Surface Morphology and Optical Properties of Hafnium Oxide Thin Films Produced by Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, X.; Li, Y.; Yang, H.; Liang, Y.; He, K.; Sun, W.; Lin, H.-H.; Yao, S.; Lu, X.; Wan, L.; et al. Investigation of HfO2 thin films on Si by X-ray Photoelectron Spectroscopy, Rutherford Backscattering, Grazing Incidence X-ray Diffraction and Variable Angle Spectroscopic Ellipsometry. Crystals 2018, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Kukli, K.; Aarik, J.; Ritala, M.; Uustare, T.; Sajavaara, T.; Lu, J.; Sundqvist, J.; Aidla, A.; Pung, L.; Hårsta, A.; et al. Effect of selected Atomic Layer Deposition parameters on the structure and dielectric properties of hafnium oxide films. J. Appl. Phys. 2004, 96, 5298–5307. [Google Scholar] [CrossRef]
- Khoshman, J.M.; Kordesch, M.E. Optical properties of a-HfO2 thin films. Surf. Coat. Technol. 2006, 201, 3530–3535. [Google Scholar] [CrossRef]
- He, G.; Liu, M.; Zhu, L.Q.; Chang, M.; Fang, Q.; Zhang, L.D. Effect of postdeposition annealing on the thermal stability and structural characteristics of sputtered HfO2 films on Si (100). Surf. Sci. 2005, 576, 67–75. [Google Scholar] [CrossRef]
- Jie, N.; Yu, Z.; Qin, Z.; Zhengjun, Z. Morphology in-design deposition of HfO2 thin films. J. Am. Ceram. Soc. 2008, 91, 3458–3460. [Google Scholar] [CrossRef]
- Laishram, D.; Shejale, K.P.; Gupta, R.; Sharma, R.K. Solution processed hafnia nanoaggregates: Influence of surface oxygen on catalytic soot oxidation. ACS Sustain. Chem. Eng. 2018, 6, 11286–11294. [Google Scholar] [CrossRef]
- Laudadio, E.; Stipa, P.; Pierantoni, L.; Mencarelli, D. Phase properties of different HfO2 polymorphs: A DFT-based study. Crystals 2022, 12, 90. [Google Scholar]
- Perevalov, T.V.; Gutakovskii, A.K.; Kruchinin, V.N.; Gritsenko, V.A.; Prosvirin, I.P. Atomic and electronic structure of ferroelectric La-doped HfO2 films. Mater. Res. Express 2019, 6, 036403. [Google Scholar] [CrossRef]
- Xu, L.; Nishimura, T.; Shibayama, S.; Yajima, T.; Migita, S.; Toriumi, A. Ferroelectric phase stabilization of HfO2 by nitrogen doping. Appl. Phys. Express 2016, 9, 091501. [Google Scholar] [CrossRef]
- Waser, R.; Aono, M. Nanoionics-based resistive switching memories. Nat. Mater. 2007, 6, 833–840. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kaneda, Y.; Ito, A. Highly self-oriented growth of (020) and (002) monoclinic HfO2 thick films using Laser Chemical Vapor Deposition. Ceram. Int. 2020, 46, 1810–1815. [Google Scholar] [CrossRef]
- Zahoor, A.; Xu, C.; Shahid, T.; Arfan Anwar, M.; Song, Z. Effects of O2 flux on structure, optical properties and hydrophobicity of highly emissive antireflective HfO2 thin films by magnetron sputtering. Vacuum 2022, 197, 110824. [Google Scholar] [CrossRef]
- Lia, S.; Zhang, Y.; Yanga, D.; Yanga, W.; Chen, X.; Zhao, H.; Hou, J.; Yang, P. Structure and optical properties of HfO2 films on Si (100) substrates prepared by ALD at different temperatures. Phys. B 2020, 584, 412065. [Google Scholar] [CrossRef]
- Khan, S.B.; Zhang, Z.; Lee, S.L. Annealing influence on optical performance of HfO2 thin films. J. Alloy. Compd. 2020, 816, 152552. [Google Scholar] [CrossRef]
- Liaoa, J.; Dai, S.; Peng, R.-C.; Yang, J.; Zeng, B.; Liao, M.; Zhoua, Y. HfO2-based ferroelectric thin film and memory device applications in the post-Moore era: A review. Fundam. Res. 2023, 3, 332–345. [Google Scholar]
- Martínez, F.L.; Toledano-Luque, M.; Gandía, J.J.; Cárabe, J.; Bohne, W.; Röhrich, J.; Strub, E.; Mártil, I. Optical properties and structure of HfO2 thin films grown by high pressure reactive sputtering. J. Phys. D Appl. Phys. 2007, 40, 5256–5265. [Google Scholar] [CrossRef] [Green Version]
- Tchenka, A.; Agdad, A.; Samba Vall, M.C.; Hnawi, S.K.; Narjis, A.; Nkhaili, L.; Ibnouelghazi, E.; Ech-Chamikh, E. Effect of RF sputtering power and deposition time on optical and electrical properties of indium tin oxide thin film. Adv. Mater. Sci. Eng. 2021, 2021, 5556305. [Google Scholar]
- Mayer, M. SIMNRA User’s Guide 7.02; Max-Planck-Institut für Plasmaphysik: Garching, Germany, 2019. [Google Scholar]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Jellison, G.E.; Merkulov, V.I.; Puretzky, A.A.; Geohegan, D.B.; Eres, G.; Lowndes, D.H.; Caughman, J.B. Characterization of thin-film amorphous semiconductors using Spectroscopic Ellipsometry. Thin Solid Film. 2000, 377–378, 68–73. [Google Scholar] [CrossRef]
- Manikantan, J.; Ramalingam, H.B.; Chandar Shekar, B.; Murugan, B.; Kumar, R.R.; Santhoshi, J.S. Physical and optical properties of HfO2 NPs—synthesis and characterization in finding its feasibility in opto-electronic devices. Adv. Powder Technol. 2017, 28, 1636–1646. [Google Scholar] [CrossRef]
- Kim, K.-M.; Jang, J.S.; Yoon, S.-G.; Yun, J.-Y.; Chung, N.-K. Structural, optical and electrical properties of HfO2 thin films deposited at low-temperature using Plasma-enhanced Atomic Layer Deposition. Materials 2020, 13, 2008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Wang, Y.-N.; Choi, C.K.; Zhou, D.Y. Facing-target mid-frequency magnetron reactive sputtered hafnium oxide film: Morphology and electrical properties. J. Korean Phys. Soc. 2016, 68, 679–685. [Google Scholar]
- Raeliarijaona, A.; Cohen, R.E. First-principles calculations of Raman and Infrared Spectroscopy for phase identification and strain calibration of hafnia. Appl. Phys. Lett. 2022, 120, 242903. [Google Scholar] [CrossRef]
- Gupta, P.; Colvin, V.L.; George, S.M. Hydrogen desorption kinetics from monohydride and dihydride species on silicon surfaces. Phys. Rev. B 1988, 37, 8234. [Google Scholar] [CrossRef]
- Gunde, M.K. Vibrational modes in amorphous silicon dioxide. Phys. B 2000, 292, 286–295. [Google Scholar] [CrossRef]
- Li, S.T.; Silvers, S.J.; El-Shall, M.S. Surface oxidation and luminescence properties of weblike agglomeration of silicon nanocrystals produced by a laser vaporization-controlled condensation technique. J. Phys. Chem. B 1997, 101, 1794–1802. [Google Scholar] [CrossRef]
- Sánchez, O.; Gómez-Aleixandre, C.; Palacio, C. IR spectra resolution in fluorinated silicon nitride films. J. Mater. Sci. 1991, 26, 6244–6248. [Google Scholar] [CrossRef]
- Bi, M.; Zhu, J.; Luo, Y.; Cai, H.; Li, X.; Wang, X.; Wei, Y.; Wang, X.; Hu, C.; Hu, J.; et al. Effect of deposition temperature on the surface, structural, and mechanical properties of HfO2 using Chemical Vapor Deposition (CVD). Coatings 2022, 12, 1731. [Google Scholar] [CrossRef]
- Nie, X.; Ma, F.; Ma, D.; Xu, K. Growth mode evolution of hafnium oxide by Atomic Layer Deposition. J. Vac. Sci. Technol. A 2014, 32, 01A109. [Google Scholar] [CrossRef]
- Cao, J.; Yuan, B.; Gong, N.; Meng, T.L.; Teo, S.L.; Yong, A.M.; Zhang, X.; Lin, M.; Karyappa, R.; Zhang, L.; et al. Insights into thin film blistering of gold coating on metal substrate. Appl. Surf. Sci. 2023, 611, 155700. [Google Scholar] [CrossRef]
- Zaharescu, M.; Teodorescu, V.S.; Gartner, M.; Blanchin, M.G.; Barau, A.; Anastasescu, M. Correlation between the method of preparation and the properties of the sol-gel HfO2 thin films. J. Non-Cryst. Solids 2008, 354, 409–415. [Google Scholar] [CrossRef]
- Kappes, M.; Frankel, G.S.; Sridhar, N. Adhesion and adhesion degradation of a pressure sensitive tape on carbon steel. Prog. Org. Coat. 2010, 69, 57–62. [Google Scholar] [CrossRef]
- Abadias, G.; Chason, E.; Keckes, J.; Sebastiani, M.; Thompson, G.B.; Barthel, E.; Doll, G.L.; Murray, C.E.; Stoessel, C.H.; Martinu, L. Review article: Stress in thin films and coatings: Current status, challenges, and prospects. J. Vac. Sci. Technol. A 2018, 36, 020801. [Google Scholar] [CrossRef] [Green Version]
- Malerba, C.; Valentini, M.; Azanza Ricardo, C.L.; Rinaldi, A.; Cappelletto, E.; Scardi, P.; Mittiga, A. Blistering in Cu2ZnSnS4 thin films: Correlation with residual stresses. Mater. Des. 2016, 108, 725–735. [Google Scholar] [CrossRef]
- Zhao, S.; Yuan, G.; Zhang, D.; Xu, P.; Li, G.; Han, W. Formation and elimination mechanism of thermal blistering in Al2O3/Si system. J. Mater. Sci. 2021, 56, 17478–17489. [Google Scholar] [CrossRef]
- Guo, T.; He, J.; Pang, X.; Volinsky, A.A.; Su, Y.; Qiao, L. High temperature brittle film adhesion measured from annealing-induced circular blisters. Acta Mater. 2017, 138, 1–9. [Google Scholar] [CrossRef]
- Eren, B.; Marot, L.; Günzburger, G.; Renault, P.-O.; Glatzel, T.; Steiner, R.; Meyer, E. Hydrogen-induced buckling of gold films. J. Phys. D Appl. Phys. 2014, 47, 025302. [Google Scholar] [CrossRef]
- Smith, D.; Baumeister, P. Refractive index of some oxide and fluoride coating materials. Appl. Opt. 1979, 18, 111–115. [Google Scholar] [CrossRef]
- Conlon, D.; Doyle, W.P. Absorption spectra of zirconium and hafnium dioxides. J. Chem. Phys. 1965, 42, 4315. [Google Scholar] [CrossRef]
- Bright, T.J.; Watjen, J.I.; Zhang, Z.M.; Muratore, C.; Voevodin, A.A. Optical properties of HfO2 thin films deposited by magnetron sputtering: From the visible to the far-infrared. Thin Solid Film. 2012, 520, 6793–6802. [Google Scholar] [CrossRef]
- Haynes, W.M. Chapter 4: Properties of the elements and inorganic compounds. In CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 4–66. [Google Scholar]
- Tauc, J. Amorphous and Liquid Semiconductors; Plenum: London, UK; New York, NY, USA,, 1974. [Google Scholar]
- Ortega, J.J.; Aguilar-Frutis, M.A.; Alarcón, G.; Falcony, C.; Méndez-García, V.H.; Araiza, J.J. Band gap engineering of indium zinc oxide by nitrogen incorporation. Mater. Sci. Eng. B 2014, 187, 83–88. [Google Scholar] [CrossRef]
- Callegari, A.; Cartier, E.; Gribelyuk, M.; Okorn-Schmidt, H.F.; Zabel, T. Physical and electrical characterization of hafnium oxide and hafnium silicate sputtered films. J. Appl. Phys. 2001, 90, 6466–6475. [Google Scholar] [CrossRef]
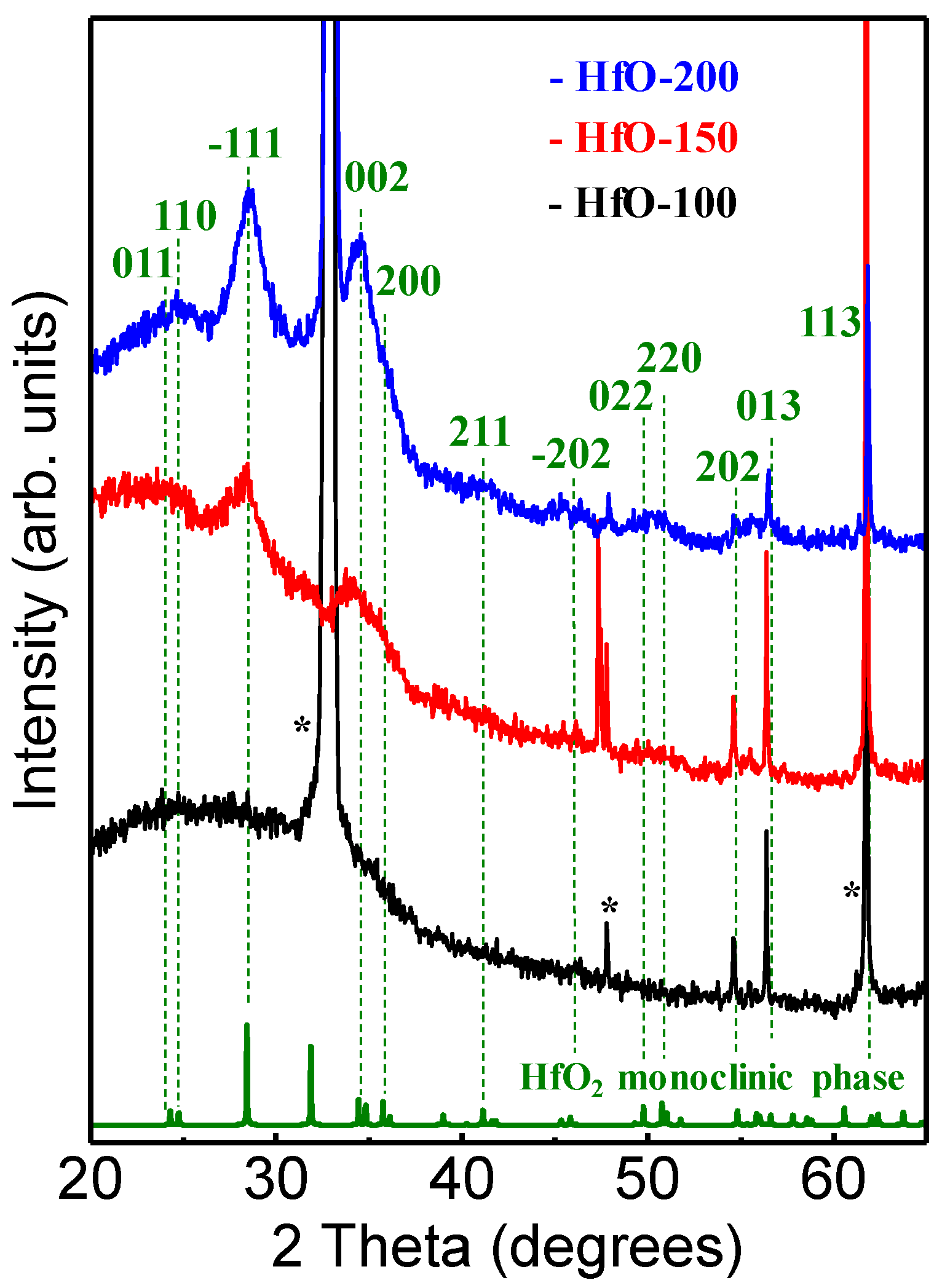
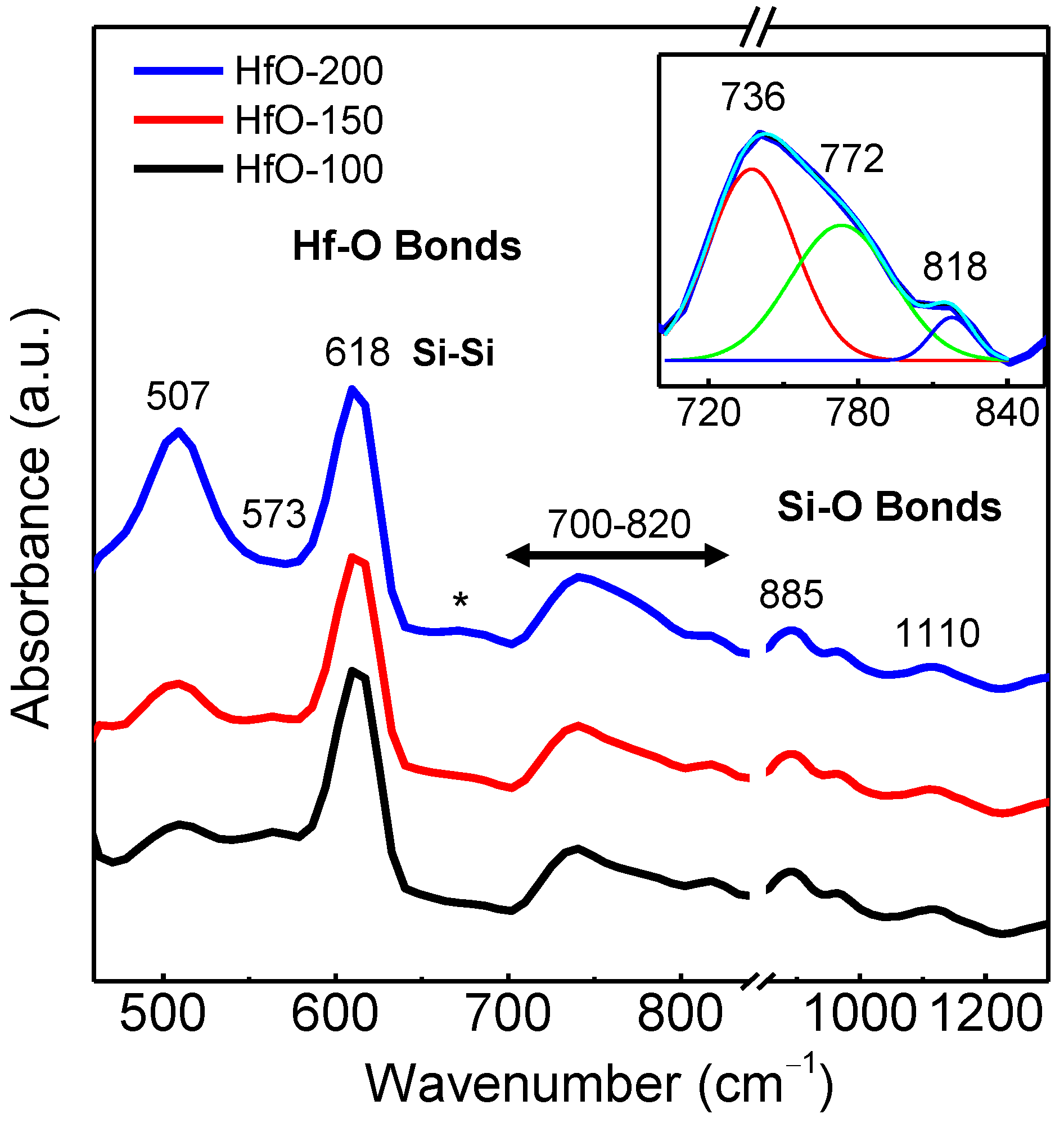
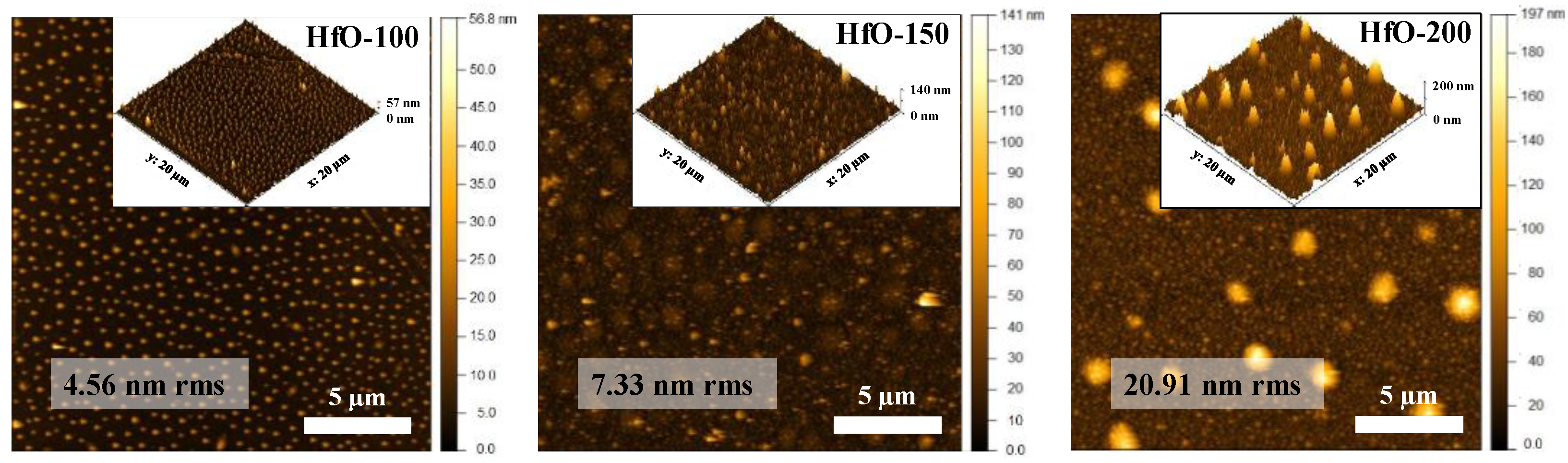
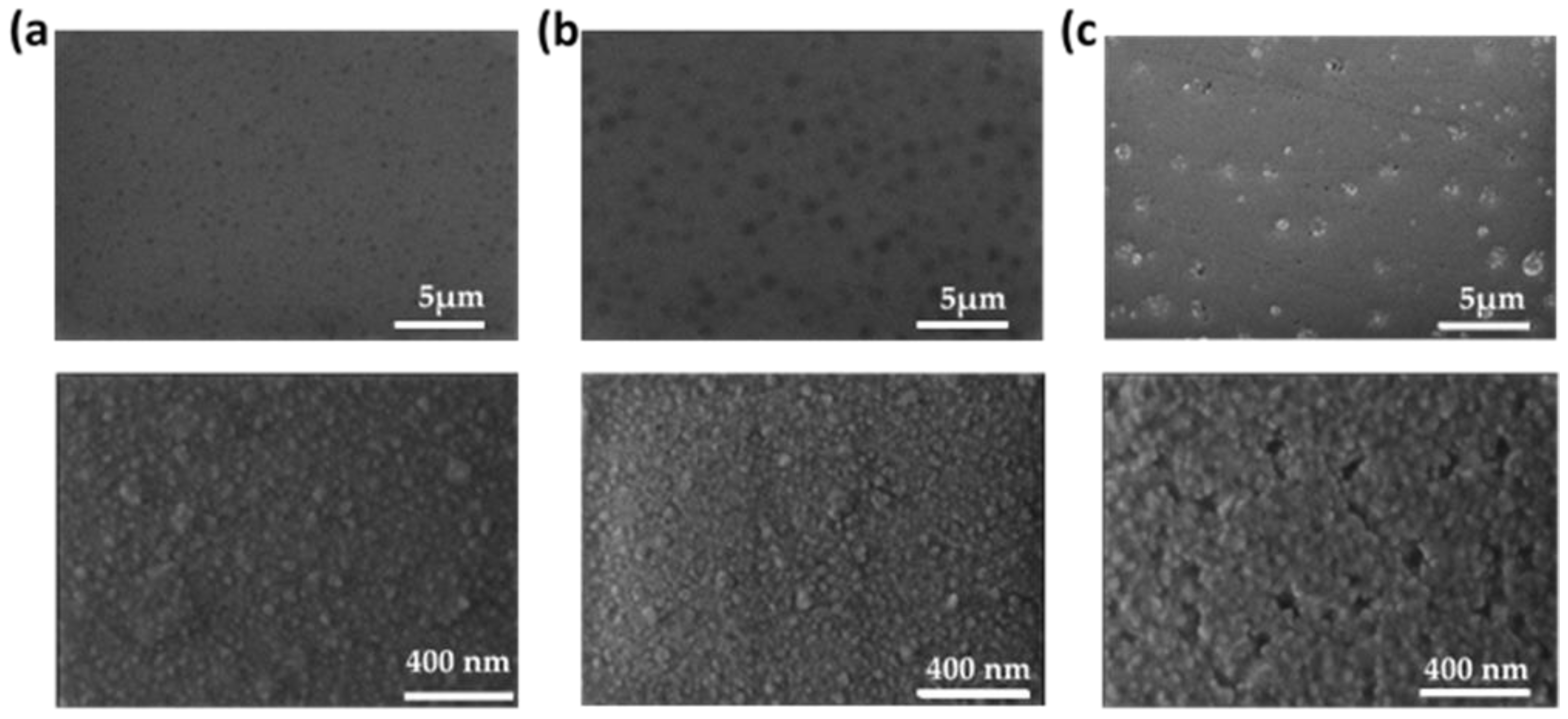
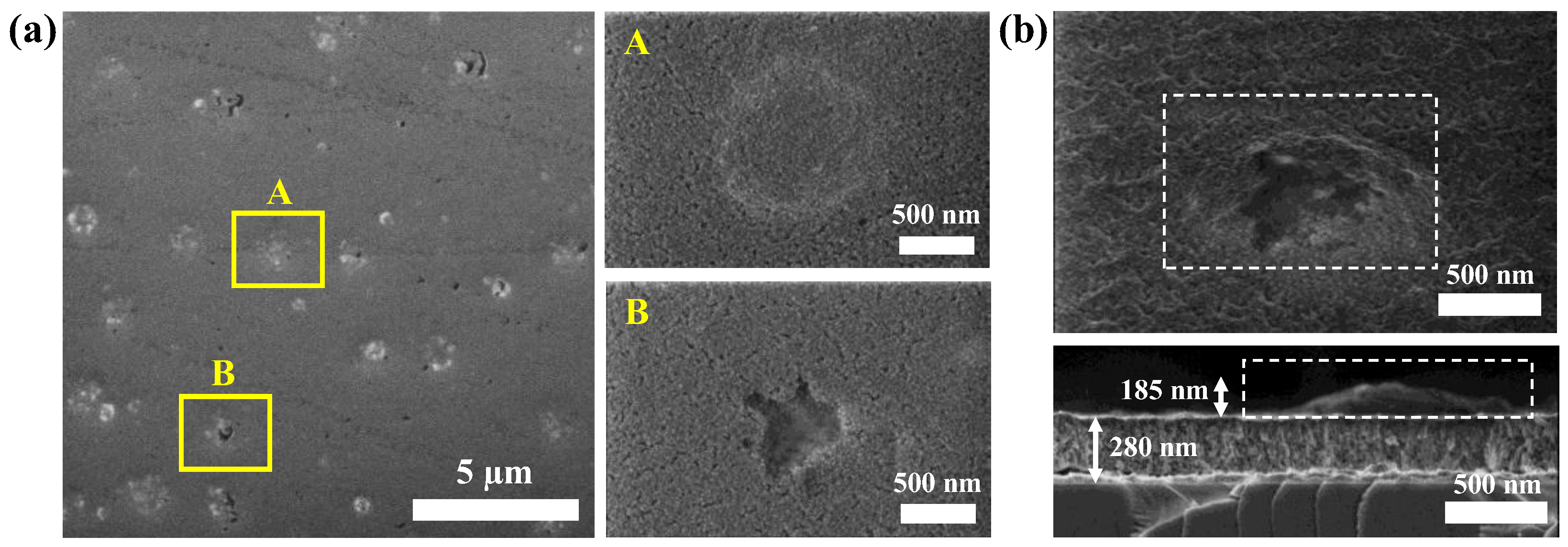

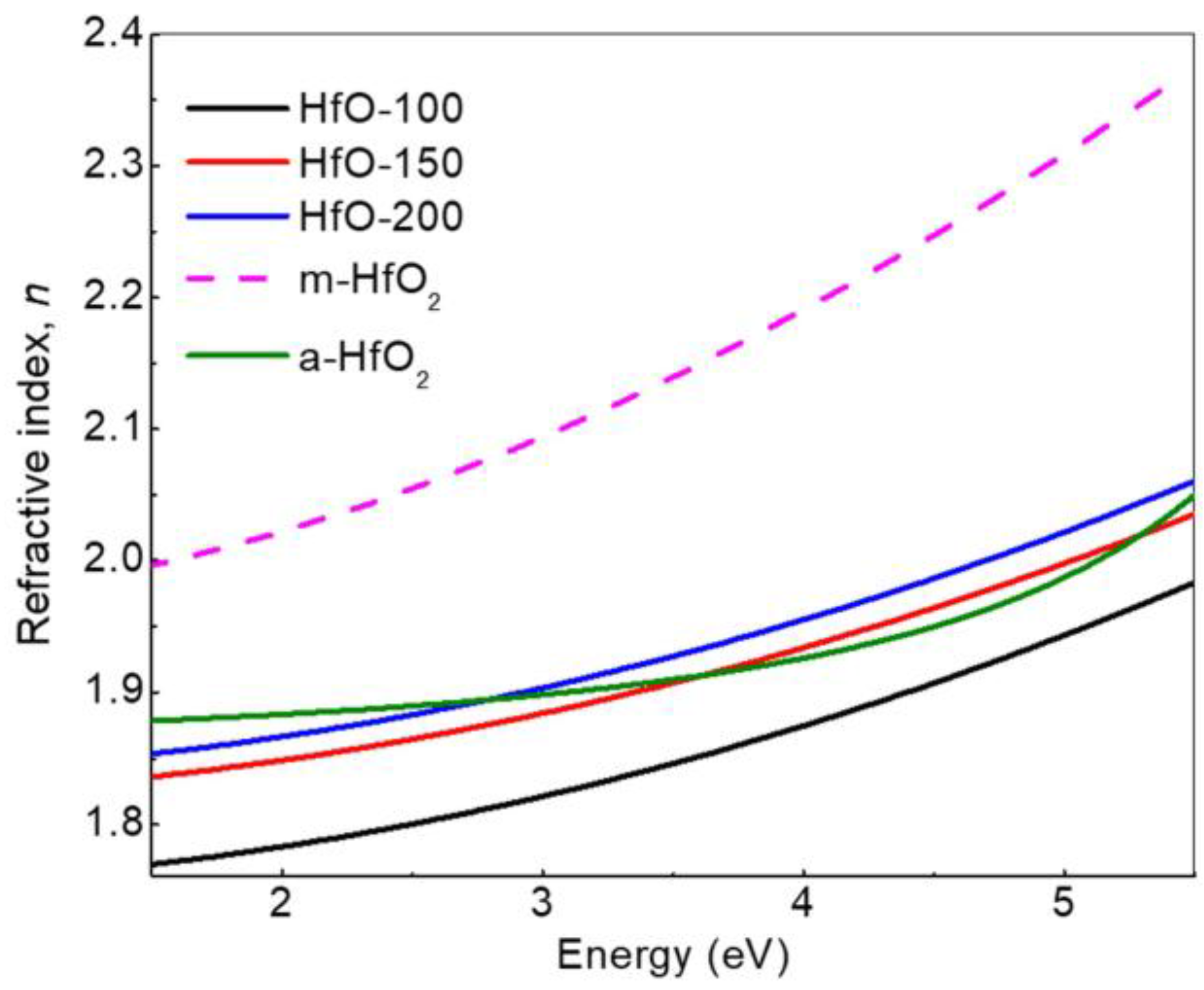
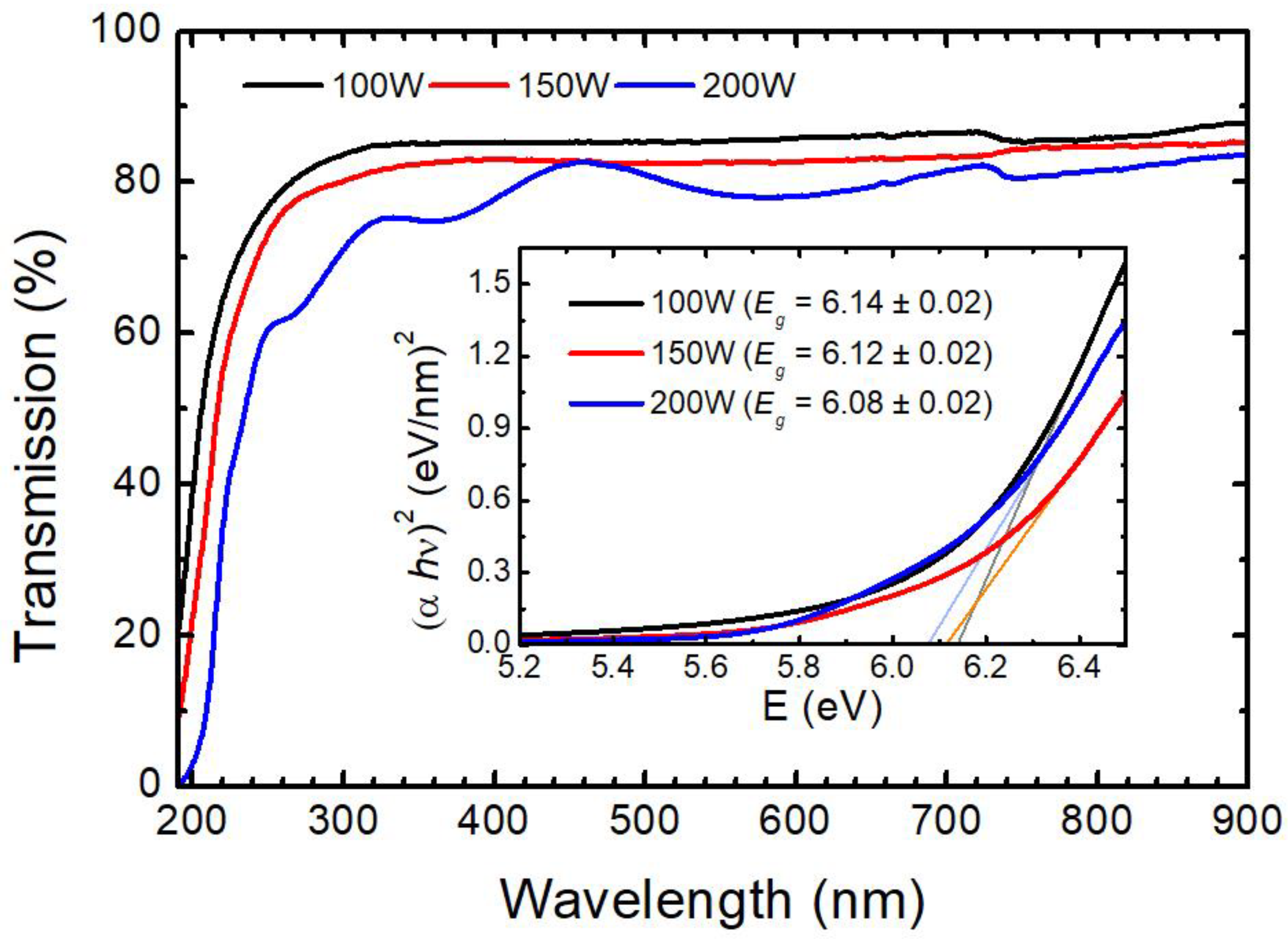
| Sample | WHf | Thickness (nm ± 10%) | Deposition Rate (nm/min) | Hf (at.%) | O2 (at.%) |
|---|---|---|---|---|---|
| HfO-100 | 100 | 80 | 2.0 ± 0.2 | 16 ± 2 | 84 ± 2 |
| HfO-150 | 150 | 150 | 3.8 ± 0.4 | 20 ± 2 | 80 ± 2 |
| HfO-200 | 200 | 300 | 7.5 ± 0.8 | 26 ± 2 | 74 ± 2 |
| Sample | 507 cm−1 m-Hf-O | 570 cm−1 a-Hf-O | 700–820 cm−1 Region | Area | |||
|---|---|---|---|---|---|---|---|
| 736 cm−1 m-Hf-O | 772 cm−1 m-Hf-O | 818 cm−1 Si-O | (arb. Units) | ||||
| Crystalline | Total | ||||||
| HfO-100 | 2.27 | 1.01 | 0.91 | 1.23 | - | 4.41 | 5.85 |
| HfO-150 | 2.70 | 0.84 | 1.03 | 0.84 | - | 4.57 | 5.91 |
| HfO-200 | 5.20 | 0.83 | 1.27 | 1.06 | - | 7.53 | 8.50 |
| Sample | Average Distance (nm) | Height (nm) | Average Diameter (nm) | Projected Area (µm2) | Volume (µm3) |
|---|---|---|---|---|---|
| HfO-100 | 650 ± 130 | 27 ± 6 | 261 ± 68 | 0.1038 ± 0.04 | 1.703 × 10−3 |
| HfO-150 | 1380 ± 275 | 62 ± 12 | 866 ± 246 | 0.7523 ± 0.56 | 1.634 × 10−2 |
| HfO-200 | 2650 ± 500 | 176 ± 36 | 1183 ± 357 | 1.6490 ± 0.48 | 1.629 × 10−1 |
| Sample | Parameter A | Parameter B | % Voids * |
|---|---|---|---|
| HfO-100 | 1.7520 | 0.01189 | 21 |
| HfO-150 | 1.8200 | 0.01107 | 15 |
| HfO-200 | 1.8366 | 0.01151 | 12 |
| m-HfO2 | 1.9627 * | 0.02200 | - |
| a-HfO2 | 1.8500 | 0.01170 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araiza, J.d.J.; Álvarez-Fraga, L.; Gago, R.; Sánchez, O. Surface Morphology and Optical Properties of Hafnium Oxide Thin Films Produced by Magnetron Sputtering. Materials 2023, 16, 5331. https://doi.org/10.3390/ma16155331
Araiza JdJ, Álvarez-Fraga L, Gago R, Sánchez O. Surface Morphology and Optical Properties of Hafnium Oxide Thin Films Produced by Magnetron Sputtering. Materials. 2023; 16(15):5331. https://doi.org/10.3390/ma16155331
Chicago/Turabian StyleAraiza, José de Jesús, Leo Álvarez-Fraga, Raúl Gago, and Olga Sánchez. 2023. "Surface Morphology and Optical Properties of Hafnium Oxide Thin Films Produced by Magnetron Sputtering" Materials 16, no. 15: 5331. https://doi.org/10.3390/ma16155331
APA StyleAraiza, J. d. J., Álvarez-Fraga, L., Gago, R., & Sánchez, O. (2023). Surface Morphology and Optical Properties of Hafnium Oxide Thin Films Produced by Magnetron Sputtering. Materials, 16(15), 5331. https://doi.org/10.3390/ma16155331







