M.; Maschmann, J.; Hartmann, M. Estimating Extra Length of Stay Due to Healthcare-Associated Infections before and after Implementation of a Hospital-Wide Infection Control Program. PLoS ONE 2019, 14, e0217159. [Google Scholar] [CrossRef] [Green Version]
- Codru, I.R.; Sava, M.; Vintilă, B.I.; Bereanu, A.S.; Bîrluțiu, V. A Study on the Contributions of Sonication to the Identification of Bacteria Associated with Intubation Cannula Biofilm and the Risk of Ventilator-Associated Pneumonia. Medicina 2023, 59, 1058. [Google Scholar] [CrossRef]
- Cotoia, A.; Spadaro, S.; Gambetti, G.; Koulenti, D.; Cinnella, G. Pathogenesis-Targeted Preventive Strategies for Multidrug Resistant Ventilator-Associated Pneumonia: A Narrative Review. Microorganisms 2020, 8, 821. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, N.; Kolodkin-Gal, I. The Matrix Reloaded: How Sensing the Extracellular Matrix Synchronizes Bacterial Communities. J. Bacteriol. 2015, 197, 2092–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial Biofilms in Nature and Disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces—Volume 8, Number 9—September 2002—Emerging Infectious Diseases Journal—CDC. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A Quorum-Sensing Inhibitor Blocks Pseudomonas Aeruginosa Virulence and Biofilm Formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal Protection of Staphylococcus Aureus against Antimicrobials by Candida Albicans Biofilm Matrix. mBio 2016, 7, e01365-16. [Google Scholar] [CrossRef] [Green Version]
- Keller, L.; Surette, M.G. Communication in Bacteria: An Ecological and Evolutionary Perspective. Nat. Rev. Microbiol. 2006, 4, 249–258. [Google Scholar] [CrossRef]
- West, S.A.; Griffin, A.S.; Gardner, A.; Diggle, S.P. Social Evolution Theory for Microorganisms. Nat. Rev. Microbiol. 2006, 4, 597–607. [Google Scholar] [CrossRef]
- Nickel, J.C.; Ruseska, I.; Wright, J.B.; Costerton, J.W. Tobramycin Resistance of Pseudomonas Aeruginosa Cells Growing as a Biofilm on Urinary Catheter Material. Antimicrob Agents Chemother. 1985, 27, 619–624. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, M.M.; Rovig, J.; Bateman, J.; Holden, B.S.; Modelzelewski, T.; Gueorguieva, I.; von Dyck, M.; Bracken, R.; Genberg, C.; Deng, S.; et al. Preclinical Testing of a Broad-Spectrum Antimicrobial Endotracheal Tube Coated with an Innate Immune Synthetic Mimic. J. Antimicrob. Chemother. 2018, 73, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Haas, C.F.; Eakin, R.M.; Konkle, M.A.; Blank, R. Endotracheal Tubes: Old and NewDiscussion. Respir. Care 2014, 59, 933–955. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-R.; Yuan, M.-K.; Pan, S.-F.; Chuang, C.-C.; So, E.C. Double-Lumen Endotracheal Tube—Predicting Insertion Depth and Tube Size Based on Patient’s Chest X-Ray Image Data and 4 Other Body Parameters. Diagnostics 2022, 12, 3162. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.C.; Kim, C.; Oh, J.; Kim, T.H.; Kim, B.; Lee, J.; Chung, J.H.; Byun, H.; Yoon, M.S.; Lee, D.K. Position Classification of the Endotracheal Tube with Automatic Segmentation of the Trachea and the Tube on Plain Chest Radiography Using Deep Convolutional Neural Network. JPM 2022, 12, 1363. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Doerfer, K.; Khampang, P.; Fathi, F.; Hong, W.; Kerschner, J.E.; Yu, B. Real-Time Optical Monitoring of Endotracheal Tube Displacement. Biosensors 2020, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Chastre, J.; Fagon, J.-Y. Ventilator-Associated Pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Efrati, S.; Deutsch, I.; Antonelli, M.; Hockey, P.M.; Rozenblum, R.; Gurman, G.M. Ventilator-Associated Pneumonia: Current Status and Future Recommendations. J. Clin. Monit. Comput. 2010, 24, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Tadié, J.-M.; Behm, E.; Lecuyer, L.; Benhmamed, R.; Hans, S.; Brasnu, D.; Diehl, J.-L.; Fagon, J.-Y.; Guérot, E. Post-Intubation Laryngeal Injuries and Extubation Failure: A Fiberoptic Endoscopic Study. Intensive Care Med. 2010, 36, 991–998. [Google Scholar] [CrossRef]
- Harless, J.; Ramaiah, R.; Bhananker, S. Pediatric Airway Management. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 65. [Google Scholar] [CrossRef] [Green Version]
- Klabusayová, E.; Klučka, J.; Kratochvíl, M.; Musilová, T.; Vafek, V.; Skříšovská, T.; Djakow, J.; Kosinová, M.; Havránková, P.; Štourač, P. Airway Management in Pediatric Patients: Cuff-Solved Problem? Children 2022, 9, 1490. [Google Scholar] [CrossRef]
- Sunder, R.A.; Haile, D.T.; Farrell, P.T.; Sharma, A. Pediatric Airway Management: Current Practices and Future Directions. Paediatr Anaesth 2012, 22, 1008–1015. [Google Scholar] [CrossRef]
- Dalal, P.G.; Murray, D.; Messner, A.H.; Feng, A.; McAllister, J.; Molter, D. Pediatric Laryngeal Dimensions: An Age-Based Analysis. Anesth Analg. 2009, 108, 1475–1479. [Google Scholar] [CrossRef]
- Tablan, O.C.; Anderson, L.J.; Besser, R.; Bridges, C.; Hajjeh, R.; CDC. Healthcare Infection Control Practices Advisory Committee Guidelines for Preventing Health-Care--Associated Pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm. Rep. 2004, 53, 1–36. [Google Scholar]
- Cernada, M.; Brugada, M.; Golombek, S.; Vento, M. Ventilator-Associated Pneumonia in Neonatal Patients: An Update. Neonatology 2014, 105, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Crivello, A.; Milazzo, M.; La Rosa, D.; Fiacchini, G.; Danti, S.; Guarracino, F.; Berrettini, S.; Bruschini, L. Experimental Assessment of Cuff Pressures on the Walls of a Trachea-Like Model Using Force Sensing Resistors: Insights for Patient Management in Intensive Care Unit Settings. Sensors 2022, 22, 697. [Google Scholar] [CrossRef]
- Dbouk, T.; Drikakis, D. Endotracheal Tubes Design: The Role of Tube Bending. Symmetry 2021, 13, 1503. [Google Scholar] [CrossRef]
- Nathe, K.E.; Mancuso, C.J.; Parad, R.; Van Marter, L.J.; Martin, C.R.; Stoler-Barak, L.; Philbin, V.J.; Phillips, M.F.; Palmer, C.D.; Levy, O. Innate Immune Activation in Neonatal Tracheal Aspirates Suggests Endotoxin-Driven Inflammation. Pediatr. Res. 2012, 72, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiesa, C.; Pacifico, L.; Natale, F.; Hofer, N.; Osborn, J.F.; Resch, B. Fetal and Early Neonatal Interleukin-6 Response. Cytokine 2015, 76, 1–12. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Jiang, Y.-Z.; Hsu, C.-M.; Chen, L.-W. Pseudomonas Aeruginosa Ventilator-Associated Pneumonia Induces Lung Injury through TNF-α/c-Jun NH2-Terminal Kinase Pathways. PLoS ONE 2017, 12, e0169267. [Google Scholar] [CrossRef] [Green Version]
- Bouadma, L.; Deslandes, E.; Lolom, I.; Le Corre, B.; Mourvillier, B.; Regnier, B.; Porcher, R.; Wolff, M.; Lucet, J.-C. Long-Term Impact of a Multifaceted Prevention Program on Ventilator-Associated Pneumonia in a Medical Intensive Care Unit. Clin. Infect. Dis. 2010, 51, 1115–1122. [Google Scholar] [CrossRef]
- Saied, W.I.; Souweine, B.; Garrouste-Orgeas, M.; Ruckly, S.; Darmon, M.; Bailly, S.; Cohen, Y.; Azoulay, E.; Schwebel, C.; Radjou, A.; et al. Respective Impact of Implementation of Prevention Strategies, Colonization with Multiresistant Bacteria and Antimicrobial Use on the Risk of Early- and Late-Onset VAP: An Analysis of the OUTCOMEREA Network. PLoS ONE 2017, 12, e0187791. [Google Scholar] [CrossRef] [Green Version]
- Rello, J.; Kollef, M.; Diaz, E.; Sandiumenge, A.; del Castillo, Y.; Corbella, X.; Zachskorn, R. Reduced Burden of Bacterial Airway Colonization with a Novel Silver-Coated Endotracheal Tube in a Randomized Multiple-Center Feasibility Study. Crit. Care Med. 2006, 34, 2766–2772. [Google Scholar] [CrossRef]
- Kollef, M.H.; Afessa, B.; Anzueto, A.; Veremakis, C.; Kerr, K.M.; Margolis, B.D.; Craven, D.E.; Roberts, P.R.; Arroliga, A.C.; Hubmayr, R.D.; et al. Silver-Coated Endotracheal Tubes and Incidence of Ventilator-Associated Pneumonia: The NASCENT Randomized Trial. JAMA 2008, 300, 805–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, E.E.; Magin, C.M.; Mettetal, M.R.; May, R.M.; Henry, M.M.; DeLoid, H.; Prater, J.; Sullivan, L.; Thomas, J.G.; Twite, M.D.; et al. Micropatterned Endotracheal Tubes Reduce Secretion-Related Lumen Occlusion. Ann. Biomed. Eng. 2016, 44, 3645–3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björling, G.; Johansson, D.; Bergström, L.; Jalal, S.; Kohn, I.; Frostell, C.; Kalman, S. Tolerability and Performance of BIP Endotracheal Tubes with Noble Metal Alloy Coating—A Randomized Clinical Evaluation Study. BMC Anesthesiol. 2015, 15, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polívková, M.; Hubáček, T.; Staszek, M.; Švorčík, V.; Siegel, J. Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology. Int. J. Mol. Sci. 2017, 18, 419. [Google Scholar] [CrossRef] [Green Version]
- Sheng, W.-H.; Ko, W.-J.; Wang, J.-T.; Chang, S.-C.; Hsueh, P.-R.; Luh, K.-T. Evaluation of Antiseptic-Impregnated Central Venous Catheters for Prevention of Catheter-Related Infection in Intensive Care Unit Patients. Diagn. Microbiol. Infect. Dis. 2000, 38, 1–5. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial Activity of the Metals and Metal Oxide Nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Gusev, I.; Ferreira, M.; Versace, D.-L.; Abbad-Andaloussi, S.; Pluczyk-Małek, S.; Erfurt, K.; Duda, A.; Data, P.; Blacha-Grzechnik, A. Electrochemically Deposited Zinc (Tetraamino)Phthalocyanine as a Light-Activated Antimicrobial Coating Effective against S. Aureus. Materials 2022, 15, 975. [Google Scholar] [CrossRef]
- Rayyif, S.M.I.; Mohammed, H.B.; Curuțiu, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.Ș.; Dițu, L.M.; Lazăr, V.; Chifiriuc, M.C.; Mihăescu, G.; et al. ZnO Nanoparticles-Modified Dressings to Inhibit Wound Pathogens. Materials 2021, 14, 3084. [Google Scholar] [CrossRef]
- Kranz, S.; Guellmar, A.; Voelpel, A.; Lesser, T.; Tonndorf-Martini, S.; Schmidt, J.; Schrader, C.; Faucon, M.; Finger, U.; Pfister, W.; et al. Bactericidal and Biocompatible Properties of Plasma Chemical Oxidized Titanium (TiOB®) with Antimicrobial Surface Functionalization. Materials 2019, 12, 866. [Google Scholar] [CrossRef] [Green Version]
- Jampilek, J.; Kralova, K. Advances in Nanostructures for Antimicrobial Therapy. Materials 2022, 15, 2388. [Google Scholar] [CrossRef]
- Toplitsch, D.; Lackner, J.M.; Schwan, A.M.; Hinterer, A.; Stögmüller, P.; Horn, K.; Fritzlar, N.; Pfuch, A.; Kittinger, C. Antimicrobial Activity of a Novel Cu(NO3)2-Containing Sol–Gel Surface under Different Testing Conditions. Materials 2021, 14, 6488. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to Combat Antimicrobial Resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, L.; Huang, X.; Lu, Y.-G.; Zheng, D.-L.; Feng, Y. Antimicrobial Properties of Metal Nanoparticles and Their Oxide Materials and Their Applications in Oral Biology. J. Nanomater. 2022, 2022, e2063265. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial Activity of Metal Oxide Nanoparticles against Gram-Positive and Gram-Negative Bacteria: A Comparative Study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [Green Version]
- Cioffi, N.; Rai, M. (Eds.) Nano-Antimicrobials: Progress and Prospects; Springer: Berlin, Heidelberg, 2012; ISBN 978-3-642-24427-8. [Google Scholar]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.-C.; Horvatovich, P.; Gies, J.-P.; Keller, V.; Keller, N.; Andre, P. TiO2 Photocatalysis Damages Lipids and Proteins in Escherichia Coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef] [Green Version]
- Idrees, Q.T.A.; Gul, N.; Fareed, M.A.; Mian, S.A.; Muzaffar, D.; Nasir, M.; Chaudhry, A.A.; Akhtar, S.; Ahmed, S.Z.; Khan, A.S. Structural, Physical, and Mechanical Analysis of ZnO and TiO2 Nanoparticle-Reinforced Self-Adhesive Coating Restorative Material. Materials 2021, 14, 7507. [Google Scholar] [CrossRef]
- Mahmoodpoor, A.; Sanaie, S.; Parthvi, R.; Shadvar, K.; Hamishekar, H.; Iranpour, A.; Nuri, H.; Rahnemayan, S.; Nader, N.D. A Clinical Trial of Silver-Coated and Tapered Cuff plus Supraglottic Suctioning Endotracheal Tubes in Preventing Ventilator-Associated Pneumonia. J. Crit. Care 2020, 56, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, M.; Valério, A.; Ulson De Souza, A.A.; De Oliveira, D.; Franco, C.V.; Serafim, R.; Guelli, U.; Souza, S.M.A. Synthesis and Application of Silver Nanoparticles as Biocidal Agent in Polyurethane Coating. J. Coat. Technol. Res. 2020, 17, 613–620. [Google Scholar] [CrossRef]
- JIS Z 2801—Test for Antimicrobial Activity of Plastics. Available online: https://www.situbiosciences.com/product/jis-z-2801-test-for-antimicrobial-activity-of-plastics/ (accessed on 24 June 2023).
- Cruz-Pacheco, A.F.; Muñoz-Castiblanco, D.T.; Gómez Cuaspud, J.A.; Paredes-Madrid, L.; Parra Vargas, C.A.; Martínez Zambrano, J.J.; Palacio Gómez, C.A. Coating of Polyetheretherketone Films with Silver Nanoparticles by a Simple Chemical Reduction Method and Their Antibacterial Activity. Coatings 2019, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Derakhshi, M.; Ashkarran, A.A.; Bahari, A.; Bonakdar, S. Shape Selective Silver Nanostructures Decorated Amine-Functionalized Graphene: A Promising Antibacterial Platform. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 101–109. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Yu, D. Synthesis of Waterborne Polyurethane–Silver Nanoparticle Antibacterial Coating for Synthetic Leather. J. Coat. Technol. Res. 2018, 15, 415–423. [Google Scholar] [CrossRef]
- Lethongkam, S.; Daengngam, C.; Tansakul, C.; Siri, R.; Chumpraman, A.; Phengmak, M.; Voravuthikunchai, S.P. Prolonged Inhibitory Effects against Planktonic Growth, Adherence, and Biofilm Formation of Pathogens Causing Ventilator-Associated Pneumonia Using a Novel Polyamide/Silver Nanoparticle Composite-Coated Endotracheal Tube. Biofouling 2020, 36, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.E.; Harmon, B.G.; Kollef, M.H. Silver-Coated Endotracheal Tubes Associated With Reduced Bacterial Burden in the Lungs of Mechanically Ventilated Dogs. Chest 2002, 121, 863–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, C.-Y.; Young, P.M.; Lee, W.-H.; Cavaliere, R.; Whitchurch, C.B.; Rohanizadeh, R. Non-Cytotoxic Silver Nanoparticle-Polyvinyl Alcohol Hydrogels with Anti-Biofilm Activity: Designed as Coatings for Endotracheal Tube Materials. Biofouling 2014, 30, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lv, B.; Shen, Q.; Wang, X. Preparation of Silicon-Modified Antimicrobial Polyethylene Endotracheal Tubes. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damas, P.; Legrain, C.; Lambermont, B.; Dardenne, N.; Guntz, J.; Kisoka, G.; Demaret, P.; Rousseau, A.-F.; Jadot, L.; Piret, S.; et al. Prevention of Ventilator-Associated Pneumonia by Noble Metal Coating of Endotracheal Tubes: A Multi-Center, Randomized, Double-Blind Study. Ann. Intensive Care 2022, 12, 1. [Google Scholar] [CrossRef]
- Tincu, R.C.; Cobilinschi, C.; Tincu, I.F.; Macovei, R.A. Efficacy of Noble Metal–Alloy Endotracheal Tubes in Ventilator-Associated Pneumonia Prevention: A Randomized Clinical Trial. Balkan Med. J 2022, 39, 167–171. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Reduced Staphylococcus Aureus Proliferation and Biofilm Formation on Zinc Oxide Nanoparticle PVC Composite Surfaces. Acta Biomater. 2011, 7, 2579–2584. [Google Scholar] [CrossRef]
- Geilich, B.M.; Webster, T.J. Reduced Adhesion of Staphylococcus aureus to ZnO/PVC Nanocomposites. IJN 2013, 8, 1177–1184. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Nguyen, K.C.; Caldwell, D.; Fine, J.H.; Lefebvre, D.E.; Tayabali, A.F. Immune Responses during Single and Repeated Murine Endotracheal Exposures of Zinc Oxide Nanoparticles. NanoImpact 2017, 7, 54–65. [Google Scholar] [CrossRef]
- Tran, P.A.; Webster, T.J. Antimicrobial Selenium Nanoparticle Coatings on Polymeric Medical Devices. Nanotechnology 2013, 24, 155101. [Google Scholar] [CrossRef] [PubMed]
- Caratto, V.; Ball, L.; Sanguineti, E.; Insorsi, A.; Firpo, I.; Alberti, S.; Ferretti, M.; Pelosi, P. Antibacterial activity of standard and N-doped titanium dioxide-coated endotracheal tubes: An in vitro study. Rev. Bras. De Ter. Intensiv. 2017, 29, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, W.; Ning, S.; Lin, Q.; Zhang, H.; Zhou, T.; Lin, H.; Long, J.; Lin, Q.; Wang, X. I-TiO2/PVC Film with Highly Photocatalytic Antibacterial Activity under Visible Light. Colloids Surf. B Biointerfaces 2016, 144, 196–202. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic Disinfection Using Titanium Dioxide: Spectrum and Mechanism of Antimicrobial Activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Wu, P.; Grainger, D.W. Drug/Device Combinations for Local Drug Therapies and Infection Prophylaxis. Biomaterials 2006, 27, 2450–2467. [Google Scholar] [CrossRef]
- Chaiban, G.; Hanna, H.; Dvorak, T.; Raad, I. A Rapid Method of Impregnating Endotracheal Tubes and Urinary Catheters with Gendine: A Novel Antiseptic Agent. J. Antimicrob. Chemother. 2005, 55, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Raad, I.I.; Mohamed, J.A.; Reitzel, R.A.; Jiang, Y.; Dvorak, T.L.; Ghannoum, M.A.; Hachem, R.Y.; Chaftari, A.-M. The Prevention of Biofilm Colonization by Multidrug-Resistant Pathogens That Cause Ventilator-Associated Pneumonia with Antimicrobial-Coated Endotracheal Tubes. Biomaterials 2011, 32, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Fowler, V.; Gaonkar, T.; Wyer, P.C.; Modak, S. Antiseptic Impregnated Endotracheal Tubes for the Prevention of Bacterial Colonization. J. Hosp. Infect. 2004, 57, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; McGovern, J.G.; Woolfson, A.D.; Adair, C.G.; Gorman, S.P. Physicochemical Characterization of Hexetidine-Impregnated Endotracheal Tube Poly(Vinyl Chloride) and Resistance to Adherence of Respiratory Bacterial Pathogens. Pharm. Res. 2002, 19, 818–824. [Google Scholar] [CrossRef]
- Venkateswaran, S.; Santos, O.D.H.D.; Scholefield, E.; Lilienkampf, A.; Gwynne, P.J.; Swann, D.G.; Dhaliwal, K.; Gallagher, M.P.; Bradley, M. Fortified Interpenetrating Polymers—Bacteria Resistant Coatings for Medical Devices. J. Mater. Chem. B 2016, 4, 5405–5411. [Google Scholar] [CrossRef] [Green Version]
- Homeyer, K.H.; Singha, P.; Goudie, M.J.; Handa, H. S-Nitroso-N-Acetylpenicillamine Impregnated Endotracheal Tubes for Prevention of Ventilator-Associated Pneumonia. Biotechnol. Bioeng. 2020, 117, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Blecha, F. Antimicrobial Peptides and Bacteriocins: Alternatives to Traditional Antibiotics. Anim. Health Res. Rev. 2008, 9, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and Host-Defense Peptides as New Anti-Infective Therapeutic Strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Aronson, M.R.; Ali Akbari Ghavimi, S.; Gehret, P.M.; Jacobs, I.N.; Gottardi, R. Drug-Eluting Endotracheal Tubes for Preventing Bacterial Inflammation in Subglottic Stenosis. Laryngoscope 2022, 132, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Dao, A.; Mills, R.J.; Kamble, S.; Savage, P.B.; Little, D.G.; Schindeler, A. The Application of Ceragenins to Orthopedic Surgery and Medicine. J. Orthop. Res. 2020, 38, 1883–1894. [Google Scholar] [CrossRef]
- Leszczyńska, K.; Namiot, A.; Cruz, K.; Byfield, F.J.; Won, E.; Mendez, G.; Sokołowski, W.; Savage, P.B.; Bucki, R.; Janmey, P.A. Potential of Ceragenin CSA-13 and Its Mixture with Pluronic F-127 as Treatment of Topical Bacterial Infections. J. Appl. Microbiol. 2011, 110, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, J.N.; Jones, R.N.; Sader, H.S.; Savage, P.B.; Rybak, M.J. Potential Synergy Activity of the Novel Ceragenin, CSA-13, against Clinical Isolates of Pseudomonas Aeruginosa, Including Multidrug-Resistant P. Aeruginosa. J. Antimicrob. Chemother. 2008, 61, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Durnaś, B.; Wnorowska, U.; Pogoda, K.; Deptuła, P.; Wątek, M.; Piktel, E.; Głuszek, S.; Gu, X.; Savage, P.B.; Niemirowicz, K.; et al. Candidacidal Activity of Selected Ceragenins and Human Cathelicidin LL-37 in Experimental Settings Mimicking Infection Sites. PLoS ONE 2016, 11, e0157242. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and Cons of Phage Therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Seitz, A.P.; Schumacher, F.; Baker, J.; Soddemann, M.; Wilker, B.; Caldwell, C.C.; Gobble, R.M.; Kamler, M.; Becker, K.A.; Beck, S.; et al. Sphingosine-Coating of Plastic Surfaces Prevents Ventilator-Associated Pneumonia. J. Mol. Med. 2019, 97, 1195–1211. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential Applications in Medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Amankwah, S.; Adisu, M.; Gorems, K.; Abdella, K.; Kassa, T. Assessment of Phage-Mediated Inhibition and Removal of Multidrug-Resistant <em>Pseudomonas Aeruginosa</Em> Biofilm on Medical Implants. IDR 2022, 15, 2797–2811. [Google Scholar] [CrossRef]
- Jones, D.S.; McMeel, S.; Adair, C.G.; Gorman, S.P. Characterisation and Evaluation of Novel Surfactant Bacterial Anti-Adherent Coatings for Endotracheal Tubes Designed for the Prevention of Ventilator-Associated Pneumonia. J. Pharm. Pharmacol. 2003, 55, 43–52. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface Characteristics Influencing Bacterial Adhesion to Polymeric Substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic Surfaces for the Reduction of Bacterial Adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Stallard, C.P.; McDonnell, K.A.; Onayemi, O.D.; O’Gara, J.P.; Dowling, D.P. Evaluation of Protein Adsorption on Atmospheric Plasma Deposited Coatings Exhibiting Superhydrophilic to Superhydrophobic Properties. Biointerphases 2012, 7, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harnett, E.M.; Alderman, J.; Wood, T. The Surface Energy of Various Biomaterials Coated with Adhesion Molecules Used in Cell Culture. Colloids Surf. B Biointerfaces 2007, 55, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Durmus, N.G.; Taylor, E.N.; Inci, F.; Kummer, K.M.; Tarquinio, K.M.; Webster, T.J. Fructose-Enhanced Reduction of Bacterial Growth on Nanorough Surfaces. Int. J. Nanomed. 2012, 7, 537–545. [Google Scholar] [CrossRef]
- Machado, M.C.; Tarquinio, K.M.; Webster, T.J. Decreased Staphylococcus aureus Biofilm Formation on Nanomodified Endotracheal Tubes: A Dynamic Airway Model. Int. J. Nanomed. 2012, 7, 3741–3750. [Google Scholar] [CrossRef] [Green Version]
- Machado, M.C.; Webster, T.J. Decreased Pseudomonas Aeruginosa Biofilm Formation on Nanomodified Endotracheal Tubes: A Dynamic Lung Model. Int. J. Nanomed. 2016, 11, 3825–3831. [Google Scholar] [CrossRef] [Green Version]
- Seil, J.T.; Rubien, N.M.; Webster, T.J.; Tarquinio, K.M. Comparison of Quantification Methods Illustrates Reduced Pseudomonas Aeruginosa Activity on Nanorough Polyvinyl Chloride. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Triandafillu, K.; Balazs, D.J.; Aronsson, B.-O.; Descouts, P.; Tu Quoc, P.; van Delden, C.; Mathieu, H.J.; Harms, H. Adhesion of Pseudomonas Aeruginosa Strains to Untreated and Oxygen-Plasma Treated Poly(Vinyl Chloride) (PVC) from Endotracheal Intubation Devices. Biomaterials 2003, 24, 1507–1518. [Google Scholar] [CrossRef]
- Loo, C.-Y.; Young, P.M.; Lee, W.-H.; Cavaliere, R.; Whitchurch, C.B.; Rohanizadeh, R. Superhydrophobic, Nanotextured Polyvinyl Chloride Films for Delaying Pseudomonas Aeruginosa Attachment to Intubation Tubes and Medical Plastics. Acta Biomater. 2012, 8, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Burgui, S.; Langheinrich, D.; Gil, C.; Solano, C.; Toledo-Arana, A.; Helbig, R.; Lasagni, A.; Lasa, I. Evaluation of Surface Microtopography Engineered by Direct Laser Interference for Bacterial Anti-Biofouling: Evaluation of Surface Microtopography Engineered by Direct Laser Interference. Macromol. Biosci. 2015, 15, 1060–1069. [Google Scholar] [CrossRef] [Green Version]
- May, R.M.; Hoffman, M.G.; Sogo, M.J.; Parker, A.E.; O’Toole, G.A.; Brennan, A.B.; Reddy, S.T. Micro-patterned Surfaces Reduce Bacterial Colonization and Biofilm Formation in Vitro: Potential for Enhancing Endotracheal Tube Designs. Clin. Transl. Med. 2014, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchma, S.L.; Brothers, K.M.; Merritt, J.H.; Liberati, N.T.; Ausubel, F.M.; O’Toole, G.A. BifA, a Cyclic-Di-GMP Phosphodiesterase, Inversely Regulates Biofilm Formation and Swarming Motility by Pseudomonas Aeruginosa PA14. J. Bacteriol. 2007, 189, 8165–8178. [Google Scholar] [CrossRef] [Green Version]
- Worthington, R.J.; Melander, C. Combination Approaches to Combat Multidrug-Resistant Bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Villani, M.; Bertoglio, F.; Restivo, E.; Bruni, G.; Iervese, S.; Arciola, C.R.; Carulli, F.; Iannace, S.; Bertini, F.; Visai, L. Polyurethane-Based Coatings with Promising Antibacterial Properties. Materials 2020, 13, 4296. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, B.; Ni, D.; Sun, Y.; Wang, G.; Jiang, H. A Novel Antibacterial and Antifouling Nanocomposite Coated Endotracheal Tube to Prevent Ventilator-Associated Pneumonia. J. Nanobiotechnology 2022, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Milenković, J.; Hrenović, J.; Goić-Barišić, I.; Tomić, M.; Rajić, N. Antibacterial Activity of Copper-Containing Clinoptilolite/ PVC Composites toward Clinical Isolate of Acinetobacter Baumannii. J. Serbian Chem. Soc. 2015, 80, 819–826. [Google Scholar] [CrossRef]
- Milenkovic, J.; Hrenovic, J.; Goic-Barisic, I.; Tomic, M.; Djonlagic, J.; Rajic, N. Synergistic Anti-Biofouling Effect of Ag-Exchanged Zeolite and D-Tyrosine on PVC Composite against the Clinical Isolate of Acinetobacter baumannii. Biofouling 2014, 30, 965–973. [Google Scholar] [CrossRef]
- Zangirolami, A.C.; Dias, L.D.; Blanco, K.C.; Vinagreiro, C.S.; Inada, N.M.; Arnaut, L.G.; Pereira, M.M.; Bagnato, V.S. Avoiding Ventilator-Associated Pneumonia: Curcumin-Functionalized Endotracheal Tube and Photodynamic Action. Proc. Natl. Acad. Sci. USA 2020, 117, 22967–22973. [Google Scholar] [CrossRef]
- Jones, D.S.; McCoy, C.P.; Andrews, G.P.; McCrory, R.M.; Gorman, S.P. Hydrogel Antimicrobial Capture Coatings for Endotracheal Tubes: A Pharmaceutical Strategy Designed to Prevent Ventilator-Associated Pneumonia. Mol. Pharm. 2015, 12, 2928–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarquinio, K. Bactericidal Effects of Silver plus Titanium Dioxide-Coated Endotracheal Tubes on Pseudomonas Aeruginosa and Staphylococcus Aureus. Int. J. Nanomed. 2010, 5, 177–183. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Yuan, X.; Xie, J.; Leong, D.T. The Influence of Lysosomal Stability of Silver Nanomaterials on Their Toxicity to Human Cells. Biomaterials 2014, 35, 6707–6715. [Google Scholar] [CrossRef] [PubMed]
- Delaval, M.; Wohlleben, W.; Landsiedel, R.; Baeza-Squiban, A.; Boland, S. Assessment of the Oxidative Potential of Nanoparticles by the Cytochrome c Assay: Assay Improvement and Development of a High-Throughput Method to Predict the Toxicity of Nanoparticles. Arch. Toxicol. 2017, 91, 163–177. [Google Scholar] [CrossRef]
- Azizi, S.; Mohamad, R.; Abdul Rahim, R.; Mohammadinejad, R.; Bin Ariff, A. Hydrogel Beads Bio-Nanocomposite Based on Kappa-Carrageenan and Green Synthesized Silver Nanoparticles for Biomedical Applications. Int. J. Biol. Macromol. 2017, 104, 423–431. [Google Scholar] [CrossRef]
- Daengngam, C.; Lethongkam, S.; Srisamran, P.; Paosen, S.; Wintachai, P.; Anantravanit, B.; Vattanavanit, V.; Voravuthikunchai, S. Green Fabrication of Anti-Bacterial Biofilm Layer on Endotracheal Tubing Using Silver Nanoparticles Embedded in Polyelectrolyte Multilayered Film. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 53–63. [Google Scholar] [CrossRef]
- Maki, D.G.; Kluger, D.M.; Crnich, C.J. The Risk of Bloodstream Infection in Adults with Different Intravascular Devices: A Systematic Review of 200 Published Prospective Studies. Mayo Clin. Proc. 2006, 81, 1159–1171. [Google Scholar] [CrossRef]
- Al-Sayed, M.F.; Tarek El-Wakad, M.; Hassan, M.A.; Soliman, A.M.; Eldesoky, A.S. Optimal Concentration and Duration of Endotracheal Tube Coating to Achieve Optimal Antimicrobial Efficacy and Safety Balance: An In Vitro Study. Gels 2023, 9, 414. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.A.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Engineering Highly Effective Antimicrobial Selenium Nanoparticles through Control of Particle Size. Nanoscale 2019, 11, 14937–14951. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Das, I.; Mehta, R.K.; Sahu, R.; Sonawane, A. Zinc-Oxide Nanoparticles Exhibit Genotoxic, Clastogenic, Cytotoxic and Actin Depolymerization Effects by Inducing Oxidative Stress Responses in Macrophages and Adult Mice. Toxicol. Sci. 2016, 150, 454–472. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ling, X.; Liu, G.; Xiao, J. Antimicrobial Coating: Tracheal Tube Application. Int. J. Nanomed. 2022, 17, 1483–1494. [Google Scholar] [CrossRef]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface Modifications for Antimicrobial Effects in the Healthcare Setting: A Critical Overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, J.F.; Levine, S.M.; Restrepo, M.I. Technologic Advances in Endotracheal Tubes for Prevention of Ventilator-Associated Pneumonia. Chest 2012, 142, 231–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahonen, M.; Kahru, A.; Ivask, A.; Kasemets, K.; Kõljalg, S.; Mantecca, P.; Vinković Vrček, I.; Keinänen-Toivola, M.M.; Crijns, F. Proactive Approach for Safe Use of Antimicrobial Coatings in Healthcare Settings: Opinion of the COST Action Network AMiCI. Int. J. Environ. Res. Public Health 2017, 14, 366. [Google Scholar] [CrossRef] [PubMed]
- Hosseinidoust, Z.; Olsson, A.L.J.; Tufenkji, N. Going Viral: Designing Bioactive Surfaces with Bacteriophage. Colloids Surf B Biointerfaces 2014, 124, 2–16. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, T.; Morent, R.; De Geyter, N.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biomedical Polymers: Influence on Cell-Material Interaction. Plasma Chem. Plasma Process 2012, 32, 1039–1073. [Google Scholar] [CrossRef]
- McEachern, R.; Campbell, G.D. Hospital-Acquired Pneumonia: Epidemiology, Etiology, and Treatment. Infect. Dis. Clin. N. Am. 1998, 12, 761–779. [Google Scholar] [CrossRef]
- Richards, M.J.; Edwards, J.R.; Culver, D.H.; Gaynes, R.P. Nosocomial Infections in Medical Intensive Care Units in the United States. National Nosocomial Infections Surveillance System. Crit. Care Med. 1999, 27, 887–892. [Google Scholar] [CrossRef]
- Kramek-Romanowska, K.; Stecka, A.M.; Zieliński, K.; Dorosz, A.; Okrzeja, P.; Michnikowski, M.; Odziomek, M. Independent Lung Ventilation-Experimental Studies on a 3D Printed Respiratory Tract Model. Materials 2021, 14, 5189. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Okada, Y.; Fujita, N.; Nagasaka, Y. A Novel Magnetic Resonance Imaging-Compatible Titanium Alloy Wire-Reinforced Endotracheal Tube. Materials 2022, 15, 5632. [Google Scholar] [CrossRef] [PubMed]
- Van Charante, F.; Wieme, A.; Rigole, P.; De Canck, E.; Ostyn, L.; Grassi, L.; Deforce, D.; Crabbé, A.; Vandamme, P.; Joossens, M.; et al. Microbial Diversity and Antimicrobial Susceptibility in Endotracheal Tube Biofilms Recovered from Mechanically Ventilated COVID-19 Patients. Biofilm 2022, 4, 100079. [Google Scholar] [CrossRef]
- Ozcelik, B.; Pasic, P.; Sangwan, P.; Be, C.L.; Glattauer, V.; Thissen, H.; Boulos, R.A. Evaluation of the Novel Antimicrobial BCP3 in a Coating for Endotracheal Tubes. ACS Omega 2020, 5, 10288–10296. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Bang, S.; Kwon, W.; Shim, J. Patient-Specific Depth of Endotracheal Intubation-from Anthropometry to the Touch and Read Method. Pak J. Med. Sci. 2016, 32, 1234–1239. [Google Scholar] [CrossRef]
- Philadelphia, T.C.H. of CHOP Researchers Develop Coating for Endotracheal Tubes That Releases Antimicrobial Peptides. Available online: https://www.chop.edu/news/chop-researchers-develop-coating-endotracheal-tubes-releases-antimicrobial-peptides (accessed on 26 June 2023).
- Machado, M.C.; Cheng, D.; Tarquinio, K.M.; Webster, T.J. Nanotechnology: Pediatric Applications. Pediatr. Res. 2010, 67, 500–504. [Google Scholar] [CrossRef] [Green Version]
- Research. V.M. Endotracheal Tube Market Size USD 3.1 Billion by 2030. Available online: https://www.vantagemarketresearch.com (accessed on 27 June 2023).
- Coated Endotracheal Tube Market. Available online: https://www.transparencymarketresearch.com/coated-endotracheal-tubes-market.html (accessed on 27 June 2023).








| Antimicrobial Metal Coating | Coating Characteristics | Base Material/Antimicrobial feature | In Vitro/In Vivo Study | Remarks | Ref. |
|---|---|---|---|---|---|
| Zinc oxide (ZnO) | ZnO exhibits antimicrobial properties. Usually, ZnO nanoparticles are used due to low manufacturing costs, high surface-to-volume ratio, and enhanced stability. ZnO has high efficiency against Gram-positive and Gram-negative bacteria. | PVC/ZnO-nanoparticles (NPs) | In vitro (S. aureus) | Through the incorporation of ZnO NPs into PVC material, a reduction of biofilm formation by 55% was noticed after 72 h. | Seil et al. [91] |
| PVC/ZnO NPs | In vitro (S. aureus) | A reduction of 87% in biofilm formation was reported after 24 h. An increase in the NPs’ concentration was linked to a high surface energy and roughness exhibiting a beneficial effect on bacteria reduction. The lower ZnO NP diameter led to increased antibacterial activity. | Geilich and Webster [92] | ||
| Commercially available coated and uncoated ZnO NPs | In vivo (male BALB/c mice) | The animal models were exposed to endotracheal instillation with one dose (5 μg/mouse), and pulmonary inflammation was noticed. After a month of weekly exposure, a higher immune response was obtained in the case of uncoated nanoparticles. | Zhang et al. [93] | ||
| Selenium (Se) | Se NPs exhibit antioxidant, anti-oncological, and antibacterial properties [14] | PVC medical grade/Se NPs | In vitro (S. aureus) | A reduction of 80% was observed in the bacterial colonization process. The biofilm formation was reduced in comparison with a silver-coated ETT. | Tran and Webster [94] |
| Titanium dioxide (TiO2) combined with photodynamic therapy | Photodynamic therapy is based on a photosensitizer material, which can be activated at a given wavelength of light. It generates an active component that has antibacterial properties. TiO2 is chemically inert, stable, and exhibits photocatalytic properties by generating reactive oxidative species (ROS) when it is exposed to a wavelength of 385 nm [97]. TiO2 is efficient against both Gram-positive and Gram-negative bacteria. | Commercial ETT from PVC/TiO2 NPs (N-doped and commercially available standard anatase) | In vitro (S. aureus and P. aeruginosa) | In the case of light absence, no antimicrobial effects were observed. For fluorescent light irradiation, both types of coating exhibited almost the same effects on P. aeruginosa, and N-doped nanoparticles proved to be more efficient against S. aureus | Caratto et al. [95] |
| PVC medical grade/iodine modified TiO2 NPs | In vitro (E. coli) and in vivo (pig model) | The modified TiO2 NPs presented photocatalytic antibacterial effects under visible light application. A decrease in bacterial attachment and biofilm formation was reported after 72 h. Reduced inflammation of the lungs was noticed in MV pigs after 72 h. | Deng et al. [96] |
| Antimicrobial Compound | Pathogen | Test Type | Remarks | Ref. |
|---|---|---|---|---|
| Lasioglossin-III | S. epidermis, S. pneumoniae | In vitro | The design of a peptide-eluting tube based on a PLGA matrix with a continuous release of Lasioglossin-III proved to be efficient against planktonic bacterial development. There were no reported side effects on epithelial and fibroblast cell lines. | Aronson et al. [107] |
| Ceragenin CSA-131 | C. auris, K. pneumoniae, C. albicans, P. aeruginosa, MRSA | In vitro, in vivo | The biofilm did not appear in the first 16 days. There was no reported multispecies biofilm formation for up to 3 days. In the pig animal model, no damages were observed to the trachea and lungs. | Hashemi et al. [40] |
| Sphingosine | S. aureus, P. aeruginosa, A. baumanii | In vitro, in vivo | In vitro efficacy against biofilm formation. In vivo tests evidenced the absence of inflammation and prevention of bacteria film. | Seitz et al. [113] |
| Phages | MDR P. aeruginosa | In vitro | The phage-coated samples were characterized by reduced bacterial colonization by a maximum of 3.2 log compared with uncoated samples. | Amankwah et al. [115] |
| Lecithin and cholesterol | S. aureus, P. aeruginosa | In vitro | After 8h, a decrease of 90% in biofilm formation was reported. | Jones et al. [116] |
| Main idea of the Combinatorial Strategy | Antimicrobial Compound | Pathogen | TEST TYPE | Remarks | Ref. |
|---|---|---|---|---|---|
| Metabolites such as fructose can enhance the antibiotics’ efficiency. | Surface with nanometric characteristics obtained after a combination of a fungal lipase and fructose. | S. aureus | In vitro | The nanoscale surface features obtained under the action of a fungal lipase determined a 45% decrease in S. aureus attachment after 24 h exposure. Soaking the unmodified surface ETT in fructose generated a 38% reduction. When the two strategies were combined, a decrease of about 60% in S. aureus attachment was reported. | Dumus et al. [121] |
| Natural polymer chitosan (CS), already used in the wound dressing domain, has a good interaction with metallic ions and nanoparticles. | CS-Ag nanoparticles @ polyacrylamide—gelatin composite | S. aureus, P. aeruginosa | In vitro (broncho-lung system); in vivo (pig model) | The complex nanocomposite coating exhibited important antibacterial properties during in vitro and in vivo tests. A reduction of 97% in lumen occlusion due to artificial mucus was observed. Antibiofouling characteristics were noticed due to reduced lumen occlusion. High biocompatibility of the coating evidenced by in vitro tests with fibroblasts | Wang et al. [132] |
| Zeolites are substances that are characterized by cavities and channels. They can trap metallic ions with antibacterial properties. | Zeolites with copper ions (CuZ) and D-Tyrosine (D-Tyr) solution. | MDR A. baumannii | In vitro | A reduction of 14% in immobilized cells was noticed after 24 h. The impregnation of composite system CuZ with D-Tyr (CuZ-Tyr) based on a synergistic effect proved to have an important antibacterial effect. | Milenković et al. [133] |
| Zeolites with micronized silver (Ag-NZ) and D-Tyr. | MDR A. baumannii | In vitro | The Ag-NZ composites (1–15 wt.% Ag-NZ) were characterized by a decrease of up to 70% (4.4 log CFU) of immobilized pathogen compared with commercially available PVC. The samples Ag-NZ coated with D-Tyr (Ag-NZ-Tyr) exhibited a 100% bactericidal effect consisting of a 6.9 log CFU reduction against immobilized bacterial cells. | Milenković et al. [134] | |
| Curcumin has an important photodynamic effect. | Curcumin combined with photodynamic action. | S. aureus, P. aeruginosa, E. coli | In vitro | Reductions of 95% (S. aureus), 72% (E. coli), and 73% (P. aeruginosa) in biofilm formation were noticed under the combined action of light and curcumin-functionalized ETT. The coating was still active after 6 light applications at a time interval of 24 h for 6 days. A pathogen decrease of 24% was reported. | Zangirolami et al. [135] |
| Hydrogel entrapped with nebulized drugs has antimicrobial and antibacterial effects. | Hydrogels made of hydroxyethylmethacrylate (HEMA): methacrylic acid (MAA) combined with nebulized gentamicin. | S. aureus, P. aeruginosa | In vitro | The most efficient combination was gentamicin-containing HEMA: MAA hydrogel. Another good combination was the 70:30 HEMA: MAA copolymer that presented a persistent effect against the tested pathogens at more than 20 days. | Jones et al. [136] |
| Antibacterial combined effect between chlorhexidine (CHX) and silver carbonate. | CHX and silver-based compound. | A. baumannii, methicilin-resistant S. aureus MRSA, S.aureus, P. aeruginosa, Enterobacter aerogenes | In vitro | The antiseptic-impregnated ETTs exhibited a reduced possibility of bacterial pathogen colonization compared with commercial ETTs. A reduction of 4–6 log of pathogen colonization of ETT after 5 days was reported. | Pacheco-Fowler et al. [101] |
| Combination of titanium dioxide (TiO2) and silver. | Silver, TiO2, and innovative metallic alloy Degussa | S. aureus, P. aeruginosa | In vitro | No positive effect was reported against S. aureus. Regarding P. aeruginosa, the silver combined with TiO2 reduced film growth after 24 h, while the combination of Degussa and TiO2 presented a diminution of pathogen growth after 48 h. | Tarquinio et al. [137] |
| Antimicrobial Approach | Strategy | Disadvantage | Selective Ref. |
|---|---|---|---|
| Active antimicrobial coatings | Antimicrobial metal coatings | Toxicity of metal ions or nanoparticles; propensity to induce bacterial resistance. | Setyawati et al. [138]; Delawal et al. [139] |
| Antimicrobial coatings based on biocide impregnation | Difficult control of release kinetics of active substances; development of antimicrobial resistance and induced drug-resistant strains when antibiotics are used; toxicity and environmental problems linked to biocides in a dose-dependent manner. | Ahonen et al. [149]; Alves et al. [14] | |
| Bio-inspired antimicrobial coatings | Proteolytic degradation; cytotoxicity and hemolysis concerns regarding AMPs; high costs of AMPs; the use of phages is characterized by moisture sensitivity and deactivation under certain conditions. | Alves et al. [14]; Hosseinidoust et al. [150] | |
| Passive coatings | Nanomodified surfaces | Surface modifications are obtained through surface chemistry manipulation, which is a difficult process, requiring the development of complex protocols and specific technological methodologies. | Alves et al. [14]; Machado et al. [122] |
| Hydrophilic/hydrophobic surface modification | High costs when plasma treatment is involved. | Jacobs et al. [151] | |
| Micropatterned surface modifications | Structural durability and stability; biocompatibility, environmental concerns, high costs. | Mann et al. [63]; May et al. [128] | |
| Combinatorial materials | Active and passive strategies | Combine the disadvantages of different involved strategies previously underlined. | Alves et al. [14]; Barnes et al. [3] |
| Antimicrobial Strategy | Antimicrobial Feature | Pathogen Strain | Selective Ref. |
|---|---|---|---|
| Active/Antimicrobial metal coatings | Ag | A. baumanii, C. albicans, K. pneumoniae, P. aeruginosa, Enterococcus faecalis, MRSA (methicilin-resistant S. aureus), S. aureus, E. coli | Lethongkam et al. [85]; Loo et al. [87]; Jiang et al. [88] |
| NMA (Au-Ag-Pd) | Enterococci spp., Neisseria spp., Haemophiles parainfluenza, Streptococcus, Staphylococci | Björling et al. [64]; Tincu et al. [90] | |
| ZnO, TiO2, Se | S. aureus, P. aeruginosa, E. coli | Seil and Webster [91]; Caratto et al. [95]; Deng et al. [96]; Tran and Webster [94] | |
| Active/Biocidal impregnation | Gardine and gendine, hexetidine | A. baumannii, E. cloacae, C. albicans, K. pneumoniae, MRSA, P. aeruginosa, S. aureus | Raad et al. [100]; Jones et al. [102] |
| Poly(lauryl acrylate)-based nanocapsules with eugenol or clove oil, styrylbenzene-based (BCP3) | MRSA, K. pneumoniae, MSSA (methicilin-sensitive S. aureus), P. aeruginosa | Venkateswaran et al. [103]; Ozcelik et al. [157] | |
| Active/Bio-inspired antimicrobials | Lasioglossin-III, ceragenin CSA-131, cholesterol and lecithin, sphingosine, phages | S. epidermis, S. pneumoniae, C. albicans, C. auris, K. pneumoniae, P. aeruginosa, MRSA, S. aureus, A. baumannii, MDR (Multidrug resistant) P. aeruginosa | Aronson et al. [107]; Hashemi et al. [40]; Jones et al. [116]; Seitz et al. [113]; Amankwah et al. [115] |
| Passive/Nanomodified surfaces | Fungal lipase | S. aureus, P. aeruginosa | Machado et al. [122]; Machado and Webster [123] |
| Passive/Hydrophilic/hydrophobic surface modification | Plasma treatments | P. aeruginosa | Triandafillu et al. [125] |
| Passive/micropatterned surfaces | Sharklet pattern design | A. baumannii, K. pneumoniae, MRSA, E. coli, P. aeruginosa | May et al. [128]; Mann et al. [63] |
| Combinatorial materials | Chitosan—AgNPs—polyacrylamide, fungal lipase and fructose, chlorhexidine and silver carbonate, copper and zeolite, hydrogels with nebulized gentamicin, curcumin and photodynamic effect | P. aeruginosa, S. aureus, A. baumannii, Enterobacter aerogenes, MRSA, MDR. A. baumannii, E. coli | Wang et al. [132]; Durmus et al. [121]; Pacheco-Fawler et al. [101]; Milenković et al. [133]; Jones et al. [136]; Zangirolami et al. [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcut, L.; Manescu, V.; Antoniac, A.; Paltanea, G.; Robu, A.; Mohan, A.G.; Grosu, E.; Corneschi, I.; Bodog, A.D. Antimicrobial Solutions for Endotracheal Tubes in Prevention of Ventilator-Associated Pneumonia. Materials 2023, 16, 5034. https://doi.org/10.3390/ma16145034
Marcut L, Manescu V, Antoniac A, Paltanea G, Robu A, Mohan AG, Grosu E, Corneschi I, Bodog AD. Antimicrobial Solutions for Endotracheal Tubes in Prevention of Ventilator-Associated Pneumonia. Materials. 2023; 16(14):5034. https://doi.org/10.3390/ma16145034
Chicago/Turabian StyleMarcut, Lavinia, Veronica Manescu (Paltanea), Aurora Antoniac, Gheorghe Paltanea, Alina Robu, Aurel George Mohan, Elena Grosu, Iuliana Corneschi, and Alin Danut Bodog. 2023. "Antimicrobial Solutions for Endotracheal Tubes in Prevention of Ventilator-Associated Pneumonia" Materials 16, no. 14: 5034. https://doi.org/10.3390/ma16145034
APA StyleMarcut, L., Manescu, V., Antoniac, A., Paltanea, G., Robu, A., Mohan, A. G., Grosu, E., Corneschi, I., & Bodog, A. D. (2023). Antimicrobial Solutions for Endotracheal Tubes in Prevention of Ventilator-Associated Pneumonia. Materials, 16(14), 5034. https://doi.org/10.3390/ma16145034



.png)




