Possibilities of Checking Water Content in Porous Geopolymer Materials Using Impedance Spectroscopy Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geopolymer Preparation
2.2. The Construction of the Measuring System
3. Results
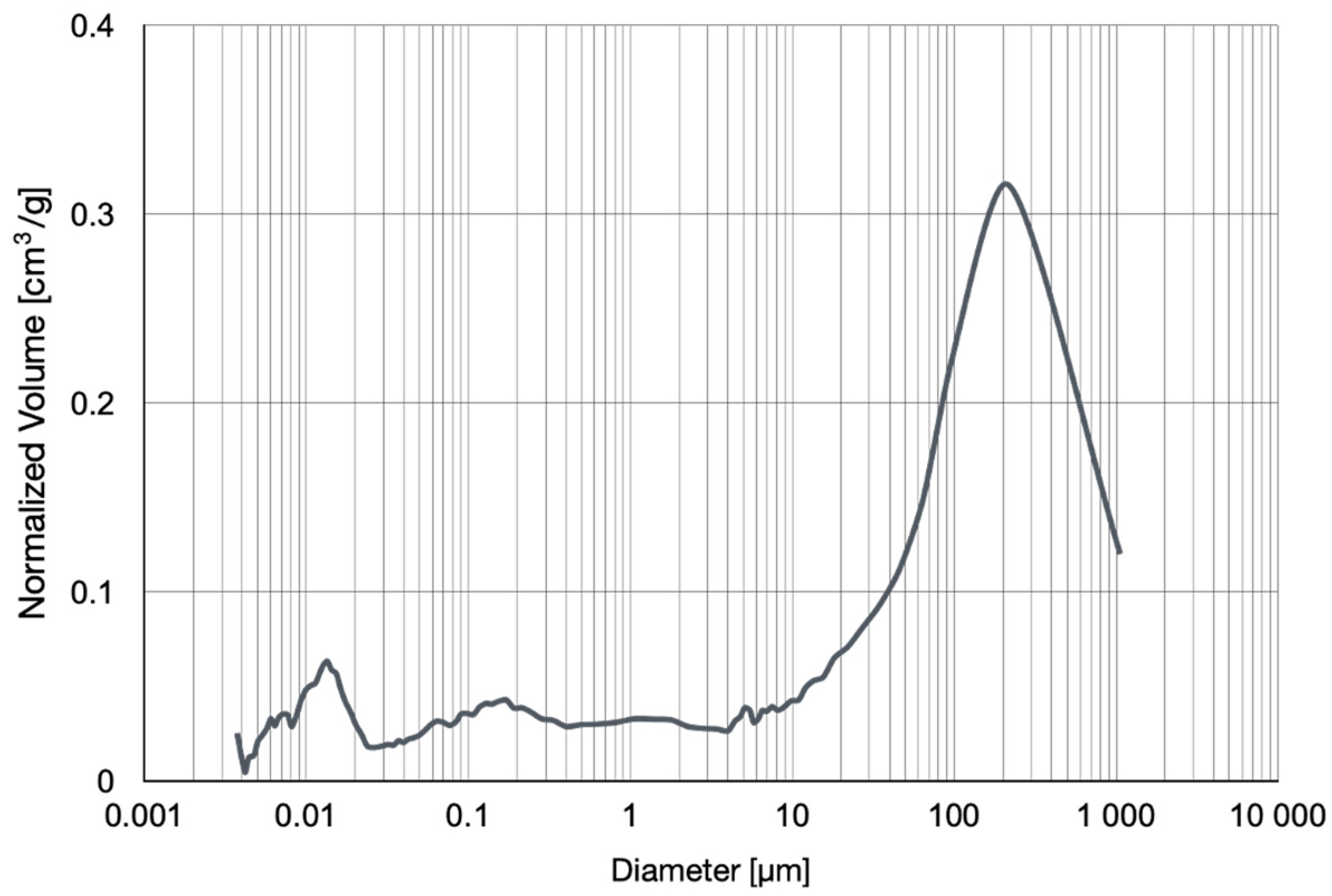
3.1. Mercury Porosity Measurements
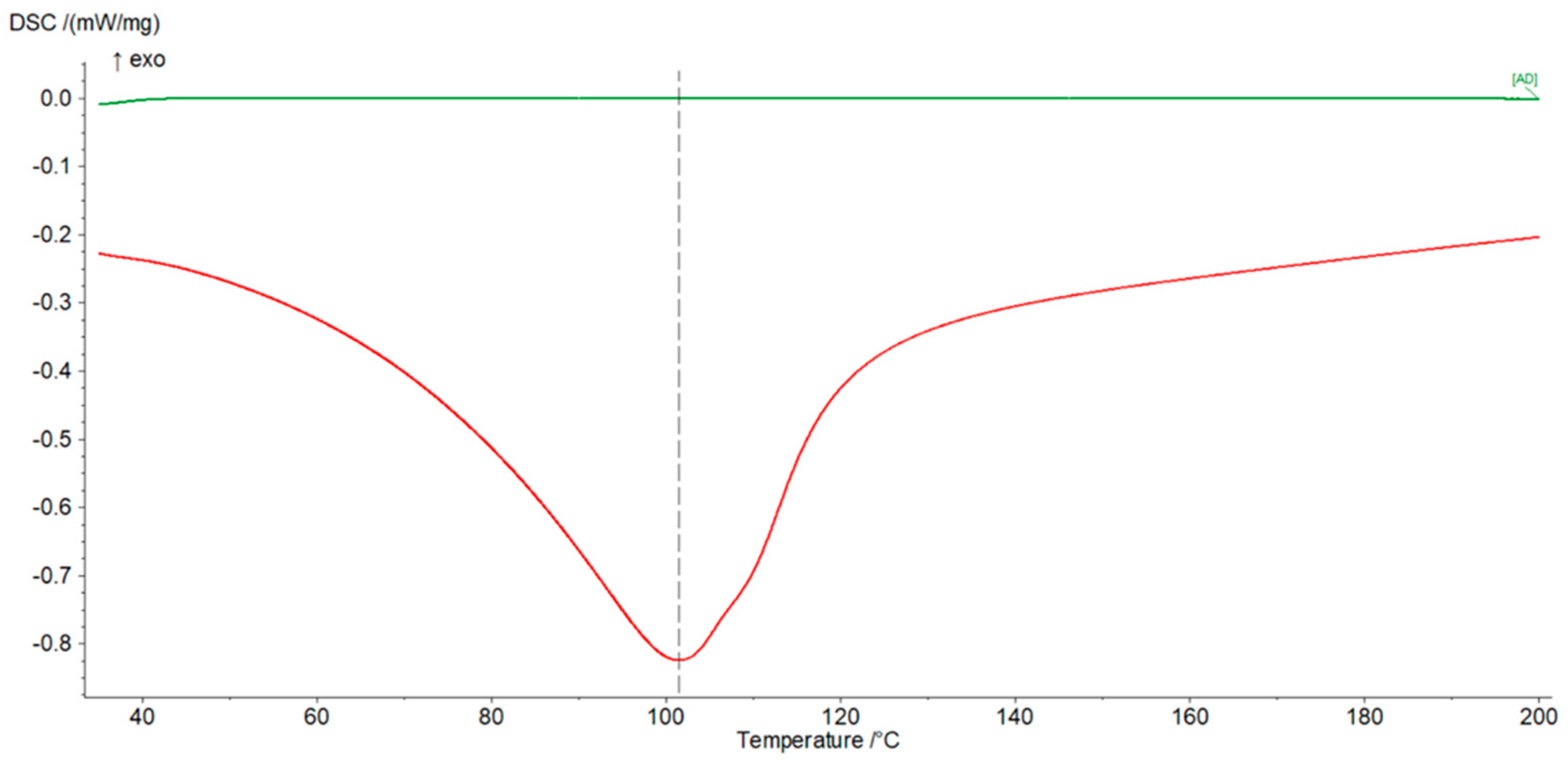
3.2. DSC Measurements
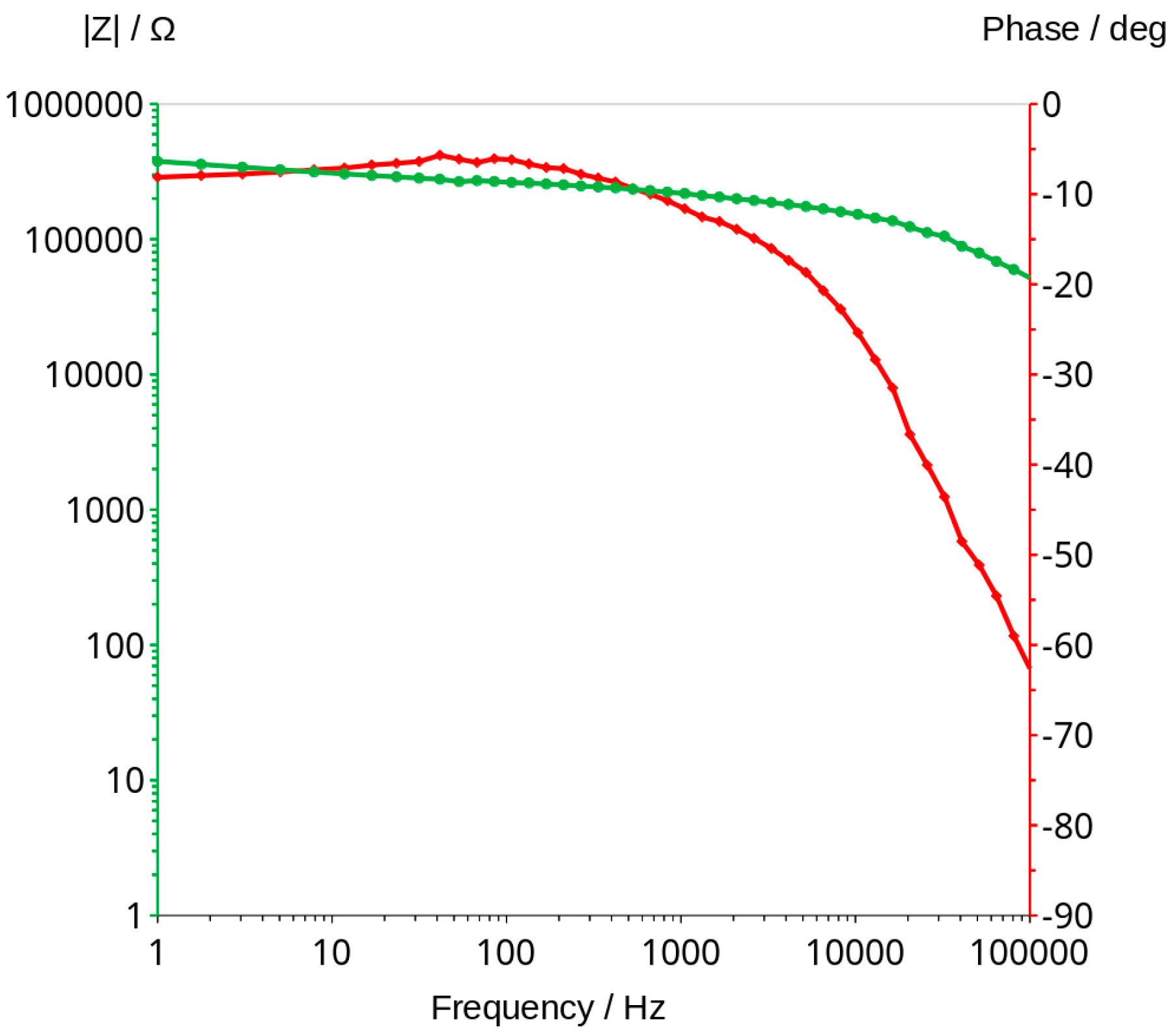
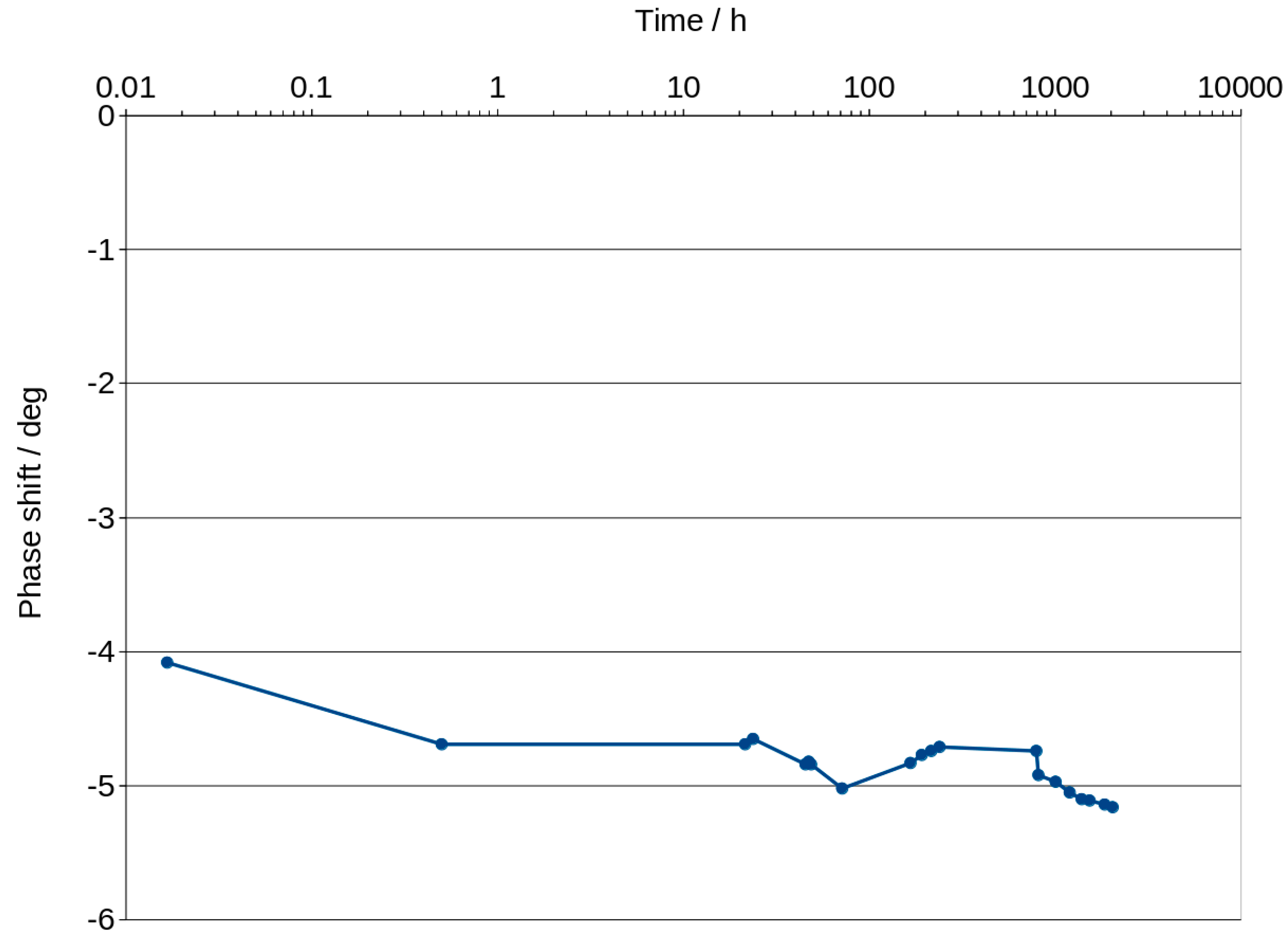
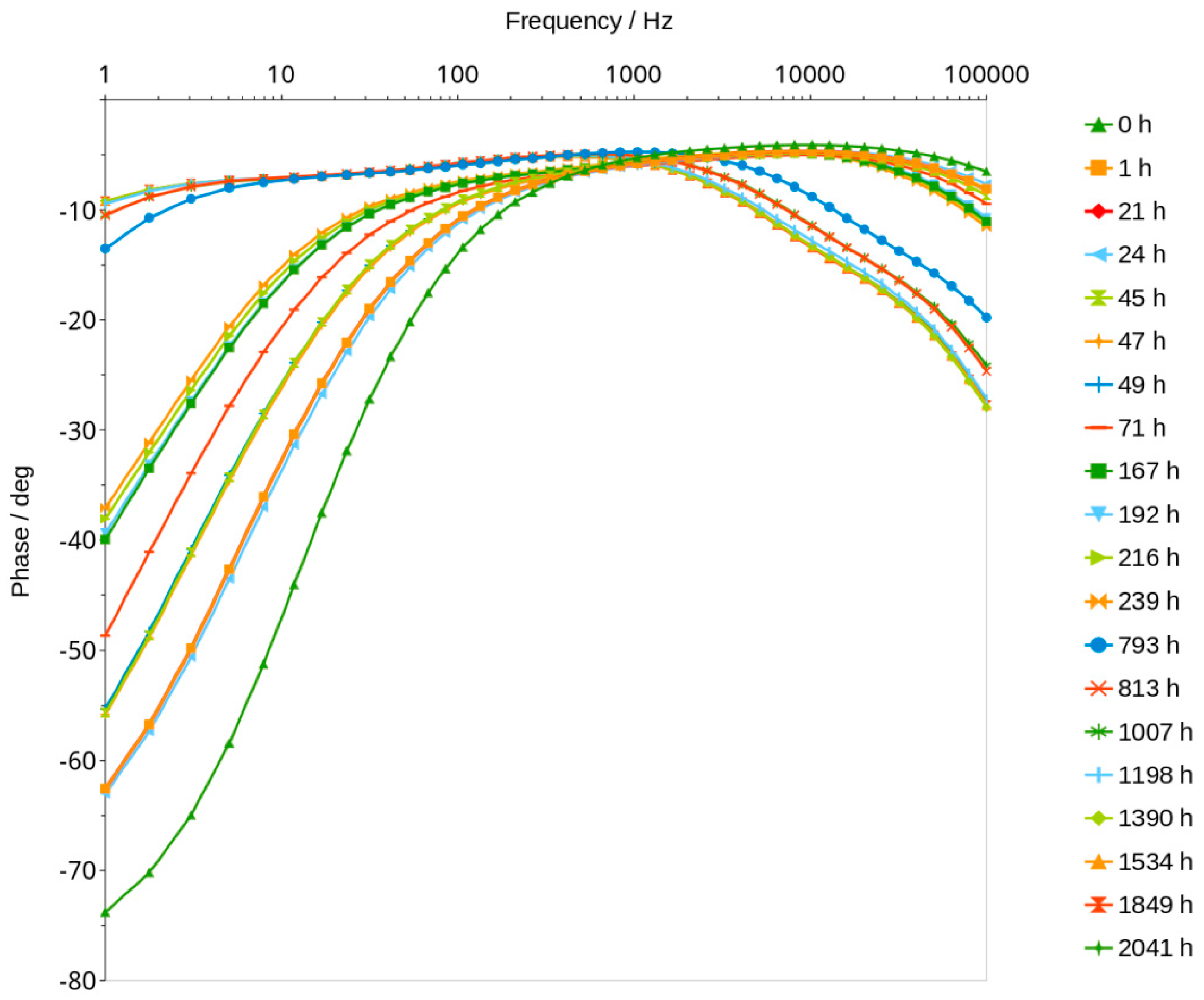
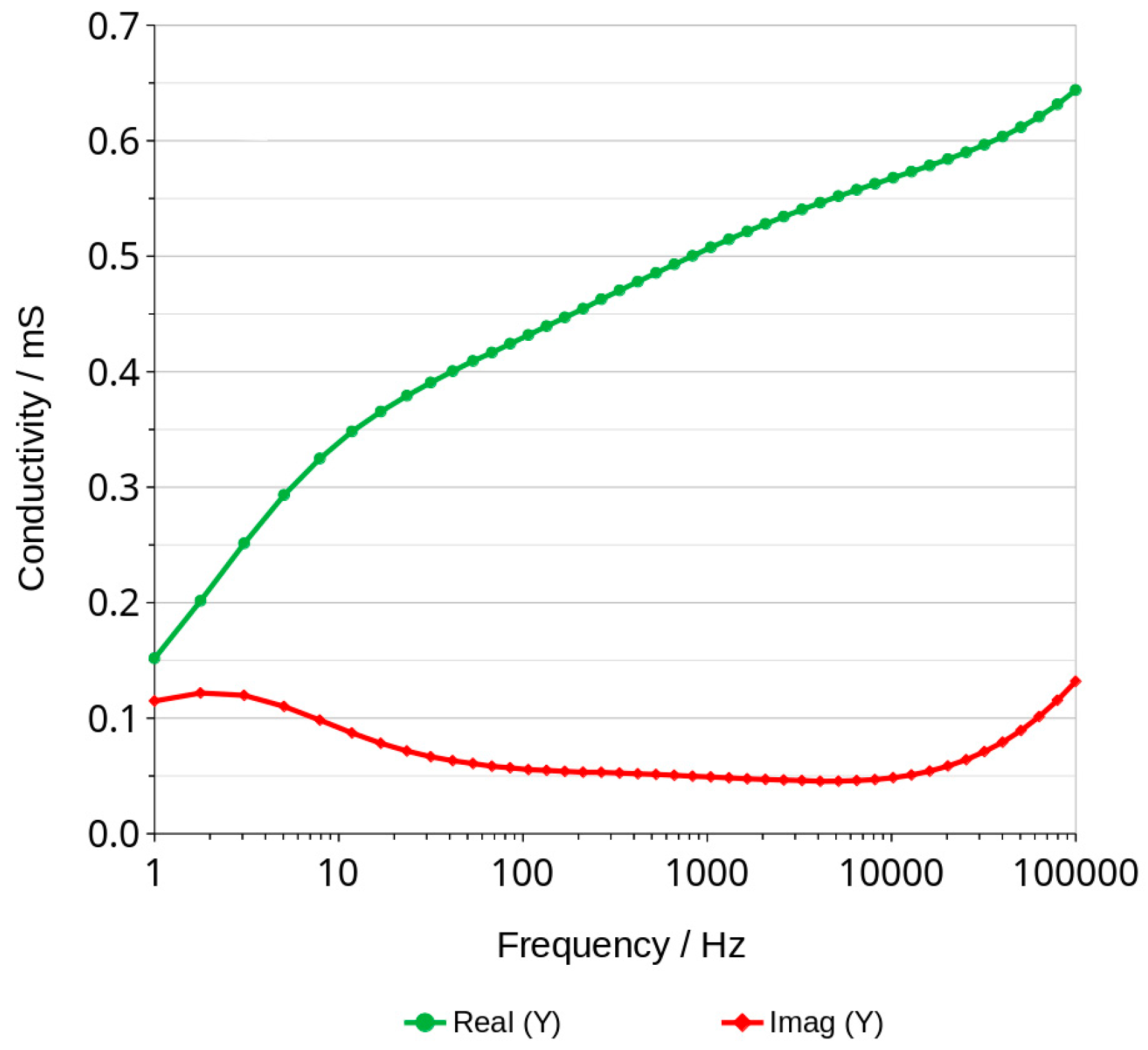
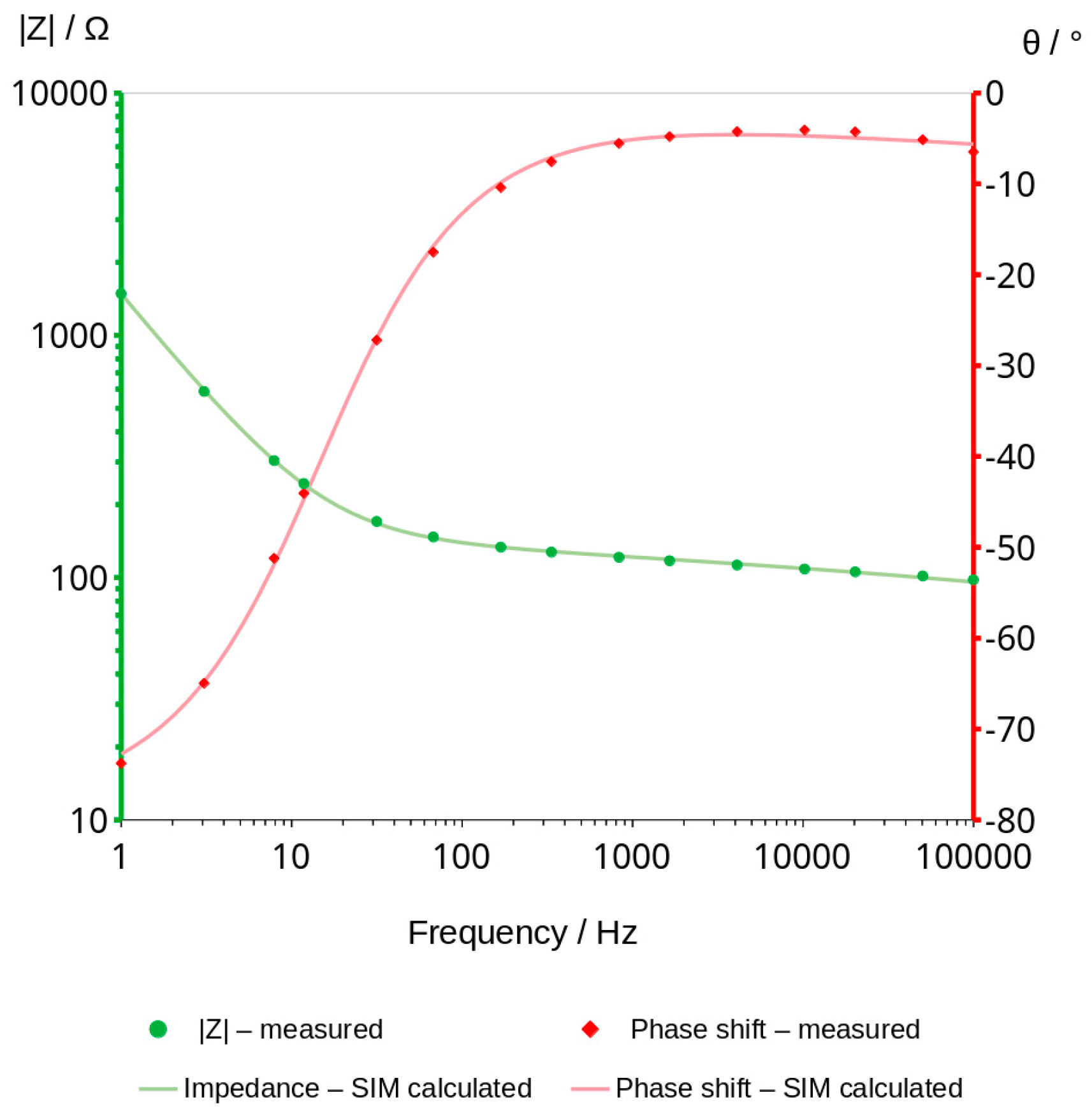
3.3. Bode Curves and Related Graphs
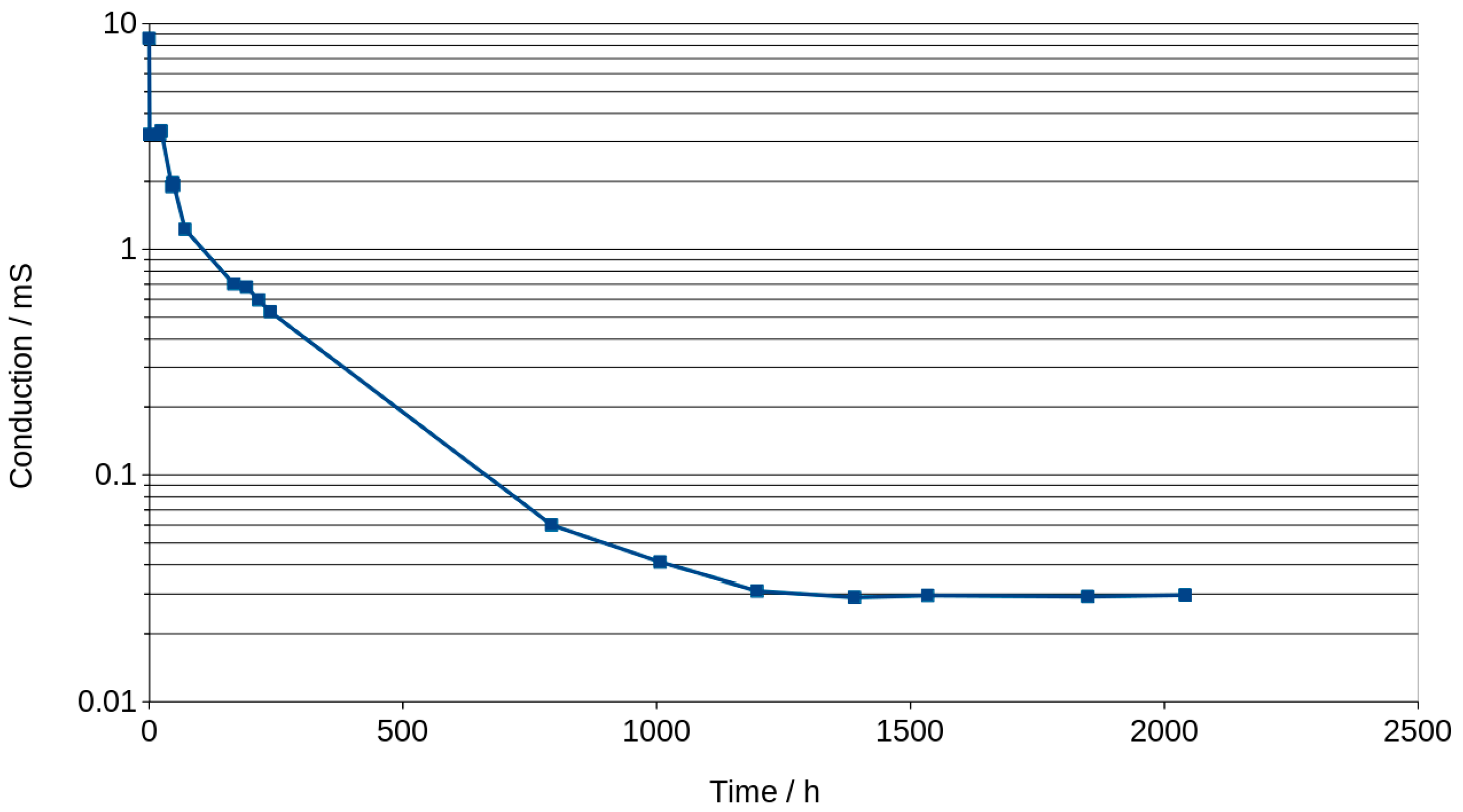
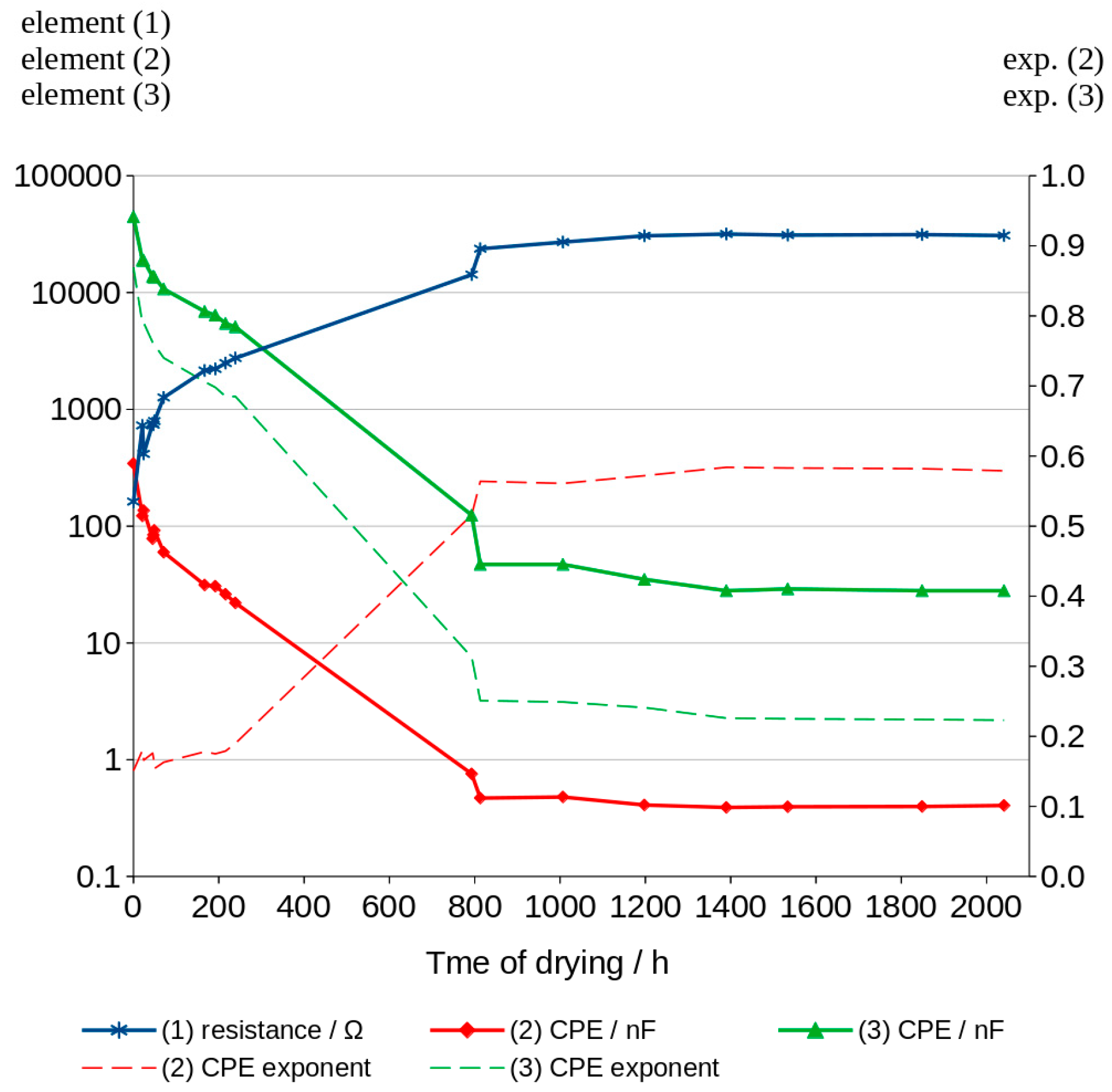
3.4. Analysis of Graphs Using SIM Module
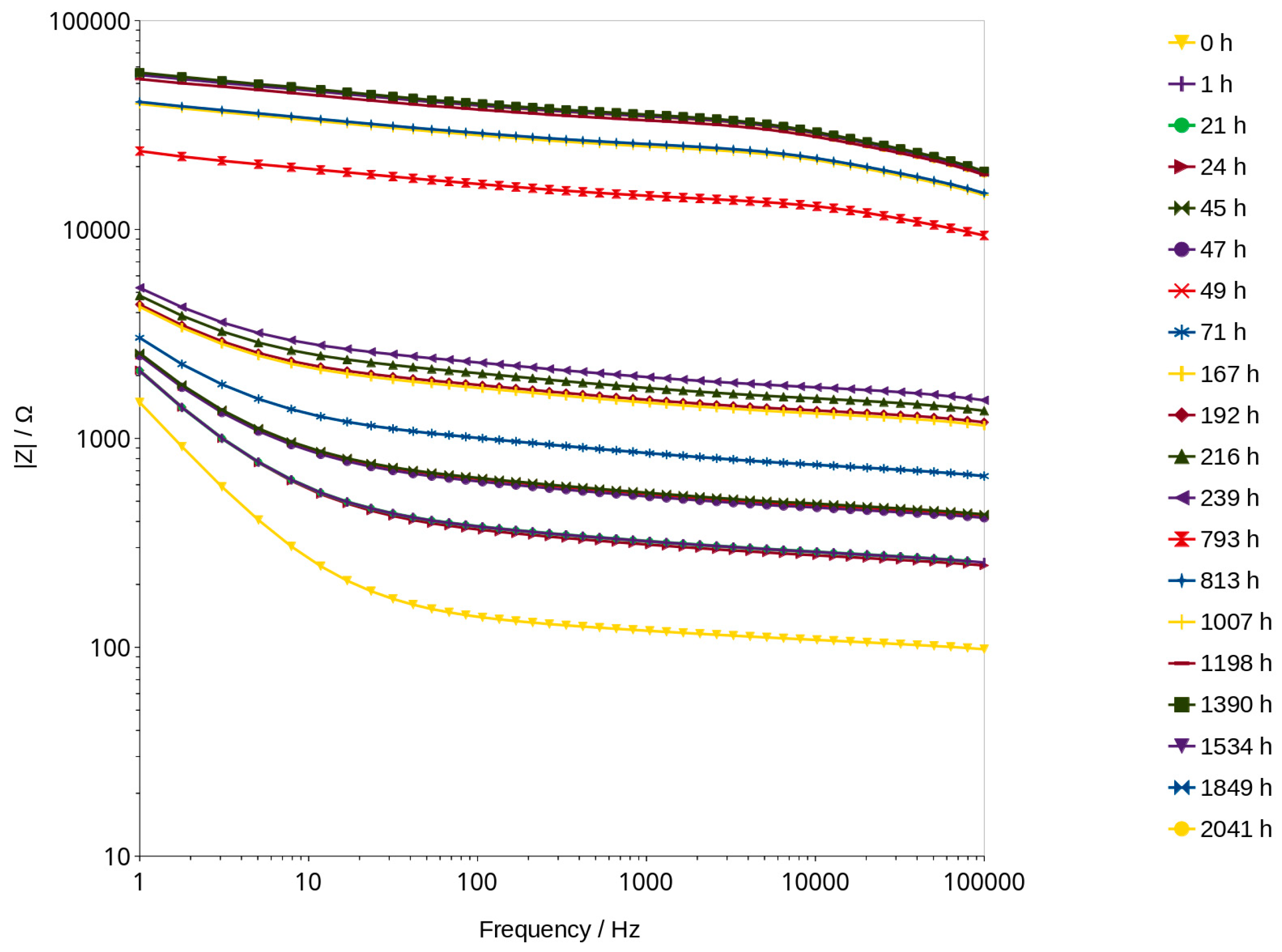
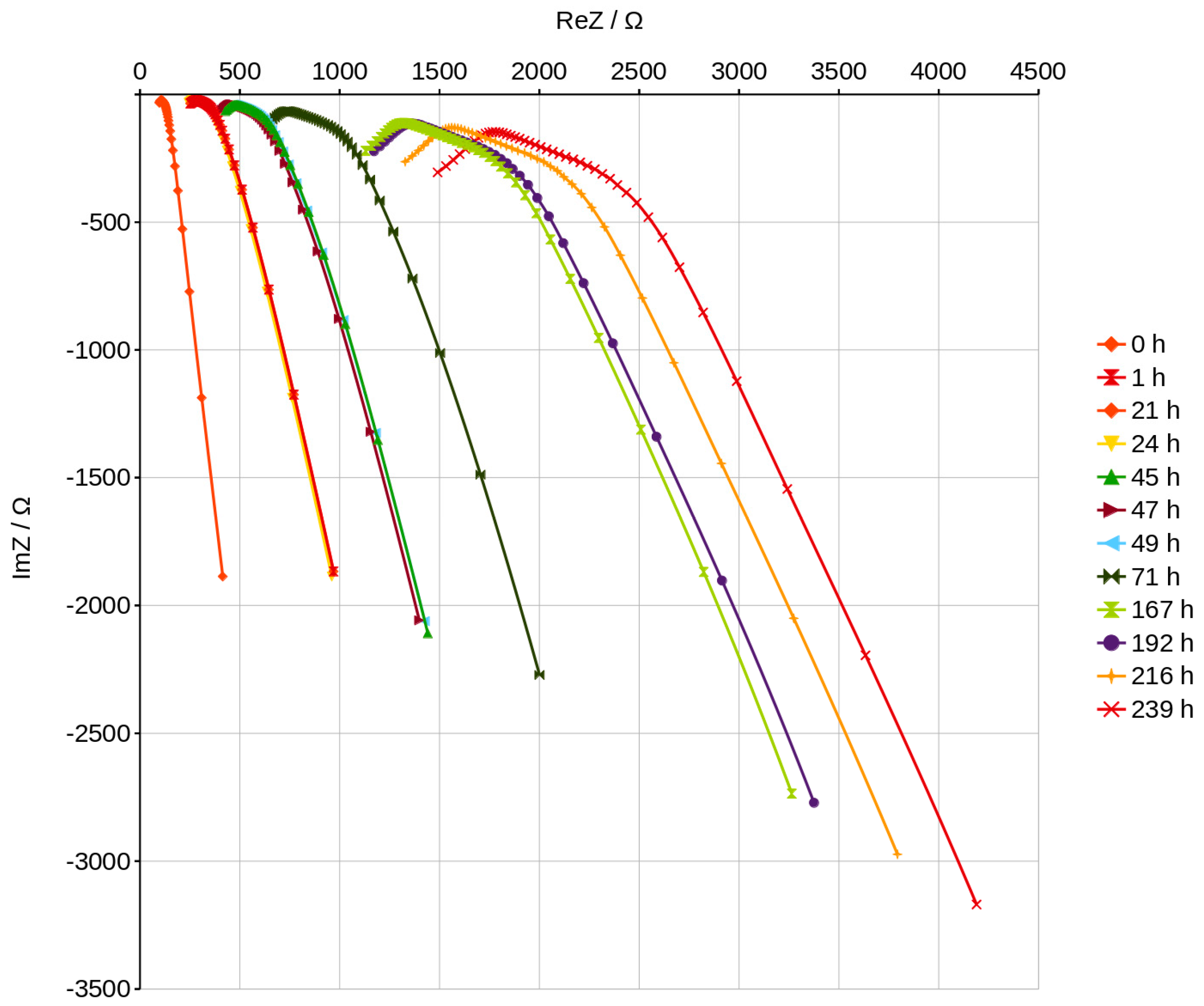
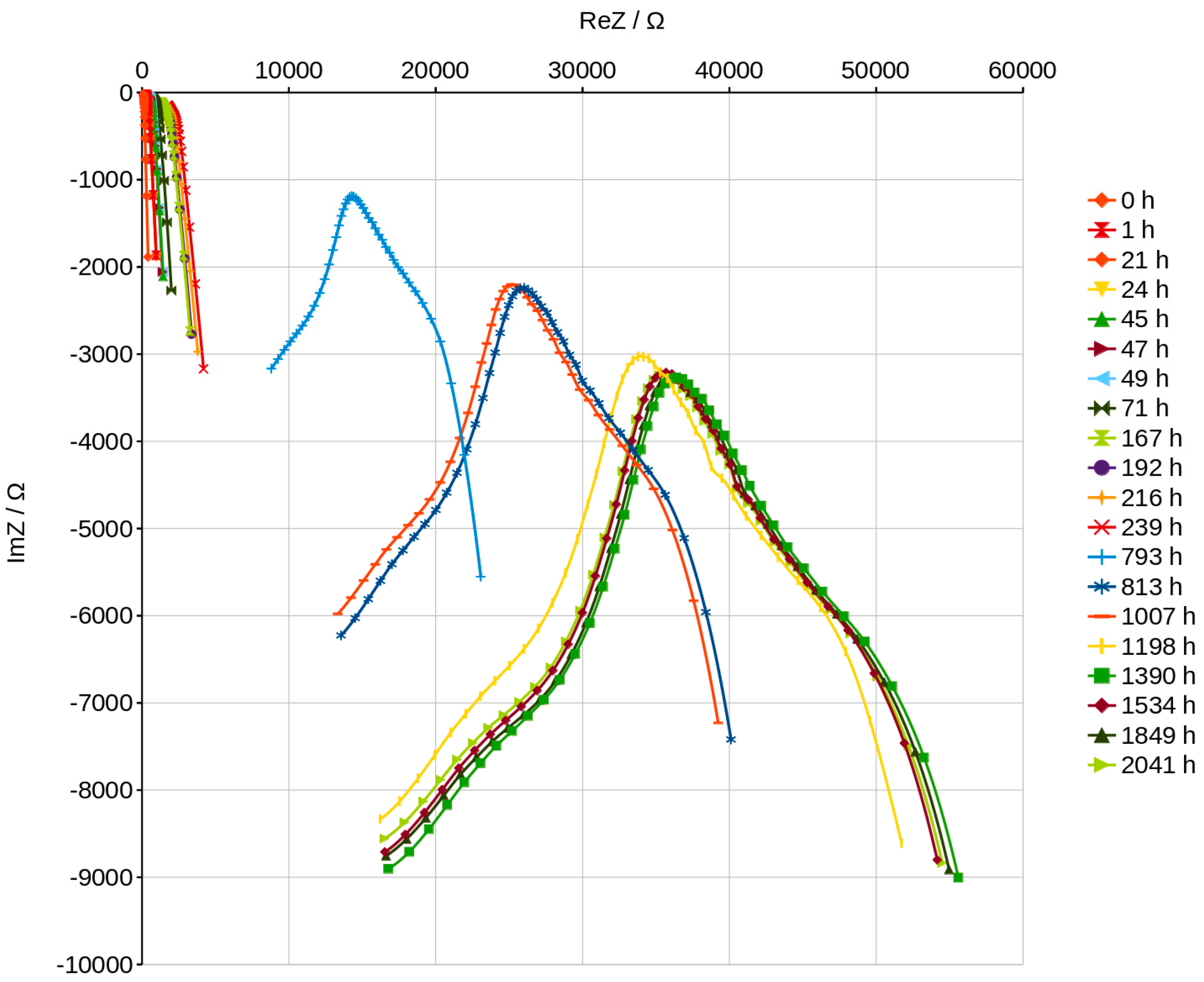
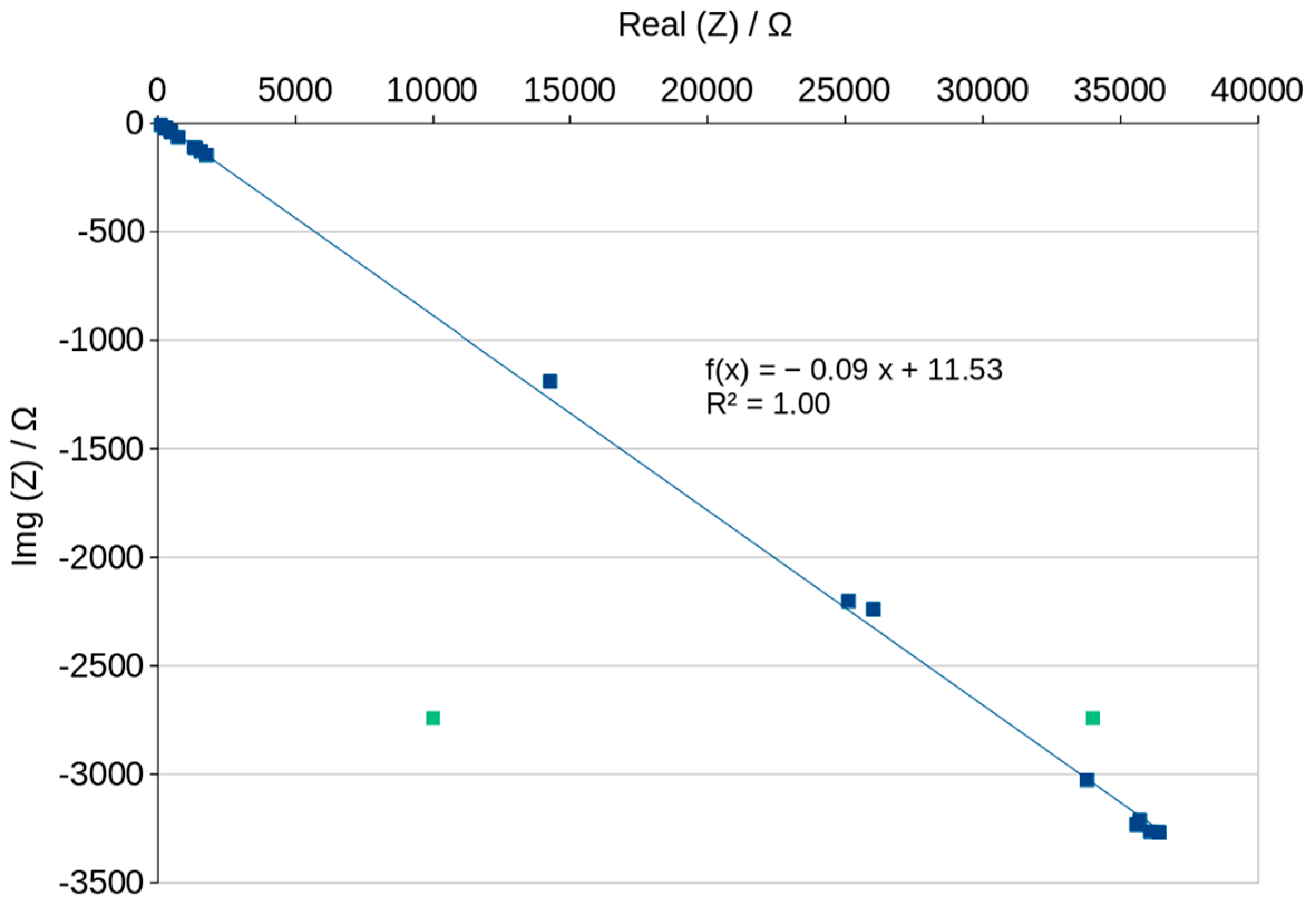
3.5. Nyquist Plots
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davidovits, J. Geopolymer Chemistry; Geopolymer Institute: Saint-Quentin, France, 2008. [Google Scholar]
- Hanjitsuwan, S.; Chindaprasirt, P.; Pimraksa, K. Electrical conductivity and dielectric property of fly ash geopolymer pastes. Int. J. Miner. Metall. Mater. 2011, 18, 94–99. [Google Scholar]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Mallikarjuna Rao, G.; Gunneswara Rao, T.D. Final Setting Time and Compressive Strength of Fly Ash and GGBS-Based Geopolymer Paste and Mortar. Arab. J. Sci. Eng. 2015, 40, 3067–3074. [Google Scholar]
- Zhanga, Z.; Wanga, H.; Provisb, J.L.; Bullena, F.; Reidc, A.; Zhua, Y. Quantitative kinetic and structural analysis of geopolymers. Part 1. The activation of metakaolin with sodium hydroxide. Thermochim. Acta 2012, 539, 23–33. [Google Scholar]
- Autef, A.; Joussein, E.; Gasgnier, G.; Rossignol, S. Role of the silica source on the geopolymerization rate: A thermal analysis study. J. Non-Cryst. Solids 2013, 366, 13–21. [Google Scholar]
- Kani, E.N.; Allahverdi, A.; Provis, J.L. Calorimetric study of geopolymer binders based on natural pozzolan. J. Therm. Anal. Calorim. 2017, 127, 2181–2190. [Google Scholar] [CrossRef]
- Aldawsari, S.; Kampmann, R.; Harnisch, J.; Rohde, C. Setting Time, Microstructure, and Durability Properties of Low Calcium Fly Ash/Slag Geopolymer: A Review. Materials 2022, 15, 876. [Google Scholar]
- Bagheri, A.; Cremonab, C. Formulation of mix design for 3D printing of geopolymers: A machine learning approach. Mater. Adv. 2020, 1, 720–727. [Google Scholar]
- Korniejenko, K.; Łach, M. Geopolymers reinforced by short and long fibres—Innovative materials for additive manufacturing. Curr. Opin. Chem. Eng. 2020, 28, 167–172. [Google Scholar]
- Łach, M.; Grela, A.; Kozub, B.; Korniejenko, K.; Azzopardi, B. The Fly-Ash Based Geopolymer Composites as an Innovative Material for Circular. MATEC Web Conf. 2020, 322, 01016. [Google Scholar]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concreto. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar]
- Verma, M.; Dev, N.; Rahman, I.; Nigam, M.; Ahmed, M.; Mallick, J. Geopolymer Concrete: A Material for Sustainable Development in Indian Construction Industries. Crystals 2022, 12, 514. [Google Scholar] [CrossRef]
- Addis, L.B.; Sendekie, Z.B.; Satheesh, N. Degradation Kinetics and Durability Enhancement Strategies of Cellulosic Fiber-Reinforced Geopolymers and Cement Composites. Adv. Mater. Sci. Eng. 2022, 2022, 1981755. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, P.; Liu, H.; Wang, X.; Ge, W.; Hua, M. Resistance to Sulfuric Acid Corrosion of Geopolymer Concrete Based on Different Binding Materials and Alkali Concentrations. Materials 2021, 14, 7109. [Google Scholar] [CrossRef] [PubMed]
- Łach, M.; Korniejenko, K.; Hebdowska-Krupa, M.; Mikuła, J. The Effect of Additives on the Properties of Metakaolin and Fly Ash Based Geopolymers. MATEC Web Conf. 2018, 163, 06005. [Google Scholar] [CrossRef] [Green Version]
- Asmara, Y.P.; Siregar, J.P.; Tezara, C.; Nurlisa, W.; Jamiluddin, J. Long Term Corrosion Experiment of Steel Rebar in Fly Ash-Based Geopolymer Concrete in NaCl Solution. Int. J. Corros. 2016, 2016, 3853045. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, D. Multifunctional structural supercapacitor based on graphene and geopolymer. Electrochim. Acta 2017, 224, 105–112. [Google Scholar] [CrossRef]
- Gill, P.; Jangra, P.; Roychand, R.; Saberian, M.; Li, J. Effects of various additives on the crumb rubber integrated geopolymer concrete. Clean. Mater. 2023, 8, 100181. [Google Scholar] [CrossRef]
- Xiao, R.; Huang, B.; Zhou, H.; Ma, Y.; Jiang, X. A state-of-the-art review of crushed urban waste glass used in OPC and AAMs (geopolymer): Progress and challenges. Clean. Mater. 2022, 4, 100083. [Google Scholar] [CrossRef]
- Łach, M.; Kluska, B.; Janus, D.; Kabat, D.; Pławecka, K.; Korniejenko, K.; Guigou, M.D.; Choińska, M. Effect of Fiber Reinforcement on the Compression and Flexural Strength of Fiber-Reinforced Geopolymers. Appl. Sci. 2021, 11, 10443. [Google Scholar] [CrossRef]
- Luna-Galiano, Y.; Fernández-Pereira, C.; Izquierdo, M. Contributions to the study of porosity in fly ash-based geopolymers. Relationship between degree of reaction, porosity and compressive strength. Mater. De Construcción 2016, 66, 098. [Google Scholar] [CrossRef]
- Kaze, C.R.; Nana, A.; Lecomte-Nana, G.L.; Deutou, J.G.N.; Kamseu, E.; Melo, U.C.; Andreola, F.; Leonelli, C. Thermal behaviour and microstructural evolution of metakaolin and meta-halloysite-based geopolymer binders: A comparative study. J. Therm. Anal. Calorim. 2022, 147, 2055–2071. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.M.Y.; Almakayeel, N.M.; Alghamdi, S. Review on the Relationship between Nano Modifications of Geopolymer Concrete and Their Structural Characteristics. Polymers 2022, 14, 1421. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yue, J.; Pang, G.; Li, R.; Zhang, P.; Xu, S. Influence of the Activator Concentration and Solid/Liquid Ratio on the Strength and Shrinkage Characteristics of Alkali-Activated Slag Geopolymer Pastes. Adv. Civ. Eng. 2021, 2021, 6631316. [Google Scholar] [CrossRef]
- Song, Q.; Wang, J.; Dong, T.; He, L.; Lu, S. Study of the Alkali activated Geopolymer by AC Impedance Spectroscopy. IOP Conf. Ser. Earth Environ. Sci. 2019, 267, 022001. [Google Scholar] [CrossRef] [Green Version]
- Biondi, L.; Perry, M.; McAlorum, J.; Vlachakis, C.; Hamilton, A. Geopolymer-based moisture sensors for reinforced concrete health T monitoring. Sens. Actuators B Chem. 2020, 309, 127775. [Google Scholar] [CrossRef]
- Dong, B.; Qiu, Q.; Gu, Z.; Xiang, J.; Huang, C.; Fang, Y.; Xing, F.; Liu, W. Characterization of carbonation behavior of fly ash blended cement materials by the electrochemical impedance spectroscopy method. Cem. Concr. Compos. 2016, 65, 118–127. [Google Scholar] [CrossRef]
- Pengou, M.; Ngouné, B.; Tchakouté, H.K.; Nanseu, C.P.N.; Ngameni, E. Utilization of geopolymer cements as supercapacitors: Influence of the hardeners on their properties. Appl. Sci. 2020, 2, 1138. [Google Scholar] [CrossRef]
- Xiong, Y.-Q. Investigation of Chloride Diffusion Behavior of Concrete Manufactured with Blended Mineral Admixtures Using Electrochemical Technique. Int. J. Electrochem. Sci. 2021, 16, 210814. [Google Scholar] [CrossRef]
- Cosoli, G.; Mobili, A.; Tittarelli, F.; Revel, G.M.; Chiariotti, P. Electrical Resistivity and Electrical Impedance Measurement in Mortar and Concrete Elements: A Systematic Review. Appl. Sci. 2020, 10, 9152. [Google Scholar] [CrossRef]
- Heifetz, A.; Shribak, D.; Bakhtiari, S.; Member, S.; Aranson, I.S.; Bentivegna, A.F. Qualification of 3-D Printed Mortar with Electrical Conductivity Measurements. IEEE Trans. Instrum. Meas. 2021, 70, 3067222. [Google Scholar] [CrossRef]
- Walter, J.; Uthayakumar, M.; Balamurugan, P.; Mierzwiński, D. The Variable Frequency Conductivity of Geopolymers during the Long Agieng Period. Materials 2021, 14, 5648. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Equivalent circuit model for AC electrochemical impedance spectroscopy of concreto. Cem. Concr. Res. 2000, 30, 1723–1730. [Google Scholar] [CrossRef]
- Yakovlev, G.; Vít, Č.; Polyanskikh, I.; Gordina, A.; Pudov, I.; Gumenyuk, A.; Smirnova, O. The Effect of Complex Modification on the Impedance of Cement Matrices. Materials 2021, 14, 557. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Yi, C.-Y. Setting Process Monitoring of Cement Paste Using Electromechanical Impedance of Piezoelectric Patch. Materials 2022, 15, 8114. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Yuan, L.; Tian, C.; Yan, Z.; Liu, Z.; Yu, J. Electrical conductivity behavior of ZrO2-MgO-Y2O3 ceramic: Effect of heat treatment temperature. J. Aust. Ceram. Soc. 2022, 58, 421–427. [Google Scholar] [CrossRef]
- Suryanto, B.; McCarter, W.J.; Starrs, G.; Chisp, T.M. Characterization of fly-ash using electrochemical impedance spectroscopy. Proceding Eng. 2017, 171, 705–714. [Google Scholar] [CrossRef]
- Madhavi, T.C.; Annamalai, S. Electrical Conductivity of Concrete. ARPN J. Eng. Appl. Sci. 2016, 11, 5979–5982. [Google Scholar]
- Kljajević, L.M.; Melichova, Z.; Stojmenović, M.D.; Todorović, B.Ž.; Pavlović, V.B.; Čitaković, N.M.; Nenadović, S.S. Structural and Electrical Properties of Geopolymer Materials Based on Different Precursors (Kaolin, Bentonite and Diatomite). Maced. J. Chem. Chem. Eng. 2019, 38, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Wu, T.; Gao, L.; He, B.; Ma, F.; Huang, Z.; Bai, X. Compressive strength and electrochemical impedance response of red mud-coal metakaolin geopolymer exposed to sulfuric acid. Constr. Build. Mater. 2021, 303, 124523. [Google Scholar] [CrossRef]
- Łach, M.; Korniejenko, K.; Walter, J.; Stefańska, A.; Mikuła, J. Decreasing of Leaching and Improvement of Geopolymer Properties by Addition of Aluminum Calcium Cements and Titanium Oxide. Materials 2020, 13, 495. [Google Scholar] [CrossRef] [Green Version]




| Class of Fly Ash | N | F | C |
|---|---|---|---|
| SiO2 + Al2O3 + Fe2O3 (min. %) | 70 | 70 | 50 |
| SO3 (max. %) | 4 | 5 | 5 |
| Humidity (max. %) | 3 | 3 | 3 |
| Loss on ignition (max. %) | 10 | 6 | 6 |
| SiO2 | Al2O3 | CaO | MgO | Na2O | K2O | |
|---|---|---|---|---|---|---|
| [wt.%] | 61.11 ± 1.20 | 26.45 ± 0.69 | 2.89 ± 0.29 | 0.98 ± 0.14 | 4.07 ± 0.26 | 4.50 ± 0.30 |
| Particle Size [μm] | >160 | 100–160 | 71–100 | 63–71 | 56–63 | <56 |
|---|---|---|---|---|---|---|
| Contents [%] | 0.3 | 3.2 | 11.9 | 9.9 | 15.4 | 59.3 |
| Tpm [°C] | ΔHm [J/g] | Tim [°C] | Tfm [°C] | DSC [mW/mg] |
|---|---|---|---|---|
| 101.5 | −184.4 | 70.9 | 123.0 | −0.6236 |
| Diameter of Pores | Mean | Median |
|---|---|---|
| 0.1269 μm | 0.0095 μm | |
| Volume of pores | 0.401 cm3/g | 0.227 cm3/g |
| Total intruded volume | 0.455 cm3/g | |
| Total surface area | 14.331 m2/g | |
| Mercury intrusion porosity | 29.83% | |
| Weight [g] | Absorbed Energy by Mass [J/K] | Specific Heat * [J/g·K] | |
|---|---|---|---|
| Wet material | 0.0255 | 0.0465 | 1.8250 |
| Dry material | 0.0221 | 0.0323 | 1.4610 |
| Water | 0.0034 | 0.0143 | 4.1900 |
| Porosity | 13.36% | ||
| Time of Drying [h] | Element (1) [Ω] | Element (2) [nF] | Exp. of Element (2) | Element (3) [nF] | Exp. of Element (3) | |Z| Error [%] | Phase Shift Error [°] |
|---|---|---|---|---|---|---|---|
| 0 | 161.7 | 342.50 | 0.151 | 44,480.0 | 0.869 | 0.3 | 0.1 |
| 21 | 427.1 | 122.30 | 0.180 | 18,860.0 | 0.790 | 0.5 | 0.2 |
| 24 | 424.8 | 136.00 | 0.166 | 18,860.0 | 0.720 | 0.5 | 0.2 |
| 45 | 758.0 | 78.10 | 0.176 | 13,660.0 | 0.762 | 0.5 | 0.2 |
| 47 | 738.3 | 84.00 | 0.169 | 13,750.0 | 0.760 | 0.5 | 0.2 |
| 49 | 793.2 | 92.18 | 0.154 | 13,540.0 | 0.758 | 0.5 | 0.2 |
| 71 | 1263.0 | 59.69 | 0.163 | 10,710.0 | 0.740 | 0.5 | 0.2 |
| 167 | 2148.0 | 31.32 | 0.178 | 6868.0 | 0.706 | 0.6 | 0.3 |
| 192 | 2216.0 | 30.59 | 0.175 | 6400.0 | 0.698 | 0.5 | 0.3 |
| 216 | 2487.0 | 26.07 | 0.179 | 5434.0 | 0.685 | 0.5 | 0.3 |
| 239 | 2747.0 | 21.94 | 0.190 | 5083.0 | 0.685 | 0.6 | 0.3 |
| 793 | 14,240.0 | 0.755 | 0.516 | 124.7 | 0.313 | 0.4 | 0.2 |
| 813 | 23,690.0 | 0.469 | 0.564 | 47.5 | 0.251 | 0.3 | 0.1 |
| 1007 | 23,020.0 | 0.479 | 0.561 | 47.4 | 0.249 | 0.3 | 0.1 |
| 1198 | 30,580.0 | 0.409 | 0.572 | 34.9 | 0.241 | 0.3 | 0.1 |
| 1390 | 31,670.0 | 0.390 | 0.584 | 28.4 | 0.226 | 0.3 | 0.1 |
| 1534 | 31,020.0 | 0.395 | 0.584 | 29.0 | 0.225 | 0.3 | 0.1 |
| 1849 | 31,320.0 | 0.397 | 0.583 | 28.4 | 0.225 | 0.3 | 0.1 |
| 2041 | 30,770.0 | 0.405 | 0.579 | 28.2 | 0.223 | 0.3 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierzwiński, D.; Walter, J.; Wanat, D. Possibilities of Checking Water Content in Porous Geopolymer Materials Using Impedance Spectroscopy Methods. Materials 2023, 16, 5190. https://doi.org/10.3390/ma16145190
Mierzwiński D, Walter J, Wanat D. Possibilities of Checking Water Content in Porous Geopolymer Materials Using Impedance Spectroscopy Methods. Materials. 2023; 16(14):5190. https://doi.org/10.3390/ma16145190
Chicago/Turabian StyleMierzwiński, Dariusz, Janusz Walter, and Dominika Wanat. 2023. "Possibilities of Checking Water Content in Porous Geopolymer Materials Using Impedance Spectroscopy Methods" Materials 16, no. 14: 5190. https://doi.org/10.3390/ma16145190
APA StyleMierzwiński, D., Walter, J., & Wanat, D. (2023). Possibilities of Checking Water Content in Porous Geopolymer Materials Using Impedance Spectroscopy Methods. Materials, 16(14), 5190. https://doi.org/10.3390/ma16145190







