Improvement of Plasterboard Properties by the Control of Polymethylhydrosiloxane Dosage, Stirring Time, and Drying Temperature Applied to the Calcium Sulfate Hemihydrate and Water Mixture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Experimental Methodology
- D: minimum 0.2%, maximum 1.0%, midpoint 0.6%;
- H: minimum 20 s, maximum 60 s, midpoint 40 s;
- T: minimum 130 °C, maximum 150 °C, midpoint 140 °C.
2.3. Experimental Design
3. Results and Discussion
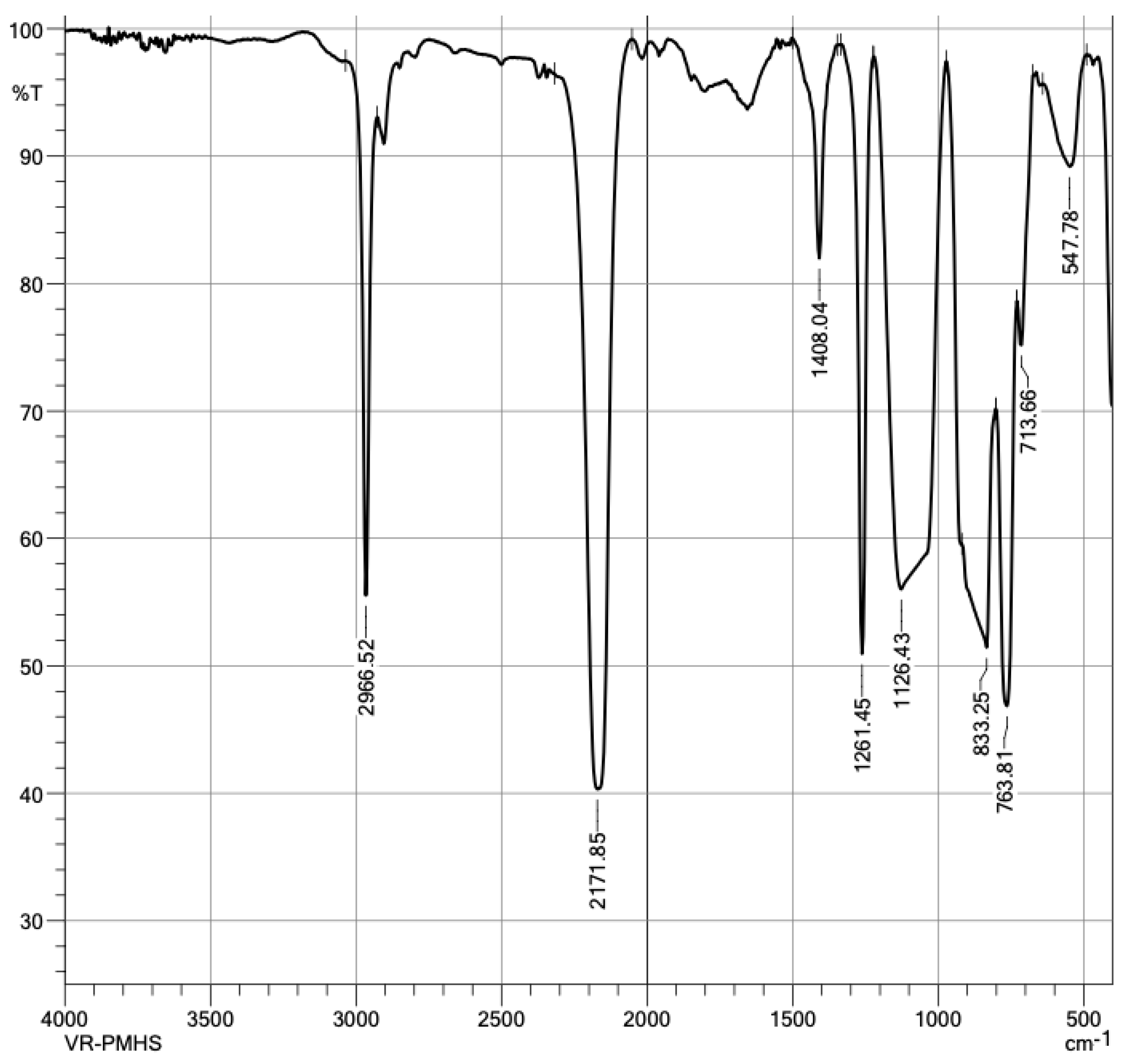
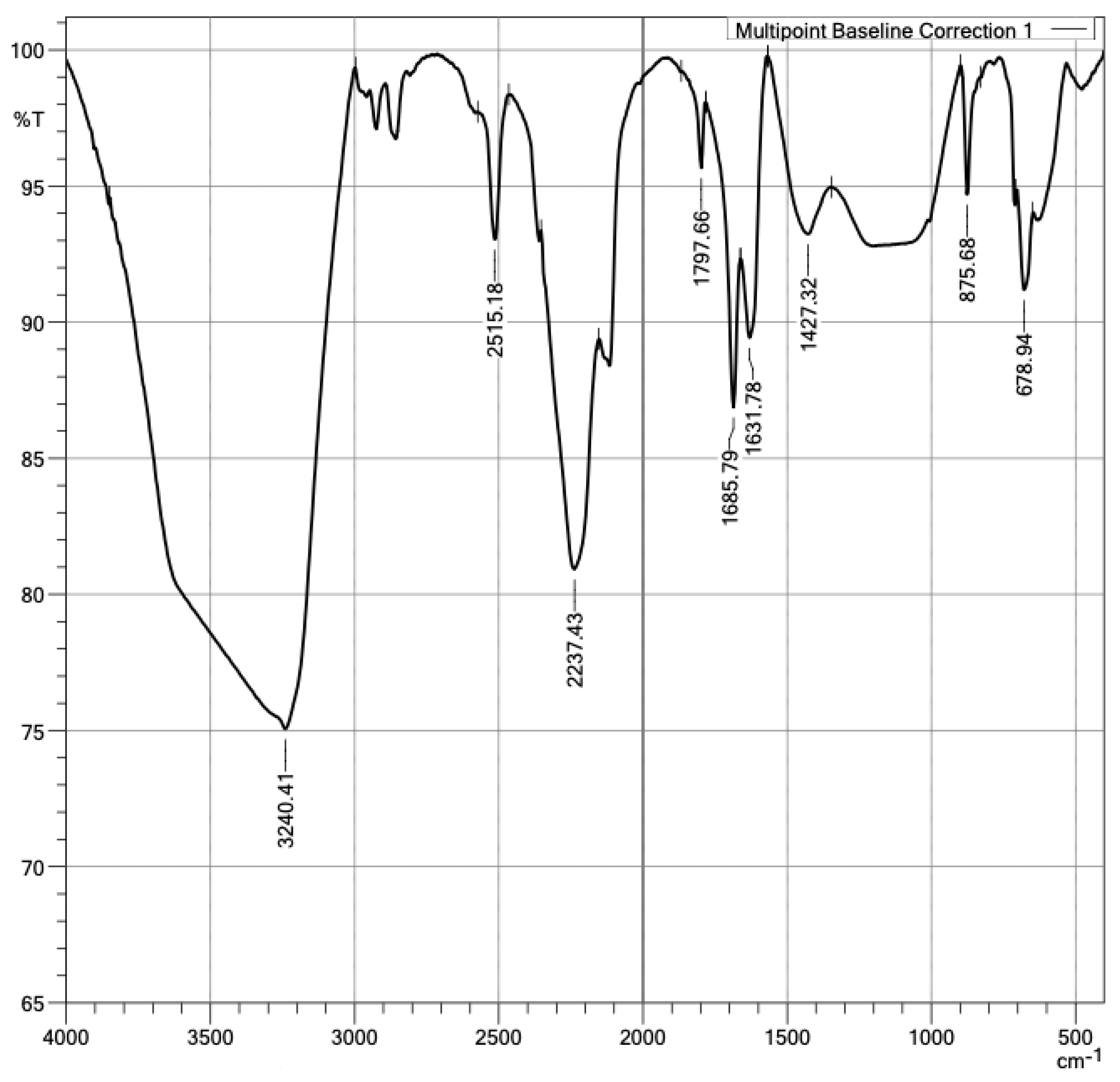
3.1. Component Characterization
3.2. Results Analysis
3.2.1. Experiment Results
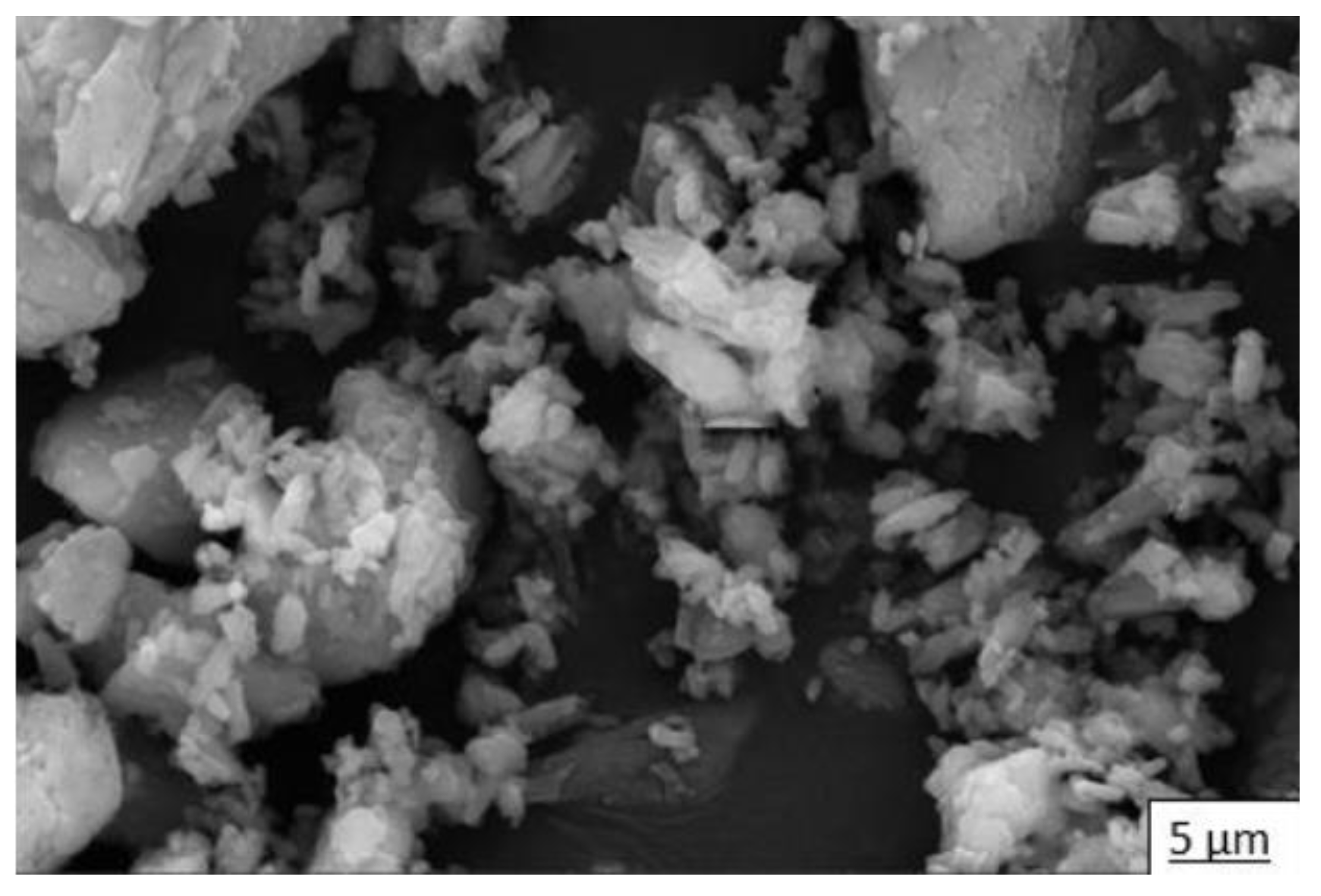
3.2.2. Crystal Morphology
- Only the stirring time (H) effect was evaluated by mixing for 20 s, 40 s, and 60 s. The reactions were stopped by adding isopropyl alcohol, washing, and drying. The DH powder obtained was analyzed by SEM, showing a length–width ratio of 50 crystals. Although it is observed that the average of this ratio decreases when stirring time increases, statistically it does not present a significant difference.
- The stirring time and drying temperature synergetic effect over the length–width ratio was also evaluated. A significant difference at medium and maximum levels in morphology with respect to the reference was observed.
- The effect of the length–width ratio was finally evaluated when all three factors (D, H, T) were changed simultaneously. In this case, a significant effect on the morphology between without and with PMHS addition at the medium and maximum levels of stirring time and temperature was observed.
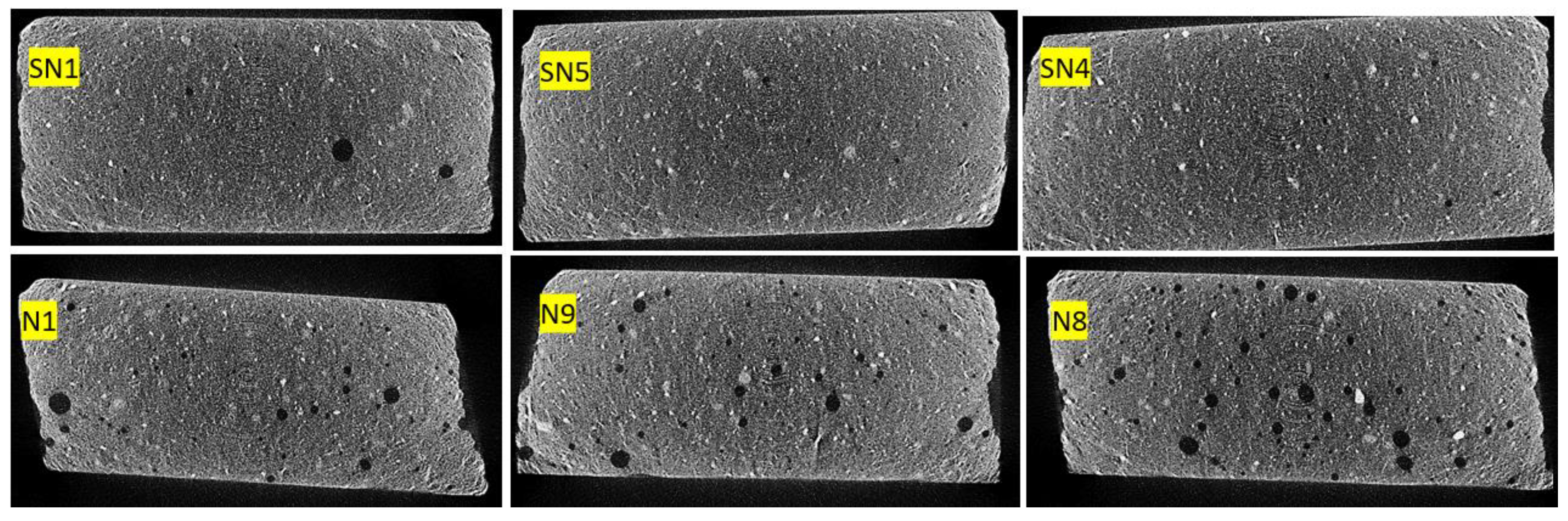
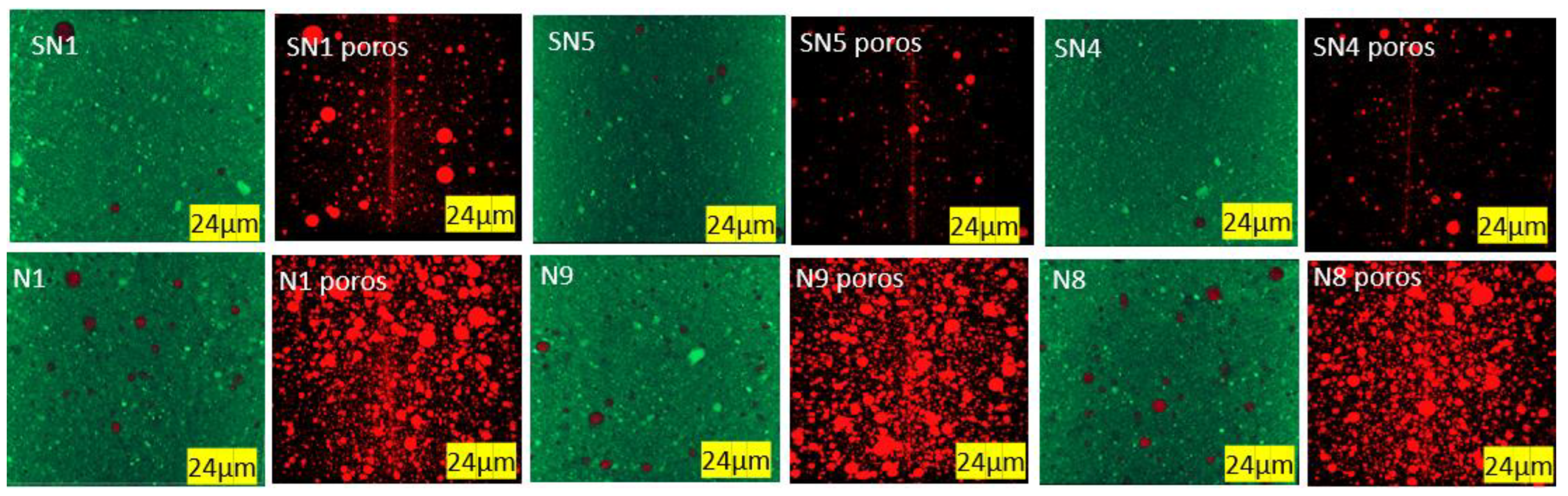
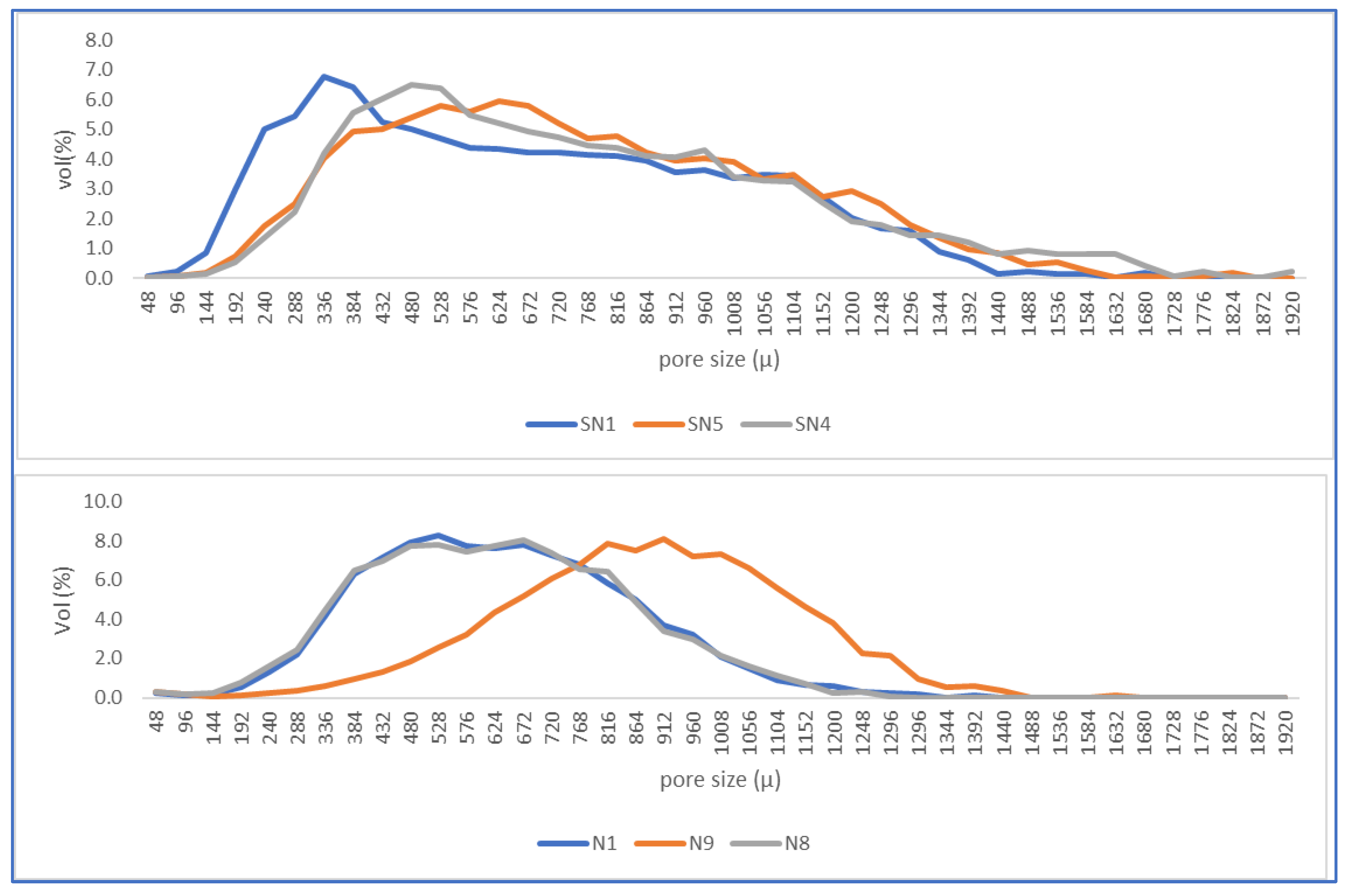
3.2.3. Porosity
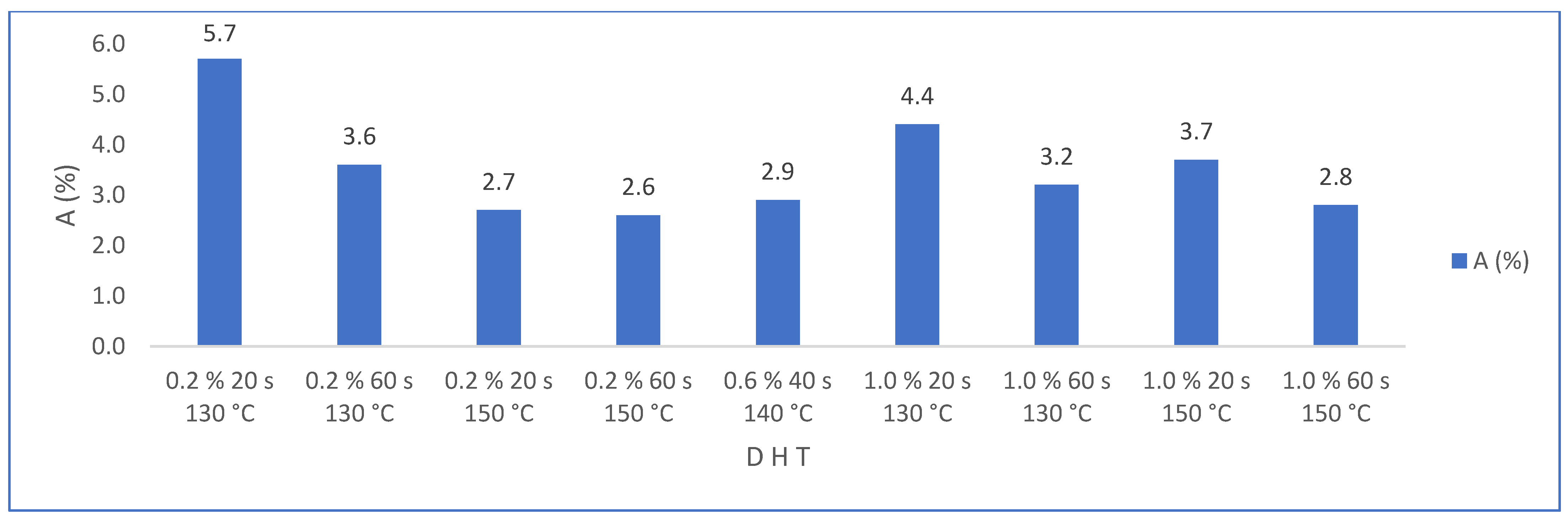
3.2.4. Water Absorption (%)
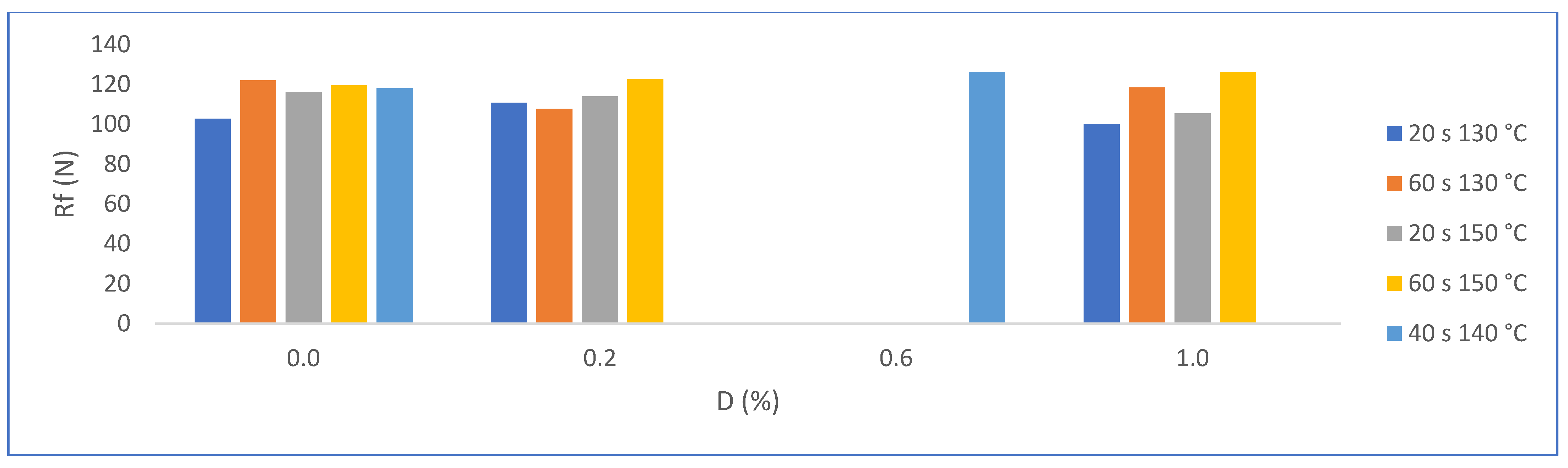
3.2.5. Flexural Strength (N)
3.2.6. Thermal Conductivity (W/(m°K))
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, N.; Middendorf, B. Calcium sulphate hemihydrate hydration leading to gypsum crystallization. Prog. Cryst. Growth Charact. Mater. 2007, 53, 57–77. [Google Scholar] [CrossRef]
- Tinkova, T.; Grigorova, I.; Nishkov, I. New Approaches on Gypsum Body Composite Materials Addition. Researchgate 2016, 2015, 10. [Google Scholar]
- Chen, X.; Wu, Q.; Gao, J.; Tang, Y. Hydration characteristics and mechanism analysis of β-calcium sulfate hemihydrate. Constr. Build. Mater. 2021, 296, 123714. [Google Scholar] [CrossRef]
- Yu, Q.; Brouwers, H. Microstructure and mechanical properties of β-hemihydrate produced gypsum: An insight from its hydration process. Constr. Build. Mater. 2011, 25, 3149–3157. [Google Scholar] [CrossRef]
- Polat, S.; Sayan, P. Ultrasonic Irradiation during the Calcium Sulfate Hemihydrate to Dihydrate Transformation Process. Chem. Eng. Technol. 2019, 42, 1466–1474. [Google Scholar] [CrossRef]
- de Korte, A.C.J.; Brouwers, H.J.H. Ultrasonic sound speed analysis of hydrating calcium sulphate hemihydrate. J. Mater. Sci. 2011, 46, 7228–7239. [Google Scholar] [CrossRef]
- De Korte, A.C.J.; Brouwers, H.J.H. Ultrasonic sound speed measurement as method for the determining the hydration degree of gypsum. In Proceedings of the 8th FIB International PhD Symposium in Civil Engineering, Lyngby, Denmark, 20–23 June 2010. [Google Scholar]
- Adrien, J.; Meille, S.; Tadier, S.; Maire, E.; Sasaki, L. In-situ X-ray tomographic monitoring of gypsum plaster setting. Cem. Concr. Res. 2016, 82, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Kamalipour, M.; Dehghani, S.A.M.; Naseri, A.; Abbasi, S. Role of agitation and temperature on calcium sulfate crystallization in water injection process. J. Pet. Sci. Eng. 2017, 151, 362–372. [Google Scholar] [CrossRef]
- Li, J.Q.; Gong, M.Z.; Li, G.Z. Relationship between Exothermic Effect and Crystal Growth in the Hydration Process of Waterproof Gypsum. Appl. Mech. Mater. 2012, 253, 308–311. [Google Scholar] [CrossRef]
- Roveri, M.; Goidanich, S.; Dotelli, G.; Toniolo, L. Semi-empirical models to describe the absorption of liquid water in natural stones employed in built heritage before and after the application of water repellent treatments. Constr. Build. Mater. 2020, 241, 117918. [Google Scholar] [CrossRef]
- Nicoleau, L.; Van Driessche, A.E.; Kellermeier, M. A kinetic analysis of the role of polymers in mineral nucleation. The example of gypsum. Cem. Concr. Res. 2019, 124, 105837. [Google Scholar] [CrossRef]
- Aquilano, D.; Otálora, F.; Pastero, L.; García-Ruiz, J.M. Three study cases of growth morphology in minerals: Halite, calcite and gypsum. Prog. Cryst. Growth Charact. Mater. 2016, 62, 227–251. [Google Scholar] [CrossRef] [Green Version]
- Mróz, P.; Mucha, M. Hydroxyethyl methyl cellulose as a modifier of gypsum properties. J. Therm. Anal. Calorim. 2018, 134, 1083–1089. [Google Scholar] [CrossRef] [Green Version]
- Czaderna, A.; Kocemba, A.; Kozanecki, M.; Mucha, M.; Mróz, P. The influence of cellulose derivatives on water structure in gypsum. Constr. Build. Mater. 2018, 160, 628–638. [Google Scholar] [CrossRef]
- Moghadam, H.A.; Mirzaei, A. Comparing the effects of a retarder and accelerator on properties of gypsum building plaster. J. Build. Eng. 2019, 28, 101075. [Google Scholar] [CrossRef]
- Polat, S.; Sayan, P. Assessment of propionic acid adsorption performance on the phase transformation of calcium sulfate hemihydrate to dihydrate. Sep. Sci. Technol. 2018, 53, 1966–1978. [Google Scholar] [CrossRef]
- Mucha, M.; Mróz, P.; Wrona, D. Chitosan applied for gypsum modification. Prog. Chem. Appl. Chitin Deriv. 2017, 22, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Mucha, M.; Mróz, P.; Kocemba, A. Polymer composites based on gypsum matrix. AIP Conf. Proc. 2016, 1736, 020119. [Google Scholar] [CrossRef]
- Garg, M.; Pundir, A.; Singh, R. Modifications in water resistance and engineering properties of β-calcium sulphate hemihydrate plaster-superplasticizer blends. Mater. Struct. 2015, 49, 3253–3263. [Google Scholar] [CrossRef]
- Zhou, P.-P.; Wu, H.-C.; Xia, Y.-M. Influence of synthetic polymers on the mechanical properties of hardened β-calcium sulfate hemihydrate plasters. J. Ind. Eng. Chem. 2016, 33, 355–361. [Google Scholar] [CrossRef]
- Huang, X.; Li, C.; Zuo, K.; Li, Q. Predominant Effect of Material Surface Hydrophobicity on Gypsum Scale Formation. Environ. Sci. Technol. 2020, 54, 15395–15404. [Google Scholar] [CrossRef] [PubMed]
- Mróz, P.; Mucha, M. Hydration kinetics of calcium sulphate hemihydrate modified by water-soluble polymers. Int. J. Eng. Res. Sci. 2017, 3, 5–13. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Yu, Y. The influences of gypsum water-proofing additive on gypsum crystal growth. Mater. Lett. 2007, 61, 872–876. [Google Scholar] [CrossRef]
- Pan, H.; Li, G. Emulsion Waterproof Agent and Its Effects on Intrinsic Properties of Gypsum. Asian J. Chem. 2013, 25, 5042–5046. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, Y.; Xue, J. Study on the improvement of the waterproof and mechanical properties of hemihydrate phosphogypsum-based foam insulation materials. Constr. Build. Mater. 2019, 230, 117014. [Google Scholar] [CrossRef]
- Kondratieva, N.; Barre, M.; Goutenoire, F.; Sanytsky, M. Study of modified gypsum binder. Constr. Build. Mater. 2017, 149, 535–542. [Google Scholar] [CrossRef]
- Ding, Y.; Fang, Y.; Ren, Q.; Fang, H.; Zhang, Q.; Oh, W.C. Study on the Waterproofing Performance of FGD Gypsum Building Products from Inorganic-Organic Composite Additives. Korean J. Mater. Res. 2015, 25, 590–597. [Google Scholar] [CrossRef]
- Li, Z.; Xu, K.; Peng, J.; Wang, J.; Zhang, J.; Li, Q. Study on mechanical strength and water resistance of organosilicon waterproofing agent blended recycled gypsum plaster. Case Stud. Constr. Mater. 2021, 14, e00546. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, H.; Chen, Q.; Gu, B.; Li, S.; Zhu, H. Effect of silane modified styrene-acrylic emulsion on the waterproof properties of flue gas desulfurization gypsum. Constr. Build. Mater. 2019, 197, 506–512. [Google Scholar] [CrossRef]
- Heim, D.; Mrowiec, A.; Pralat, K.; Mucha, M. Influence of Tylose MH1000 Content on Gypsum Thermal Conductivity. J. Mater. Civ. Eng. 2018, 30, 04018002. [Google Scholar] [CrossRef]
- Rahmanian, I.; Wang, Y. A combined experimental and numerical method for extracting temperature-dependent thermal conductivity of gypsum boards. Constr. Build. Mater. 2012, 26, 707–722. [Google Scholar] [CrossRef]
- Kondratieva, N.; Barre, M.; Goutenoire, F.; Sanytsky, M.; Rousseau, A. Effect of additives SiC on the hydration and the crystallization processes of gypsum. Constr. Build. Mater. 2020, 235, 117479. [Google Scholar] [CrossRef]
- Capasso, I.; Pappalardo, L.; Romano, R.A.; Iucolano, F. Foamed gypsum for multipurpose applications in building. Constr. Build. Mater. 2021, 307, 124948. [Google Scholar] [CrossRef]
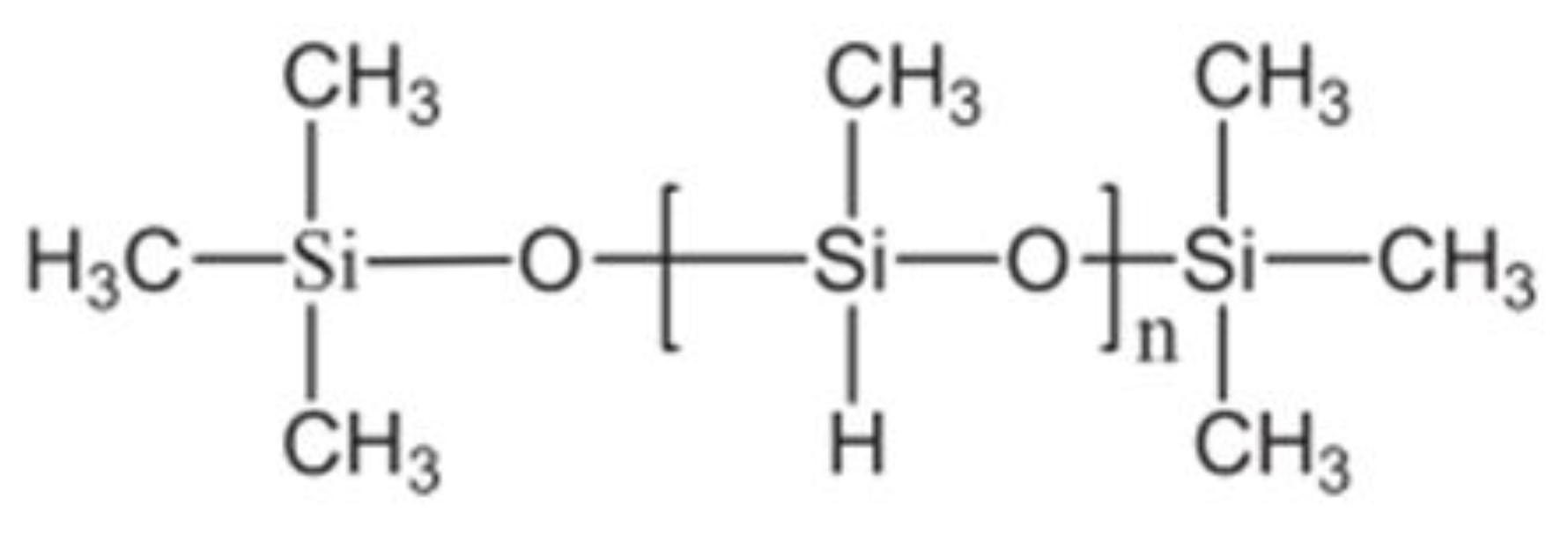
- Tönjes, J.; Longwitz, L.; Werner, T. Poly(methylhydrosiloxane) as a reductant in the catalytic base-free Wittig reaction. Green Chem. 2021, 23, 4852–4857. [Google Scholar] [CrossRef]
- Nagappan, S.; Choi, M.-C.; Sung, G.; Park, S.S.; Moorthy, M.S.; Chu, S.-W.; Lee, W.-K.; Ha, C.-S. Highly transparent, hydrophobic fluorinated polymethylsiloxane/silica organic-inorganic hybrids for anti-stain coating. Macromol. Res. 2013, 21, 669–680. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [Green Version]
- Keller, W. The Waterproofing of Gypsum with Organosilicon Compounds; Wacker-Chemie GmbH: Burghausen, Germany, 1999. [Google Scholar]
- Su, D.; Huang, C.; Hu, Y.; Jiang, Q.; Zhang, L.; Zhu, Y. Preparation of superhydrophobic surface with a novel sol–gel system. Appl. Surf. Sci. 2011, 258, 928–934. [Google Scholar] [CrossRef]
- Jakobsmeier, L. Reaktivitat und Wechselwirkungen Siliciumorganischer Verbindungen in Einer CaSO4·2H2O—Matrix; Technischen Universitas Munchen: Munich, Germany, 2000. [Google Scholar]
- Chen, C.; Ma, F.; He, T.; Kang, Z.; Wang, Y.; Shi, C. Improved water and efflorescence resistance of flue gas desulfurization gypsum-based composites by generating hydrophobic coatings. J. Clean. Prod. 2022, 371, 133711. [Google Scholar] [CrossRef]
- Hajakian, P.; Reed, P.W. Water-Resistant Gypsum Products and Methods. U.S. Patent US2017/0107151A1, 20 April 2017. [Google Scholar]
- Hajakian, P.; Reed, P.W. Water-Resistant Gypsum Products and Methods. WO2017069989A1, 27 April 2017. [Google Scholar]
- Tilford, R. Base-Mediated Hydrophobing Compositions and Processes. U.S. Patent US9879426B2, 30 January 2018. [Google Scholar]
- Instituto Nacional de Normalización-INN-Chile. NCh 143. of 1999 Yeso Calcinado-Requisitos; INN: Santiago, Chile, 1999; pp. 1–7. Available online: https://ecommerce.inn.cl/nch143199941159 (accessed on 30 May 2023).
- Lin, C.L.; Videla, A.R.; Yu, Q.; Miller, J.D. Characterization and analysis of Porous, Brittle solid structures by X-ray micro computed tomography. JOM 2010, 62, 86–89. [Google Scholar] [CrossRef]
- Videla, A.; Lin, C.; Miller, J. Simulation of saturated fluid flow in packed particle beds—The lattice-Boltzmann method for the calculation of permeability from XMT images. J. Chin. Inst. Chem. Eng. 2008, 39, 117–128. [Google Scholar] [CrossRef]
- Videla, A.; Lin, C.; Miller, J. 3D characterization of individual multiphase particles in packed particle beds by X-ray microtomography (XMT). Int. J. Miner. Process. 2006, 84, 321–326. [Google Scholar] [CrossRef]
- Kuntze, R.A. Gypsum: Connecting Science and Technology; ASTM: West Conshohocken, PA, USA, 2009. [Google Scholar] [CrossRef]
- Lootens, D.; Ampudia, M.; Hampel, C.; Mueller, M. Laboratory scaling of gypsum board production. ZKG Int. 2012, 65, 62–70. [Google Scholar]
- Seck, M.D.; Van Landeghem, M.; Faure, P.; Rodts, S.; Combes, R.; Cavalié, P.; Keita, E.; Coussot, P. The mechanisms of plaster drying. J. Mater. Sci. 2015, 50, 2491–2501. [Google Scholar] [CrossRef] [Green Version]
- Seck, M.D.; Keita, E.; Coussot, P. Some Observations on the Impact of a Low-Solubility Ionic Solution on Drying Characteristics of a Model Porous Medium. Transp. Porous Media 2018, 128, 915–928. [Google Scholar] [CrossRef]
- Seck, M.D.; Keita, E.; Faure, P.; Cavalié, P.; Van Landeghem, M.; Rodts, S.; Coussot, P. Subflorescence and plaster drying dynamics. Chem. Eng. Sci. 2016, 148, 203–211. [Google Scholar] [CrossRef]
- Mohammed, W.; Alabidalkreem, O.; Hallosh, A. Calculating the Effective Moisture Diffusivity during Drying Process of Gypsum Board. Int. Res. J. Innov. Eng. Technol. 2022, 6, 31–42. [Google Scholar]
- UNE-EN 520:2005+A1 2010; Gypsum plasterboards—Definitions, requirements and test methods. Asociacion Española de Normalizacion y Certificacion (AENOR): Madrid, Spain, 2010.
- UNE-EN ISO 22007-2:2016; AENOR (Asociacion Española de Normalizacion y Certificacion), Plásticos-Determinación de la Conductividad Térmica y la Difusividad Térmica-Parte 2: Método de la Fuente de Calor Transitoria (Disco Caliente). Asociacion Española de Normalizacion y Certificacion (AENOR): Madrid, Spain, 2016; p. 27.
- Anbalagan, G.; Mukundakumari, S.; Murugesan, K.S.; Gunasekaran, S. Infrared, optical absorption, and EPR spectroscopic studies on natural gypsum. Vib. Spectrosc. 2009, 50, 226–230. [Google Scholar] [CrossRef]
- Hajji, S.; Turki, T.; Boubakri, A.; Ben Amor, M.; Mzoughi, N. Study of cadmium adsorption onto calcite using full factorial experiment design. Desalin. Water Treat. 2017, 83, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Preite, M. Interpretación de Espectros Infrarrojos—Tabla de Espectroscopía Infrarroja QPG 3310—2do Semestre 2019; Facultad de Química y de Farmacia: Santiago, Chile, 2019; pp. 1–6. [Google Scholar]
- Ricci, C.; Miliani, C.; Brunetti, B.G.; Sgamellotti, A. Non-invasive identification of surface materials on marble artifacts with fiber optic mid-FTIR reflectance spectroscopy. Talanta 2006, 69, 1221–1226. [Google Scholar] [CrossRef]
- Bishop, J.L.; Lane, M.D.; Dyar, M.D.; King, S.J.; Brown, A.J.; Swayze, G.A. Spectral Properties of Ca-sulfates: Gypsum, Bassanite and Anhydrite. Am. Mineral. 2013, 99, 2105–2115. [Google Scholar] [CrossRef]
- Launer, P.J.; Arkles, B. Infrared Analysis of Organosilicon Compounds: Spectra-Structure Correlations; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]









| Trial | D (%) | H (s) | T (°C) | Water/HH | Number of Replicates | |
|---|---|---|---|---|---|---|
| Trials without PMHS | SN1 | 0.0 | 20 | 130 | 0.8 | 8 |
| SN2 | 0.0 | 60 | 130 | 0.8 | 8 | |
| SN3 | 0.0 | 20 | 150 | 0.8 | 8 | |
| SN4 | 0.0 | 60 | 150 | 0.8 | 8 | |
| SN5 | 0.0 | 40 | 140 | 0.8 | 8 | |
| Trials with PMHS | N1 | 0.2 | 20 | 130 | 0.8 | 8 |
| N2 | 1.0 | 20 | 130 | 0.8 | 8 | |
| N3 | 0.2 | 60 | 130 | 0.8 | 8 | |
| N4 | 1.0 | 60 | 130 | 0.8 | 8 | |
| N5 | 0.2 | 20 | 150 | 0.8 | 8 | |
| N6 | 1.0 | 20 | 150 | 0.8 | 8 | |
| N7 | 0.2 | 60 | 150 | 0.8 | 8 | |
| N8 | 1.0 | 60 | 150 | 0.8 | 8 | |
| N9 | 0.6 | 40 | 140 | 0.8 | 8 |
| SO3 | CaO | SiO2 | Al2O3 | Fe2O3 | SrO | K | LOI |
|---|---|---|---|---|---|---|---|
| 45.00 | 39.83 | 1.10 | 0.50 | 0.18 | 0.17 | 0.05 | 13.18 |
| Name | Formula | Presence (wt%) |
|---|---|---|
| Bassanite | CaSO4·0.5H2O | 83.2 |
| Calcite | CaCO3 | 10.8 |
| Anhydrite | CaSO4 | 6.0 |
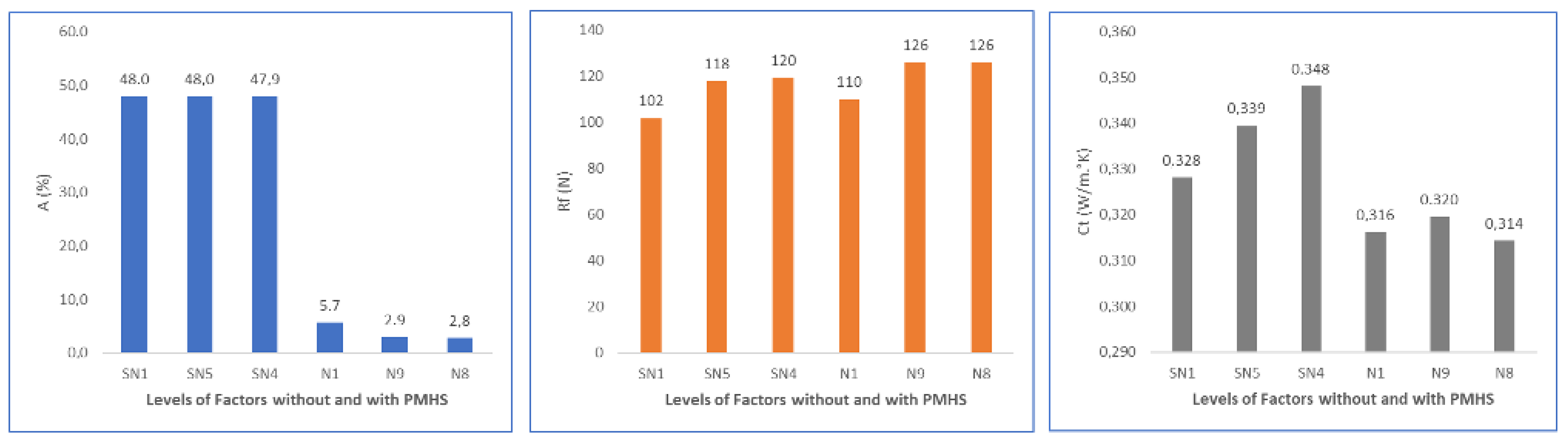
| Matrix without PMHS | Trial | D (%) | H (s) | T (°C) | A (%) | Rf (N) | Ct (W/m°K) | |||
| Avg. | Dev. | Avg. | Dev. | Avg. | Dev. | |||||
| SN1 | 0.0 | 20 | 130 | 48.0 | 0.6 | 102 | 26 | 0.328 | 0.018 | |
| SN2 | 0.0 | 60 | 130 | 47.7 | 0.6 | 122 | 28 | 0.340 | 0.012 | |
| SN3 | 0.0 | 20 | 150 | 48.5 | 0.7 | 116 | 24 | 0.332 | 0.020 | |
| SN4 | 0.0 | 60 | 150 | 47.9 | 0.6 | 120 | 40 | 0.348 | 0.008 | |
| SN5 | 0.0 | 40 | 140 | 48.0 | 0.5 | 118 | 21 | 0.339 | 0.013 | |
| Matrix with PMHS | Trial | D (%) | H (s) | T (°C) | A (%) | Rf (N) | Ct (W/m°K) | |||
| Avg. | Dev. | Avg. | Dev. | Avg. | Dev. | |||||
| N1 | 0.2 | 20 | 130 | 5.7 | 1.6 | 110 | 15 | 0.316 | 0.015 | |
| N2 | 1.0 | 20 | 130 | 4.4 | 3.5 | 100 | 18 | 0.315 | 0.010 | |
| N3 | 0.2 | 60 | 130 | 3.6 | 2.2 | 108 | 16 | 0.329 | 0.005 | |
| N4 | 1.0 | 60 | 130 | 3.2 | 1.1 | 118 | 27 | 0.322 | 0.011 | |
| N5 | 0.2 | 20 | 150 | 2.7 | 1.1 | 114 | 24 | 0.316 | 0.016 | |
| N6 | 1.0 | 20 | 150 | 3.7 | 2.2 | 105 | 6 | 0.332 | 0.037 | |
| N7 | 0.2 | 60 | 150 | 2.6 | 1.2 | 123 | 26 | 0.319 | 0.018 | |
| N8 | 1.0 | 60 | 150 | 2.8 | 1.7 | 126 | 18 | 0.314 | 0.028 | |
| N9 | 0.6 | 40 | 140 | 2.9 | 1.9 | 126 | 26 | 0.320 | 0.012 | |
| Trial | D (%) | H (s) | T (°C) | L/W | Po (%vol) | |
|---|---|---|---|---|---|---|
| Avg. | Dev. | |||||
| SN1 | 0.0 | 20 | 130 | 3.38 | 1.93 | 0.513 |
| SN5 | 0.0 | 40 | 140 | 3.04 | 2.10 | 0.274 |
| SN4 | 0.0 | 60 | 150 | 2.81 | 1.48 | 0.282 |
| N1 | 0.2 | 20 | 130 | 2.93 | 1.30 | 1.494 |
| N9 | 0.6 | 40 | 140 | 2.16 | 1.30 | 1.158 |
| N8 | 1.0 | 60 | 150 | 1.94 | 1.01 | 1.918 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano-Matos, V.; Tundidor-Camba, A.; Vera, S.; Videla, A.R. Improvement of Plasterboard Properties by the Control of Polymethylhydrosiloxane Dosage, Stirring Time, and Drying Temperature Applied to the Calcium Sulfate Hemihydrate and Water Mixture. Materials 2023, 16, 5084. https://doi.org/10.3390/ma16145084
Romano-Matos V, Tundidor-Camba A, Vera S, Videla AR. Improvement of Plasterboard Properties by the Control of Polymethylhydrosiloxane Dosage, Stirring Time, and Drying Temperature Applied to the Calcium Sulfate Hemihydrate and Water Mixture. Materials. 2023; 16(14):5084. https://doi.org/10.3390/ma16145084
Chicago/Turabian StyleRomano-Matos, Victoria, Alain Tundidor-Camba, Sergio Vera, and Alvaro R. Videla. 2023. "Improvement of Plasterboard Properties by the Control of Polymethylhydrosiloxane Dosage, Stirring Time, and Drying Temperature Applied to the Calcium Sulfate Hemihydrate and Water Mixture" Materials 16, no. 14: 5084. https://doi.org/10.3390/ma16145084






