Corrosion Resistance of Zinc and Zinc-Aluminum-Magnesium Coatings in Atmosphere on the Territory of Russia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Testing Conditions
2.3. Dose–Response Functions
3. Results
3.1. Meteorological and Chemical Parameters of the Atmospheres
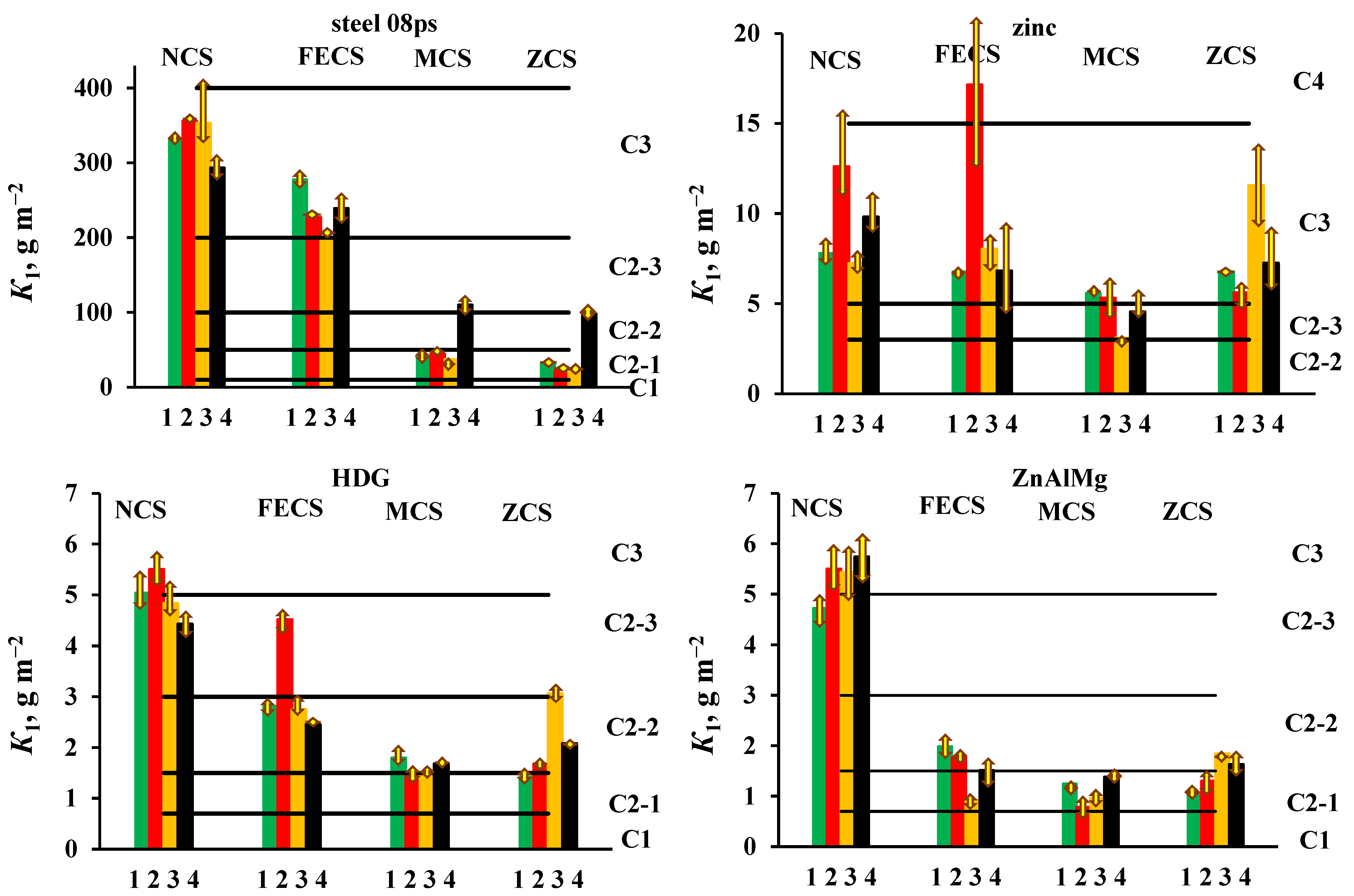
3.2. Determination of the Atmospheric Corrosivity at a CS with Respect to Materials at Open Areas and under a Shelter
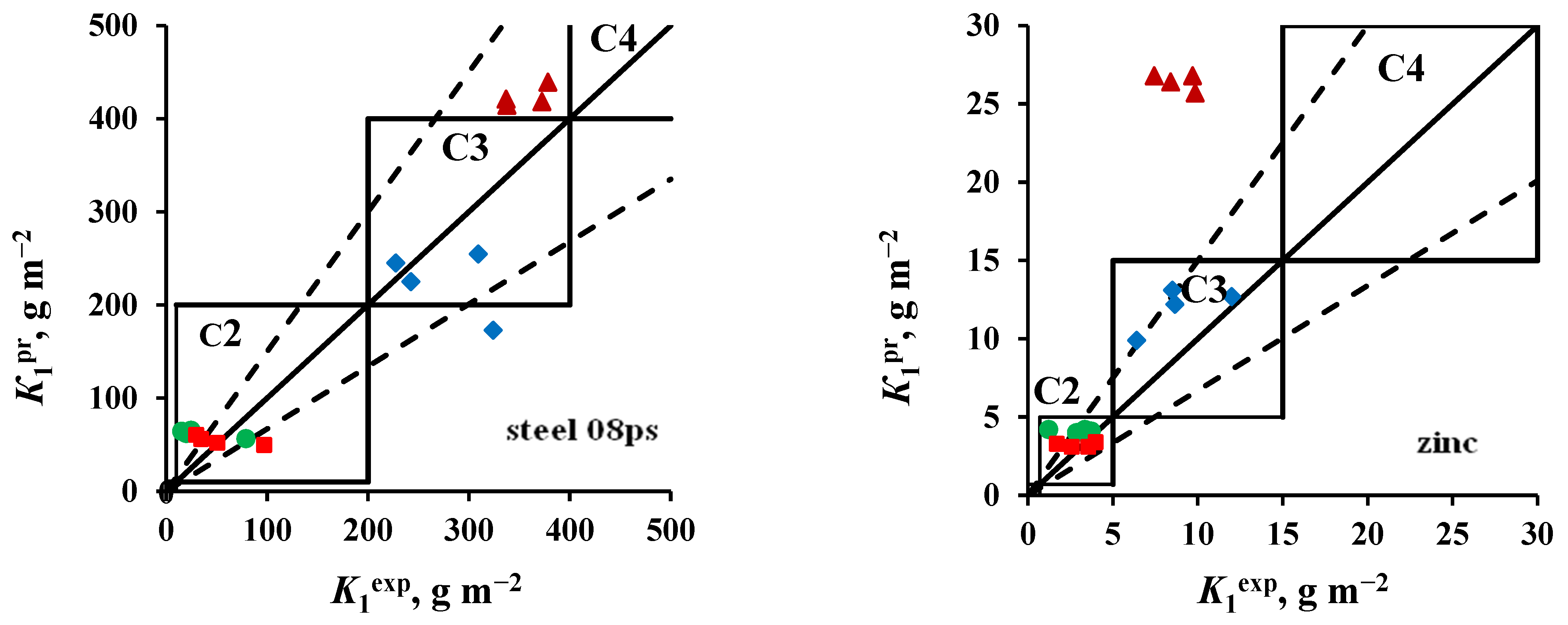
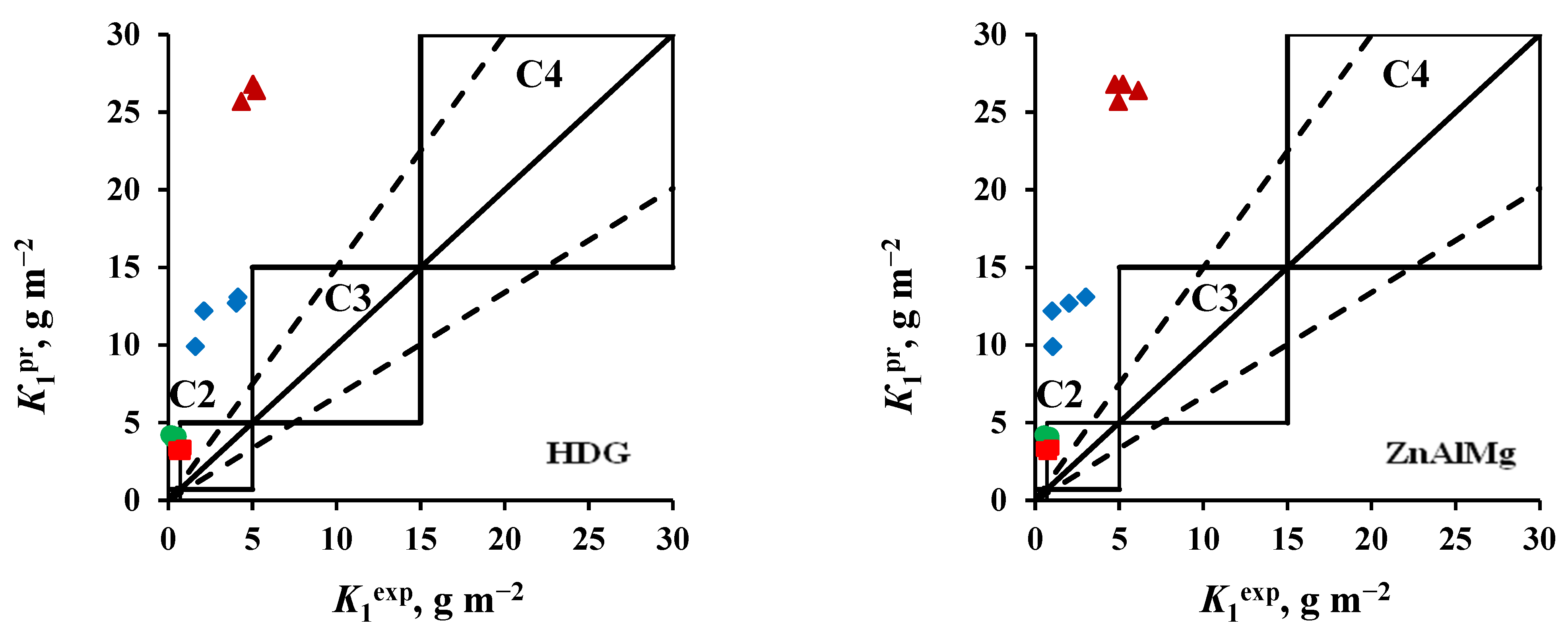
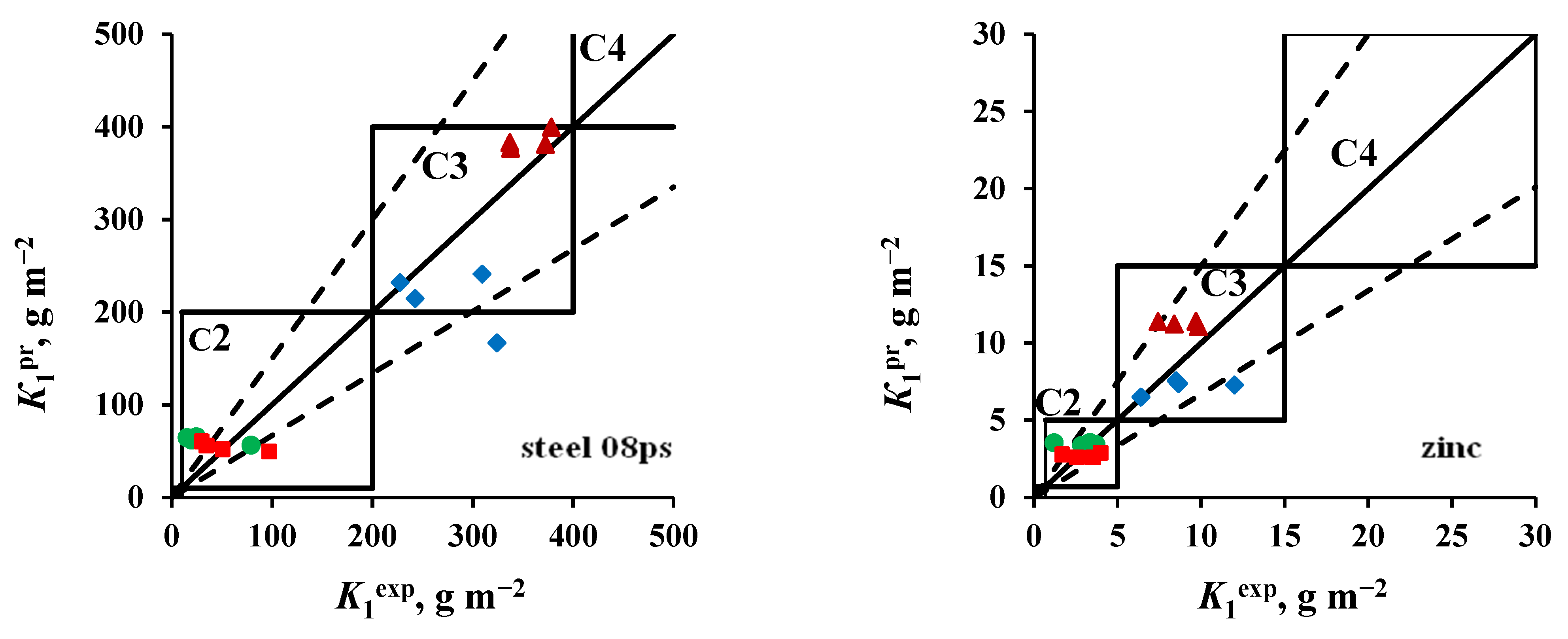
3.3. Accessing the Atmosphere Corrosivity Categories
- −
- Steel 08ps. Most of the K1pr values correspond to the atmosphere corrosivity categories determined from K1ex.
- −
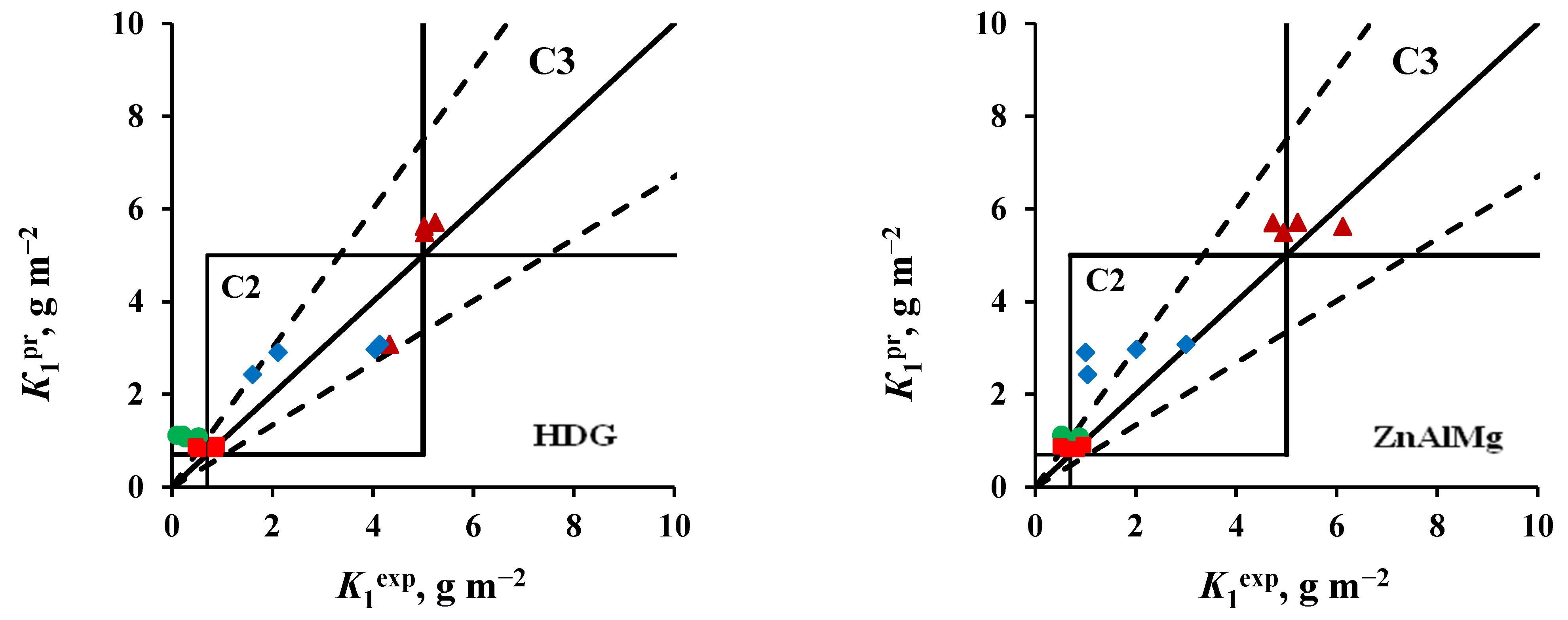
- Zinc. The values of K1pr calculated for tests in an open area and under a shelter at the NCS are overestimated, according to which the atmosphere category is C4 instead of the category C3 determined experimentally. The K1pr values calculated for the OA conditions at the ZCS are underestimated, as a result of which, according to the predictive estimate, the C2 category is obtained instead of C3.
- −
- Coatings. The K1pr values calculated for test conditions in an open area and under a shelter in the marine atmosphere are greatly overestimated, as a result of which the atmosphere category C3 was obtained at FECS instead of C2, and at NCS, category C4 was found instead of C3. In the non-marine atmospheres (MCS and ZCS), the atmospheric category is correctly assessed as C2.
3.4. Corrosion Rate of Coatings for 2 Years of Exposure
3.5. Approximate Service Life of Coatings
4. Conclusions
- The values of four one-year corrosion losses (K1) of steel 08ps, zinc, ZnAlMg, and HDG coatings with the setting of samples at the beginning of each season in two locations with marine atmospheres and two locations with non-marine atmospheres in an open area and under a shelter differ significantly, though the average annual atmospheric parameters are almost the same. To clarify the reasons for this effect, additional studies are needed that would take the meteorological and aerochemical parameters of the first months of testing into account.
- It has been shown that the average corrosion losses of materials for the four batches of samples for the first year in an open area is greater than under a shelter, except for steel 08ps at NCS and FECS, as well as ZnAlMg coatings at NCS. After two years in an open area, the corrosion losses at the CS with a marine atmosphere are smaller than or comparable to the corrosion losses under a shelter. This may be due to the accumulation of sea salts on the surface of materials in the absence of the wash-off effect of rains.
- The first-year corrosion losses of materials were used to determine the atmosphere corrosivity categories in relation to each material in open area and under a shelter. For ZnAlMg and HDG coatings, the atmosphere corrosivity categories developed for zinc were used. It has been shown that the corrosiveness of the atmosphere defined based on four values of K1 may correspond to two different categories or different subcategories of category C2.
- To determine the K1 values without conducting repeated one-year tests, dose–response functions for zinc, ZnAlMg, and HDG coatings for exposure conditions in an open area and under a shelter have been suggested. The errors in the K1 values calculated for each material do not exceed the allowable uncertainty ranges set by ISO 9223:2012.
- Based on the corrosion rates of ZnAlMg and HDG coatings over 2 years, an approximate estimate of their service life has been found.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMurray, H.N.; Parry, G.; Jeffs, B.D. Corrosion resistance of Zn-Al alloy coated steels investigated using electrochemical impedance spectroscopy. Iron Steelmak. 1998, 25, 210–215. [Google Scholar]
- Schmidt, D.P.; Shaw, B.A.; Sikora, E.; Shaw, W.W. Corrosion Protection Assessment of Barrier Properties of Several Zinc-Containing. Coating Systems on Steel in Artificial Seawater. Corrosion 2006, 62, 323–339. [Google Scholar] [CrossRef]
- Fujita, S.; Mizuno, D. Corrosion and corrosion test methods of zinc coated steel sheets on automobiles. Corros. Sci. 2007, 49, 211–219. [Google Scholar] [CrossRef]
- Kuroda, S.; Kawakita, J.; Takemoto, M. An 18-Year Exposure Test of Thermal-Sprayed Zn, Al, and Zn-Al Coatings in Marine Environment. Corrosion 2006, 62, 635–647. [Google Scholar] [CrossRef]
- Prosek, T.; Larche, N.; Vlot, M.; Goodwin, F.; Thierry, D. Corrosion performance of Zn–Al–Mg coatings in open and confined zones in conditions simulating automotive applications. Mater. Corros. 2010, 61, 412–420. [Google Scholar] [CrossRef]
- Zhang, X.G. Corrosion and Electrochemistry of Zinc; Plenum Press: New York, NY, USA, 1996; p. 241. ISBN 10: 0306453347. [Google Scholar]
- Palma, E.; Puente, J.M.; Morcillo, M. The atmospheric corrosion mechanism of 55%Al-Zn coating on steel. Corros. Sci. 1998, 40, 61–68. [Google Scholar] [CrossRef]
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- de Rincón, O.; Rincón, A.; Sánchez, M.; Romero, N.; Salas, O.; Delgado, R.; Panosian, Z. Evaluating Zn, Al and Al-Zn coatings on carbon steel in a special atmosphere. Constr. Build. Mater. 2009, 23, 1465–1471. [Google Scholar] [CrossRef]
- Schouller-Guinnet, P.; Alltly, C.; Volovich, P. ZnAlMg: An innovative metallic coaling that offers protection in the harshest environments. Genova 2011, 11. [Google Scholar]
- LeBozec, N.; Thierry, D.; Peltola, A.; Luxem, L.; Luckeneder, G.; Marchiaro, G.; Rohwerder, M. Corrosion performance of Zn–Mg–Al coated steel in accelerated corrosion tests used in the automotive industry and field exposures. Mater. Corros. 2013, 64, 969–978. [Google Scholar] [CrossRef]
- Bozec, N.L.; Thierry, D.; Rohwerder, M.; Kovacs, A.; Peltola, A.; Luckeneder, G.; Luxem, L.; Marchiaro, G.; Autocoat—European Comission. Advanced Zinc-Based Hot Dip Coatings for the Automotive Application; European Comission Report; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar] [CrossRef]
- Nordic Galvanizers. Corrosion Performance—Real Performance Evaluations in Infraestructure Applications; Nordic Galvanizers: Stockholm, Sweden, 2017. [Google Scholar]
- Kimab, S. Alternative Materials for Cable Tray Systems; Kista, Sweden, 2014. [Google Scholar]
- Salgueiro Azevedo, M.; Allély, C.; Ogle, K.; Volovitch, P. Corrosion mechanisms of Zn(Mg, Al) coated steel in accelerated tests and natural exposure: 1. The role of electrolyte composition in the nature of corrosion products and relative corrosion rate. Corros. Sci. 2015, 90, 472–481. [Google Scholar] [CrossRef]
- LeBozec, N.; Thierry, D.; Persson, D.; Riener, C.K.; Luckeneder, G. Influence of microstructure of zinc-aluminium-magnesium alloy coated steel on the corrosion behavior in outdoor marine atmosphere. Surf. Coat. Technol. 2019, 374, 897–909. [Google Scholar] [CrossRef]
- Thierry, D.; Persson, D.; Luckeneder, G.; Stellnberger, K.-H. Atmospheric corrosion of ZnAlMg coated steel during long-term atmospheric weathering at different worldwide exposure sites. Corros. Sci. 2019, 148, 338–354. [Google Scholar] [CrossRef]
- Thierry, D.; LeBozec, N.; Gac, A.L.; Persson, D. Long-term atmospheric corrosion rates of hot dip galvanised steel and zinc-aluminium-magnesium coated steel. Mater. Corros. 2019, 70, 2200–2227. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I.; Nikolaeva, L.A.; Nenasheva, T.A.; Ivanenko, A.A. Assessment of the corrosion resistance in atmosphere and service life of zinc-aluminum and zinc–aluminum–magnesium coatings in various regions of the world. Int. J. Corros. Scale Inhib. 2022, 11, 1238–1268. [Google Scholar] [CrossRef]
- Mitrofanov, A.V.; Kuzyuk, M.V.; Ivanenko, A.A.; Zhidkova, M.A. Increasing the corrosion resistance of zinc-based coatings by zinc melt alloying. Probl. Ferr. Metall. Mater. Sci. 2022, 4, 90–96. [Google Scholar] [CrossRef]
- Schurz, S.; Luckeneder, G.H.; Fleischanderl, M.; Mack, P.; Gsaller, H.; Kneissl, A.C.; Mori, G. Chemistry of corrosion products on Zn–Al–Mg alloy coated steel. Corros. Sci. 2010, 52, 3271–3279. [Google Scholar] [CrossRef]
- Tomandl, A.; Labrenz, E. The corrosion behavior of ZnAlMg alloys in maritime environments. Mater. Corros. 2016, 67, 1286–1293. [Google Scholar] [CrossRef]
- ISO 9226:2012; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Determination of Corrosion Rate of Standard Specimens for the Evaluation of Corrosivity, IDT. International Standards Organization: Geneva, Switzerland, 2012.
- ISO 9225:2012(E); Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Measurement of Environmental Parameters Affecting Corrosivity of Atmospheres, 2012. Classification, Determination and Estimation. International Standards Organization: Geneva, Switzerland, 2012.
- ISO 8407 2021; Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens, ASTM G1 Standard Practice for Preparing, Cleaning, and Evaluation Corrosion Test Specimens. International Standards Organization: Geneva, Switzerland, 2021.
- ISO 9223:2012(E); Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation. International Standards Organization: Geneva, Switzerland, 2012.
- Panchenko, Y.M.; Marshakov, A.I.; Nikolaeva, L.A.; Igonin, T.N. Estimating the First-year Corrosion Losses of Structural Metals for Continental Regions of the World. Civ. Eng. J. 2020, 6, 1503–1519. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I.; Nikolaeva, L.A.; Igonin, T.N. Development of models for the prediction of first-year corrosion losses of standard metals for territories with a coastal atmosphere in various climatic regions of the world. Corros. Eng. Sci. Technol. 2020, 55, 655–669. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I. Prediction of First-Year Corrosion Losses of Carbon Steel and Zinc in Continental Regions. Materials 2017, 10, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchenko, Y.M.; Marshakov, A.A.; Igonin, T.N.; Nikolaeva, L.A.; Kovtanyuk, V.V. Corrosivity of atmosphere toward structural metals and mapping the continental Russian territory. Corros. Eng. Sci. Technol. 2019, 54, 369–378. [Google Scholar] [CrossRef]
- Han, Y.; Hao, W.; Xu, L.; Chen, X. Classification and spatial mapping of atmospheric corrosion of China. NPJ Mater. Degrad. 2022, 6, 100. [Google Scholar]
- Knotkova, D.; Boschek, P.; Kreislova, K. Results of ISO CORRAG Program: Processing of One Year Data in Respect to Corrosivity Classification; ASTM Special Technical Publications: West Conshohocken, PA, USA, 1995; pp. 38–55. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.A.; Igonin, T.N.; Nikolaeva, L.A. Estimation of long-term corrosion resistance of structural metals and mapping the continental territory of the Russian Federation for various time periods. Corros. Eng. Sci. Technol. 2021, 56, 363–371. [Google Scholar] [CrossRef]



| Coating | Approx. Composition (wt %) | Nominal Coating Mass, g/m² | |
|---|---|---|---|
| Al | Mg | ||
| ZnAlMg | 1.0–1.4 | 1.0–1.4 | 275 |
| HDG | 0.18–0.30 | - | 120 |
| Corrosion Station | Designation | Longitude/Latitude | Classification |
|---|---|---|---|
| Moscow | MCS | 55°65′ N/37°54′ E | urban |
| Zvenigorod (Moscow region) | ZCS | 55°04′ N/37°57′ E | rural |
| Dal’nie Zelentsy (Barents Sea shore) | NCS | 69°07′ N/36°04′ E | marine |
| Vladivostok (Sea of Japan shore) | FECS | 43°04′ N/131°57′ E | marine |
| Parameter | Batch No. | Average Value | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| NCS | |||||
| T, °C | 2.1 | 2.1 | 2.7 | 2.7 | 2.4 |
| RH, % | 78 | 78 | 79 | 80 | 79 |
| Prec, mm/year | 389 | 374 | 317 | 220 | 325 |
| [SO2], mg/(m2·day) | 2.6 | 2.53 | 2.55 | 2.50 | 2.55 |
| [Cl−], mg/(m2·day) | 187 | 184 | 183 | 170 | 181 |
| FECS | |||||
| T, °C | 7.1 | 7.1 | 6.8 | 6.5 | 6.9 |
| RH, % | 66 | 66 | 66 | 67 | 66 |
| Prec, mm/year | 666 | 625 | 884 | 771 | 737 |
| [SO2], mg/(m2·day) | 3.63 | 3.27 | 3.28 | 3.33 | 3.38 |
| [Cl−], mg/(m2·day) | 36.3 | 34.8 | 24.8 | 14.4 | 27.6 |
| MCS | |||||
| T, °C | 8.2 | 7.6 | 7.5 | 7.0 | 7.6 |
| RH, % | 67 | 67 | 66 | 68 | 67 |
| Prec, mm/year | 665 | 559 | 438 | 421 | 521 |
| [SO2], mg/(m2·day) | 2.85 | 3.0 | 2.96 | 2.70 | 2.89 |
| ZCS | |||||
| T, °C | 6.4 | 6.0 | 5.9 | 4.9 | 5.8 |
| RH, % | 82 | 81 | 80 | 81 | 81 |
| Prec, mm/year | 533 | 479 | 459 | 456 | 482 |
| [SO2], mg/(m2·day) | 2.46 | 2.88 | 2.91 | 2.71 | 2.74 |
| Material | Batch | K1, g m−2 | Categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | NCS | FECS | MCS | ZCS | NCS | FECS | MCS | ZCS | |||||||||
| K1 | −∆K1 | +∆K1 | K1 | −∆K1 | +∆K1 | K1 | −∆K1 | +∆K1 | K1 | −∆K1 | +∆K1 | ||||||
| Steel 08ps | 1 | 331.9 | −4.3 | +6.7 | 277.8 | −8.7 | +9.7 | 40.4 | −4.7 | +2.8 | 32.8 | −0.9 | +1.4 | C3 | C3 | C2-1 | C2-1 |
| 2 | 355.8 | −1.4 | +1.8 | 226.8 | −1.4 | +0.8 | 44.5 | −0.5 | +0.4 | 22.1 | −0.9 | +1.6 | C3 | C3 | C2-1 | C2-1 | |
| 3 | 352.8 | −28.2 | +52.1 | 198.1 | −2.0 | +2.9 | 36.9 | −0.5 | +0.7 | 24.6 | −0.3 | +0.2 | C3 | C2-3 | C2-1 | C2-1 | |
| 4 | 293.1 | −13.2 | +10.5 | 239 | −26.4 | +16.7 | 110 | −6.7 | +6.3 | 97.8 | −16.9 | +9.1 | C3 | C3 | C2-3 | C2-2 | |
| Average | 333.4 | 235.4 | 57.9 | 44.3 | C3 | C3 | C2-2 | C2-1 | |||||||||
| Zn | 1 | 7.81 | −0.62 | +0.82 | 6.78 | −0.40 | +0.21 | 5.58 | −0.31 | +0.22 | 6.79 | −0.11 | +0.06 | C3 | C3 | C3 | C3 |
| 2 | 12.6 | −1.52 | +2.89 | 17.14 | −3.79 | +4.59 | 5.31 | −1.07 | +1.20 | 5.6 | −0.50 | +0.95 | C3 | C4 | C3 | C3 | |
| 3 | 7.23 | −0.54 | +0.77 | 8.03 | −1.13 | +0.96 | 3.09 | −0.26 | +0.16 | 11.55 | −2.78 | +1.68 | C3 | C3 | C2-3 | C3 | |
| 4 | 9.81 | −1.09 | +1.24 | 6.82 | −2.05 | +2.55 | 4.54 | −0.57 | +1.07 | 7.25 | −1.32 | +1.96 | C3 | C3 | C2-3 | C3 | |
| Average | 9.36 | 9.69 | 4.63 | 7.80 | C3 | C3 | C2-3 | C3 | |||||||||
| HDG | 1 | 5.04 | −0.27 | +0.30 | 2.82 | −0.13 | +0.11 | 1.8 | −0.14 | +0.17 | 1.42 | −0.16 | +0.12 | C3 | C2-2 | C2-2 | C2-1 |
| 2 | 5.5 | −0.28 | +0.24 | 4.52 | −0.19 | +0.22 | 1.44 | −0.13 | +0.12 | 1.68 | −0.10 | +0.05 | C3 | C2-3 | C2-1 | C2-2 | |
| 3 | 4.83 | −0.22 | +0.33 | 2.75 | −0.09 | +0.21 | 1.47 | −0.10 | +0.10 | 3.06 | −0.13 | +0.10 | C2-3 | C2-2 | C2-1 | C2-3 | |
| 4 | 4.43 | −0.27 | +0.13 | 2.45 | −0.05 | +0.04 | 1.68 | −0.07 | +0.08 | 2.08 | −0.03 | +0.03 | C2-3 | C2-2 | C2-1 | C2-2 | |
| Average | 4.95 | 3.14 | 1.60 | 2.06 | C2-3 | C2-3 | C2-2 | C2-2 | |||||||||
| ZnAlMg | 1 | 4.73 | −0.30 | +0.18 | 1.99 | −0.22 | +0.16 | 1.25 | −0.15 | +0.09 | 1.04 | −0.13 | +0.07 | C2-3 | C2-2 | C2-1 | C2-1 |
| 2 | 5.5 | −0.34 | +0.36 | 1.77 | −0.19 | +0.10 | 0.78 | −0.16 | +0.16 | 1.3 | −0.19 | +0.24 | C3 | C2-2 | C2-1 | C2-1 | |
| 3 | 5.43 | −0.31 | +0.56 | 0.92 | −0.11 | +0.16 | 0.96 | −0.10 | +0.14 | 1.84 | −0.08 | +0.07 | C3 | C2-1 | C2-1 | C2-2 | |
| 4 | 5.74 | −0.38 | +0.35 | 1.51 | −0.21 | +0.22 | 1.38 | −0.11 | +0.13 | 1.63 | −0.18 | +0.18 | C3 | C2-2 | C2-1 | C2-2 | |
| Average | 5.35 | 1.55 | 1.09 | 1.45 | C3 | C2-2 | C2-1 | C2-1 | |||||||||
| Material | Batch | K1, g m−2 | Categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | NCS | FECS | MCS | ZCS | NCS | FECS | MCS | ZCS | |||||||||
| K1 | −∆K1 | +∆K1 | K1 | −∆K1 | +∆K1 | K1 | −∆K1 | +∆K1 | K1 | −∆K1 | +∆K1 | ||||||
| Steel 08ps | 1 | 336.9 | −4.2 | +3.5 | 309.3 | −1.3 | +1.1 | 29.6 | −0.59 | +0.81 | 24.8 | −6.23 | +6.12 | C3 | C3 | C2-1 | C2-1 |
| 2 | 337.7 | −8.9 | +8.6 | 227.5 | −5.9 | +5.6 | 34.6 | −0.64 | +1.16 | 15.5 | −0.82 | +0.53 | C3 | C3 | C2-1 | C2-1 | |
| 3 | 378.3 | −7.8 | +4.7 | 242.5 | −2.9 | +1.5 | 50.6 | −1.41 | +1.52 | 20.0 | −0.10 | +0.06 | C3 | C3 | C2-2 | C2-1 | |
| 4 | 372.2 | −5.7 | +7.5 | 323.9 | −11.1 | +16.9 | 97.0 | −17.7 | +10.6 | 79.2 | −18.5 | +13.9 | C3 | C3 | C2-2 | C2-2 | |
| Average | 356.3 | 275.8 | 53.0 | 34.9 | C3 | C3 | C2-2 | C2-1 | |||||||||
| Zn | 1 | 7.43 | −0.50 | +0.59 | 8.51 | −0.07 | +0.08 | 3.98 | −0.11 | +0.14 | 3.36 | −0.46 | +0.31 | C3 | C3 | C2-3 | C2-3 |
| 2 | 8.40 | −0.88 | +1.67 | 11.99 | −0.61 | +0.96 | 1.69 | −0.57 | +0.39 | 1.22 | −0.37 | +0.64 | C3 | C3 | C2-2 | C2-1 | |
| 3 | 9.69 | −1.24 | +2.46 | 8.64 | −0.62 | +0.76 | 2.57 | −0.17 | +0.30 | 3.73 | −0.17 | +0.26 | C3 | C3 | C2-2 | C2-3 | |
| 4 | 9.84 | −1.85 | +2.47 | 6.40 | −1.81 | +1.35 | 3.54 | −1.08 | +0.71 | 2.86 | −0.25 | +0.32 | C3 | C3 | C2-3 | C2-2 | |
| Average | 8.84 | 8.89 | 2.95 | 2.79 | C3 | C3 | C2-2 | C2-2 | |||||||||
| HDG | 1 | 5.02 | −0.26 | +0.26 | 4.13 | −0.21 | +0.32 | 0.89 | −0.05 | +0.03 | 0.21 | −0.01 | +0.02 | C3 | C2-3 | C2-1 | C1 |
| 2 | 5.20 | −0.26 | +0.19 | 4.04 | −0.26 | +0.24 | 0.47 | −0.05 | +0.05 | 0.1 | −0.04 | +0.04 | C3 | C2-3 | C1 | C1 | |
| 3 | 5.03 | −0.34 | +0.38 | 2.11 | −0.12 | +0.09 | 0.52 | −0.05 | +0.07 | 0.53 | −0.07 | +0.11 | C3 | C2-2 | C1 | C1 | |
| 4 | 4.33 | −0.23 | +0.12 | 1.6 | −0.18 | +0.13 | 0.86 | −0.08 | 0.05 | 0.27 | −0.04 | +0.03 | C2-3 | C2-2 | C2-1 | C1 | |
| Average | 4.90 | 2.97 | 0.69 | 0.28 | C2-3 | C2-2 | C1 | C1 | |||||||||
| ZnAlMg | 1 | 4.73 | −0.13 | +0.24 | 3.0 | −0.12 | +0.20 | 0.96 | −0.04 | +0.06 | 0.53 | −0.08 | +0.09 | C2-3 | C2-2 | C2-1 | C1 |
| 2 | 6.10 | −0.31 | +0.26 | 2.01 | −0.21 | +0.16 | 0.5 | −0.12 | +0.14 | 0.53 | −0.04 | +0.07 | C3 | C2-2 | C1 | C1 | |
| 3 | 5.22 | −0.16 | +0.13 | 1.0 | −0.12 | +0.04 | 0.65 | −0.06 | +0.07 | 0.88 | −0.05 | +0.03 | C3 | C2-1 | C1 | C2-1 | |
| 4 | 4.94 | −0.24 | +0.16 | 1.04 | −0.11 | +0.09 | 0.83 | −0.04 | +0.04 | 0.89 | −0.03 | +0.01 | C2-3 | C2-1 | C2-1 | C2-1 | |
| Average | 5.25 | 1.76 | 0.74 | 0.71 | C3 | C2-2 | C2-1 | C2-1 | |||||||||
| Coefficient | Zn | HDG | ZnAlMg |
|---|---|---|---|
| Open Area | |||
| A | 0.55 | 0.22 | 0.17 |
| β | 0.27 | 0.30 | 0.38 |
| Shelter | |||
| A | 0.38 | 0.12 | 0.12 |
| β | 0.35 | 0.46 | 0.46 |
| Error | Open Area | Shelter | ||||||
|---|---|---|---|---|---|---|---|---|
| Steel 08ps | Zn | HDG | ZnAlMg | Steel 08ps | Zn | HDG | ZnAlMg | |
| In comparison with K1ex values obtained for each of 4 batches | ||||||||
| −δ% | 35.1 | 29.3 | 12.9 | 10.9 | 31.9 | 18.2 | 18.8 | 4.8 |
| +δ% | 29.2 | 40.4 | 24.7 | 42.6 | 41.0 | 21.0 | 33.1 | 41.6 |
| In comparison with the average K1ex values obtained for 4 batches | ||||||||
| −δ% | 7.0 | 17.5 | 2.4 | - | 21.3 | 8.1 | 2.7 | - |
| +δ% | 27.6 | 44 | 15.5 | 8.1 | 29.4 | 12.5 | 19.0 | 25.6 |
| Material | NCS | FECS | MCS | ZCS | ||||
|---|---|---|---|---|---|---|---|---|
| OA | Shelter | OA | Shelter | OA | Shelter | OA | Shelter | |
| Steel 08ps | 194.1 | 224.4 | 177.7 | 245.6 | 35.5 | 28.2 | 24.7 | 21.6 |
| ZnO | 4.95 | 5.81 | 4.99 | 4.70 | 4.14 | 2.75 | 4.26 | 1.93 |
| HDG | 3.83 | 3.76 | 2.42 | 2.54 | 1.43 | 0.56 | 1.31 | 0.26 |
| ZnAlMg120 | 3.52 | 3.40 | 1.77 | 2.42 | 0.90 | 0.60 | 0.75 | 0.44 |
| References | Coating | σ2, μm year−1 | |
|---|---|---|---|
| C2 | C3 | ||
| [12] | Zn1%Al1%Mg Zn1.5%Al1.5%Mg | 0.22–0.26 Average 0.24 | 0.39–0.81 Average 0.6 |
| [12] | Zn2%Al2%Mg | 0.15 | 0.4–0.64 Average 0.52 |
| [16] | Zn2.67%Al1.51%Mg Zn2.71%Al1.51%Mg Zn2.72%Al1.49%Mg Zn2.9%Al1.6%Mg | - | 0.34–0.54 Average 0.41 |
| [17,18] | Zn3.7%Al3%Mg | 0.09–0.36 Average 0.23 | 0.42–0.81 Average 0.62 |
| Testing Site | Category | Testing Conditions | σ2, μm year−1 | |
|---|---|---|---|---|
| HDG | ZnAlMg | |||
| ZCS, MCS FECS | C2 | OA | 0.19–0.35 Average 0.27 | 0.12–0.27 Average 0.20 |
| Shelter | 0.04–0.36 Average 0.20 | 0.07–0.38 Average 0.23 | ||
| NCS | C3 | OA | 0.55 | 0.54 |
| Shelter | 0.54 | 0.53 | ||
| Coating | ZCS | MCS | FECS | NCS | ||||
|---|---|---|---|---|---|---|---|---|
| Category | T | Category | T | Category | T | Category | T | |
| Open area | ||||||||
| HDG | C2 | ˃200 | C2 | 190 | C2 | 113 | C3 | 72 |
| ZnAlMg | C2 | 160 | C2 | 130 | C2 | 68 | C3 | 34 |
| Shelter | ||||||||
| HDG | C2 | ˃˃200 | C2 | ˃˃200 | C2 | 108 | C3 | 73 |
| ZnAlMg | C2 | ˃200 | C2 | 200 | C2 | 50 | C3 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchenko, Y.M.; Marshakov, A.I.; Igonin, T.N.; Nenasheva, T.A.; Nikolaeva, L.A.; Ivanenko, A.A. Corrosion Resistance of Zinc and Zinc-Aluminum-Magnesium Coatings in Atmosphere on the Territory of Russia. Materials 2023, 16, 5214. https://doi.org/10.3390/ma16155214
Panchenko YM, Marshakov AI, Igonin TN, Nenasheva TA, Nikolaeva LA, Ivanenko AA. Corrosion Resistance of Zinc and Zinc-Aluminum-Magnesium Coatings in Atmosphere on the Territory of Russia. Materials. 2023; 16(15):5214. https://doi.org/10.3390/ma16155214
Chicago/Turabian StylePanchenko, Yulia M., Andrey I. Marshakov, Timofey N. Igonin, Tatyana A. Nenasheva, Ludmila A. Nikolaeva, and Artem A. Ivanenko. 2023. "Corrosion Resistance of Zinc and Zinc-Aluminum-Magnesium Coatings in Atmosphere on the Territory of Russia" Materials 16, no. 15: 5214. https://doi.org/10.3390/ma16155214
APA StylePanchenko, Y. M., Marshakov, A. I., Igonin, T. N., Nenasheva, T. A., Nikolaeva, L. A., & Ivanenko, A. A. (2023). Corrosion Resistance of Zinc and Zinc-Aluminum-Magnesium Coatings in Atmosphere on the Territory of Russia. Materials, 16(15), 5214. https://doi.org/10.3390/ma16155214






