Deterioration of Cement-Based Materials in Low-Temperature Seawater
Abstract
1. Introduction
2. Experimental Method
2.1. Materials and Sample Preparation
2.2. Laboratory Immersion Tests
2.3. Characterization Methods
2.3.1. XRD Analysis
2.3.2. EPMA
2.3.3. SEM-EDS Analysis
2.3.4. Magic-Angle Spinning (MAS) NMR Spectroscopy
3. Results
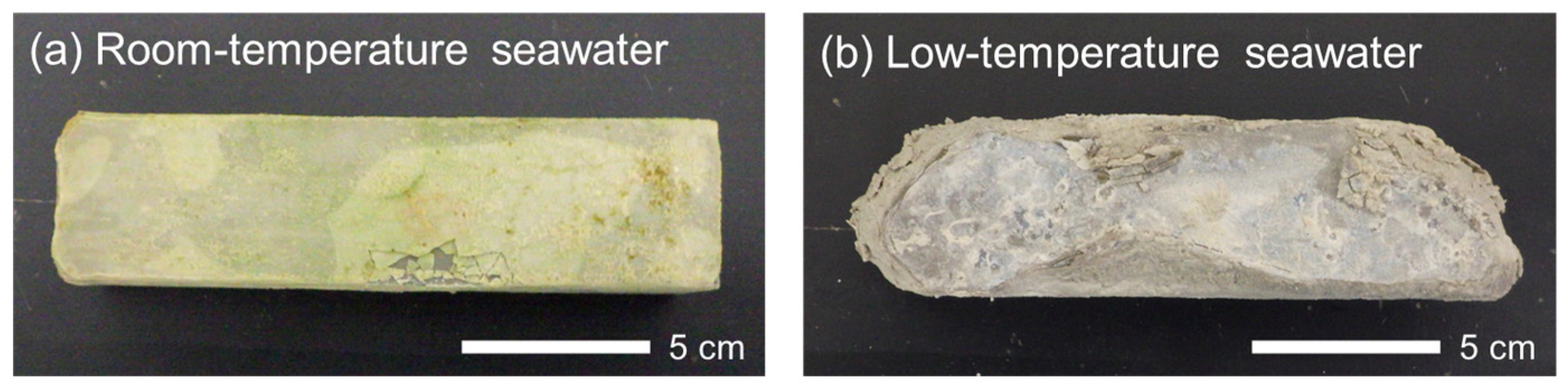
3.1. Visual Changes
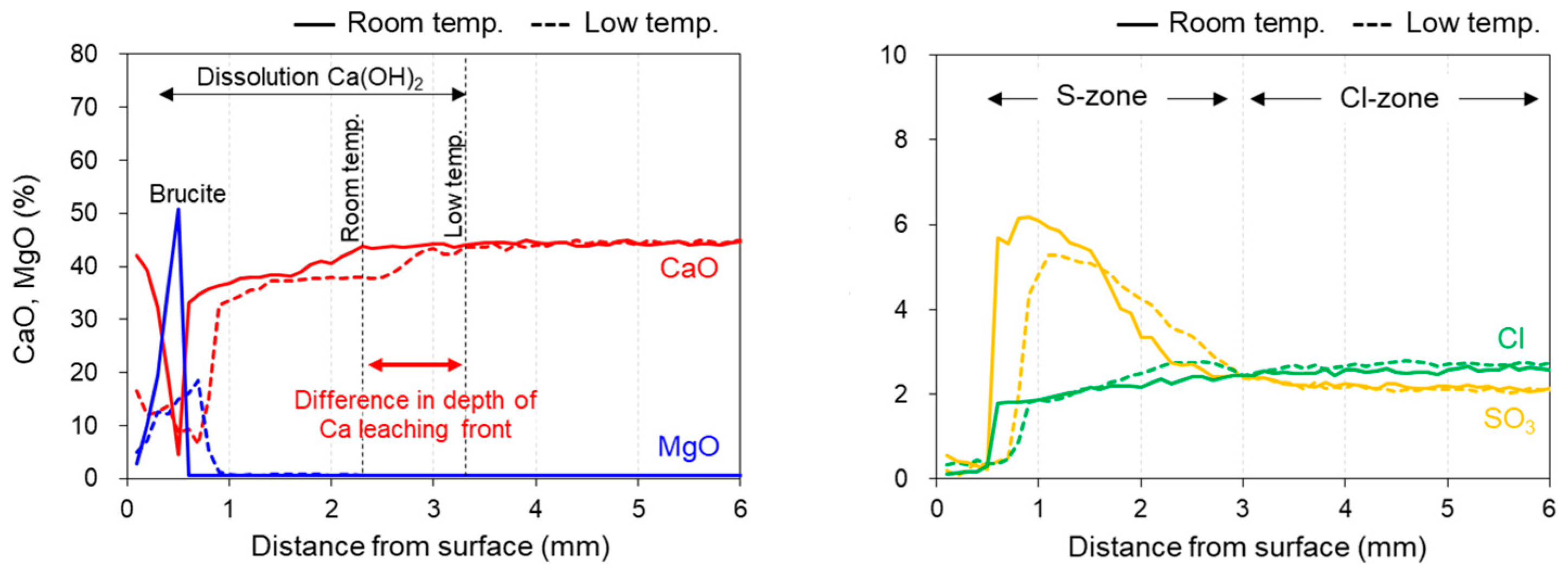
3.2. Elemental Mapping by EPMA
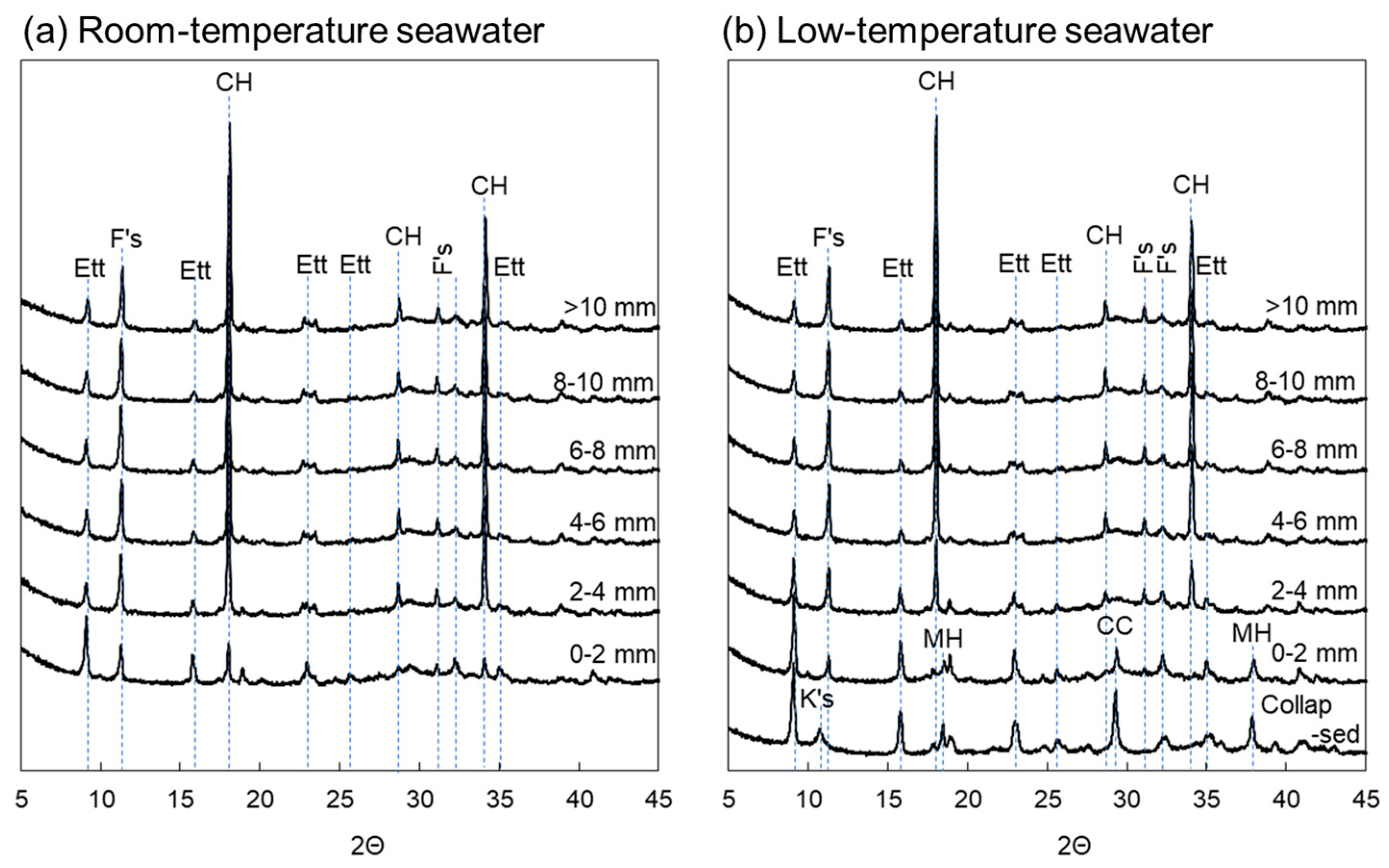
3.3. XRD Analysis
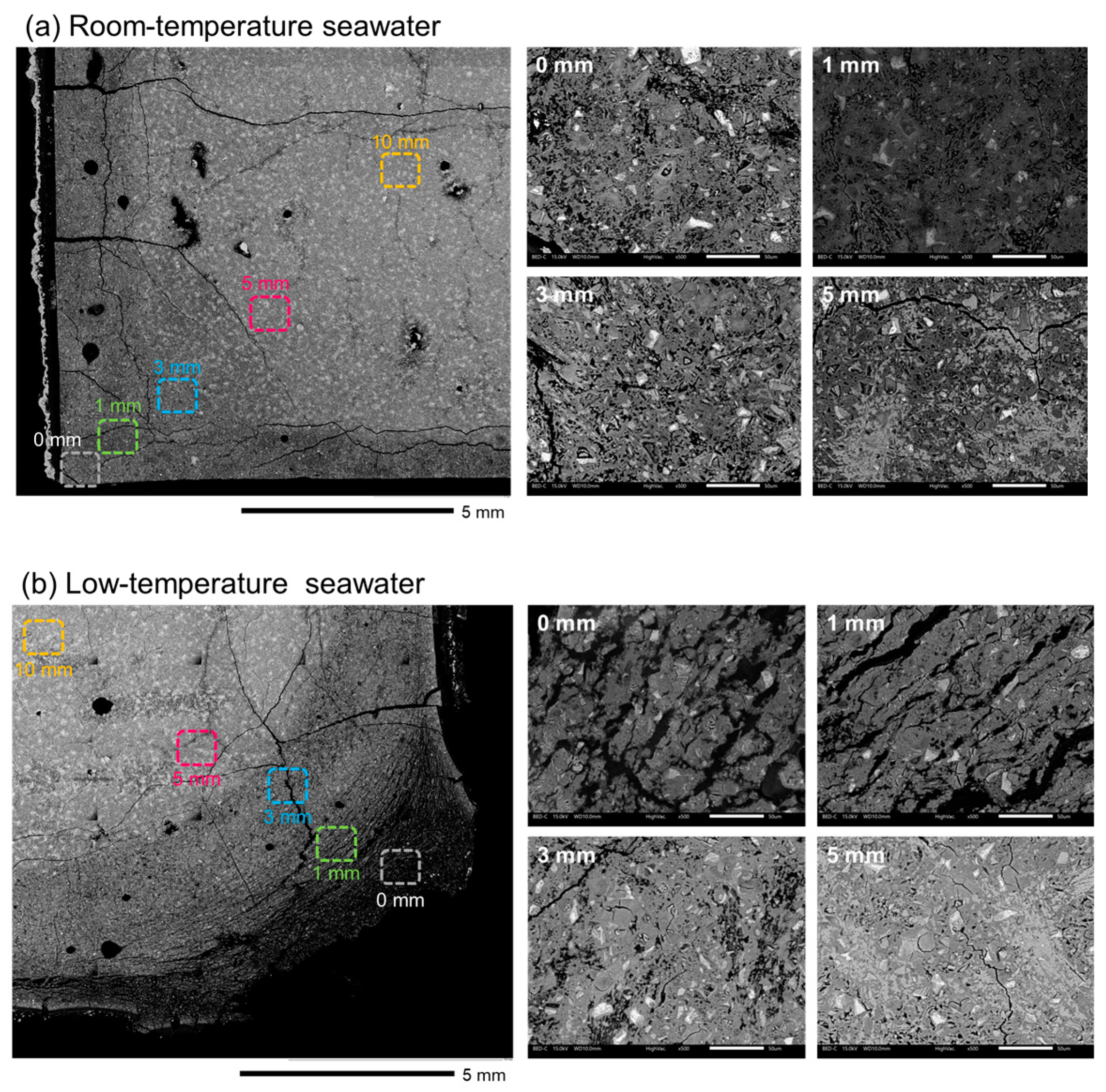
3.4. SEM Imaging
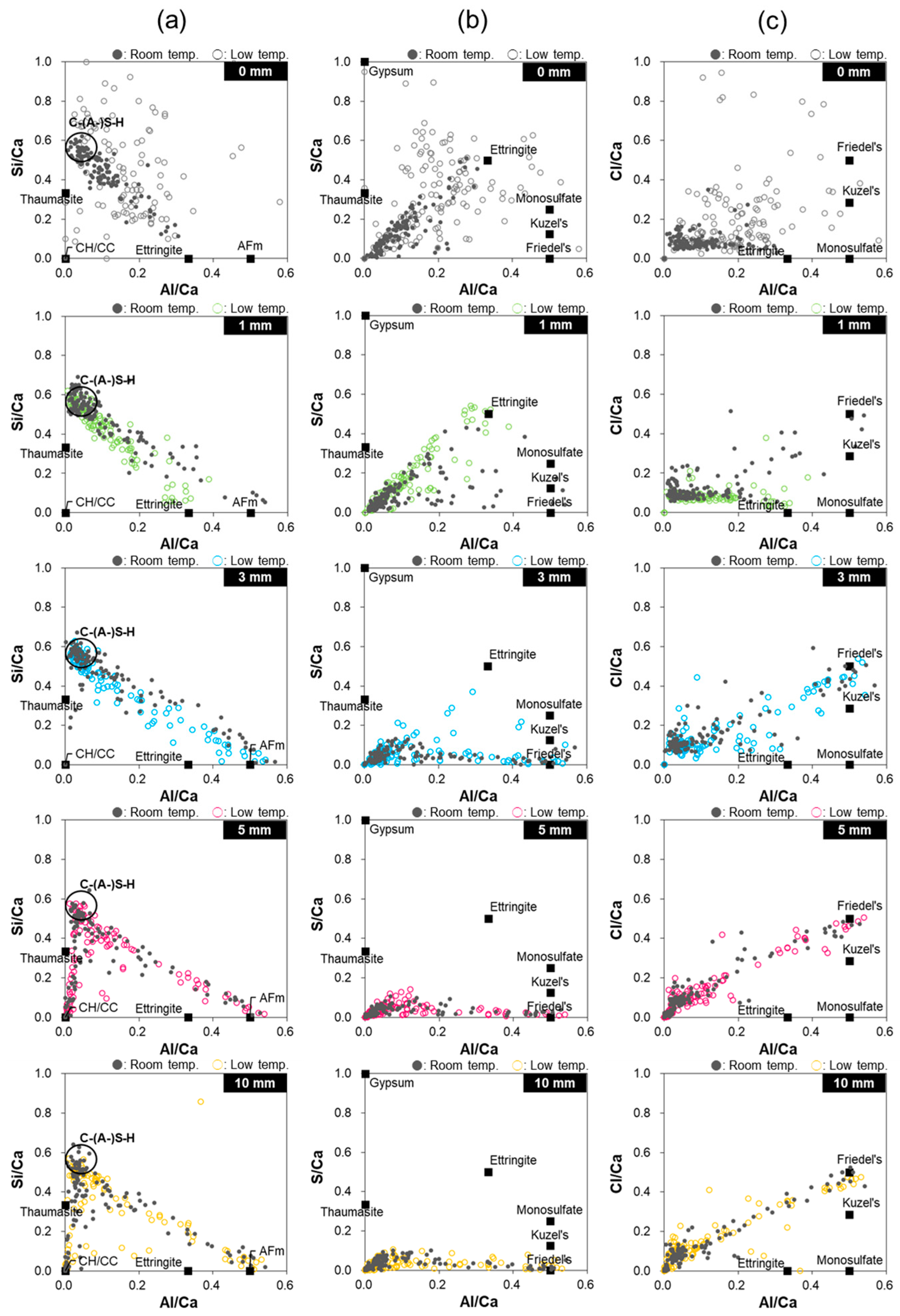
3.5. EDS Analysis
3.6. 29Si-NMR Analysis
4. Discussion
5. Conclusions
- The Ca dissolution front was deep within the sample, and portlandite dissolution was more pronounced than in the specimen immersed in room-temperature seawater;
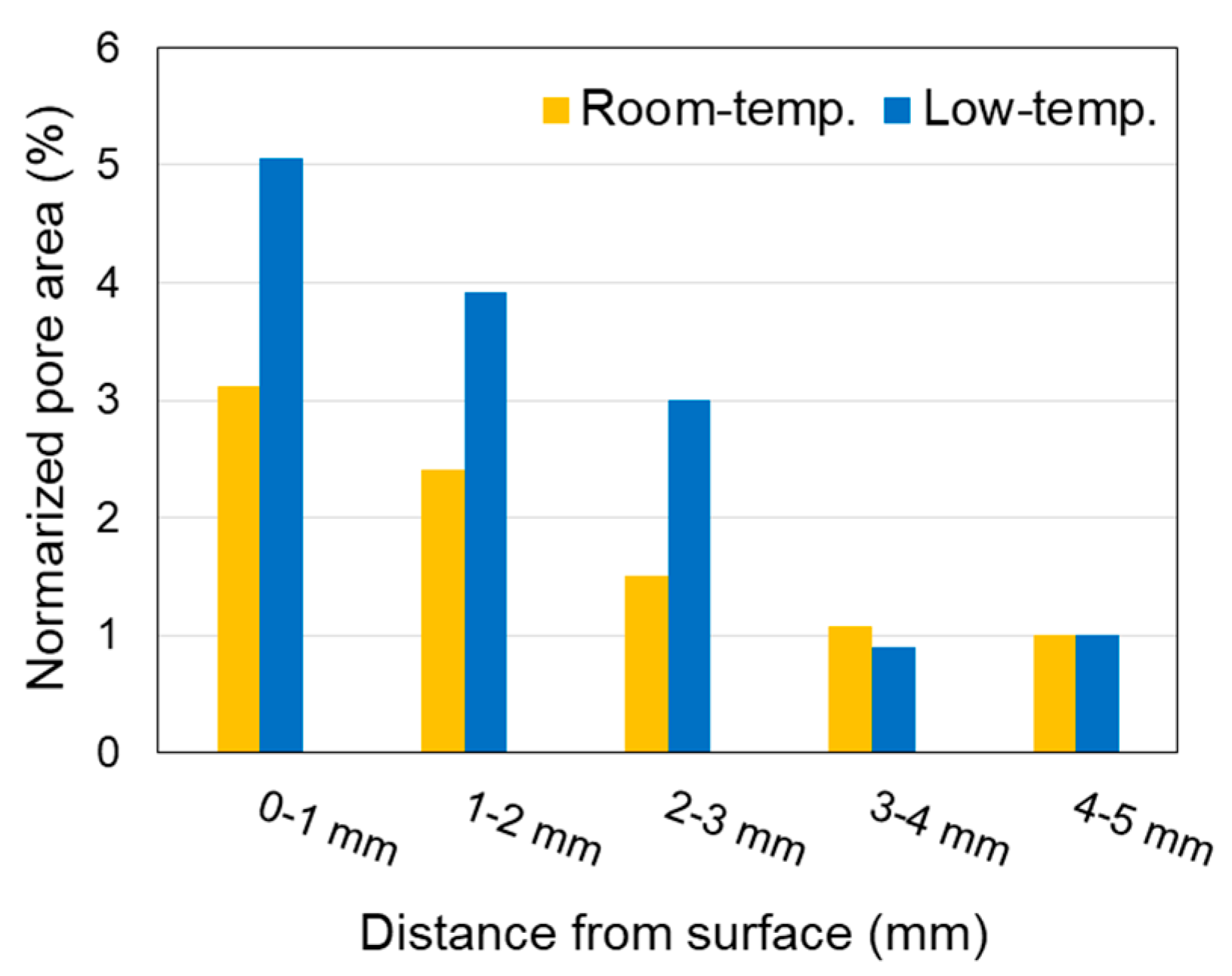
- The porosity of the specimen increased with the dissolution of portlandite;
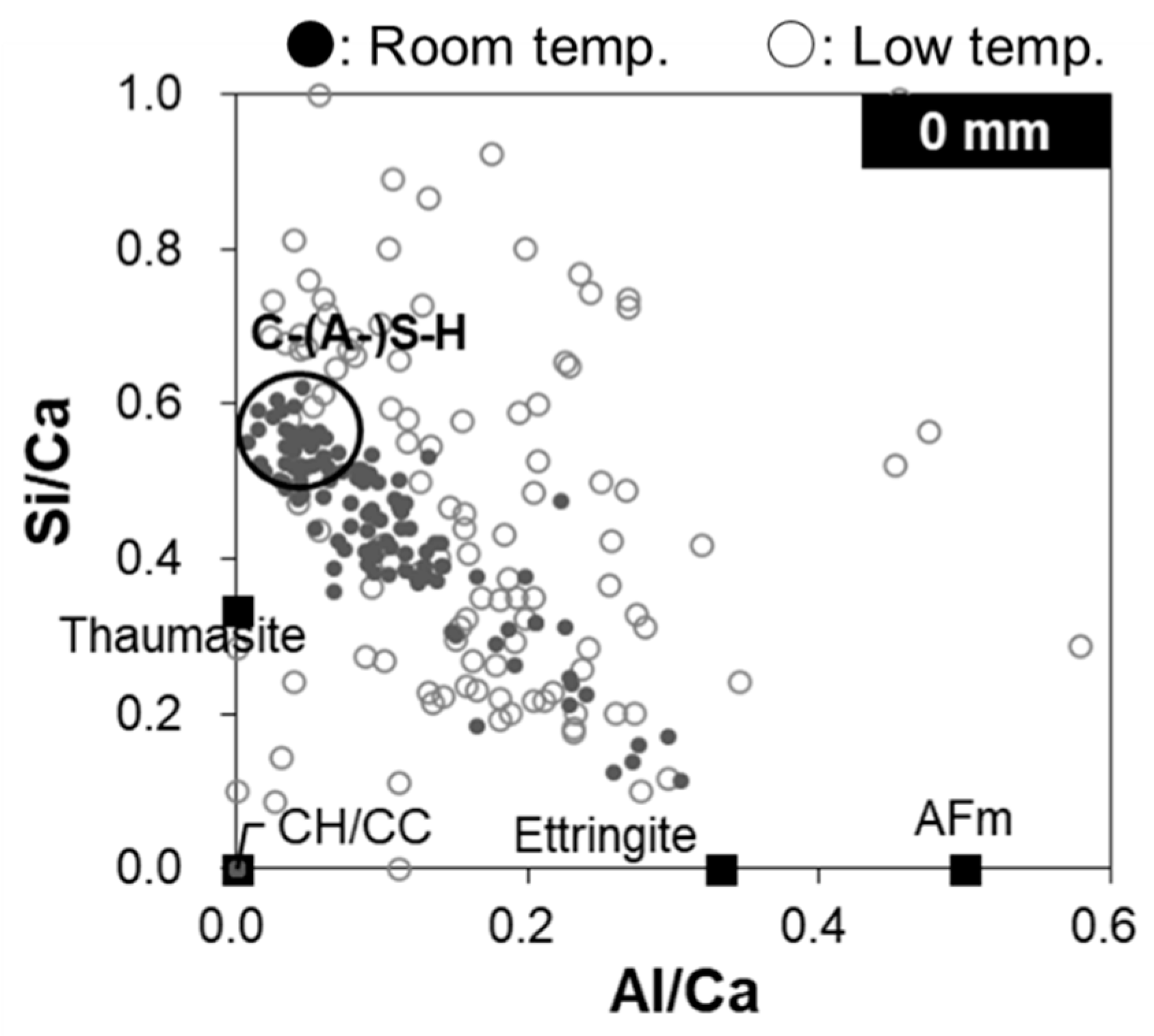
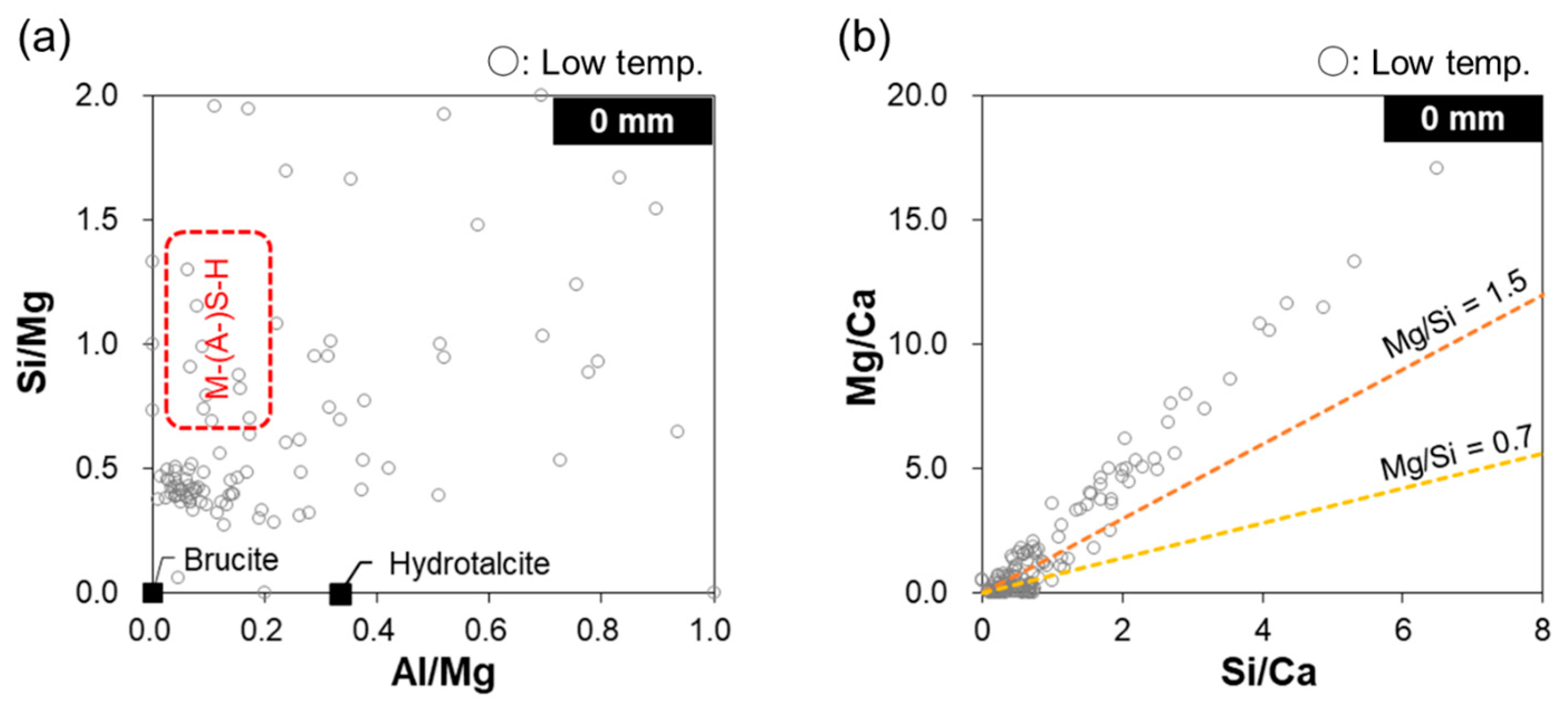
- On the collapsed mashy surface, C–(A-)S–H was decalcified and Mg-based hydrates (e.g., brucite and M–(A-)S–H) and thaumasite formed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
References
- Gjorv, O.E. Long-time durablity of concrete in seawater. J. Proc. 1971, 68, 60–67. [Google Scholar] [CrossRef]
- Buenfeld, N.R.; Newman, J.B. The development and stability of surface layers on concrete exposed to sea-water. Cem. Concr. Res. 1986, 16, 721–732. [Google Scholar] [CrossRef]
- O’Farrell, M.; Wild, S.; Sabir, B.B. Resistance to chemical attack of ground brick–PC mortar: Part II. Synthetic seawater. Cem. Concr. Res. 2000, 30, 757–765. [Google Scholar] [CrossRef]
- Mohammed, T.U.; Hamada, H.; Yamaji, T. Marine durability of 30-year old concrete made with different cements. J. Adv. Concr. Technol. 2003, 1, 63–75. [Google Scholar] [CrossRef]
- Ganjian, E.; Pouya, H.S. Effect of magnesium and sulfate ions on durability of silica fume blended mixes exposed to the seawater tidal zone. Cem. Concr. Res. 2005, 35, 1332–1343. [Google Scholar] [CrossRef]
- Shekarchi, M.; Rafiee, A.; Layssi, H. Long-term chloride diffusion in silica fume concrete in harsh marine climates. Cem. Concr. Compos. 2009, 31, 769–775. [Google Scholar] [CrossRef]
- De Weerdt, K.; Justnes, H.; Geiker, M.R. Changes in the phase assemblage of concrete exposed to sea water. Cem. Concr. Compos. 2014, 47, 53–63. [Google Scholar] [CrossRef]
- Ragab, A.M.; Elgammal, M.A.; Hodhod, O.A.; Ahmed, T.E. Evaluation of field concrete deterioration under real conditions of seawater attack. Constr. Build. Mater. 2016, 119, 130–144. [Google Scholar] [CrossRef]
- Jakobsen, U.H.; De Weerdt, K.; Geiker, M.R. Elemental zonation in marine concrete. Cem. Concr. Res. 2016, 85, 12–27. [Google Scholar] [CrossRef]
- Moffatt, E.G.; Thomas, M.D.A. Performance of 25-year-old silica fume and fly ash lightweight concrete blocks in a harsh marine environment. Cem. Concr. Res. 2018, 113, 65–73. [Google Scholar] [CrossRef]
- De Weerdt, K.; Lothenbach, B.; Geiker, M.R. Comparing chloride ingress from seawater and NaCl solution in Portland cement mortar. Cem. Concr. Res. 2019, 115, 80–89. [Google Scholar] [CrossRef]
- Val, D.V.; Stewart, M.G. Life-cycle cost analysis of reinforced concrete structures in marine environments. Struct. Saf. 2003, 25, 343–362. [Google Scholar] [CrossRef]
- Jin, Z.; Zhao, X.; Zhao, T.; Liu, Y. Corrosion behavior of steel bar and corrosive cracking of concrete induced by magnesium-sulfate-chloride ions. J. Adv. Concr. Technol. 2016, 14, 172–182. [Google Scholar] [CrossRef]
- Glasser, F.P.; Kindness, A.; Stronach, S.A. Stability and solubility relationships in AFm phases: Part I. Chloride, sulfate and hydroxide. Cem. Concr. Res. 1999, 29, 861–866. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Deng, D. Chloride binding of cement-based materials subjected to external chloride environment—A review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Balonis, M.; Lothenbach, B.; Le Saout, G.; Glasser, F.P. Impact of chloride on the mineralogy of hydrated Portland cement systems. Cem. Concr. Res. 2010, 40, 1009–1022. [Google Scholar] [CrossRef]
- Mesbah, A.; Rapin, J.-P.; François, M.; Cau-dit-Coumes, C.; Frizon, F.; Leroux, F.; Renaudin, G. Crystal structures and phase transition of cementitious bi-anionic AFm-(Cl−, CO32−) compounds. J. Am. Ceram. Soc. 2011, 94, 261–268. [Google Scholar] [CrossRef]
- De Weerdt, K.; Orsáková, D.; Geiker, M.R. The impact of sulphate and magnesium on chloride binding in Portland cement paste. Cem. Concr. Res. 2014, 65, 30–40. [Google Scholar] [CrossRef]
- De Weerdt, K.; Colombo, A.; Coppola, L.; Justnes, H.; Geiker, M.R. Impact of the associated cation on chloride binding of Portland cement paste. Cem. Concr. Res. 2015, 68, 196–202. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Le Goff, F.; Pochard, I.; Dauzères, A. Effect of magnesium on calcium silicate hydrate (C-S-H). Cem. Concr. Res. 2017, 97, 61–72. [Google Scholar] [CrossRef]
- Jia, S.; Richardson, I.G. Micro- and nano-structural evolutions in white Portland cement/pulverized fuel ash cement pastes due to deionized-water leaching. Cem. Concr. Res. 2018, 103, 191–203. [Google Scholar] [CrossRef]
- Bonen, D. Composition and appearance of magnesium silicate hydrate and its relation to deterioration of cement-based materials. J. Am. Ceram. Soc. 1992, 75, 2904–2906. [Google Scholar] [CrossRef]
- Kurdowski, W. The protective layer and decalcification of C-S-H in the mechanism of chloride corrosion of cement paste. Cem. Concr. Res. 2004, 34, 1555–1559. [Google Scholar] [CrossRef]
- Roosz, C.; Grangeon, S.; Blanc, P.; Montouillout, V.; Lothenbach, B.; Henocq, P.; Giffaut, E.; Vieillard, P.; Gaboreau, S. Crystal structure of magnesium silicate hydrates (M-S-H): The relation with 2:1 Mg–Si phyllosilicates. Cem. Concr. Res. 2015, 73, 228–237. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Chlique, C.; Wyrzykowski, M.; Dauzères, A.; Pochard, I.; Cau-Dit-Coumes, C. Characterization of magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2019, 116, 309–330. [Google Scholar] [CrossRef]
- De Weerdt, K.; Justnes, H. The effect of sea water on the phase assemblage of hydrated cement paste. Cem. Concr. Compos. 2015, 55, 215–222. [Google Scholar] [CrossRef]
- Hewlett, P.C. Lea’s Chemistry of Cement and Concrete; Arnold: London, UK, 1998. [Google Scholar]
- Kunther, W.; Lothenbach, B.; Scrivener, K.L. On the relevance of volume increase for the length changes of mortar bars in sulfate solutions. Cem. Concr. Res. 2013, 46, 23–29. [Google Scholar] [CrossRef]
- Bizzozero, J.; Gosselin, C.; Scrivener, K.L. Expansion mechanisms in calcium aluminate and sulfoaluminate systems with calcium sulfate. Cem. Concr. Res. 2014, 56, 190–202. [Google Scholar] [CrossRef]
- Sibbick, T.; Fenn, D.; Crammond, N. The occurrence of thaumasite as a product of seawater attack. Cem. Concr. Compos. 2003, 25, 1059–1066. [Google Scholar] [CrossRef]
- Sea Temperature Info. Available online: https://seatemperature.info/new-brunswick-water-temperature.html (accessed on 24 May 2023).
- Kobayashi, M.; Takahashi, K.; Kawabata, Y. Physicochemical properties of the Portland cement-based mortar exposed to deep seafloor conditions at a depth of 1680 m. Cem. Concr. Res. 2021, 142, 106335. [Google Scholar] [CrossRef]
- Takahashi, K.; Kawabata, Y.; Iwanami, M.; Kobayashi, M.; Kasaya, T.; Yamanaka, T.; Nomura, S.; Makita, H. In-situ deep-sea monitoring of cement mortar specimen at a depth of 3515 m and changes in mechanical properties after exposure to deep sea condition. J. Adv. Concr. Technol. 2022, 20, 254–266. [Google Scholar] [CrossRef]
- JIS R 5204; Chemical analysis method of cement by X-ray fluorescence. Japanese Standards Association (JSA): Tokyo, Japan, 2019.
- Liu, Z.; Deng, D.; De Schutter, G.; Yu, Z. The effect of MgSO4 on thaumasite formation. Cem. Concr. Compos. 2013, 35, 102–108. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Yamada, K.; Johannesson, B.; Nilsson, L.-O. Development of a multi-species mass transport model for concrete with account to thermodynamic phase equilibriums. Mater. Struct. 2011, 44, 1577–1592. [Google Scholar] [CrossRef]
- Kobayashi, M.; Takahashi, K.; Kawabata, Y.; Bier, T.A. Physicochemical properties of Portland cement/calcium aluminate cement/calcium sulfate ternary binder exposed to long-term deep-sea conditions. Mater. Struct. 2022, 55, 182. [Google Scholar] [CrossRef]
- van Aardt, J.H.P.; Visser, S. Thaumasite formation: A cause of deterioration of Portland cement and related substances in the presence of sulphates. Cem. Concr. Res. 1975, 5, 225–232. [Google Scholar] [CrossRef]
- Richardson, I.G. The nature of C-S-H in hardened cements. Cem. Concr. Res. 1999, 29, 1131–1147. [Google Scholar] [CrossRef]
- Faucon, P.; Delagrave, A.; Richet, C.; Marchand, J.M.; Zanni, H. Aluminum incorporation in calcium silicate hydrates (C–S–H) depending on their Ca/Si ratio. J. Phys. Chem. B 1999, 103, 7796–7802. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Cau-Dit-Coumes, C.; Pochard, I.; Rentsch, D. Aluminum incorporation into magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2020, 128, 105931. [Google Scholar] [CrossRef]
- San Román, M.S.; Holgado, M.J.; Jaubertie, C.; Rives, V. Synthesis, characterisation and delamination behaviour of lactate-intercalated Mg,Al-hydrotalcite-like compounds. Solid State Sci. 2008, 10, 1333–1341. [Google Scholar] [CrossRef]
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. Characterization of white Portland cement hydration and the C-S-H structure in the presence of sodium aluminate by 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2004, 34, 857–868. [Google Scholar] [CrossRef]
- Richardson, I.G. Model structures for C-(A)-S-H(I). Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 903–923. [Google Scholar] [CrossRef] [PubMed]
- Haga, K.; Shibata, M.; Hironaga, M.; Tanaka, S.; Nagasaki, S. Silicate anion structural change in calcium silicate hydrate gel on dissolution of hydrated cement. J. Nucl. Sci. Technol. 2002, 39, 540–547. [Google Scholar] [CrossRef]
- Brew, D.R.M.; Glasser, F.P. Synthesis and characterisation of magnesium silicate hydrate gels. Cem. Concr. Res. 2005, 35, 85–98. [Google Scholar] [CrossRef]
- Nied, D.; Enemark-Rasmussen, K.; L’Hopital, E.; Skibsted, J.; Lothenbach, B. Properties of magnesium silicate hydrates (M-S-H). Cem. Concr. Res. 2016, 79, 323–332. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Rentsch, D.; Pochard, I.; Dauzères, A. Formation of magnesium silicate hydrates (M-S-H). Phys. Chem. Earth Parts A/B/C 2017, 99, 142–157. [Google Scholar] [CrossRef]
- Skibsted, J.; Hjorth, L.; Jakobsen, H.J. Quantification of thaumasite in cementitious materials by 29Si {1H} cross-polarization magic-angle spinning NMR spectroscopy. Adv. Cem. Res. 1995, 7, 69–83. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bassuoni, M.T. Thaumasite sulfate attack on concrete: Mechanisms, influential factors and mitigation. Constr. Build. Mater. 2014, 73, 652–662. [Google Scholar] [CrossRef]
- Boynton, R.S. Chemistry and Technology of Lime and Limestone, 2nd ed.; Wiley-Interscience: New York, NY, USA, 1980. [Google Scholar]
- ChemBK. Calcium Hydroxide. Available online: https://www.chembk.com/en/chem/Calcium%20hydroxide (accessed on 27 June 2023).
- Santhanam, M.; Cohen, M.; Olek, J. Differentiating seawater and groundwater sulfate attack in Portland cement mortars. Cem. Concr. Res. 2006, 36, 2132–2137. [Google Scholar] [CrossRef]
- Flatt, R.J.; Scherer, G.W. Thermodynamics of crystallization stresses in DEF. Cem. Concr. Res. 2008, 38, 325–336. [Google Scholar] [CrossRef]
- Lothenbach, B.; Matschei, T.; Möschner, G.; Glasser, F. Thermodynamic modelling of the effect of temperature on the hydration and porosity of Portland cement. Cem. Concr. Res. 2008, 38, 1–18. [Google Scholar] [CrossRef]
- Maruyama, I.; Ohkubo, T.; Haji, T.; Kurihara, R. Dynamic microstructural evolution of hardened cement paste during first drying monitored by 1H NMR relaxometry. Cem. Concr. Res. 2019, 122, 107–117. [Google Scholar] [CrossRef]



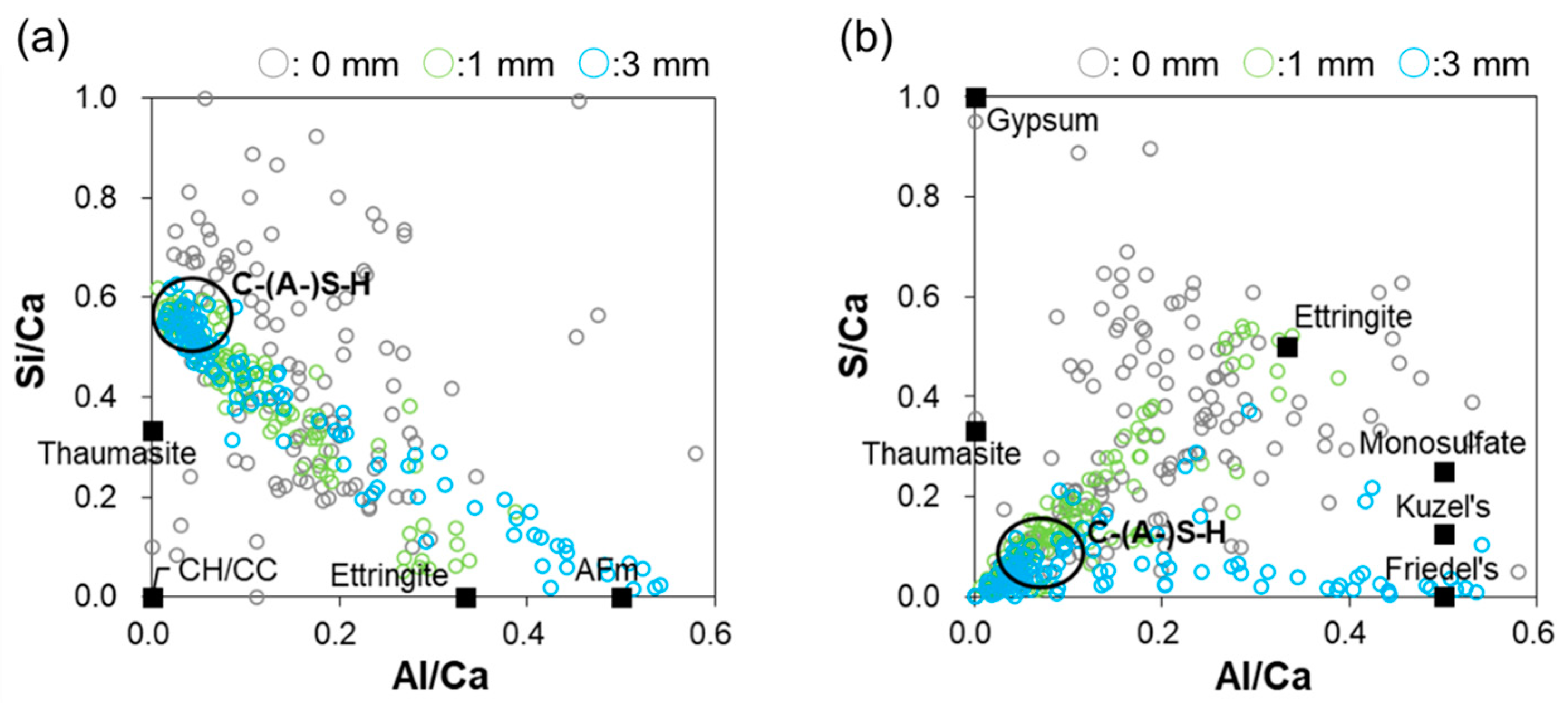
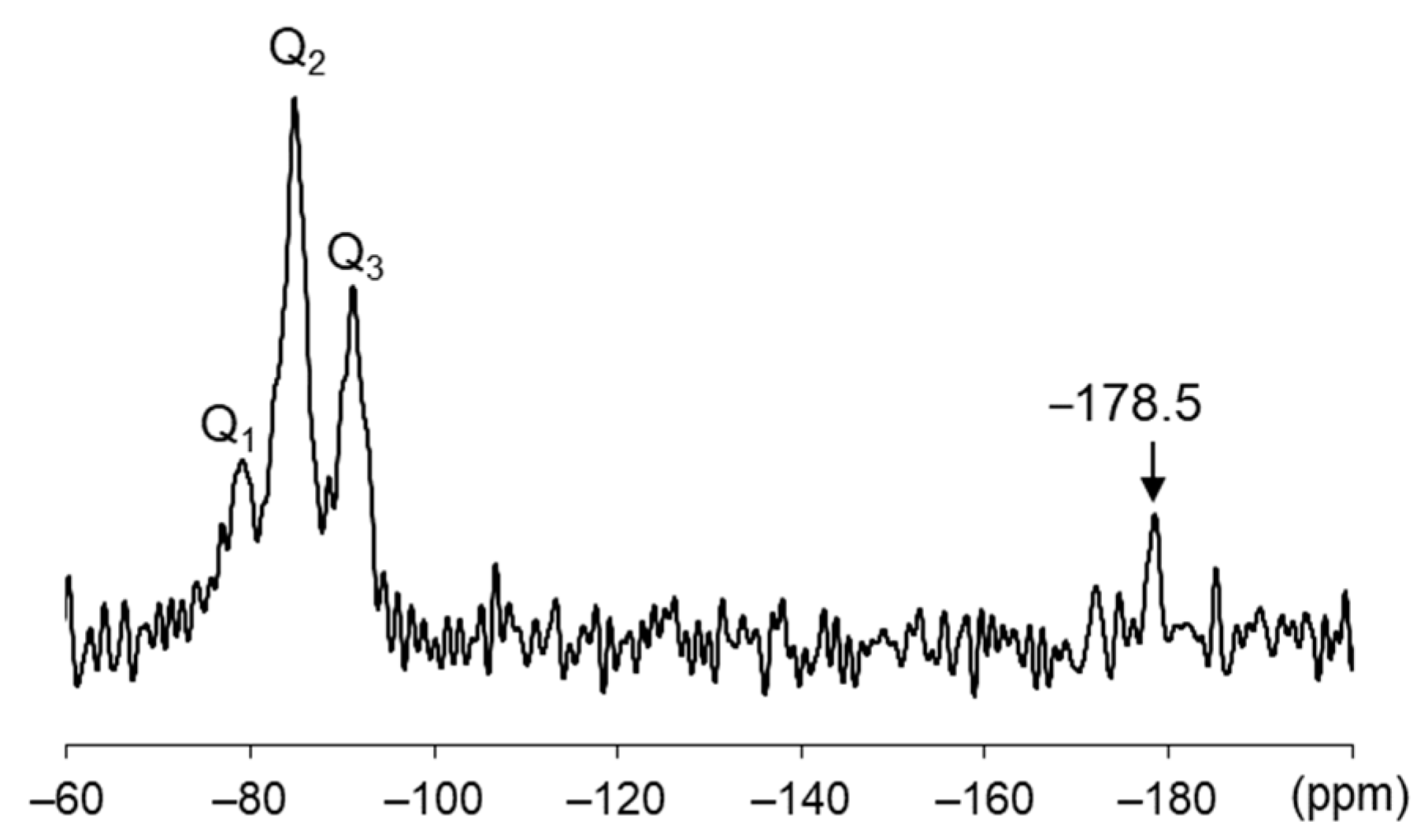
| Site | Zone | Seawater Temp (°C) | Time Period | Specimen |
|---|---|---|---|---|
| Kish Island, Iran [5] | Tidal zone | 22–33 a | up to 180 days | Paste and concrete, w/b c 0.2–0.4, mixed with SF and BFS |
| North coast of the Mediterranean Sea [8] | Splash zone | 15–25 a | 4 to >60 years | Concrete, w/c c 0.34–0.65 |
| Kurihama, Japan [4] | In seawater | 15–28 a | 30 years | Concrete, w/c 0.52–0.55, with five different cements |
| Trondheim, Norway [1] | In seawater | 5–15 a | up to 30 years | Concrete, 2500 different mixes |
| Trondheim, Norway [7] | Tidal zone | 5–15 a | 10 years | Concrete, w/b 0.4 |
| Nine different locations in Norway and Denmark [9] | Mainly in seawater | minimum 5–15 a | 2–34 years | Concrete with 21 different mixes |
| Treat Island, USA [10] | In seawater | minimum −3 a | 24–25 years | Concrete, w/b 0.26–0.6, FA and SF containing lightweight blocks |
| Bandar-Abbas [6] | Tidal zone | 3 years | Concrete, w/c 0.35, 0.40, 0.45, and 0.50, SF mixed with 5%, 7.5, 10, and 12.5% | |
| Lab [3] | In seawater b | 20 | 560 days | Mortar, w/b 0.5, mixed with ground brick |
| Lab [2] | In seawater b | 20 | 33 weeks | Concrete, w/c 0.4 |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | LOI a |
|---|---|---|---|---|---|---|---|---|
| 20.29 | 5.31 | 2.72 | 65.20 | 1.20 | 2.90 | 0.23 | 0.29 | 1.03 |
| Component | Amount (g) |
|---|---|
| Portland cement (PC) | 1200 |
| Polycarboxylic ether (PCE) | 9.6 |
| Hydroxypropyl methylcellulose (HPMC) | 5.8 |
| Defoamer | 2.4 |
| Tap water | 720 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, M.; Takahashi, K.; Kawabata, Y. Deterioration of Cement-Based Materials in Low-Temperature Seawater. Materials 2023, 16, 5278. https://doi.org/10.3390/ma16155278
Kobayashi M, Takahashi K, Kawabata Y. Deterioration of Cement-Based Materials in Low-Temperature Seawater. Materials. 2023; 16(15):5278. https://doi.org/10.3390/ma16155278
Chicago/Turabian StyleKobayashi, Mari, Keisuke Takahashi, and Yuichiro Kawabata. 2023. "Deterioration of Cement-Based Materials in Low-Temperature Seawater" Materials 16, no. 15: 5278. https://doi.org/10.3390/ma16155278
APA StyleKobayashi, M., Takahashi, K., & Kawabata, Y. (2023). Deterioration of Cement-Based Materials in Low-Temperature Seawater. Materials, 16(15), 5278. https://doi.org/10.3390/ma16155278






