Corrosion and Erosion Wear Behaviors of HVOF-Sprayed Fe-Based Amorphous Coatings on Dissolvable Mg-RE Alloy Substrates
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
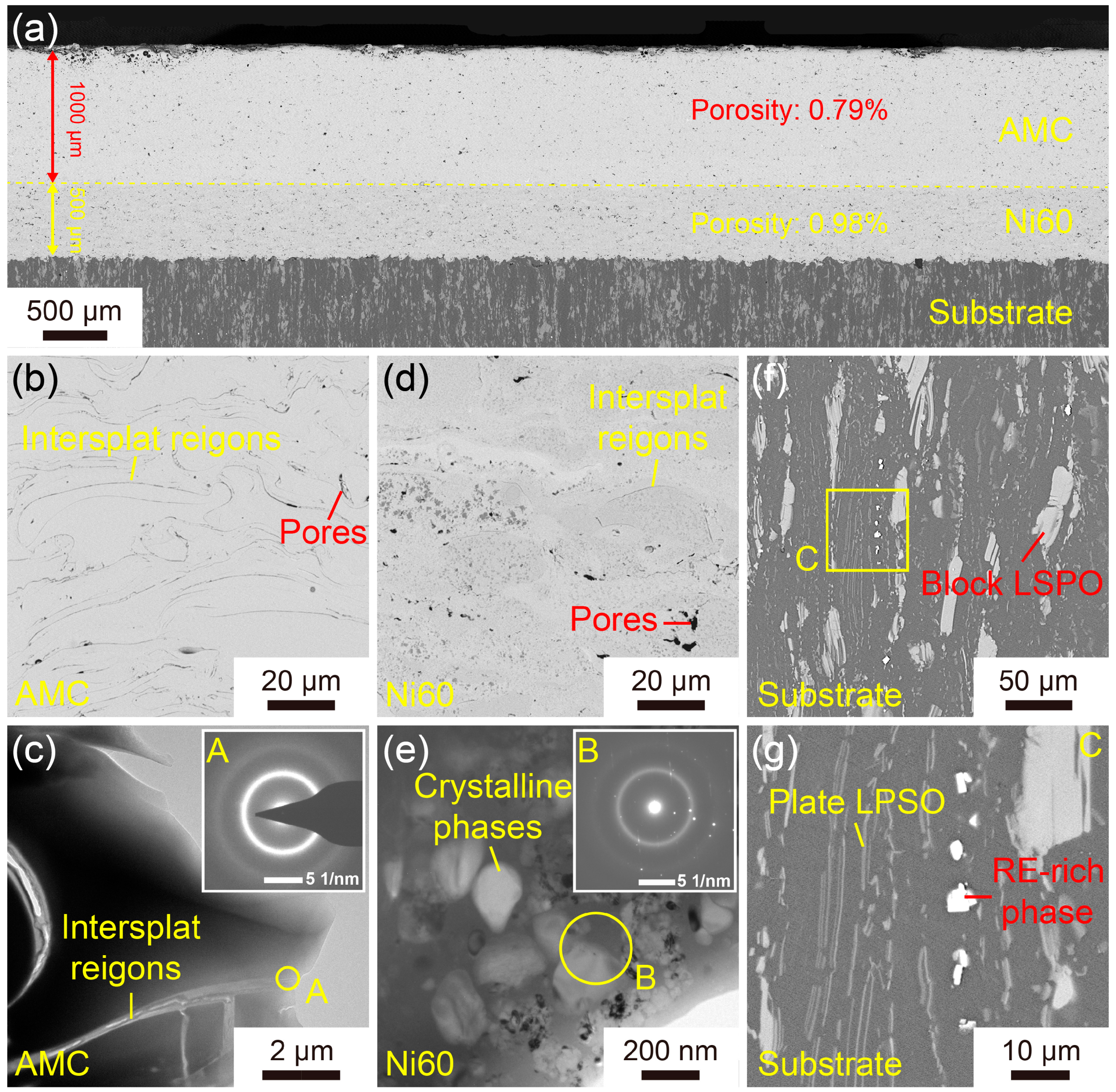
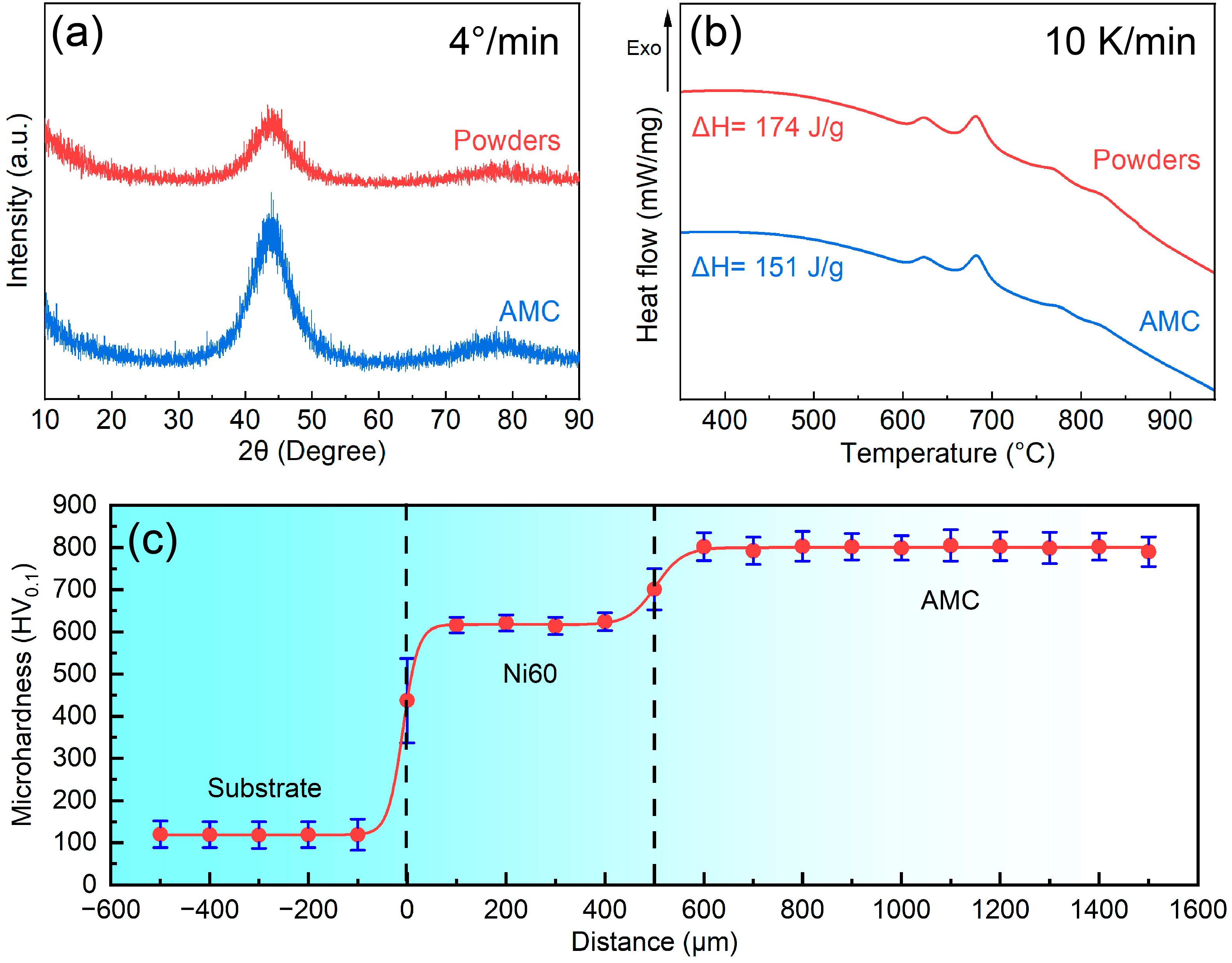
3.1. Microstructure of the Fe-Based AMC
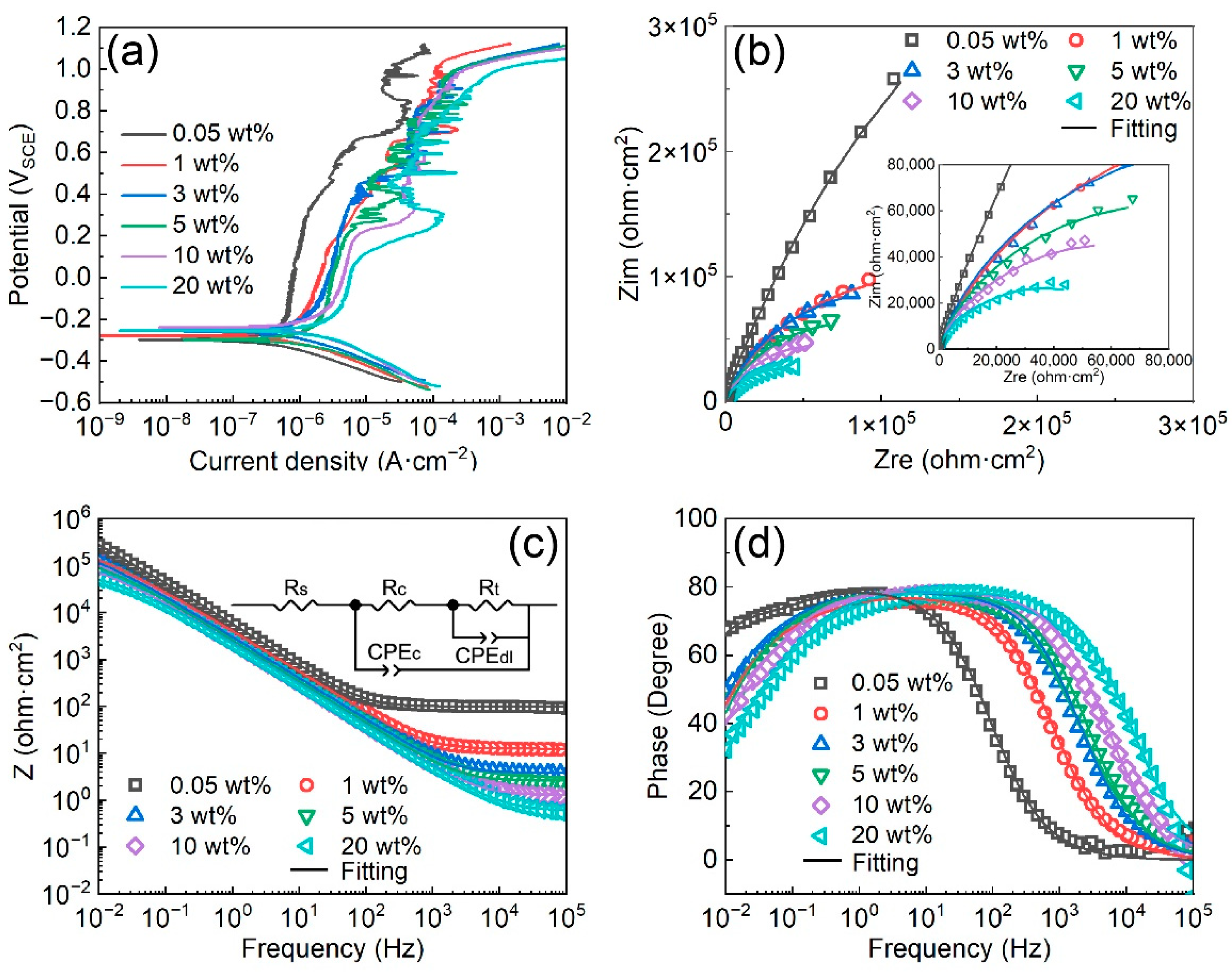
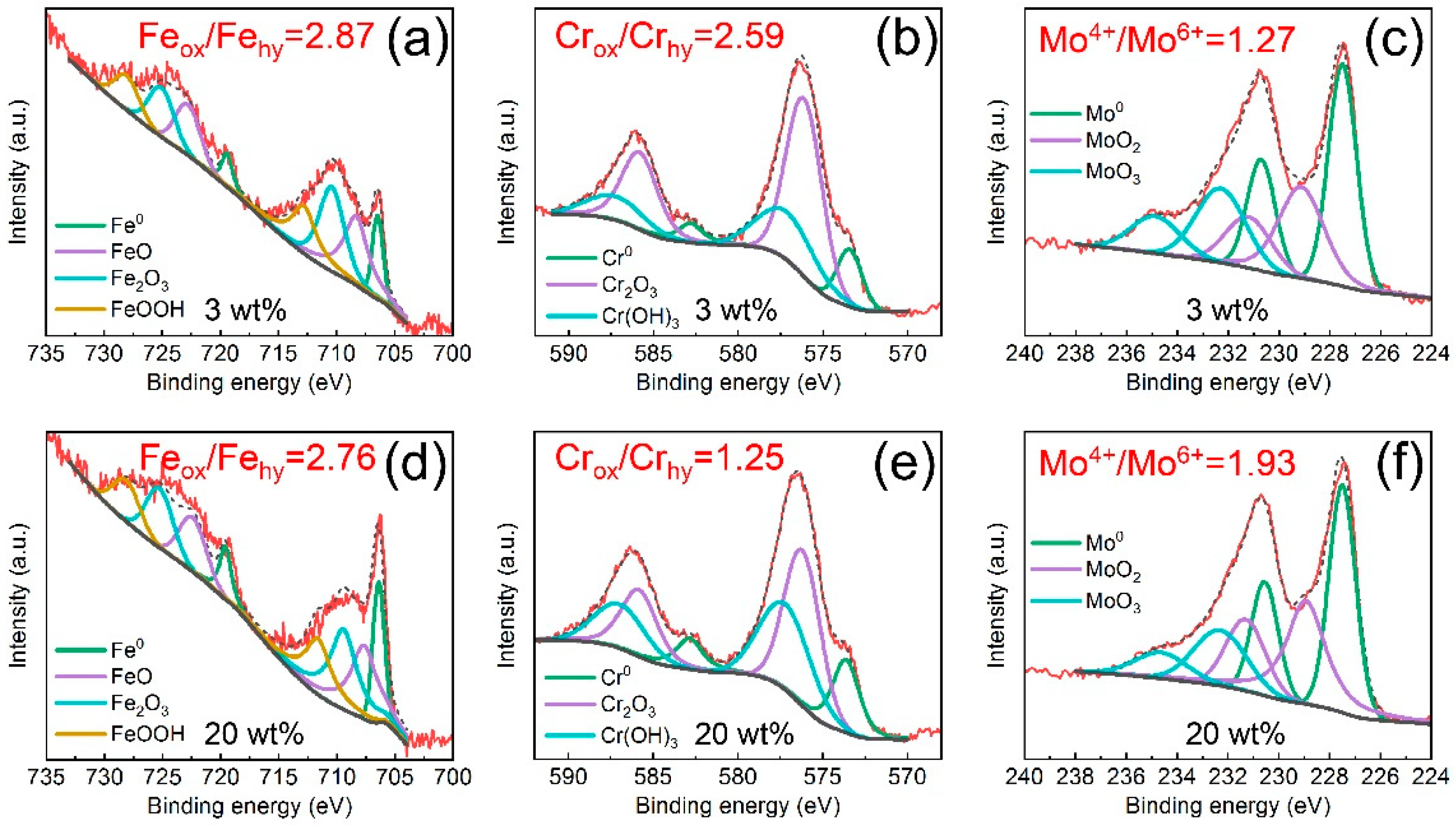
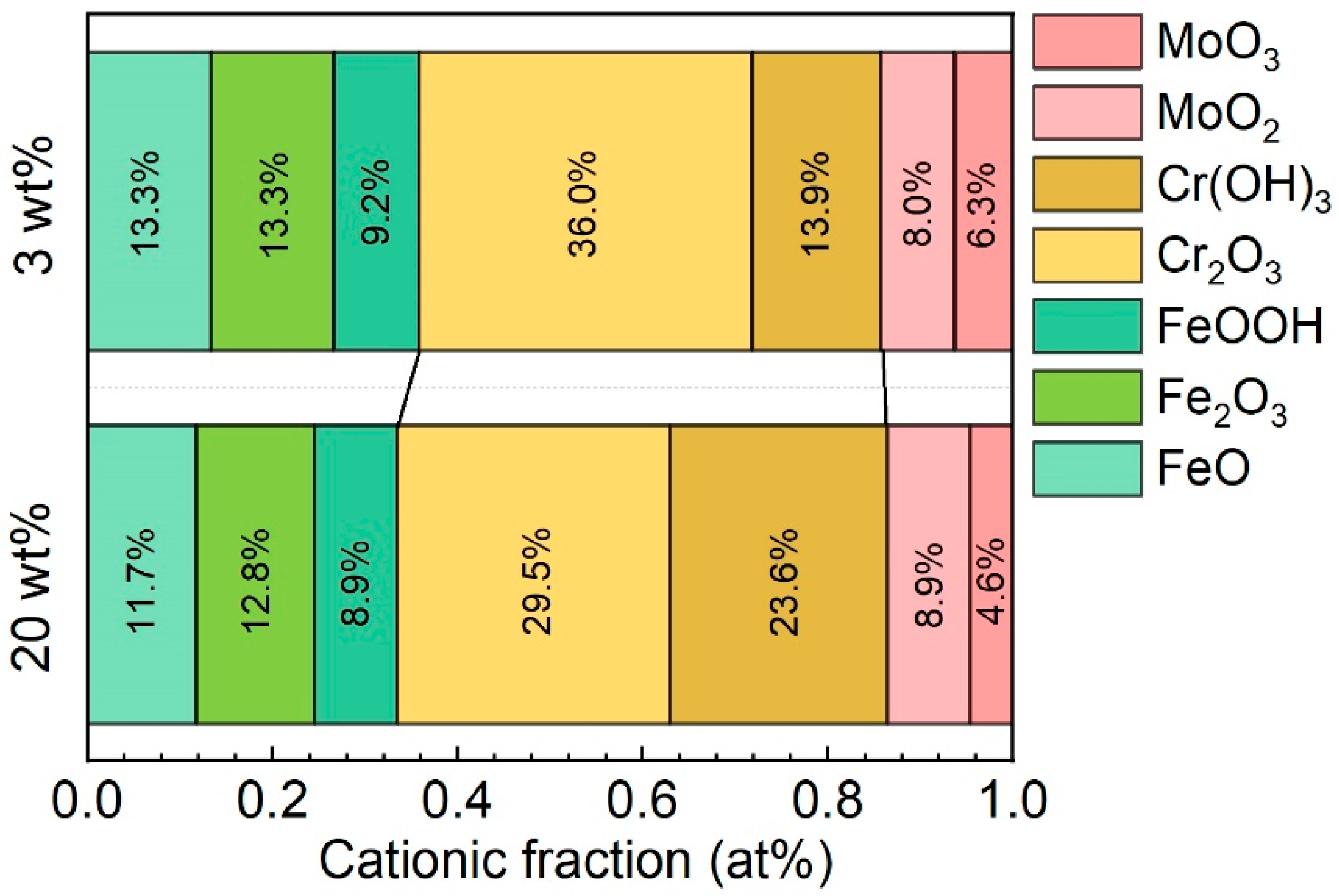
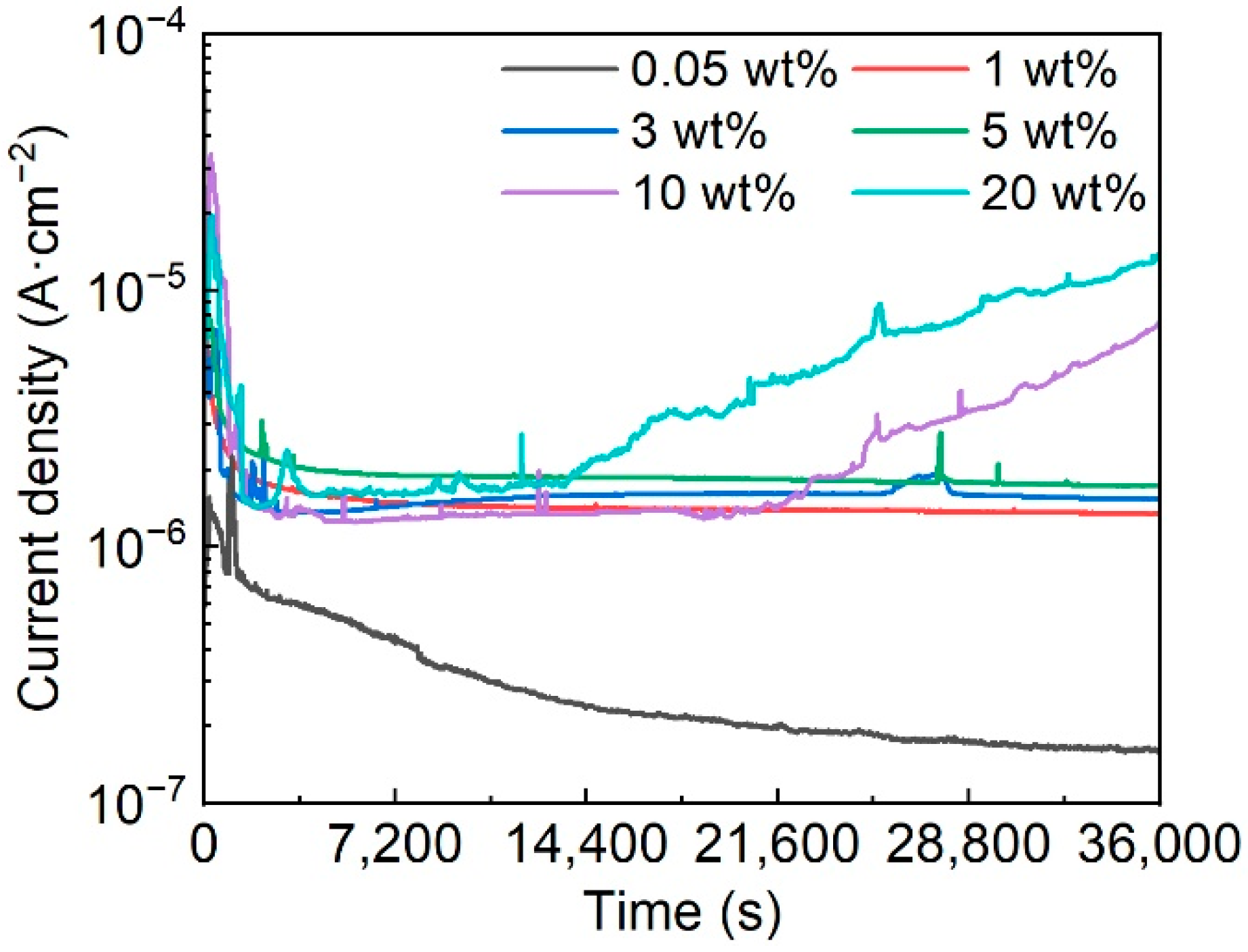
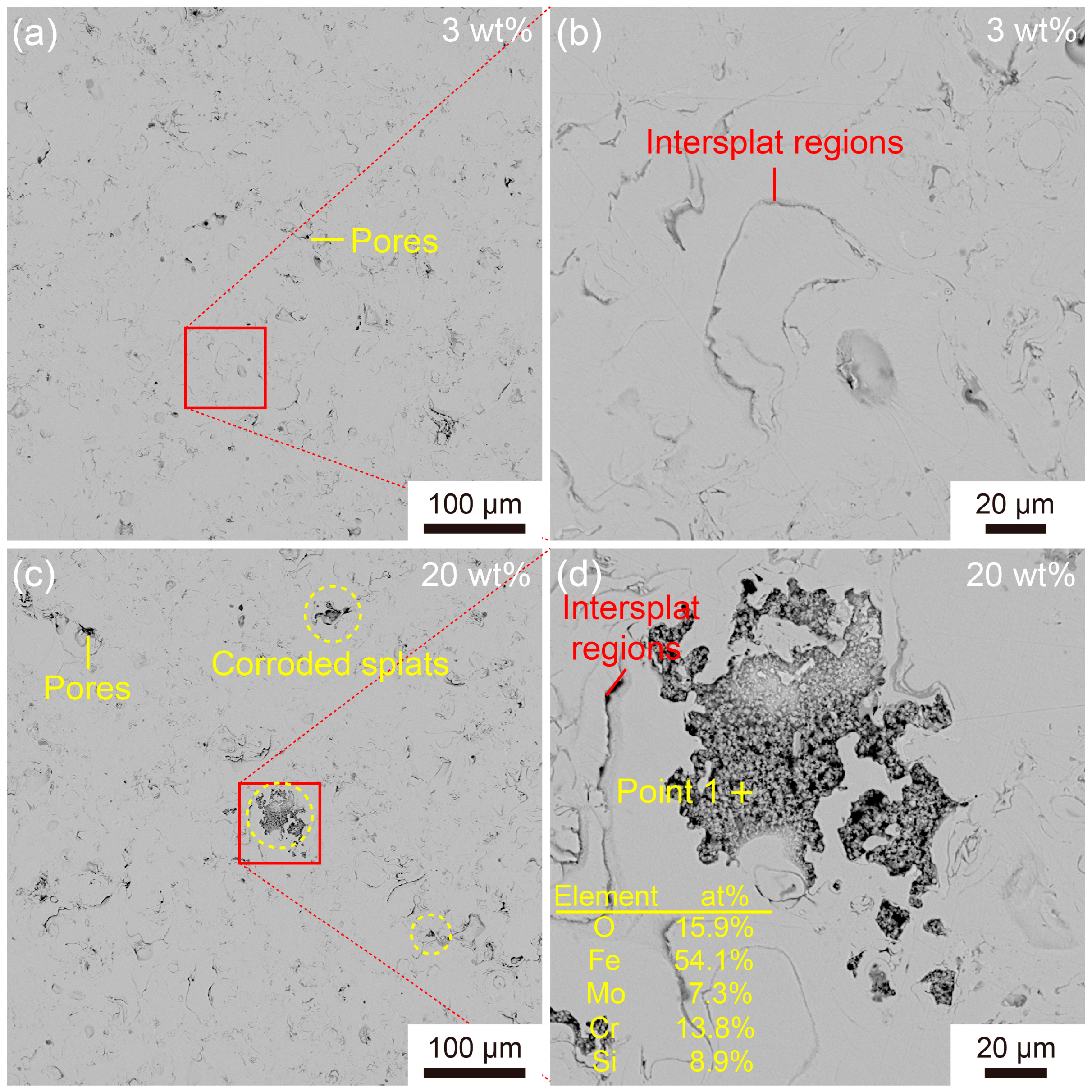
3.2. Corrosion Behavior of the Fe-Based AMC
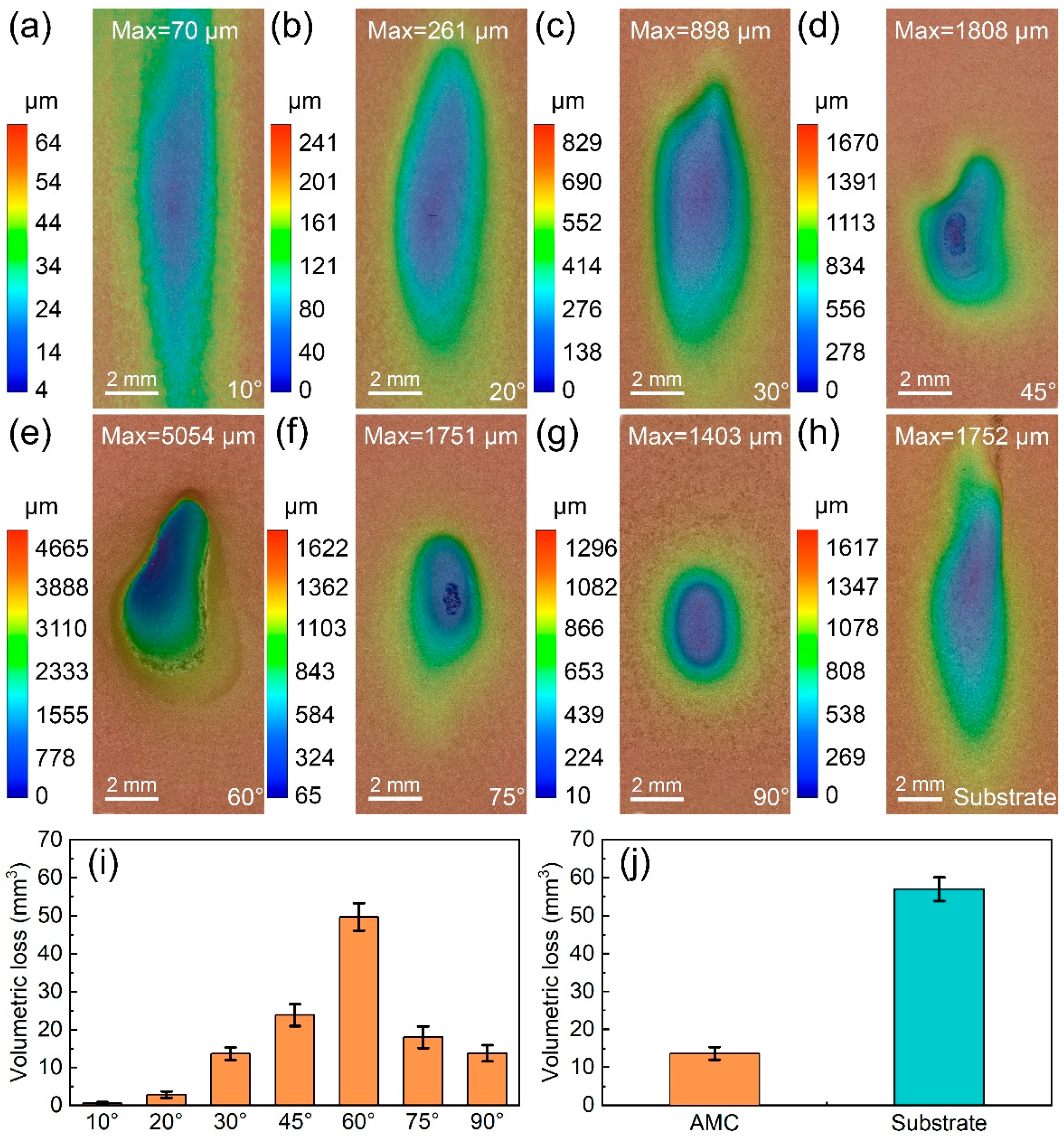
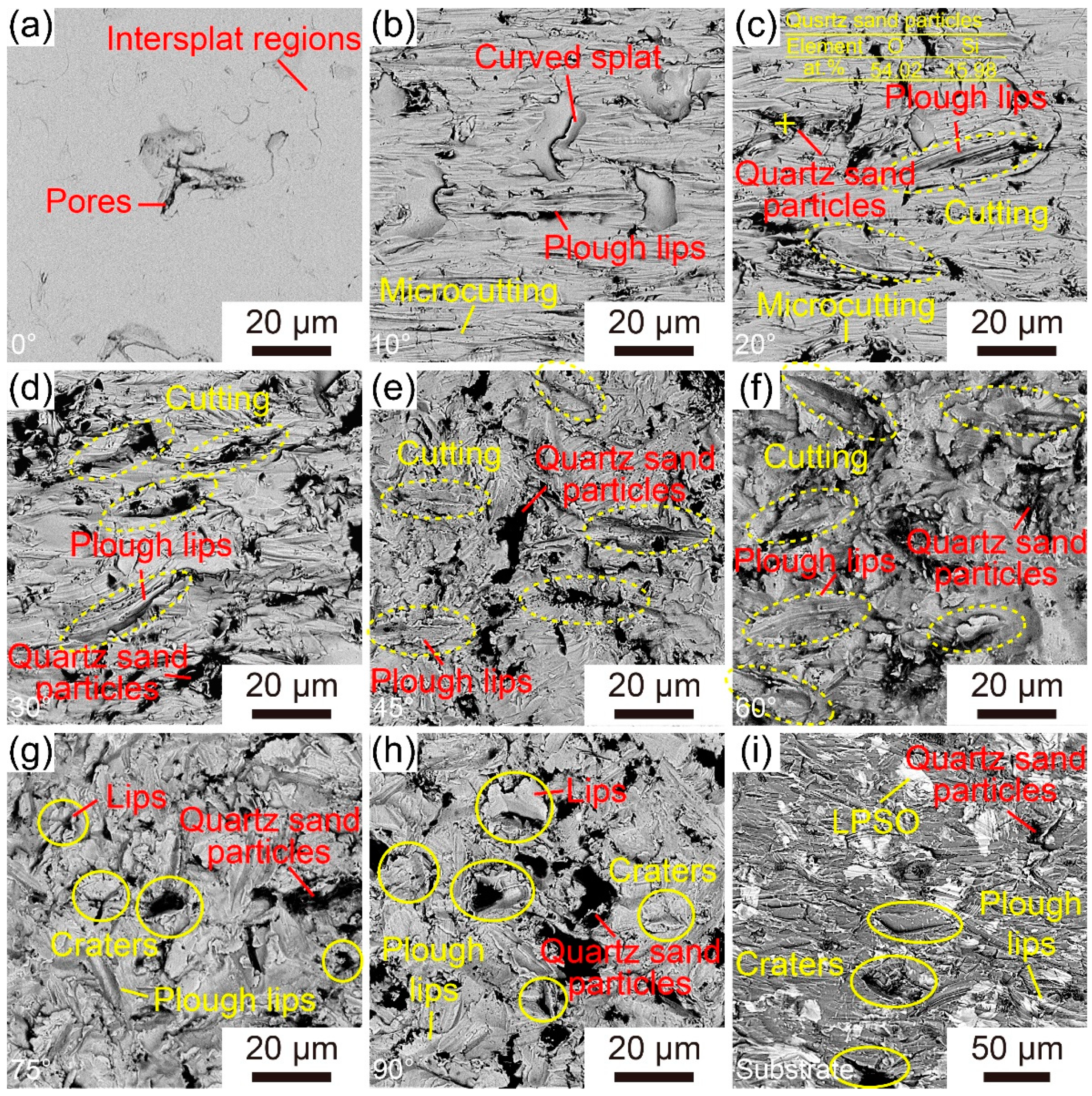
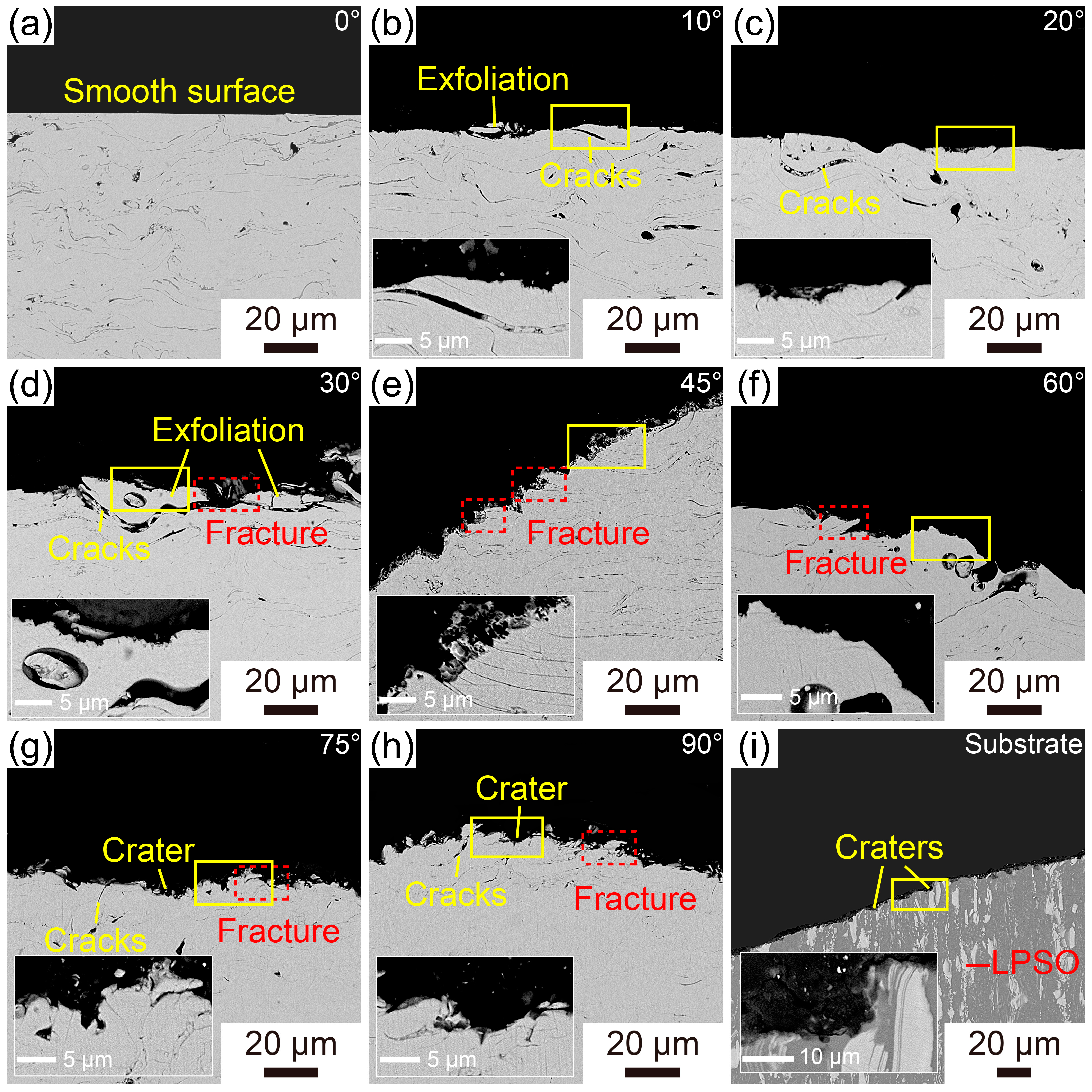
3.3. Erosion Wear Behavior of the Fe-Based AMCs
4. Conclusions
- Fe-based AMCs on dissolvable Mg-RE alloy substrates can have a thickness of 1000 μm without any cracks, and the porosity and amorphous contents of Fe-based AMCs are 0.79% and 86.8%, respectively.
- The corrosion resistance and pitting resistance of Fe-based AMCs decrease with the increase in KCl concentration, but Fe-based AMCs exhibit a low corrosion current density of only 3.31 μA/cm2 and a high pitting potential of 1 VSCE in 20 wt% KCl solution.
- Cl− ions can promote the transformation of oxides into hydroxides in the passive films on Fe-based AMCs, resulting in decreases in the compactness and protective efficacy of the passive films.
- The erosion rates of Fe-based AMCs have a nonlinear relationship with the impact angle, initially increasing before decreasing. Specifically, the Fe-based AMCs experience the highest erosion rate when the impact angle is 60°.
- At an impact angle of 30°, the erosion wear resistance of Fe-based AMCs is about 4.16 times higher than that of dissolvable Mg-RE alloy substrates. The erosion wear mechanisms of Fe-based AMCs vary with the impact angles, including cutting, delamination, splat fracture, and deformation wear.
- Fe-based AMCs can provide excellent protection against corrosion and erosion for dissolvable Mg-RE alloy substrates. This work proposes a potential solution to protect dissolvable magnesium alloy plugging tools by thermal spraying of Fe-based AMCs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Du, W.; Fu, J.; Liu, K.; Li, S.; Wang, Z.; Liang, H. A review on magnesium alloys for application of degradable fracturing tools. J. Magnes. Alloy 2022, 10, 2649–2672. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S.; Liu, Y.; Liu, D.; Gao, M.; Tian, Y.; Wang, J. A review on shale oil and gas characteristics and molecular dynamics simulation for the fluid behavior in shale pore. J. Mol. Liq. 2023, 376, 121507. [Google Scholar] [CrossRef]
- Jiao, F. Re-recognition of “unconventional” in unconventional oil and gas. Petrol. Explor. Dev. 2019, 46, 847–855. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Y.; Wang, H.; Chen, C.; Qin, J.; Liu, Z.; Shen, Y. Numerical simulation of the conveyance characteristics of fracturing ball in the horizontal section. J. Nat. Gas Sci. Eng. 2016, 34, 401–411. [Google Scholar] [CrossRef]
- Yao, B.; Ding, Q.; Hou, Y.; Liu, S.; Zhang, S. A new openhole multistage hydraulic fracturing system and the ball plug motion in a horizontal pipe. J. Nat. Gas Sci. Eng. 2018, 50, 11–21. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Y.; Wang, H.; Zhu, H.; Ji, R.; Liu, Z.; Shen, Y. Research on the effect of gas nitriding treatment on the wear resistance of ball seat used in multistage fracturing. Mater. Des. 2015, 70, 45–52. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Yang, J.; Zhang, J. Effects of Temperature and Pressure on Corrosion Behavior of HVOF-Sprayed Fe-Based Amorphous Coating on the Mg-RE Alloy for Dissolvable Plugging Tools. Materials 2023, 16, 1313. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Li, H.X.; Ma, Y.Z.; Zhao, K.N.; Yang, C.L.; Zhang, J.S. Effect of trace Ni addition on microstructure, mechanical and corrosion properties of the extruded Mg–Gd–Y–Zr–Ni alloys for dissoluble fracturing tools. J. Magnes. Alloy 2021, 9, 1632–1643. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Y.; Wang, H.; Qin, J.; Chen, C.; Liu, Z.; Shen, Y. Finite element analysis and experimental study on the deformation characteristics of an aluminum alloy fracturing ball. J. Nat. Gas Sci. Eng. 2016, 35, 203–210. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Y.; Wang, H.; Zhu, H.; Liu, Z.; Cai, B.; Shen, Y. Numerical study on improving the erosion life of ball seat for oil and gas reservoir fracturing. Eng. Fail. Anal. 2016, 60, 188–198. [Google Scholar] [CrossRef]
- Pereira, G.S.; Prada, R.O.M.; Avila, P.R.T.; Avila, J.A.; Pinto, H.C.; Miyazaki, M.H.; de Melo, H.G.; Bose Filho, W.W. Cerium conversion coating and sol-gel coating for corrosion protection of the WE43 Mg alloy. Corros. Sci. 2022, 206, 110527. [Google Scholar] [CrossRef]
- Wan, H.; Song, D. Corrosion failure process of organic conductive coating on Mg-RE alloy with PEO in the simulated Xisha atmospheric solution. Mater. Chem. Phys. 2022, 291, 126771. [Google Scholar] [CrossRef]
- Jothi, V.; Adesina, A.Y.; Kumar, A.M.; Rahman, M.M.; Ram, J.S.N. Enhancing the biodegradability and surface protective performance of AZ31 Mg alloy using polypyrrole/gelatin composite coatings with anodized Mg surface. Surf. Coat. Technol. 2020, 381, 125139. [Google Scholar] [CrossRef]
- Singh, C.; Tiwari, S.K.; Singh, R. Development of corrosion-resistant electroplating on AZ91 Mg alloy by employing air and water-stable eutectic based ionic liquid bath. Surf. Coat. Technol. 2021, 428, 127881. [Google Scholar] [CrossRef]
- Witecka, A.; Yamamoto, A.; Idaszek, J.; Chlanda, A.; Swieszkowski, W. Influence of biodegradable polymer coatings on corrosion, cytocompatibility and cell functionality of Mg-2.0Zn-0.98Mn magnesium alloy. Colloids Surf. B 2016, 144, 284–292. [Google Scholar] [CrossRef]
- Ishizaki, T.; Hieda, J.; Saito, N.; Saito, N.; Takai, O. Corrosion resistance and chemical stability of super-hydrophobic film deposited on magnesium alloy AZ31 by microwave plasma-enhanced chemical vapor deposition. Electrochim. Acta 2010, 55, 7094–7101. [Google Scholar] [CrossRef]
- Liu, L.; Yu, S.; Zhu, G.; Li, Q.; Liu, E.; Xiong, W.; Wang, B.; Yang, X. Corrosion and wear resistance of micro-arc oxidation coating on glass microsphere reinforced Mg alloy composite. J. Mater. Sci. 2021, 56, 15379–15396. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Y.; Qin, J.; Chen, C.; Ji, R. Wear behavior of HVOF sprayed WC coating under water-in-oil fracturing fluid condition. Tribol. Int. 2017, 115, 28–34. [Google Scholar] [CrossRef]
- Bhosale, D.G.; Rathod, W.S. Investigation on wear behaviour of SS 316L, atmospheric plasma and high velocity oxy-fuel sprayed WC-Cr3C2-Ni coatings for fracturing tools. Surf. Coat. Technol. 2020, 390, 125679. [Google Scholar] [CrossRef]
- Nayak, S.K.; Kumar, A.; Laha, T. Fe-based metallic glass coatings by thermal spraying: A focused review on corrosion properties and related degradation mechanisms. Int. Mater. Rev. 2022, 68, 404–485. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.W.; Chen, Q.; Liu, L. Effect of hydrostatic pressure on the corrosion behavior of HVOF-sprayed Fe-based amorphous coating. J. Alloys Compd. 2018, 758, 108–115. [Google Scholar] [CrossRef]
- Zhang, L.M.; Yan, M.C.; Zhang, S.D.; Zhu, L.Y.; Umoh, A.J.; Ma, A.L.; Zheng, Y.G.; Wang, J.Q. Significantly enhanced resistance to SRB corrosion via Fe-based amorphous coating designed with high dose corrosion-resistant and antibacterial elements. Corros. Sci. 2020, 164, 108305. [Google Scholar] [CrossRef]
- Gan, Z.; Zhang, C.; Zhang, Z.-R.; Chen, Z.-J.; Liu, L. Crystallization-dependent transition of corrosion resistance of an Fe-based bulk metallic glass under hydrostatic pressures. Corros. Sci. 2021, 179, 109098. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.G.; Ke, W.; Sun, W.H.; Hou, W.L.; Chang, X.C.; Wang, J.Q. Slurry erosion–corrosion behaviour of high-velocity oxy-fuel (HVOF) sprayed Fe-based amorphous metallic coatings for marine pump in sand-containing NaCl solutions. Corros. Sci. 2011, 53, 3177–3185. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G.; Sun, W.H.; Wang, J.Q. Effect of applied potential on passivation and erosion–corrosion of a Fe-based amorphous metallic coating under slurry impingement. Corros. Sci. 2014, 82, 115–124. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G.; Zhou, X.; He, S.Y.; Sun, W.H.; Wang, J.Q. Determination of the critical flow velocities for erosion–corrosion of passive materials under impingement by NaCl solution containing sand. Corros. Sci. 2014, 88, 187–196. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G.; Sun, W.H.; Wang, J.Q. Erosion–corrosion of HVOF-sprayed Fe-based amorphous metallic coating under impingement by a sand-containing NaCl solution. Corros. Sci. 2013, 76, 337–347. [Google Scholar] [CrossRef]
- Wu, T.; Shi, W.; Xie, L.; Gong, M.; Huang, J.; Xie, Y.; He, K. Study on the effect of Ni60 transition coating on microstructure and mechanical properties of Fe/WC composite coating by laser cladding. Opt. Laser Technol. 2023, 163, 109387. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Zhang, J.; Li, Y.; Jia, L.; Liu, B.; Li, H. A dissolvable Mg-Gd-Y-Zn-Cu alloy possessing the highest yield strength for the fabrication of fracturing plugging tools. Mater. Lett. 2023, 338, 134053. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Zhang, Z.; Li, Y.; Jia, L.; Wang, J.; Wang, J.; Zhang, J. Effect of Cu micro-alloying on the microstructure, mechanical and corrosion properties of Mg-Gd-Y-Zn based alloy applied as plugging tools. J. Alloys Compd. 2023, 939, 168768. [Google Scholar] [CrossRef]
- Tian, W.P.; Yang, H.W.; Zhang, S.D. Synergistic Effect of Mo, W, Mn and Cr on the Passivation Behavior of a Fe-Based Amorphous Alloy Coating. Acta Metall. Sin. 2017, 31, 308–320. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S.D.; Sun, W.H.; Wang, J.Q. Influence of oxidation related structural defects on localized corrosion in HVAF-sprayed Fe-based metallic coatings. Surf. Coat. Technol. 2018, 335, 205–218. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Zuo, X.; Guo, P.; Liu, L.; Lee, K.R.; Wang, A.; Ke, P. Cr/GLC multilayered coating in simulated deep-sea environment: Corrosion behavior and growth defect evolution. Corros. Sci. 2021, 188, 109528. [Google Scholar] [CrossRef]
- Veluchamy, A.; Sherwood, D.; Emmanuel, B.; Cole, I.S. Critical review on the passive film formation and breakdown on iron electrode and the models for the mechanisms underlying passivity. J. Electroanal. Chem. 2017, 785, 196–215. [Google Scholar] [CrossRef]
- Xu, J.; Liu, L.; Li, Z.; Munroe, P.; Xie, Z.H. Niobium addition enhancing the corrosion resistance of nanocrystalline Ti5Si3 coating in H2SO4 solution. Acta Mater. 2014, 63, 245–260. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Z.; Wang, Q.; Wang, Z.; Zhang, X.; Liu, Y. Role of passive film in dominating the electrochemical corrosion behavior of FeCrMoCBY amorphous coating. J. Alloys Compd. 2019, 811, 151962. [Google Scholar] [CrossRef]
- Wang, L.; Tian, H.; Gao, H.; Xie, F.; Zhao, K.; Cui, Z. Electrochemical and XPS analytical investigation of the accelerative effect of bicarbonate/carbonate ions on AISI 304 in alkaline environment. Appl. Surf. Sci. 2019, 492, 792–807. [Google Scholar] [CrossRef]
- Liang, D.D.; Wei, X.S.; Wang, Y.; Jiang, H.R.; Shen, J. Electrochemical behaviors and passive film properties of Fe-based bulk metallic glass in Cl−-containing acetic acid solutions under high temperature. J. Alloys Compd. 2018, 766, 964–972. [Google Scholar] [CrossRef]
- Ogunsanya, I.G.; Hansson, C.M. The semiconductor properties of passive films and corrosion behavior of stainless steel reinforcing bars in simulated concrete pore solution. Materialia 2019, 6, 100321. [Google Scholar] [CrossRef]
- Meng, G.; Li, Y.; Shao, Y.; Zhang, T.; Wang, Y.; Wang, F. Effect of Cl− on the Properties of the Passive Films Formed on 316L Stainless Steel in Acidic Solution. J. Mater. Sci. Technol. 2014, 30, 253–258. [Google Scholar] [CrossRef]
- Sun, Y.J.; Yang, R.; Xie, L.; Li, Y.B.; Wang, S.L.; Li, H.X.; Wang, W.R.; Zhang, J.S. Interfacial bonding and corrosion behaviors of HVOF-sprayed Fe-based amorphous coating on 8090 Al-Li alloy. Surf. Coat. Technol. 2022, 436, 128316. [Google Scholar] [CrossRef]
- Singh, B.; Zafar, S. Effect of microwave exposure time on microstructure and slurry erosion behavior of Ni + 20% Cr7C3 composite clads. Wear 2019, 426–427, 491–500. [Google Scholar] [CrossRef]


| Coatings | Fe-Based AMCs | Ni60 Interlayers |
|---|---|---|
| Kerosene flow rate (L/s) | 0.0074 | 0.0063 |
| Oxygen flow rate (m3/s) | 0.0149 | 0.0146 |
| Spray distance (mm) | 320 | 330 |
| Powder feed rate (g/s)) | 0.6667 | 1.3333 |
| Scanning velocity (mm/s) | 300 | 300 |
| Samples | Ecorr (VSCE) | Icorr (μA/cm2) | Epit (VSCE) | Ipit (μA/cm2) |
|---|---|---|---|---|
| 0.05 wt% | −0.296 | 0.64 | 1.002 | 29.71 |
| 1 wt% | −0.288 | 1.16 | 1.008 | 162.56 |
| 3 wt% | −0.287 | 1.66 | 1.003 | 189.23 |
| 5 wt% | −0.292 | 2.06 | 1.002 | 193.51 |
| 10 wt% | −0.257 | 2.68 | 1.007 | 266.45 |
| 20 wt% | −0.274 | 3.31 | 1.002 | 648.78 |
| Samples | 0.05 wt% | 1 wt% | 3 wt% | 5 wt% | 10 wt% | 20 wt% |
|---|---|---|---|---|---|---|
| Rs (Ω·cm2) | 99.54 | 12.43 | 3.96 | 2.72 | 1.43 | 0.77 |
| Rc (Ω·cm2) | 337,440 | 107,500 | 98,060 | 93,491 | 59,629 | 38,112 |
| CPEc (S·sn·cm−2) | 3.38 × 10−5 | 5.03 × 10−5 | 6.53 × 10−5 | 7.61 × 10−5 | 8.58 × 10−5 | 9.09 × 10−5 |
| CPEc-n | 0.891 | 0.865 | 0.879 | 0.879 | 0.882 | 0.877 |
| Rt (Ω·cm2) | 999,910 | 144,420 | 112,630 | 66,516 | 56,394 | 31,655 |
| CPEdl (S·sn·cm−2) | 1.06 × 10−5 | 3.24 × 10−5 | 3.89 × 10−5 | 7.51 × 10−5 | 9.68 × 10−5 | 1.42 × 10−4 |
| CPEdl-n | 0.869 | 0.841 | 0.834 | 0.824 | 0.846 | 0.845 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Sun, Y.; Su, M.; Yin, X.; Li, H.; Zhang, J. Corrosion and Erosion Wear Behaviors of HVOF-Sprayed Fe-Based Amorphous Coatings on Dissolvable Mg-RE Alloy Substrates. Materials 2023, 16, 5170. https://doi.org/10.3390/ma16145170
Yang J, Sun Y, Su M, Yin X, Li H, Zhang J. Corrosion and Erosion Wear Behaviors of HVOF-Sprayed Fe-Based Amorphous Coatings on Dissolvable Mg-RE Alloy Substrates. Materials. 2023; 16(14):5170. https://doi.org/10.3390/ma16145170
Chicago/Turabian StyleYang, Jun, Yijiao Sun, Minwen Su, Xueming Yin, Hongxiang Li, and Jishan Zhang. 2023. "Corrosion and Erosion Wear Behaviors of HVOF-Sprayed Fe-Based Amorphous Coatings on Dissolvable Mg-RE Alloy Substrates" Materials 16, no. 14: 5170. https://doi.org/10.3390/ma16145170
APA StyleYang, J., Sun, Y., Su, M., Yin, X., Li, H., & Zhang, J. (2023). Corrosion and Erosion Wear Behaviors of HVOF-Sprayed Fe-Based Amorphous Coatings on Dissolvable Mg-RE Alloy Substrates. Materials, 16(14), 5170. https://doi.org/10.3390/ma16145170







