Drop-Dry Deposition of SnO2 Using a Complexing Agent and Fabrication of Heterojunctions with Co3O4
Abstract
:1. Introduction
2. Experimental Design
3. Results and Discussion
3.1. Deposition Reactions
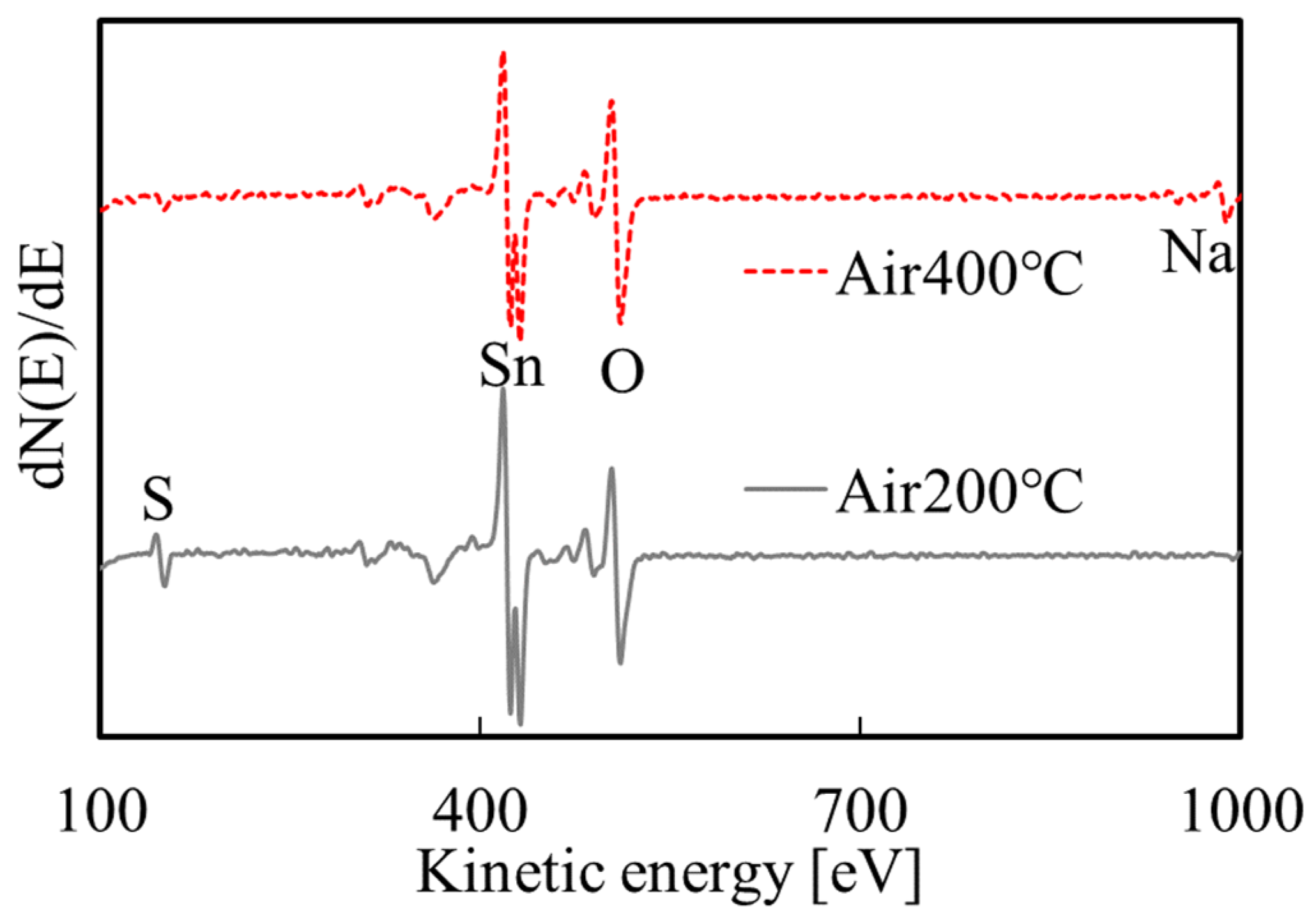
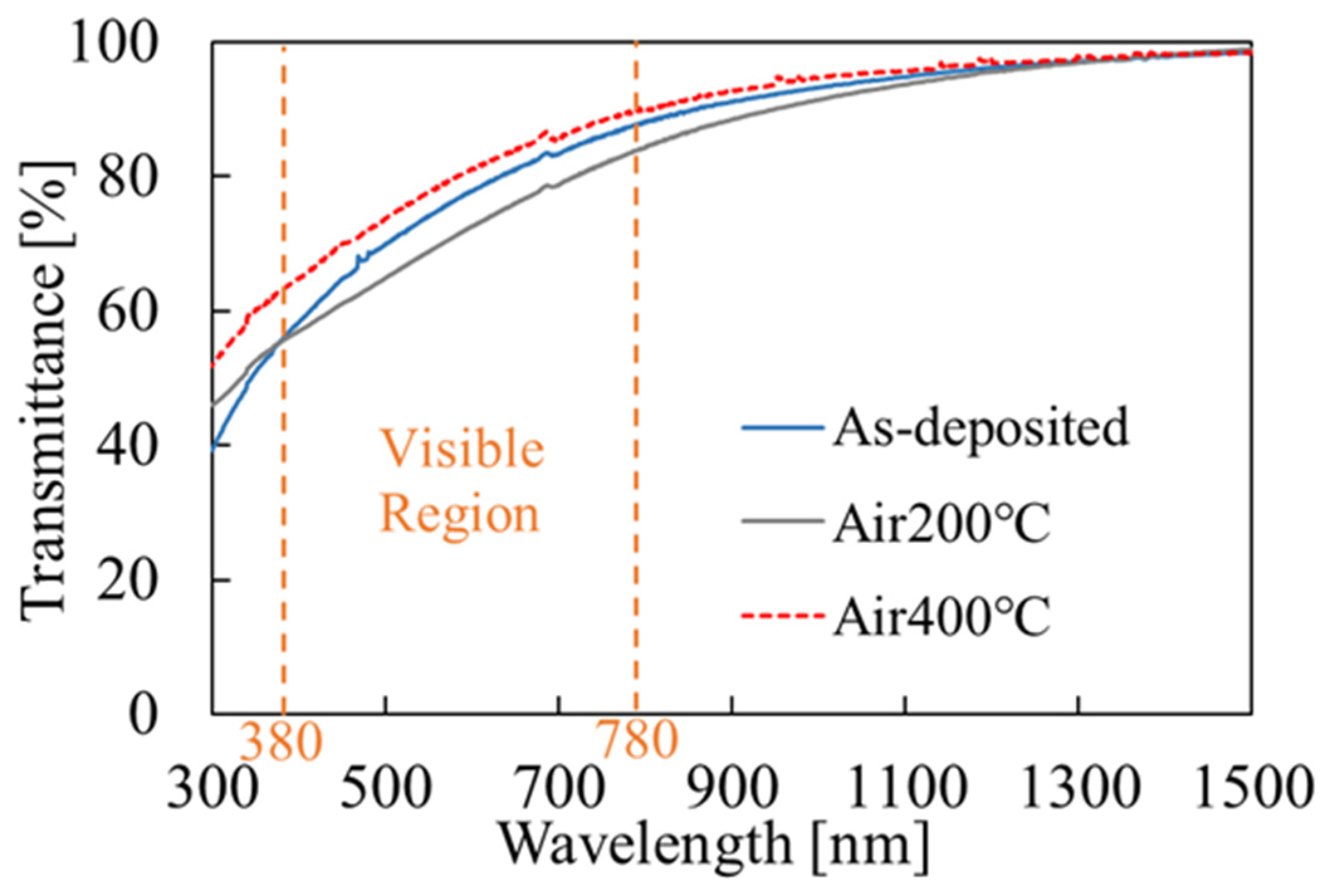
3.2. SnO2 Film Properties
3.3. Co3O4/SnO2 Heterojunction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gevorgyan, V.A.; Mangasaryan, N.R.; Gremenok, V.F.; Tivanov, M.S.; Thakur, P.; Thakur, A.; Trukhanov, S.V.; Zubar, T.I.; Sayyed, M.I.; Tishkevich, D.I.; et al. Morphology and structure of CdCl2-Containing CdTe films deposited by discrete vacuum thermal evaporation. Vacuum 2023, 214, 112248. [Google Scholar] [CrossRef]
- Knechtli, R.C.; Loo, R.Y.; Kamath, G.S. High-efficiency GaAs solar cells. IEEE Trans. Electron Devices 1984, 31, 577–588. [Google Scholar] [CrossRef]
- Schock, H.W.; Noufi, R. CIGS-based solar cells for the next millennium. Prog. Photovolt. Res. Appl. 2000, 8, 151–160. [Google Scholar] [CrossRef]
- Fröhlich, D.; Klenkies, R.; Helbig, R. Band-Gap Assignment in SnO2 by Two-Photon Spectroscopy. Phys. Rev. Lett. 1978, 41, 1750. [Google Scholar] [CrossRef]
- Sanon, G.; Rup, R.; Mansingh, A. Band-gap narrowing and band structure in degenerate tin oxide (SnO2) films. Phys. Rev. B 1991, 44, 5672. [Google Scholar]
- Kane, J.; Schweizer, H.P.; Kern, W. Chemical Vapor Deposition of Transparent, Electrically Conductive Tin Oxide Films Formed from Dibutyl Tin Diacetate. J. Electrochem. Soc. 1975, 122, 1144. [Google Scholar] [CrossRef]
- Popova, L.I.; Michailov, M.G.; Gueorguiev, V.K.; Shopov, A. Structure and morphology of thin SnO2 films. Thin Solid Films 1990, 186, 107–112. [Google Scholar] [CrossRef]
- Czapla, A.; Kusior, E.; Bucko, M. Optical properties of non-stoichiometric tin oxide films obtained by reactive sputtering. Thin Solid Films 1989, 182, 15–22. [Google Scholar] [CrossRef]
- Rakhshani, A.E.; Makdisi, Y.; Ramazaniyan, H.A. Electronic and optical properties of fluorine-doped tin oxide films. J. Appl. Phys. 1998, 83, 1049–1057. [Google Scholar] [CrossRef]
- Karlsson, T.; Roos, A.; Ribbing, C.G. Influence of spray conditions and dopants on highly conducting tin dioxide films. Sol. Energy Mater. 1985, 11, 469–478. [Google Scholar] [CrossRef]
- Vasiliev, R.B.; Rumyantseva, M.N.; Yakovlev, N.V.; Gaskov, A.M. CuO/SnO2 thin film heterostructures as chemical sensors to H2S. Sens. Actuators B Chem. 1998, 50, 186–193. [Google Scholar] [CrossRef]
- Tatsuyama, C.; Ichimura, S. Electrical and Optical Properties of GaSe-SnO2 Heterojunctions. Jpn. J. Appl. Phys. 1976, 15, 843. [Google Scholar] [CrossRef]
- Vequizo, J.J.M.; Ichimura, M. Fabrication of Electrodeposited SnS/SnO2 Heterojunction Solar Cells. Jpn. J. Appl. Phys. 2012, 51, 10NC38. [Google Scholar] [CrossRef]
- Qin, C.; Wang, Y.; Lou, Z.; Yue, S.; Niu, W.; Zhu, L. Surface modification and stoichiometry control of Cu2O/SnO2 heterojunction solar cell by an ultrathin MgO tunneling layer. J. Alloys Compd. 2019, 779, 387–393. [Google Scholar] [CrossRef]
- Anaraki, E.H.; Kermanpur, A.; Mayer, M.T.; Steier, L.; Ahmed, T.; Turren-Cruz, S.H.; Seo, J.Y.; Luo, J.S.; Zakeeruddin, S.M.; Tress, W.R.; et al. Low-temperature Nb-doped SnO2 electron-selective contact yields over 20% efficiency in planar perovskite solar cells. ACS Energy Lett. 2018, 3, 773–778. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.Q.; Wang, H.L.; Yang, X.L.; Meng, J.H.; Liu, H.; Yin, Z.G.; Wu, J.L.; Zhang, X.W.; You, J.B. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2017, 2, 16177. [Google Scholar] [CrossRef]
- Roose, B.; Baena, J.P.C.; Gödel, K.C.; Graetzel, M.; Hagfeldt, A.; Steiner, U.; Abate, A. Mesoporous SnO2 electron selective contact enables UV-stable perovskite solar cells. Nano Energy 2016, 30, 517–522. [Google Scholar] [CrossRef] [Green Version]
- El-Etre, A.Y.; Reda, S.M. Characterization of nanocrystalline SnO2 thin film fabricated by electrodeposition method for dye-sensitized solar cell application. Appl. Surf. Sci. 2010, 256, 6601–6606. [Google Scholar] [CrossRef]
- Panayotatos, P.; Parikh, D.; Sauers, R.; Bird, G.; Piechowski, A.; Husain, S. Improved p-n heterojunction solar cells employing thin film organic semiconductors. Sol. Cells 1986, 18, 71–84. [Google Scholar] [CrossRef]
- Xiong, L.; Guo, Y.; Wen, J.; Liu, H.; Yang, G.; Qin, P.; Fang, G. Review on the Application of SnO2 in Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1802757. [Google Scholar] [CrossRef]
- Tennakone, K.; Bandara, J.; Bandaranayake, P.K.M.; Kumara, G.R.A.; Konno, A. Enhanced Efficiency of a Dye-Sensitized Solar Cell Made from MgO-Coated Nanocrystalline SnO2. Jpn. J. Appl. Phys. 2001, 40, L732. [Google Scholar] [CrossRef]
- Cai, N.; Cho, J. Low temperature processed SnO2 films using aqueous precursor solutions. Ceram. Int. 2013, 39, 143–151. [Google Scholar] [CrossRef]
- Ohgi, H.; Maeda, T.; Hosono, E.; Fujihara, S.; Imai, H. Evolution of Nanoscale SnO2 Grains, Flakes, and Plates into Versatile Particles and Films through Crystal Growth in Aqueous Solutions. Cryst. Growth Des. 2005, 5, 1079–1083. [Google Scholar] [CrossRef]
- Chatelon, J.P.; Terrier, C.; Bernstein, E.; Berjoan, R.; Roger, J.A. Morphology of SnO2 thin films obtaibed by the sol-gel technique. Thin Solid Films 1994, 247, 162–168. [Google Scholar] [CrossRef]
- Racheva, T.M.; Critchlow, G.W. SnO2 thin films prepared by the sol-gel process. Thin Solid Films 1997, 292, 299–302. [Google Scholar] [CrossRef]
- Terrier, C.; Chatelon, J.P.; Roger, J.A. Electrical and optical properties of Sb:SnO2 thin films obtained by the sol-gel method. Thin Solid Films 1997, 295, 95–100. [Google Scholar] [CrossRef]
- Vequizo, J.J.M.; Wang, J.; Ichimura, M. Electrodeposition of SnO2 Thin Films from Aqueous Tin Sulfate Solutions. Jpn. J. Appl. Phys. 2010, 49, 125502. [Google Scholar] [CrossRef]
- Chu, D.; Masuda, Y.; Ohji, T.; Kato, K. Fast synthesis, optical and bio-sensor properties of SnO2 nanostructures by electrochemical deposition. Chem. Eng. J. 2011, 168, 955–958. [Google Scholar] [CrossRef]
- Li, T.; Ichimura, M. Fabrication of Transparent Mg(OH)2 Thin Films by Drop-Dry Deposition. Materials 2021, 14, 724. [Google Scholar] [CrossRef]
- Li, T.; Ichimura, M. Drop-dry deposition of Co3O4 and fabrication of heterojunction solar cells with electrochemically deposited ZnO. Semicond. Sci. Technol. 2021, 36, 095030. [Google Scholar] [CrossRef]
- Li, T.; Okada, T.; Ichimura, M. Drop–Dry Deposition of Ni(OH)2 Precursors for Fabrication of NiO Thin Films. Materials 2022, 15, 4513. [Google Scholar] [CrossRef] [PubMed]
- Turan, E.; Zeybekoğlu, E.; Kul, M. Effects of bath temperature and deposition time on Co3O4 thin films produced by chemical bath deposition. Thin Solid Films 2019, 692, 137632. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Aboud, A.A.; Irfan, M.; Alam, S. Effect of substrate temperature on structure and optical properties of Co3O4 films prepared by spray pyrolysis technique. IOP Conf. Ser. Mater. Sci. Eng. 2014, 60, 012058. [Google Scholar] [CrossRef]
- Pettine, M.; Millero, F.J.; Macchi, G. Hydrolysis of tin(II) in aqueous solutions. Anal. Chem. 1981, 53, 1039–1043. [Google Scholar] [CrossRef]
- Trachevskii, V.V.; Zimina, S.V.; Rodina, E.P. Thiosulfate metal complexes. Russ. J. Coord. Chem. 2008, 34, 664–669. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p−n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Kupfer, B.; Majhi, K.; Keller, D.A.; Bouhadana, Y.; Rühle, S.; Barad, H.N.; Anderson, A.Y.; Zaban, A. Thin Film Co3O4/TiO2 Heterojunction Solar Cells. Adv. Energy Mater. 2015, 5, 1401007. [Google Scholar] [CrossRef]
- Majhi, K.; Bertoluzzi, L.; Keller, D.A.; Barad, H.N.; Ginsburg, A.; Anderson, A.Y.; Vidal, R.; Lopez-Varo, P.; Mora-Sero, I.; Bisquert, J.; et al. Co3O4 Based All-Oxide PV: A Numerical Simulation Analyzed Combinatorial Material Science Study. J. Phys. Chem. C 2016, 120, 9053–9060. [Google Scholar] [CrossRef]
- Lohaus, C.; Morasch, J.; Brotz, J.; Klein, A.; Jaegermann, W. Investigations on RF-magnetron sputtered Co3O4 thin films regarding the solar energy conversion properties. J. Phys. D Appl. Phys. 2016, 49, 155306. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, M.; Kim, H.S.; Park, W.H.; Choi, E.H.; Kim, J. Reactive sputtering growth of Co3O4 thin films for all metal oxide device: A semitransparent and self-powered ultraviolet photodetector. Mater. Sci. Semicond. Proc. 2018, 74, 74–79. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, S.X.; Tang, W. SnO2/Co3O4 nanofibers using double jets electrospinning as low operating temperature gas sensor. Chin. Phys. B 2022, 31, 028101. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Optimization and gas sensing mechanism of n-SnO2-p-Co3O4 composite nanofibers. Sens. Actuators B Chem. 2017, 248, 500–511. [Google Scholar] [CrossRef]
- Meng, D.; Si, J.; Wang, M.; Wang, G.; Shen, Y.; San, X.; Meng, F. One-step synthesis and the enhanced trimethylamine sensing properties of Co3O4/SnO2 flower-like structures. Vacuum 2020, 171, 108994. [Google Scholar] [CrossRef]
- Bai, S.; Liu, H.; Luo, R.; Chen, A.; Li, D. SnO2@Co3O4 p–n heterostructures fabricated by electrospinning and mechanism analysis enhanced acetone sensing. RSC Adv. 2014, 4, 62862–62868. [Google Scholar] [CrossRef]
- Ni, T.; Yang, Z.; Zhang, H.; Zhou, L.; Guo, W.; Pan, L.; Yang, Z.; Chang, K.; Ge, C.; Liu, D. Peroxymonosulfate activation by Co3O4/SnO2 for efficient degradation of ofloxacin under visible light. J. Colloid Interface Sci. 2022, 615, 650–662. [Google Scholar] [CrossRef]
- Huang, R.; Huang, S.; Chen, D.; Zhang, Q.; Le, T.T.; Wang, Q.; Hu, Z.; Chen, Z. Environmentally benign synthesis of Co3O4-SnO2 heteronanorods with efficient photocatalytic performance activated by visible light. J. Colloid Interface Sci. 2019, 542, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Tyagi, I.; Gupta, V.K.; Sohrabi, M.; Mohammadi, S.; Golikand, A.N.; Fakhri, A. Iron doped SnO2/Co3O4 nanocomposites synthesized by sol-gel and precipitation method for metronidazole antibiotic degradation. Mater. Sci. Eng. C 2017, 70, 178–183. [Google Scholar] [CrossRef]
- Koyano, H. Studies in Electroless Tin Plating Reclamation of Used Bath. Hyomen Gijutsu 1993, 44, 518–524. [Google Scholar]
- Lokhande, C.D. A chemical method for tin disulphide thin film deposition. J. Phys. D Appl. Phys. 1990, 23, 1703. [Google Scholar] [CrossRef]
- Valiukonis, G.; Gusenova, D.A.; Krivate, G.; Sileika, A. Optical spectra and energy band structure of layer-type AIVBVI compounds. Phys. Stat. Sol. 1986, 135, 299–307. [Google Scholar] [CrossRef]
- Ristov, M.; Sinadinovski, G.J.; Grozdanov, I.; Mitreski, M. Chemical deposition of TIN(II) sulphide thin films. Thin Solid Films 1989, 173, 53–58. [Google Scholar] [CrossRef]
- Nair, P.K.; Nair, M.T.S.; Campos, J.; Sanchez, A. SnS--SnO2 conversion of chemically deposited SnS thin films. Adv. Mater. Opt. Electron. 1992, 1, 117–121. [Google Scholar] [CrossRef]
- Nair, P.K.; Nair, M.T.S.; Zingaro, R.A.; Meyers, E.A. XRD, XPS, optical and electrical studies on the conversion of SnS thin films to SnO2. Thin Solid Films 1994, 239, 85–92. [Google Scholar] [CrossRef]
- Khan, A.F.; Mehmood, M.; Aslam, M.; Ashraf, M. Characteristics of electron beam evaporated nanocrystalline SnO2 thin films annealed in air. Appl. Surf. Sci. 2010, 256, 2252–2258. [Google Scholar] [CrossRef]
- Lee, J. Effects of oxygen concentration on the properties of sputtered SnO2:Sb films deposited at low temperature. Thin Solid Films 2008, 516, 1386–1390. [Google Scholar] [CrossRef]
- Um, J.; Kim, S.E. Homo-Junction pn Diode Using p-Type SnO and n-Type SnO2 Thin Films. ECS Solid State Lett. 2014, 3, P94. [Google Scholar] [CrossRef]
- Dattoli, E.N.; Wan, Q.; Guo, W.; Chen, Y.; Pan, X.; Lu, W. Fully Transparent Thin-Film Transistor Devices Based on SnO2 Nanowires. Nano Lett. 2007, 7, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Kantareddy, S.N.R.; Mathews, I.; Sun, S.; Layurova, M.; Thapa, J.; Correa-Baena, J.P.; Bhattacharyya, R.; Buonassisi, T.; Sarma, S.E.; Peters, I.M. Perovskite PV-Powered RFID: Enabling Low-Cost Self-Powered IoT Sensors. IEEE Sens. J. 2019, 20, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Ko, K.Y.; Lee, S.; Park, K.; Kim, Y.; Woo, W.J.; Kim, D.; Song, J.G.; Park, J.; Kim, J.H.; Lee, Z.; et al. High-Performance Gas Sensor Using a Large-Area WS2xSe2–2x Alloy for Low-Power Operation Wearable Applications. ACS Appl. Mater. Interfaces 2018, 10, 34163–34171. [Google Scholar] [CrossRef]
- Tanuma, R.; Sugiyama, M. Polycrystalline SnO2 Visible-Light-Transparent CO2 Sensor Integrated with NiO/ZnO Solar Cell for Self-Powered Devices. Phys. Status Solidi A 2019, 216, 1800749. [Google Scholar] [CrossRef]
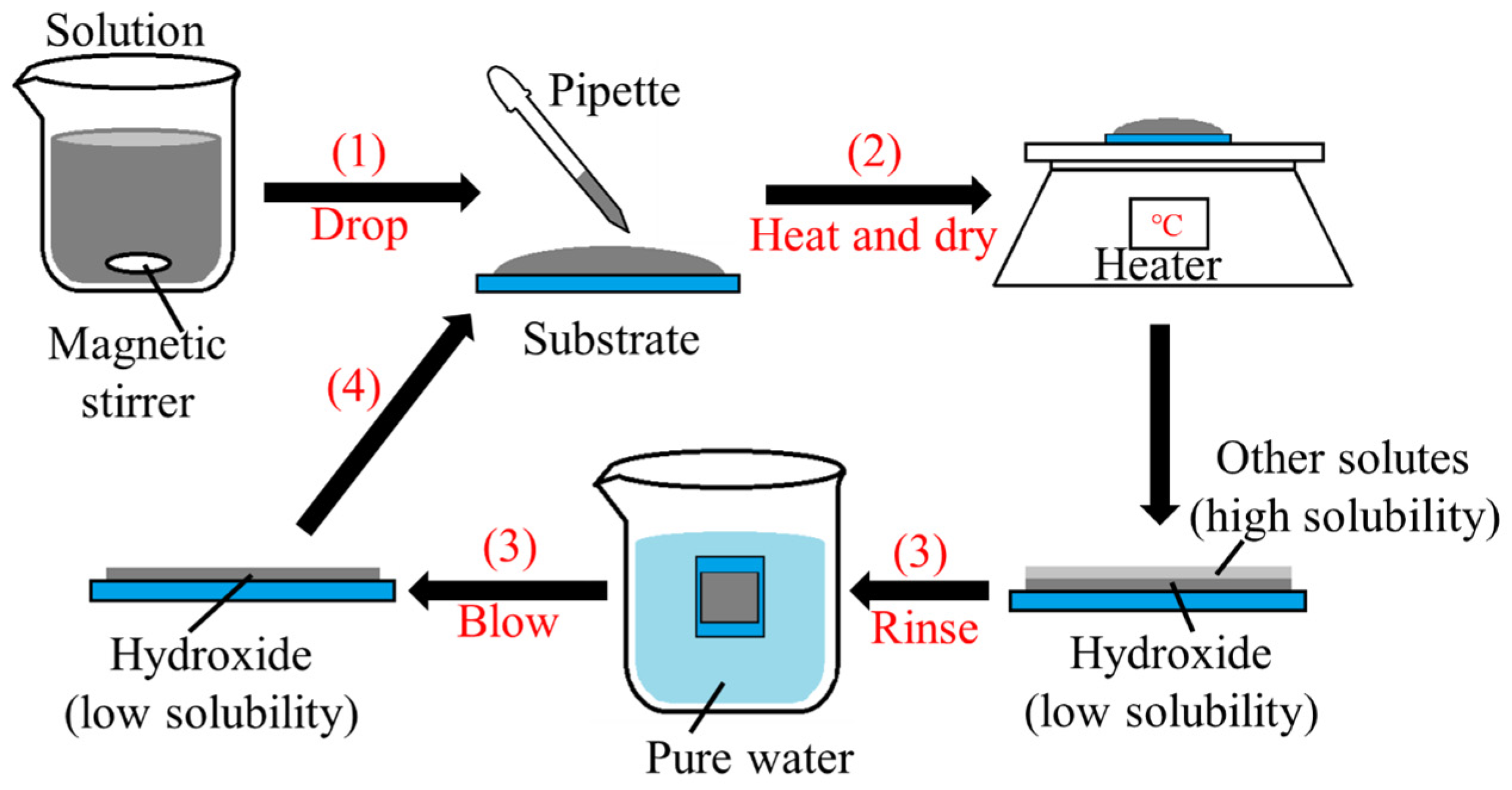
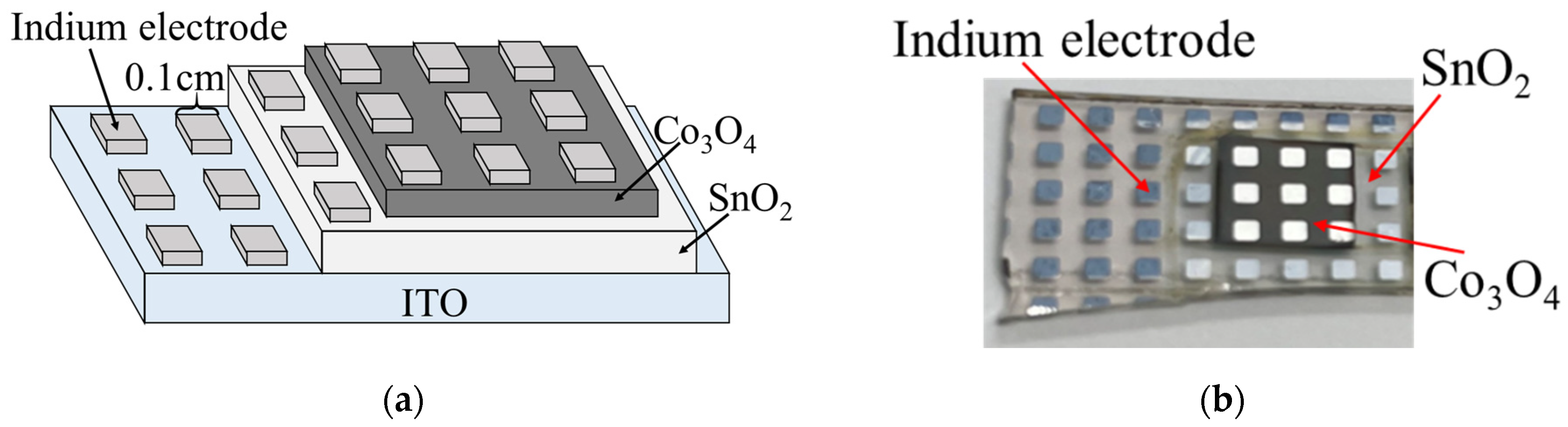





| Condition | SnSO4 [mM] | Na2S2O3 [mM] | NaOH [mM] | pH | Precipitate | Solution Color | Thickness [μm] |
|---|---|---|---|---|---|---|---|
| I | 10 | 50 | — | 3.8 | none | yellowish-white | 0.2 |
| II | 10 | 100 | — | 3.5 | none | white | 0.2 |
| III | 10 | 200 | — | 3.4 | none | Colorless | — |
| IV | 20 | 50 | — | 4.4 | yellow | yellow | — |
| V | 10 | 50 | 2.5 | 4.7 | yellow | yellow | — |
| VI | 10 | 50 | 25 | 11.7 | white→black | white→dark | — |
| As-Deposited | 200 °C | 400 °C | |
|---|---|---|---|
| O/Sn | 1.11 | 1.65 | 2.16 |
| resistivity (Ωcm) | 1.4 × 106 | 3.4 × 104 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Ichimura, M. Drop-Dry Deposition of SnO2 Using a Complexing Agent and Fabrication of Heterojunctions with Co3O4. Materials 2023, 16, 5273. https://doi.org/10.3390/ma16155273
Li T, Ichimura M. Drop-Dry Deposition of SnO2 Using a Complexing Agent and Fabrication of Heterojunctions with Co3O4. Materials. 2023; 16(15):5273. https://doi.org/10.3390/ma16155273
Chicago/Turabian StyleLi, Tong, and Masaya Ichimura. 2023. "Drop-Dry Deposition of SnO2 Using a Complexing Agent and Fabrication of Heterojunctions with Co3O4" Materials 16, no. 15: 5273. https://doi.org/10.3390/ma16155273
APA StyleLi, T., & Ichimura, M. (2023). Drop-Dry Deposition of SnO2 Using a Complexing Agent and Fabrication of Heterojunctions with Co3O4. Materials, 16(15), 5273. https://doi.org/10.3390/ma16155273







