Iron Selenide Particles for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. FeSe Preparation
2.2. Characterization
3. Results and Discussion
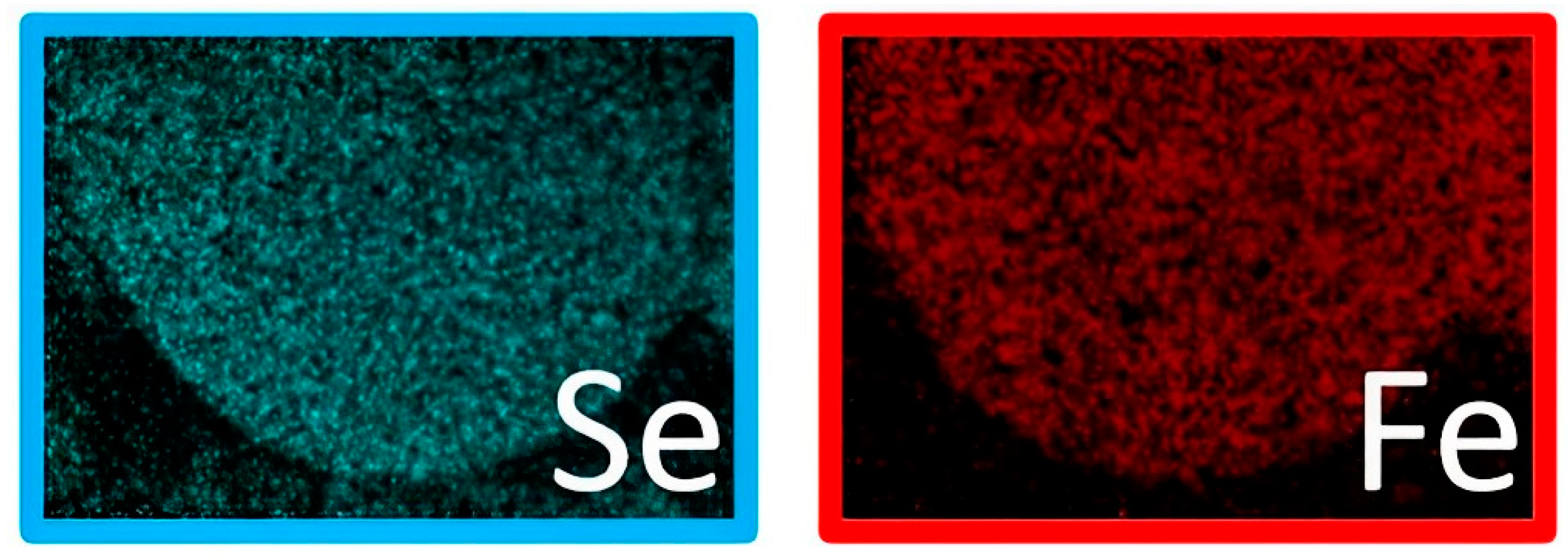
3.1. Morphological Characterization
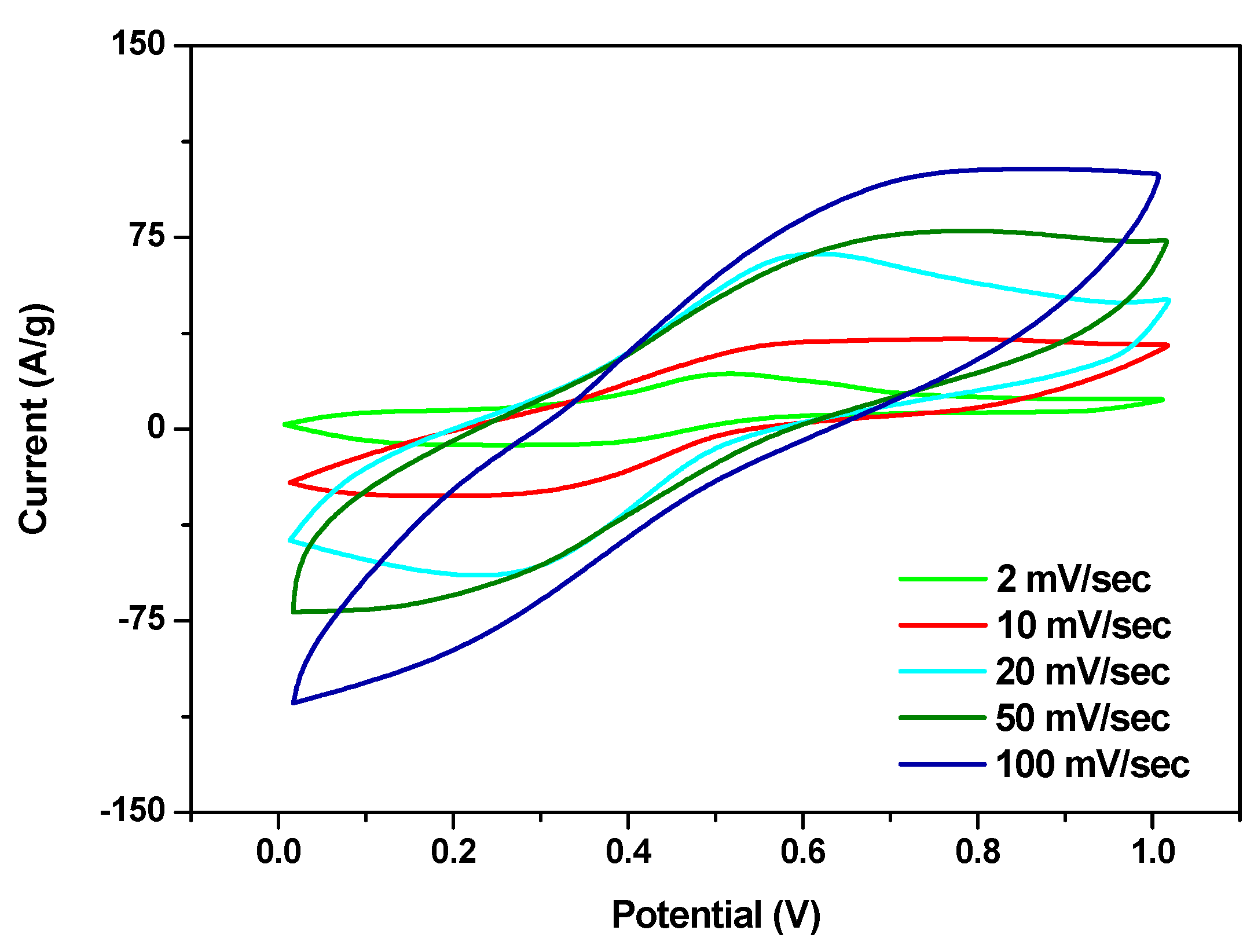
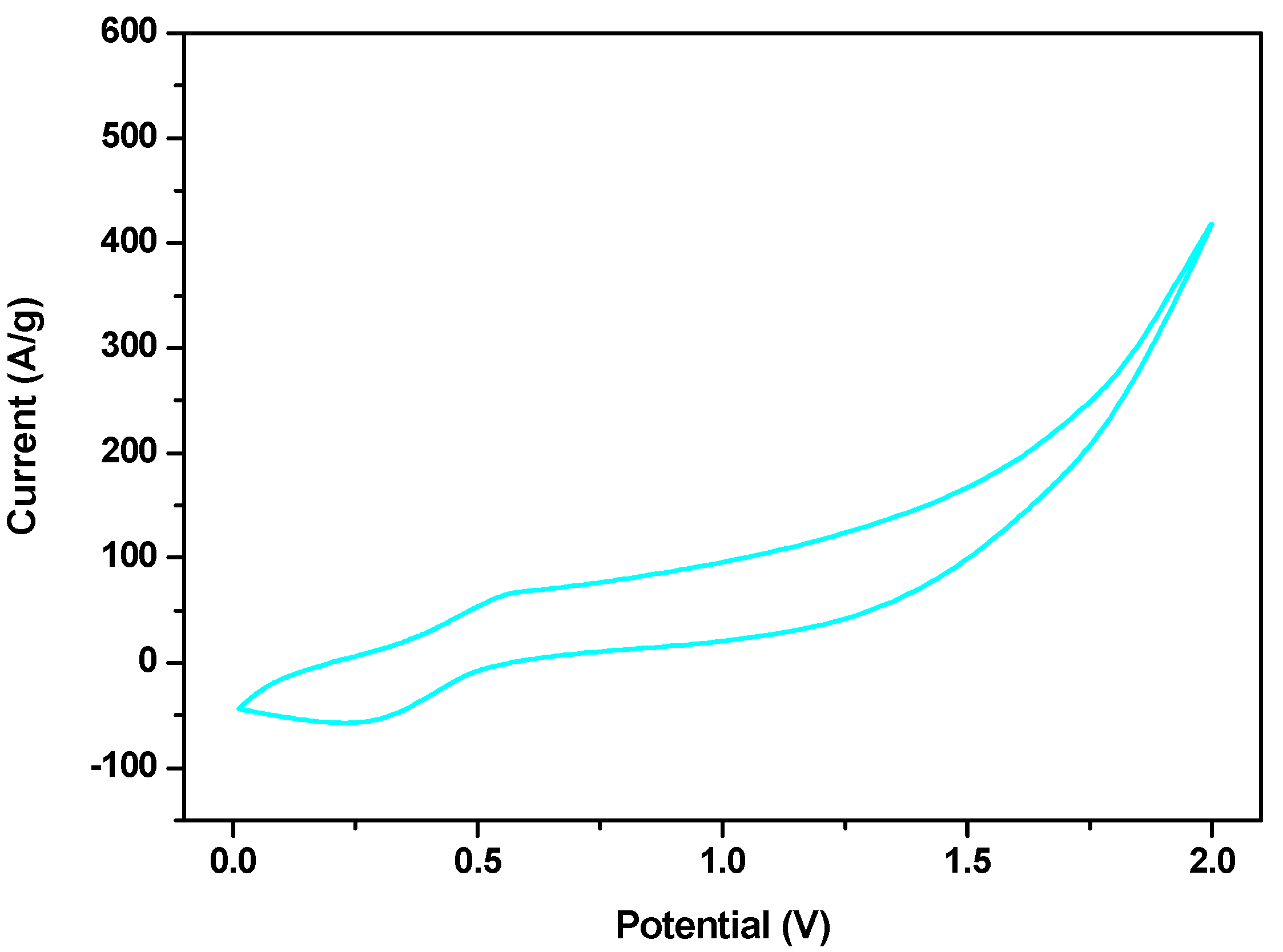
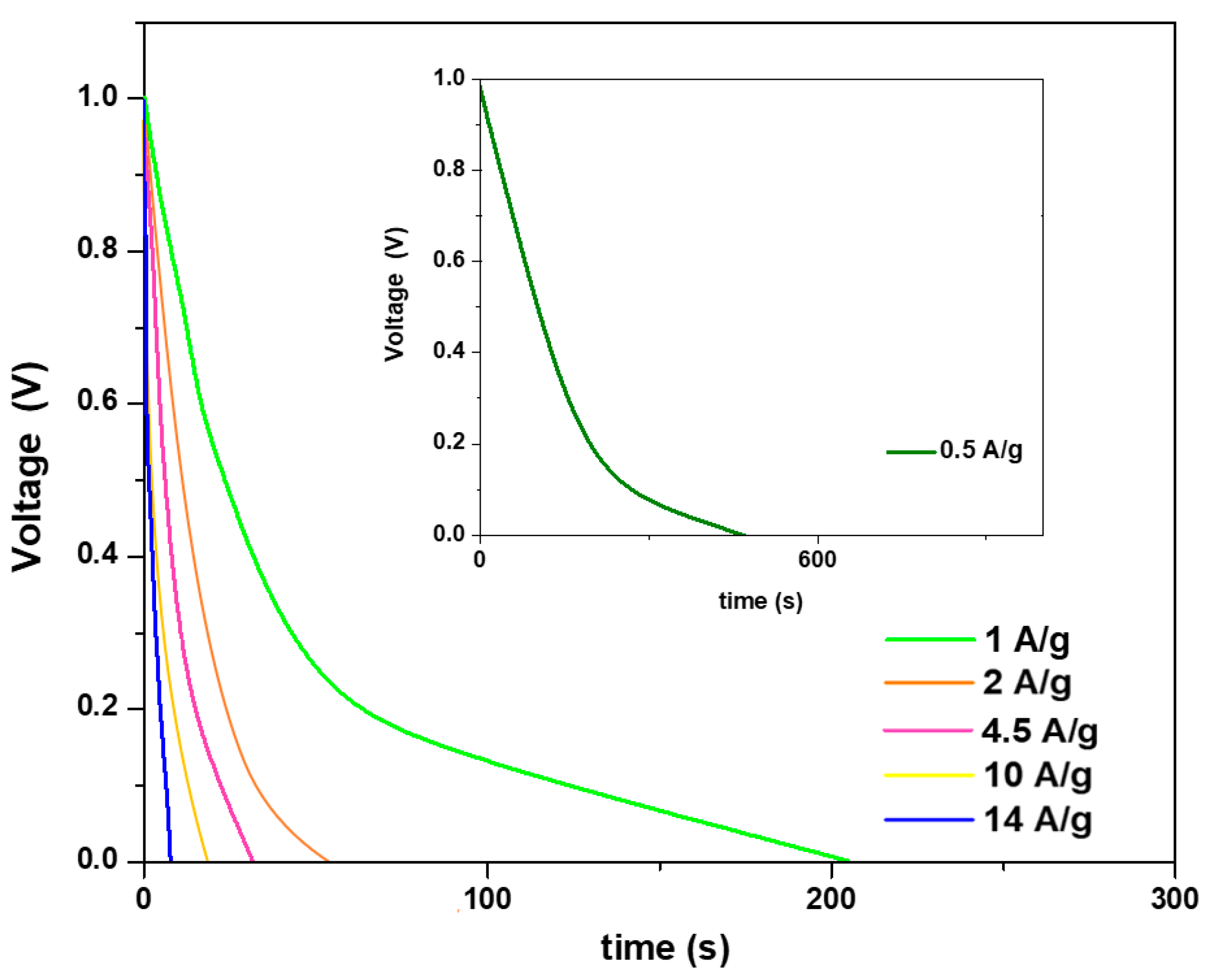
3.2. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernão Pires, V.; Romero-Cadaval, E.; Vinnikov, D.; Roasto, I.; Martins, J.F. Power converter interfaces for electrochemical energy storage systems—A review. Energy Convers. Manag. 2014, 86, 453–475. [Google Scholar] [CrossRef]
- Bhojane, P. Recent advances and fundamentals of Pseudocapacitors: Materials, mechanism, and its understanding. J. Energy Storage 2022, 45, 103654. [Google Scholar] [CrossRef]
- Xu, B.; Wu, F.; Chen, S.; Zhang, C.; Cao, G.; Yang, Y. Activated carbon fiber cloths as electrodes for high performance electric double layer capacitors. Electrochim. Acta 2007, 52, 4595–4598. [Google Scholar] [CrossRef]
- Evanko, B.; Boettcher, S.W.; Yoo, S.J.; Stucky, G.D. Redox-Enhanced Electrochemical Capacitors: Status, Opportunity, and Best Practices for Performance Evaluation. ACS Energy Lett. 2017, 2, 2581–2590. [Google Scholar] [CrossRef]
- Chun, S.E.; Evanko, B.; Wang, X.; Vonlanthen, D.; Ji, X.; Stucky, G.D.; Boettcher, S.W. Design of aqueous redox-enhanced electrochemical capacitors with high specific energies and slow self-discharge. Nat. Commun. 2015, 7, 7818. [Google Scholar] [CrossRef]
- Sarno, M.; Ponticorvo, E.; Scarpa, D. Ru and Os based new electrode for electrochemical flow supercapacitors. Chem. Eng. J. 2019, 3771, 120050. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185. [Google Scholar] [CrossRef]
- Petrucci, E.; Porcelli, F.; Orsini, M.; De Santis, S.; Sotgiu, G. Mixed Oxide Electrodes Based on Ruthenium and Copper: Electrochemical Properties as a Function of the Composition and Method of Manufacture. Metals 2022, 12, 316. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal oxide-based supercapacitors: Progress and prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef]
- Lu, T.; Dong, S.; Zghang, C.; Cui, G. Fabrication of transition metal selenides and their applications in energy storage. Coord. Chem. Rev. 2017, 332, 75–99. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Li, Y.; Zheng, M.; Pang, H. Applications of MxSey (M = Fe, Co, Ni) and Their Composites in Electrochemical Energy Storage and Conversion. Nano-Micro Lett. 2019, 11, 40. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Balamurugan, J.; Aravindan, V.; Kim, N.H.; Lee, J.H. Boosting the Energy Density of Flexible Solid-State Supercapacitors via Both Ternary NiV2Se4 and NiFe2Se4 Nanosheet Arrays. Chem. Mater. 2019, 31, 4490–4504. [Google Scholar] [CrossRef]
- Song, X.; Huang, C.; Qin, Y.; Li, H.; Chen, H.C. Hierarchical hollow, sea-urchin-like and porous Ni0.5Co0.5Se2 as advanced battery material for hybrid supercapacitors. J. Mater. Chem. 2018, 6, 16205–16212. [Google Scholar] [CrossRef]
- Hou, L.; Shi, Y.; Wu, C.; Zhang, Y.; Ma, Y.; Sun, X.; Sun, J.; Zhang, X.; Yuan, C. Monodisperse Metallic NiCoSe2 Hollow Sub-Microspheres: Formation Process, Intrinsic Charge-Storage Mechanism, and Appealing Pseudocapacitance as Highly Conductive Electrode for Electrochemical Supercapacitors. Adv. Funct. Mater. 2018, 28, 1705921. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, Y.; Zhang, J.; Wang, Y.; Zhang, X.; Zhang, R.; Huang, L.L.; Zhang, D. Synthesis of bimetallic nickel cobalt selenide particles for high-performance hybrid supercapacitors. RSC Adv. 2022, 12, 1471. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.W.; Ge, Y.C.; Wang, M.; Zhang, Z.Q.; Dong, P.; Baines, R.; Ye, M.X.; Shen, J.F. Cobalt-Doped FeSe2-RGO as Highly Active and Stable Electrocatalysts for Hydrogen Evolution Reactions. ACS Appl. Mater. Interfaces 2016, 8, 18036–18042. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, L.; Cheng, Z.; Qiu, W. Induced Superconducting Transition in Ultra-Thin Iron-Selenide Films by a Mg-Coating. Process. Mater. 2021, 14, 6383. [Google Scholar] [CrossRef] [PubMed]
- Sarno, M.; Iuliano, M. Biodiesel production from waste cooking oil. Green Process. Synth. 2019, 8, 828–836. [Google Scholar] [CrossRef]
- Sarno, M.; Baldino, L.; Scudieri, C.; Cardea, S.; Ciambelli, P.; Reverchon, E. SC-CO2-assisted process for a high energy density aerogel supercapacitor: The effect of GO loading. Nanotechnology 2017, 28, 204001. [Google Scholar] [CrossRef] [PubMed]
- Sarno, M.; Ponticorvo, E.; Scarpa, D. Controlled PtIr nanoalloy as an electro-oxidation platform for methanol reaction and ammonia detection. Nanotechnology 2019, 30, 394004. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Shi, X.; Yu, J.; Huang, J.; Chen, B.; Fang, L.; Shao, L.; Sun, Z. Metal-organic framework derived high-content N, P and O-codoped Co/C composites as electrode materials for high performance supercapacitors. J. Power Sources 2020, 467, 228304. [Google Scholar] [CrossRef]
- Sarno, M.; Ponticorvo, E. Effect of the amount of nickel sulphide, molybdenum disulphide and carbon nanosupport on a Tafel slope and overpotential optimization. Nanotechnology 2017, 28, 214003. [Google Scholar] [CrossRef]
- Sarno, M.; Ponticorvo, E.; Piotto, S.; Nardiello, A.M.; De Pasquale, S.; Funicello, N. AuAg/ZnO nanocatalyst for CO2 valorization and H2 and CO electrochemical production. J. CO2 Util. 2020, 39, 101179. [Google Scholar] [CrossRef]
- Gnatchenko, S.L.; Chareev, D.A.; Volkova, O.S.; Vasiliev, A.N. Magnetic properties of superconducting FeSe in the normal state. J. Phys. Condens. Matter 2013, 25, 046004. [Google Scholar]
- Pandit, B.; Rondiya, S.R.; Shegokar, S.; Bommineedi, L.K.; Cross, R.W.; Dzade, N.Y.; Sankapal, B.R. Combined electrochemical and DFT investigations of iron selenide: A mechanically bendable solidstate symmetric supercapacitor. Sustain. Energy Fuels 2021, 5, 5001–5012. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, H.; Han, M.; Dai, Z.; Pang, H.; Zheng, Y.; Lan, Y.Q.; Bao, J.; Zhu, J. Two-Dimensional Tin Selenide Nanostructures for Flexible All-Solid-State Supercapacitors. ACS Nano 2014, 8, 3761–3770. [Google Scholar] [CrossRef]
- Kirubasankar, B.; Murugadoss, V.; Lin, J.; Ding, T.; Dong, M.; Liu, H.; Zhang, J.; Li, T.; Wang, N.; Guo, Z.; et al. In situ grown nickel selenide on graphene nanohybrid electrodes for high energy density asymmetric supercapacitors. Nanoscale 2018, 10, 20414–20425. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, X.; Bai, M.; Wang, H.; Xue, D.; Wang, W.; Cheng, J. Flexible full-solid-state supercapacitors based on self-assembly of mesoporous MoSe2 nanomaterials. Inorg. Chem. Front. 2017, 4, 675–682. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Wang, Q.; Song, W.; Hou, X.; Chen, D.; Cheng, Y.B.; Shen, G. Three-Dimensional Hierarchical GeSe2 Nanostructures for High Performance Flexible All-Solid-State Supercapacitors. Adv. Mater. 2013, 25, 1479–1486. [Google Scholar] [CrossRef]
- Li, L.; Gong, J.; Liu, C.; Tian, Y.; Han, M.; Wang, Q.; Hong, X.; Ding, Q.; Zhu, W.; Bao, J. Vertically Oriented and Interpenetrating CuSe Nanosheet Films with Open Channels for Flexible All-Solid-State Supercapacitors. ACS Omega 2017, 3, 1089–1096. [Google Scholar] [CrossRef]
- Pawar, S.A.; Patil, D.S.; Shin, J.C. Cadmium selenide microspheres as an electrochemical supercapacitor. Mater. Today Chem. 2017, 4, 164–171. [Google Scholar] [CrossRef]
- Sahoo, S.; Pazhamalai, P.; Krishnamoorthy, K.; Kim, S.G. Hydrothermally prepared α-MnSe nanoparticles as a new pseudocapacitive electrode material for supercapacitor. Electrochim. Acta 2018, 268, 403–410. [Google Scholar] [CrossRef]
- Pandit, B.; Sankapal, R.B. Cerium Selenide Nanopebble/Multiwalled Carbon Nanotube Composite Electrodes for Solid-State Symmetric Supercapacitors. ACS Appl. Nano Mater 2022, 5, 3007–3017. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, Y.; Shu, L.; Li, A.; Zhang, J.; Wang, R. Structure related RuSe2 nanoparticles and their application in supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129702. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Lee, D.K.; Kim, C.W.; Cha, H.G.; Kim, Y.H.; Kang, Y.S. Synthesis of Magnetic Nanoparticles of Fe3O4 and CoFe2O4 and Their Surface Modification by Surfactant Adsorption. Bull. Korean Chem. Soc. 2006, 27, 237–242. [Google Scholar]


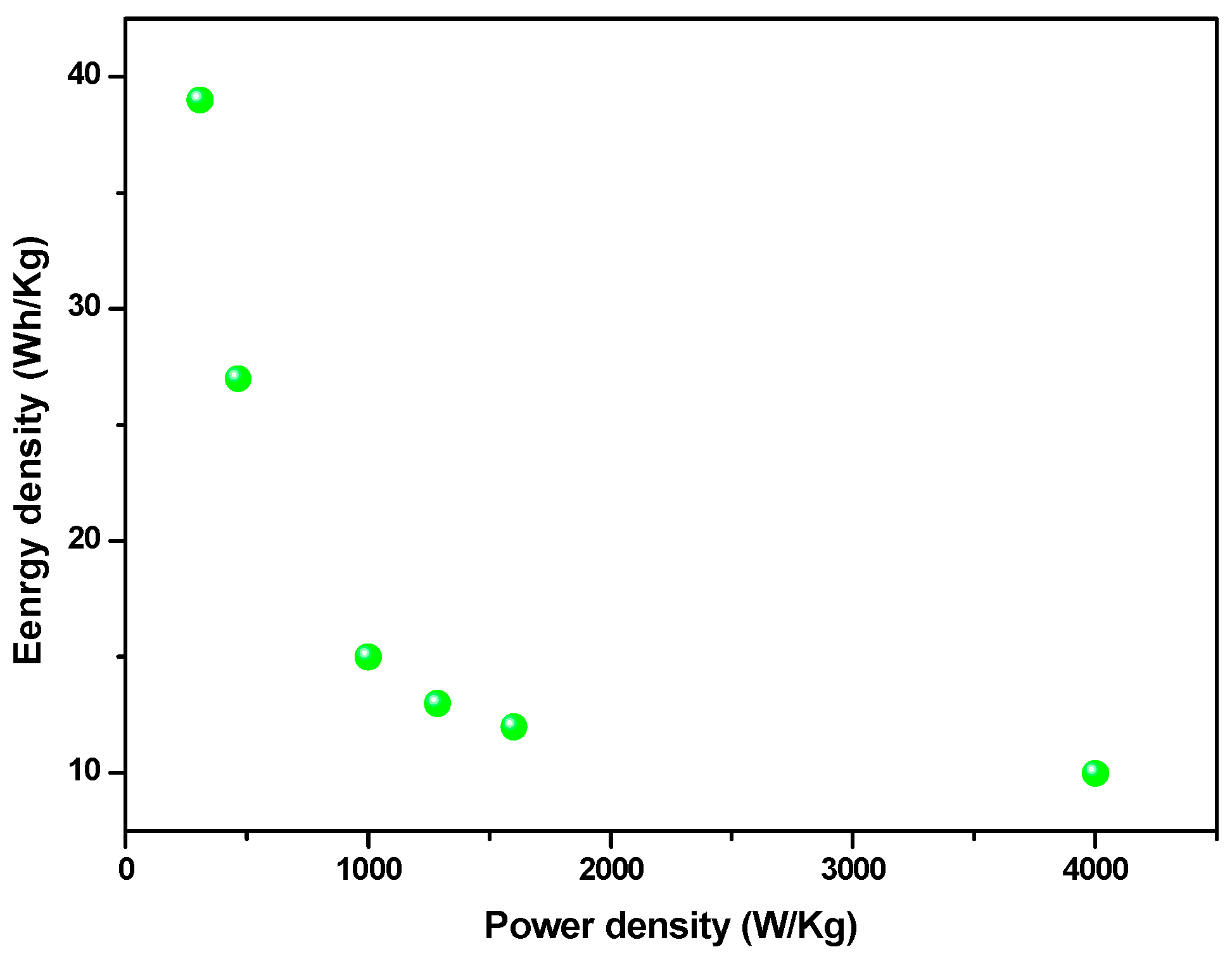
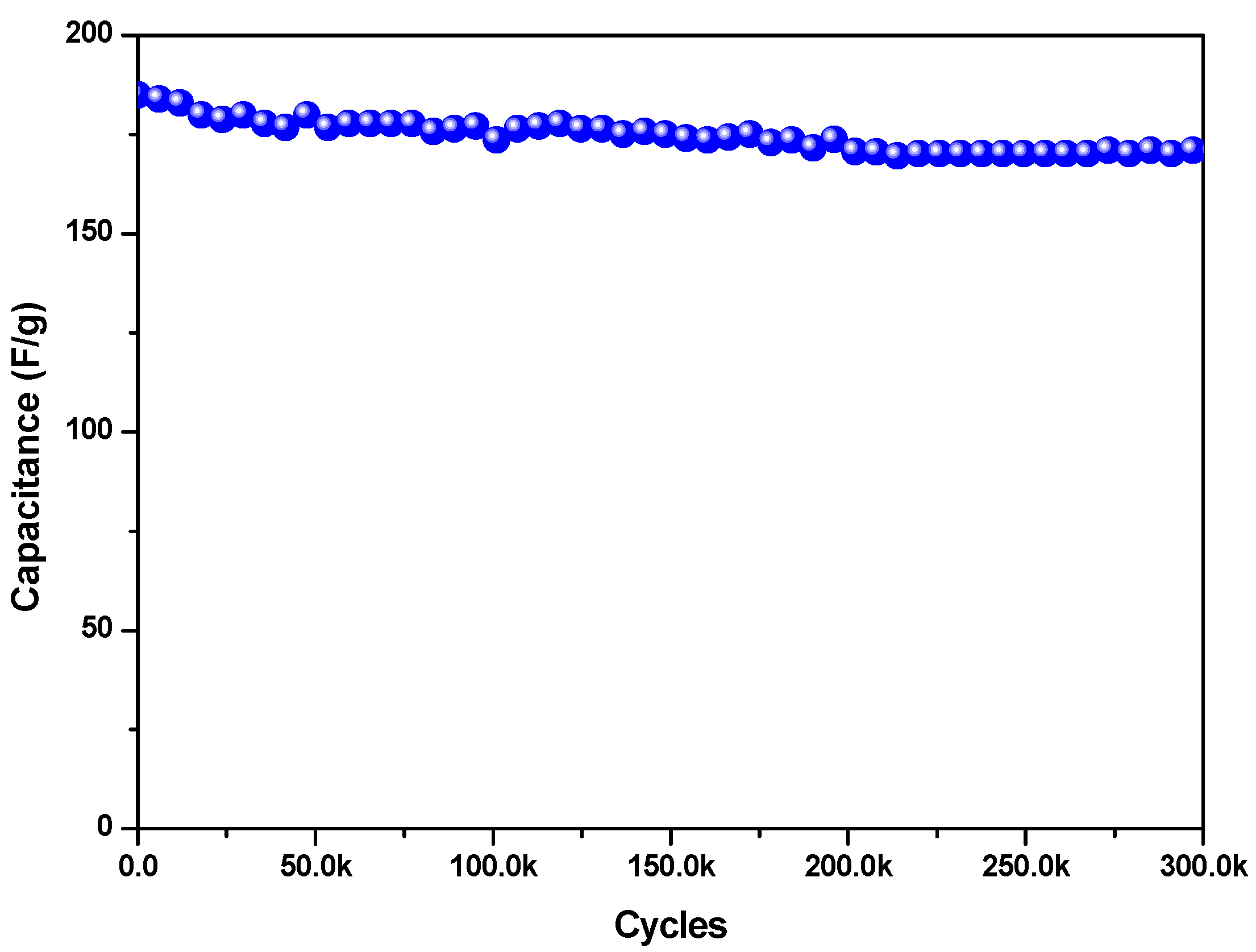
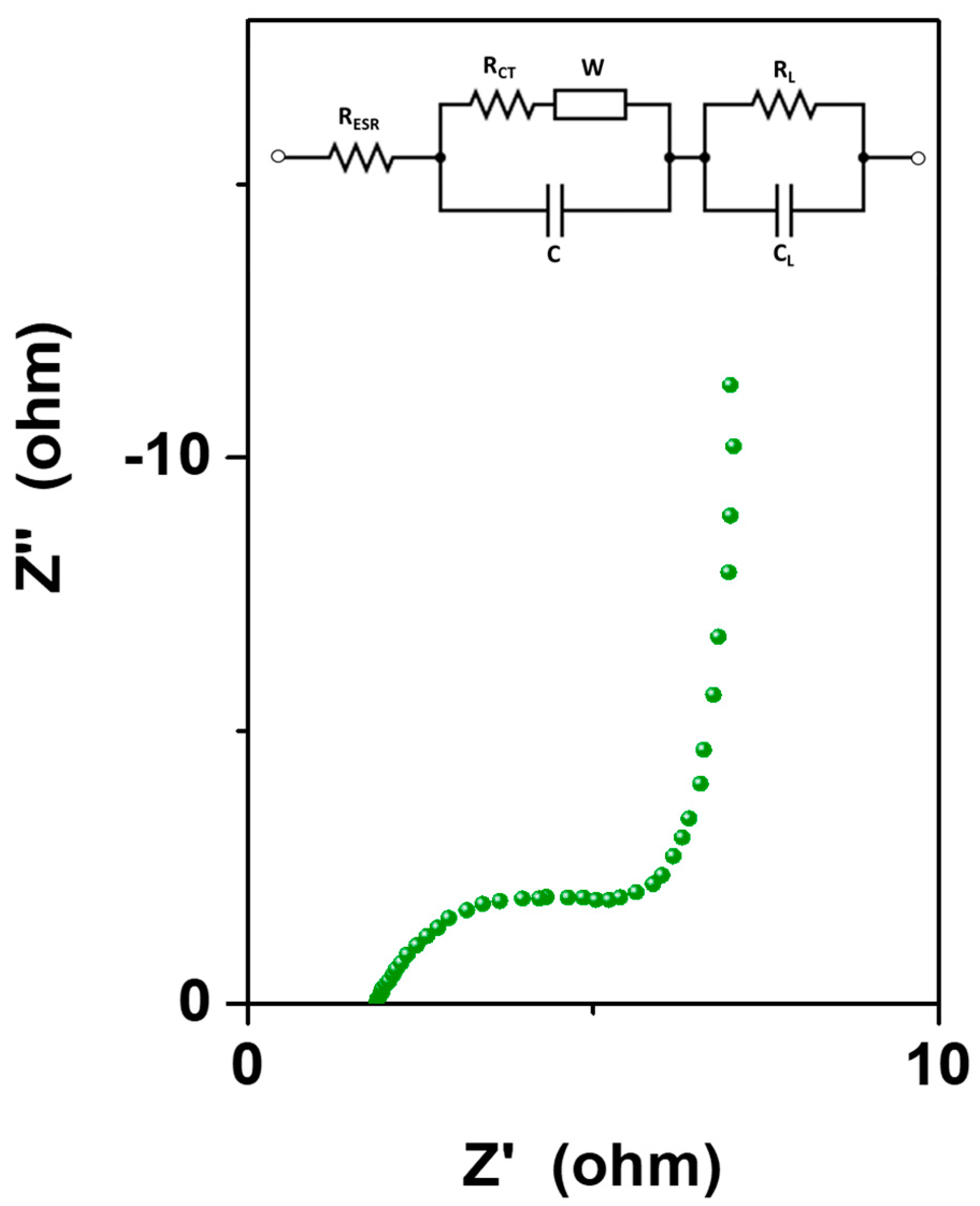
| Se-Based Material (with Average Size) | CV Potential Window (V) | GCD Current Density (A/g) | Specific Capacitance (F/gsample) | Energy Density (Wh/kg) | Power Density (W/kg) | Capacitance Retention | Ref. |
|---|---|---|---|---|---|---|---|
| SnSe2 nanodisks (8 nm) | 0 ÷ 0.45 vs. SCE | 0.5 | 168 | - | - | retains about 99.0% after 1000 cycles at 1 A/g | [27] |
| SnSe2 nanosheets (2 nm) | 0 ÷ 0.6 vs. SCE | 0.5 | 228 | - | - | retains about 99.2% after 1000 cycles at 1 A/g | [27] |
| NiSe nanoparticles (23 nm) | −0.2 ÷ 0.4 vs. SCE | 1 | 705 | - | - | retains about 92% after 2500 cycles at 5 A/g | [28] |
| MoSe2 nanoflakes /Mo Se2 nanorods (500 nm) | 0 ÷ 0.6 vs. Hg/HgO | 2 | 133 | 36.2 (MoSe2 NRs ∥ MoSe2 NFs device) | 1400 (MoSe2 NRs ∥ MoSe2 NFs device) | retains about 92% after 2000 cycles at 2 A/g | [29] |
| GeS2 nanobelts (125 nm) | 0.1 ÷ 0.65 vs. Hg/HgO | 1 | 300 | - | - | retains about 99.3% after 2000 cycles at 1 A/g | [30] |
| CuSe nanosheets (15 nm) | −0.4 ÷ 0.2 vs. SCE | 0.2 | 209 | - | - | retains about 90% after 10,000 cycles at 1 A/g | [31] |
| CdSe microspheres (300 nm) | 0 ÷ 0.8 vs. SCE | 0.2 mA | 1.285 mFcm−2 | 4.015 | - | retains about 83.7% after 2000 cycles | [32] |
| MnSe nanoparticles (25 nm) | 0 ÷ 0.8 vs. Ag/AgCl | 0.1 mA/cm−2 | 96.76 | 8.60 | 47.05 | Specific capacitance increases slightly (103.40%) after 2000 cycles | [33] |
| CeSe2 nanopebbles/MWCNTs (23.7 nm) | −0.9 ÷ 0 vs. Ag/AgCl | 2 mA/cm−2 | 451.4 | 36.3 (CeSe2/MWCNTs ∥ CeSe2/MWCNTs device) | 2800 (CeSe2/MWCNTs ∥ CeSe2/MWCNTs device) | retains about 70.7% after 4000 cycles at 100 mV/s | [34] |
| RuSe2@550 nanoparticles (7 nm) | 0 ÷ 0.7 vs. SCE | 0.5 | 786.4 | 44.8 (RuSe2@500 ∥ carbon powder device) | 400 (RuSe2@500 ∥ carbon powder device) | retains about 78.2% after 10,000 cycles | [35] |
| very small FeSe particles (235 nm) | 0 ÷ 1 vs. SCE | 0.5 | 280 | 39 | 306 | retains about 92% after 30,000 cycles at 1 A/g | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpa, D.; Cirillo, C.; Ponticorvo, E.; Cirillo, C.; Attanasio, C.; Iuliano, M.; Sarno, M. Iron Selenide Particles for High-Performance Supercapacitors. Materials 2023, 16, 5309. https://doi.org/10.3390/ma16155309
Scarpa D, Cirillo C, Ponticorvo E, Cirillo C, Attanasio C, Iuliano M, Sarno M. Iron Selenide Particles for High-Performance Supercapacitors. Materials. 2023; 16(15):5309. https://doi.org/10.3390/ma16155309
Chicago/Turabian StyleScarpa, Davide, Claudia Cirillo, Eleonora Ponticorvo, Carla Cirillo, Carmine Attanasio, Mariagrazia Iuliano, and Maria Sarno. 2023. "Iron Selenide Particles for High-Performance Supercapacitors" Materials 16, no. 15: 5309. https://doi.org/10.3390/ma16155309






