Narrow-Band Deep-Blue Emission and Superior Thermal Stability of Fluoroaluminate Phosphor Based on Tungsten Bronze-Type Mineral Structure
Abstract
1. Introduction
2. Experimental Section
2.1. Material Synthesis
2.2. Material Characterization
3. Results and Discussion
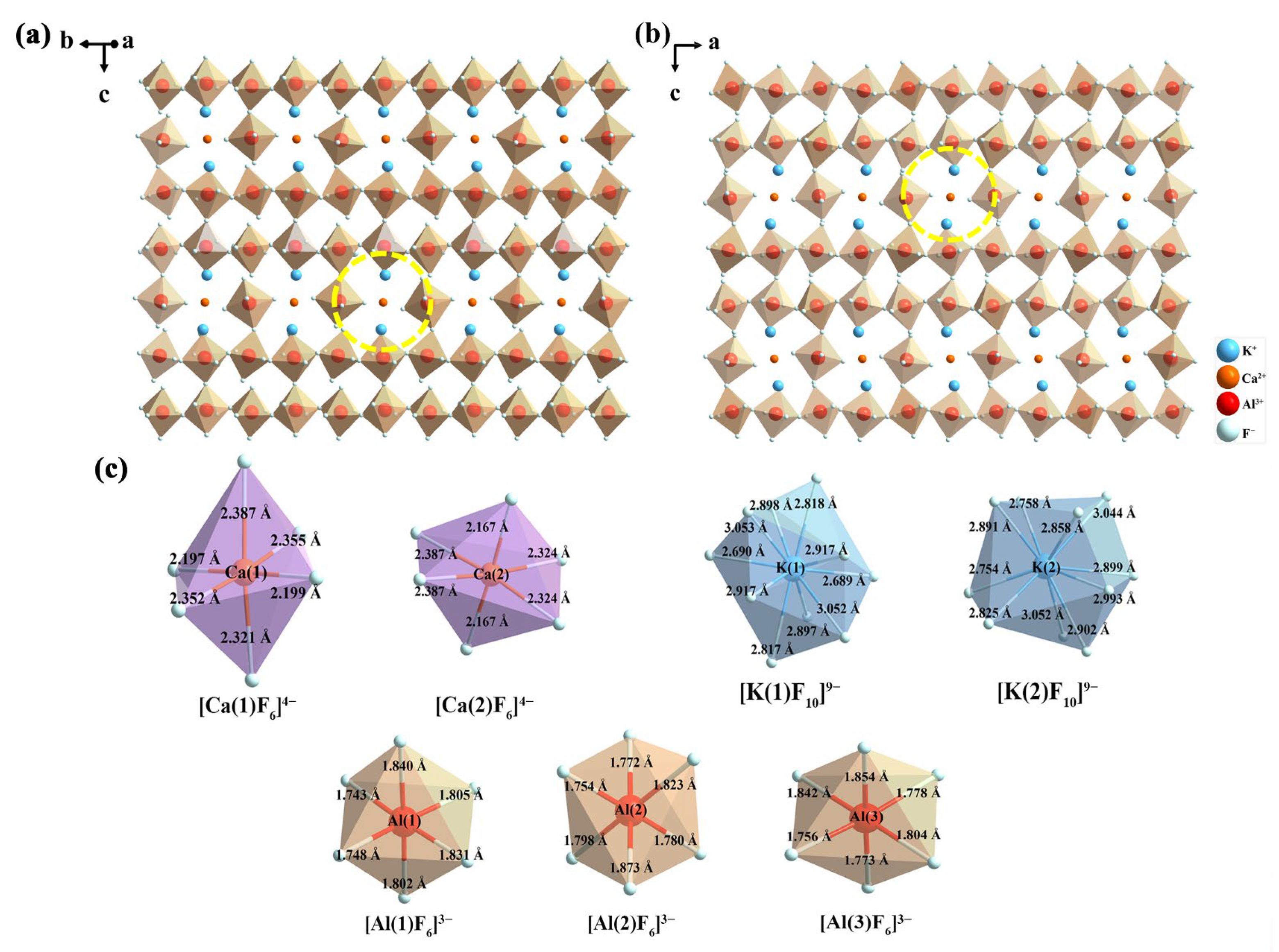
3.1. Phase Identification and Crystal Structure
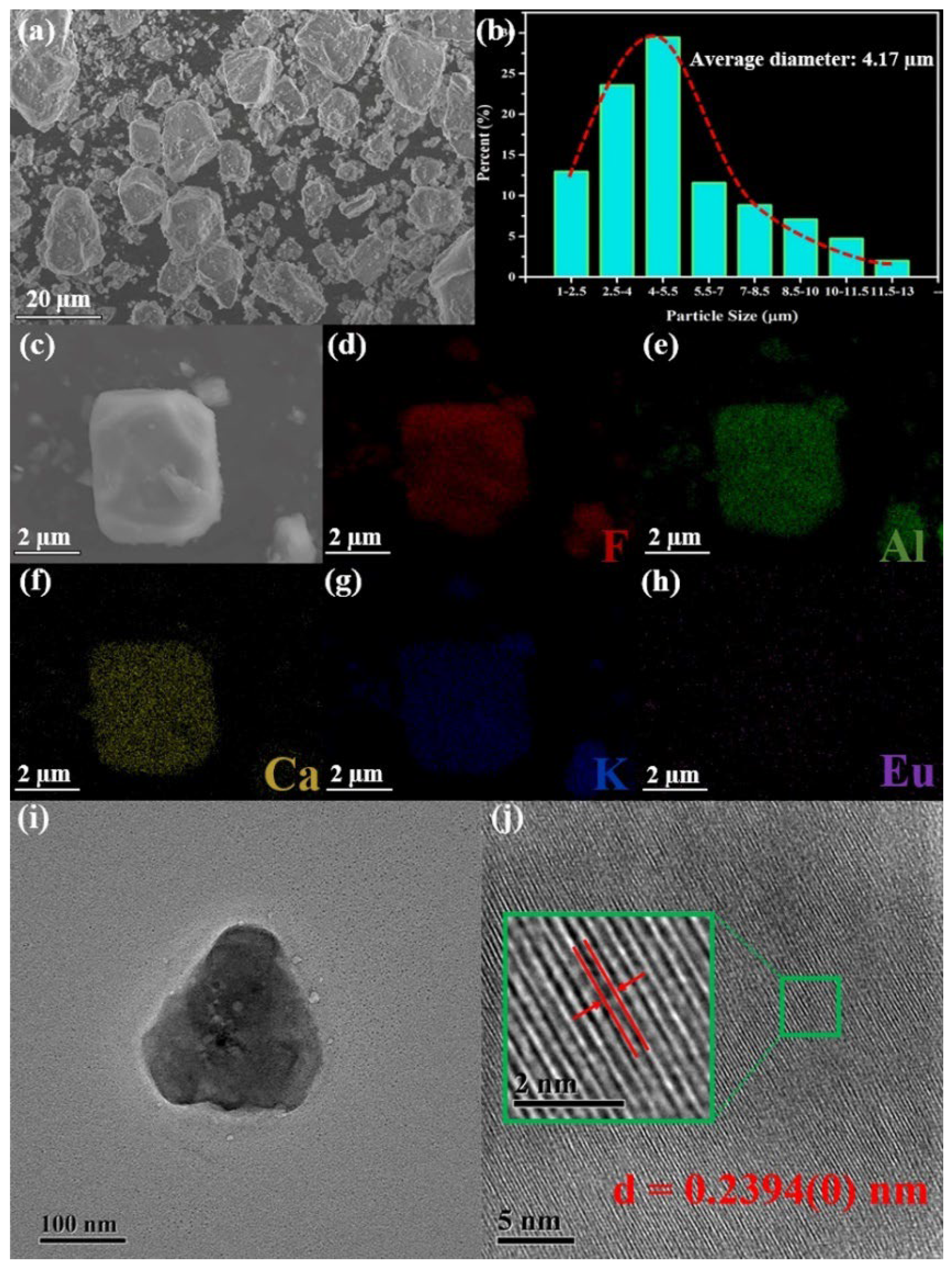
3.2. Morphology and Composition of KCAF:Eu2+
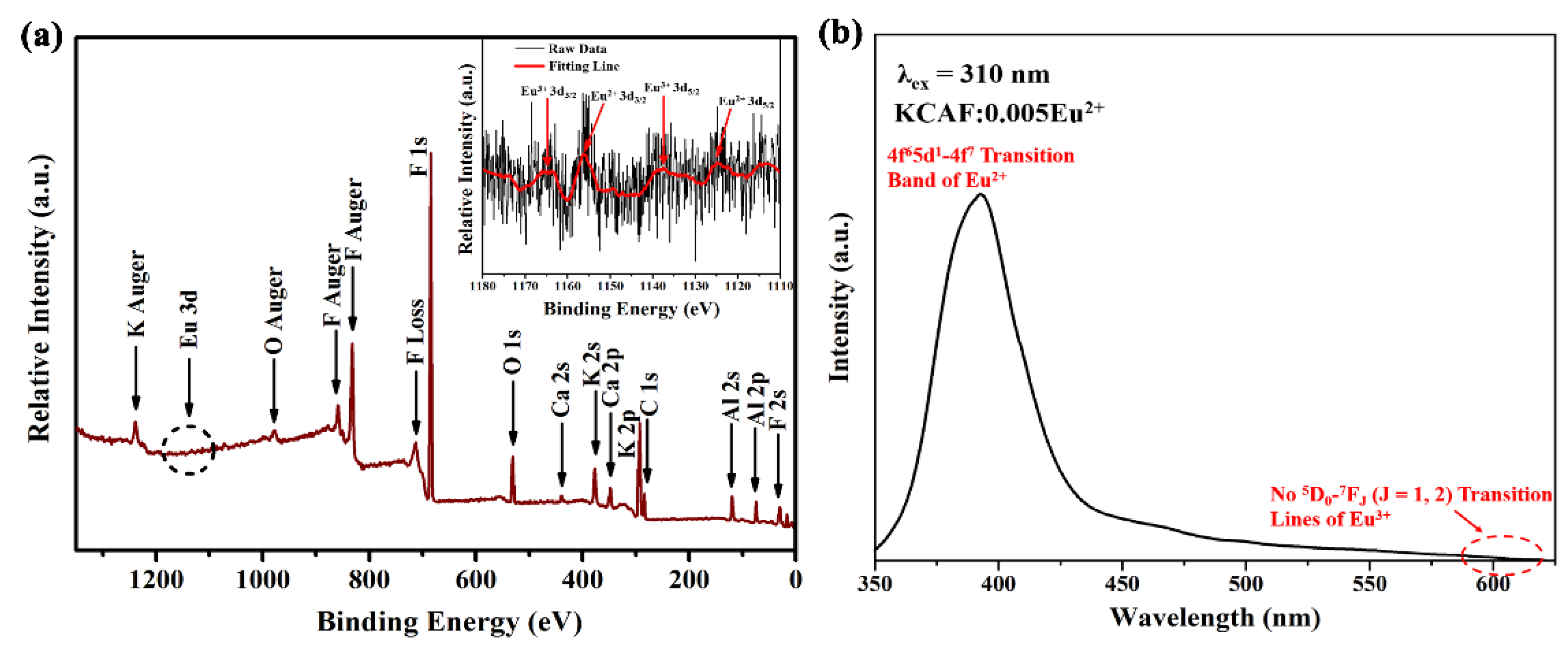
3.3. Valence State of Europium Ion
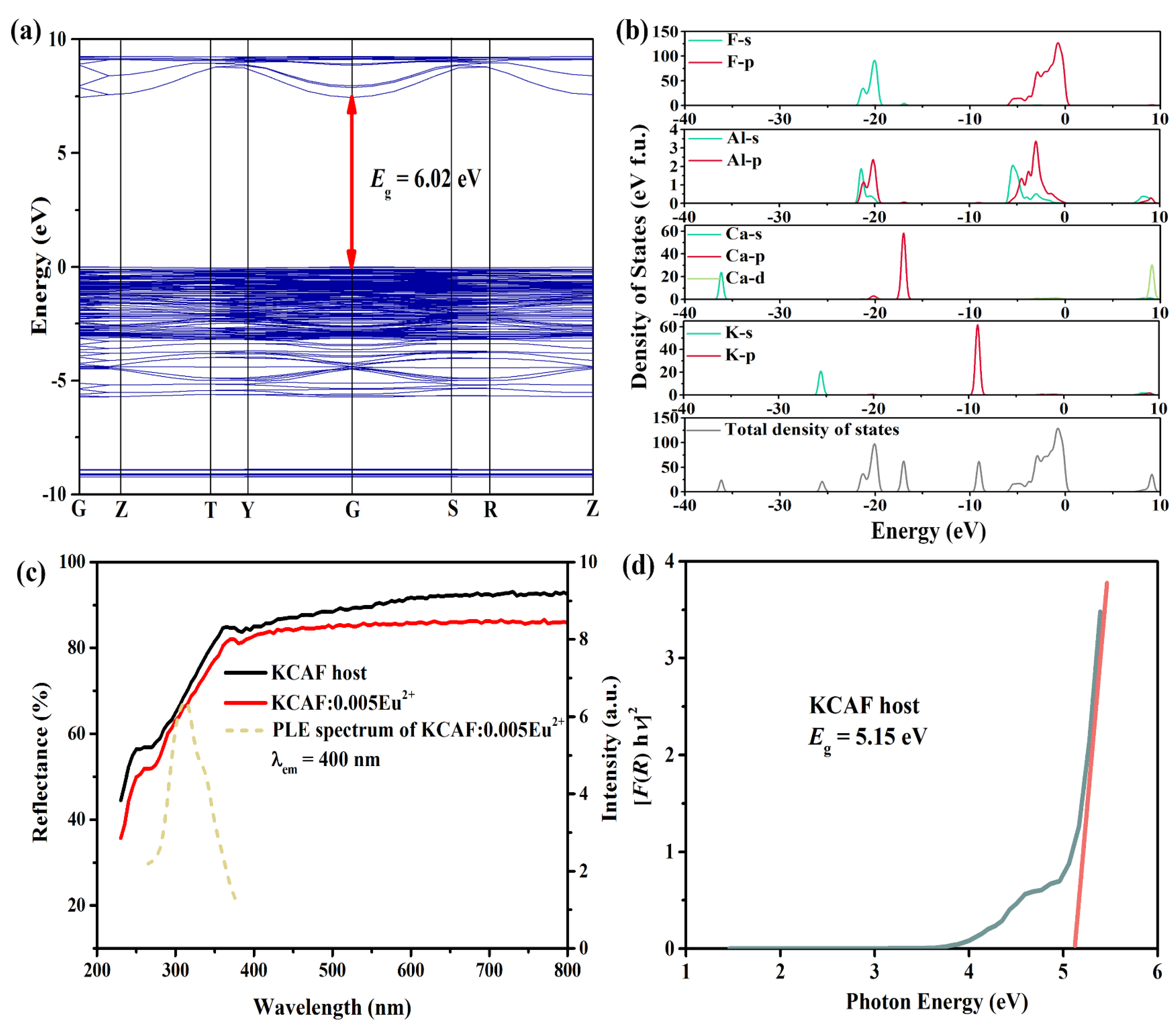
3.4. Electronic Band Structure
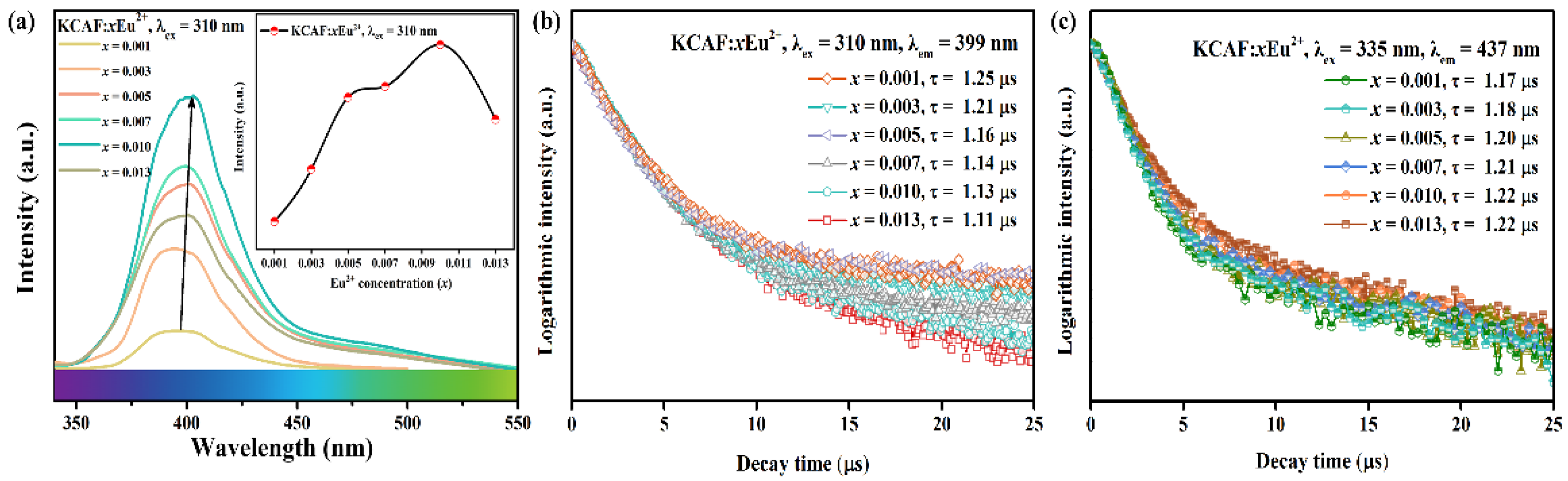
3.5. PL Properties
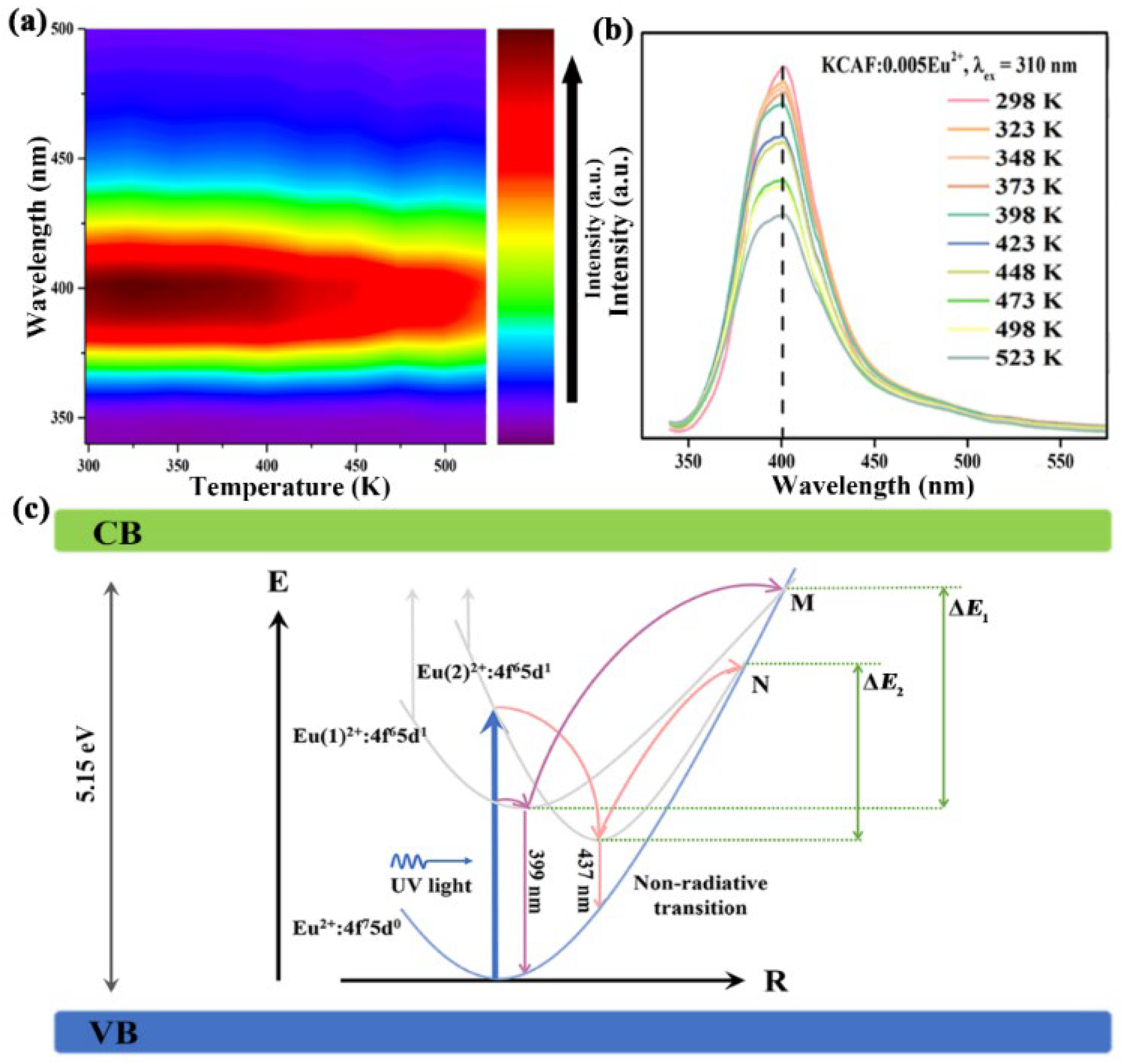
3.6. Thermal Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, P.; Ding, G.; Xie, R.J. Research progress on optical quenching of Ce3+- and Eu2+-doped luminescent materials. Chin. J. Lumin. 2021, 42, 1447–1457. [Google Scholar] [CrossRef]
- Wang, L.; Ding, G.Z.; Li, S.X.; Funahashi, S.; Takeda, T.; Yin, L.; Liang, P.; Hirosaki, N.; Xie, R.J. Broadband orange-emitting Sr3Si8O4N10:Eu2+ phosphor discovered by a modified single-particle-diagnosis approach. J. Adv. Ceram. 2023, 12, 734–746. [Google Scholar] [CrossRef]
- Zhou, Q.; Dolgov, L.; Srivastava, A.M.; Zhou, L.; Wang, Z.L.; Shi, J.X.; Dramićanin, M.D.; Brik, M.G.; Wu, M.M. Mn2+ and Mn4+ red phosphors: Synthesis, luminescence and applications in WLEDs. A review. J. Mater. Chem. C 2018, 6, 2652–2671. [Google Scholar] [CrossRef]
- Prayag, A.S.; Münch, M.; Aeschbach, D.; Chellappa, S.L.; Gronfier, C. Light modulation of human clocks, wake, and sleep. Clocks Sleep 2019, 1, 193–208. [Google Scholar] [CrossRef]
- Zheng, C.B.; Xiong, P.X.; Peng, M.Y.; Liu, H.L. Discovery of a novel rare-earth free narrow-band blue-emitting phosphor Y3Al2Ga3O12:Bi3+ with strong NUV excitation for LCD LED backlights. J. Mater. Chem. C 2020, 8, 13668–13675. [Google Scholar] [CrossRef]
- Liu, L.; Peng, S.S.; Fu, L.; Guo, Y.X.; Sun, X.; Song, L.; Shi, J.P.; Zhang, Y. Closing the deep-blue gap: Realizing narrow-band deep-blue emission with strong n-UV excitation by cationic substitution for full-spectrum warm w-LED lighting. ACS Sustain. Chem. Eng. 2022, 10, 6190–6195. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Devakumar, B.; Wang, S.Y.; Sun, L.L.; Ma, N.; Li, W.; Huang, X.Y. Using an excellent near-UV-excited cyan-emitting phosphor for enhancing the color rendering index of warm-white LEDs by filling the cyan gap. Mater. Today Chem. 2021, 20, 100471. [Google Scholar] [CrossRef]
- Liang, J.; Devakumar, B.; Sun, L.L.; Wang, S.Y.; Sun, Q.; Huang, X.Y. Full-visible-spectrum lighting enabled by an excellent cyan-emitting garnet phosphor. J. Mater. Chem. C 2020, 8, 4934–4943. [Google Scholar] [CrossRef]
- Hariyani, S.; Brgoch, J. Local structure distortion induced broad band emission in the all-inorganic BaScO2F:Eu2+ perovskite. Chem. Mater. 2020, 32, 6640–6649. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, J.F. An efficient perovskite-type Rb2CaPO4F:Eu2+ phosphor with high brightness towards closing the cyan gap. J. Alloys Compd. 2021, 872, 159698. [Google Scholar] [CrossRef]
- Liao, H.X.; Zhao, M.; Zhou, Y.Y.; Molokeev, M.S.; Liu, Q.L.; Zhang, Q.Y.; Xia, Z.G. Polyhedron transformation toward stable narrow-band green phosphors for wide-color-gamut liquid crystal display. Adv. Funct. Mater. 2019, 29, 1901988. [Google Scholar] [CrossRef]
- Liao, H.X.; Zhao, M.; Molokeev, M.S.; Liu, Q.L.; Xia, Z.G. Learning from a mineral structure toward an ultra-narrow-band blue-emitting silicate phosphor RbNa3[Li3SiO4]4:Eu2+. Angew. Chem. Int. Ed. 2018, 57, 11728–11731. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, Y.Y.; Molokeev, M.S.; Zhang, Q.Y.; Liu, Q.L.; Xia, Z.G. Discovery of new narrow-band phosphors with the UCr4C4-related type structure by alkali cation effect. Adv. Opt. Mater. 2019, 7, 1801631. [Google Scholar] [CrossRef]
- Dutzler, D.; Seibald, M.; Baumann, D.; Philipp, F.; Peschke, S.; Huppertz, H. RbKLi2[Li3SiO4]4:Eu2+ an ultra narrow-band phosphor. Z. Naturforsch. B 2019, 74, 535–546. [Google Scholar] [CrossRef]
- Zhao, M.; Liao, H.X.; Molokeev, M.S.; Zhou, Y.Y.; Zhang, Q.Y.; Liu, Q.L.; Xia, Z.G. Emerging ultra-narrow-band cyan-emitting phosphor for white LEDs with enhanced color rendition. Light-Sci. Appl. 2019, 8, 38. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Sun, J.F. Intense blue emission of perovskite-type fluoride phosphor Cs4Mg3CaF12:Eu2+ as a promising pc-WLEDs material. J. Alloys Compd. 2020, 835, 155225. [Google Scholar] [CrossRef]
- Leng, Z.H.; Zhang, D.; Bai, H.; He, H.B.; Qing, Q.; Zhao, J.; Tang, Z.B. A zero-thermal-quenching perovskite-like phosphor with an ultra-narrow-band blue-emission for wide-color-gamut backlight display applications. J. Mater. Chem. C 2021, 9, 13722–13732. [Google Scholar] [CrossRef]
- Wei, Y.; Cao, L.; Lv, L.M.; Li, G.G.; Hao, J.R.; Gao, J.S.; Su, C.C.; Lin, C.C.; Jang, H.S.; Dang, P.P.; et al. Highly efficient blue emission and superior thermal stability of BaAl12O19:Eu2+ phosphors based on highly symmetric crystal structure. Chem. Mater. 2018, 30, 2389–2399. [Google Scholar] [CrossRef]
- Leng, Z.H.; Bai, H.; Qing, Q.; He, H.B.; Hou, J.Y.; Li, B.Y.; Tang, Z.B.; Song, F.; Wu, H.Y. A zero-thermal-quenching blue phosphor for sustainable and human-centric WLED lighting. ACS Sustain. Chem. Eng. 2022, 10, 10966–10977. [Google Scholar] [CrossRef]
- Amidani, L.; Korthout, K.; Joos, J.J.; van der Linden, M.; Sijbom, H.F.; Meijerink, A.; Poelman, D.; Smet, P.F.; Glatzel, P. Oxidation and luminescence quenching of europium in BaMgAl10O17 blue phosphors. Chem. Mater. 2017, 29, 10122–10129. [Google Scholar] [CrossRef]
- Guo, Q.F.; Zhao, C.L.; Liao, L.B.; Lis, S.; Liu, H.K.; Mei, L.F. Novel apatite KLaSr3(PO4)3F:Eu2+ phosphors: Synthesis, structure, and luminescence properties. J. Mater. Res. 2016, 31, 3489–3497. [Google Scholar] [CrossRef]
- Zhang, W.B.; Shi, C.; Ye, S.S.; Zhou, J.C.; Wu, D.W.; Li, Y.W.; Chen, M.T.; Ding, J.Y.; Wu, Q.S. A novel narrow band blue-emitting phosphor Rb2ZrSi3O9:Eu2+ with low thermal quenching and high quantum efficiency. Ceram. Int. 2021, 47, 22786–22793. [Google Scholar] [CrossRef]
- Xie, R.J.; Hirosaki, N. Silicon-based oxynitride and nitride phosphors for white LEDs-a review. Sci. Technol. Adv. Mater. 2007, 8, 588–600. [Google Scholar] [CrossRef]
- Li, J.H.; Xu, S.D.; Deng, Z.; Wei, L.L.; Yang, Z.P. Photoluminescence and ferroelectric behaviors related to Sr/Ba ratio in Eu3+-doped (SrxBa1−x)0.98Eu0.02Nb2O6 multifunctional tungsten bronze ceramics. J. Alloys Compd. 2019, 780, 355–363. [Google Scholar] [CrossRef]
- Ekmekçi, M.K.; İlhan, M.; Başak, A.S.; Deniz, S. Structural and luminescence properties of Sm3+-doped TTB-type BaTa2O6 ceramic phosphors. J. Fluoresc. 2015, 25, 1757–1762. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Liu, R.Y.; Geng, X.; Hu, X.P.; Chen, W.S.; Deng, B.; Yu, R.J.; Geng, H.L. Synthesis and spectroscopic analysis of Eu3+-doped tungsten bronze Sr5YTi3Nb7O30 phosphors for w-LED and visualization of latent fingerprint. Ceram. Int. 2022, 48, 4080–4089. [Google Scholar] [CrossRef]
- Hao, S.L.; Li, J.H.; Yang, P.; Wei, L.L.; Yang, Z.P. Enhanced electrical properties and strong red light-emitting in Eu3+-doped Sr1.90Ca0.15Na0.9Nb5O15 ceramics. J. Am. Ceram. Soc. 2017, 100, 5620–5628. [Google Scholar] [CrossRef]
- Hemon, A.; Le Bail, A.; Courbion, G. Crystal structure approach of KCaAl2F9-a new hexagonal-tungsten-bronze related structure. Eur. J. Solid State Inorg. Chem. 1993, 30, 415–426. [Google Scholar] [CrossRef]
- Toby, B.H. A graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Sun, J.F.; Zhang, X.Y.; Li, T.Y. New Eu2+-activated borophosphate phosphors Ba3(ZnB5O10)PO4 for near-ultraviolet-pumped white light-emitting diodes. Mater. Lett. 2017, 212, 343–345. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Hume-Rothery, W.; Smallman, R.E.; Haworth, C.W. The Structure of Metals and Alloys; Metals and Metallurgy Trust: London, UK, 1969. [Google Scholar]
- Yin, X.; Shi, L.; Wei, A.; Wan, D.Y.; Wang, Y.M.; Huang, F.Q. Effect of structural packing on the luminescence properties in tungsten bronze compounds M2KNb5O15 (M = Ca, Sr, Ba). J. Solid State Chem. 2012, 192, 182–185. [Google Scholar] [CrossRef]
- Fang, M.H.; Leaño, J.L.; Liu, R.S. Control of narrow-band emission in phosphor materials for application in light-emitting diodes. ACS Energy Lett. 2018, 3, 2573–2586. [Google Scholar] [CrossRef]
- Crist, B.V. Handbook of Monochromatic XPS Spectra: The Elements of Native Oxides; John Wiley & Sons Inc.: New York, NY, USA, 2000. [Google Scholar]
- Leng, Z.H.; Li, R.F.; Li, L.P.; Xue, D.K.; Zhang, D.; Li, G.S.; Chen, X.Y.; Zhang, Y. Preferential neighboring substitution-triggered full visible spectrum emission in single-phased Ca10.5−xMgx(PO4)7:Eu2+ phosphors for high color-rendering white LEDs. ACS Appl. Mater. Interfaces 2018, 10, 33322–33334. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, L.; Tang, D.M.; Cho, Y.J.; Liu, X.J.; Zhou, X.T.; Lu, L.; Zhang, L.; Takeda, T.; Hirosaki, N.; et al. Achieving high quantum efficiency narrow-band beta-sialon: Eu2+ phosphors for high-brightness LCD backlights by reducing the Eu3+ luminescence killer. Chem. Mater. 2018, 30, 494–505. [Google Scholar] [CrossRef]
- Li, H.H.; Li, R.Z.; Chang, C.K. Red-shift and improved afterglow of Sr3Al2-xBxO5Cl2:Eu2+, Dy3+ phosphors via a cation substitution strategy. Ceram. Int. 2022, 48, 1814–1819. [Google Scholar] [CrossRef]
- Hoerder, G.J.; Seibald, M.; Baumann, D.; Schröder, T.; Peschke, S.; Schmid, P.C.; Tyborski, T.; Pust, P.; Stoll, I.; Bergler, M.; et al. Sr[Li2Al2O2N2]:Eu2+—A high performance red phosphor to brighten the future. Nat. Commun. 2019, 10, 1824. [Google Scholar] [CrossRef]
- Hu, T.; Gao, Y.; Ji, X.H.; Xia, Z.G.; Zhang, Q.Y. Thermal quenching properties of narrow-band blue-emitting MBe2(PO4)2:Eu2+ (M = Ca, Sr) phosphors towards backlight display applications. Inorg. Chem. Front. 2020, 7, 2685–2691. [Google Scholar] [CrossRef]
- Zhang, N.M.; Guo, C.F.; Zheng, J.M.; Su, X.Y.; Zhao, J. Synthesis, electronic structures and luminescent properties of Eu3+-doped KGdTiO4. J. Mater. Chem. C 2014, 2, 3988–3994. [Google Scholar] [CrossRef]
- Qin, X.; Liu, X.W.; Huang, W.; Bettinelli, M.; Liu, X.G. Lanthanide-activated phosphors based on 4f-5d optical transitions: Theoretical and experimental aspects. Chem. Rev. 2017, 117, 4488–4527. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, Z.X.; Zhang, J.L.; Ji, X.Y.; Zhang, X.G.; Liao, S.Z.; Zhou, W.L.; Yu, L.P.; Lian, S.X. Highly efficient and thermally stable single-activator white-emitting phosphor K2Ca(PO4)F:Eu2+ for white light-emitting diodes. J. Mater. Chem. C 2019, 7, 8982–8991. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhao, Z.Y.; Wu, Q.S.; Wang, C.; Wang, Q.; Li, Y.Y.; Wang, Y.H. Structure, photoluminescence and abnormal thermal quenching behavior of Eu2+-doped Na3Sc2(PO4)3: A novel blue-emitting phosphor for n-UV LEDs. J. Mater. Chem. C 2016, 4, 8795–8801. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Y.; Yan, X.D.; Zuo, F.; Cheng, J. Preparation and luminescent properties of SrAl2Si2O8:Eu2+ phosphor with high thermal stability. J. Synth. Cryst. 2020, 49, 1625–1630. [Google Scholar]
- Zhang, X.T.; An, Z.C.; Dong, R.J.; Song, Y.H.; Zheng, K.Y.; Sheng, Y.; Zhan, S.; Zou, H.F. Properties and application of single Eu2+-activated color tuning phosphors. ACS Sustain. Chem. Eng. 2019, 7, 10724–10733. [Google Scholar] [CrossRef]
- Kubelka, P. New contributions to the optics of intensely light-scattering materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef]
- Pace, C.N.; Shaw, K.L. Linear extrapolation method of analyzing solvent denaturation curves. Proteins 2000, 4, 1–7. [Google Scholar] [CrossRef]
- Strobel, P.; Maak, C.; Weiler, V.; Schmidt, P.J.; Schnick, W. Ultra-narrow-band blue-emitting oxoberyllates AELi2[Be4O6]:Eu2+ (AE = Sr, Ba) paving the way to efficient RGB pc-LEDs. Angew. Chem. Int. Ed. 2018, 57, 8739–8743. [Google Scholar] [CrossRef]
- Mooney, J.; Kambhampati, P. Get the basics right: Jacobian conversion of wavelength and energy scales for quantitative analysis of emission spectra. J. Phys. Chem. Lett. 2013, 4, 3316–3318. [Google Scholar] [CrossRef]
- Huang, C.H.; Wu, P.J.; Lee, J.F.; Chen, T.M. (Ca,Mg,Sr)9Y(PO4)7:Eu2+, Mn2+: Phosphors for white-light near-UV LEDs through crystal field tuning and energy transfer. J. Mater. Chem. 2011, 21, 10489–10495. [Google Scholar] [CrossRef]
- Liu, H.K.; Liao, L.B.; Molokeev, M.S.; Guo, Q.F.; Zhang, Y.Y.; Mei, L.F. A novel single-phase white light emitting phosphor Ca9La(PO4)5(SiO4)F2:Dy3+: Synthesis, crystal structure and luminescence properties. RSC Adv. 2016, 6, 24577–24583. [Google Scholar] [CrossRef]
- Zhang, M.L.; Xia, Z.G.; Liu, Q.L. Thermally stable KxCs1-xAlSi2O6:Eu2+ phosphors and their photoluminescence tuning. J. Mater. Chem. C 2017, 5, 7489–7494. [Google Scholar] [CrossRef]
- Wu, Q.S.; Li, Y.Y.; Wang, Y.J.; Liu, H.; Ye, S.S.; Zhao, L.; Ding, J.Y.; Zhou, J.C. A novel narrow-band blue-emitting phosphor of Bi3+-activated Sr3Lu2Ge3O12 based on a highly symmetrical crystal structure used for WLEDs and FEDs. Chem. Eng. J. 2020, 401, 126130. [Google Scholar] [CrossRef]
- Sun, W.Z.; Jia, Y.L.; Pang, R.; Li, H.F.; Ma, T.F.; Li, D.; Fu, J.P.; Zhang, S.; Jiang, L.H.; Li, C.Y. Sr9Mg1.5(PO4)7:Eu2+: A novel broadband orange-yellow-emitting phosphor for blue light-excited warm white LEDs. ACS Appl. Mater. Interfaces 2015, 7, 25219–25226. [Google Scholar] [CrossRef]
- Fang, M.H.; Mariano, C.O.M.; Chen, P.Y.; Hu, S.F.; Liu, R.S. Cuboid-size-controlled color-tunable Eu-doped alkali-lithosilicate phosphors. Chem. Mater. 2020, 32, 1748–1759. [Google Scholar] [CrossRef]
- Dexter, D.L. A theory of sensitized luminescence in solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Huang, Y.M.; Zhang, X.G.; Pan, P.L. Synthesis and photoluminescence of violet-blue phosphor Ba10(PO4)6F2:Eu2+. Solid State Sci. 2017, 63, 9–15. [Google Scholar] [CrossRef]
- Jia, Y.C.; Huang, Y.J.; Zheng, Y.H.; Guo, N.; Qiao, H.; Zhao, Q.; Lv, W.Z.; You, H.P. Color point tuning of Y3Al5O12:Ce3+ phosphor via Mn2+-Si4+ incorporation for white light generation. J. Mater. Chem. 2012, 22, 15146–15152. [Google Scholar] [CrossRef]
- Inokuti, M.; Hirayama, F.J. Influence of energy transfer by the exchange mechanism on donor luminescence. J. Chem. Phys. 1965, 43, 1978–1989. [Google Scholar] [CrossRef]
- Song, E.H.; Zhou, Y.Y.; Wei, Y.; Han, X.X.; Tao, Z.R.; Qiu, R.L.; Xia, Z.G.; Zhang, Q.Y. A thermally stable narrow-band green-emitting phosphor MgAl2O4:Mn2+ for wide color gamut backlight display application. J. Mater. Chem. C 2019, 7, 8192–8198. [Google Scholar] [CrossRef]
- Sun, J.F.; Shi, Y.M.; Zhang, W.L.; Shen, D.Z.; Sun, J.Y. Luminescent and thermal properties of a new green-emitting Ca6Sr4(Si2O7)3Cl2:Eu2+ phosphor for near-UV light-emitting diodes. Mater. Res. Bull. 2012, 47, 400–404. [Google Scholar] [CrossRef]
- Liao, M.; Wang, Q.; Lin, Q.M.; Xiong, M.X.; Zhang, X.; Dong, H.F.; Lin, Z.P.; Wen, M.R.; Zhu, D.Y.; Mu, Z.F.; et al. Na replaces Rb towards high-performance narrow-band green phosphors for backlight display applications. Adv. Opt. Mater. 2021, 9, 2100465. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, J.; Huang, L.; Pan, F.J.; Chen, Y.; Lei, B.F.; Peng, M.Y.; Wu, M.M. Tunable luminescent properties and concentration-dependent, site-preferable distribution of Eu2+ ions in silicate glass for white LEDs applications. ACS Appl. Mater. Interfaces 2015, 7, 10044–10054. [Google Scholar] [CrossRef]

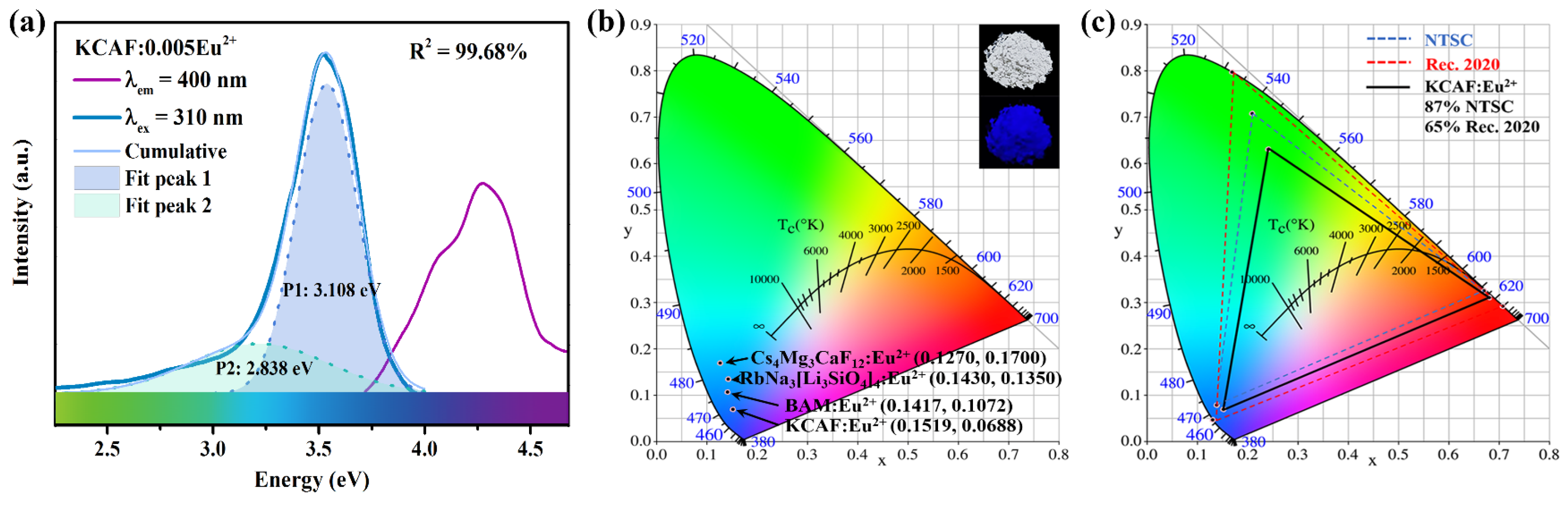

| Phosphors | Peak (nm) | FWHM (nm) | Thermal Stability | Color Purity (%) | Color Gamut (%NTSC) | Color Gamut (%Rec. 2020) | IQE (%) | EQE (%) |
|---|---|---|---|---|---|---|---|---|
| Gd2Si2O7:Bi3+ [6] | 422 (deep blue) | 46 | 58% (423 K) | – | – | – | – | – |
| Y2Si2O7:Bi3+ [6] | 412 (deep blue) | 41 | 70% (423 K) | – | – | – | – | – |
| Lu2Si2O7:Bi3+ [6] | 408 (deep blue) | 39 | 53% (423 K) | – | – | – | – | – |
| Sc2Si2O7:Bi3+ [6] | 403 (deep blue) | 38 | 81% (423 K) | – | – | – | 41 | – |
| RbNa3[Li3SiO4]4:Eu2+ [12] | 471 (blue) | 22 | 96% (423 K) | 83 | 75 | 56 | 53 | 13 |
| RbNa2K[Li3SiO4]4:Eu2+ [13] | 480 (blue) | 26 | 99% (423 K) | – | – | – | – | – |
| RbKLi2[Li3SiO4]4:Eu2+ [14] Na0.5K0.5[Li3SiO4]:Eu2+ [15] | 474 (blue) 486 (blue) | 25 21 | 88% (423 K) 93% (423 K) | – 67 | – – | – – | 50 76 | – 30 |
| Cs4Mg3CaF12:Eu2+ [16] | 474 (blue) | 55 | 82% (423 K) | 85 | – | – | 64 | – |
| K2BaPO4F:Eu2+ [17] | 439 (blue) | 25 | 98% (423 K) | 99 | 83 | 62 | 50 | 25 |
| BaAl12O19:Eu2+ [18] | 443 (blue) | 52 | 92% (473 K) | 90 | 90 | 67 | 90 | – |
| Na3KMg7(PO4)6:Eu2+ [19] | 446 (blue) | 41 | 97% (497 K) | 95 | – | – | 93 | 40 |
| BAM:Eu2+ [20] | 453 (blue) | 55 | 91% (373 K) | 91 | 82 | 61 | 89 | – |
| KLaSr3(PO4)3F:Eu2+ [21] Rb2ZrSi3O9:Eu2+ [22] | 461 (blue) 470 (blue) | 53 60 | 51% (473 K) 82% (423 K) | – – | 79 – | 59 – | 65 75 | – – |
| KCAF:Eu2+ | 400 (deep blue) | 45 | 90% (423 K) | 92 | 87 | 65 | 63 | 34 |
| Formula | KCAF | KCAF:0.005Eu2+ | KCAF:0.013Eu2+ |
|---|---|---|---|
| Crystal system | Orthorhombic | Orthorhombic | Orthorhombic |
| Profile range (o) | 5–130 | 5–130 | 5–130 |
| Radiation type; λ (Å) | X-ray; 1.5418 | X-ray; 1.5418 | X-ray; 1.5418 |
| Temperature (K) | 298 | 298 | 298 |
| Space group (Z) | C2221 (20); (12) | C2221 (20); (12) | C2221 (20); (12) |
| a (Å) | 12.329 (6) | 12.329 (8) | 12.331 (7) |
| b (Å) | 7.152 (7) | 7.154 (6) | 7.156 (3) |
| c (Å) | 22.618 (8) | 22.619 (3) | 22.623 (8) |
| Unit cell volume V (Å3) | 1994.750 (4) | 1995.356 (7) | 1996.535 (5) |
| Weighted profile R-factor, Rwp (%) | 6.32 | 6.57 | 6.78 |
| Profile R-factor, Rp (%) | 4.36 | 4.25 | 4.37 |
| χ2 | 2.14 | 2.43 | 2.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, R.; Sun, J. Narrow-Band Deep-Blue Emission and Superior Thermal Stability of Fluoroaluminate Phosphor Based on Tungsten Bronze-Type Mineral Structure. Materials 2023, 16, 5053. https://doi.org/10.3390/ma16145053
Lu R, Sun J. Narrow-Band Deep-Blue Emission and Superior Thermal Stability of Fluoroaluminate Phosphor Based on Tungsten Bronze-Type Mineral Structure. Materials. 2023; 16(14):5053. https://doi.org/10.3390/ma16145053
Chicago/Turabian StyleLu, Rui, and Jianfeng Sun. 2023. "Narrow-Band Deep-Blue Emission and Superior Thermal Stability of Fluoroaluminate Phosphor Based on Tungsten Bronze-Type Mineral Structure" Materials 16, no. 14: 5053. https://doi.org/10.3390/ma16145053
APA StyleLu, R., & Sun, J. (2023). Narrow-Band Deep-Blue Emission and Superior Thermal Stability of Fluoroaluminate Phosphor Based on Tungsten Bronze-Type Mineral Structure. Materials, 16(14), 5053. https://doi.org/10.3390/ma16145053






