Recovery of Zinc from Metallurgical Slag and Dust by Ammonium Acetate Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Composition of MSD Materials
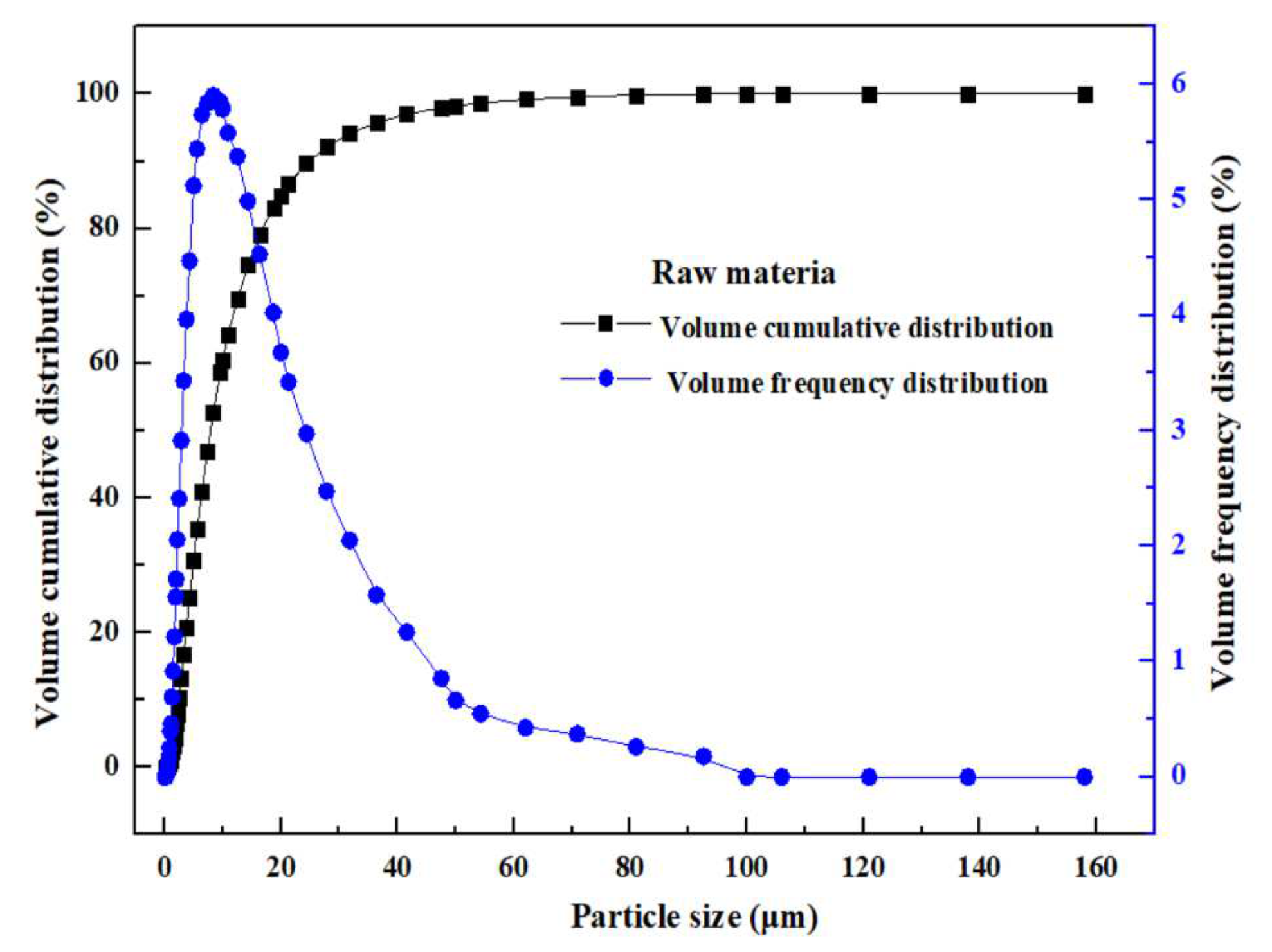
2.2. Particle Size Distribution of MSD Materials
2.3. Experimental Design of Response Surfaces and Leaching Experimental Methods
2.3.1. Experimental Design of Response Surfaces
2.3.2. Leaching Experimental Methods
2.4. Leaching Reaction of Zinc Extraction Process
3. Results and Discussion
3.1. Results of the Optimization of Response Surfaces
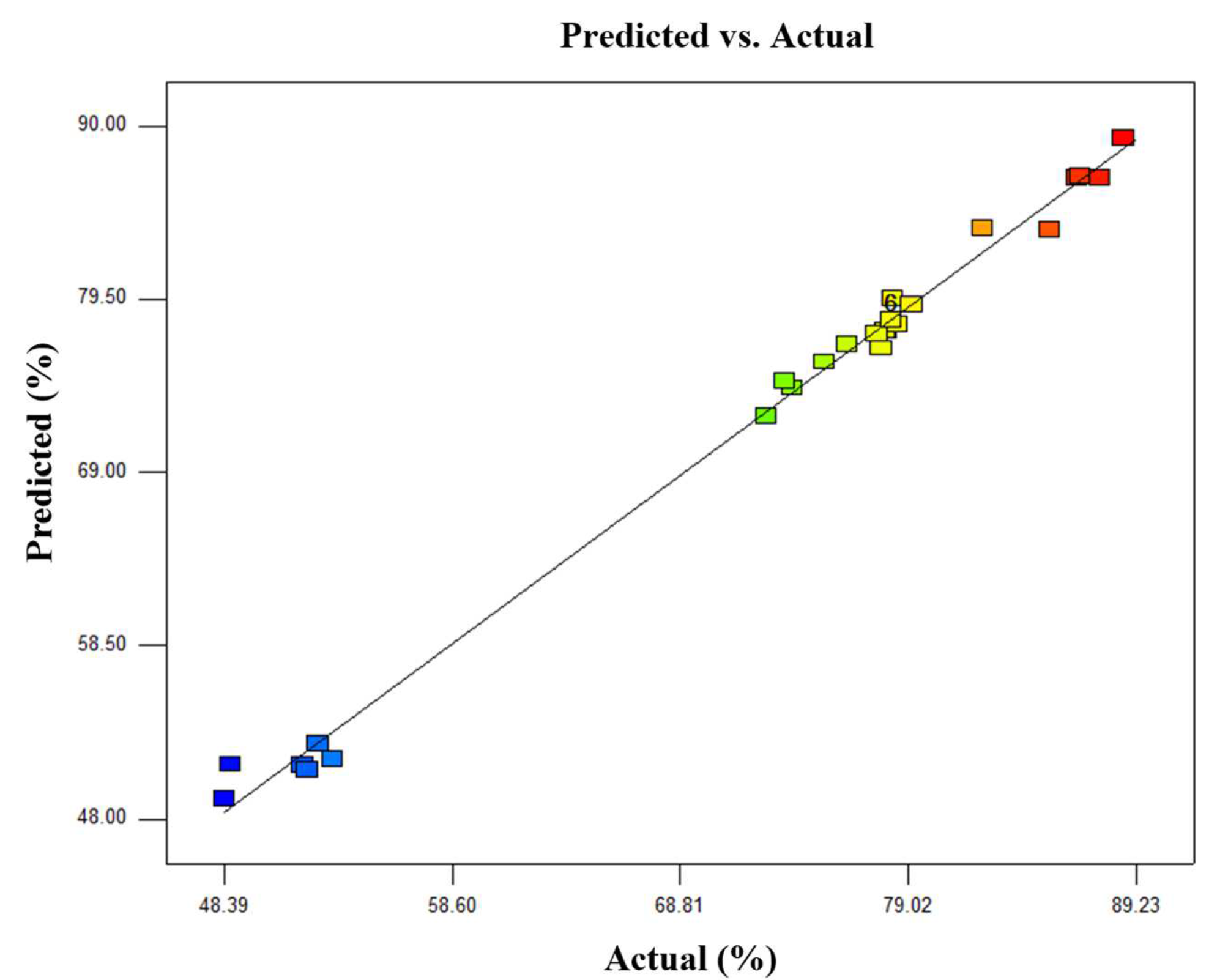
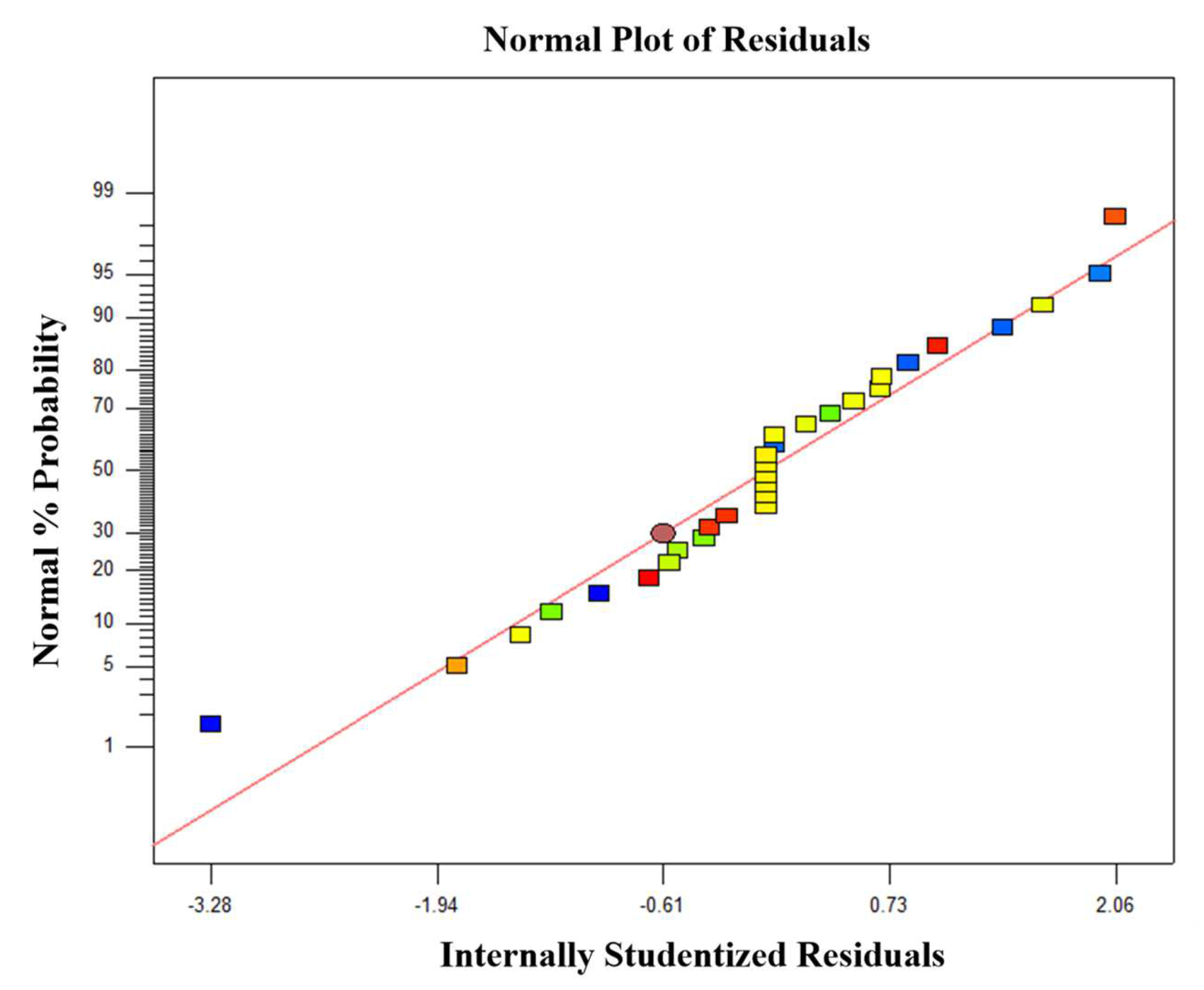
3.2. Model Verification and Statistical Analysis
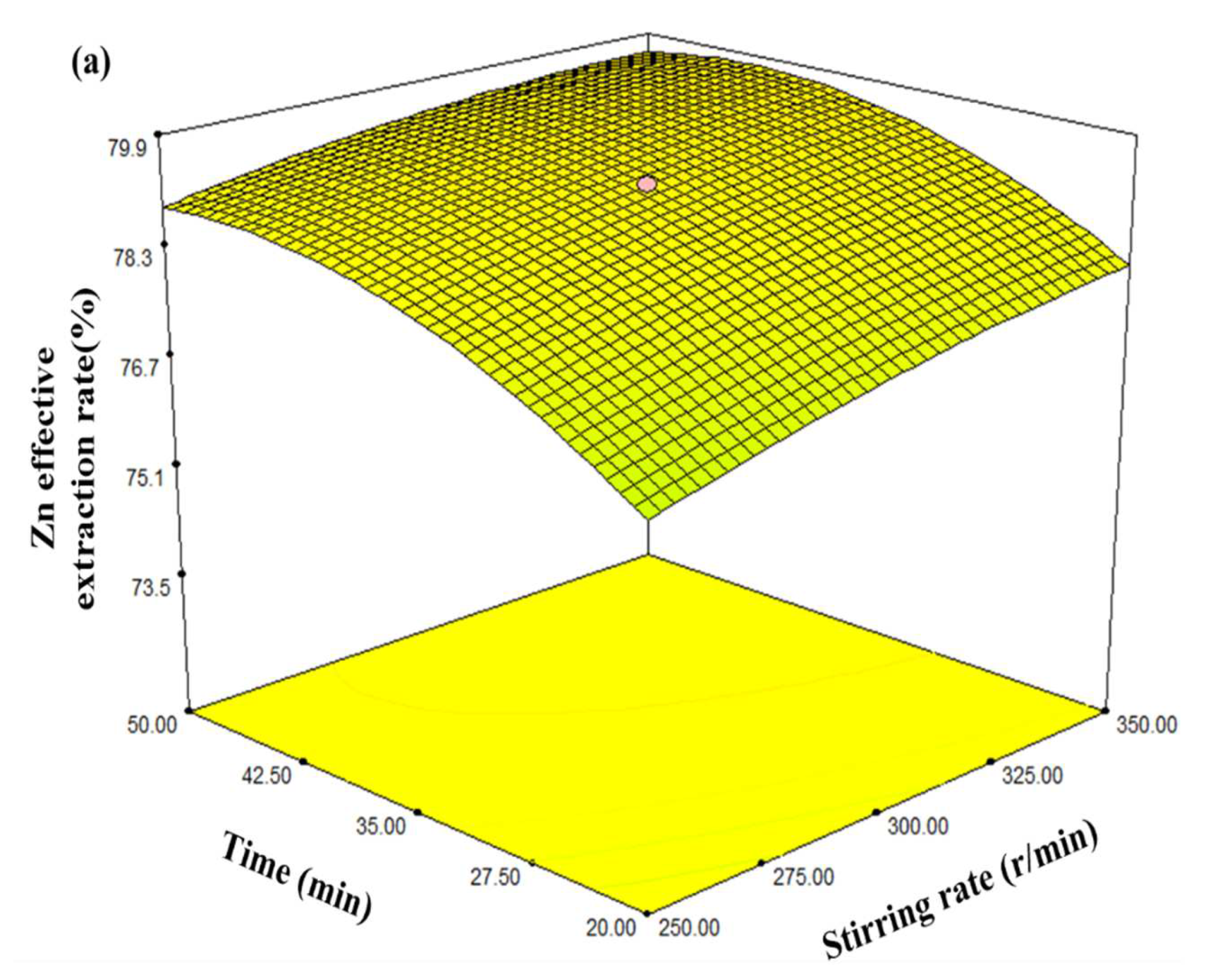
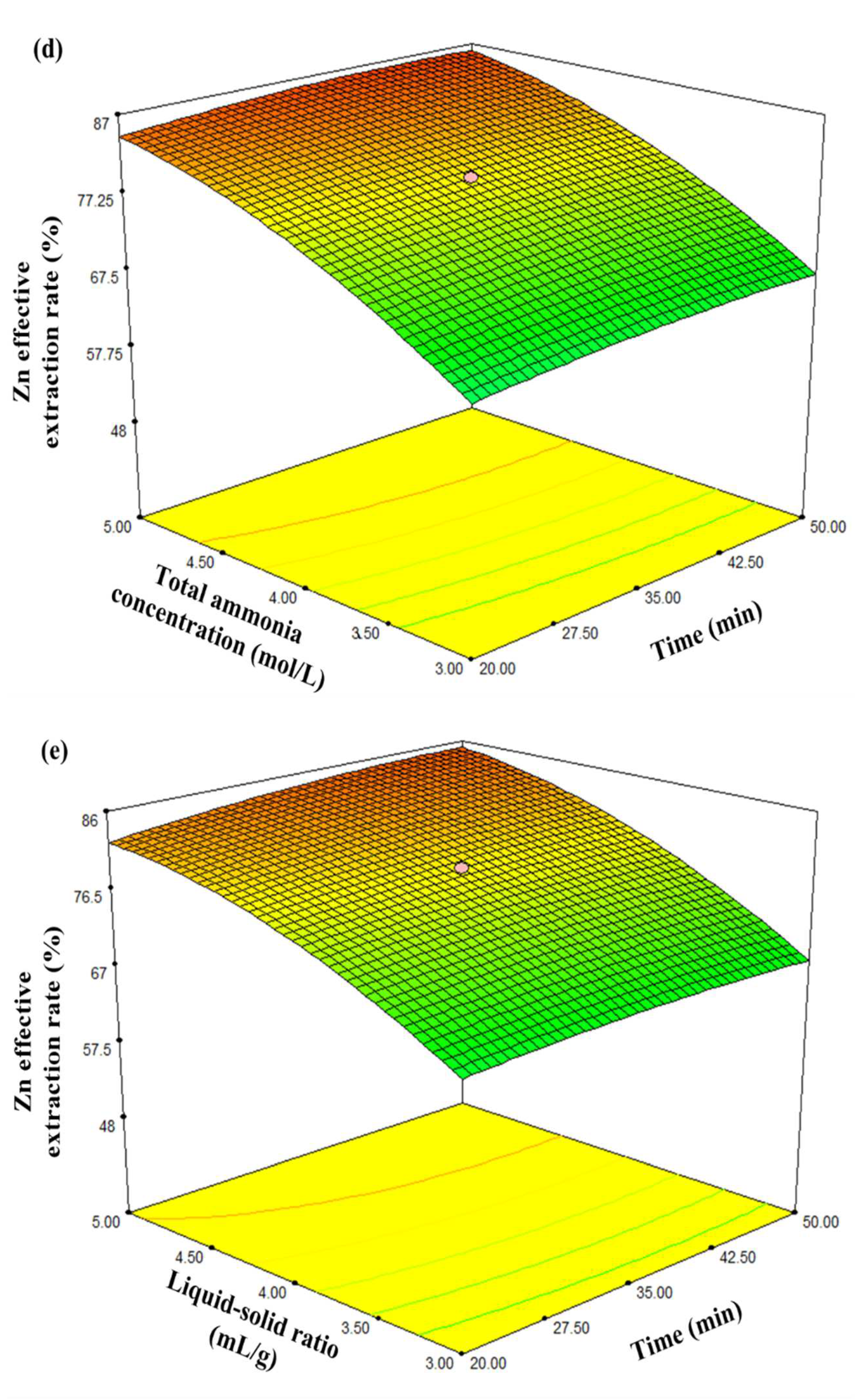
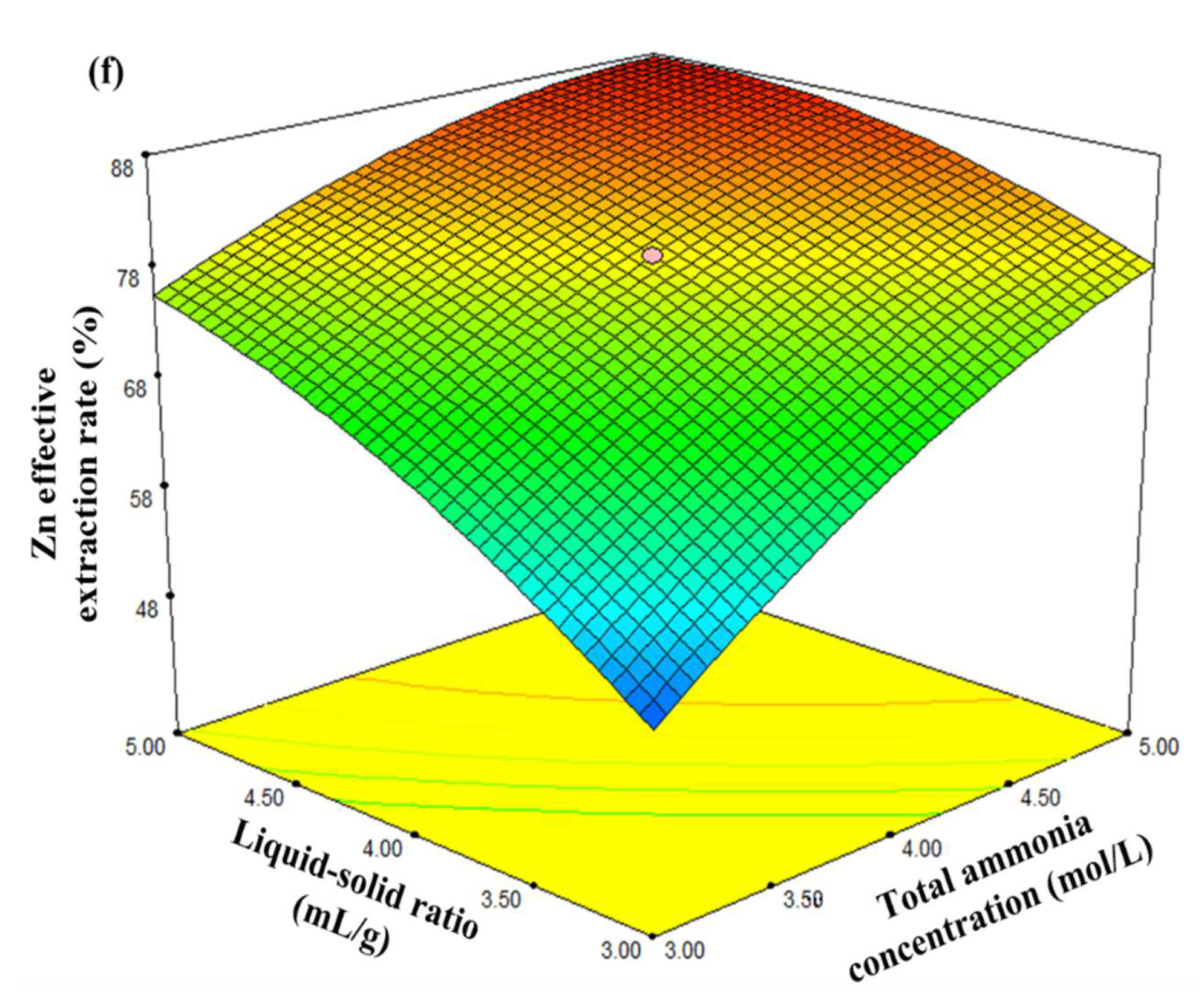
3.3. Analysis of the Response Surface Model
3.4. Condition Optimisation and Verification
3.5. Characterization Analysis
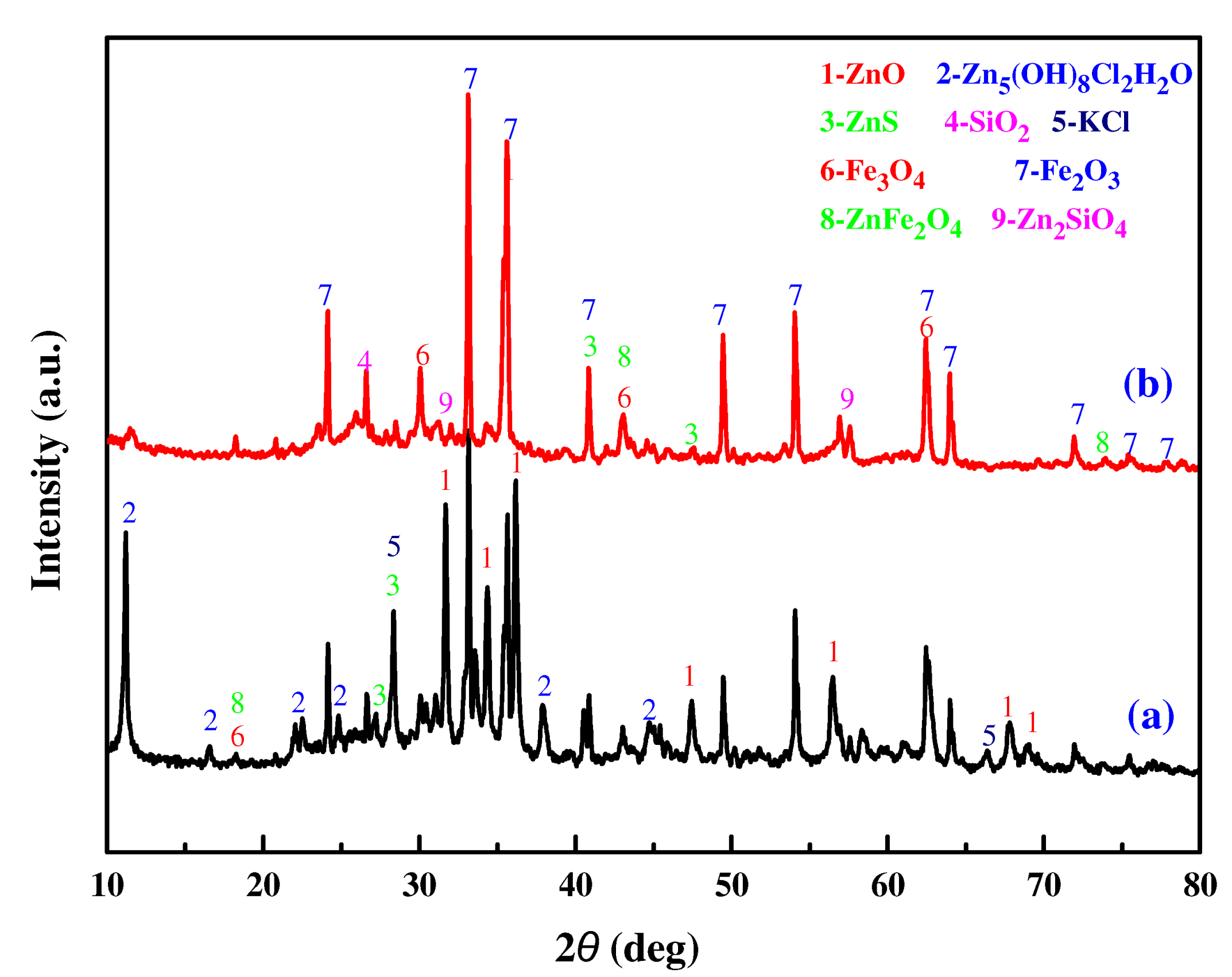
3.5.1. XRD Analysis
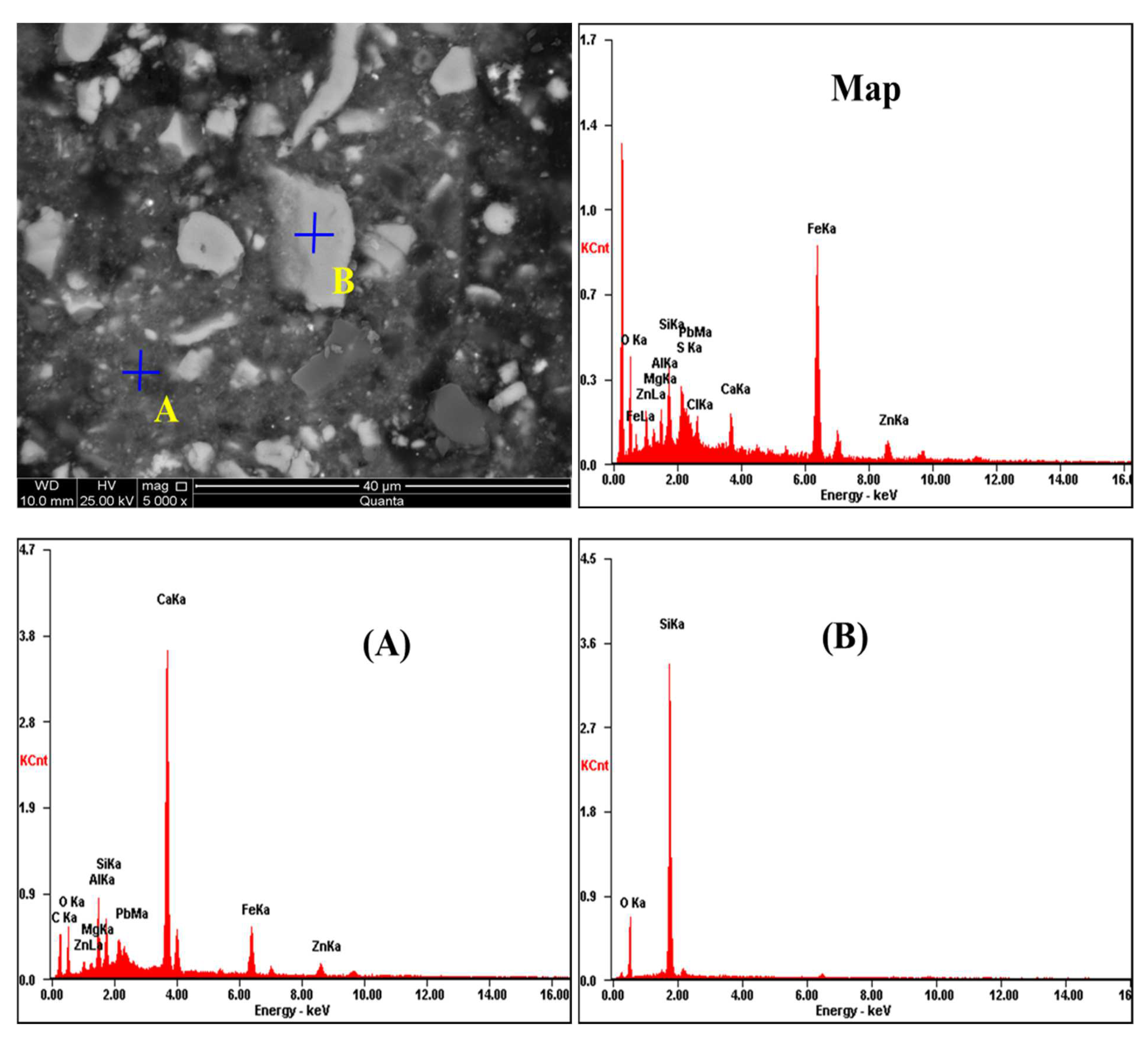
3.5.2. SEM-EDS Analysis
4. Conclusions
- (1)
- At a constant leaching temperature and ammonia/ammonium ratio, the influences of liquid/solid ratio, stirring speed, leaching time, total ammonia concentration, and interactions between them on the Zn effective extraction rate were investigated using the central composite design. The mathematic model of the factors affecting the Zn effective extraction rate was established as:Y = 79.19 + 0.38X1 + 0.84X2 + 8.55X3 + 7.25X4 − 0.012X1X2 + 0.10X1X3 + 0.28X1X4 −0.20X2X3 + 0.36X2X4 − 4.30X3X4 − 0.16X12 − 0.69X22 − 3.27X32 − 3.34X42
- (2)
- The optimised parameters for the leaching experiments were obtained: the leaching temperature was 25 °C, the total ammonia concentration was 4.8 mol/L, the leaching time was 46 min, the liquid/solid ratio was 4.3:1, [NH3]/[NH4]+ was 1:1, the stirring speed was 345 r/min, the measured Zn effective extraction rate was 84.67%, and the Zn predicted effective extraction rate was 85.25%. The experimental and predicted values of the Zn effective extraction rate were similar, indicating the reliability of the proposed model and the suitability of the optimised process parameters.
- (3)
- The XRD and SEM-EDS analysis results showed that Zn2SiO4, ZnS, and ZnFe2O4 were among the main factors affecting the low extraction rate of zinc from metallurgical slag dust under the CH3COONH4 system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hua, X.; Yang, Q.; Zhang, D.; Meng, F.; Chen, C.; You, Z.; Zhang, J.; Lv, S.; Meng, J. Microstructures and mechanical properties of a newly developed high-pressure die casting Mg-Zn-RE alloy. J. Mater. Sci. Technol. 2020, 53, 174–184. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Liu, C.; Mai, Y.; Zhang, Y.; Jie, X. Deposition of Zn-G/Al composite coating with excellent cathodic protection on low-carbon steel by low-pressure cold spraying. J. Alloys Compd. 2020, 821, 153483. [Google Scholar] [CrossRef]
- Dong, L.; Yang, W.; Yang, W.; Tian, H.; Huang, Y.; Wang, X.; Xu, C.; Wang, C.; Kang, F.; Wang, G. Flexible and conductive scaffold-stabilized zinc metal anodes for ultralong-life zinc-ion batteries and zinc-ion hybrid capacitors. Chem. Eng. J. 2020, 384, 123355. [Google Scholar] [CrossRef]
- Abkhoshk, E.; Jorjani, E.; Al-Harahsheh, M.; Rashchi, F.; Naazeri, M. Review of the hydrometallurgical processing of non-sulfide zinc ores. Hydrometallurgy 2014, 149, 153–167. [Google Scholar] [CrossRef]
- Matsukevich, I.; Kulinich, N.; Romanovski, V. Direct reduced iron and zinc recovery from electric arc furnace dust. J. Chem. Technol. Biotechnol. 2022, 97, 3453–3458. [Google Scholar] [CrossRef]
- Zhu, X.-L.; Xu, C.-Y.; Tang, J.; Hua, Y.-X.; Zhang, Q.-B.; Liu, H.; Wang, X.; Huang, M.-T. Selective recovery of zinc from zinc oxide dust using choline chloride based deep eutectic solvents. Trans. Nonferrous Met. Soc. China 2019, 29, 2222–2228. [Google Scholar] [CrossRef]
- Ma, A.; Zheng, X.; Li, S.; Wang, Y.; Zhu, S. Zinc recovery from metallurgical slag and dust by coordination leaching in NH3–CH3COONH4–H2O system. R. Soc. Open Sci. 2018, 5, 180660. [Google Scholar] [CrossRef]
- Trinkel, V.; Mallow, O.; Aschenbrenner, P.; Rechberger, H.; Fellner, J. Characterization of Blast Furnace Sludge with Respect to Heavy Metal Distribution. Ind. Eng. Chem. Res. 2016, 55, 5590–5597. [Google Scholar] [CrossRef]
- Trinkel, V.; Mallow, O.; Thaler, C.; Schenk, J.; Rechberger, H.; Fellner, J. Behavior of Chromium, Nickel, Lead, Zinc, Cadmium, and Mercury in the Blast Furnace—A Critical Review of Literature Data and Plant Investigations. Ind. Eng. Chem. Res. 2015, 54, 11759–11771. [Google Scholar] [CrossRef]
- Lanzerstorfer, C.; Bamberger-Strassmayr, B.; Pilz, K. Recycling of Blast Furnace Dust in the Iron Ore Sintering Process: Investigation of Coke Breeze Substitution and the Influence on Off-gas Emissions. ISIJ Int. 2015, 55, 758–764. [Google Scholar] [CrossRef]
- Ma, N. Recycling of basic oxygen furnace steelmaking dust by in-process separation of zinc from the dust. J. Clean. Prod. 2016, 112, 4497–4504. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, E.-D.; Zhang, L.-B.; Peng, J.-H.; Zhou, J.-W.; Srinivasakannan, C.; Yang, C.-J. A comparison of ultrasound-augmented and conventional leaching of silver from sintering dust using acidic thiourea. Ultrason. Sonochem. 2017, 34, 222–231. [Google Scholar] [CrossRef]
- Li, H.; Ma, A.; Srinivasakannan, C.; Zhang, L.; Li, S.; Yin, S. Investigation on the recovery of gold and silver from cyanide tailings using chlorination roasting process. J. Alloys Compd. 2018, 763, 241–249. [Google Scholar] [CrossRef]
- Cantarino, M.V.; Filho, C.d.C.; Mansur, M.B. Selective removal of zinc from basic oxygen furnace sludges. Hydrometallurgy 2012, 111, 124–128. [Google Scholar] [CrossRef]
- Miki, T.; Chairaksa-Fujimoto, R.; Maruyama, K.; Nagasaka, T. Hydrometallurgical extraction of zinc from CaO treated EAF dust in ammonium chloride solution. J. Hazard. Mater. 2016, 302, 90–96. [Google Scholar] [CrossRef]
- Kukurugya, F.; Vindt, T.; Havlík, T. Behavior of zinc, iron and calcium from electric arc furnace (EAF) dust in hydrometallurgical processing in sulfuric acid solutions: Thermodynamic and kinetic aspects. Hydrometallurgy 2015, 154, 20–32. [Google Scholar] [CrossRef]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- Tsakiridis, P.; Papadimitriou, G.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef]
- Safari, V.; Arzpeyma, G.; Rashchi, F.; Mostoufi, N. A shrinking particle—Shrinking core model for leaching of a zinc ore containing silica. Int. J. Miner. Process. 2009, 93, 79–83. [Google Scholar] [CrossRef]
- Dutra, A.; Paiva, P.; Tavares, L. Alkaline leaching of zinc from electric arc furnace steel dust. Miner. Eng. 2006, 19, 478–485. [Google Scholar] [CrossRef]
- Brunelli, K.; Dabalà, M. Ultrasound effects on zinc recovery from EAF dust by sulfuric acid leaching. Int. J. Miner. Met. Mater. 2015, 22, 353–362. [Google Scholar] [CrossRef]
- Xu, H.; Wei, C.; Li, C.; Fan, G.; Deng, Z.; Zhou, X.; Qiu, S. Leaching of a complex sulfidic, silicate-containing zinc ore in sulfuric acid solution under oxygen pressure. Sep. Purif. Technol. 2012, 85, 206–212. [Google Scholar] [CrossRef]
- Halli, P.; Agarwal, V.; Partinen, J.; Lundström, M. Recovery of Pb and Zn from a citrate leach liquor of a roasted EAF dust using precipitation and solvent extraction. Sep. Purif. Technol. 2019, 236, 116264. [Google Scholar] [CrossRef]
- Steer, J.M.; Griffiths, A.J. Investigation of carboxylic acids and non-aqueous solvents for the selective leaching of zinc from blast furnace dust slurry. Hydrometallurgy 2013, 140, 34–41. [Google Scholar] [CrossRef]
- Sethurajan, M.; Huguenot, D.; Jain, R.; Lens, P.N.L.; Horn, H.A.; Figueiredo, L.H.A.; van Hullebusch, E.D. Leaching and selective zinc recovery from acidic leachates of zinc metallurgical leach residues. J. Hazard. Mater. 2017, 324, 71–82. [Google Scholar] [CrossRef]
- Ma, A.; Zhang, L.; Peng, J.; Zheng, X.; Li, S.; Yang, K.; Chen, W. Extraction of zinc from blast furnace dust in ammonia leaching system. Green Process. Synth. 2016, 5, 23–30. [Google Scholar] [CrossRef]
- Wang, R.-X.; Tang, M.-T.; Yang, S.-H.; Zhagn, W.-H.; Tang, C.-B.; He, J.; Yang, J.-G. Leaching kinetics of low grade zinc oxide ore in NH3-NH4Cl-H2O system. J. Cent. South Univ. Technol. 2008, 15, 679–683. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Cao, Z.; Yang, T. Dissolution behavior of willemite in the (NH4)2SO4–NH3–H2O system. Hydrometallurgy 2012, 125, 50–54. [Google Scholar] [CrossRef]
- Ma, A.; Zheng, X.; Zhang, L.; Peng, J.; Li, Z.; Li, S.; Li, S. Clean recycling of zinc from blast furnace dust with ammonium acetate as complexing agents. Sep. Sci. Technol. 2018, 53, 1327–1341. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, D.; Jie, Y.; Tang, C.; He, J.; Chen, Y. Hydrometallurgical Process for Zinc Recovery from C.Z.O. Generated by the Steelmaking Industry with Ammonia–Ammonium Chloride Solution. Metals 2019, 9, 83. [Google Scholar] [CrossRef]
- Rao, S.; Yang, T.; Zhang, D.; Liu, W.; Chen, L.; Hao, Z.; Xiao, Q.; Wen, J. Leaching of low grade zinc oxide ores in NH4Cl–NH3 solutions with nitrilotriacetic acid as complexing agents. Hydrometallurgy 2015, 158, 101–106. [Google Scholar] [CrossRef]
- Li, Z.; Yu, D.; Liu, X.; Wang, Y. The Fate of Heavy Metals and Risk Assessment of Heavy Metal in Pyrolysis Coupling with Acid Washing Treatment for Sewage Sludge. Toxics 2023, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, C.; Lei, Z.; Li, J.; Lu, J.; Xiang, Q.; Chen, X.; Hua, Y.; Li, Y. Recycling of zinc oxide dust using ChCl-urea deep eutectic solvent with nitrilotriacetic acid as complexing agents. Miner. Eng. 2022, 175, 107295. [Google Scholar] [CrossRef]
- Javanshir, S.; Qashoqchi, S.S.; Mofrad, Z.H. Silver production from spent zinc–silver oxide batteries via leaching–cementation technique. Sep. Sci. Technol. 2021, 56, 1956–1964. [Google Scholar] [CrossRef]

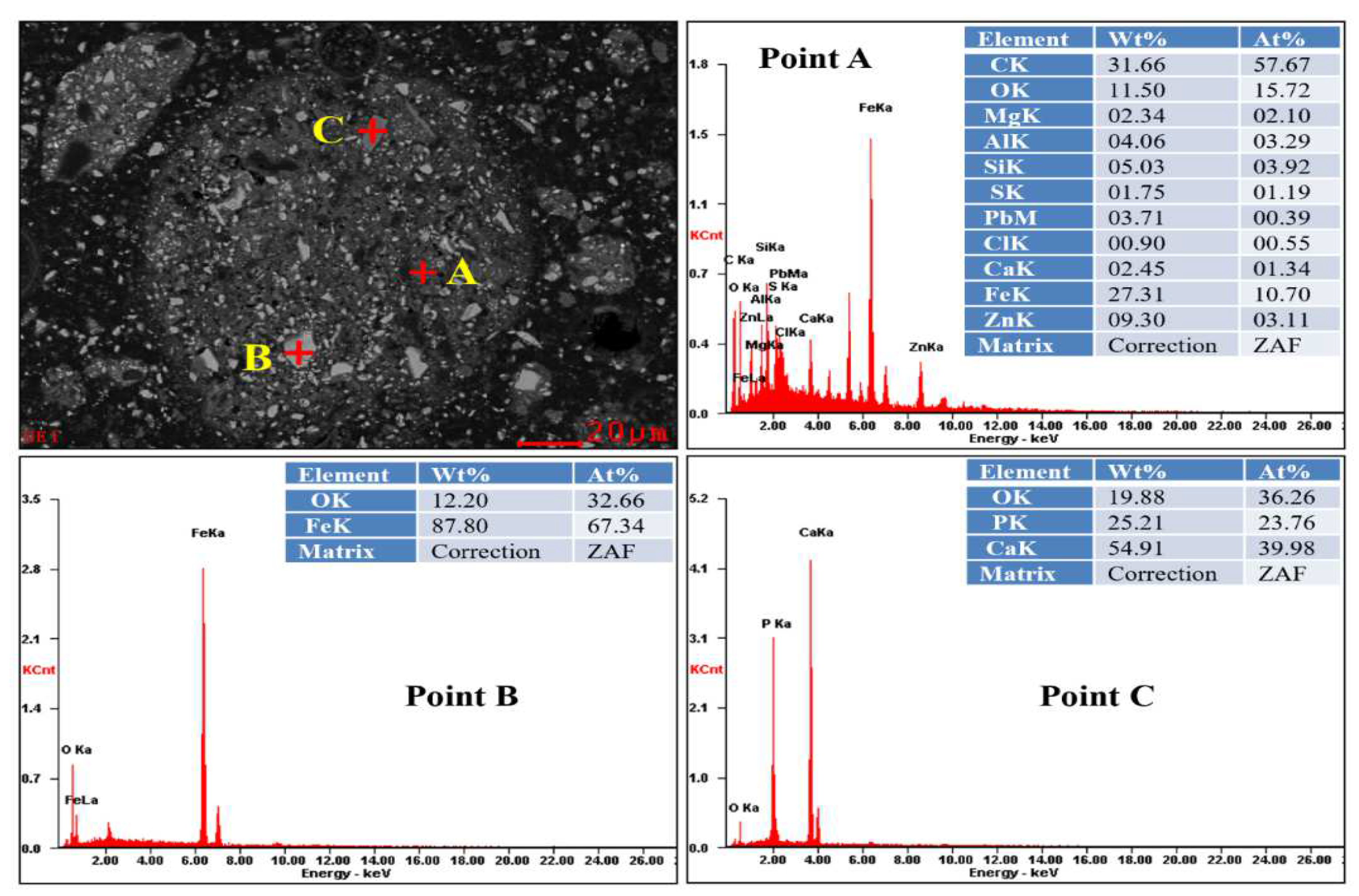
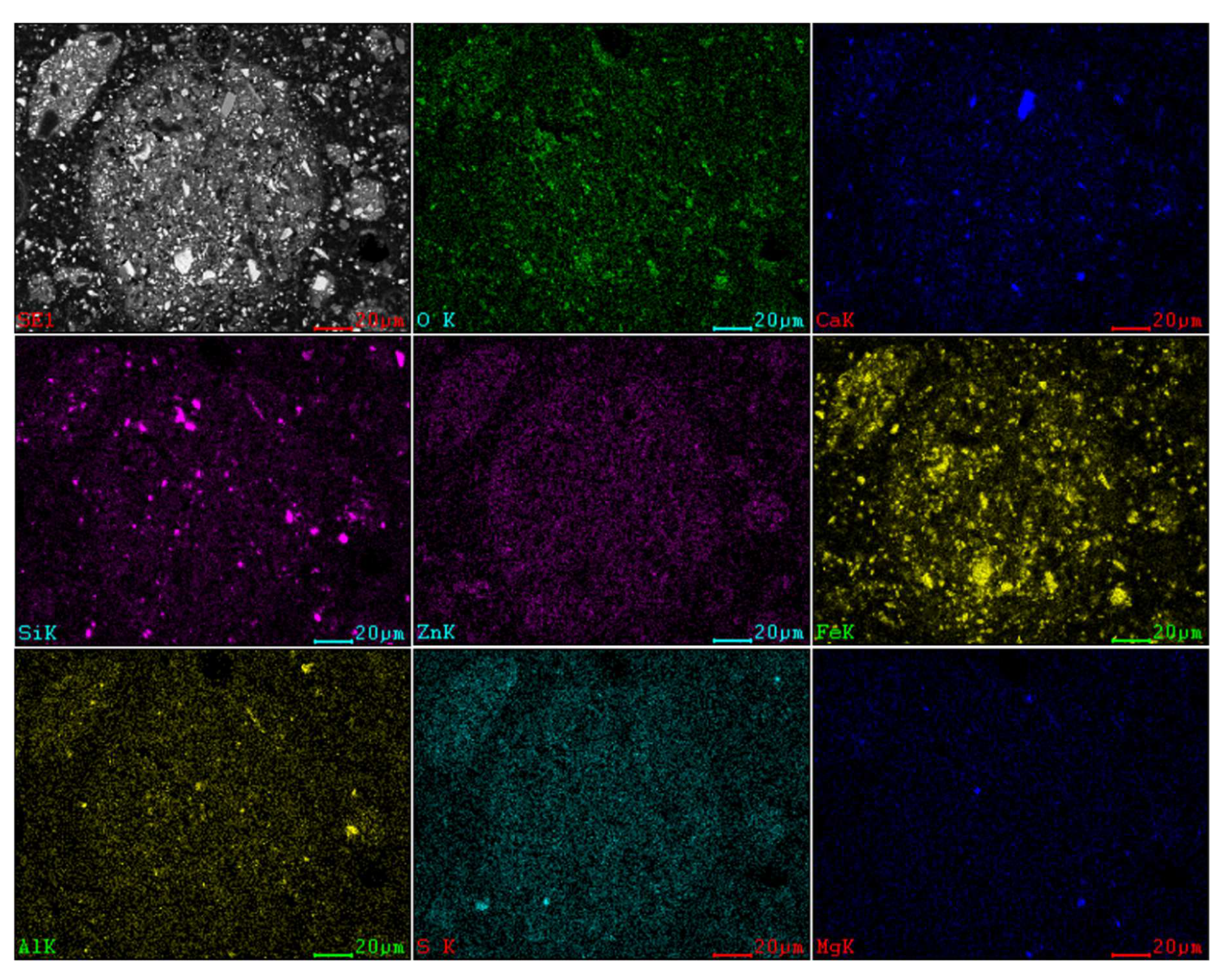
| Compositions | Zn | Fe | C | Si | Cl | S |
| Mass (w%) | 24.74 | 21.66 | 9.14 | 2.66 | 2.94 | 1.39 |
| Compositions | Mg | Bi | Pb | In (g/t) | ||
| Mass (w%) | 1.14 | 0.97 | 1.13 | 354 |
| D10 (μm) | D50 (μm) | D90 (μm) | D98 (μm) | Volume Average Particle Size (μm) | Area Average Particle Size (μm) | Surface Area to Volume Ratio (m2/cm3) |
|---|---|---|---|---|---|---|
| 2.47 | 7.89 | 24.85 | 47.91 | 10.78 | 5.59 | 6.42 |
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Stirring speed (X1, r/min) | 250 | 300 | 350 |
| Leaching time (X2, min) | 20 | 35 | 50 |
| Total ammonia concentration (X3, mol/L) | 3 | 4 | 5 |
| Liquid/solid ratio (X4, mL/g) | 3 | 4 | 5 |
| Number | X1 (r/min) | X2 (min) | X3 (mol/L) | X4 (L/S) | Y (%) |
|---|---|---|---|---|---|
| 1 | 300 | 35 | 4 | 6 | 81.88 |
| 2 | 300 | 35 | 6 | 4 | 82.75 |
| 3 | 300 | 35 | 4 | 4 | 79.19 |
| 4 | 350 | 20 | 5 | 3 | 78.08 |
| 5 | 300 | 35 | 4 | 4 | 79.19 |
| 6 | 300 | 35 | 4 | 4 | 79.19 |
| 7 | 350 | 20 | 5 | 5 | 83.33 |
| 8 | 250 | 20 | 5 | 5 | 81.03 |
| 9 | 350 | 50 | 3 | 5 | 76.30 |
| 10 | 300 | 35 | 4 | 4 | 79.19 |
| 11 | 350 | 50 | 5 | 3 | 78.51 |
| 12 | 350 | 20 | 3 | 5 | 73.82 |
| 13 | 250 | 50 | 5 | 5 | 82.98 |
| 14 | 250 | 20 | 3 | 5 | 72.69 |
| 15 | 250 | 20 | 5 | 3 | 77.83 |
| 16 | 200 | 35 | 4 | 4 | 77.62 |
| 17 | 250 | 50 | 3 | 5 | 75.27 |
| 18 | 250 | 50 | 5 | 3 | 78.01 |
| 19 | 350 | 50 | 5 | 5 | 84.50 |
| 20 | 350 | 50 | 3 | 3 | 53.21 |
| 21 | 300 | 65 | 4 | 4 | 78.28 |
| 22 | 300 | 35 | 4 | 2 | 48.67 |
| 23 | 350 | 20 | 3 | 3 | 52.09 |
| 24 | 250 | 50 | 3 | 3 | 52.59 |
| 25 | 300 | 35 | 4 | 4 | 79.19 |
| 26 | 300 | 5 | 4 | 4 | 73.50 |
| 27 | 400 | 35 | 4 | 4 | 78.36 |
| 28 | 250 | 20 | 3 | 3 | 51.91 |
| 29 | 300 | 35 | 4 | 4 | 79.19 |
| 30 | 300 | 35 | 2 | 4 | 48.39 |
| The Sequential Model Sum of Squares | ||||||
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Probd > F | |
| Mean vs. total | 160,900 | 1 | 1.609 × 105 | |||
| Linear vs. mean | 3036.32 | 4 | 759.08 | 22.16 | <0.0001 | |
| 2FI vs. linear | 300.53 | 6 | 50.09 | 1.71 | 0.1726 | |
| Quadratic vs. 2FI | 536.69 | 4 | 134.17 | 104.59 | <0.0001 | Suggested |
| Cubic vs. quadratic | 15.57 | 8 | 1.95 | 3.71 | 0.0505 | Aliased |
| Residual | 3.67 | 7 | 0.52 | |||
| Total | 1.647 × 105 | 30 | 5491.61 | |||
| Lack of fit tests | ||||||
| Source | Sum of squares | df | Mean square | |||
| Linear | 856.46 | 20 | 42.82 | |||
| 2FI | 555.93 | 14 | 39.71 | |||
| Quadratic | 19.24 | 10 | 1.92 | Suggested | ||
| Cubic | 3.67 | 2 | 1.84 | Aliased | ||
| Pure error | 0.00 | 5 | 0.00 | |||
| Model summary statistics | ||||||
| Source | Std. | Adjusted R-squared | Predicted R-squared | PRESS | ||
| Dev. | R-squared | |||||
| Linear | 5.85 | 0.7800 | 0.7448 | 0.6832 | 1233.10 | |
| 2FI | 5.41 | 0.8572 | 0.7820 | 0.7741 | 879.23 | |
| Quadratic | 1.13 | 0.9951 | 0.9904 | 0.9715 | 110.84 | Suggested |
| Cubic | 0.72 | 0.9991 | 0.9961 | 0.8641 | 528.85 | Aliased |
| Std. Dev. | 1.13 | R-Squared | 0.9951 |
| Mean | 73.22 | Adj R-Squared | 0.9904 |
| C.V.% | 1.55 | Pred R-Squared | 0.9715 |
| PRESS | 110.84 | Adeq Precision | 44.8820 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value (Probd > F) | Significance |
|---|---|---|---|---|---|---|
| Model | 3873.53 | 14 | 276.68 | 215.67 | <0.0001 | significant |
| X1 | 3.38 | 1 | 3.38 | 2.64 | 0.1252 | |
| X2 | 16.92 | 1 | 16.92 | 13.19 | 0.0025 | significant |
| X3 | 1752.92 | 1 | 1752.92 | 1366.39 | <0.0001 | significant |
| X4 | 1263.10 | 1 | 1263.10 | 984.57 | <0.0001 | significant |
| X1X2 | 2.26 × 10−3 | 1 | 2.26 × 10−3 | 1.76 × 10−3 | 0.9671 | |
| X1X3 | 0.16 | 1 | 0.16 | 0.13 | 0.7273 | |
| X1X4 | 1.23 | 1 | 1.23 | 0.96 | 0.3437 | |
| X2X3 | 0.61 | 1 | 0.61 | 0.48 | 0.5002 | |
| X2X4 | 2.08 | 1 | 2.08 | 1.62 | 0.2222 | |
| X3X4 | 296.44 | 1 | 296.44 | 231.07 | <0.0001 | significant |
| X12 | 0.72 | 1 | 0.72 | 0.56 | 0.4655 | |
| X22 | 12.94 | 1 | 12.94 | 10.09 | 0.0063 | significant |
| X32 | 292.75 | 1 | 292.75 | 228.20 | <0.0001 | significant |
| X42 | 306.12 | 1 | 306.12 | 238.61 | <0.0001 | significant |
| Residual | 19.24 | 15 | 1.28 | |||
| Lack of Fit | 19.24 | 10 | 1.92 | |||
| Pure Error | 0 | 5 | 0 | |||
| Cor Total | 3892.78 | 29 |
| Total Ammonia Concentration (mol/L) | [NH3]/[NH4]+ Mole Ratio | Temperature (°C) | Time (min) |
|---|---|---|---|
| 4.78 | 1:01 | 25 | 46.20 |
| Liquid/solid ratio (mL/g) | Stirring speed (r/min) | Zn effective leaching rate (%) | |
| Predicted value | Experimental value | ||
| 4.29 | 344.78 | 85.25 | 84.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Li, J.; Ma, A.; Liu, B. Recovery of Zinc from Metallurgical Slag and Dust by Ammonium Acetate Using Response Surface Methodology. Materials 2023, 16, 5132. https://doi.org/10.3390/ma16145132
Zheng X, Li J, Ma A, Liu B. Recovery of Zinc from Metallurgical Slag and Dust by Ammonium Acetate Using Response Surface Methodology. Materials. 2023; 16(14):5132. https://doi.org/10.3390/ma16145132
Chicago/Turabian StyleZheng, Xuemei, Jinjing Li, Aiyuan Ma, and Bingguo Liu. 2023. "Recovery of Zinc from Metallurgical Slag and Dust by Ammonium Acetate Using Response Surface Methodology" Materials 16, no. 14: 5132. https://doi.org/10.3390/ma16145132
APA StyleZheng, X., Li, J., Ma, A., & Liu, B. (2023). Recovery of Zinc from Metallurgical Slag and Dust by Ammonium Acetate Using Response Surface Methodology. Materials, 16(14), 5132. https://doi.org/10.3390/ma16145132






