Composite Fe-Cr-V-C Coatings Prepared by Plasma Transferred-Arc Powder Surfacing
Abstract
:1. Introduction
2. Experimental Part
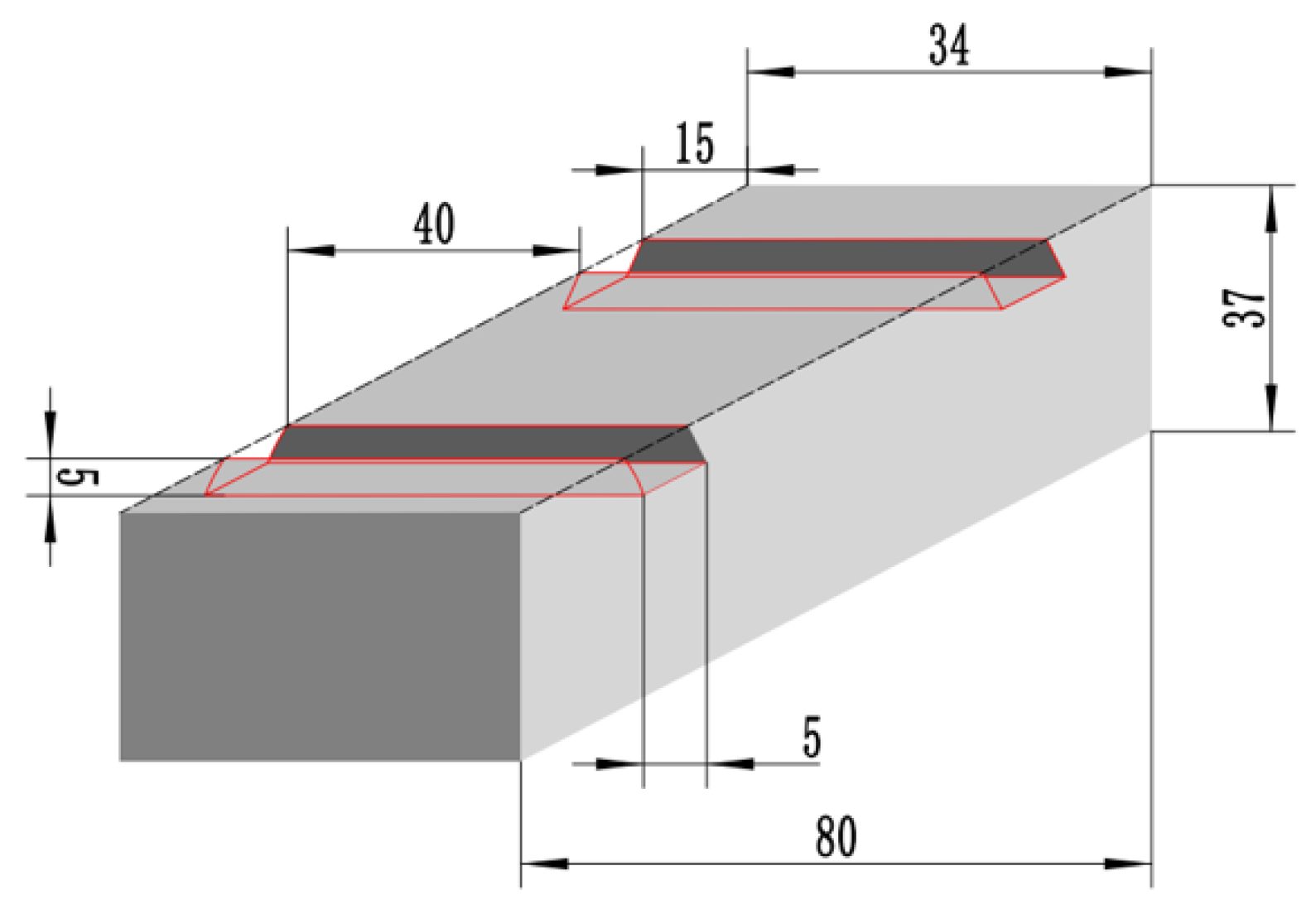
2.1. Materials Preparation
2.2. PTA Powder Surfacing Process
2.3. Microanalysis
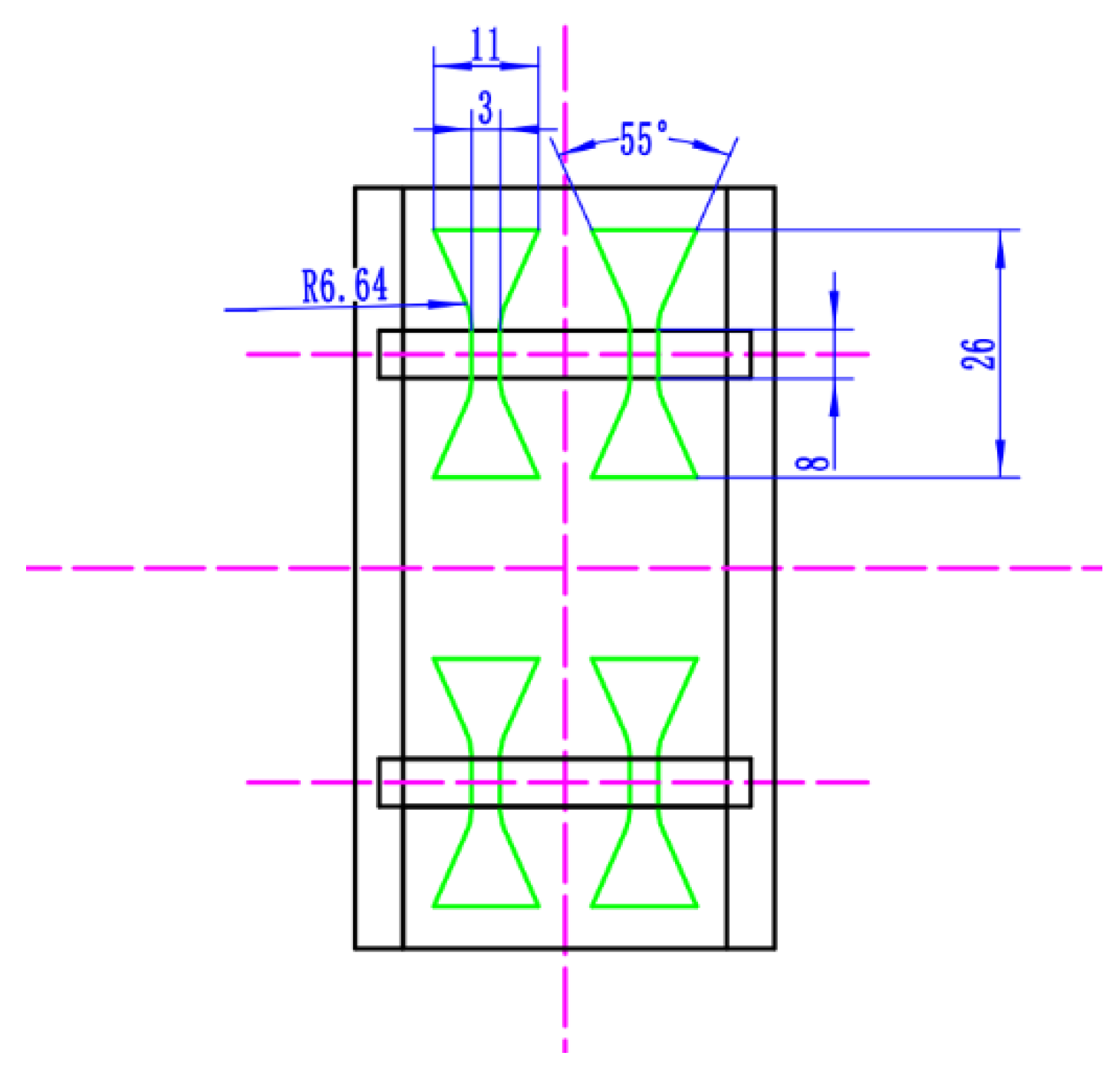
2.4. Tensile Strength and Hardness Tests
3. Results and Discussions
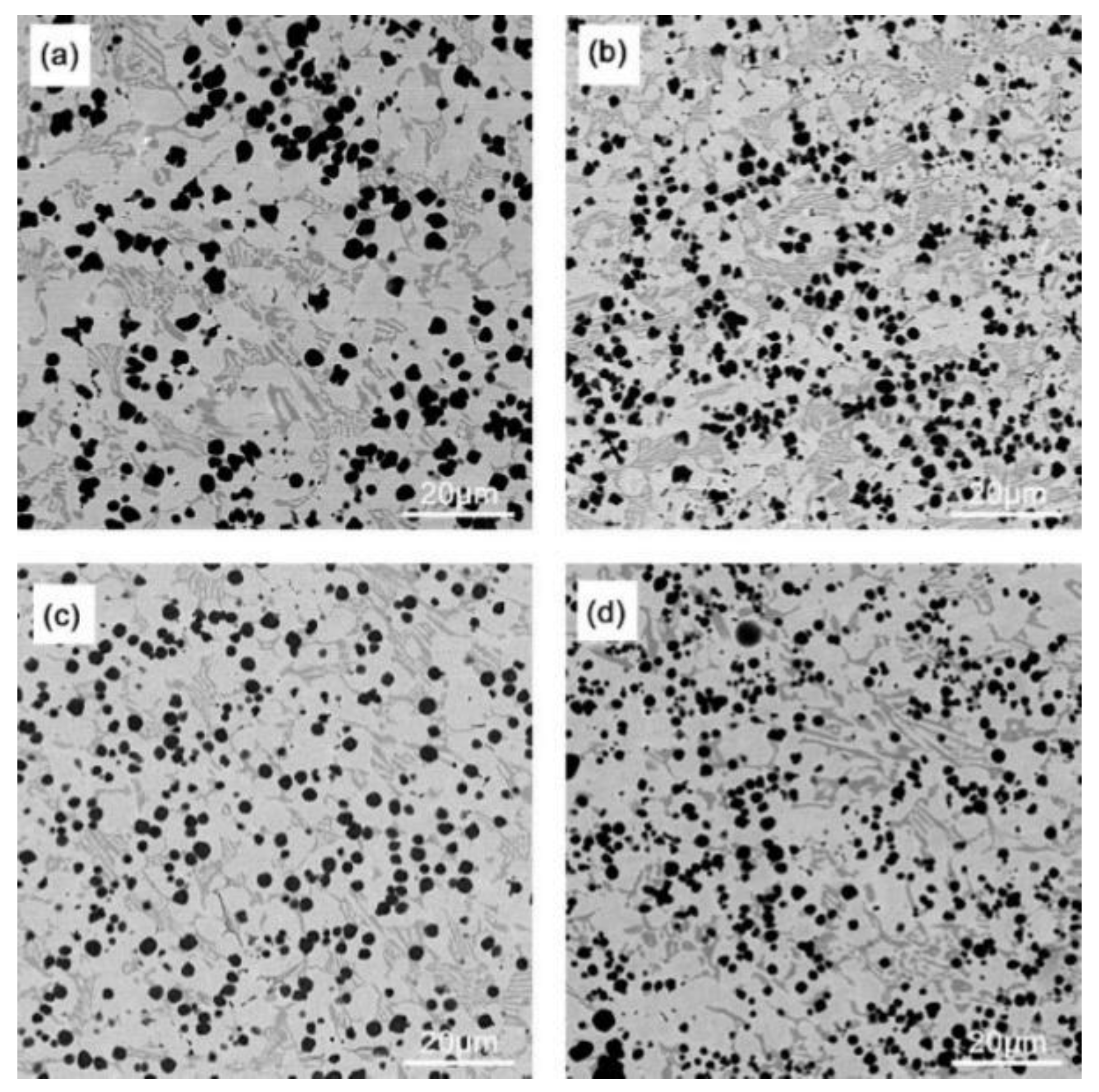
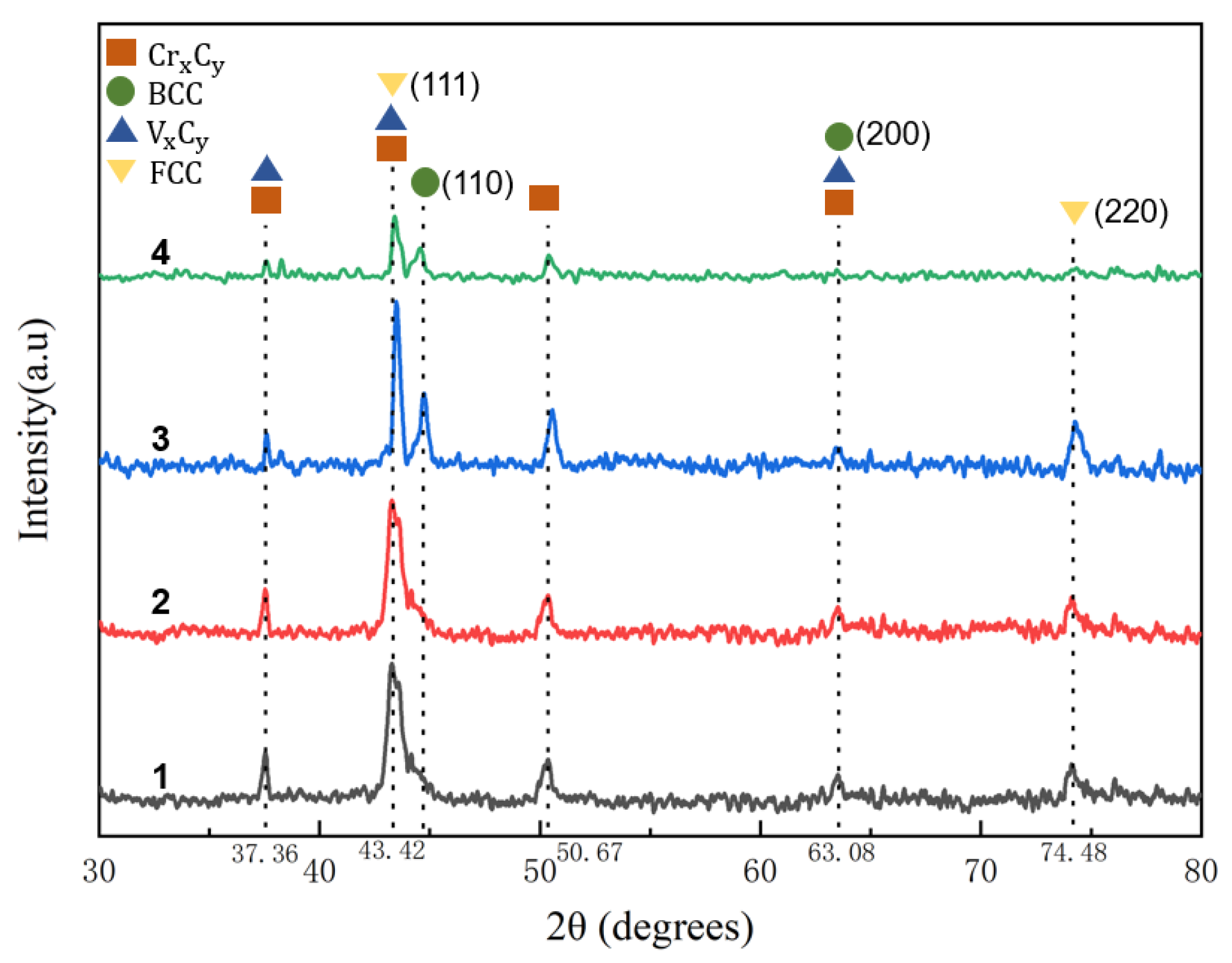
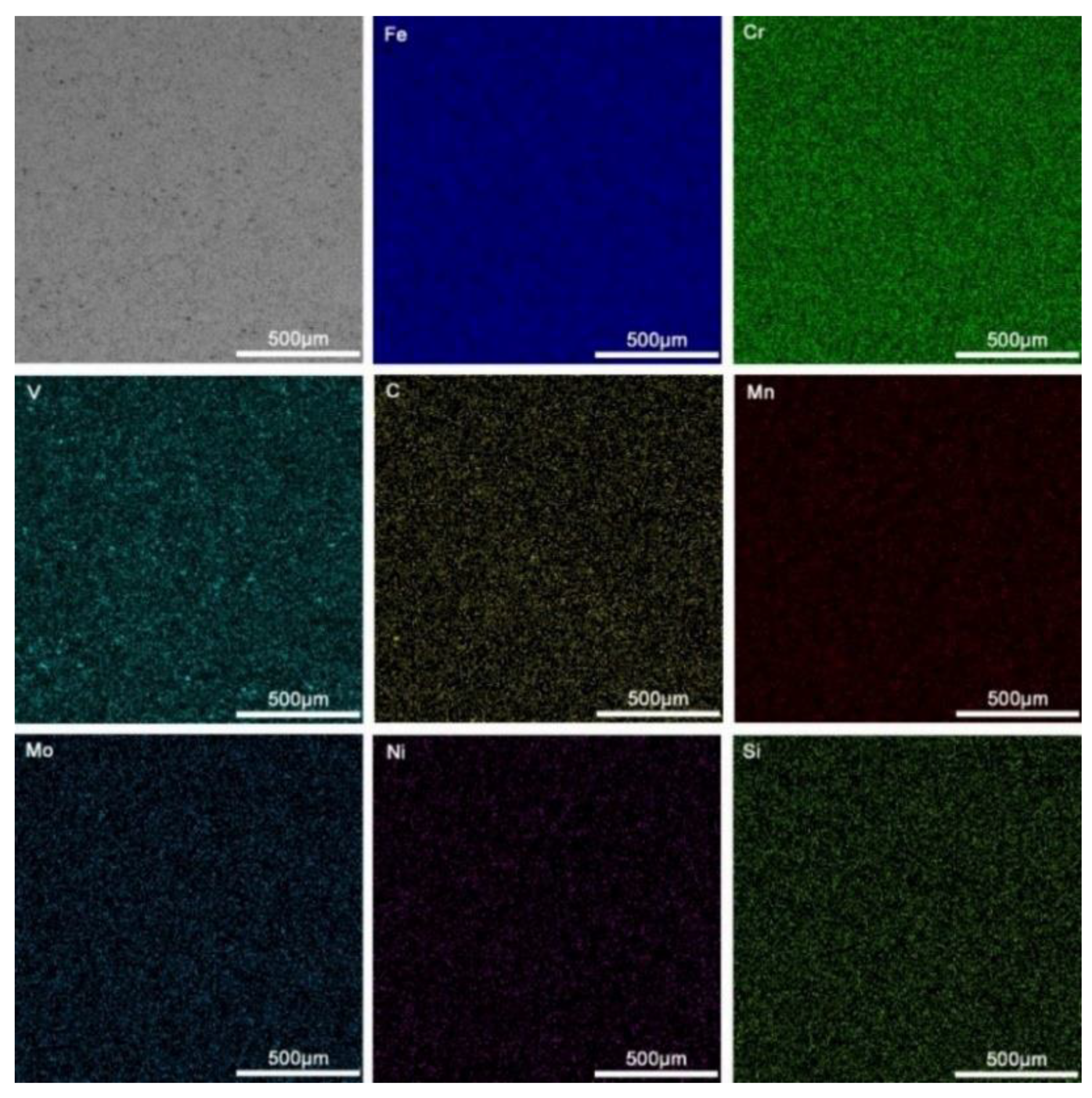
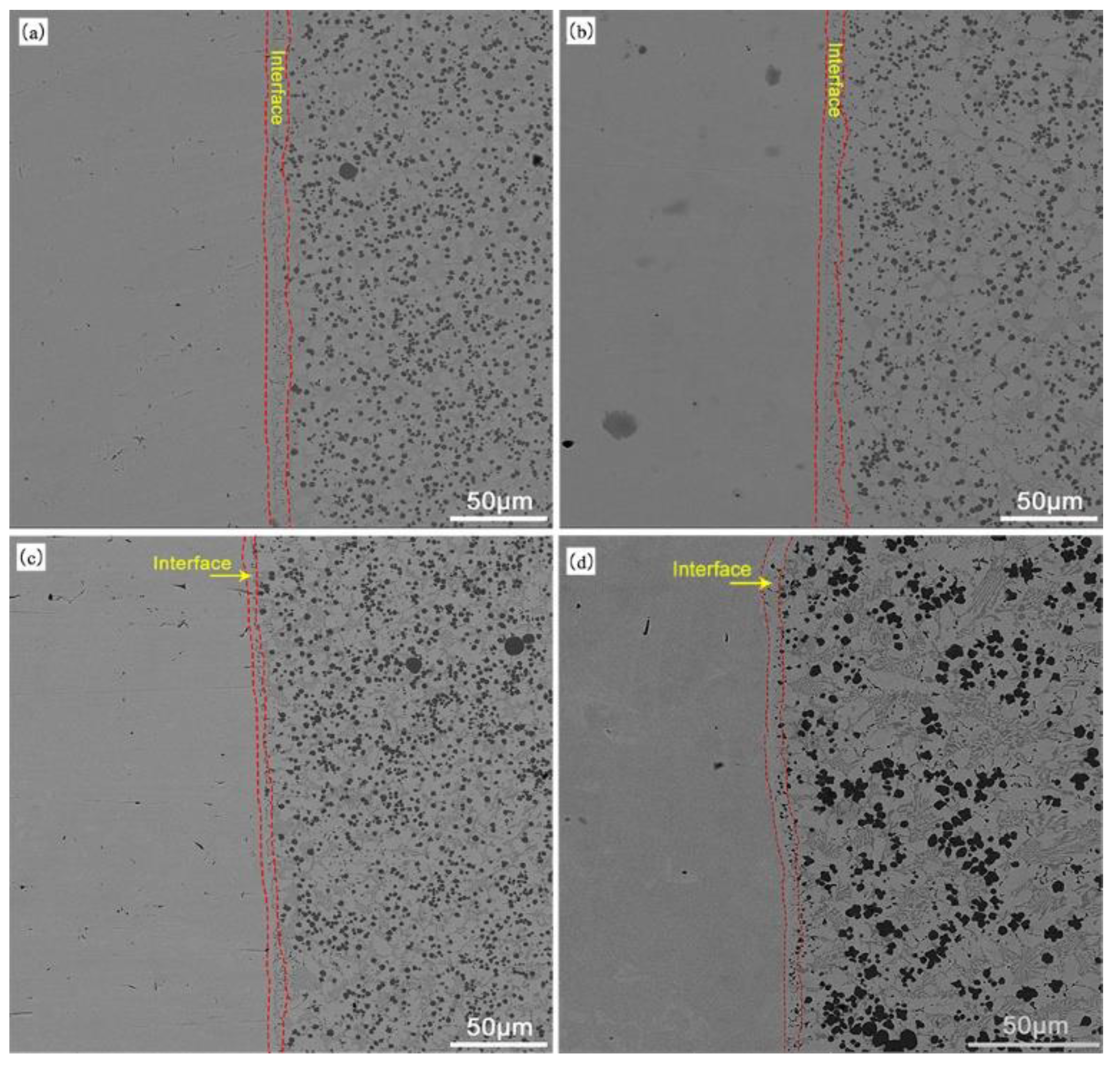
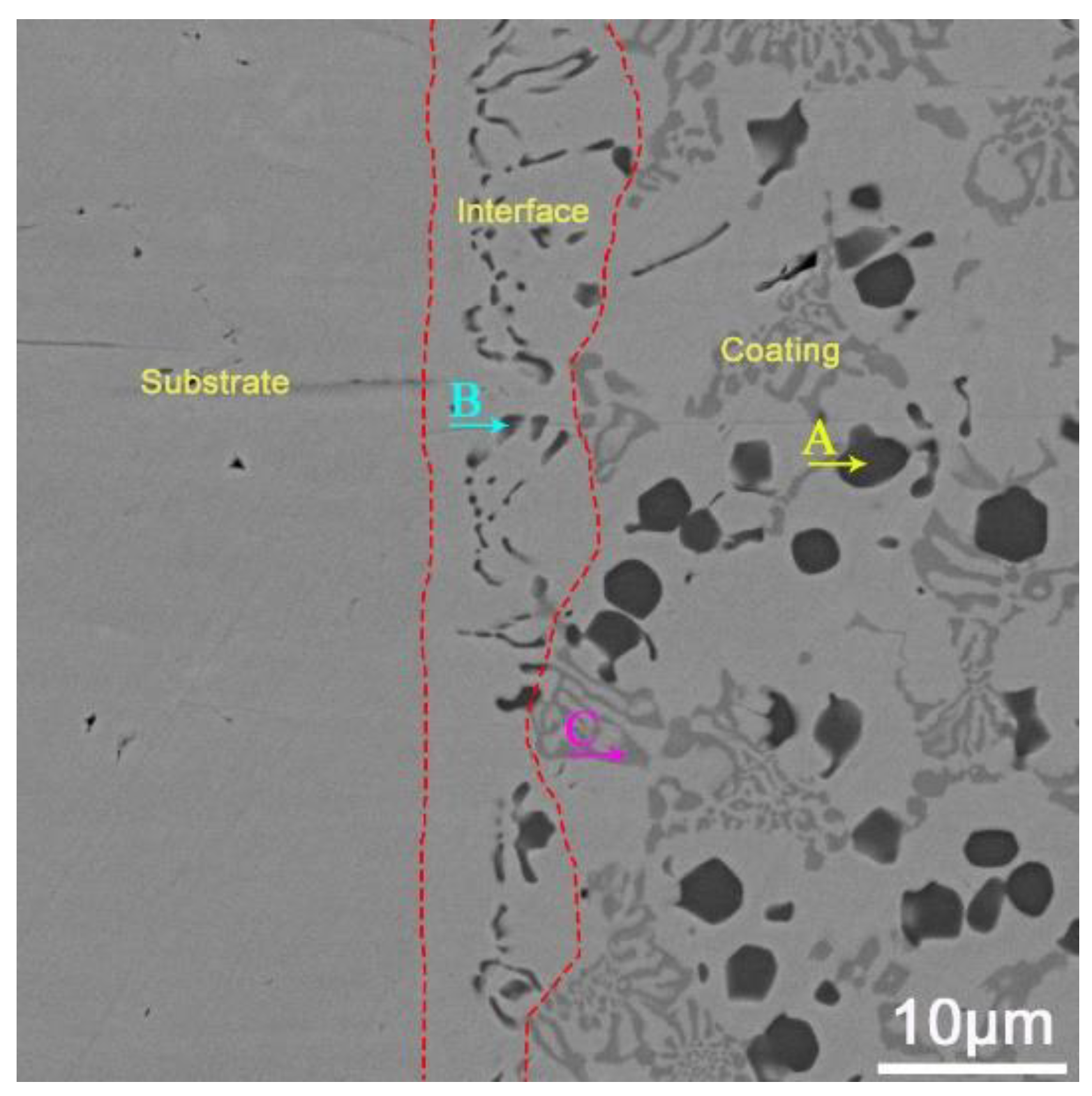
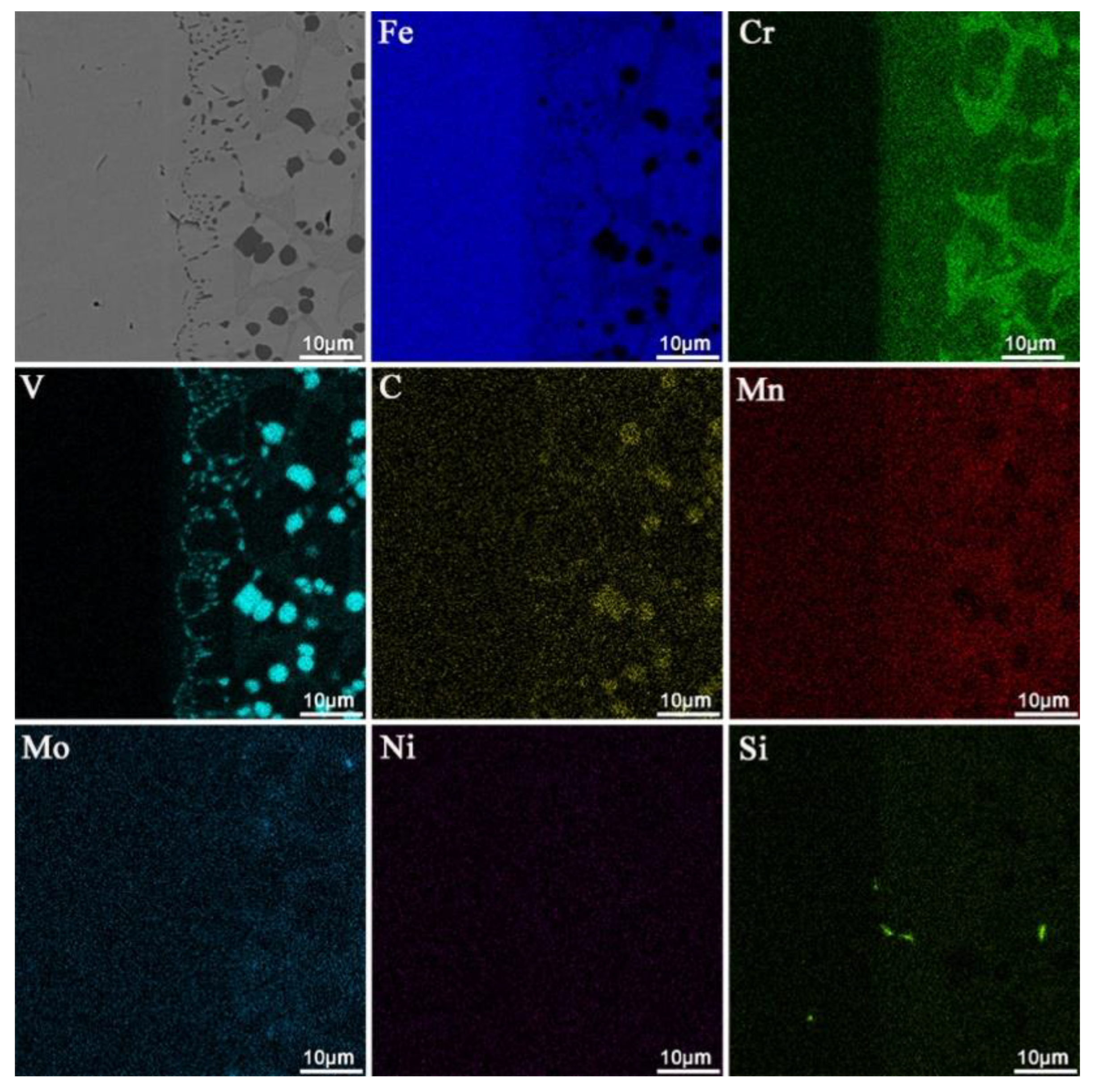
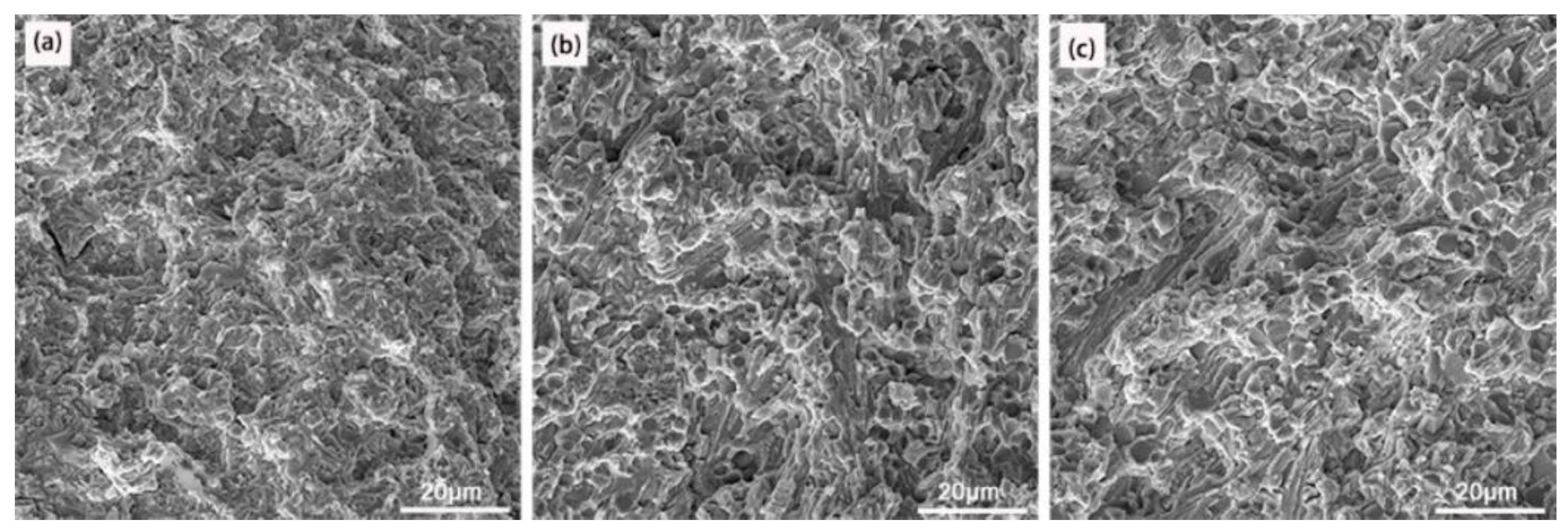
3.1. Microstructural Characterization
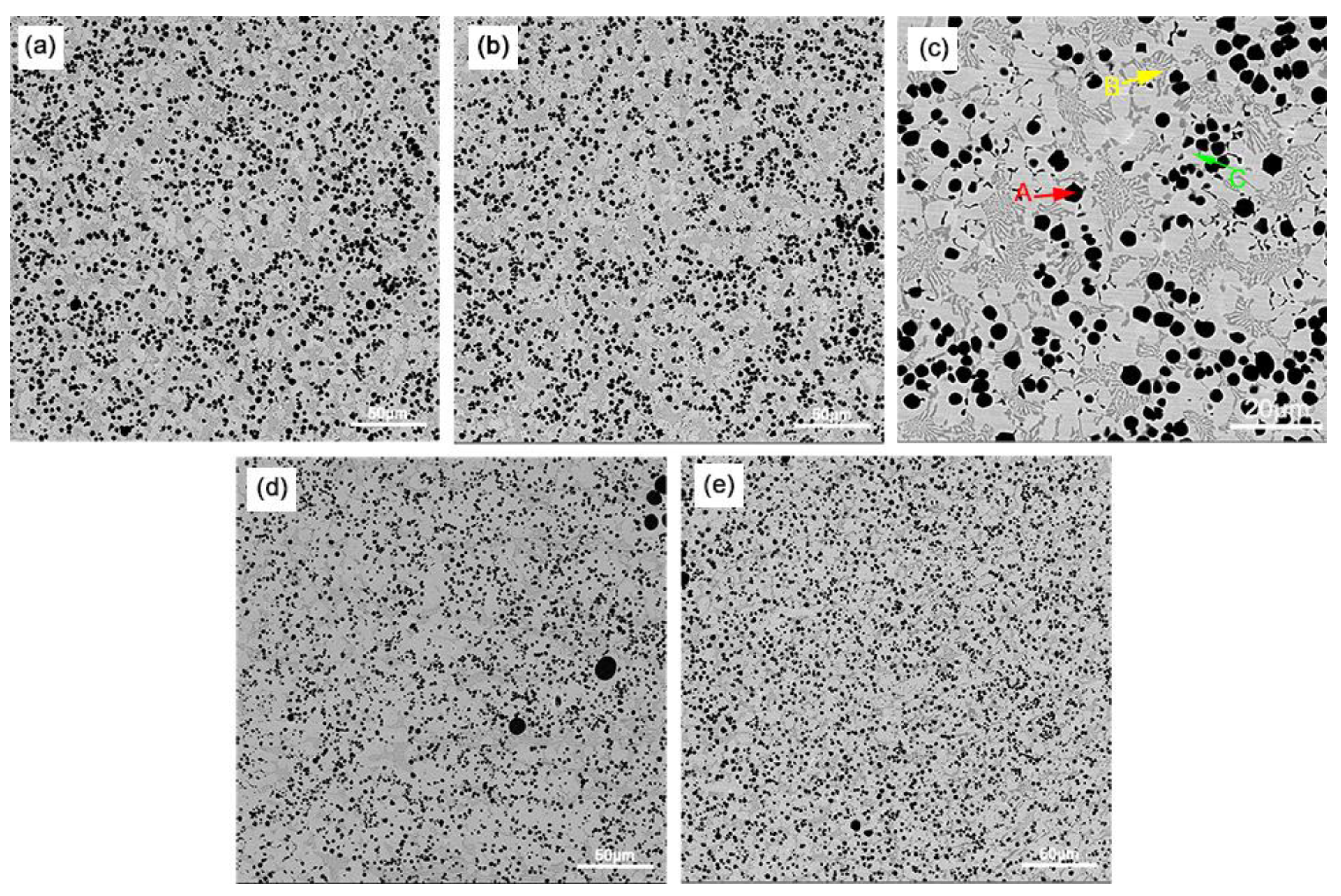
| Black A | Dark Gray B | Light Gray C | |
|---|---|---|---|
| Element | wt.% | wt.% | wt.% |
| C | 13.18 | 10.09 | 4.28 |
| V | 74.31 | 10.03 | 4.22 |
| Cr | 7.11 | 27.42 | 9.98 |
| Mn | 0.09 | 1.15 | 1.08 |
| Fe | 2.72 | 48.32 | 77.51 |
| Si | 0.82 | 1.14 | 1.20 |
| Ni | 0.25 | 0.18 | 0.28 |
| Mo | 1.52 | 1.67 | 1.45 |
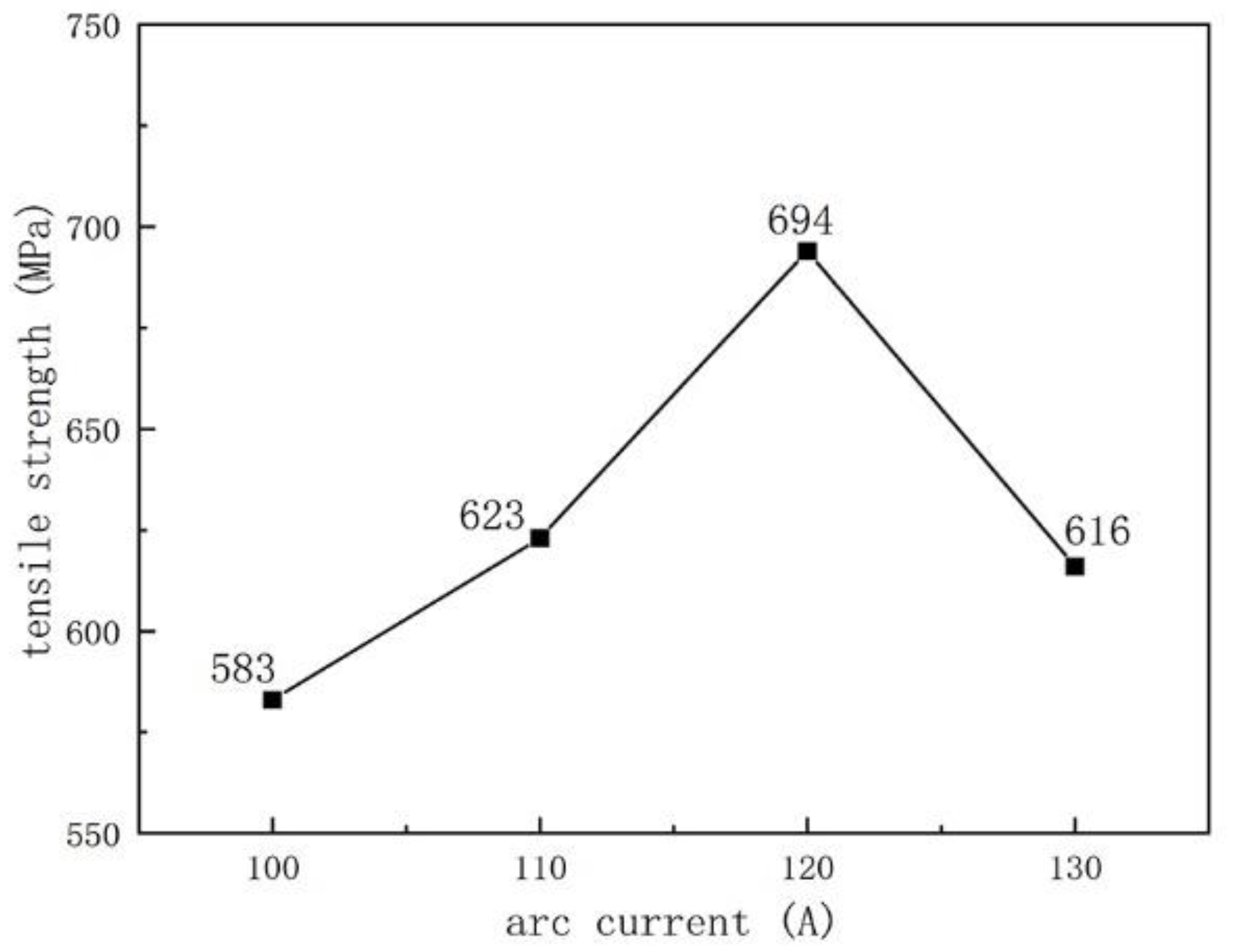
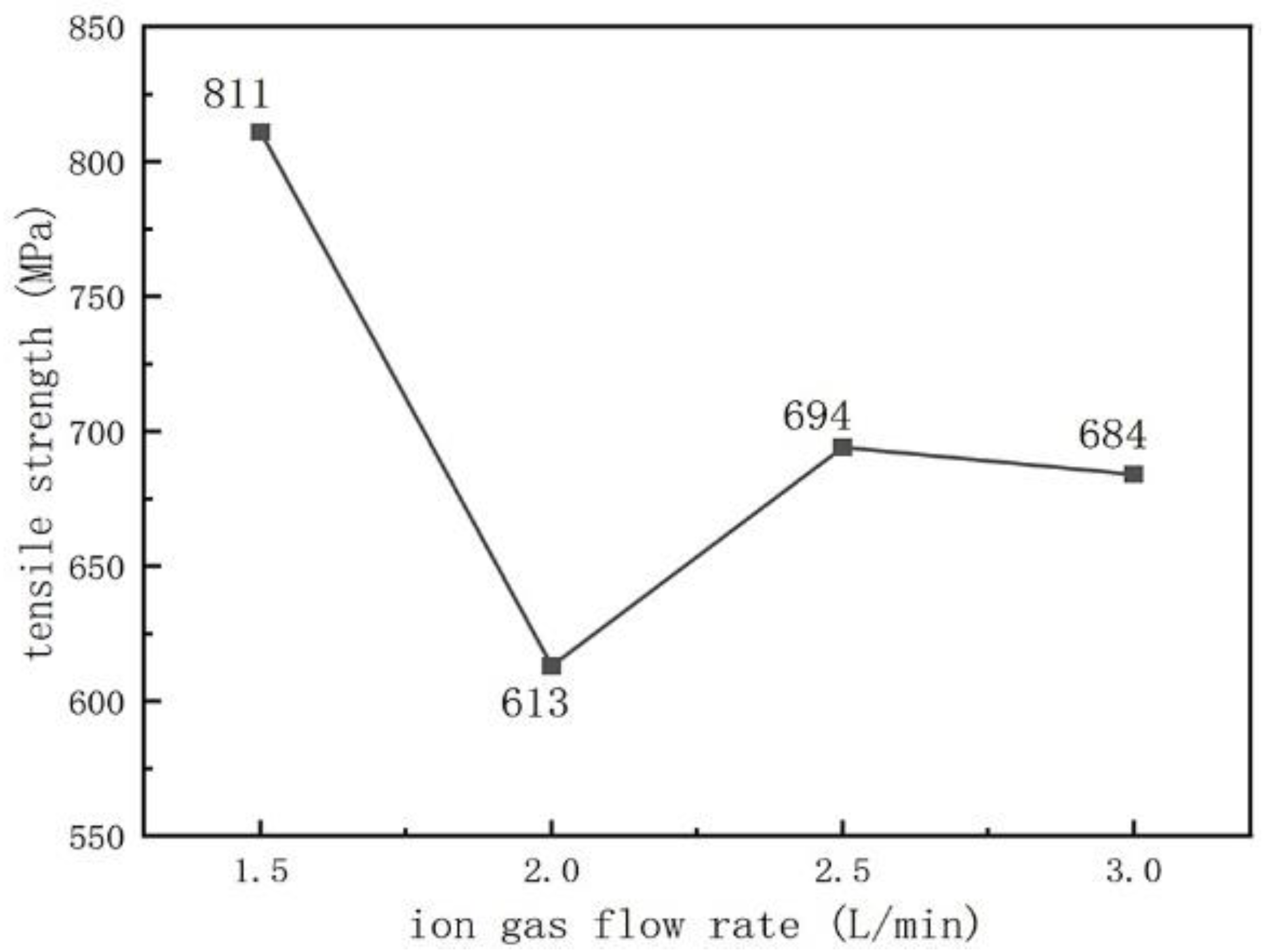
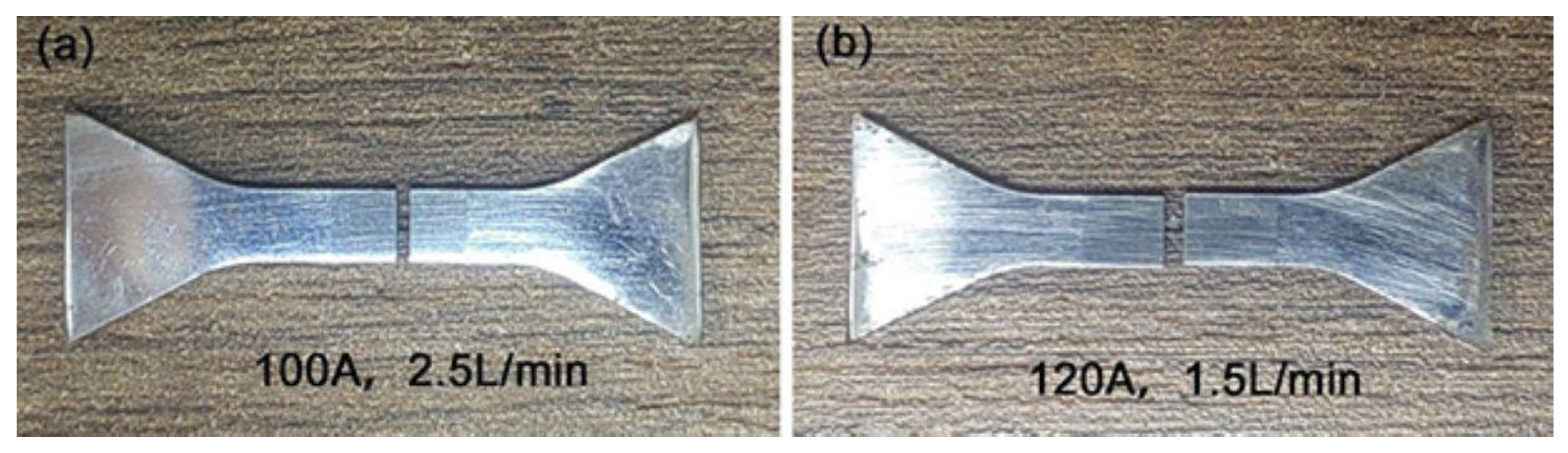
3.2. Tensile Strength
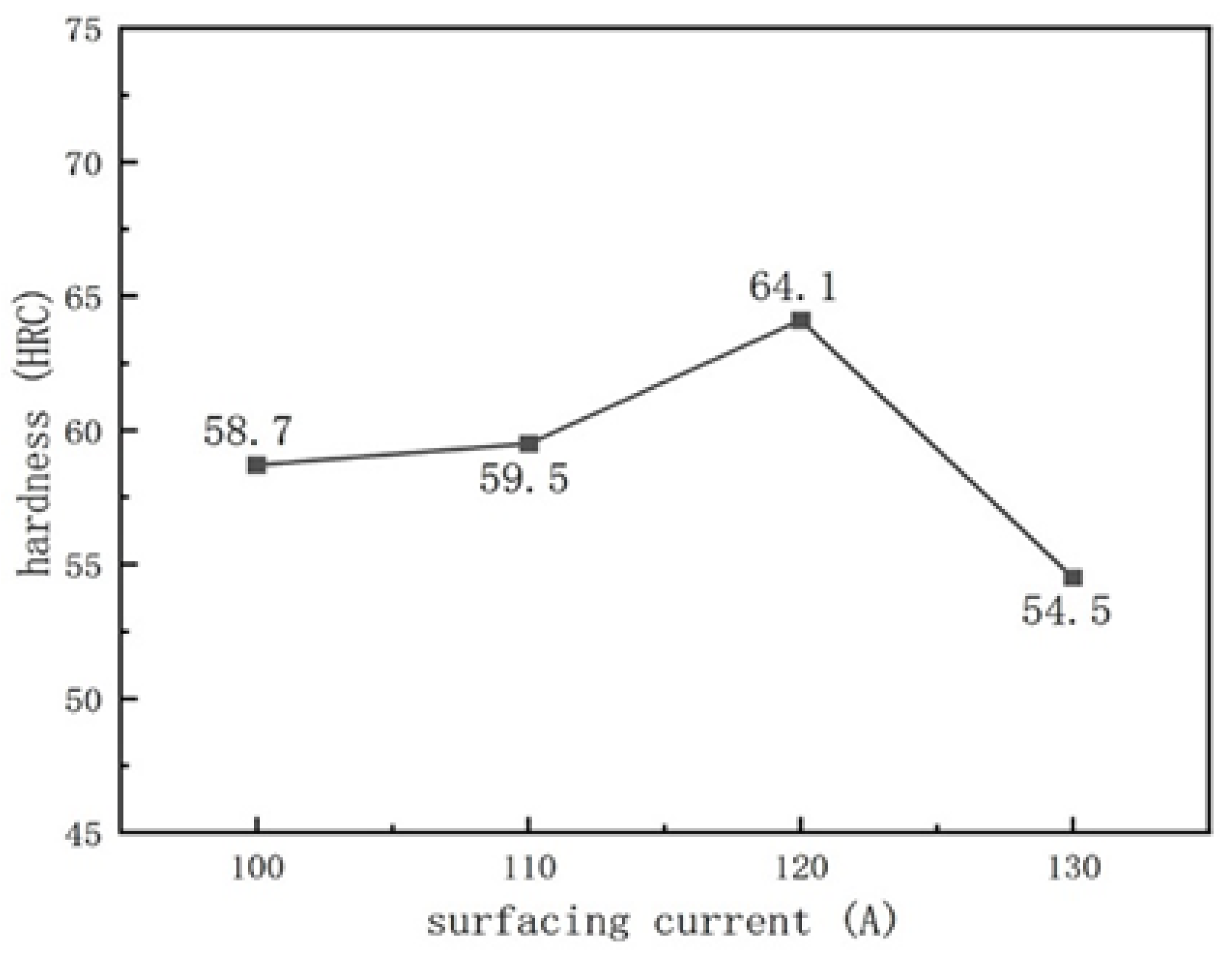
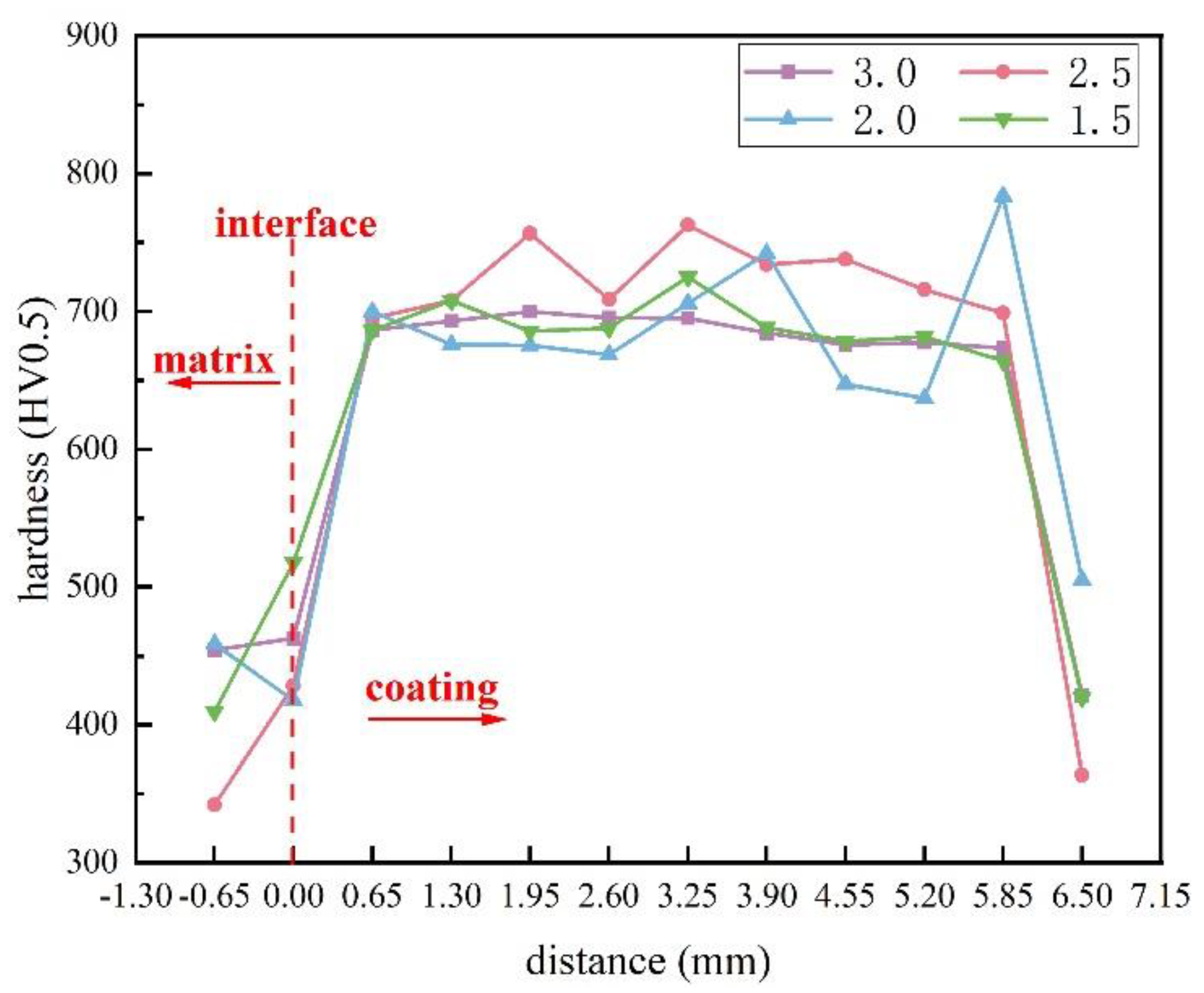
3.3. Hardness
4. Conclusions
- 1
- The overlay layer is mainly composed of α-Fe, γ-Fe, VxCy (VC,V6C5,VC0.845,VC0.75), M7C3 (M=Fe,Cr) and other phases, which can significantly improve the hardness of the surface coating.
- 2
- The Fe-Cr-V-C plasma surface layer exhibits good metallurgical bonding with the substrate, and the tensile fractures do not occur at the junction. There are no fractures and a good connection between the welding layer and the substrate. The surface layer is free of defects, e.g., pores and slag inclusions.
- 3
- We used tensile strength to characterize the bonding strength between the substrate and the surface layer. The tensile strength varies with different welding process parameters. As the arc current increases, the tensile strength and surface hardness first increase and then decrease. With the increase in the ion gas flow rate, the tensile strength first decreases and then increases, while the surface hardness first increases and then decreases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.B.; Wang, X.F.; Shi, Z.Q. The composite Fe–Ti–B–C Coatings by PTA powder surfacing process. Surf. Coat. Technol. 2005, 192, 257–262. [Google Scholar]
- Correa, E.O.; Costa, S.C.; Santos, J.N. Weldability of iron-based powder metal materials using pulsed plasma arc welding process. J. Mater. Process. Technol. 2008, 198, 323–329. [Google Scholar] [CrossRef]
- Liu, Z.M.; Cui, S.; Luo, Z.; Zhang, C.Z.; Wang, Z.M.; Zhang, Y.C. Plasma arc welding: Process variants and its recent developments of sensing, controlling and modeling. J. Manuf. Process. 2016, 23, 315–327. [Google Scholar] [CrossRef]
- Lyu, Y.; Sun, Y.; Jing, F. On the microstructure and wear resistance of Fe-based composite coatings processed by plasma cladding with B4C injection. Ceram. Int. 2015, 41, 10934–10939. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, D.; Wang, Q.; Liu, H.; Cheng, G.; Ge, S. Preparation and properties of a new cutting pick of coal shearers. Min. Sci. Technol. 2010, 20, 794–796. [Google Scholar] [CrossRef]
- Cheluszka, P.; Mikuła, S.; Mikuła, J. Theoretical consideration of fatigue strengthening of conical picks for rock cutting. Tunn. Undergr. Space Technol. 2022, 125, 104481. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Li, C.; Xiao, Y.; Yang, M.; Yuan, H.; Li, H.; Li, S. Effect of Current Variation on Microstructure and Properties of B4C Nickel-base Alloy by Plasma Surfacing. IOP Conf. Ser. Earth Environ. Sci. 2019, 332, 032052. [Google Scholar] [CrossRef]
- Zong, L.; Zhao, Y.; Long, S.; Guo, N. Effect of Nb Content on the Microstructure and Wear Resistance of Fe-12Cr-xNb-4C Coatings Prepared by Plasma-Transferred Arc Welding. Coatings 2020, 10, 585. [Google Scholar] [CrossRef]
- Qi, K.; Yang, Y.; Sun, R.; Hu, G.; Lu, X.; Li, J.; Liang, W.; Jin, K.; Xiong, L. Effect of magnetic field on crack control of Co-based alloy laser cladding. Opt. Laser Technol. 2021, 141, 107129. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Y.; Yang, L.; Sun, R.; Zhang, T.; Yang, X. Research and progress of laser cladding on engineering alloys: A review. J. Manuf. Process. 2021, 66, 341–363. [Google Scholar] [CrossRef]
- Czupryński, A.; Wyględacz, B. Comparative Analysis of Laser and Plasma Surfacing by Nickel-Based Superalloy of Heat Resistant Steel. Materials 2020, 13, 2367. [Google Scholar] [CrossRef]
- Sahoo, A.; Tripathy, S. Development in plasma arc welding process: A review. Mater. Today Proc. 2021, 41, 363–368. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, X.-S.; Chen, B.; Zuo, J.-Y.; Ma, T.-C.; Shen, J. Parameter optimization for tungsten carbide/Ni-based composite coating deposited by plasma transferred arc hardfacing. Trans. Nonferrous Met. Soc. China 2018, 28, 2511–2519. [Google Scholar] [CrossRef]
- Appiah, A.N.S.; Bialas, O.; Czupryński, A.; Adamiak, M. Powder Plasma Transferred Arc Welding of Ni-Si-B+60 wt.%WC and Ni-Cr-Si-B+45 wt.%WC for Surface Cladding of Structural Steel. Materials 2022, 15, 4956. [Google Scholar] [CrossRef]
- Hou, Q.Y.; Luo, L.M.; Huang, Z.Y.; Wang, P.; Ding, T.T.; Wu, Y.C. Comparison of three kinds of MC-type carbide modified thick W coatings fabricated by plasma transferred arc surfacing. Surf. Coat. Technol. 2015, 283, 52–60. [Google Scholar] [CrossRef]
- Garcia, R.P.; Canobre, S.C.; Costa, H.L. Microabrasion-corrosion resistance of Ni–Cr superalloys deposited by plasma transferred arc (PTA) welding. Tribol. Int. 2020, 143, 106080. [Google Scholar] [CrossRef]
- D’oliveira, A.; Paredes RS, C.; Santos RL, C. Pulsed current plasma transferred arc hardfacing. J. Mater. Process. Technol. 2006, 171, 167–174. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Ye, H.; Yao, Y. Study on plasma arc welding technology and properties of metal materials. In Proceedings of the 2019 International Conference on Advanced Electronic Materials, Computers and Materials Engineering (AEMCME 2019), Changsha, China, 19–21 April 2019; Iop Publishing Ltd.: Bristol, UK, 2019; Volume 563, p. 022003. [Google Scholar]
- Ranjan, R.; Kumar Das, A. Protection from corrosion and wear by different weld cladding techniques: A review. Mater. Today Proc. 2022, 57, 1687–1693. [Google Scholar] [CrossRef]
- Bratberg, J.; Frisk, K. An experimental and theoretical analysis of the phase equilibria in the Fe-Cr-V-C system. Metall. Mater. Trans. A 2004, 35, 3649–3663. [Google Scholar] [CrossRef]
- Chang, C.-M.; Chen, L.-H.; Lin, C.-M.; Chen, J.-H.; Fan, C.-M.; Wu, W. Microstructure and wear characteristics of hypereutectic Fe–Cr–C cladding with various carbon contents. Surf. Coat. Technol. 2010, 205, 245–250. [Google Scholar] [CrossRef]
- Branagan, D.J.; Marshall, M.C.; Meacham, B.E. High toughness high hardness iron based PTAW weld materials. Mater. Sci. Eng. A 2006, 428, 116–123. [Google Scholar] [CrossRef]
- Wang, X.B.; Li, C.G.; Peng, X.M.; Shi, L.B.; Zhang, H. The powder’s thermal behavior on the surface of the melting pool during PTA powder surfacing. Surf. Coat. Technol. 2006, 201, 2648–2654. [Google Scholar]
- Gao, Y.; Liu, Y.; Wang, L.; Yang, X.; Zeng, T.; Sun, L.; Wang, R. Microstructure evolution and wear resistance of laser cladded 316L stainless steel reinforced with in-situ VC-Cr7C3. Surf. Coat. Technol. 2022, 435, 128264. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, J.; Zhang, M.; Wang, K.; Peng, P. Laser in-situ preparation and mechanical properties of VC reinforced Fe-based wear-resistant composite cladding. Ceram. Int. 2022, 48, 28240–28249. [Google Scholar] [CrossRef]
- Gnyusov, S.F.; Degterev, A.S.; Tarasov, S.Y. The effect of plasma torch weaving on microstructural evolution in multiple-pass plasma-transferred arc Fe-Cr-V-Mo-C coating. Surf. Coat. Technol. 2018, 344, 75–84. [Google Scholar] [CrossRef]
- Pan, Z.; Dong, X.; Cao, H.; Huang, Q. The Role of Distribution Forms of Fe–Cr–C Cladding Layer in the Impact Abrasive Wear Performance of Hadfield Steel. Materials 2020, 13, 1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Fu, S. Production of in situ Vanadium Carbide Particulate Reinforced Iron Matrix Composite. Mater. Sci. 2014, 20, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhou, Z.; Wu, L.; Ding, Y.; Xu, F.; Wang, Z. Statistical Optimization of Reactive plasma cladding to synthesize a WC-reinforced Fe-based alloy coating. J. Therm. Spray Technol. 2018, 27, 769–777. [Google Scholar] [CrossRef]
- Xu, H.; Huang, H.; Liu, Z. Influence of Plasma Transferred Arc Remelting on Microstructure and Properties of PTAW-Deposited Ni-Based Overlay Coating. J. Therm. Spray Technol. 2021, 30, 946–958. [Google Scholar] [CrossRef]
- Wilden, J.; Bergmann, J.P.; Frank, H. Plasma transferred arc welding—Modeling and experimental optimization. J. Therm. Spray Technol. 2006, 15, 779–784. [Google Scholar] [CrossRef]




| Elements | C | Mn | Mo | Cr | Ni | Fe |
|---|---|---|---|---|---|---|
| Content (wt.%) | 0.45 | 0.60 | 0.20 | 1.00 | 0.25 | Bal. |
| Elements | C | Mn | Mo | Ni | Si | Cr | V | Fe |
|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 4.12 | 1.05 | 1.32 | 0.21 | 0.91 | 13.50 | 14.50 | Bal. |
| Number | Arc Current (A) | Ion Gas Flow Rate (L/min) | Protective Gas Flow Rate (L/min) | Powder Feeding Gas Flow Rate (L/min) | Powder Feed Rate (g/min) | Speed (rpm) |
|---|---|---|---|---|---|---|
| 1 | 130 | 2.5 | 1.0 | 2.0 | 65 | 1.0 |
| 2 | 120 | |||||
| 3 | 110 | |||||
| 4 | 100 | |||||
| 5 | 120 | 3.0 | ||||
| 6 | 2.0 | |||||
| 7 | 1.5 |
| Black A | Dark Gray B | Light Gray C | |
|---|---|---|---|
| Element | wt.% | wt.% | wt.% |
| C | 16.62 | 10.40 | 10.61 |
| V | 41.60 | 8.28 | 9.49 |
| Cr | 10.18 | 18.73 | 13.62 |
| Mn | 0.43 | 1.32 | 1.18 |
| Fe | 28.91 | 58.49 | 62.59 |
| Si | 0.44 | 0.91 | 1.16 |
| Ni | 0.22 | 0.19 | 0.28 |
| Mo | 1.60 | 1.68 | 1.07 |
| Point | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Average Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Arc Current | ||||||||||
| 130 A | 10.66 | 11.48 | 9.29 | 12.84 | 10.38 | 12.02 | 10.93 | 10.38 | 11.00 | |
| 120 A | 7.92 | 9.56 | 7.92 | 11.75 | 11.48 | 11.20 | 12.02 | 10.36 | 10.28 | |
| 110 A | 6.63 | 5.52 | 4.97 | 6.35 | 7.10 | 6.08 | 9.39 | 8.29 | 6.79 | |
| 100 A | 7.09 | 5.26 | 5.67 | 6.48 | 5.06 | 6.28 | 6.48 | 5.55 | 5.98 | |
| A | B | C | |
|---|---|---|---|
| Element | wt.% | wt.% | wt.% |
| C | 0.03 | 0.01 | 0.02 |
| V | 83.72 | 24.15 | 13.51 |
| Cr | 8.72 | 10.41 | 31.70 |
| Mn | 0.15 | 0.98 | 1.40 |
| Fe | 5.21 | 62.22 | 50.26 |
| Si | 0.14 | 0.78 | 0.33 |
| Ni | 0.08 | 0.24 | 0.16 |
| Mo | 1.96 | 1.20 | 2.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, Y.; Cheng, H.; Li, K.; Qian, C.; Li, W. Composite Fe-Cr-V-C Coatings Prepared by Plasma Transferred-Arc Powder Surfacing. Materials 2023, 16, 5059. https://doi.org/10.3390/ma16145059
Zhang X, Liu Y, Cheng H, Li K, Qian C, Li W. Composite Fe-Cr-V-C Coatings Prepared by Plasma Transferred-Arc Powder Surfacing. Materials. 2023; 16(14):5059. https://doi.org/10.3390/ma16145059
Chicago/Turabian StyleZhang, Xin, Yong Liu, Huichao Cheng, Kun Li, Cheng Qian, and Wei Li. 2023. "Composite Fe-Cr-V-C Coatings Prepared by Plasma Transferred-Arc Powder Surfacing" Materials 16, no. 14: 5059. https://doi.org/10.3390/ma16145059





