Study on Enhancing the Thermoelectric Stability of the β-Cu2Se Phase by Mn Doping
Abstract
1. Introduction
2. Experiment
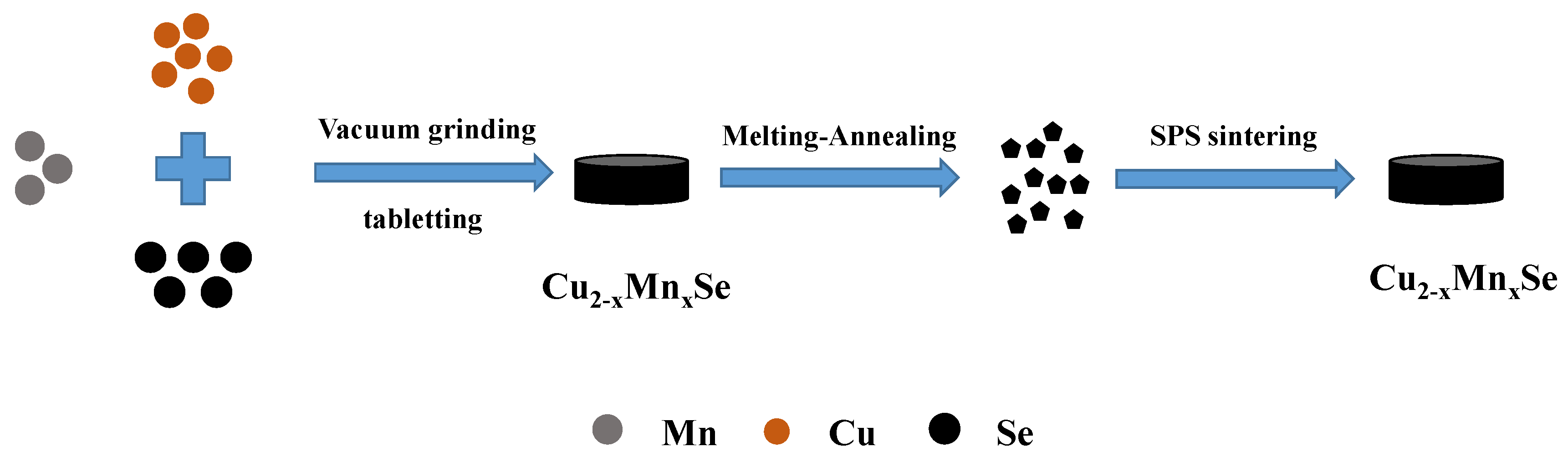
2.1. Fabrication
2.2. Testing and Characterization
3. Results and Discussion
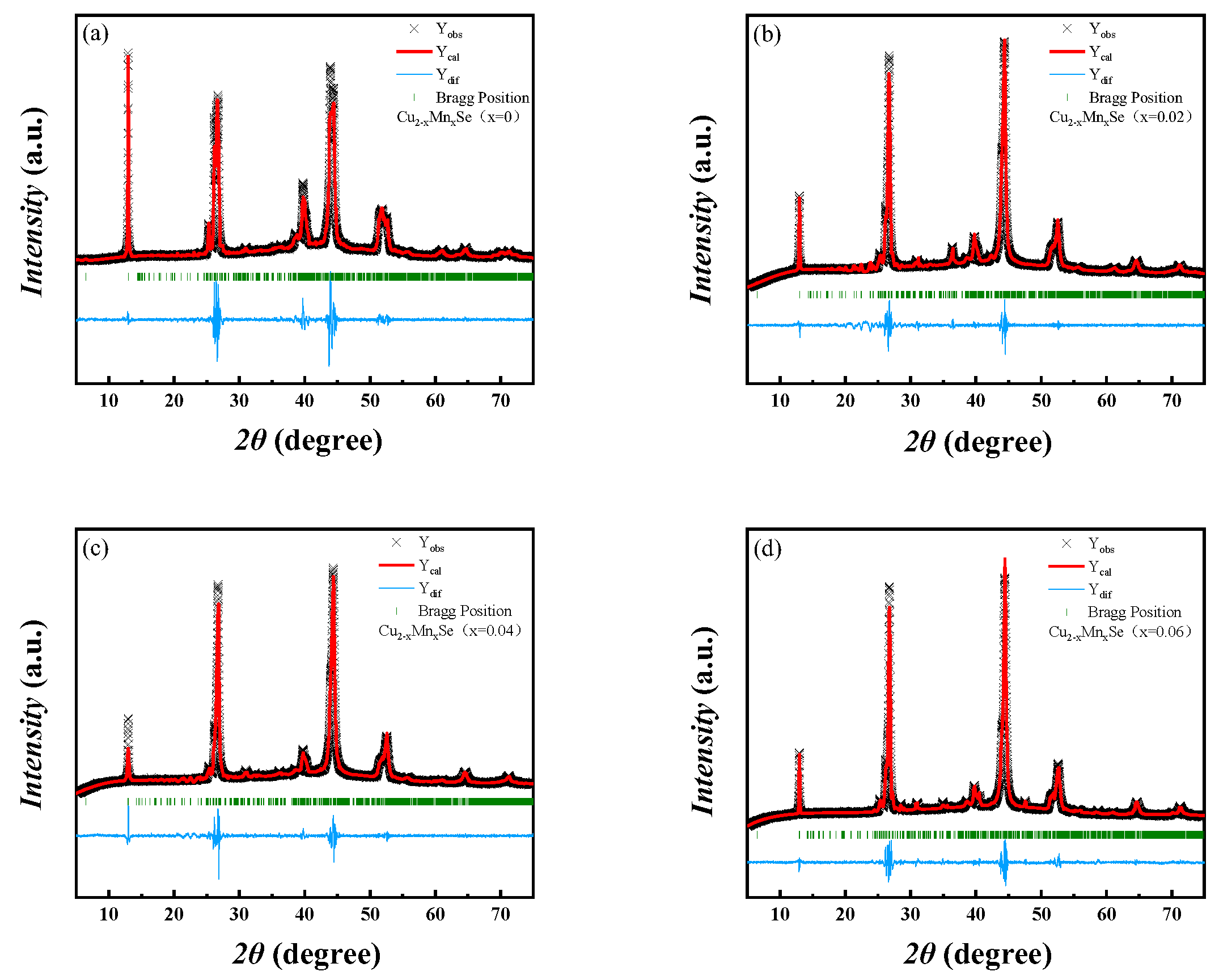
3.1. Crystal Structure Analysis
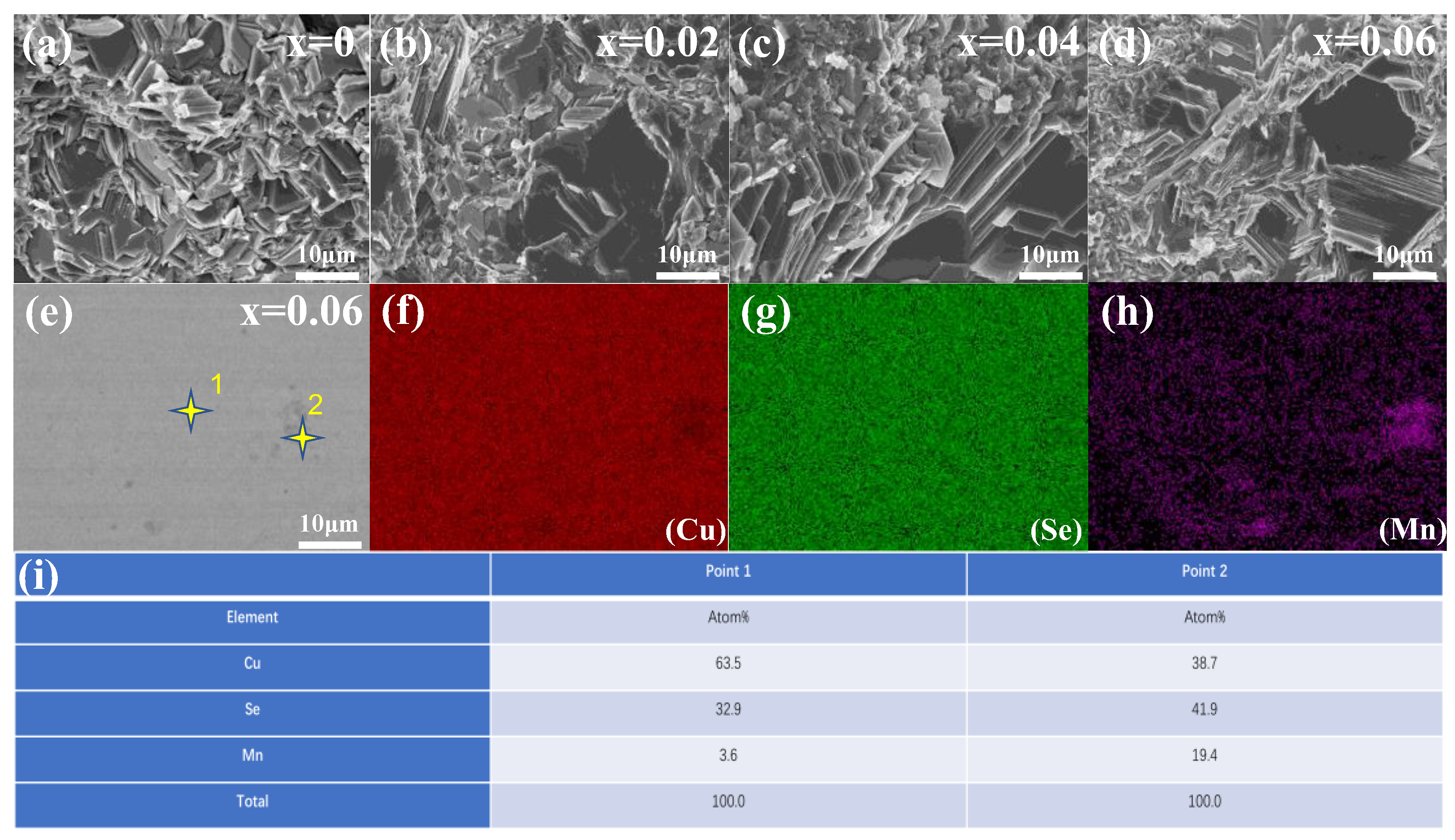
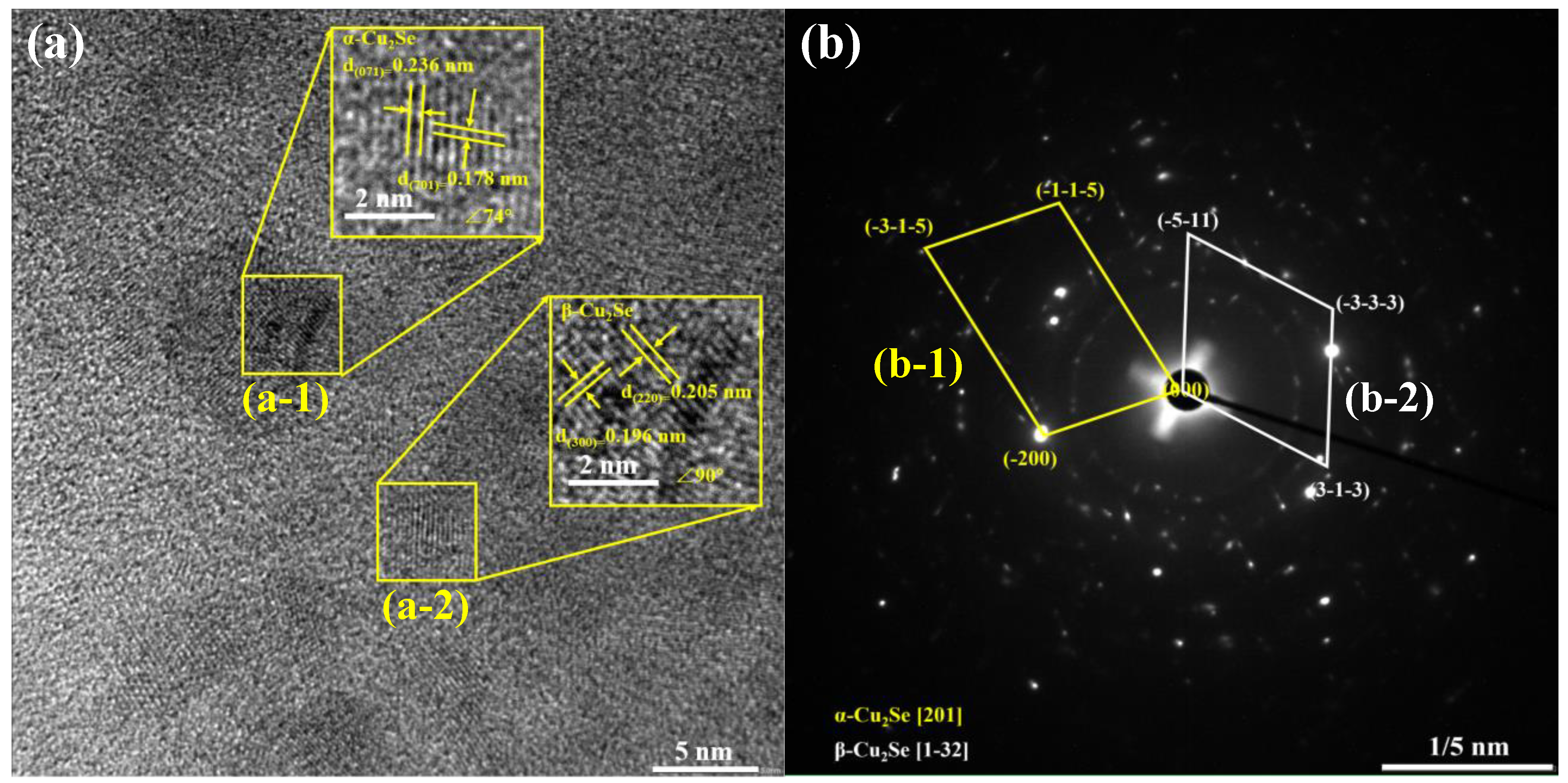
3.2. Micromorphology Characterization
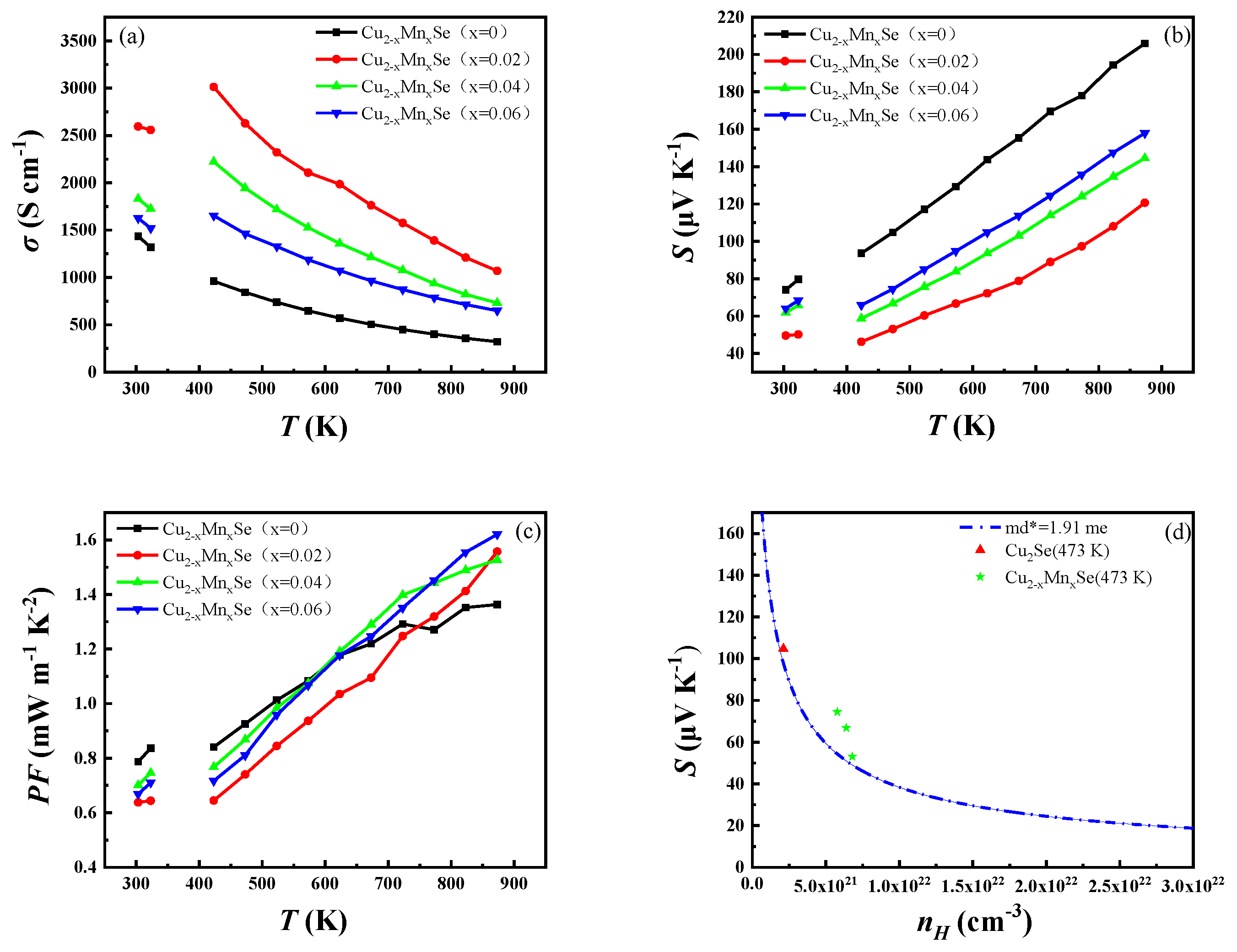
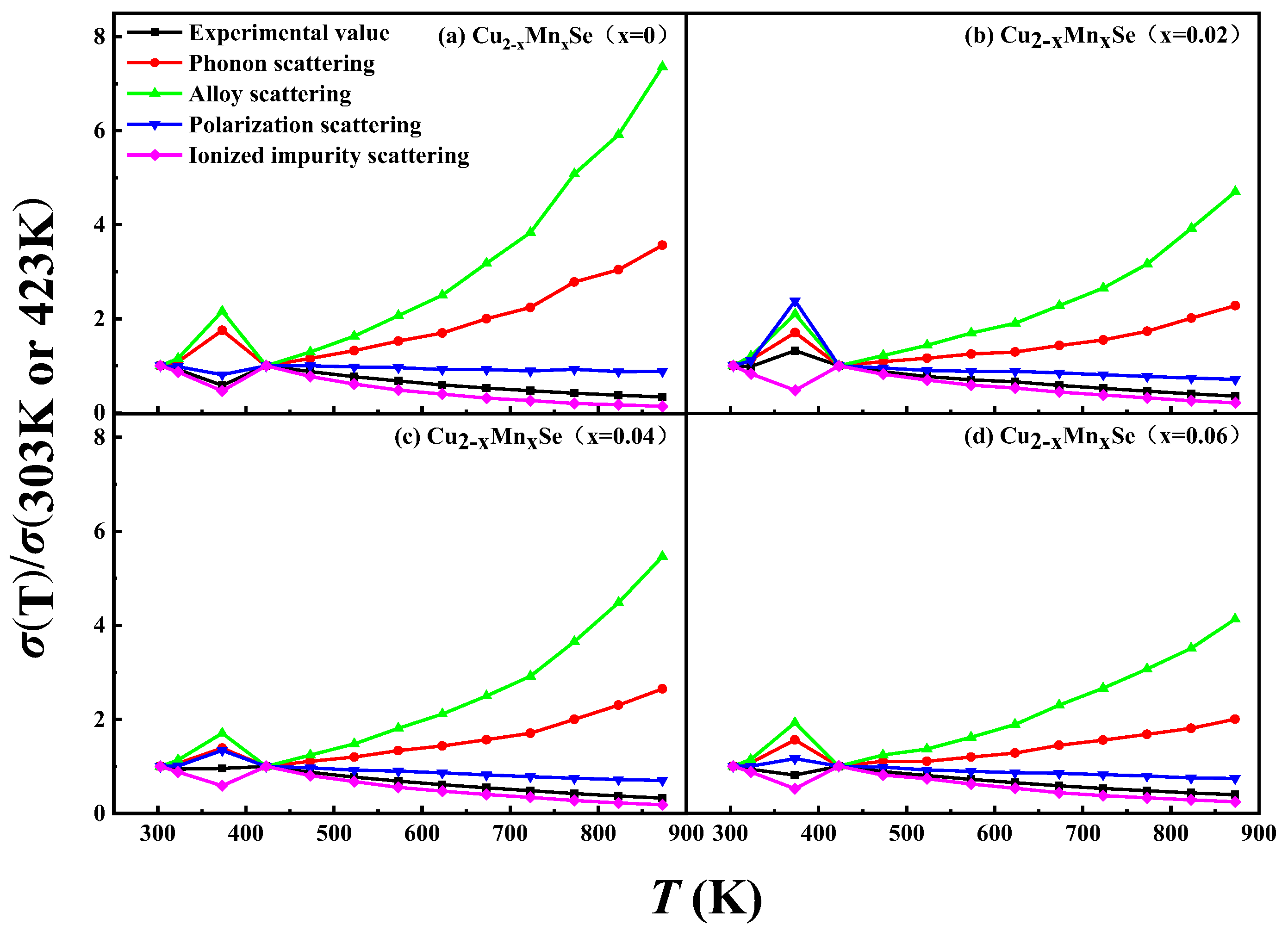
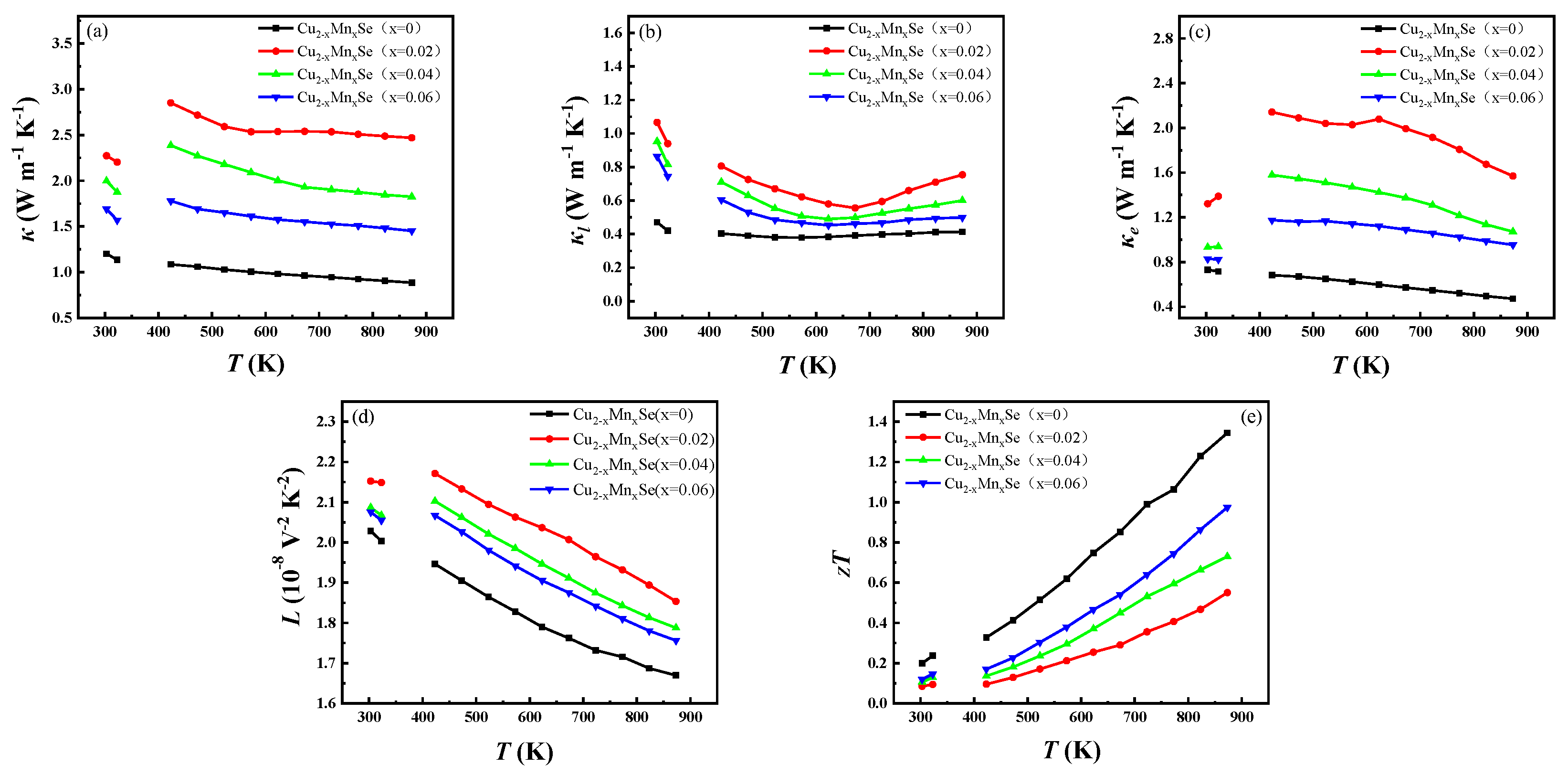
3.3. Thermoelectric Performance Discussion
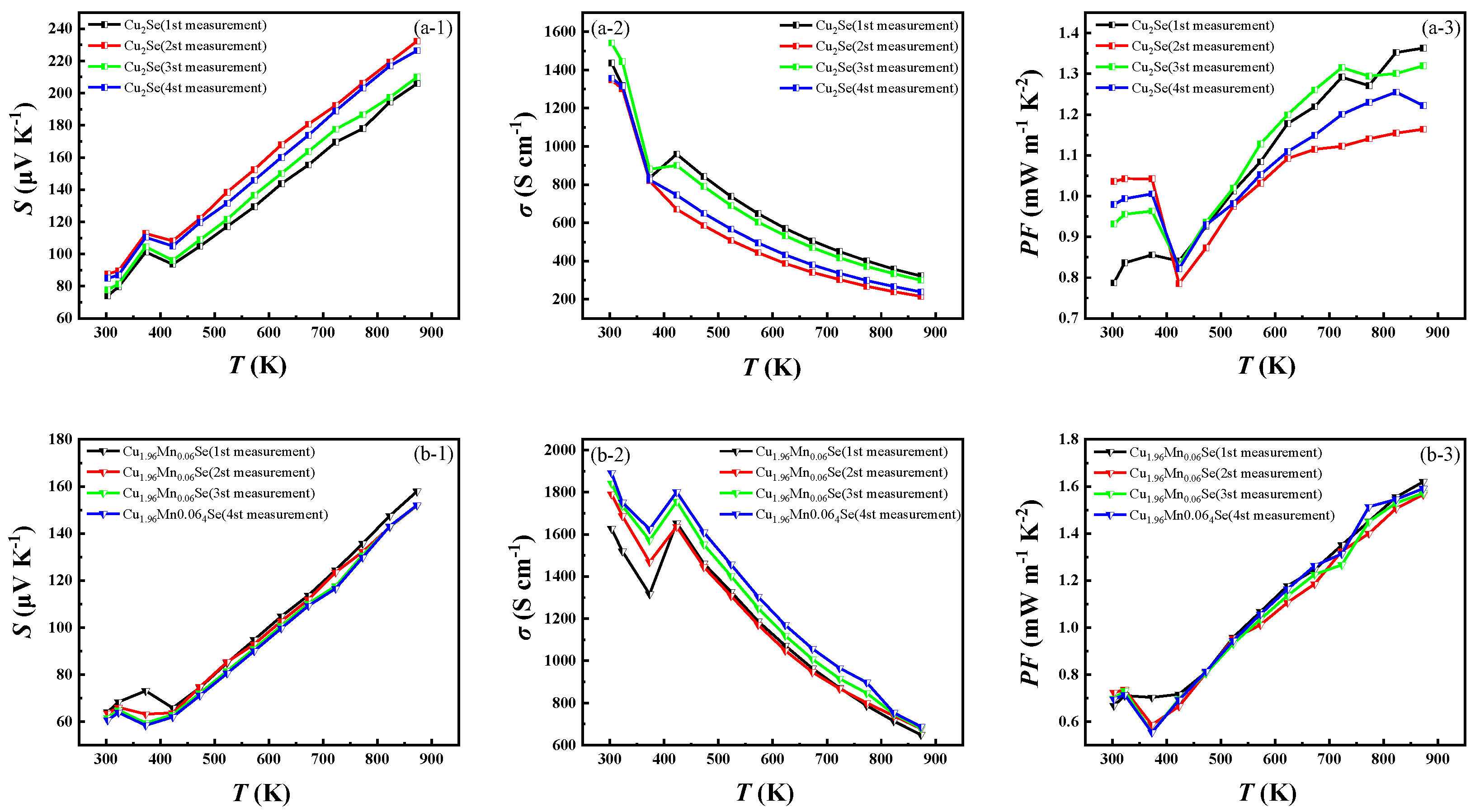
3.4. Cyclic Test of Thermoelectric Properties
4. Conclusions
- XRD analysis shows that the Mn-doped samples are a mixture of α-Cu2Se and Cu2Se. With the increase in Mn content, the cell parameters of the material decrease, and the β-Cu2Se phase content increases. Specifically, with the increase in Mn content, the β-Cu2Se phase can be stabilized theoretically. DSC analysis showed that the heat absorption peak moves to low temperatures, and the phase transition temperature decreases with the increase in Mn doping content.
- SEM and HRTEM analysis showed that the sample is composed of flaky crystal piles. the flaky particles decreased with the increase in Mn doping amount; the shape of the segregation region of the sample was irregular; the size was 5–10 nm; and there were many dislocations in each segregation micro-interval, which enhanced the scattering of carriers and phonons.
- The σ and S showed opposite test temperature and Mn doping dependence. The conductivity decreases with the increase in temperature, and S increases with the increase in temperature. The PF value of Cu1.94Mn0.06Se at 873 K was 1.62 mW m−1 K−2, which is 1.2 times higher than that of Cu2Se without Mn at 873 K.
- Conductivity ratio analysis showed that the Mn impure sample and pure phase Cu2Se have the exact carrier scattering mechanism, the scattering characteristic of ionized impurities. This is consistent with the result that the carrier mobility increases with temperature, so the scattering factor value is 3/2. This feature could be one of the reasons for Cu2Se’s high Seebeck coefficient and thermoelectric properties.
- Due to Mn’s inclusion of electrons, its bipolar diffusion thermal conductivity significantly increases. The minority carrier concentration introduced at x = 0.02 is the highest for the lattice thermal conductivity of the sample, which does not reach a high zT value.
- The results of four high-temperature cycle tests showed that the thermoelectric properties of Cu1.94Mn0.06Se show little change, and the high-temperature cycle stability is better than that of Cu2Se. The ionic radius of Mn2+ is smaller than that of Cu+. Therefore, as a dopant, Mn is easily dissolved into the structure of Cu2Se. The electronegativity of Mn is less than that of Cu. Theoretically speaking, the manganese VIIB element has a strong reducing ability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, J.; Chen, G.; Ren, Z. Thermoelectric cooling materials. Nat. Mater. 2021, 20, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Qiu, P.; Shi, X.; Chen, L. Recent Advances in Liquid-Like Thermoelectric Materials. Adv. Funct. Mater. 2020, 30, 1903867. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, K.; Wei, T.-R.; Qiu, P.; Chen, L.; Shi, X. Cu2Se-Based liquid-like thermoelectric materials: Looking back and stepping forward. Energy Environ. Sci. 2020, 13, 3307–3329. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, H.; Snyder, G.J. Band Engineering of Thermoelectric Materials. Adv. Mater. 2012, 24, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Olvera, A.; Bailey, T.P.; Uher, C.; Poudeu, P.F. Nanoscale Engineering of Polymorphism in Cu2Se-Based Composites. ACS Appl. Mater. Interfaces 2020, 12, 31601–31611. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, X.; Xu, F.; Zhang, L.; Zhang, W.; Chen, L.; Li, Q.; Uher, C.; Day, T.; Jeffrey, G.S. Copper ion liquid-like thermoelectrics. Nat. Mater. 2012, 11, 422–425. [Google Scholar] [CrossRef]
- Wei, T.-R.; Wu, C.-F.; Li, F.; Li, J.-F. Low-cost and environmentally benign selenides as promising thermoelectric materials. J. Mater. 2018, 4, 304–320. [Google Scholar] [CrossRef]
- Bo, L.; Zhang, R.; Zhao, H.; Hou, Y.; Wang, X.; Zhu, J.; Zhao, L.; Zuo, M.; Zhao, D. Achieving High Thermoelectric Properties of Cu2Se via Lattice Softening and Phonon Scattering Mechanism. ACS Appl. Energy Mater. 2022, 5, 6453–6461. [Google Scholar] [CrossRef]
- Byeon, D.; Sobota, R.; Delime-Codrin, K.; Choi, S.; Hirata, K.; Adachi, M.; Kiyama, M.; Matsuura, T.; Yamamoto, Y.; Matsunami, M.; et al. Discovery of colossal Seebeck effect in metallic Cu2Se. Nat. Commun. 2019, 10, 72. [Google Scholar] [CrossRef]
- Zhao, K.; Blichfeld, A.B.; Eikeland, E.; Qiu, P.; Ren, D.; Iversen, B.B.; Shi, X.; Chen, L. Extremely Low Thermal Conductivity and High Thermoelectric Performance in Liquid-like Cu2Se 1−xSx Polymorph Materials. J. Mater. Chem. A 2017, 5, 18148–18156. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Zhu, T.; Yan, Y.; Su, X.; Tang, X. Mechanical Properties and Thermal Stability of the High-Thermoelectric-Performance Cu2Se Compound. ACS Appl. Mater. Interfaces 2021, 13, 45736–45743. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Gong, Z.N.; Liu, F.S.; Huang, M.J.; Ao, W.Q.; Li, Y.; Li, J.Q. Structure and thermoelectric performance of β-Cu2Se doped with Fe, Ni, Mn, In, Zn or Sm. Intermetallics 2016, 75, 72–78. [Google Scholar] [CrossRef]
- Wang, N.; Song, G.; Li, G.; Wu, Y.; You, J. Thermoelectric properties of β-(Cu,Mn)2Se films with high (111) preferred orientation. Vacuum 2022, 197, 110845. [Google Scholar] [CrossRef]
- Ducka, A.; Trawiński, B.; Bochentyn, B.; Dubiel, A.; Kusz, B. Structure and thermoelectric properties of nickel-doped copper selenide synthesised in a hydrogen atmosphere. Mater. Res. Bull. 2021, 133, 111042. [Google Scholar] [CrossRef]
- Day, T.W.; Borup, K.A.; Zhang, T.; Drymiotis, F.; Brown, D.R.; Shi, X.; Chen, L.; Iversen, B.B.; Snyder, G.J. High-temperature thermoelectric properties of Cu1.97Ag0.30Se1+y. Mater. Renew. Sustain. Energy 2014, 3, 111042. [Google Scholar] [CrossRef]
- Hu, Q.; Zhu, Z.; Zhang, Y.; Li, X.-J.; Song, H.; Zhang, Y. Remarkably high thermoelectric performance ofCu2−xLixSe bulks with nanopores. J. Mater. Chem. A 2018, 6, 23417–23424. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Song, H.; Li, X.-J. High thermoelectric performance and low thermal conductivity in Cu2−xNaxSe bulk materials with micro-pores. Appl. Phys. A 2019, 125, 572. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Song, H.; Li, X.-J. Enhancement of thermoelectric performance of Cu2Se by K doping. Appl. Phys. A 2018, 124, 871. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Bhattacharya, A.; Tyagi, K.; Gahtori, B.; Chauhan, N.S.; Vishwakarma, A.; Johari, K.K.; Bathula, S.; Auluck, S.; Dhar, A. Enhancement in thermoelectric performance of single step synthesized Mg doped Cu2Se: An experimental and theoretical study. Intermetallics 2019, 112, 106541. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Miao, N.; Zhou, J.; Sun, Z. I-doped Cu2Se nanocrystals for high-performance thermoelectric applications. J. Alloys Compd. 2019, 772, 366–370. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, G.-G.; Kim, W.; Kim, K.; Tak, J.-Y.; Shin, W.H.; Seo, W.-S.; Hong, J.; Lim, Y.S. Effects of Cl-Doping on Thermoelectric Transport Properties of Cu2Se Prepared by Spark Plasma Sintering. J. Electron. Mater. 2018, 48, 1958–1964. [Google Scholar] [CrossRef]
- Zhao, K.; Blichfeld, A.B.; Chen, H.; Song, Q.; Zhang, T.; Zhu, C.; Ren, D.; Hanus, R.; Qiu, P.; Iversen, B.B.; et al. Enhanced Thermoelectric Performance through Tuning Bonding Energy in Cu2Se1−xSx Liquid-like Materials. Chem. Mater. 2017, 29, 6367–6377. [Google Scholar] [CrossRef]
- Butt, S.; Xu, W.; Farooq, M.U.; Ren, G.K.; Zhang, Q.; Zhu, Y.; Khan, S.U.; Liu, L.; Yu, M.; Mohmed, F.; et al. Enhanced Thermoelectricity in High-Temperature β-Phase Copper(I) Selenides Embedded with Cu2Te Nanoclusters. ACS Appl. Mater. Interfaces 2016, 8, 15196–15204. [Google Scholar] [CrossRef] [PubMed]
- James, G.; Speight, P.D. Lange’s Handbook of Chemistry; McGraw-Hill Professional: Chicago, IL, USA, 1972. [Google Scholar]
- Gordy, W.; Thomas, W.J.O. Electronegativities of the Elements. J. Chem. Phys. 1956, 24, 439–444. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Lin, S.; Chang, Y.; Ge, B.; Chen, Y.; Pei, Y. Band and scattering tuning for high performance thermoelectric Sn1−xMnxTe alloys. J. Mater. 2015, 1, 307–315. [Google Scholar] [CrossRef]
- Das, S.; Valiyaveettil, S.M.; Chen, K.-H.; Suwas, S.; Mallik, R.C. Thermoelectric properties of Mn doped BiCuSeO. Mater. Res. Express 2019, 6, 086305. [Google Scholar] [CrossRef]
- Xiang, S.; Liang, Y.; Zhang, X. Thermoelectric properties and thermal stability of metal-doped cuprous sulfide thermoelectrics. J. Eur. Ceram. Soc. 2022, 42, 7468–7474. [Google Scholar] [CrossRef]
- Xu, G.; Ren, P.; Lin, T.; Wu, X.; Zhang, Y.; Niu, S.; Bailey, T.P. Mechanism and application method to analyze the carrier scattering factor by electrical conductivity ratio based on thermoelectric property measurement. J. Appl. Phys. 2018, 123, 015101. [Google Scholar] [CrossRef]




| Element | Cu | Mn | Cu+ | Cu2+ | Mn2+ |
|---|---|---|---|---|---|
| radius/Å [24] | 1.28 | 1.32 | 0.77 | 0.73 | 0.67 |
| electronegativity/eV [25] | 1.90 | 1.55 | 1.8 | 2.0 | 1.4 |
| Sample | a (Å) | b (Å) | c (Å) | Vol (Å3) | χ2 |
|---|---|---|---|---|---|
| x = 0 | 7.159418 | 12.002411 | 27.771622 | 2345.075 | 4.151 |
| x = 0.02 | 7.005779 | 12.408270 | 27.327600 | 2344.639 | 4.165 |
| x = 0.04 | 6.988073 | 12.514909 | 27.393709 | 2244.077 | 4.626 |
| x = 0.06 | 7.373002 | 12.079905 | 27.417471 | 2243.847 | 4.028 |
| Carrier Scattering Mechanism | Scattering Factor | Electrical Conductivity |
|---|---|---|
| Acoustical phonon scattering | −1/2 | (4) |
| Alloy scattering | −1/2 | (5) |
| Polar optical phonon scattering | 1/2 | (6) |
| Ionized impurity scattering | 3/2 | (7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tie, J.; Xu, G.; Li, Y.; Fan, X.; Yang, Q.; Nan, B. Study on Enhancing the Thermoelectric Stability of the β-Cu2Se Phase by Mn Doping. Materials 2023, 16, 5204. https://doi.org/10.3390/ma16145204
Tie J, Xu G, Li Y, Fan X, Yang Q, Nan B. Study on Enhancing the Thermoelectric Stability of the β-Cu2Se Phase by Mn Doping. Materials. 2023; 16(14):5204. https://doi.org/10.3390/ma16145204
Chicago/Turabian StyleTie, Jian, Guiying Xu, Yawei Li, Xian Fan, Quanxin Yang, and Bohang Nan. 2023. "Study on Enhancing the Thermoelectric Stability of the β-Cu2Se Phase by Mn Doping" Materials 16, no. 14: 5204. https://doi.org/10.3390/ma16145204
APA StyleTie, J., Xu, G., Li, Y., Fan, X., Yang, Q., & Nan, B. (2023). Study on Enhancing the Thermoelectric Stability of the β-Cu2Se Phase by Mn Doping. Materials, 16(14), 5204. https://doi.org/10.3390/ma16145204






