Exploring Ln(III)-Ion-Based Luminescent Species as Down-Shifters for Photovoltaic Solar Cells
Abstract
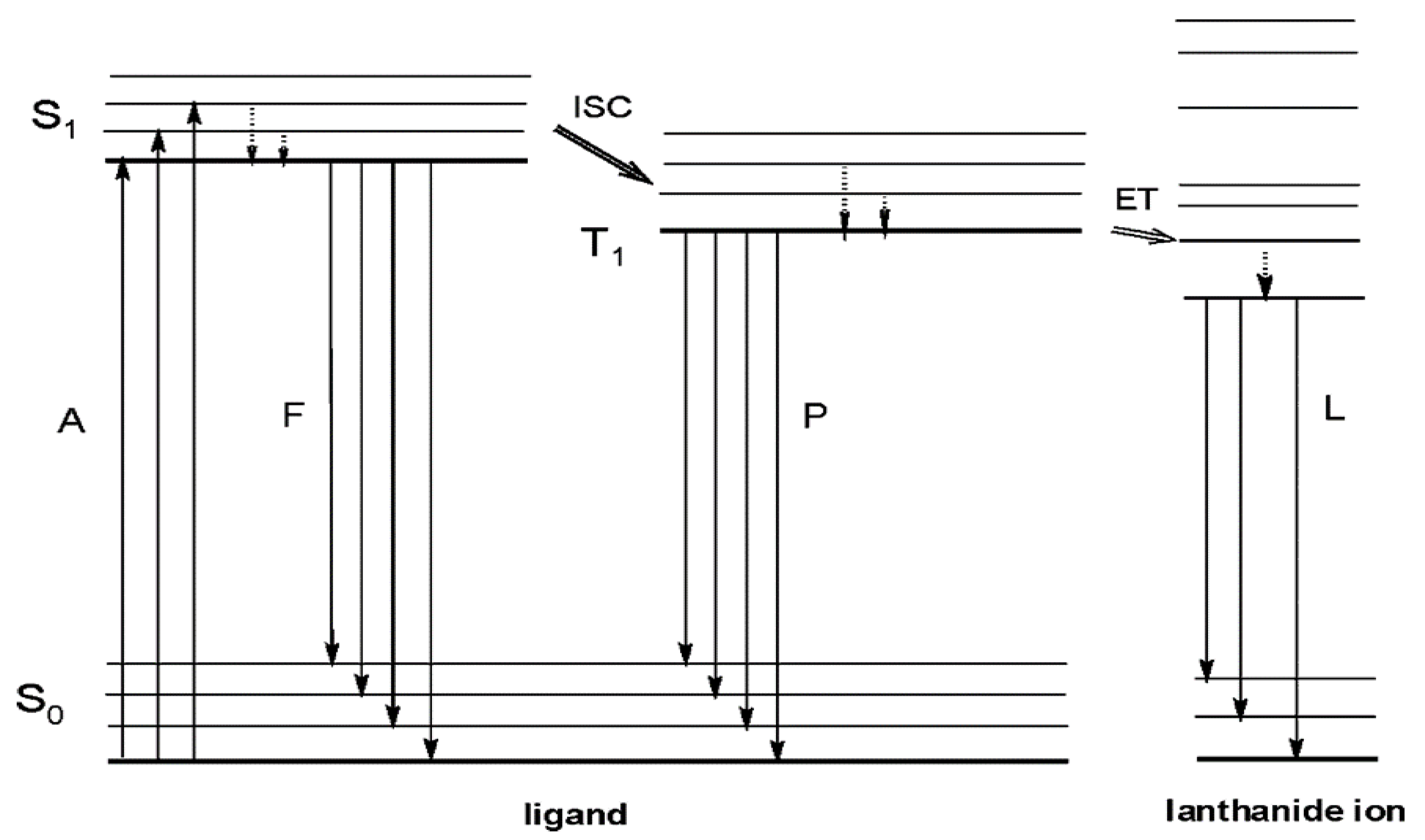
1. Introduction
2. Materials and Methods
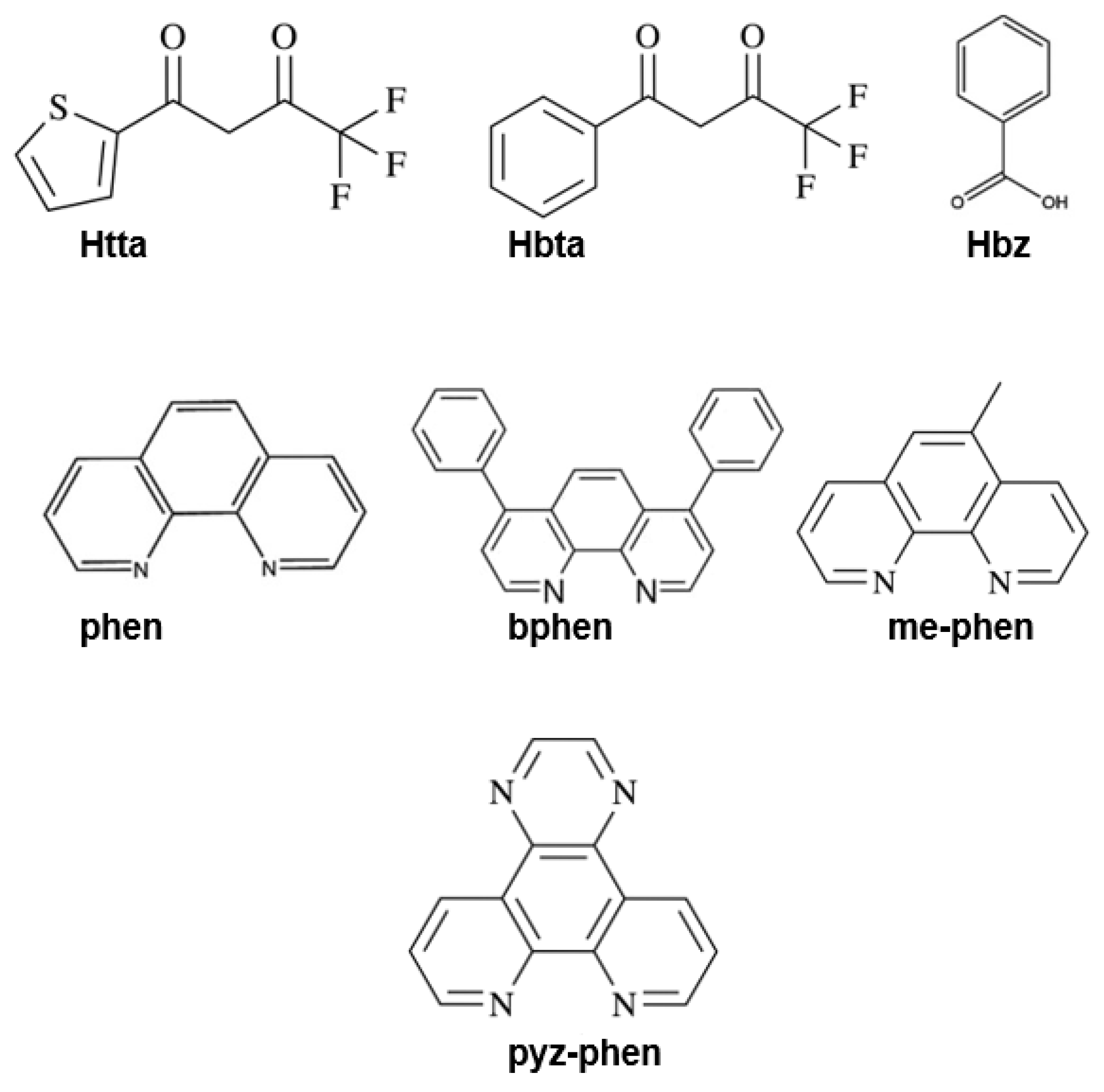
2.1. Materials
2.2. General Methods
2.3. Photoluminescence and EQE Measurements
2.4. Accelerated Aging
3. Results and Discussion
3.1. Synthesis and Characterization of the Complexes
3.2. Structure of the Complexes
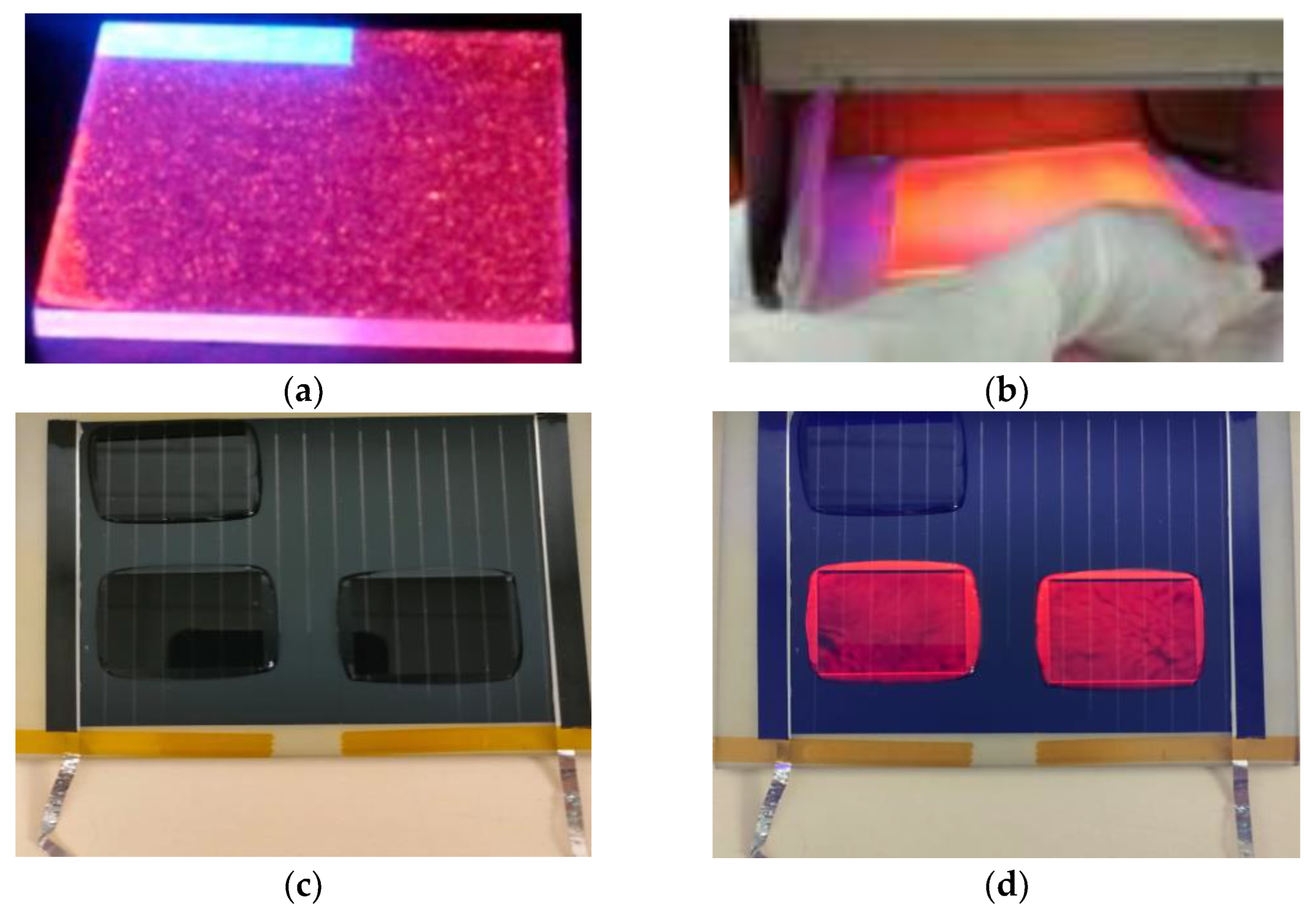
3.3. Preparation of the Luminescent Down-Shifting Layers
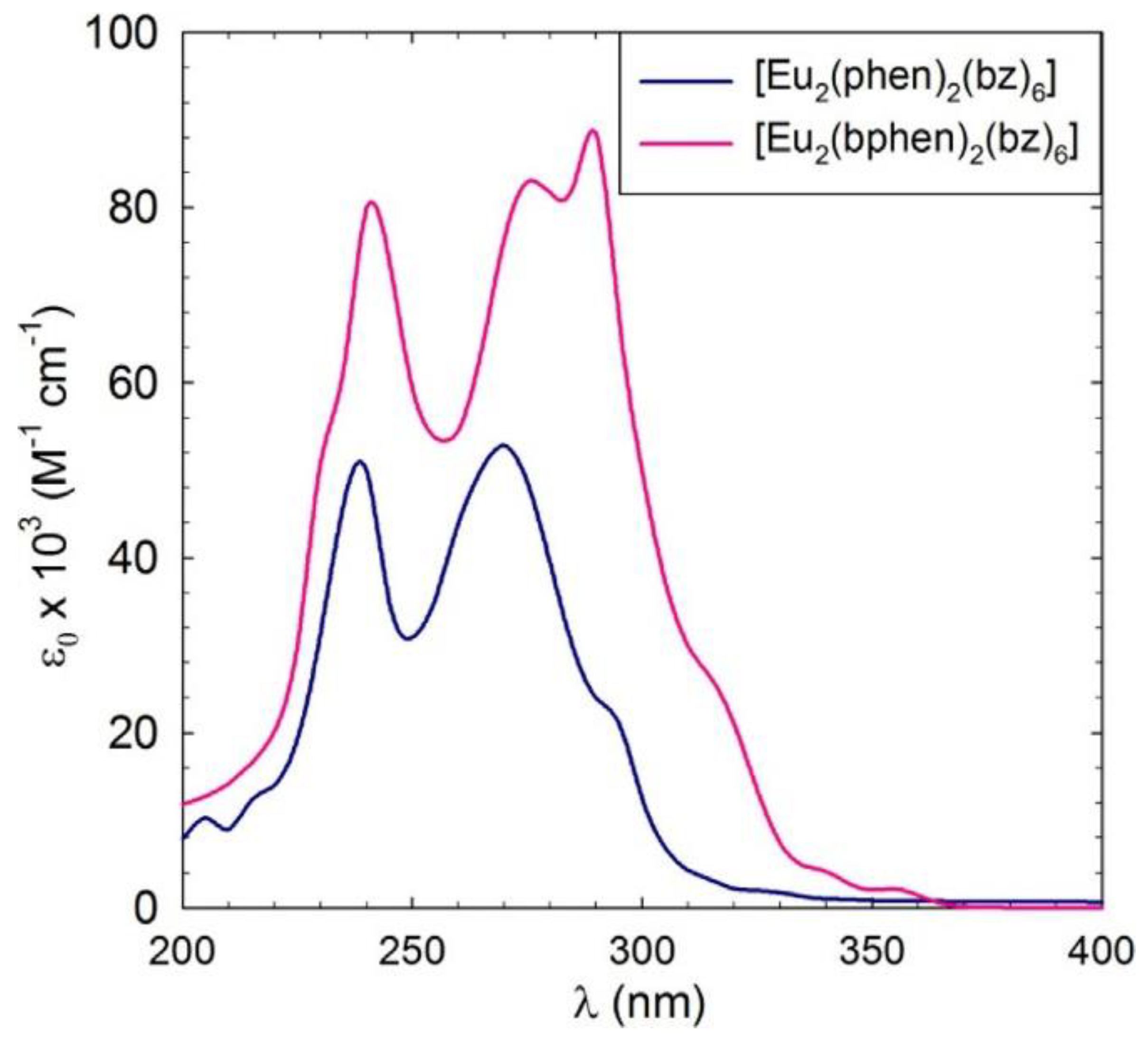
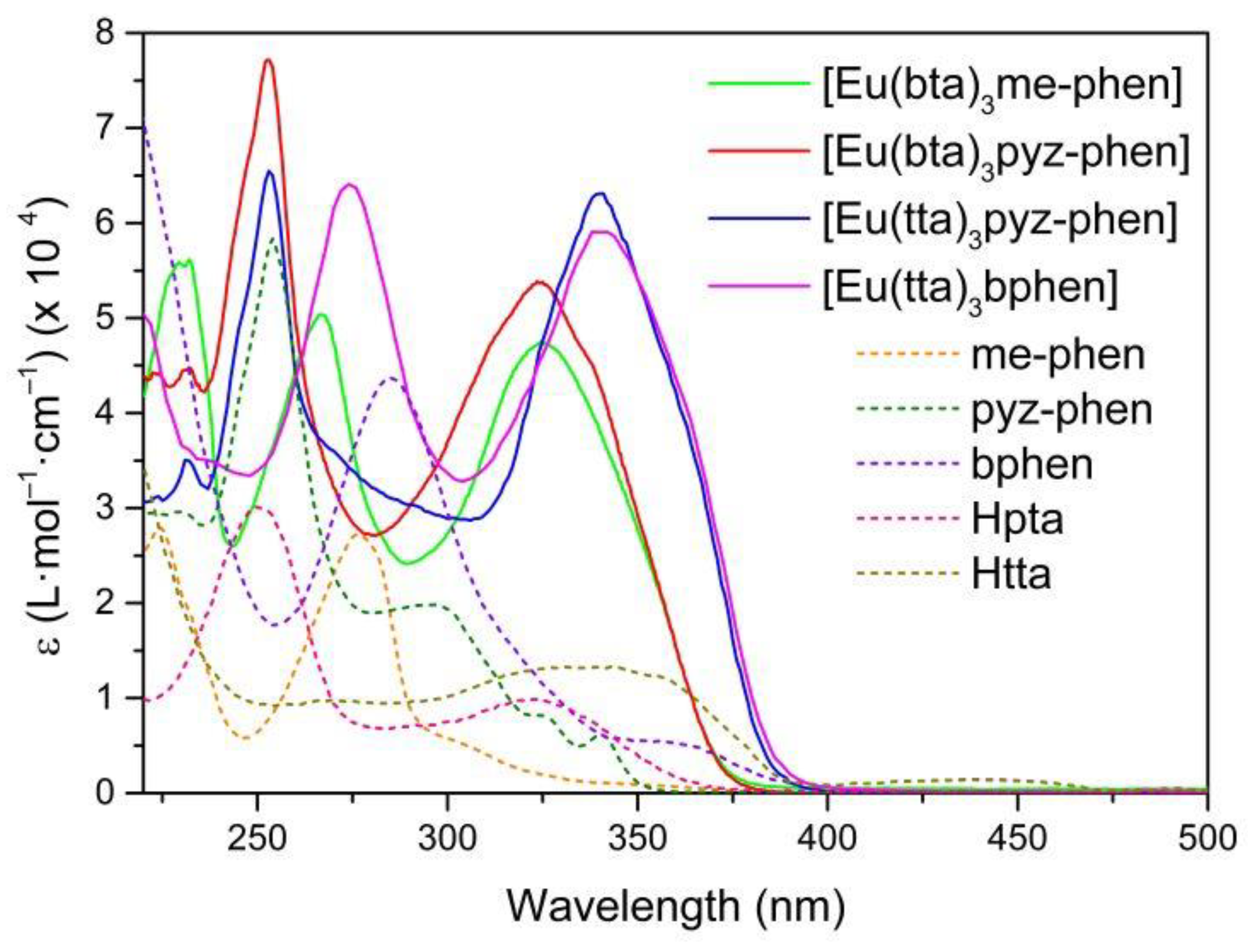
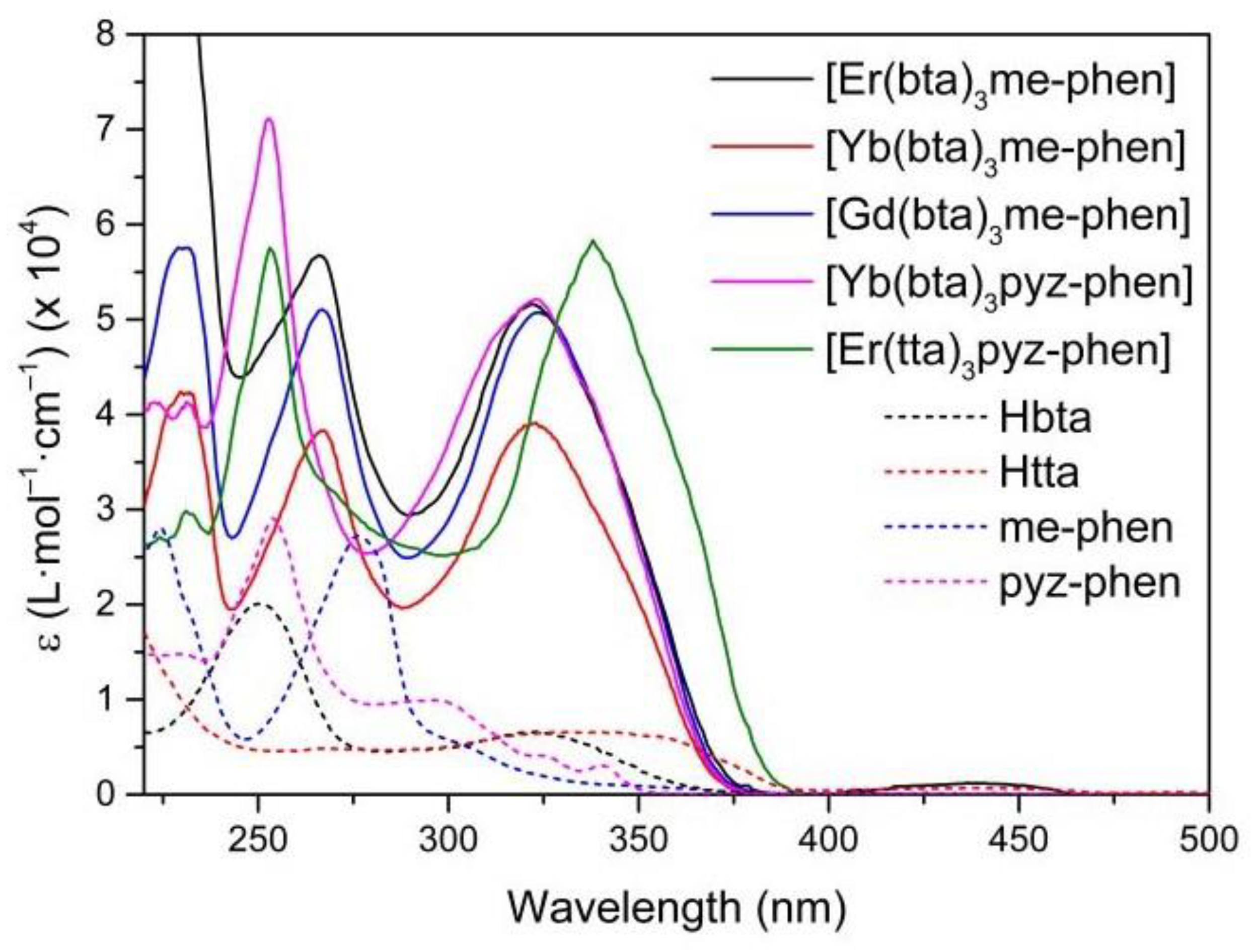
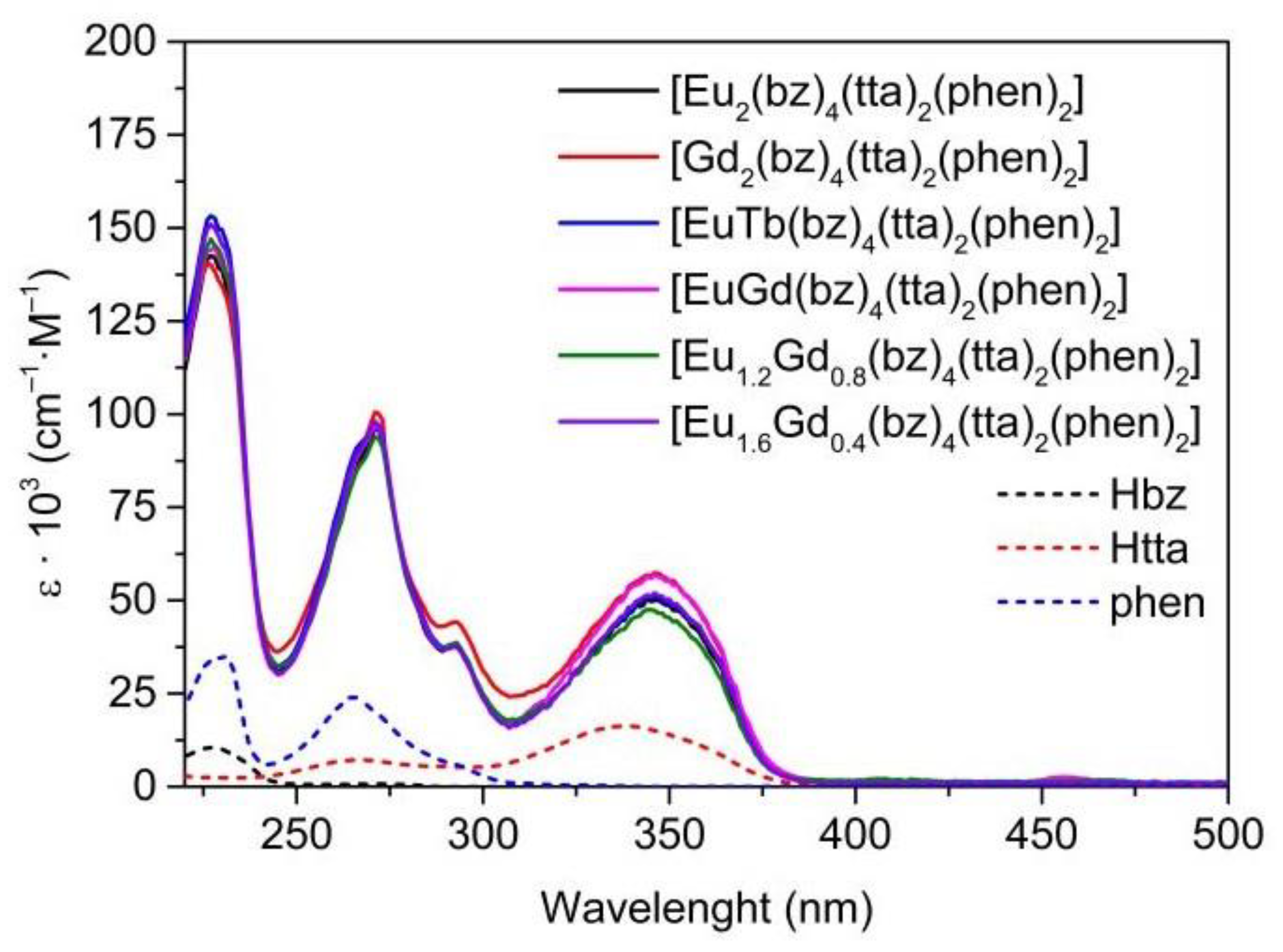
3.4. UV-Vis Absorption Spectra
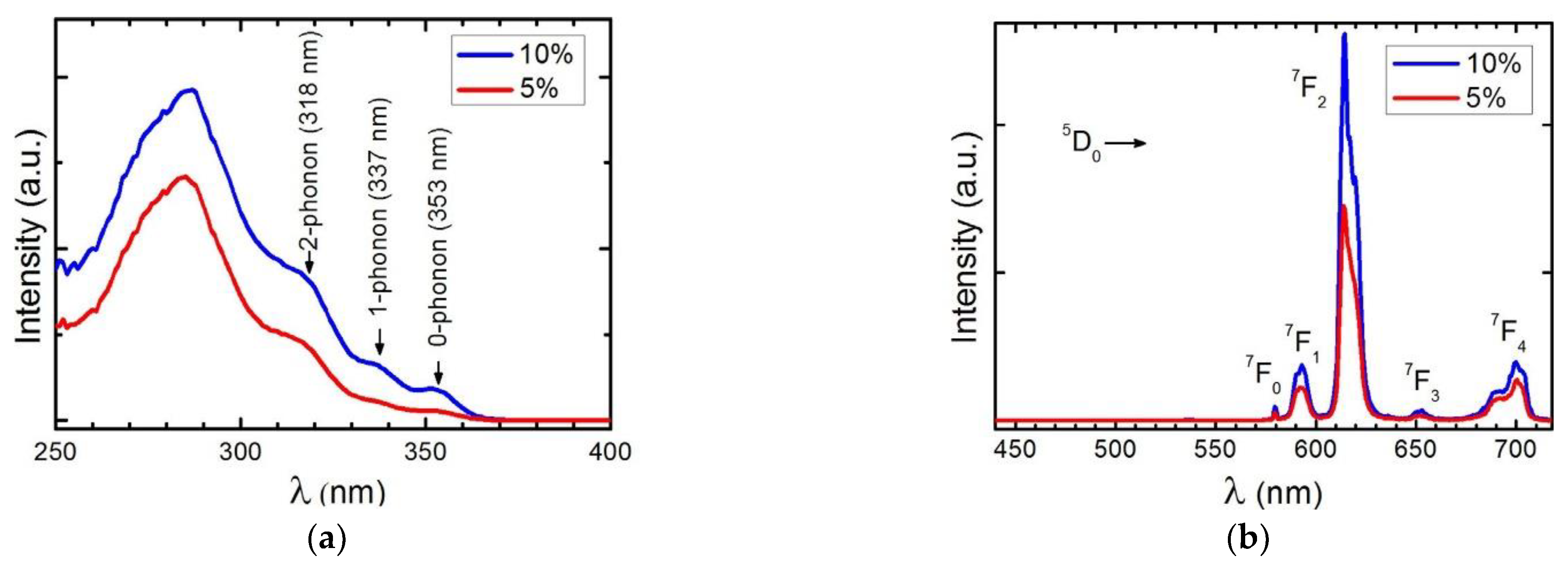
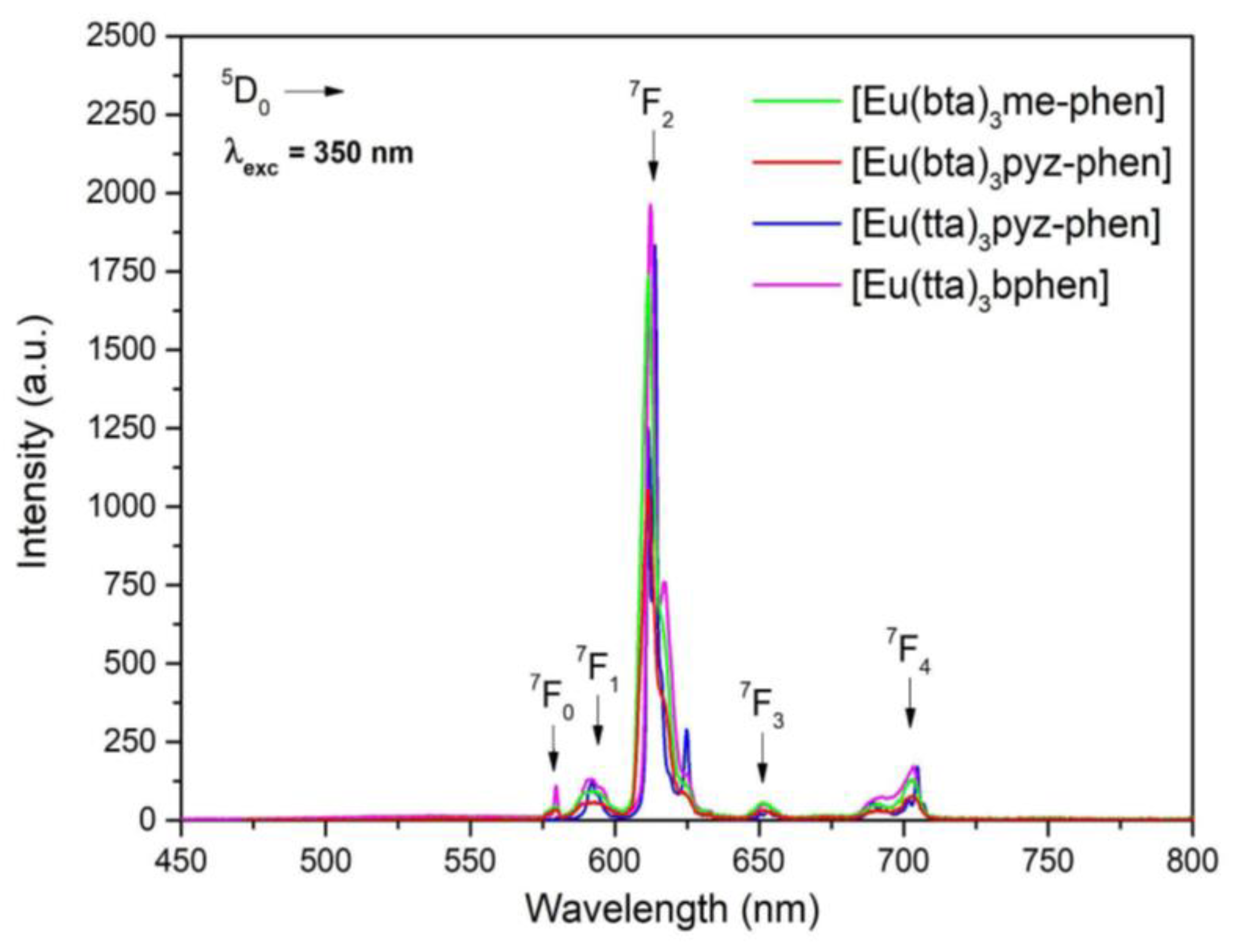
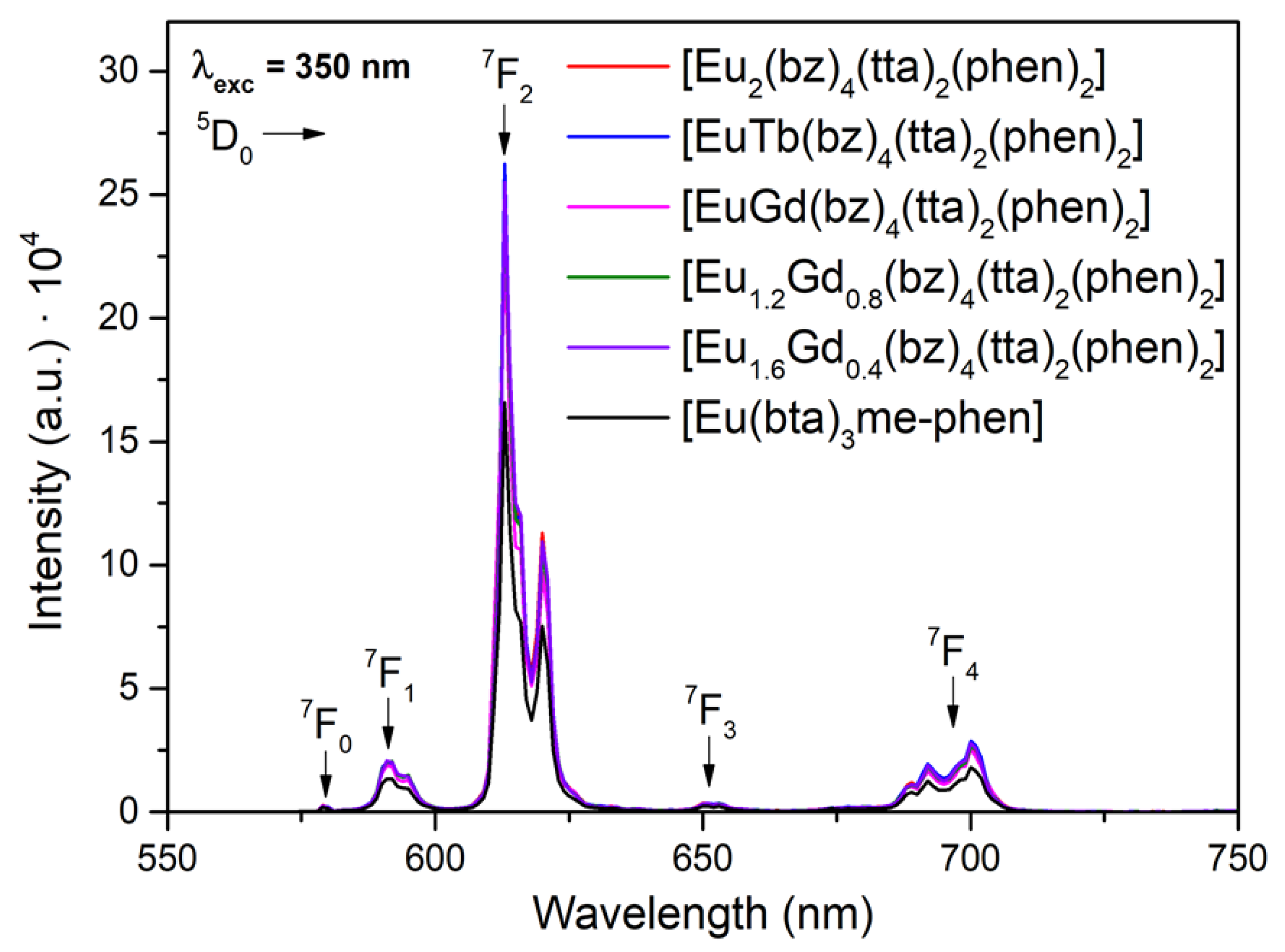
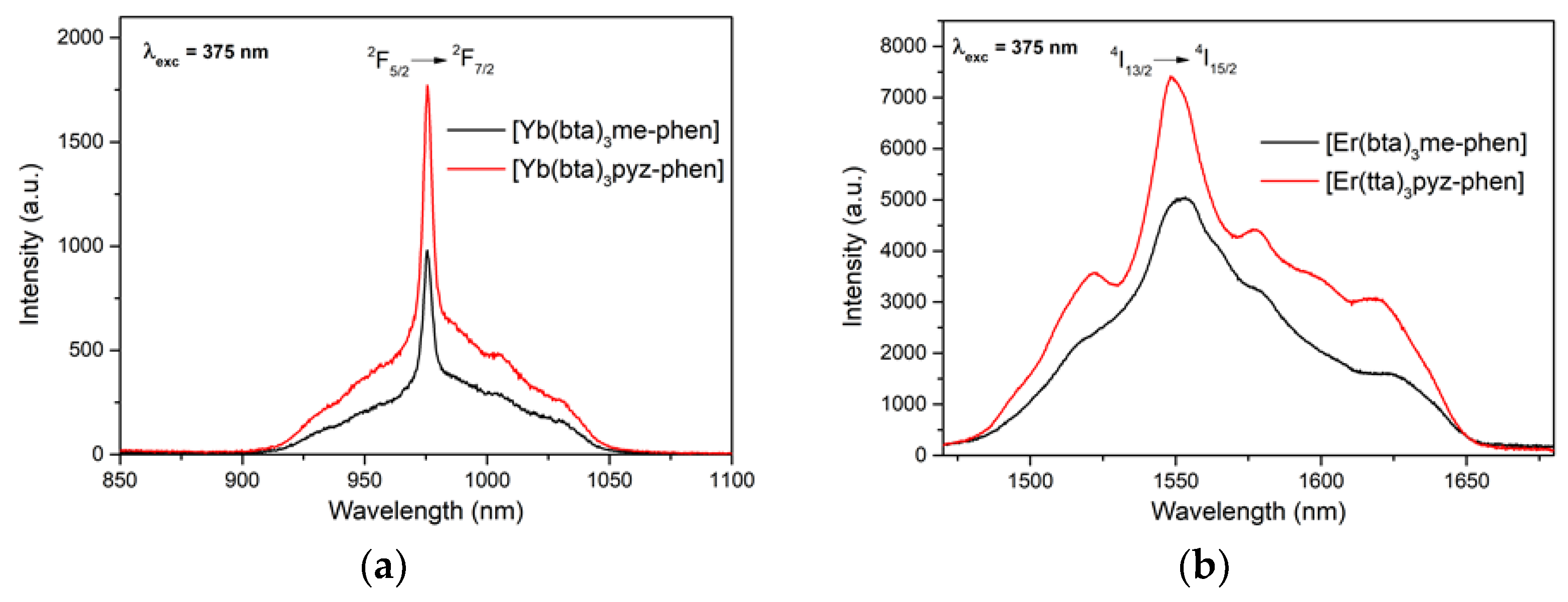
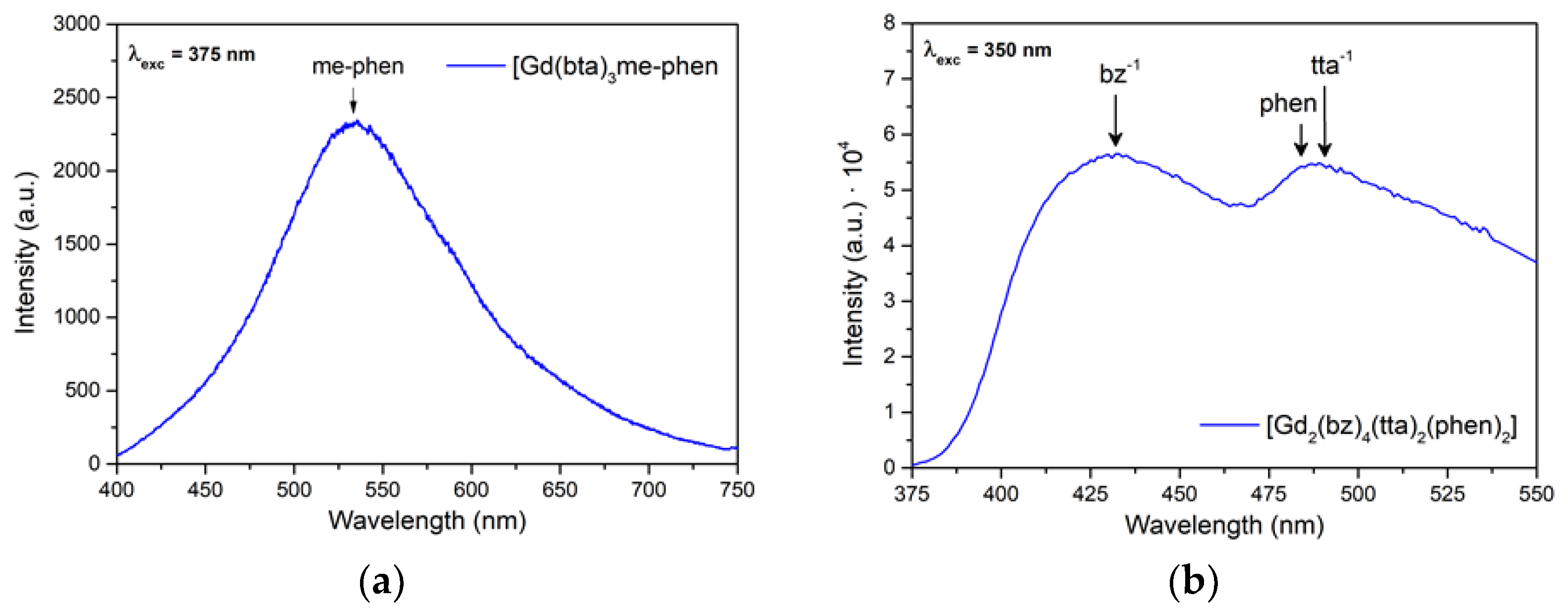
3.5. Photoluminescence
3.6. EQE Measurements
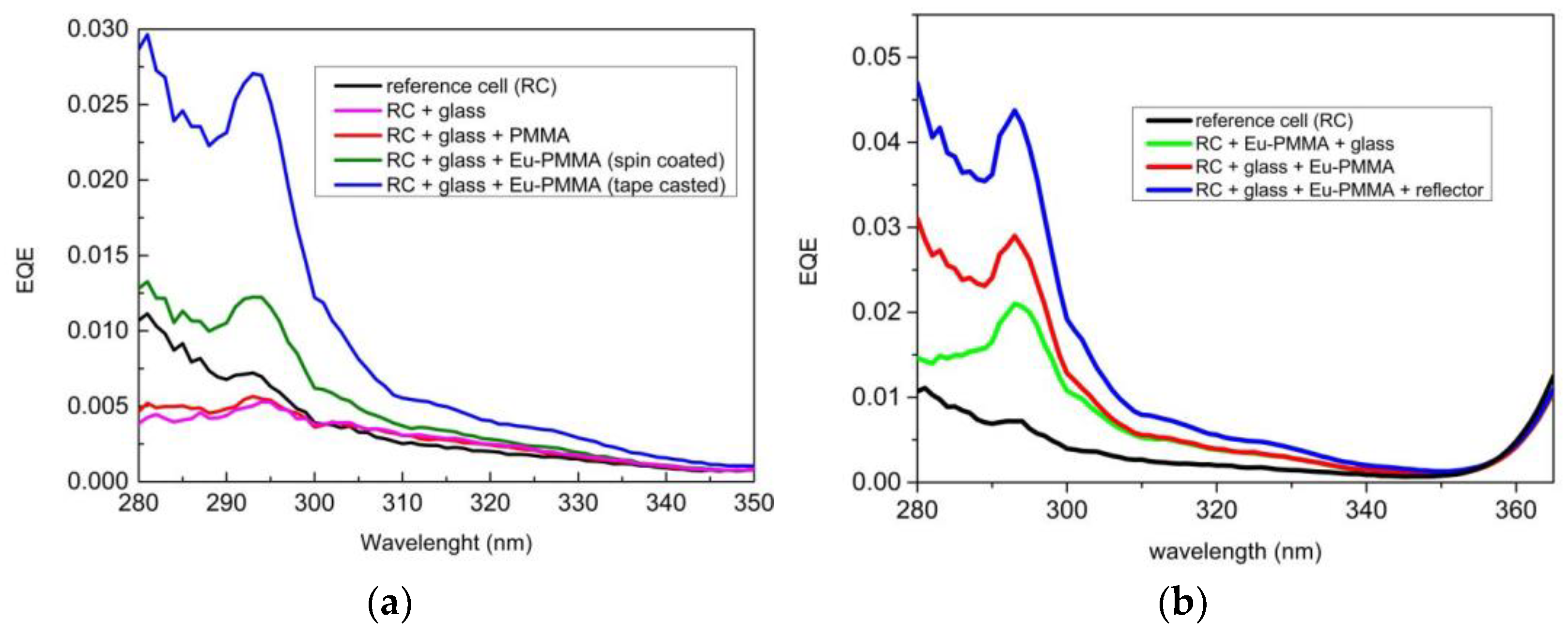
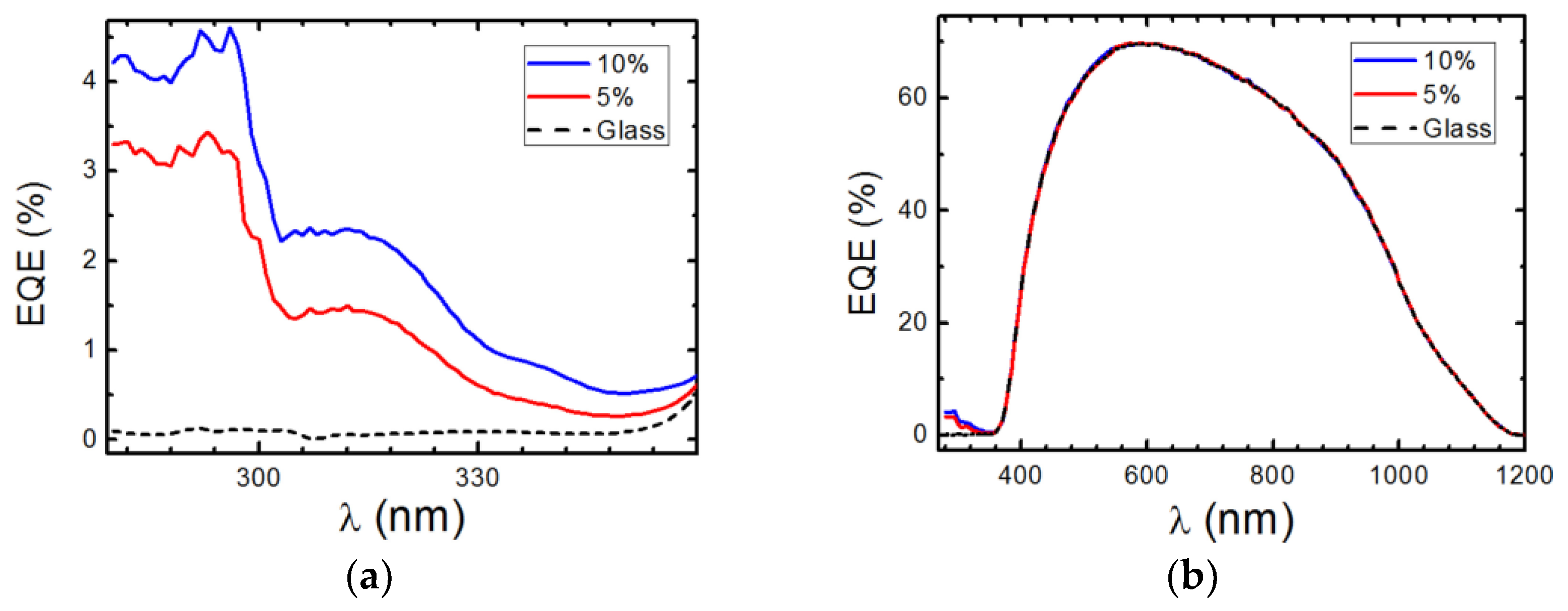
3.6.1. DS Layers Using Different Concentrations of Complexes C1 and C2
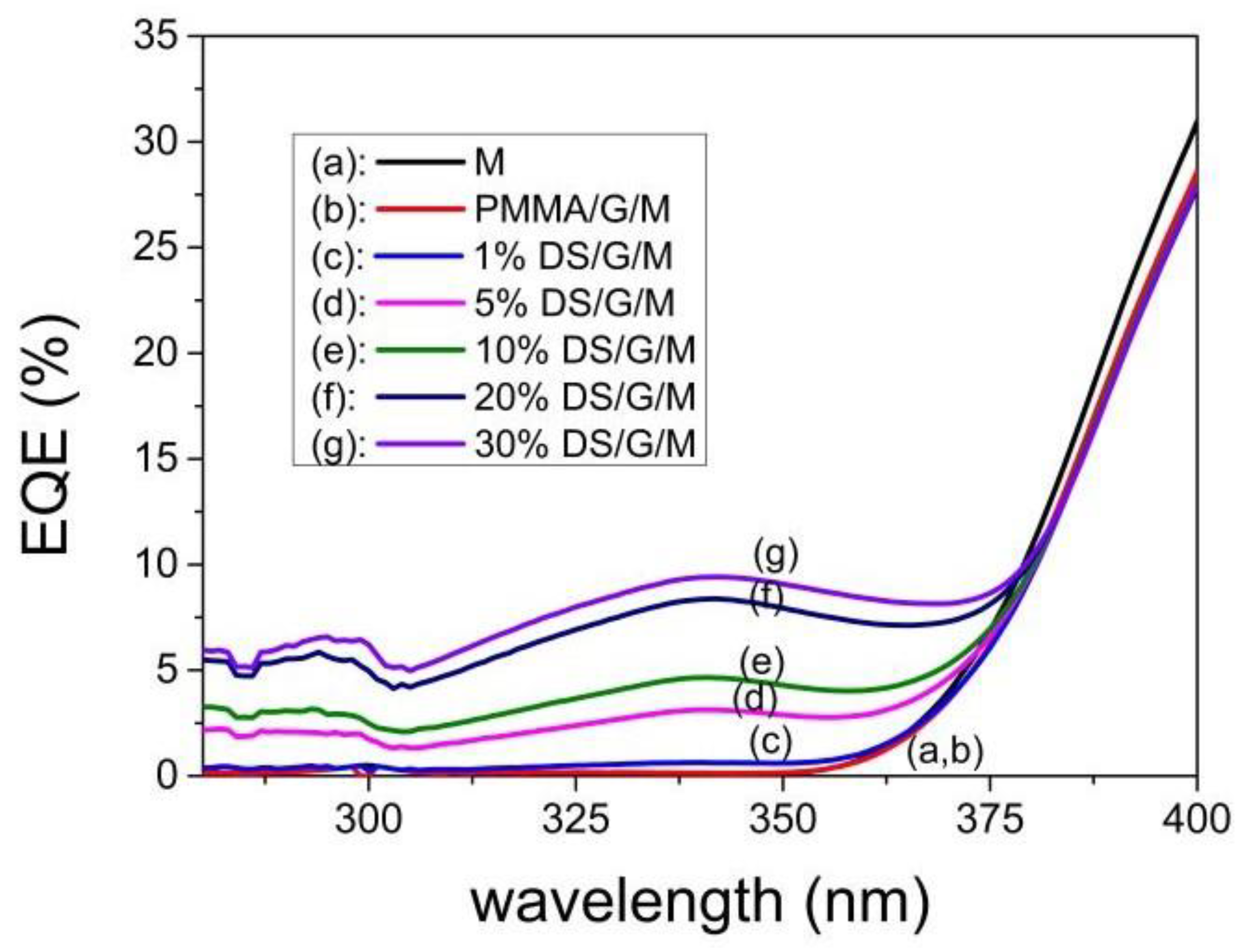
3.6.2. DS Layers Using Different Concentrations of Complex C3
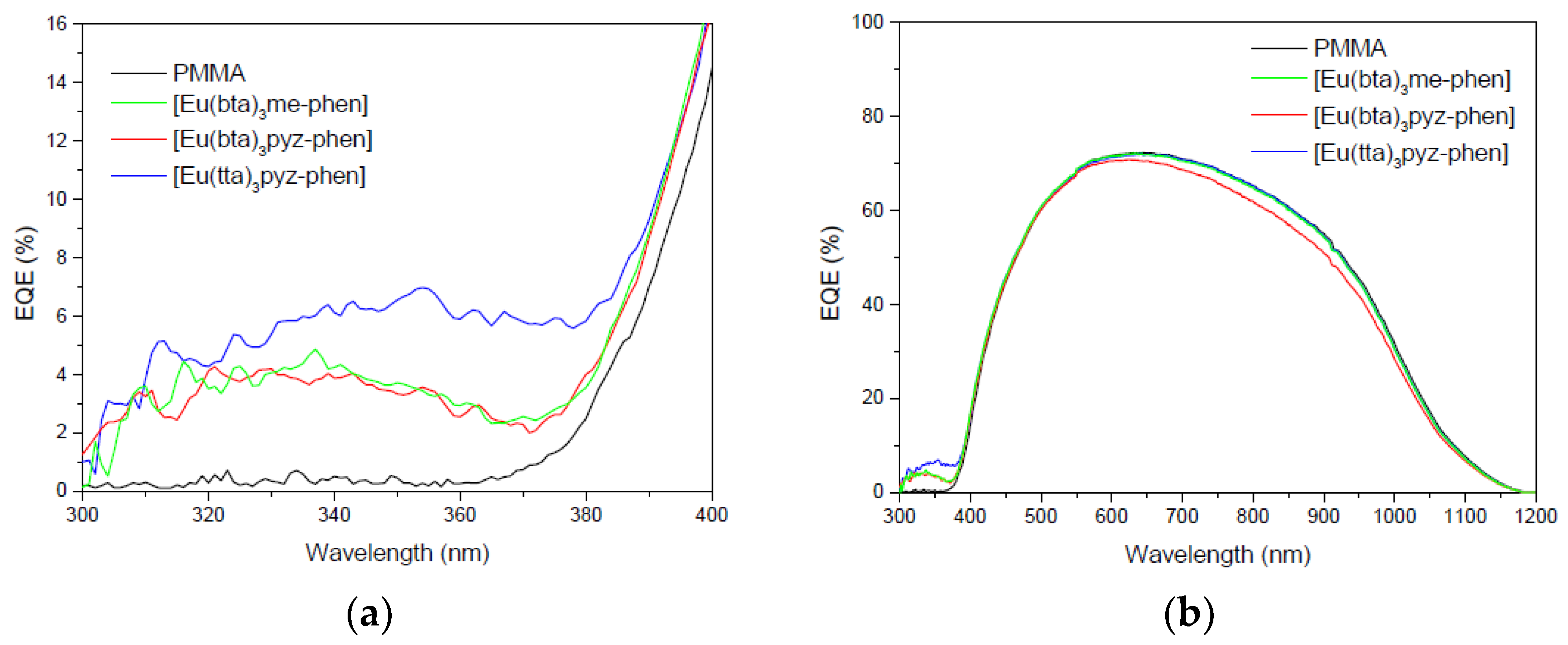
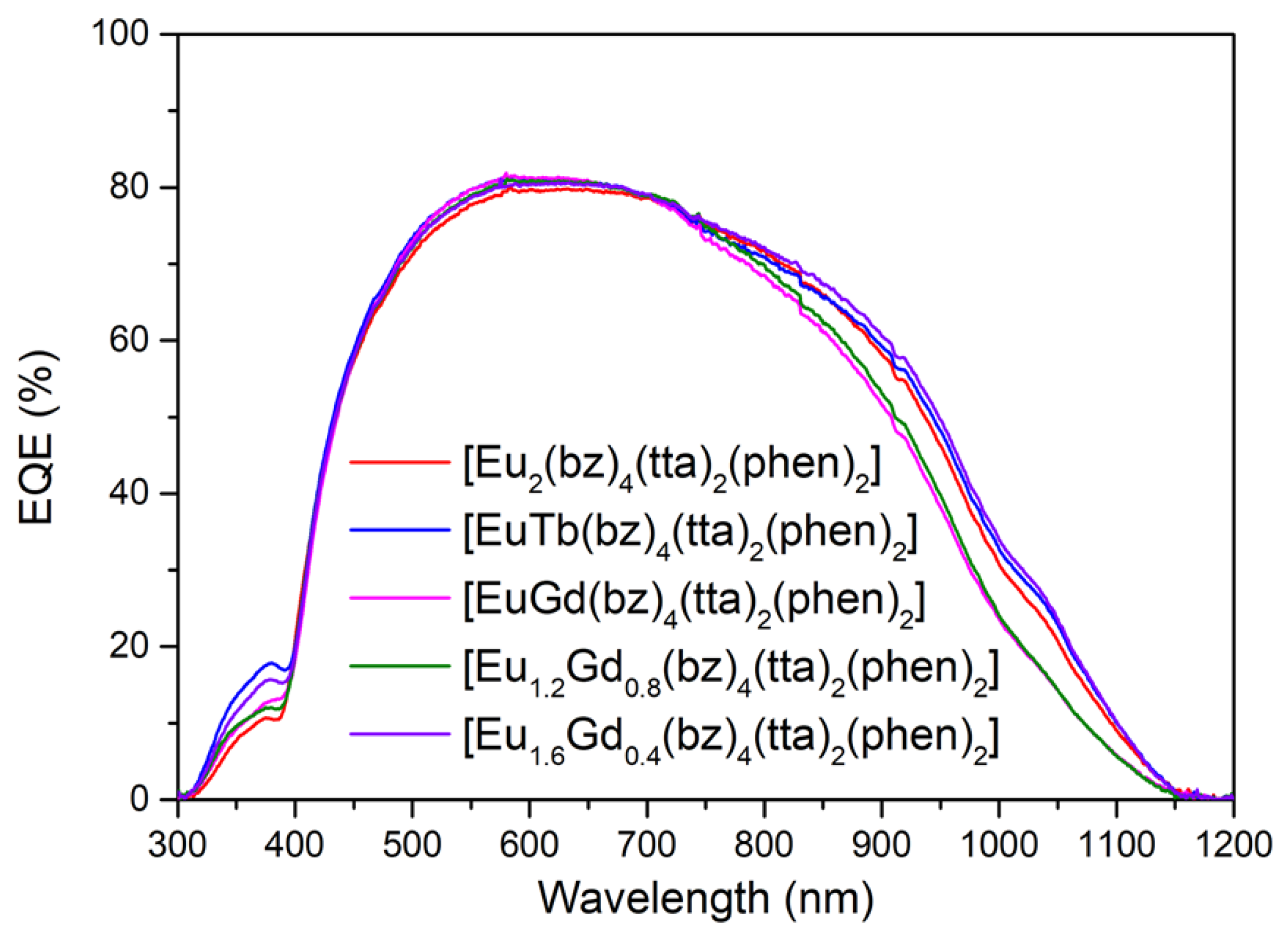
3.6.3. DS Layers Using Complexes C4–6
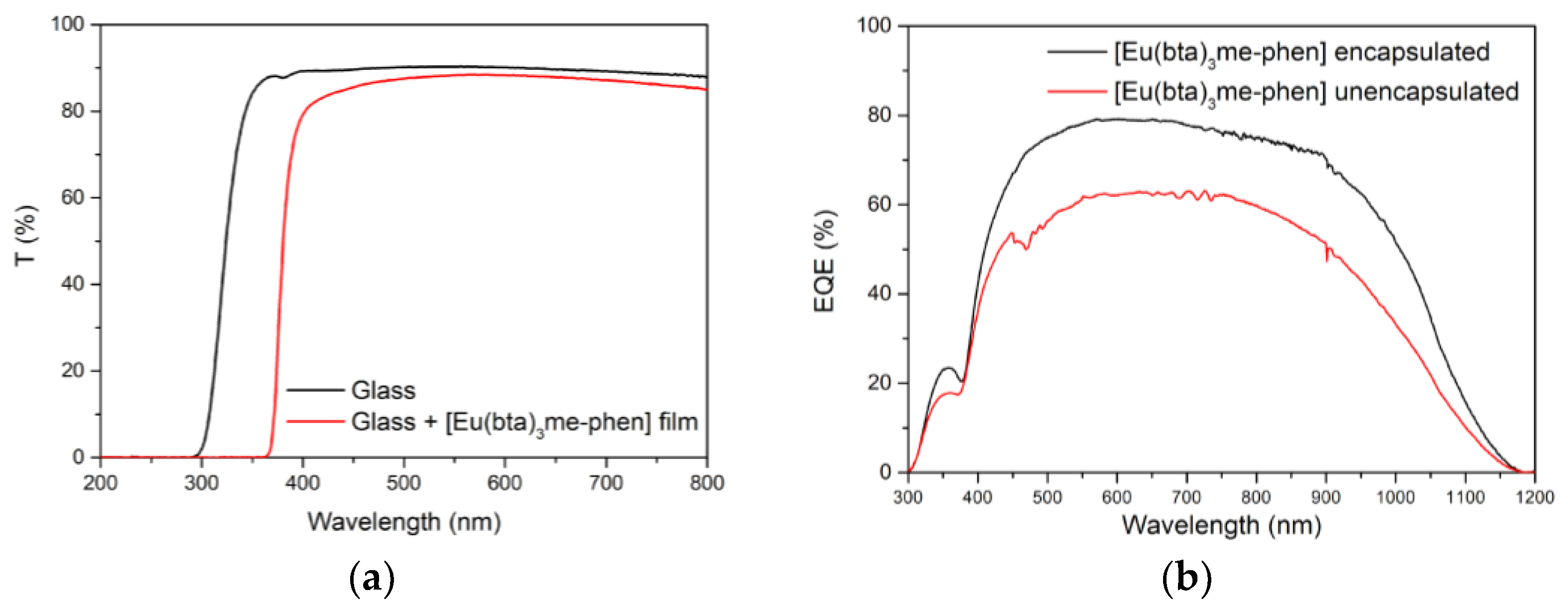
3.6.4. DS Layers Encapsulated Using Complexes C6 and C12–17
3.6.5. Accelerate Aging of DS Layers Encapsulated Using Complex C6
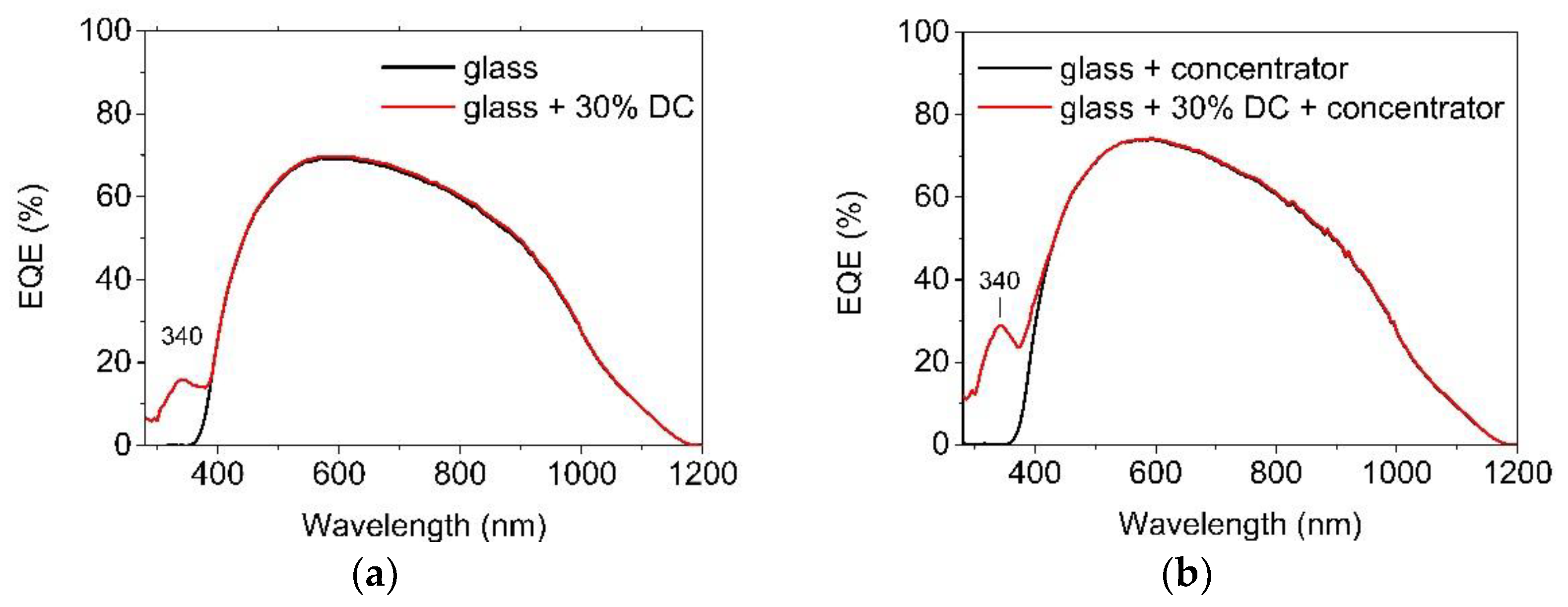
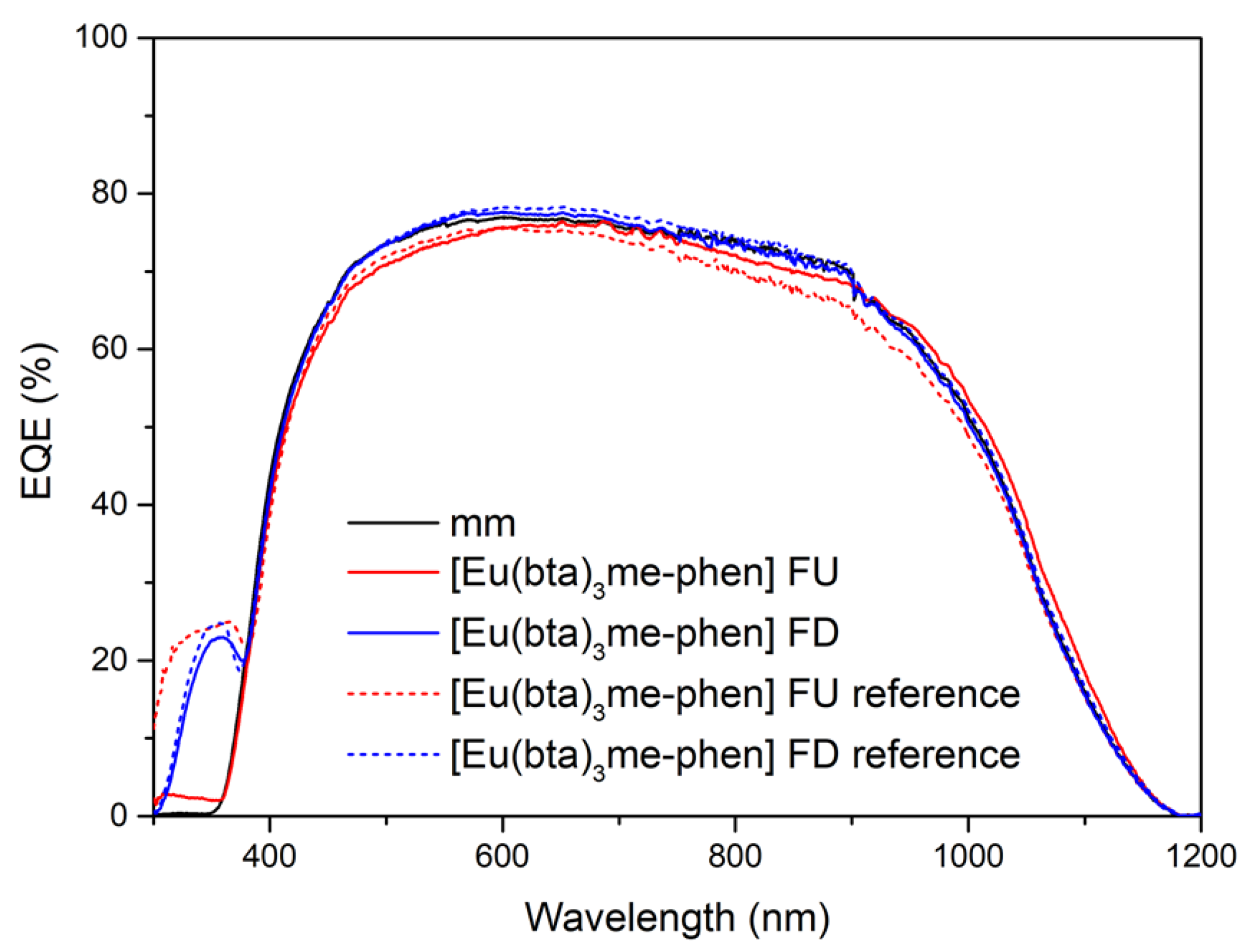
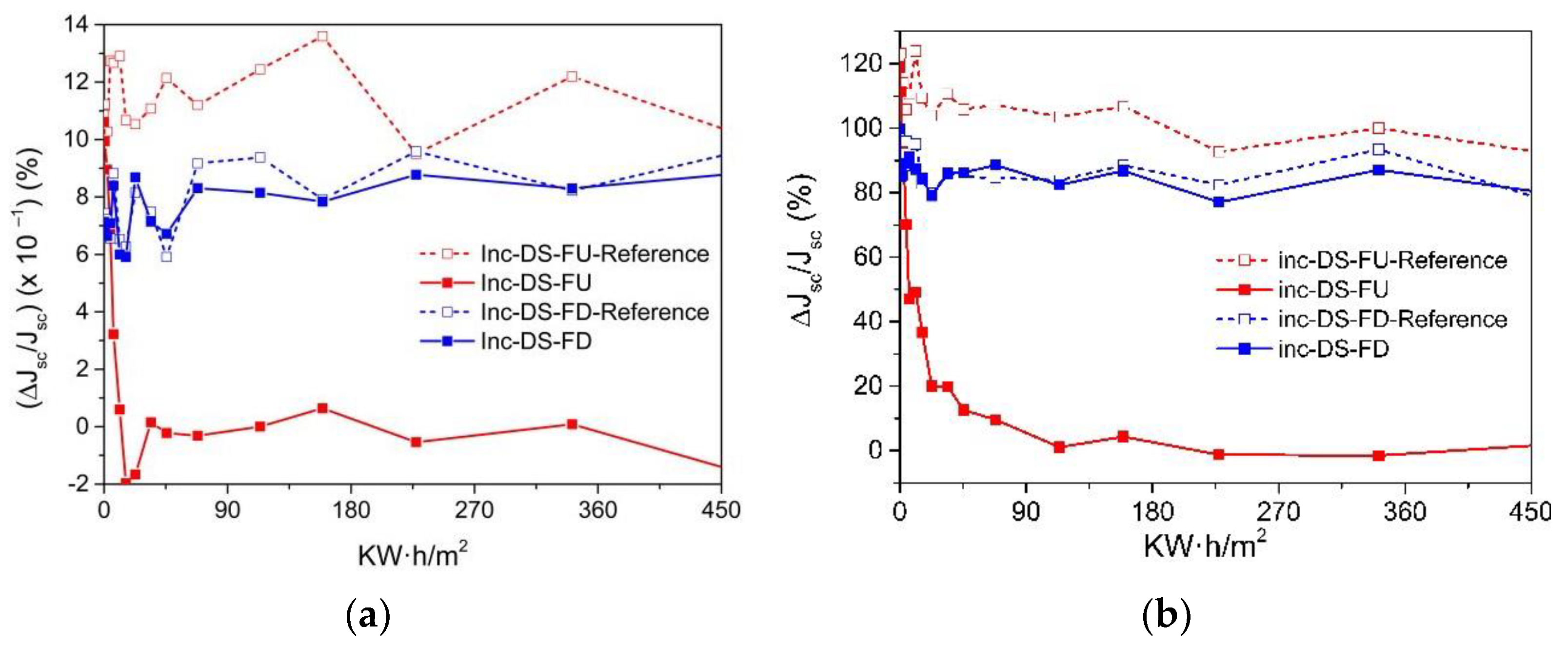
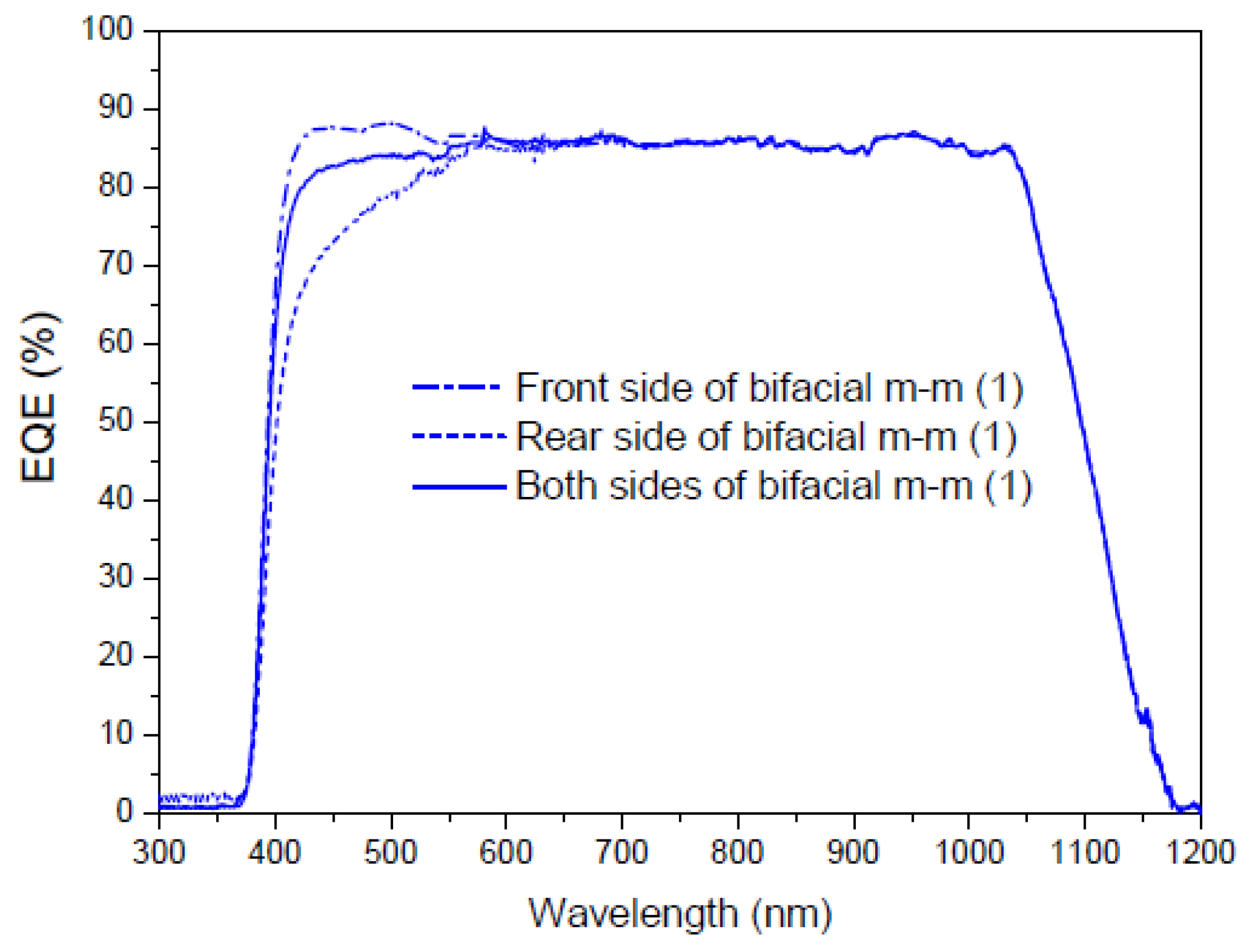
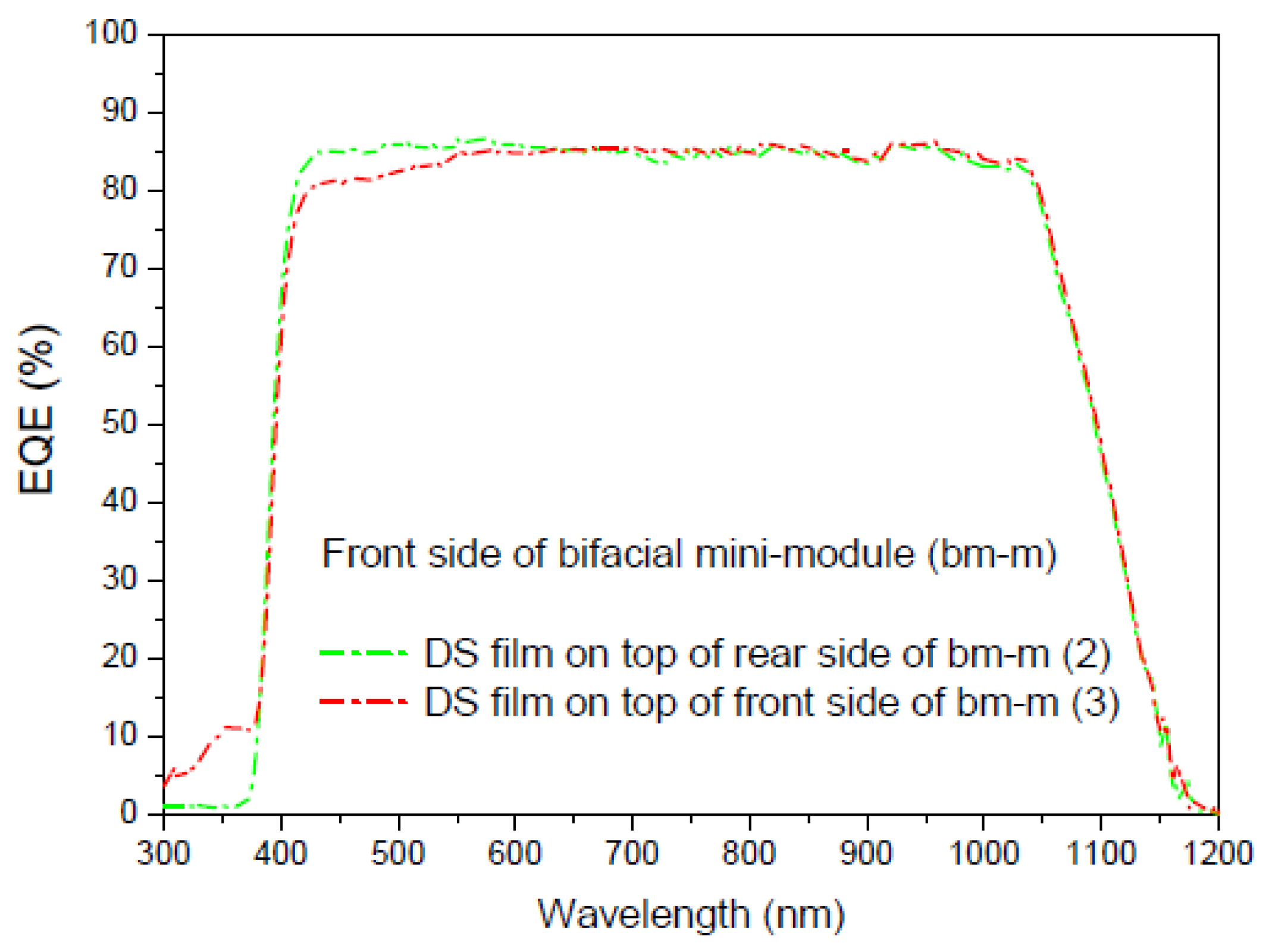
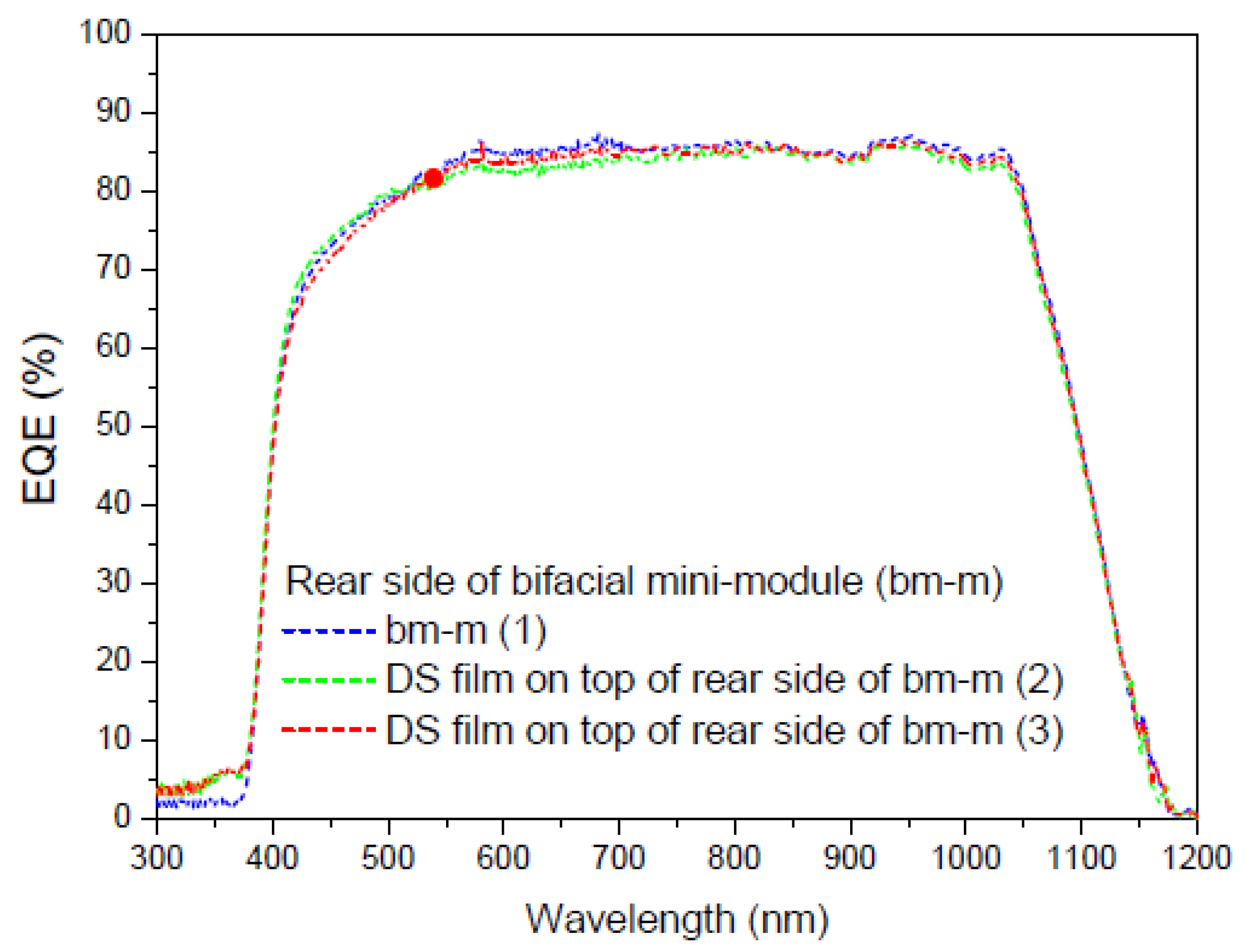
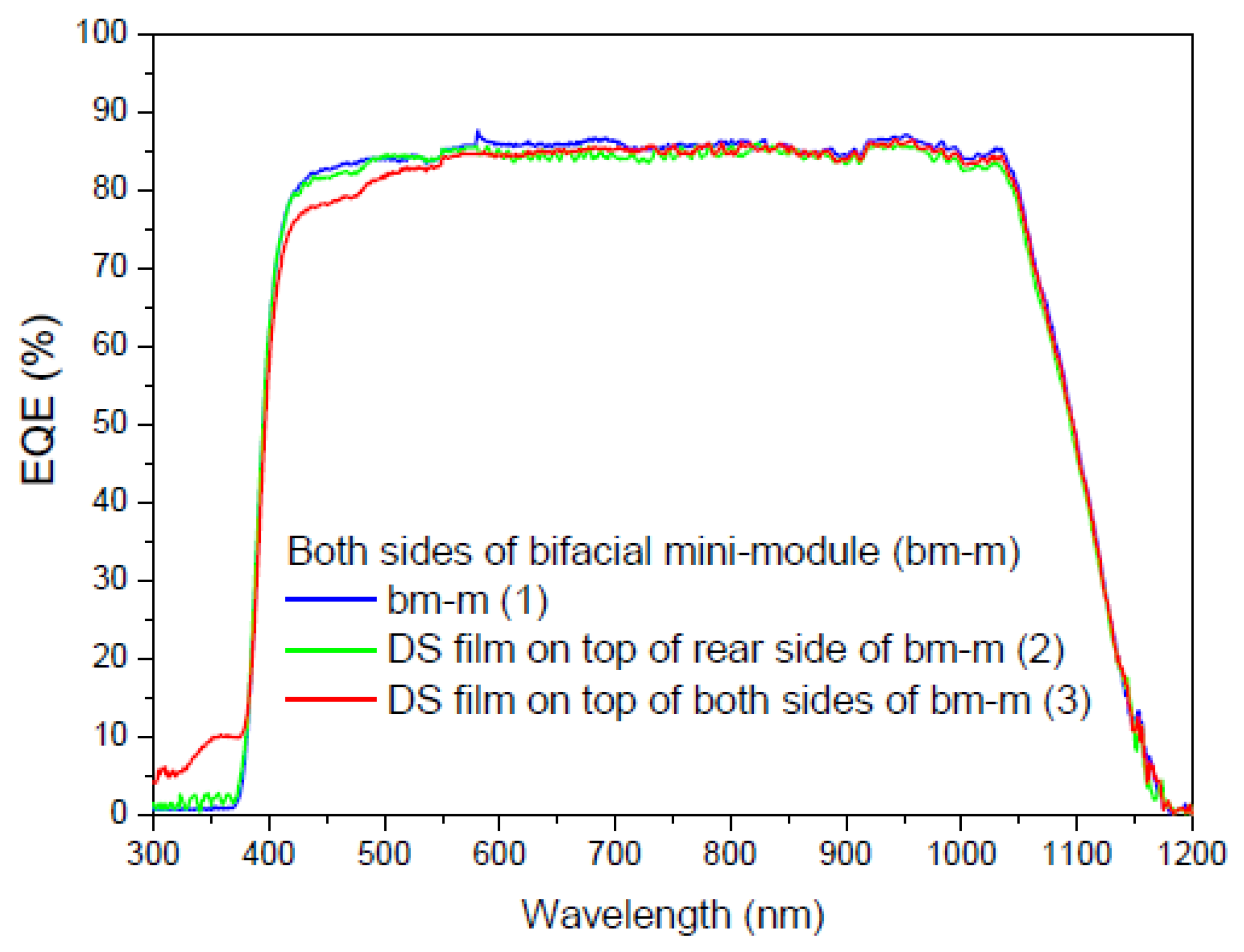
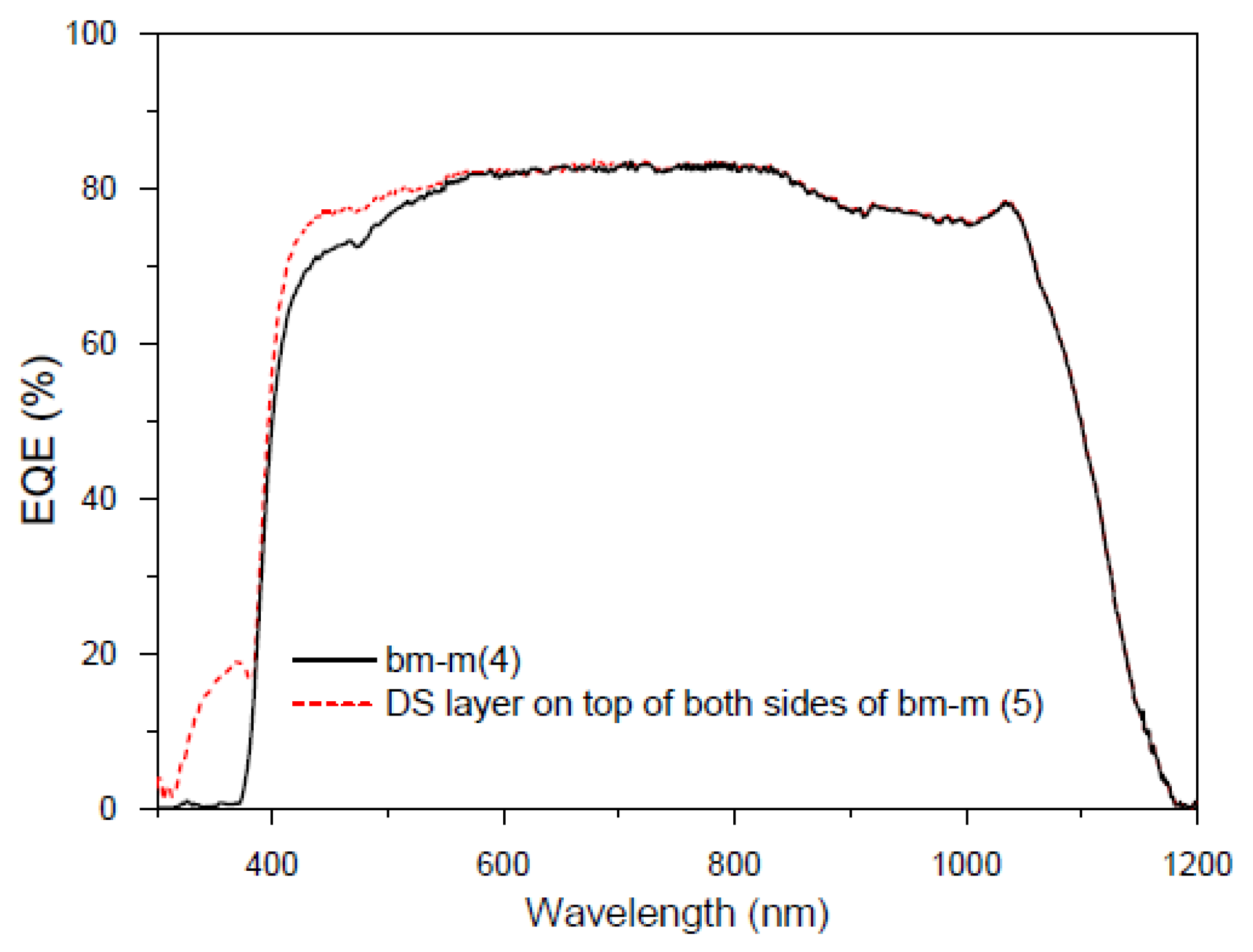
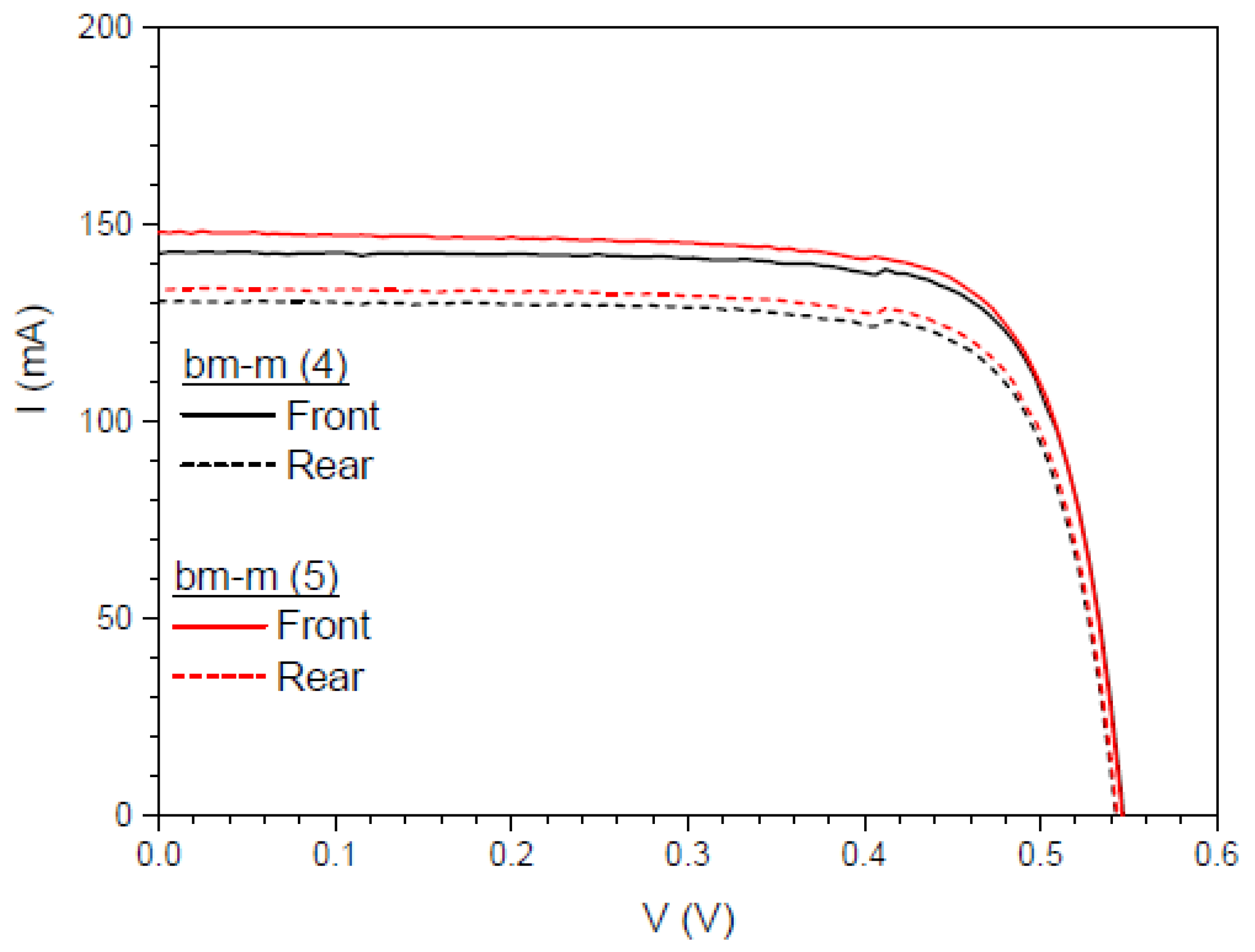
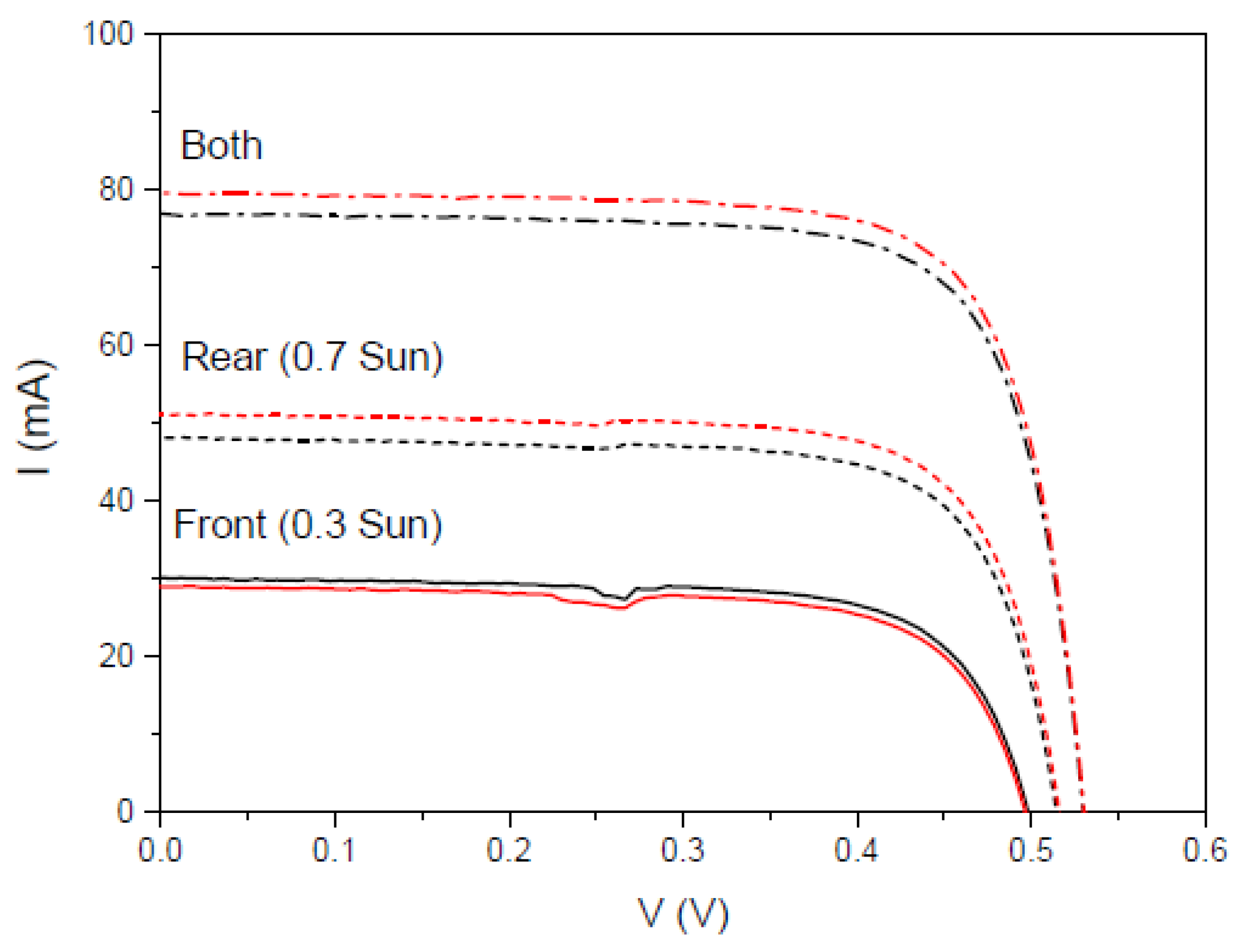
3.6.6. Application of Complex C6 to a Bifacial Solar Cell
First Experiment
Second Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Griffiths, D.J.; Schroeter, D.F. Introduction to Quantum Mechanics, 3rd ed.; Cambridge University Press: Cambridge, UK, 2018; pp. 279–313. [Google Scholar] [CrossRef]
- Tanner, P.A. Lanthanide Luminescence in Solids. In Lanthanide Luminescence: Photophysical, Analytical and Biological Aspects; Hänninen, P., Härmä, H., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 7, pp. 183–214. [Google Scholar] [CrossRef]
- Buenzli, J.C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293, 19–47. [Google Scholar] [CrossRef]
- SeethaLekshmi, S.; Ramya, A.R.; Reddy, M.L.P.; Varughese, S. Lanthanide complex-derived white-light emitting solids: A survey on design strategies. J. Photochem. Photobiol. A 2017, 33, 109–131. [Google Scholar] [CrossRef]
- Monteiro, J.H.S.K. Recent advances in luminescence imaging of biological systems using lanthanide (III) luminescent complexes. Molecules 2020, 25, 2089. [Google Scholar] [CrossRef]
- Wu, J.; Fujii, K.; Yashima, M.; Staykov, A.; Akbay, T.; Ishihara, T.; Kilner, J.A. A systematic evaluation of the role of lanthanide elements in functional complex oxides; implications for energy conversion devices. J. Mater. Chem. A 2018, 6, 11819–11829. [Google Scholar] [CrossRef]
- Bunzli, J.C.G.; Chauvin, A.S. Lanthanides in Solar Energy Conversion. In Handbook on the Physics and Chemistry of Rare Earths; Bunzli, J.C.G., Pecharsky, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 44, pp. 169–281. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, X.; Liu, S.; Zhang, B.; Lin, K.; Zhang, J.; Conibeer, G. A review on thermalization mechanisms and prospect absorber materials for the hot carrier solar cells. Sol. Energy Mater. Sol. Cells 2021, 225, 111073. [Google Scholar] [CrossRef]
- Wan, X.; Xu, Y.; Guo, H.; Shehzad, K.; Ali, A.; Liu, Y.; Luo, J. A self-powered high-performance graphene/silicon ultraviolet photodetector with ultra-shallow junction: Breaking the limit of silicon? 2D Mater. Appl. 2017, 1, 4. [Google Scholar] [CrossRef]
- Richards, B.S. Enhancing the performance of silicon solar cells via the application of passive luminescence conversion layers. Sol. Energy Mater. Sol. Cells 2006, 90, 2329–2337. [Google Scholar] [CrossRef]
- Grzeszkiewicz, K.; Marciniak, L.; Stręk, W.; Hreniak, D. Downconversion in Y2Si2O7: Pr3+, Yb3+ polymorphs for its possible application as luminescent concentrators in photovoltaic solar-cells. J. Lumin. 2016, 177, 172–177. [Google Scholar] [CrossRef]
- Hovel, H.J.; Hodgson, R.T.; Woodall, J.M. The effect of fluorescent wavelength shifting on solar cell spectral response. Sol. Energy Mater. Sol. Cells 1979, 2, 19–29. [Google Scholar] [CrossRef]
- Wegh, R.T.; Donker, H.; Oskam, K.D.; Meijerink, A. Visible quantum cutting in LiGdF4: Eu3+ through downconversion. Science 1999, 283, 663–666. [Google Scholar] [CrossRef]
- Dexter, D.L. Possibility of luminescent quantum yields greater than unity. Phys. Rev. 1957, 108, 630–633. [Google Scholar] [CrossRef]
- Trupke, T.; Green, M.A.; Würfel, P. Improving solar cell efficiencies by up-conversion of sub-band-gap light. J. Appl. Phys. 2002, 92, 4117–4122. [Google Scholar] [CrossRef]
- Badescu, V.; De Vos, A. Influence of some design parameters on the efficiency of solar cells with down-conversion and down shifting of high-energy photons. J. Appl. Phys. 2007, 102, 073102. [Google Scholar] [CrossRef]
- Green, A.P.; Buckley, A.R. Solid state concentration quenching of organic fluorophores in PMMA. Phys. Chem. Chem. Phys. 2015, 17, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Yahia-Ammar, A.; Nonat, A.M.; Boos, A.; Rehspringer, J.L.; Asfari, Z.; Charbonnière, L.J. Thin-coated water soluble CdTeS alloyed quantum dots as energy donors for highly efficient FRET. Dalton Trans. 2014, 43, 15583–15592. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef]
- Dieke, G.H.; Crosswhite, H.M.; Dunn, B. Emission spectra of the doubly and triply ionized rare earths. J. Opt. Soc. Am. 1961, 51, 820–827. [Google Scholar] [CrossRef]
- Peijzel, P.S.; Meijerink, A.; Wegh, R.T.; Reid, M.F.; Burdick, G.W. A complete 4fn energy level diagram for all trivalent lanthanide ions. J. Solid State Chem. 2005, 178, 448–453. [Google Scholar] [CrossRef]
- Nehra, K.; Dalal, A.; Hooda, A.; Bhagwan, S.; Saini, R.K.; Mari, B.; Singh, D. Lanthanides β-diketonate complexes as energy-efficient emissive materials: A review. J. Mol. Struct. 2022, 1249, 131531. [Google Scholar] [CrossRef]
- Saloutin, V.I.; Edilova, Y.O.; Kudyakova, Y.S.; Burgart, Y.V.; Bazhin, D.N. Heterometallic molecular architectures based on fluorinated β-diketone ligands. Molecules 2022, 27, 7894. [Google Scholar] [CrossRef]
- Monzón-Hierro, T.; Sanchiz, J.; González-Pérez, S.; González-Díaz, B.; Holinski, S.; Borchert, D.; Guerrero-Lemus, R. A new cost-effective polymeric film containing an Eu (III) complex acting as UV protector and down-converter for Si-based solar cells and modules. Sol. Energy Mater. Sol. Cells 2015, 136, 187–192. [Google Scholar] [CrossRef]
- González-Pérez, S.; Sanchiz, J.; Rodríguez, V.D.; Cañadillas-Ramallo, D.; González-Platas, J.; Borchert, D.; González-Díaz, B.; Hernández-Rodríguez, C.; Guerrero-Lemus, R. Highly luminescent film as enhancer of photovoltaic devices. J. Lumin. 2018, 201, 148–155. [Google Scholar] [CrossRef]
- Guerrero-Lemus, R.; Sanchiz, J.; Sierra, M.; Martín, I.R.; Hernández-Rodríguez, C.; Borchert, D. Alternative and fully experimental procedure for characterizing down-shifters placed on photovoltaic devices. Sol. Energy Mater. Sol. Cells 2018, 185, 312–317. [Google Scholar] [CrossRef]
- Guerrero-Lemus, R.; Sanchiz, J.; Sierra-Ramos, M.; Martín, I.R.; Hernández-Rodríguez, C.; Borchert, D. Downshifting maximization procedure applied to [Eu(bphen)(tta)3] at different concentrations applied to a photovoltaic device and covered with a hemispherical reflector. Sens. Actuators A Phys. 2018, 271, 60–65. [Google Scholar] [CrossRef]
- González-Díaz, B.; Saw, M.H.; Hernández-Rodríguez, C.; Sanchiz, J.; Khoo, Y.S.; Guerrero-Lemus, R. Evolution of the external quantum efficiency of Si-based PV minimodules with encapsulated down-shifters and aged under UV radiation. Mater. Sci. Eng. B 2020, 261, 114763. [Google Scholar] [CrossRef]
- González-Díaz, B.; Hernández-Rodríguez, C.; Cañadillas-Ramallo, D.; Sanchiz, J.; Guerrero-Lemus, R. Ageing evaluation of different polymeric Er 3+ down-shifters exposed to harsh outdoor conditions. In Proceedings of the 2018 IEEE 7th World Conference on Photovoltaic Energy Conversion (WCPEC) (A Joint Conference of 45th IEEE PVSC, 28th PVSEC & 34th EU PVSEC), Waikoloa, HI, USA, 10–15 June 2018; pp. 2882–2885. [Google Scholar] [CrossRef]
- Brito-Santos, G.; Gil-Hernández, B.; Hernández-Rodríguez, C.; González-Díaz, B.; Guerrero-Lemus, R.; Sanchiz, J. Degradation analysis of highly UV-resistant down-shifting layers for silicon-based PV module applications. Mater. Sci. Eng. B 2023, 288, 116207. [Google Scholar] [CrossRef]
- González-Díaz, B.; Sierra-Ramos, M.; Sanchiz, J.; Guerrero-Lemus, R. Durability analysis of the [Eu(bphen)(tta)3] down-shifter on Si-based PV modules exposed to extreme outdoor conditions. Sens. Actuators A Phys. 2018, 276, 312–319. [Google Scholar] [CrossRef]
- Brito-Santos, G.; Gil-Hernández, B.; Martín, I.R.; Guerrero-Lemus, R.; Sanchiz, J. Visible and NIR emitting Yb (iii) and Er (iii) complexes sensitized by β-diketonates and phenanthroline derivatives. RSC Adv. 2020, 10, 27815–27823. [Google Scholar] [CrossRef]
- Brito-Santos, G.; Hernández-Rodríguez, C.; Gil-Hernández, B.; González-Díaz, B.; Martín, I.R.; Guerrero-Lemus, R.; Sanchiz, J. Highly luminescent mixed-ligand bimetallic lanthanoid (III) complexes for photovoltaic applications. Dalton Trans. 2022, 51, 3146–3158. [Google Scholar] [CrossRef]
- Ho, W.-J.; Liu, J.-J.; Ke, B.-X. Characterization of Luminescent Down-Shifting Spectral Conversion Effects on Silicon Solar Cells with Various Combinations of Eu-Doped Phosphors. Materials 2022, 15, 452. [Google Scholar] [CrossRef]
- Correia, S.F.; Bastos, A.R.; Fu, L.; Carlos, L.D.; André, P.S.; Ferreira, R.A. Lanthanide-based downshifting layers tested in a solar car race. Opto-Electron. Adv. 2019, 2, 190006. [Google Scholar] [CrossRef]
- Le Donne, A.; Acciarri, M.; Narducci, D.; Marchionna, S.; Binetti, S. Encapsulating Eu3+ complex doped layers to improve Si-based solar cell efficiency. Prog. Photovolt. Res. Appl. 2009, 17, 519–525. [Google Scholar] [CrossRef]
- Leyre, S.; Proost, K.; Cappelle, J.; Durinck, G.; Hofkens, J.; Deconinck, G.; Hanselaer, P. Experimental validation of adding-doubling modeling of solar cells including luminescent down-shifting layers. J. Renew. Sustain. Energy. 2015, 7, 043130. [Google Scholar] [CrossRef]
- Klampaftis, E.; Congiu, M.; Robertson, N.; Richards, B.S. Luminescent ethylene vinyl acetate encapsulation layers for enhancing the short wavelength spectral response and efficiency of silicon photovoltaic modules. IEEE J. Photovolt. 2011, 1, 29–36. [Google Scholar] [CrossRef]
- Klampaftis, E.; Richards, B.S. Improvement in multi-crystalline silicon solar cell efficiency via addition of luminescent material to EVA encapsulation layer. Prog. Photovolt. Res. Appl. 2011, 19, 345–351. [Google Scholar] [CrossRef]
- Sayed, H.; Matar, Z.S.; Al-Dossari, M.; Amin, A.F.; Abd El-Gawaad, N.S.; Aly, A.H. The design and optimization of an anti-reflection coating and an intermediate reflective layer to enhance tandem solar cell photons capture. Crystals 2022, 12, 57. [Google Scholar] [CrossRef]
- Leyre, S.; Cappelle, J.; Durinck, G.; Abass, A.; Hofkens, J.; Deconinck, G.; Hanselaer, P. The use of the adding-doubling method for the optical optimization of planar luminescent down shifting layers for solar cells. Opt. Express. 2014, 22, A765–A778. [Google Scholar] [CrossRef]
- Mohan, B.V.; Mayandi, J.; Muniasamy, K.; Veerapandy, V. Assessment of the design efficacy of Eu2+ ion embedded thick scattering layers for operational photovoltaics. Appl. Surf. Sci. Adv. 2021, 4, 100067. [Google Scholar] [CrossRef]
- McIntosh, K.R.; Lau, G.; Cotsell, J.N.; Hanton, K.; Bätzner, D.L.; Bettiol, F.; Richards, B.S. Increase in external quantum efficiency of encapsulated silicon solar cells from a luminescent down-shifting layer. Prog. Photovolt. Res. Appl. 2009, 17, 191–197. [Google Scholar] [CrossRef]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef]
- Farah Khaleda, M.Z.; Vengadaesvaran, B.; Rahim, N.A. Spectral response and quantum efficiency evaluation of solar cells: A review. In Energy Materials; Dhoble, S.J., Kalyani, N.T., Vengadaesvaran, B., Arof, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 525–566. [Google Scholar] [CrossRef]
- De la Mora, M.B.; Amelines-Sarria, O.; Monroy, B.M.; Hernández-Pérez, C.D.; Lugo, J.E. Materials for downconversion in solar cells: Perspectives and challenges. Sol. Energy Mater. Sol. Cells 2017, 165, 59–71. [Google Scholar] [CrossRef]
- Satpute, N.S.; Mehare, C.M.; Tiwari, A.; Swart, H.C.; Dhoble, S.J. Synthesis and Luminescence Characterization of Downconversion and Downshifting Phosphor for Efficiency Enhancement of Solar Cells: Perspectives and Challenges. ACS Appl. Electron. Mater. 2022, 4, 3354–3391. [Google Scholar] [CrossRef]
- Lian, H.; Hou, Z.; Shang, M.; Geng, D.; Zhang, Y.; Lin, J. Rare earth ions doped phosphors for improving efficiencies of solar cells. Energy 2013, 57, 270–283. [Google Scholar] [CrossRef]
- Fix, T.; Ferblantier, G.; Rinnert, H.; Slaoui, A. Evaluation of the effective quantum efficiency of photon conversion layers placed on solar cells. Sol. Energy Mater. Sol. Cells 2015, 132, 191–195. [Google Scholar] [CrossRef]
- Kennedy, M.; Ahmed, H.; Doran, J.; Norton, B.; Bosch-Jimenez, P.; Pirriera, M.D.; Puigdollers, J. Large Stokes shift downshifting Eu (III) films as efficiency enhancing UV blocking layers for dye sensitized solar cells. Phys. Status Solidi (A) 2015, 212, 203–210. [Google Scholar] [CrossRef]
- Wei, C.; Ma, L.; Wei, H.; Liu, Z.; Bian, Z.; Huang, C. Advances in luminescent lanthanide complexes and applications. Sci. China Technol. Sci. 2018, 61, 1265–1285. [Google Scholar] [CrossRef]
- Lopez-Delgado, R.; Zhou, Y.; Zazueta-Raynaud, A.; Zhao, H.; Pelayo, J.E.; Vomiero, A.; Ayon, A. Enhanced conversion efficiency in Si solar cells employing photoluminescent down-shifting CdSe/CdS core/shell quantum dots. Sci. Rep. 2017, 7, 14104. [Google Scholar] [CrossRef] [PubMed]
- Dumont, L.; Cardin, J.; Labbé, C.; Frilay, C.; Anglade, P.M.; Yu, I.S.; Gourbilleau, F. First down converter multilayers integration in an industrial Si solar cell process. Prog. Photovolt. Res. Appl. 2019, 27, 152–162. [Google Scholar] [CrossRef]
- Debnath, G.H.; Mukherjee, P.; Waldeck, D.H. Optimizing the key variables to generate host sensitized lanthanide doped semiconductor nanoparticle luminophores. J. Phys. Chem. C 2020, 124, 26495–26517. [Google Scholar] [CrossRef]
- Tawalare, P.K. Optimizing photovoltaic conversion of solar energy. AIP Adv. 2021, 11, 100701. [Google Scholar] [CrossRef]
- Devi, R.; Rajendran, M.; Singh, K.; Pal, R.; Vaidyanathan, S. Smart luminescent molecular europium complexes and their versatile applications. J. Mater. Chem. C 2021, 9, 6618–6633. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.; Sung, S.J.; Chang, C.H. Downshifting and antireflective thin films for solar module power enhancement. Mater. Des. 2021, 201, 109454. [Google Scholar] [CrossRef]
- Li, P.; Li, H. Recent progress in the lanthanide-complexes based luminescent hybrid materials. Coord. Chem. Rev. 2021, 441, 213988. [Google Scholar] [CrossRef]
- Datt, R.; Bishnoi, S.; Lee HK, H.; Arya, S.; Gupta, S.; Gupta, V.; Tsoi, W.C. Down-conversion materials for organic solar cells: Progress, challenges, and perspectives. Aggregate 2022, 3, e185. [Google Scholar] [CrossRef]
- Yang, L.; Luo, J.; Gao, L.; Song, B.; Tang, J. Inorganic Lanthanide Compounds with f–d Transition: From Materials to Electroluminescence Devices. J. Phys. Chem. Lett. 2022, 13, 4365–4373. [Google Scholar] [CrossRef]
- Sekar, R.; Ravitchandiran, A.; Angaiah, S. Recent Advances and Challenges in Light Conversion Phosphor Materials for Third-Generation Quantum-Dot-Sensitized Photovoltaics. ACS Omega 2022, 7, 35351–35360. [Google Scholar] [CrossRef]
- Yu, D.; Yu, T.; Lin, H.; Zhuang, S.; Zhang, D. Recent advances in luminescent downconversion: New materials, techniques, and applications in solar cells. Adv. Opt. Mater. 2022, 10, 2200014. [Google Scholar] [CrossRef]
- Galico, D.A.; Calado, C.; Murugesu, M. Lanthanide Molecular Cluster-Aggregates as the Next Generation of Optical Materials. Chem. Sci. 2023, 14, 5827–5841. [Google Scholar] [CrossRef]
- Zhang, J.; Ming, C.; Song, F. Spectrum conversion by luminescent glass for improving efficiency of solar cells. J. Non-Cryst. Solids 2023, 606, 122217. [Google Scholar] [CrossRef]
- Ramanujam, J.; Verma, A.; González-Díaz, B.; Guerrero-Lemus, R.; Del Cañizo, C.; Garcia-Tabares, E.; Cirlin, G.E. Inorganic photovoltaics–planar and nanostructured devices. Prog. Mater. Sci. 2016, 82, 294–404. [Google Scholar] [CrossRef]
- International Energy Agency. Available online: https://www.iea.org/reports/solar-pv (accessed on 13 June 2023).
- Giannouli, M. Current status of emerging PV technologies: A comparative study of dye-sensitized, organic, and perovskite solar cells. Int. J. Photoenergy 2021, 2021, 6692858. [Google Scholar] [CrossRef]
- GM Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- ASTM G173-03; Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020. [CrossRef]
- Hu, M.-L.; Huang, Z.-Y.; Cheng, Y.-Q.; Wang, S.; Lin, J.-J.; Hu, Y.; Xu, D.-J.; Xu, Y.-Z. Crystal structure and fluorescence spectrum of the complex [Eu(III)(TTA)3(phen)]. Chin. J. Chem. 1999, 17, 637–643. [Google Scholar] [CrossRef]
- Blieske, U.; Stollwerck, G. Glass and Other Encapsulation Materials. In Semiconductors and Semimetals; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 89, pp. 199–258. [Google Scholar] [CrossRef]
- Sun, Q.; Yan, P.; Niu, W.; Chu, W.; Yao, X.; An, G.; Li, G. NIR luminescence of a series of benzoyl trifluoroacetone erbium complexes. RSC Adv. 2015, 5, 65856–65861. [Google Scholar] [CrossRef]
- Gavriluta, A.; Fix, T.; Nonat, A.; Slaoui, A.; Guillemoles, J.F.; Charbonnière, L.J. EuIII-Based Nanolayers as Highly Efficient Down-shifters for CIGS Solar Cells. Eur. J. Inorg. Chem. 2017, 44, 5318–5326. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Y.; Wei, Y.; Chen, L.; Zhou, X.; Liu, S. Determining the Energy Gap between the S1 and T1 States of Thermally Activated Delayed Fluorescence Molecular Systems Using Transient Fluorescence Spectroscopy. J. Phys. Chem. Lett. 2022, 13, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Lahiri, D.; Ameerunisha Begum, M.S.; Saha, S.; Majumdar, R.; Dighe, R.R.; Chakravarty, A.R. Photocytotoxic lanthanum (III) and gadolinium (III) complexes of phenanthroline bases showing light-induced DNA cleavage activity. Inorg. Chem. 2010, 49, 4036–4045. [Google Scholar] [CrossRef]
- Li, H.F.; Yan, P.F.; Chen, P.; Wang, Y.; Xu, H.; Li, G.M. Highly luminescent bis-diketone lanthanide complexes with triple-stranded dinuclear structure. Dalton Trans. 2012, 41, 900–907. [Google Scholar] [CrossRef]
- Dawson, W.R.; Kropp, J.L.; Windsor, M.W. Internal-Energy-Transfer Efficiencies in Eu3+ and Tb3+ Chelates Using Excitation to Selected Ion Levels. J. Chem. Phys. 1966, 45, 2410–2418. [Google Scholar] [CrossRef]
- González-Pérez, S.; Martín, I.R.; Lahoz, F.; HAro-González, P.; Herreros, J. Local crystallization in an oxyfluoride glass doped with Er3+ ions using a continuous argon laser. Appl. Phys. A 2008, 93, 983–986. [Google Scholar] [CrossRef]
- Alexander, O.T.; Kroon, R.E.; Brink, A.; Visser, H.G. Symmetry correlations between crystallographic and photoluminescence study of ternary β-diketone europium (III) based complexes using 1, 10-phenanthroline as the ancillary ligand. Dalton Trans. 2019, 48, 16074–16082. [Google Scholar] [CrossRef]
- Silva, F.G.E.; Menezes, J.F.S.; Rocha, G.B.; Alves, S.; Brito, H.F.D.; Longo, R.L.; Malta, O.L. Emission quantum yield of europium (III) mixed complexes with thenoyltrifluoroacetonate and some aromatic ligands. J. Alloys Compd. 2000, 303, 364–370. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Zheng, W.; Huang, W.; LI, C.-H.; You, X.-Z. Improving spectral response of monocrystalline silicon photovoltaic modules using high efficient luminescent down-shifting Eu3+ complexes. Prog. Photovolt. Res. Appl. 2013, 21, 668–675. [Google Scholar] [CrossRef]
- Liang, T.S.; Pravettoni, M.; Deline, C.; Stein, J.S.; Kopecek, R.; Singh, J.P.; Khoo, Y.S. A review of crystalline silicon bifacial photovoltaic performance characterisation and simulation. Energy Environ. Sci. 2019, 12, 116–148. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Sakamoto, M.; Koyama, M.; Tsutsui, K.; Uematsu, T.; Yazawa, Y. Characteristics of bifacial solar cells under bifacial illumination with various intensity levels. Prog. Photovolt. Res. Appl. 2001, 9, 1–13. [Google Scholar] [CrossRef]

| Complex | Molecular Structure | Schematic View |
|---|---|---|
| [Eu2(phen)2(bz)6] (C1) | 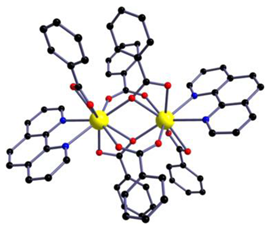 | 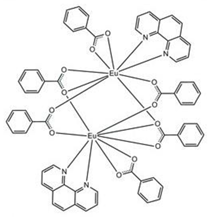 |
| [Eu2(bphen)2(bz)6] (C2) | 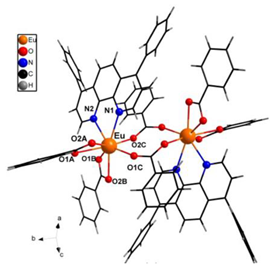 | (*) |
| [Eu(tta)3bphen] (C3) | (**) | 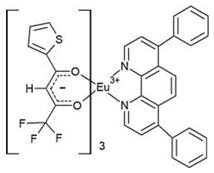 |
| [Eu(bta)3pyz-phen] (C4) | 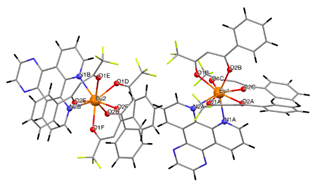 | (***) |
| [Eu(tta)3pyz-phen] (C5) | 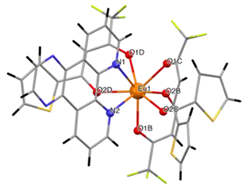 | |
| [Eu(bta)3(me-phen] (C6) | 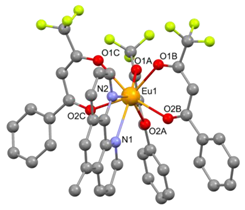 | 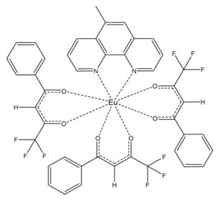 |
| Complex | Molecular Structure |
|---|---|
| [Yb(bta)3(me-phen)] (C8) (*) | 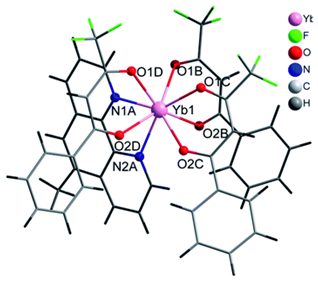 |
| [Yb(bta)3(pyz-phen)] (C10) | 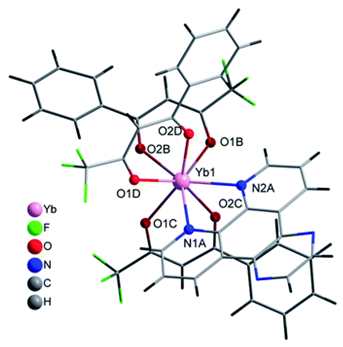 |
| [Er(tta)3(pyz-phen)] (C11) | 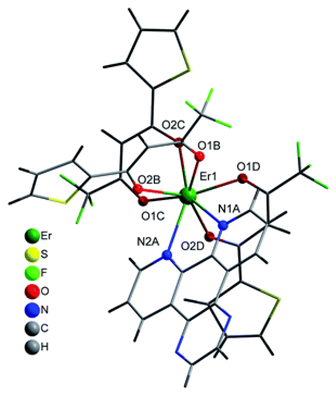 |
| Complex | Molecular Structure |
|---|---|
| [Eu2(bz)4(tta)2(phen)2] (C12) (*) | 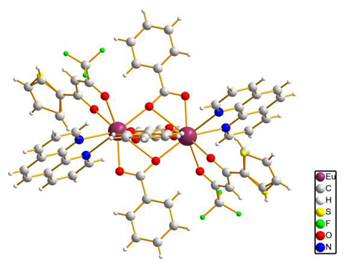 |
| Complex | λexc (nm) | λem (nm) | τ (ms) | PLQY (%) | Reference |
|---|---|---|---|---|---|
| C1 | 340 | 614 | 1.02 | 48 | [25] |
| C2 | 280 | 0.96 | 50 | this work, [79] | |
| C3 | 340 | 612 | 0.98 | 69 | this work, [26,80] |
| C4 | 375 | 0.53 | this work | ||
| C5 | 375 | 0.81 | |||
| C6 | 350 | 0.54 | |||
| C7 | 375 | 1550 | 1.6 × 10−3 | this work, [32] | |
| C8 | 980 | 7.2 × 10−3 | |||
| C10 | 980 | 6.1 × 10−3 | |||
| C11 | 1550 | 1.3 × 10−3 | |||
| C12 | 350 | 612 | 0.60 | 63 | this work, [33] |
| C14 | 0.75 | 66 | |||
| C15 | 0.65 | 50 | |||
| C16 | 0.72 | 70 | |||
| C17 | 0.74 | 74 |
| Complex Used | (Δη/η) (%) (285–350 nm) | (Δη/η) (%) (285–1200 nm) |
|---|---|---|
| C1 (tape-cast) | 279.0 | 0.1 |
| C1 (spin-coated) | 143.0 | <0.1 |
| C1 (tape-cast and reflector) | 347.0 | 0.18 |
| Complex | ΔEQE (%) a | Jsc (mA/cm2) b | ΔJsc (mA/cm2) b | (Δη/η) (%) b | Jsc (mA/cm2) c | ΔJsc (mA/cm2) c | (Δη/η) (%) c | |
|---|---|---|---|---|---|---|---|---|
| m-m used for C6 | — | 28.40 | 0.18 | — | ||||
| C6 | 10.0 | 28.70 | 0.30 | 1.05 | 0.29 | 0.11 | 61.1 | |
| m-m used for C12–17 | — | d 25.80 | e 26.86 | 0.05 | — | |||
| C12 | 9.7 | 25.53 | 26.46 | — | — | 0.13 | 0.08 | 160 |
| C13 | 11.4 | 24.79 | 25.60 | — | — | 0.15 | 0.10 | 200 |
| C14 | 16.3 | 26.69 | 26.95 | 0.89; 0.09 | 3.45; 0.33 | 0.20 | 0.15 | 300 |
| C16 | 10.9 | 25.40 | 25.78 | — | — | 0.14 | 0.09 | 180 |
| C17 | 14.3 | 26.04 | 27.04 | 0.24; 0.18 | 0.93; 0.67 | 0.17 | 0.12 | 240 |
| Device | Jsc (mA/cm2) Front | Jsc (mA/cm2) Rear | Bifaciality Factor (%) |
|---|---|---|---|
| 36.18 | 35.15 | 97.15 |
| 19.00 | 14.49 | 76.26 |
| 35.71 | 34.69 | 97.14 |
| 19.56 | 17.99 | 91.97 |
| 35.60 | 34.85 | 97.89 |
| 25.04 | 17.16 | 68.53 |
| Device | Isc (mA) Front | Isc (mA) Rear | BF (%) | Pmax (mW) Front | Pmax (mW) Rear | η (%) Front | η (%) Rear |
|---|---|---|---|---|---|---|---|
| bm-m(4) | 142.96 | 130.58 | 91.34 | 60.22 | 54.37 | 15.05 | 13.59 |
| bm-m(5) | 148.18 | 133.80 | 90.29 | 61.39 | 55.66 | 15.35 | 13.91 |
| Device | Isc (mA) Front | Isc (mA) Rear | Isc (mA) Both | SR (%) | Pmax (mW) Front | Pmax (mW) Rear | Pmax (mW) Both |
|---|---|---|---|---|---|---|---|
| bm-m(4) | 30.04 | 48.09 | 76.85 | −1.6 | 10.66 | 18.20 | 30.66 |
| bm-m(5) | 28.95 | 51.13 | 79.53 | −0.7 | 10.16 | 19.46 | 31.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito-Santos, G.; Hernández-Rodríguez, C.; Gil-Hernández, B.; Sanchiz, J.; Martín, I.R.; González-Díaz, B.; Guerrero-Lemus, R. Exploring Ln(III)-Ion-Based Luminescent Species as Down-Shifters for Photovoltaic Solar Cells. Materials 2023, 16, 5068. https://doi.org/10.3390/ma16145068
Brito-Santos G, Hernández-Rodríguez C, Gil-Hernández B, Sanchiz J, Martín IR, González-Díaz B, Guerrero-Lemus R. Exploring Ln(III)-Ion-Based Luminescent Species as Down-Shifters for Photovoltaic Solar Cells. Materials. 2023; 16(14):5068. https://doi.org/10.3390/ma16145068
Chicago/Turabian StyleBrito-Santos, Gabriela, Cecilio Hernández-Rodríguez, Beatriz Gil-Hernández, Joaquín Sanchiz, Inocencio R. Martín, Benjamín González-Díaz, and Ricardo Guerrero-Lemus. 2023. "Exploring Ln(III)-Ion-Based Luminescent Species as Down-Shifters for Photovoltaic Solar Cells" Materials 16, no. 14: 5068. https://doi.org/10.3390/ma16145068
APA StyleBrito-Santos, G., Hernández-Rodríguez, C., Gil-Hernández, B., Sanchiz, J., Martín, I. R., González-Díaz, B., & Guerrero-Lemus, R. (2023). Exploring Ln(III)-Ion-Based Luminescent Species as Down-Shifters for Photovoltaic Solar Cells. Materials, 16(14), 5068. https://doi.org/10.3390/ma16145068










