A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles
Abstract
:1. Introduction
2. Synthesis Methods of Selenium Nanoparticles
2.1. Influence of the Method of Synthesis of Selenium Nanoparticles on the Resulting Size and Shape of Nanoparticles
2.2. Influence of Selenium Nanoparticle Synthesis Method on the Minimum Inhibitory Concentration in Antibacterial Studies
3. Effective Concentration/Minimum Inhibitory Concentration of Selenium Nanoparticles Depending on Their Size
3.1. Dependence of the Effective Concentration of Selenium Nanoparticles on Their Size for the Study of Antiviral Activity
3.2. Dependence of the Minimum Inhibitory Concentration of Selenium Nanoparticles on Their Size and Shape in the Study of Antibacterial Activity
| № | Precursor | Composition | Method of the Synthesis | Particle Size, nm | Microorganism Strains | Effect | MIC | Results | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Na2SeO3 | Lysozyme SeNPs | Chemical reduction | 35.6 | Escherichia coli, Staphylococcus aureus | BS | 82 μg/mL | SeNPs and lysozyme demonstrated synergetic bacteriostatic activity. | [121] |
| 2 | Na2SeO3 | Propolis SeNPs | Bioorganic chemical reduction | 159, 151.9, 11.2 and 169.3 | Salmonella typhimurium ATCC 14028, Escherichia coli ATCC 25922, Staphylococcus aureus- ATCC 25923 | BC | 25 mg/L 27.5 mg/L 30 mg/L | BNCt/Pro/SeNPs were the most effective against all bacterial strains. | [168] |
| 3 | Na2SeO3 | SeNPs | Chemical reduction | 32.3 | Staphylococcus aureus (MSSA), Staphylococcus aureus (MRSA), Staphylococcus aureus (VRSA), Enterococci (VRE) | BC; BS | 20 µg/mL, 80 µg/mL, 320 µg/mL, and >320 µg/mL | SeNPs showed a synergistic effect with linezolid (LZD) through protein degradation against MSSA and MRSA. | [112] |
| 4 | Na2SeO3 | SeNPs | Biosynthesis of SeNPs by Providencia sp. | 120 | P. aeruginosa, E. coli, V. parahemolyticus, S. aureus, B. cereus, B. subtilis | BC; BS | 500 mg/L | Bio-SeNPs showed strong antibacterial effects on the five of pathogens at 100 mg/L. It was shown that most of G-bacteria (P. aeruginosa, E. coli and V. parahemolyticus) were locally killed by 500 mg/L of the bio-SeNPs after 12 h, which was better than G+-bacteria (S. aureus and B. cereus, except for B. subtilis). | [188] |
| 5 | Se (solid) | SeNPs | Pulsed laser ablation in liquids | ~80 and ~10 | E. coli (MDR-EC) ATCC BAA-2471, P. aeruginosa (PA) ATCC 27853, S. aureus (MRSA) ATCC 4330 Staphylococcus epidermidis ATCC 35984 | BC; BS | 25 µg/mL | SeNPs showed a dose-dependent antibacterial effect toward both standard and antibiotic-resistant phenotypes of Gram-negative and Gram-positive bacteria. | [113] |
| 6 | NaHSeO3 | SeNPs with polyester fabrics | Chemical reduction | 40–60 | Salmonella typhi, Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa | BC | 1980 µg/mL | The treated fabric under study showed excellent killing potentiality against Gram-positive and Gram-negative bacteria. | [174] |
| 7 | NaHSeO3 | Leather material/ SeNPs | Chemical reduction | 36–77 and 41–149 | Bacillus cereus, Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli | BC | 1980 µg/mL | Potential application to the footwear industry to color the leather as well as prevent the spread of bacterial infection promoted by humidity, poor breathability and temperature. | [122] |
| 8 | Na2SeO3 | SeNPs/ orange peel waste extract | Bioorganic chemical reduction | 16–95 | Pseudomonas aeruginosa PAO1, MDR, S. aureus ATCC 29213 | BS | 25 µg/mL | The biosynthesized SeNPs had a promising antibiofilm activity, where the largest inhibition of biofilm was noticed in MDR K. pneumonia. | [169] |
| 9 | Na2SeO3 | bacterial cellulose/ gelatin/ SeNPs hydrogels | Chemical reduction | 75 | E. coli, S. aureus | BC | 65.44 μg | BC/Gel/SeNPs nanocomposite hydrogel: potential wound dressing for preventing wound infection and accelerating skin regeneration in clinic. | [189] |
| 10 | H2SeO3 | rGO-S/Se composite | Chemical reduction | 12 | Staphylococcus aureus, Enterococcus faecalis | BS | 200 µg/mL | Concentration and time-dependent BS activity of the rGO (Reduced graphene oxide)-S/Se NP against S. aureus cells | [170] |
| 11 | Na2SeO3 | SeNPs | Biosynthesis of SeNPs by cyanobacteria Anabaena sp. | 25 | Staphylococcus aureus Escherichia coli | BS | 50 µg/mL | These biogenic SeNPs demonstrated significant antibacterial and anti-biofilm activity against bacterial pathogens. | [131] |
| 12 | Na2SeO3 | Ag-SeNPs | Biosynthesis of SeNPs by Aureobasidium pullulans | 50 and 70 | Staphylococcus aureus F1557 E. coli WT F1693 | BC; BS | - | The Ag–Se coating reduced 81.2% and 59.7% of viable bacterial adhesion. The antibacterial mechanism of Ag–Se coatings works through effective contact-killing activity against S. aureus. | [146] |
| 13 | Na2SeO3 | SeNP-chitosan, SeNPs-carboxymethyl cellulose | Chemical reduction | 55–500 50–300 | Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, Staphylococcus epidermidis | BS | 5 µg/mL | The SeNP-modified collagenous scaffolds at SeNP concentrations low as 5 µg/mL showed a strong antibacterial effect (up to 94% of bacterial growth inhibition) toward laboratory and clinical isolates of Gram-positive bacteria from the genus Staphylococcus. | [171] |
| 14 | Na2SeO3 | SeNPs | Biosynthesis from Stenotrophomonas maltophilia SeI TE02 | 181 | P. aeruginosa PAO1, INT, BR1 and BR2, S. maltophilia VR10 and VR20, Achromobacter xyloxidans strain C, Burkholderia cenocepacia strain LMG 16656, Staphylococcus aureus Mu50 strain, S. aureus UR1, Staphylococcus epidermidis ET024, Staphylococcus hemolitycus UST1 | BS | 4–128 μg/mL | The progressive loss in protein and carbohydrate content of the organic cap determines a decrease in nanoparticle stability. This leads to an alteration of size and electrical properties of SeNPs along with a gradual attenuation of their antibacterial efficacy. | [190] |
| 15 | Na2SeO4 | SeNPs | Biosynthesis of SeNPs from Aspergillus quadrilineatus and Aspergillus ochraceus isolated from the twigs and leaves of Ricinus communis | 45–75 | Pseudomonas aeruginosa ATCC 15442, Bacillus cereus ATCC 10876, Staphylococcus aureus ATCC 6538, Klebsiella pneumoniae ATCC 13883, Bacillus subtilis TCC 6633, Escherichia coli ATCC 11229 | BS | 62.5–1000 µg/mL | SeNPs showed potent antifungal and antibacterial potentials against different human and phyto-pathogens. | [175] |
| 16 | Na2SeO3 | “Green” SeNPs | Biosynthesis using aqueous leaf extract of U. dioica | 10–87.4 | Staphylococcus aureus ATCC 25923, Bacillus subtilis ATCC605, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 Fungi: Candida albicans, Cryptococcus neoformans | BS, FS | 125, 62.5 and 15.62 µg/mL 3.9 and 7.81 µg/mL | SeNPs exhibited promising antibacterial activity against Gram-negative and Gram-positive bacteria and antifungal activity. | [150] |
| 17 | SeO2 | SeNPs/tree gum | Chemical reduction | 105.6 | Bacillus subtilis, Micrococcus luteus | BS | 12 μg/mL | The synthesized SeNPs inhibited the growth of the Gram-positive bacteria B. subtilis only. | [123] |
| 18 | Na2SeO3 | SeNPs/chitosan | Chemical reduction | 100 | Staphylococcus aureus, Escherichia coli | BS | 158 μg/mL | The antibacterial activity of CS(H)-SeNPs markedly decreased owing to the aggregation of NPs. | [166] |
| 19 | Na2SeO3 | SeNPs | Phytofabrication of SeNPs from aqueous Spirulina platensis | 79.4 | Salmonella abony NCTC 6017, Klebsiella pneumonia ATCC 700603, E. coli ATCC 8739 | BS | 25–200 µg/mL | SeNPs have shown potent antimicrobial activity against Gram-negative bacteria. No toxic effect was observed for SeNPs on normal kidney and liver cell lines. | [191] |
| 20 | Na2SeO3 | SeNPs | Biosynthesis of SeNPs from Nepeta plant powder | 75 | P. aeruginosa: ATCC 27853 and A. baumannii: ATCC BAA-747 | BS | 4 μg/mL 8 μg/mL | The inhibition of bacterial growth demonstrated in the presence of lower concentrations of SeNPs than common antibiotics. | [117] |
| 21 | Na2SeO3 | Collagen/Chitosan/SeNPs | Chemical reduction | 100–200 | Staphylococcus aureus NCTC 8511, MRSA CCM 7110 and Escherichia coli NCTC 13216 | BS | 0.5–5 µg/mL | SeNPs are able to enhance the scaffold’s antibacterial properties toward S. aureus and MRSA at concentrations between 0.5 µg/mL and 5 µg/mL. | [172] |
| 22 | Na2SeO3 | B-SeNPs | Biosynthesis of SeNPs from Anabaena variabilis (cyanobacteria) | 10.8 | Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae | BS | 20 µg/mL | Cyanobacteria mediated synthesis can be considered as safe and nontoxic way to synthesize SeNPs. | [116] |
| 23 | Na2SeO3 | SeNPs | Biosynthesis of SeNPs-S by Bacillus sp. Q33 | 159.2 | E. coli, P. aeruginosa, S. aureus, L. monocytogenes | BS | 200 µg/mL | SeNPs-S (product of whole cells) and SeNPs-E (product of the extracellular extract) exhibited obvious inhibitory effects on the four pathogenic bacteria. | [115] |
| 24 | Se (wafer) | SeNPs | Chemical reduction | 42 | E. coli, S. aureus | BS | 0.2 mg/mL | The synergistic antibacterial effect of SeNPs and microstructured parylene-C. | [192] |
| 25 | H2Se | Arabinogalactan/ SeNPs | Bioorganic synthesis with AG from Larix Sibirica isolated | 94 | bacterial phytopathogen Clavibacter michiganensis sepedonicus (Cms) | BS | 6.25 μg/mL | Antimicrobial activity of AG/SeNPs is due to their ability to inhibit the dehydrogenase activity of Cms cells, to disrupt the integrity of the cell membrane, resulting in a decrease of transmembrane potential and reduction of cellular respiration. | [125] |
| 26 | Na2SeO3 | Cefotaxime/Ag–SeNPs | Gamma irradiation | 34.5; 24.9 | E. coli, P. aeruginosa, K. pneumoniae, S. aureus, Enterococcus sp. | BS; BC | 2.5–5 μg/mL; 0.625–2.5 μg/mL (with CFM) | Ag NPs-CFM, SeNPs-CFM and Ag–SeNPs-CFM possessed antimicrobial activity against Staphylococcus aureus, Escherichia coli. | [193] |
| 27 | Na2SeO3s | SeNPs | Chemical reduction | 70 | Porphyromonas gingivalis | BS; BC | 4–16 μg/mL | The growth of P. gingivalis was significantly inhibited by SeNPs. | [114] |
| 28 | SeO2 | Se NP-ε-poly-L-lysine | chemical reduction | 82 | S. aureus ATCC 29213, S. aureus (MRSA) ATCC 43300, E. faecalis ATCC 29212, E. coli ATCC 25922, A. baumannii 2208, ATCC 19606, P. aeruginosa strain PAO1-LAC ATCC 47085, K. pneumoniae ATCC 13883, and K. pneumoniae (MDR) FADDI-KP628 | BS; BC | 6−26 μg/mL | The MICs of Se NP-ε-PL against the eight different types of bacteria tested are approximately 6–26 μg/mL. | [173] |
| 29 | Na2SeO3 | SeNPs | Biosynthesis by Se-resistant Bacillus subtilis AS12 | 77 | Aeromonas hydrophilia Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, Escherichia coli, Aeromonas hydrophilia, Klebsiella pneumonia | BS; BC | 3–5 μg/mL | Bio-SeNPs can mitigate the accumulation of heavy metals and reduce the bacterial load in a concentration-dependent manner. | [194] |
| 30 | Na2SeO3 | TiO2 nanotube with SeNPs | chemical reduction | 88.93 | E. coli | BS | - | Selenium nanoparticles improved antibacterial properties of titanium dioxide nanotubes. | [176] |
| 31 | Na2SeO3 | SeNPs | microwave technique in the presence of citric acid | 10.5–20 | P. aeruginosa, E. coli, B. subtilis, S. aureus | BS | 100 mg/mL | SeNPs had the highest activity against E. coli, with a zone of inhibition (ZOI) of 25.2 ± 1.5 mm compared to 16.0 ± 0.6 mm for the standard antibiotic. | [136] |
| 32 | Na2SeO3 | SeNPs | biosynthesis of SeNPs by endophytic fungal strain Penicillium crustosum EP-1 | 3–22 | Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 9022 | BS | 12.5 µg/mL 50 µg/mL 50 µg/mL 25 µg/mL (in the presence of light) 50 µg/mL, 100 µg/mL (under dark conditions) | The effect of SeNPs was dose-dependent, and higher activities against bacteria were attained in the presence of light than were attained under dark conditions. | [147] |
| 33 | Na2SeO3 | Mk-SeNPs | chemical reduction with the presence of aqueous berry extract of Murraya koenigii (Mk-SeNPs) | 50–150 | Streptococcus mutans (HQ 693279.1 & ATCC 25175), Enterococcus faecalis Shigella sonnei, Pseudomonas aeruginosa (K 7769531 & HQ 693272 | BS; BC | 40 μg/mL 50 μg/mL | Mk-SeNPs are considered to be a prospective antibacterial agent with effective antioxidant capacity at 25 and 50 μg/mL, which is target-specific only for the bacterial cells and not for the erythrocytes and macrophages at the same concentration. | [195] |
| 34 | Na2SeO3 | SeNPs | The abiotic reduction of selenite with the use of Enterococcus spp. cell-free extract (biotic and abiotic stages) | 200 | E. coli | BS | 3.2 g/L | The obtained nanoparticles exhibited antimicrobial properties by directly inhibiting the viability of an E. coli bacterial strain. The results demonstrate not only the potential of abiotic production of SeNPs but also the potential for these particles as microbial inhibitors in medical or similar fields. | [196] |
| 35 | Na2SeO3 | SeNPs | chemical reduction with PVA as a stabilizer | 30–70 | S. aureus (ATCC 29213), E. coli (ATCC 25922) | BS | 1 μg/mL | The growth of S. aureus was inhibited by the nanoparticles at concentrations as low as 1 μg/mL. | [197] |
| 36 | Na2SeO3 | Artemisia annua extract/SeNPs | Biosynthesized using Artemisia annua, and subsequently, the surface of the biogenic SeNPs was functionally modified with starch. | <200 | Staphylococcus aureus, Bacillus cereus, Salmonella enterica, Escherichia coli | BS; BC | 5–100 μg/mL | StAaSeNPs showed the highest antibacterial activity against tested strains S. enterica (23.26 ± 0.35 mm). Based on the findings, it can be inferred that surface chemistry is the most influential factor in determining the antibacterial activity of SeNPs. | [148] |
| 37 | Na2SeO3 | Hollow SeNPs | Bioorganic synthesis of SeNPs with the potato extract | 115 | B. subtilis (MTCC441), E. coli (MTCC40) | BC | 10–20 μg/mL | The hSeNPs showed good antibacterial activity against tested bacteria. | [198] |
| 38 | SeO2 | SeNPs | chemical reduction with the presence of PVA | 43–205 | Staphylococcus aureus (MSSA) ATCC 29213, Staphylococcus aureus (MRSA) ATCC 43300 | BC | 16 µg/mL | The SeNPs were shown to have multimodal mechanisms of action that depended on their size, including depleting internal ATP, inducing ROS production, and disrupting membrane potential. | [199] |
| 39 | Na2SeO3 | BSA/ SeNPs | chemical reduction method in the presence of the BSA | 20–30 | Escherichia coli (ATCC no. 25922), Escherichia coli (ATCC no. BAA-2471), Staphylococcus aureus (ATCC no. 25923) | BC | 1 mg/mL | SeNPs achieved a 10-fold reduction for S. aureus. | [200] |
| 40 | SeO2 | eADF4(κ16)/SeNPs PVA/ SeNPs | chemical reduction method in the presence of the spider silk protein eADF4(κ16) and PVA (Polyvinyl alcohol) | 46 | Escherichia coli | BC | 8 ± 1 µg/mL 405 ± 80 µg/mL | The eADF4(κ16)-coated SeNPs demonstrated a much higher bactericidal efficacy against the E. coli, with a minimum bactericidal concentration (MBC) approximately 50 times lower than that of PVA/SeNPs. | [201] |
| 41 | Na2SeO3 | mycogenic SeNPs SeNPs-CN | 2 methods: a biogenic process using Penicillium chrysogenum filtrate and by utilizing gentamicin drug (CN) following the application of gamma irradiation | 33.84 22.37 | Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae Fungi: Candida albicans | BC, FC | 0.490 μg/mL 0.245 μg/mL | The synthesized SeNPs-CN possesses an encouraging antimicrobial potential with respect to the biogenic SeNPs against all examined microbes. Remarkably, SeNPs-CN showed antimicrobial potential toward 23.0 mm ZOI for Escherichia coli and 20.0 mm ZOI against Staphylococcus aureus. It also inhibited the expansion and invasion of C. albicans suggested the use of gentamycin as antifungal agent after the combination with the synthesized SeNPs. | [202] |
| 42 | Se (pellets) | SeNPs | Pulsed laser ablation in liquids | 115 | Escherichia coli, Staphylococcus aureus | BC | 50 µg/mL | The pure selenium nanoparticles determined the minimal concentration required for ~50% inhibition of either E. coli or S. aureus after 24 h to be at least ~50 µg/mL. | [203] |
| 43 | H2SeO3 | Green Orange Peel extract/SeNPs | Chemical reduction in the presence of BSA—stabilizer | 18.3 | S. aureus (ATCC 25923), S. epidermidis (ATCC 1228) | BS | 4.94 μg/L | The SeNP sample demonstrated excellent antibacterial activity with an average diameter of inhibition zones of 20.0 mm and an MIC of 4.94 μg/L. | [124] |
| 44 | Na2SeO3 | SeNPs | Bioorganic synthesis of SeNPs with the use of Penicillium corylophilum As-1 biomass filtrate, in presence of ascorbic acid as a reducing agent | 29.1–48.9 | Staphylococcus aureus ATCC 6538, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 9027 | BS | 9.37 μg/mL 18.75 μg/mL 37.5 μg/mL 37.5 μg/mL | The formed SeNPs showed a prominent antimicrobial activity at different concentrations against the pathogens Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa and E. coli. | [132] |
| 45 | SeO2 | Penicillium expansum/SeNPs | biosynthesis with Penicillium expansum ATTC 36200 | 4–12.7 | Bacillus subtilis ATCC 6051, Staphylococcus aureus ATCC 23235, Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 9027 Fungi: Candida albicans ATCC 90028, Aspergillus fumigatus RCMB 02568, A. niger RCMB 02724 | BS, FS | 62.5 μg/mL 62.5 μg/mL 125 μg/mL 125 μg/mL 125 μg/mL 125 μg/mL 125 μg/mL | The inhibitory effect against Gram-positive bacteria was more pronounced than against Gram-negative bacteria and fungi. | [149] |
| 46 | Na2SeO3 | Chitosan/SeNPs | chemical reduction | 77 | Streptococcus mutans | BS | 128 and 64 µg/mL | The comparison between the treated and untreated groups showed that combining therapy with SeNPs and PDT markedly decreased colony-forming units of one-day-old S. mutans biofilm. | [204] |
| 47 | SeO2 | SeNPs | solvothermal method using Moringa oleifera leaf extract as a reducing agent | 82.86 | Listeria innocua ATCC 33090, Bacillus cereus ATCC 10876, Escherichia coli ATCC 43888, Salmonella typhimurium ATCC 14028 | BS | 100 μg/mL | Zones of inhibition were observed only in S. typhimurium (12.5 ± 0.5 mm), E. coli (10.1 ± 0.7 mm) and B. cereus (9.8 ± 0.7 mm). | [167] |
| 48 | Na2SeO3 | SeNPs | green synthesis using ascorbic acid as a reducing agent and methanolic extract of Calendula officinalis L. flowers as a stabilizer | 40–60 | Serratia marcescens, Enterobacter cloacae, Alcaligenes faecalis | BS | - | The antibacterial activity of the extract, AsAc, and Na2SeO3 was enhanced by producing the SeNPs, which significantly inhibited the growth of S. marcescens, E. cloacae, and A. faecalis bacterial strains. | [205] |
| 49 | Na2SeO3 | SeNPs | Chemical reduction synthesis from extracts of three plants: Allium cepa (onion), Malpighia emarginata (acerola), and Gymnanthemum amygdalinum (boldo) | 245–321 | Streptococcus agalactiae, Staphylococcus aureus, S. aureus, Pseudomonas aeruginosa, Escherichia coli | BS | 6.125 to 98 μg/mL | The antimicrobial activity and low hemolytic concentration indicate the possibility of use against Gram-positive bacteria, including multidrug-resistant ones, opening a wide variety of options for their application | [151] |
| 50 | SeO2 | Algae/ SeNPs | Microwave-assisted synthesis of SeNPs | 40 | V. harveyi (PTCC 1755) | BS | 200 μg/mL | The presence of different functional groups of Sargassum angustifolium on the surface of the algae-coated SeNPs might be responsible for the more effective reaction of these nanoparticles with the cell walls and/or membrane of V. harveyi. | [206] |
| 51 | Na2SeO3 | SeNPs | chemical reduction | 71 | V. cholerae O1 ATCC 14035 strain | BS | 50–200 μg/mL | SeNPs are safe as an antibacterial and antibiofilm agent against V. cholerae O1 ATCC 14035 strain. | [207] |
| 52 | Na2SeO3 | SeNPs, NCT/GA, NCT/GA/Eug and NCT/GA/Eug/SeNPs | chemical reduction | 9.7, 124.8, 132.6 and 134.2 nm | Escherichia coli, Staphylococcus aureus | BS; BC | 15.0 μg/mL 20 μg/mL | The entire fabricated nanocomposite exhibited potent antibacterial activity and cell destruction capability within 5–10 h of exposure. | [208] |
| 53 | Na2SeO3 | SeNPs | synthesis and purification, in the presence of pepper extract; chemical reduction method, plus microwave | 79–90 nm | Escherichia coli ATCC BAA-2471, Staphylococcus aureus ATCC 4330 | BS | 72,2 μg/mL 85,1 μg/mL | Selenium nanoparticles were biocompatible and showed bacteriostatic activity | [152] |
| 54 | Na2SeO3 | SeNPs | biosynthesized with a standard strain of C. albicans | 38 | Fungi: Candida albicans, Candida glabrata | FS | 1 and 0.5 µg/mL | SeNPs showed much better fungistatic activity compared to itraconazole, amphotericin B and anidulafungin. | [209] |
| 55 | Na2SeO3 | SeNPs | Biosynthesis from lactic acid bacteria (LAB) | 56 | Fungi: Candida and Fusarium species | FC | 80–130 µg/mL | The LAB-SeNPs MFC was in the range of 80–130 µg/mL, which ensured the complete killing of all tested fungi. | [210] |
| 56 | Na2SeO4 | SeNPs | Biosynthesis with standard strains of A. Flavus and C. albicans | 37 and 38 | Fungi: Candida and Aspergillus species | FS | 0.5, and 0.25 μg/mL | The utilization of SeNPs at concentrations of 1, 0.5 and 0.25 μg/mL or in, some strains, even lesss than 0.125 μg/mL, resulted in zero growth of fungal agents. | [126] |
| 57 | Na2SeO3 | SeNPs | “green” method using the Halomonas elongata bacterium | 11 | Fungi: Candida albicans | FS | - | The synthesized NPs in optimal situation stopped the growth of Candida albicans up to 72%. | [211] |
| 58 | Se (pellets) | Chitosan/SeNPs | laser ablation in liquids | 100 | Fungi: C. albicans TW17 and 6486 strains | FC, FS | 3.5 μg/mL | Taken separately, SeNPs and CS have shown fungicidal properties, but when combined (CS-SeNPs), achieved a potent inhibitory effect against the mature biofilm in a dose–response manner. | [212] |
| 59 | Na2SeO3 | SeNPs | biosynthesis with the leaf extract of Melia azedarach | 74 | Fungi: Fusarium mangiferae | FC | 300 μg/mL | Biogenic selenium NPs are widely expected to be efficient and cost-effective treatments for fungal plant diseases. | [213] |
| 60 | Na2SeO3 | SeNPs | green synthesis using extracts from A. glaucum leaves and C. officinalis flowers | 8 and 133 | Fungi: Fusarium oxysporum, Colletotrichum gloeosporioides | FS | 0.25 mg/mL | It was observed that both SeNPs had antifungal activity against both plant pathogens at concentrations of 0.25 mg/mL and above. SeNPs-AGL demonstrated better antifungal activity and smaller size (approximately 8.0 nm) than SeNPs-COF (134.0 nm). | [214] |
| 61 | Na2SeO3 | SeNPs | biosynthesis by Lactobacillus aci- dophilus ML1 | 46 | Fungi: Fusarium culmorum, Fusarium graminearum | FC | 100 mg/mL | Under greenhouse conditions, the wheat supplemented with BioSeNPs (100 mg/mL) experienced significantly incidence of crown and root rot diseases by 75% and considerably enhanced plant growth, grain quantity and quality by 5–40%. | [215] |
| 62 | SeCl4 | SeNPs | biosynthesis using endophytic fungus Fusarium oxysporum | 42 | Fungi: Aspergillus niger | FS | 8 mg/mL; diluted to 4, 2, 1, 0.5, and 0.25 mg/mL | SeNPs showed excellent antifungal and antisporulant activity against black fungus Aspergillus niger, which has become life-threatening to SARS-CoV-2 patients during the pandemic. | [127] |
| 63 | Na2SeO4 | SeNPs | biosynthesis with the use of Aspergillus strains | 64.8 | Fungi: Aspergillus fumigatus, Aspergillus flavus | FS | 0.5 µg/mL | The MIC of itraconazole and amphotericin B against A. fumigatus and A. flavus was 4 μg/mL, whereas the MIC values for treated samples with SeNPs have decreased to 0.5 μg/mL. | [216] |
| 64 | Na2SeO3 | SeNPs | biosynthesis using the extract of Melia azedarach leaves | 61 | Fungi: Puccinia striformis | FS | 30 mg/L | SeNPs at a concentration of 30 mg/L reduced the disease severity and enhanced the morphological, physiological, biochemical and antioxidant parameters. | [217] |
| 65 | Na2SeO3 | PPE/SeNPs and NCT/PPE/SeNPs | Pomegranate peel extract (PPE) used for biosynthesis | 9.4 85 | Fungi: Penicillium digitatum | FC | 22.5 15 mg/mL | NCT/PPE/SeNPs nanocomposite was the most effective and significantly exceeded the fungicidal action of standard fungicide. The direct treatment of fungal mycelia with NCT/PPE/SeNPs nanocomposite led to remarkable lysis and deformations of P. digitatum hyphae within 12 h of treatment. | [218] |
| 66 | H2SeO3 | SeNPs | Biosynthesis by Bacillus megaterium ATCC 55000 | 41.2 | Fungi: Rhizoctonia solani RCMB 031001 | FS, FC | 0.0625 and 1 mM | SeNPs improve morphological and metabolic indicators and yield significantly compared with infected control. | [219] |
| 67 | Na2SeO3 | SeNPs | chemical reduction method with the use of the Trichoderma atroviride cell culture lysate | 93.2–98.5 | Fungi: Pyricularia grisea, Colletotrichum capsici, Alternaria solani on chili and tomato leaves | FS | 50 μg/mL 100 μg/mL 100 μg/mL | The synthesized nanoparticles displayed excellent in vitro antifungal activity against Pyricularia grisea and inhibited the infection of Colletotrichum capsici and Alternaria solani on chili and tomato leaves. | [220] |
| 68 | Na2SeO3 | bovine serum albumin (BSA)/ SeNP, ascorbic acid/)/ SeNP, chitosan/SeNP, glucose/SeNP | chemical reduction method | 70–300 | Staphylococcus aureus (ATCC 6538), Enterococcus faecalis (ATCC 29212), Bacillus subtilis (ATCC 6633), and Kocuria rhizophila (ATCC 9341), Escherichia coli (ATCC 8739), Salmonella sp. (NCTC 6017), Klebsiella pneumoniae (NCIMB 9111), Pseudomonas aeruginosa (ATCC 9027), Fungi: Candida albicans (ATCC 10231) | BS, BC, FC | 100 μg/mL 100 μg/mL 200 μg/mL 200 μg/mL 400 μg/mL 400 μg/mL 200 μg/mL 400 μg/mL 25 μg/mL | Chitosan/SeNPs had greater antibacterial and antifungal activity than BSA/SeNPs and glucose/SeNPs. The MIC for Gram-positive bacteria was higher. | [221] |
| 69 | Na2SeO3 | TiO2-nanotubes/ SeNPs, AgNPs or Ag2SeNP | electrolysis of Na2SO3 | <10 | Staphylococcus epidermidis | BS | - | Nanocomposite reduced bacterial growth and biofilm formation. In comparison with the non-modifed control, the TiO2-nanotubes/SeNPs surfaces showed a signifcantly higher coverage area with osteoblastic MG-63-cells. | [177] |
| 70 | Na2SeO3 | TiO2-nanotubes/ SeNPs | Chemical reduction in presence of TiO2-nanotubes | <10 | Escherichia coli, Staphylococcus aureus | BS | - | Samples reduced the density of E. coli by 94.6% and of S. aureus by 89.6% compared to titanium controls. | [178] |
| 71 | Na2SeO3 | polycarbonate films/SeNPs | Chemical reduction by glutation | 50–100 | Staphylococcus aureus | BS | Polycarbonate films/SeNPs inhibited bacterial growth to 8.9% and 27% when compared with an uncoated polycarbonate surface after 24 and 72 h, respectively. |
3.3. Dependence of the Minimum Inhibitory Concentration of Selenium Nanoparticles on Their Size in the Study of Antifungal Activity
4. Mechanisms of Selenium Nanoparticle Antimicrobial Action
- (1)
- Degradation of proteins due to the bactericidal action of selenium nanoparticles [112].
- (2)
- Slow emission of selenium ions from the surface of nanoparticles can lead to their interaction with -SH, -NH or -COOH functional groups of proteins and enzymes and the subsequent loss of their tertiary and quaternary structure and functions [125].
- (3)
- SeNPs contribute to the inactivation of the natural mechanisms of membrane transport of ions and nutrients through the cell walls, which blocks the vital activity of the cell [225].
- (4)
- Hyperproduction of ROS, disturbance of membrane potential, and depletion of internal ATP [199].
- (5)
- Inhibition of the activity of the dehydrogenase enzyme, as well as destruction of the integrity of the cell membrane [125].
- (6)
- Inhibition of the ability of bacteria to attach to the surface and form bacterial films [146].
- (7)
- Photocatalytic action against bacteria [226].
5. Methods for Studying the Characteristics of Selenium Nanoparticles
6. Cytotoxicity to Eukaryotic Cells
7. Biomedical Applications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Minasyan, H. Sepsis: Mechanisms of bacterial injury to the patient. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-R.; Lee, J.H.; Park, K.S.; Kim, Y.B.; Jeong, B.C.; Lee, S.H. Global Dissemination of Carbapenemase-Producing Klebsiella pneumoniae: Epidemiology, Genetic Context, Treatment Options, and Detection Methods. Front. Microbiol. 2016, 7, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozgonyi, F.; Valenta, B.; Brátovics, A.; Csire, B. The sensitivity of “polyresistant” microorganisms to new antibiotics. Changes in the resistance to antibiotics of the more important pathogenic bacteria isolated from clinical specimens during 1962–1965. Orvosi Hetil. 1967, 108, 337–342. [Google Scholar]
- Abraham, E.P.; Chain, E. An Enzyme from Bacteria able to Destroy Penicillin. Nature 1940, 146, 837. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Gusyakova, O.A.; Lyamin, A.V.; Kezko, J.L.; Khaliulin, A.V.; Ereshchenko, A.A. Polyresistent microflora in the structure of microorganisms divided from blood of patients of the general hospital. Klin. Lab. Diagn. 2018, 63, 574–578. [Google Scholar]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef] [Green Version]
- Rowe, S.Y.; Rocourt, J.R.; Shiferaw, B.; Kassenborg, H.D.; Segler, S.D.; Marcus, R.; Daily, P.J.; Hardnett, F.P.; Slutsker, L. Breast-feeding decreases the risk of sporadic salmonellosis among infants in FoodNet sites. Clin. Infect. Dis. 2004, 38 (Suppl. 3), S262–S270. [Google Scholar] [CrossRef]
- Batz, M.B.; Henke, E.; Kowalcyk, B. Long-term consequences of foodborne infections. Infect. Dis. Clin. N. Am. 2013, 27, 599–616. [Google Scholar] [CrossRef]
- Kalyoussef, S.; Feja, K.N. Foodborne Illnesses. Adv. Pediatr. 2014, 61, 287–312. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, N.C.; Dambrosio, A. Helicobacter pylori: A foodborne pathogen? World J. Gastroenterol. 2018, 24, 3472–3487. [Google Scholar] [CrossRef]
- Posfay-Barbe, K.M.; Wald, E.R. Listeriosis. Semin. Fetal Neonatal Med. 2009, 14, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Salhotra, A.; Fleisher, J.; Uslan, D.Z. Listeria endocarditis in a patient with psoriatic arthritis on infliximab: Are biologic agents as treatment for inflammatory arthritis increasing the incidence of Listeria infections? J. Infect. 2010, 60, 386–396. [Google Scholar] [CrossRef]
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, Salmonellosis, Yersiniosis, and Listeriosis as Zoonotic Foodborne Diseases: A Review. Int. J. Environ. Res. Public Health 2018, 15, 863. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Khan, A.U. Global economic impact of antibiotic resistance: A review. J. Glob. Antimicrob. Resist. 2019, 19, 313–316. [Google Scholar] [CrossRef]
- Gebreyes, W.A.; Thakur, S. Multidrug-Resistant Salmonella enterica Serovar Muenchen from Pigs and Humans and Potential Interserovar Transfer of Antimicrobial Resistance. Antimicrob. Agents Chemother. 2005, 49, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endimiani, A.; Hujer, K.M.; Hujer, A.M.; Bertschy, I.; Rossano, A.; Koch, C.; Gerber, V.; Francey, T.; Bonomo, R.A.; Perreten, V. Acinetobacter baumannii isolates from pets and horses in Switzerland: Molecular characterization and clinical data. J. Antimicrob. Chemother. 2011, 66, 2248–2254. [Google Scholar] [CrossRef]
- Fodor, A.; Varga, I.; Hevesi, M.; Mathe-Fodor, A.; Racsko, J.; Hogan, A.J. Novel Anti-Microbial Peptides of Xenorhabdus Origin against Multidrug Resistant Plant Pathogens. In A Search for Antibacterial Agents; InTech: Singapore, 2012. [Google Scholar]
- Ahmed, S.A.; Barış, E.; Go, D.S.; Lofgren, H.; Osorio-Rodarte, I.; Thierfelder, K. Assessing the global poverty effects of antimicrobial resistance. World Dev. 2018, 111, 148–160. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bhullar, K.; Waglechner, N.; Pawlowski, A.; Koteva, K.; Banks, E.D.; Johnston, M.D.; Barton, H.A.; Wright, G.D. Antibiotic Resistance Is Prevalent in an Isolated Cave Microbiome. PLoS ONE 2012, 7, e34953. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Richmond, G.E.; Piddock, L.J.V. Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol. 2014, 9, 1165–1177. [Google Scholar] [CrossRef]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb. Perspect. Med. 2015, 5, a019752. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Pang, Z.; Li, M.; Lou, F.; An, X.; Zhu, S.; Song, L.; Tong, Y.; Fan, H.; Fan, J. Molnupiravir and Its Antiviral Activity against COVID-19. Front. Immunol. 2022, 13, 855496. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. Do Iron Oxide Nanoparticles Have Significant Antibacterial Properties? Antibiotics 2021, 10, 884. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Smirnova, V.V.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles. Nanomaterials 2022, 12, 2635. [Google Scholar] [CrossRef] [PubMed]
- Giedraitienė, A.; Ruzauskas, M.; Šiugždinienė, R.; Tučkutė, S.; Milcius, D. Antimicrobial Properties of CuO Particles Deposited on a Medical Mask. Materials 2022, 15, 7896. [Google Scholar] [CrossRef]
- Li, R.; Mansukhani, N.D.; Guiney, L.M.; Ji, Z.; Zhao, Y.; Chang, C.H.; French, C.T.; Miller, J.F.; Hersam, M.C.; Nel, A.E.; et al. Identification and Optimization of Carbon Radicals on Hydrated Graphene Oxide for Ubiquitous Antibacterial Coatings. ACS Nano 2016, 10, 10966–10980. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Ma, R.; Gao, M.; Tian, X.; Li, Y.-Q.; Zeng, L.; Li, R. Antibacterial applications of graphene oxides: Structure-activity relationships, molecular initiating events and biosafety. Sci. Bull. 2018, 63, 133–142. [Google Scholar] [CrossRef]
- Zheng, H.; Ji, Z.; Roy, K.R.; Gao, M.; Pan, Y.; Cai, X.; Wang, L.; Li, W.; Chang, C.H.; Kaweeteerawat, C.; et al. Engineered Graphene Oxide Nanocomposite Capable of Preventing the Evolution of Antimicrobial Resistance. ACS Nano 2019, 13, 11488–11499. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, H.; Li, G.; Li, Y.; Jiang, J.; Chen, J.; Xie, Q.; Wu, D.; Ma, R.; Liu, X.; et al. Antibiotic-Like Activity of Atomic Layer Boron Nitride for Combating Resistant Bacteria. ACS Nano 2022, 16, 7674–7688. [Google Scholar] [CrossRef]
- Xie, M.; Gao, M.; Yun, Y.; Malmsten, M.; Rotello, V.M.; Zboril, R.; Akhavan, O.; Kraskouski, A.; Amalraj, J.; Cai, X.; et al. Antibacterial Nanomaterials: Mechanisms, Impacts on Antimicrobial Resistance and Design Principles. Angew. Chem. Int. Ed. 2023, 62, e202217345. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Kuo, T.-R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Yougbare, S.; Chang, T.-K.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Kuo, T.-R. Antimicrobial Gold Nanoclusters: Recent Developments and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 2924. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.-H.; Wang, Y.-H.; Kuo, C.-H.; Ou, S.-F.; Huang, P.-Z.; Song, T.-Y.; Chen, Y.-C.; Chen, S.-T.; Wu, C.-H.; Hsueh, Y.-H.; et al. Hybrid ZnO/chitosan antimicrobial coatings with enhanced mechanical and bioactive properties for titanium implants. Carbohydr. Polym. 2021, 257, 117639. [Google Scholar] [CrossRef]
- Butler, K.S.; Peeler, D.J.; Casey, B.J.; Dair, B.J.; Elespuru, R.K. Silver nanoparticles: Correlating nanoparticle size and cellular uptake with genotoxicity. Mutagenesis 2015, 30, 577–591. [Google Scholar] [CrossRef]
- Shuguang, W.; Lawson, R.; Ray, P.C.; Hongtao, Y. Toxic effects of gold nanoparticles on Salmonella typhimurium bacteria. Toxicol. Ind. Health 2011, 27, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2017, 13, 65–71. [Google Scholar] [CrossRef]
- Li, X.Z.; Nikaido, H.; Williams, K.E. Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J. Bacteriol. 1997, 179, 6127–6132. [Google Scholar] [CrossRef] [Green Version]
- Niño-Martínez, N.; Salas Orozco, M.F.; Martínez-Castañón, G.-A.; Torres Méndez, F.; Ruiz, F. Molecular Mechanisms of Bacterial Resistance to Metal and Metal Oxide Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808. [Google Scholar] [CrossRef] [Green Version]
- Amaro, F.; Morón, Á.; Díaz, S.; Martín-González, A.; Gutiérrez, J.C. Metallic Nanoparticles-Friends or Foes in the Battle against Antibiotic-Resistant Bacteria? Microorganisms 2021, 9, 364. [Google Scholar] [CrossRef]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.; Köller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef] [Green Version]
- Napierska, D.; Thomassen, L.C.J.; Lison, D.; Martens, J.A.; Hoet, P.H. The nanosilica hazard: Another variable entity. Part. Fibre Toxicol. 2010, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Wang, J.; Jing, L.; Ma, R.; Liu, X.; Gao, L.; Cao, L.; Duan, J.; Zhou, X.; Li, Y.; et al. Mitochondrial dysfunction, perturbations of mitochondrial dynamics and biogenesis involved in endothelial injury induced by silica nanoparticles. Environ. Pollut. 2018, 236, 926–936. [Google Scholar] [CrossRef]
- Farooq, A.; Whitehead, D.; Azzawi, M. Attenuation of endothelial-dependent vasodilator responses, induced by dye-encapsulated silica nanoparticles, in aortic vessels. Nanomedicine 2014, 9, 413–425. [Google Scholar] [CrossRef]
- Maltseva, V.N.; Gudkov, S.; Turovsky, E. Modulation of the Functional State of Mouse Neutrophils by Selenium Nanoparticles In Vivo. Int. J. Mol. Sci. 2022, 23, 13651. [Google Scholar] [CrossRef]
- Varlamova, E.; Plotnikov, E.; Gudkov, S.; Turovsky, E. Size-Dependent Cytoprotective Effects of Selenium Nanoparticles during Oxygen-Glucose Deprivation in Brain Cortical Cells. Int. J. Mol. Sci. 2022, 23, 7464. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Khabatova, V.V.; Gudkov, S.V.; Plotnikov, E.Y.; Turovsky, E.A. Cytoprotective Properties of a New Nanocomplex of Selenium with Taxifolin in the Cells of the Cerebral Cortex Exposed to Ischemia/Reoxygenation. Pharmaceutics 2022, 14, 2477. [Google Scholar] [CrossRef]
- Varlamova, E.; Goltyaev, M.; Simakin, A.; Gudkov, S.; Turovsky, E. Comparative Analysis of the Cytotoxic Effect of a Complex of Selenium Nanoparticles Doped with Sorafenib, “Naked” Selenium Nanoparticles, and Sorafenib on Human Hepatocyte Carcinoma HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 6641. [Google Scholar] [CrossRef]
- Malyugina, S.; Skalickova, S.; Skladanka, J.; Slama, P.; Horky, P. Biogenic Selenium Nanoparticles in Animal Nutrition: A Review. Agriculture 2021, 11, 1244. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Xu, T. Elemental Selenium at Nano Size (Nano-Se) as a Potential Chemopreventive Agent with Reduced Risk of Selenium Toxicity: Comparison with Se-Methylselenocysteine in Mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, S.V.; Staroverov, S.A.; Skvortsova, N.I.; Soldatov, D.A.; Chekunov, M.A.; Silina, E.V.; Kozlov, E.S.; Artemev, D.A. Method of Obtaining a Veterinary Drug Based on Non-Specific Immunoglobulins and Colloidal Particles of Selenium for the Correction of the Immune System. RU Patent 2798268C1, 20 June 2023. [Google Scholar]
- Chen, C.; Hu, H.; Li, X.; Zheng, Z.; Wang, Z.; Wang, X.; Zheng, P.; Cui, F.; Li, G.; Wang, Y.; et al. Rapid Detection of Anti-SARS-CoV-2 Antibody Using a Selenium Nanoparticle-Based Lateral Flow Immunoassay. IEEE Trans. NanoBiosci. 2022, 21, 37–43. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Z.; Hu, H.; Zhou, Q.; Liu, W.; Li, X.; Liu, Z.; Wang, Y.; Ma, Y. A point-of-care selenium nanoparticle-based test for the combined detection of anti-SARS-CoV-2 IgM and IgG in human serum and blood. Lab A Chip 2020, 20, 4255–4261. [Google Scholar] [CrossRef]
- Wang, Q.; Webster, T.J. Nanostructured selenium for preventing biofilm formation on polycarbonate medical devices. J. Biomed. Mater. Res. Part A 2012, 100A, 3205–3210. [Google Scholar] [CrossRef]
- Wa, N.J. Method for Producing Hydrous Tissue Paper Having Antibacterial and Antifungal Functions. JP Patent 2011501977A, 12 April 2007. [Google Scholar]
- Webster, T.J.; Tran, P.A. Antipathogenic Surfaces Having Selenium Nanoclusters. U.S. Patent 9259005B2, 16 February 2016. [Google Scholar]
- Yeee, A.F.; Liang, L.; Ing, N.; Gibbs, M.; Dickson, M.N. Bactericidal Surface Patterns. U.S. Patent 10875235B2, 29 December 2020. [Google Scholar]
- Fang, M.; Zhang, H.; Wang, Y.; Zhang, H.; Zhang, D.; Xu, P. Biomimetic selenium nanosystems for infectious wound healing. Eng. Regen. 2023, 4, 152–160. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Tehmasebi-Foolad, A.; Rajabzadeh, A.; Beigi-Brojeni, N.; Zarei, L. Effects of Chitosan/Nano Selenium Biofilm on Infected Wound Healing in Rats; An Experimental Study. Bull. Emerg. Trauma 2019, 7, 284–291. [Google Scholar] [CrossRef]
- Huang, W.; Hu, B.; Yuan, Y.; Fang, H.; Jiang, J.; Li, Q.; Zhuo, Y.; Yang, X.; Wei, J.; Wang, X. Visible Light-Responsive Selenium Nanoparticles Combined with Sonodynamic Therapy to Promote Wound Healing. ACS Biomater. Sci. Eng. 2023, 9, 1341–1351. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef]
- Dipak, N.; Loveleen, K.; Harvinder, S.S.; Dharambeer, M.S.; Sonali, G.; Deepa, T. Application of Selenium Nanoparticles in Localized Drug Targeting for Cancer Therapy. Anti-Cancer Agents Med. Chem. 2022, 22, 2715–2725. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Liu, W.; Chen, T.; Li, Y.; Zheng, W.; Man, C.W.-Y.; Wong, M.-K.; Wong, K.-H. Surface decoration of selenium nanoparticles by mushroom polysaccharides–protein complexes to achieve enhanced cellular uptake and antiproliferative activity. J. Mater. Chem. 2012, 22, 9602–9610. [Google Scholar] [CrossRef]
- Tang, S.; Wang, T.; Jiang, M.; Huang, C.; Lai, C.; Fan, Y.; Yong, Q. Construction of arabinogalactans/selenium nanoparticles composites for enhancement of the antitumor activity. Int. J. Biol. Macromol. 2019, 128, 444–451. [Google Scholar] [CrossRef]
- Sun, D.; Liu, Y.; Yu, Q.; Qin, X.; Yang, L.; Zhou, Y.; Chen, L.; Liu, J. Inhibition of tumor growth and vasculature and fluorescence imaging using functionalized ruthenium-thiol protected selenium nanoparticles. Biomaterials 2014, 35, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhang, L.; Li, R.; Feng, F.; Wang, W.; Li, D.; Xiang, Q.; Yan, P. Folic Acid-Chitosan-Nano-Selenium Tumor Targeted Drug Delivery System and Preparation Method Thereof. CN Patent 111214460A, 02 June 2020. [Google Scholar]
- Xia, Y.; Zhao, M.; Chen, Y.; Hua, L.; Xu, T.; Wang, C.; Li, Y.; Zhu, B. Folate-targeted selenium nanoparticles deliver therapeutic siRNA to improve hepatocellular carcinoma therapy. RSC Adv. 2018, 8, 25932–25940. [Google Scholar] [CrossRef]
- Zou, J.; Su, S.; Chen, Z.; Liang, F.; Zeng, Y.; Cen, W.; Zhang, X.; Xia, Y.; Huang, D. Hyaluronic acid-modified selenium nanoparticles for enhancing the therapeutic efficacy of paclitaxel in lung cancer therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3456–3464. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, X.; Huang, Z.; Zheng, W.; Fan, C.; Chen, T. Enhancement of cell permeabilization apoptosis-inducing activity of selenium nanoparticles by ATP surface decoration. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 74–84. [Google Scholar] [CrossRef]
- Goltyaev, M.V.; Varlamova, E.G. The Role of Selenium Nanoparticles in the Treatment of Liver Pathologies of Various Natures. Int. J. Mol. Sci. 2023, 24, 10547. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Khabatova, V.V.; Gudkov, S.V.; Turovsky, E.A. Ca2+-Dependent Effects of the Selenium-Sorafenib Nanocomplex on Glioblastoma Cells and Astrocytes of the Cerebral Cortex: Anticancer Agent and Cytoprotector. Int. J. Mol. Sci. 2023, 24, 2411. [Google Scholar] [CrossRef]
- Jiang, P.I.; Cai, J.; Hua, J. Oridonin Functionalized Nanoparticles and Method of Preparation Thereof Selenium. U.S. Patent 962,423,7B2, 18 April 2017. [Google Scholar]
- Walsh, S.K.; Kamali, N.; McGrath, J.; Hogan, J.J.; Hanrahan, J.P. Multidimensional Application of Selenium Nanoparticles. 2023. Available online: https://glantreo.com/multidimensional-application-of-selenium-nanoparticles/ (accessed on 12 May 2023).
- Wu, A.; Hu, D.; Na, L.I.U.; Yu, S.; Yu, D.; Tang, Y.; Wang, Y. Trichoderma-Derived Selenium Nanoparticles Foliar Fertilizer for Reducing Crop Fungal Diseases and Toxin Contamination. U.S. Patent 10807920B2, 20 October 2020. [Google Scholar]
- Usmani, Z.; Kumar, A.; Tripti; Ahirwal, J.; Prasad, M.N.V. Chapter 20—Scope for Applying Transgenic Plant Technology for Remediation and Fortification of Selenium. In Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids; Prasad, M.N.V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 429–461. [Google Scholar]
- Wang, Q.; Yu, Y.; Li, J.; Wan, Y.; Huang, Q.; Guo, Y.; Li, H. Effects of Different Forms of Selenium Fertilizers on Se Accumulation, Distribution, and Residual Effect in Winter Wheat–Summer Maize Rotation System. J. Agric. Food Chem. 2017, 65, 1116–1123. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and Use of Selenium Nanoparticles as Fertilizers. ACS Omega 2020, 5, 17767–17774. [Google Scholar] [CrossRef]
- Shafeev, G.; Barmina, E.; Valiullin, L.; Simakin, A.; Ovsyankina, A.; Demin, D.; Kosolapov, V.; Korshunov, A.; Denisov, R. Soil fertilizer based on selenium nanoparticles. IOP Conf. Ser. Earth Environ. Sci. 2019, 390, 012041. [Google Scholar] [CrossRef]
- Hak, K.; Jong, L.; Hyo, K.; Gwang, L.; Jun, P.; Chan, L.; St, Y. Method for Cultivating High Quality and Functional Vegetable Fruit. KR Patent 101120635B1, 16 March 2012. [Google Scholar]
- Fouda, A.; Al-Otaibi, W.A.; Saber, T.; AlMotwaa, S.M.; Alshallash, K.S.; Elhady, M.; Badr, N.F.; Abdel-Rahman, M.A. Antimicrobial, Antiviral, and In-Vitro Cytotoxicity and Mosquitocidal Activities of Portulaca oleracea-Based Green Synthesis of Selenium Nanoparticles. J. Funct. Biomater. 2022, 13, 157. [Google Scholar] [CrossRef]
- Ahmed, F.; Dwivedi, S.; Shaalan, N.M.; Kumar, S.; Arshi, N.; Alshoaibi, A.; Husain, F.M. Development of Selenium Nanoparticle Based Agriculture Sensor for Heavy Metal Toxicity Detection. Agriculture 2020, 10, 610. [Google Scholar] [CrossRef]
- Dumore, N.S.; Mukhopadhyay, M. Sensitivity enhanced SeNPs-FTO electrochemical sensor for hydrogen peroxide detection. J. Electroanal. Chem. 2020, 878, 114544. [Google Scholar] [CrossRef]
- Mostafavi, E.; Medina-Cruz, D.; Truong, L.B.; Kaushik, A.; Iravani, S. Selenium-based nanomaterials for biosensing applications. Mater. Adv. 2022, 3, 7742–7756. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Habibey, R.; Shokri, F.; Hajmiresmail, S.J.; Akhavan, O.; Mashaghi, A.; Pazoki-Toroudi, H. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci. Rep. 2019, 9, 6044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ghazaly, M.A.; Fadel, N.; Rashed, E.; El-Batal, A.; Kenawy, S.A. Anti-inflammatory effect of selenium nanoparticles on the inflammation induced in irradiated rats. Can. J. Physiol. Pharmacol. 2017, 95, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, J.; Zhang, L. Hollow Sphere Selenium Nanoparticles: Their In-Vitro Anti Hydroxyl Radical Effect. Adv. Mater. 2002, 14, 290–293. [Google Scholar] [CrossRef]
- Torres, S.K.; Campos, V.L.; León, C.G.; Rodríguez-Llamazares, S.M.; Rojas, S.M.; González, M.; Smith, C.; Mondaca, M.A. Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J. Nanoparticle Res. 2012, 14, 1236. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Abd El-Maksoud, M.D.; Abdel Moneim, A.E.; Aglan, H.A. Pre-Clinical Study for the Antidiabetic Potential of Selenium Nanoparticles. Biol. Trace Elem. Res. 2016, 177, 267–280. [Google Scholar] [CrossRef]
- Gao, X.; Sun, Y. Selenium Nanoparticles with Improved Biological Effects. U.S. Patent 844,502,6B2, 21 May 2013. [Google Scholar]
- Peng, Y.; Peng, L.; Liu, T. Protein-Bound Nano-Selenium and Preparation Method and Application Thereof. CN Patent 108484715B, 11 March 2022. [Google Scholar]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Wilayat Husain, S. Synthesis of silica nanoparticles from sodium silicate under alkaline conditions. J. Sol-Gel Sci. Technol. 2016, 77, 753–758. [Google Scholar] [CrossRef]
- Kuddus, A.; Islam, R.; Tabassum, S.; Ismail, A.B. Synthesis of Si NPs from River Sand Using the Mechanochemical Process and its Applications in Metal Oxide Heterojunction Solar Cells. Silicon 2019, 12, 1723–1733. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Bai, X.; Jiang, T.; Zhang, Q.; Wang, S. Mesoporous Silica Nanoparticles for Increasing the Oral Bioavailability and Permeation of Poorly Water Soluble Drugs. Mol. Pharm. 2012, 9, 505–513. [Google Scholar] [CrossRef]
- Makarovsky, I.; Boguslavsky, Y.; Alesker, M.; Lellouche, J.; Banin, E.; Lellouche, J.-P. Novel Triclosan-Bound Hybrid-Silica Nanoparticles and their Enhanced Antimicrobial Properties. Adv. Funct. Mater. 2011, 21, 4295–4304. [Google Scholar] [CrossRef]
- Wu, S.-H.; Lin, Y.-S.; Hung, Y.; Chou, Y.-H.; Hsu, Y.-H.; Chang, C.; Mou, C.-Y. Multifunctional Mesoporous Silica Nanoparticles for Intracellular Labeling and Animal Magnetic Resonance Imaging Studies. ChemBioChem 2008, 9, 53–57. [Google Scholar] [CrossRef]
- Pandey, S.; Mewada, A.; Thakur, M.; Pillai, S.; Dharmatti, R.; Phadke, C.; Sharon, M. Synthesis of mesoporous silica oxide/C-dot complex (meso-SiO2/C-dots) using pyrolysed rice husk and its application in bioimaging. RSC Adv. 2014, 4, 1174–1179. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Wu, X.; Wu, X.; Maudgal, R.; Zhang, H.; Han, G. In Vivo Repeatedly Charging Near-Infrared-Emitting Mesoporous SiO2/ZnGa2O4:Cr3+ Persistent Luminescence Nanocomposites. Adv. Sci. 2015, 2, 1500001. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Tan, Y.; Xu, K.; Zhang, L.; Qiu, B.; Guo, L.; Lin, Z.; Chen, G. Stimulus-response mesoporous silica nanoparticle-based chemiluminescence biosensor for cocaine determination. Biosens. Bioelectron. 2016, 75, 8–14. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, H.; Yang, W.; Li, Y.; Sun, C. Gold nanoparticles-mesoporous silica composite used as an enzyme immobilization matrix for amperometric glucose biosensor construction. Sens. Actuators B Chem. 2007, 124, 179–186. [Google Scholar] [CrossRef]
- Moon, J.H.; McDaniel, W.; Hancock, L.F. Facile fabrication of poly(p-phenylene ethynylene)/colloidal silica composite for nucleic acid detection. J. Colloid Interface Sci. 2006, 300, 117–122. [Google Scholar] [CrossRef]
- Boora, R.; Sheoran, P.; Rani, N.; Kumari, S.; Thakur, R.; Grewal, S. Biosynthesized Silica Nanoparticles (Si NPs) Helps in Mitigating Drought Stress in Wheat Through Physiological Changes and Upregulation of Stress Genes. Silicon 2023, 1–13. [Google Scholar] [CrossRef]
- Li, L.-L.; Wang, H. Enzyme-Coated Mesoporous Silica Nanoparticles as Efficient Antibacterial Agents In Vivo. Adv. Healthc. Mater. 2013, 2, 1351–1360. [Google Scholar] [CrossRef]
- González, B.; Colilla, M.; Díez, J.; Pedraza, D.; Guembe, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous silica nanoparticles decorated with polycationic dendrimers for infection treatment. Acta Biomater. 2018, 68, 261–271. [Google Scholar] [CrossRef]
- Díaz-García, D.; Ardiles, P.R.; Díaz-Sánchez, M.; Mena-Palomo, I.; del Hierro, I.; Prashar, S.; Rodríguez-Diéguez, A.; Páez, P.L.; Gómez-Ruiz, S. Copper-functionalized nanostructured silica-based systems: Study of the antimicrobial applications and ROS generation against gram positive and gram negative bacteria. J. Inorg. Biochem. 2020, 203, 110912. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.-H.; Jeong, H.; Hong, J.; Choi, W.S.; Lee, B.-H.; Park, C.Y. An Evaluation of the in vivo Safety of Nonporous Silica Nanoparticles: Ocular Topical Administration versus Oral Administration. Sci. Rep. 2017, 7, 8238. [Google Scholar] [CrossRef] [Green Version]
- An, S.S.A.; Ryu, H.J.; Seong, N.-W.; So, B.J.; Seo, H.-S.; Kim, J.-H.; Hong, J.-S.; Park, M.-K.; Kim, M.-S.; Kim, Y.-R.; et al. Evaluation of silica nanoparticle toxicity after topical exposure for 90 days. Int. J. Nanomed. 2014, 9, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Han, H.-W.; Patel, K.D.; Kwak, J.-H.; Jun, S.-K.; Jang, T.-S.; Lee, S.-H.; Knowles, J.C.; Kim, H.-W.; Lee, H.-H.; Lee, J.-H. Selenium Nanoparticles as Candidates for Antibacterial Substitutes and Supplements against Multidrug-Resistant Bacteria. Biomolecules 2021, 11, 1028. [Google Scholar] [CrossRef]
- Geoffrion, L.D.; Hesabizadeh, T.; Medina-Cruz, D.; Kusper, M.; Taylor, P.; Vernet-Crua, A.; Chen, J.; Ajo, A.; Webster, T.J.; Guisbiers, G. Naked Selenium Nanoparticles for Antibacterial and Anticancer Treatments. ACS Omega 2020, 5, 2660–2669. [Google Scholar] [CrossRef]
- Hou, J.; Tamura, Y.; Lu, H.-Y.; Takahashi, Y.; Kasugai, S.; Nakata, H.; Kuroda, S. An In Vitro Evaluation of Selenium Nanoparticles on Osteoblastic Differentiation and Antimicrobial Properties against Porphyromonas gingivalis. Nanomaterials 2022, 12, 1850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Zhang, L.; Lei, Z.; Jin, L.; Cao, J.; Quan, C. High-Efficiency Reducing Strain for Producing Selenium Nanoparticles Isolated from Marine Sediment. Int. J. Mol. Sci. 2022, 23, 11953. [Google Scholar] [CrossRef] [PubMed]
- Afzal, B.; Yasin, D.; Naaz; Sami, N.; Zaki, A.; Kumar, R.; Srivastava, P.; Fatma, T. Biomedical potential of Anabaena variabilis NCCU-44 based Selenium nanoparticles and their comparison with commercial SeNPs. Sci. Rep. 2021, 11, 13507. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, S.; Tahan, M.; Sadeghian, H.; Nazari, R.; Behmadi, M.; Hosseini Bafghi, M. Effect of biosynthesized selenium nanoparticles using Nepeta extract against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. J. Basic Microbiol. 2023, 63, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, J.; Xu, J.-F.; Pi, J. The Advancing of Selenium Nanoparticles Against Infectious Diseases. Front. Pharmacol. 2021, 12, 682284. [Google Scholar] [CrossRef]
- Martínez-Esquivias, F.; Guzmán-Flores, J.M.; Pérez-Larios, A.; González Silva, N.; Becerra-Ruiz, J.S. A Review of the Antimicrobial Activity of Selenium Nanoparticles. J. Nanosci. Nanotechnol. 2021, 21, 5383–5398. [Google Scholar] [CrossRef]
- Kopel, J.; Fralick, J.; Reid, T.W. The Potential Antiviral Effects of Selenium Nanoparticles and Coated Surfaces. Antibiotics 2022, 11, 1683. [Google Scholar] [CrossRef]
- Vahdati, M.; Tohidi Moghadam, T. Synthesis and Characterization of Selenium Nanoparticles-Lysozyme Nanohybrid System with Synergistic Antibacterial Properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef] [Green Version]
- Abou Elmaaty, T.; Sayed-Ahmed, K.; Mohamed Ali, R.; El-Khodary, K.; Abdeldayem, S.A. Simultaneous Sonochemical Coloration and Antibacterial Functionalization of Leather with Selenium Nanoparticles (SeNPs). Polymers 2022, 14, 74. [Google Scholar] [CrossRef]
- Kora, A.J. Tree gum stabilised selenium nanoparticles: Characterisation and antioxidant activity. IET Nanobiotechnol. 2018, 12, 658–662. [Google Scholar] [CrossRef]
- Dang-Bao, T.; Ho, T.G.-T.; Do, B.L.; Phung Anh, N.; Phan, T.D.T.; Tran, T.B.Y.; Duong, N.L.; Hong Phuong, P.; Nguyen, T. Green Orange Peel-Mediated Bioinspired Synthesis of Nanoselenium and Its Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus. ACS Omega 2022, 7, 36037–36046. [Google Scholar] [CrossRef]
- Lesnichaya, M.; Perfileva, A.; Nozhkina, O.; Gazizova, A.; Graskova, I. Synthesis, toxicity evaluation and determination of possible mechanisms of antimicrobial effect of arabinogalactane-capped selenium nanoparticles. J. Trace Elem. Med. Biol. 2022, 69, 126904. [Google Scholar] [CrossRef]
- Hosseini Bafghi, M.; Darroudi, M.; Zargar, M.; Zarrinfar, H.; Nazari, R. Biosynthesis of selenium nanoparticles by Aspergillus flavus and Candida albicans for antifungal applications. Micro Nano Lett. 2021, 16, 12096. [Google Scholar] [CrossRef]
- Islam, S.N.; Naqvi, S.M.A.; Raza, A.; Jaiswal, A.; Singh, A.K.; Dixit, M.; Barnwal, A.; Gambhir, S.; Ahmad, A. Mycosynthesis of highly fluorescent selenium nanoparticles from Fusarium oxysporum, their antifungal activity against black fungus Aspergillus niger, and in-vivo biodistribution studies. 3 Biotech 2022, 12, 309. [Google Scholar] [CrossRef]
- Jadhav, A.A.; Khanna, P.K. Impact of microwave irradiation on cyclo-octeno-1,2,3-selenadiazole: Formation of selenium nanoparticles and their polymorphs. RSC Adv. 2015, 5, 44756–44763. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Turovsky, E.A.; Blinova, E.V. Therapeutic Potential and Main Methods of Obtaining Selenium Nanoparticles. Int. J. Mol. Sci. 2021, 22, 10808. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Bisht, N.; Singh, P. Selenium Nanoparticles: A Review on Synthesis and Biomedical Applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Pandey, S.; Awasthee, N.; Shekher, A.; Rai, L.C.; Gupta, S.C.; Dubey, S.K. Biogenic synthesis and characterization of selenium nanoparticles and their applications with special reference to antibacterial, antioxidant, anticancer and photocatalytic activity. Bioprocess Biosyst. Eng. 2021, 44, 2679–2696. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Fouda, M.M.G.; Fouda, A.; Awad, M.A.; Al-Olayan, E.M.; Allam, A.A.; Shaheen, T.I. Antibacterial, Cytotoxicity and Larvicidal Activity of Green Synthesized Selenium Nanoparticles Using Penicillium corylophilum. J. Clust. Sci. 2021, 32, 351–361. [Google Scholar] [CrossRef]
- Kis-Csitári, J.; Kónya, Z.; Kiricsi, I. Sonochemical Synthesis of Inorganic Nanoparticles. In Functionalized Nanoscale Materials, Devices and Systems; NATO Science for Peace and Security Series B: Physics and Biophysics; Springer: Berlin/Heidelberg, Germany, 2008; pp. 369–372. [Google Scholar]
- Shar, A.H.; Lakhan, M.N.; Wang, J.; Ahmed, M.; Alali, K.T.; Ahmed, R.; Ali, I.; Dayo, A.Q. Facile synthesis and characterization of selenium nanoparticles by the hydrothermal approach. Dig. J. Nanomater. Biostruct. 2019, 14, 867–872. [Google Scholar]
- Aditha, S.K.; Kurdekar, A.D.; Chunduri, L.A.A.; Patnaik, S.; Kamisetti, V. Aqueous based reflux method for green synthesis of nanostructures: Application in CZTS synthesis. MethodsX 2016, 3, 35–42. [Google Scholar] [CrossRef]
- Alhawiti, A. Citric acid-mediated green synthesis of selenium nanoparticles: Antioxidant, antimicrobial, and anticoagulant potential applications. Biomass Convers. Biorefinery 2022, 1–10. [Google Scholar] [CrossRef]
- Mellinas, C.; Jiménez, A.; Garrigós, M.D.C. Microwave-Assisted Green Synthesis and Antioxidant Activity of Selenium Nanoparticles Using Theobroma cacao L. Bean Shell Extract. Molecules 2019, 24, 4048. [Google Scholar] [CrossRef] [Green Version]
- Hien, N.Q.; Tuan, P.D.; Phu, D.V.; Quoc, L.A.; Lan, N.T.K.; Duy, N.N.; Hoa, T.T. Gamma Co-60 ray irradiation synthesis of dextran stabilized selenium nanoparticles and their antioxidant activity. Mater. Chem. Phys. 2018, 205, 29–34. [Google Scholar] [CrossRef]
- Clifford, D.M.; Castano, C.E.; Rojas, J.V. Supported transition metal nanomaterials: Nanocomposites synthesized by ionizing radiation. Radiat. Phys. Chem. 2017, 132, 52–64. [Google Scholar] [CrossRef]
- Amin, B.H.; Ahmed, H.Y.; El Gazzar, E.M.; Badawy, M.M.M. Enhancement the Mycosynthesis of Selenium Nanoparticles by Using Gamma Radiation. Dose-Response 2021, 19, 15593258211059323. [Google Scholar] [CrossRef]
- Mosallam, F.M.; El-Sayyad, G.S.; Fathy, R.M.; El-Batal, A.I. Biomolecules-mediated synthesis of selenium nanoparticles using Aspergillus oryzae fermented Lupin extract and gamma radiation for hindering the growth of some multidrug-resistant bacteria and pathogenic fungi. Microb. Pathog. 2018, 122, 108–116. [Google Scholar] [CrossRef]
- Ayyyzhy, K.; Voronov, V.; Gudkov, S.; Rakov, I.; Simakin, A.; Shafeev, G. Laser Fabrication and Fragmentation of Selenium Nanoparticles in Aqueous Media. Phys. Wave Phenom. 2019, 27, 113–118. [Google Scholar] [CrossRef]
- Shafeev, G.A.; Barmina, E.V.; Pimpha, N.; Rakov, I.I.; Simakin, A.V.; Sharapov, M.G.; Uvarov, O.V.; Gudkov, S.V. Laser generation and fragmentation of selenium nanoparticles in water and their testing as an additive to fertilisers. Quantum Electron. 2021, 51, 615–618. [Google Scholar] [CrossRef]
- Vasileiadis, T.; Dracopoulos, V.; Kollia, M.; Sygellou, L.; Yannopoulos, S.N. Synthesis of t-Te and a-Se nanospheres using continuous wave visible light. J. Nanoparticle Res. 2019, 21, 218. [Google Scholar] [CrossRef]
- Vorozhtsov, A.; Goncharova, D.; Gavrilenko, E.; Nemoykina, A.; Svetlichnyi, V. Antibacterial activity of zinc oxide nanoparticles obtained by pulsed laser ablation in water and air. MATEC Web Conf. 2018, 243, 00017. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Zhang, S.; Gadd, G.M.; McGrath, J.; Rooney, D.W.; Zhao, Q. Fungal-derived selenium nanoparticles and their potential applications in electroless silver coatings for preventing pin-tract infections. Regen. Biomater. 2022, 9, rbac013. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.; Eid, A.; Abdel-Rahman, M.; Hamza, M. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, Penicillium crustosum EP-1. Sci. Rep. 2022, 12, 11834. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Naveen, K.V.; Wang, M.-H. Enhancement of anti-bacterial potential of green synthesized selenium nanoparticles by starch encapsulation. Microb. Pathog. 2022, 167, 105544. [Google Scholar] [CrossRef]
- Hashem, A.H.; Khalil, A.M.A.; Reyad, A.M.; Salem, S.S. Biomedical Applications of Mycosynthesized Selenium Nanoparticles Using Penicillium expansum ATTC 36200. Biol. Trace Elem. Res. 2021, 199, 3998–4008. [Google Scholar] [CrossRef]
- Hashem, A.H.; Salem, S.S. Green and ecofriendly biosynthesis of selenium nanoparticles using Urtica dioica (stinging nettle) leaf extract: Antimicrobial and anticancer activity. Biotechnol. J. 2022, 17, 2100432. [Google Scholar] [CrossRef]
- Souza, L.M.d.S.; Dibo, M.; Sarmiento, J.J.P.; Seabra, A.B.; Medeiros, L.P.; Lourenço, I.M.; Kobayashi, R.K.T.; Nakazato, G. Biosynthesis of selenium nanoparticles using combinations of plant extracts and their antibacterial activity. Curr. Res. Green Sustain. Chem. 2022, 5, 100303. [Google Scholar] [CrossRef]
- Shah, V.; Medina-Cruz, D.; Vernet-Crua, A.; Truong, L.B.; Sotelo, E.; Mostafavi, E.; González, M.U.; García-Martín, J.M.; Cholula-Díaz, J.L.; Webster, T.J. Pepper-Mediated Green Synthesis of Selenium and Tellurium Nanoparticles with Antibacterial and Anticancer Potential. J. Funct. Biomater. 2023, 14, 24. [Google Scholar] [CrossRef]
- Nikam, P.B.; Salunkhe, J.D.; Minkina, T.; Rajput, V.D.; Kim, B.S.; Patil, S.V. A review on green synthesis and recent applications of red nano Selenium. Results Chem. 2022, 4, 100581. [Google Scholar] [CrossRef]
- ElSaied, B.E.F.; Diab, A.M.; Tayel, A.A.; Alghuthaymi, M.A.; Moussa, S.H. Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract. Green Process. Synth. 2021, 10, 49–60. [Google Scholar] [CrossRef]
- Mulla, N.A.; Otari, S.V.; Bohara, R.A.; Yadav, H.M.; Pawar, S.H. Rapid and size-controlled biosynthesis of cytocompatible selenium nanoparticles by Azadirachta indica leaves extract for antibacterial activity. Mater. Lett. 2020, 264, 127353. [Google Scholar] [CrossRef]
- Pansare, A.V.; Kulal, D.K.; Shedge, A.A.; Patil, V.R. hsDNA groove binding, photocatalytic activity, and in vitro breast and colon cancer cell reducing function of greener SeNPs. Dalton Trans. 2016, 45, 12144–12155. [Google Scholar] [CrossRef] [PubMed]
- Vyas, J.; Rana, S. Antioxidant activity and green synthesis of selenium nanoparticles using allium sativum extract. Int. J. Phytomed. 2017, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Qiao, L.; Guo, Y.; Ma, L.; Cheng, Y. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr. Polym. 2018, 195, 576–585. [Google Scholar] [CrossRef]
- Khiralla, G.M.; El-Deeb, B.A. Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT-Food Sci. Technol. 2015, 63, 1001–1007. [Google Scholar] [CrossRef]
- Ali, E.N.; El-Sonbaty, S.M.; Salem, F.M. Evaluation of selenium nanoparticles as a potential chemopreventive agent against lung carcinoma. Int. J. Pharmacol. Biol. Sci. 2013, 2, 38–46. [Google Scholar]
- Zhang, W.; Chen, Z.; Liu, H.; Zhang, L.; Gao, P.; Li, D. Biosynthesis and structural characteristics of selenium nanoparticles by Pseudomonas alcaliphila. Colloids Surf. B Biointerfaces 2011, 88, 196–201. [Google Scholar] [CrossRef]
- Yazdi, M.H.; Mahdavi, M.; Faghfuri, E.; Faramarzi, M.A.; Sepehrizadeh, Z.; Mohammad Hassan, Z.; Gholami, M.; Shahverdi, A.R. Th1 Immune Response Induction by Biogenic Selenium Nanoparticles in Mice with Breast Cancer: Preliminary Vaccine Model. Iran. J. Biotechnol. 2015, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhou, H.; Bai, J.; Li, Y.; Yang, J.; Ma, Q.; Qu, Y. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surf. A Physicochem. Eng. Asp. 2019, 571, 9–16. [Google Scholar] [CrossRef]
- Hariharan, N.; Al-Harbi, P.; Karuppiah, S.R. Microbial synthesis of selenium nanocomposite using Saccharomyces cerevisiae and its antimicrobial activity against pathogens causing nosocomial infection. Chalcogenide Lett. 2012, 9, 509–515. [Google Scholar]
- Lian, S.; Diko, C.S.; Yan, Y.; Li, Z.; Zhang, H.; Ma, Q.; Qu, Y. Characterization of biogenic selenium nanoparticles derived from cell-free extracts of a novel yeast Magnusiomyces ingens. 3 Biotech 2019, 9, 221. [Google Scholar] [CrossRef]
- Song, X.; Chen, Y.; Sun, H.; Liu, X.; Leng, X. Physicochemical and functional properties of chitosan-stabilized selenium nanoparticles under different processing treatments. Food Chem. 2020, 331, 127378. [Google Scholar] [CrossRef] [PubMed]
- Ndwandwe, B.K.; Malinga, S.P.; Kayitesi, E.; Dlamini, B.C. Solvothermal synthesis of selenium nanoparticles with polygonal-like nanostructure and antibacterial potential. Mater. Lett. 2021, 304, 130619. [Google Scholar] [CrossRef]
- Youssef, D.M.; Alshubaily, F.A.; Tayel, A.A.; Alghuthaymi, M.A.; Al-Saman, M.A. Application of Nanocomposites from Bees Products and Nano-Selenium in Edible Coating for Catfish Fillets Biopreservation. Polymers 2022, 14, 2378. [Google Scholar] [CrossRef]
- Salem, S.S.; Badawy, M.S.E.M.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Hashem, A.H. Green Biosynthesis of Selenium Nanoparticles Using Orange Peel Waste: Characterization, Antibacterial and Antibiofilm Activities against Multidrug-Resistant Bacteria. Life 2022, 12, 893. [Google Scholar] [CrossRef]
- Niranjan, R.; Zafar, S.; Lochab, B.; Priyadarshini, R. Synthesis and Characterization of Sulfur and Sulfur-Selenium Nanoparticles Loaded on Reduced Graphene Oxide and Their Antibacterial Activity against Gram-Positive Pathogens. Nanomaterials 2022, 12, 191. [Google Scholar] [CrossRef]
- Dorazilová, J.; Muchová, J.; Šmerková, K.; Kočiová, S.; Diviš, P.; Kopel, P.; Veselý, R.; Pavliňáková, V.; Adam, V.; Vojtová, L. Synergistic Effect of Chitosan and Selenium Nanoparticles on Biodegradation and Antibacterial Properties of Collagenous Scaffolds Designed for Infected Burn Wounds. Nanomaterials 2020, 10, 1971. [Google Scholar] [CrossRef]
- Muchová, J.; Hearnden, V.; Michlovská, L.; Vištejnová, L.; Zavaďáková, A.; Šmerková, K.; Kočiová, S.; Adam, V.; Kopel, P.; Vojtová, L. Mutual influence of selenium nanoparticles and FGF2-STAB® on biocompatible properties of collagen/chitosan 3D scaffolds: In vitro and ex ovo evaluation. J. Nanobiotechnol. 2021, 19, 103. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.A.; Reynolds, E.C.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Multifunctional Antimicrobial Polypeptide-Selenium Nanoparticles Combat Drug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2020, 12, 55696–55709. [Google Scholar] [CrossRef] [PubMed]
- Abou Elmaaty, T.; Sayed-Ahmed, K.; Elsisi, H.; Ramadan, S.M.; Sorour, H.; Magdi, M.; Abdeldayem, S.A. Novel Antiviral and Antibacterial Durable Polyester Fabrics Printed with Selenium Nanoparticles (SeNPs). Polymers 2022, 14, 955. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.G.; El-Sayed, E.-S.R.; Younis, N.A.; Hamdy, A.E.H.A.; Easa, S.M. Harnessing endophytic fungi for biosynthesis of selenium nanoparticles and exploring their bioactivities. AMB Express 2022, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Bilek, O.; Fohlerová, Z.; Hubalek, J. Enhanced antibacterial and anticancer properties of Se-NPs decorated TiO2 nanotube film. PLoS ONE 2019, 14, 0214066. [Google Scholar] [CrossRef] [Green Version]
- Staats, K.; Pilz, M.; Sun, J.; Boiadjieva-Scherzer, T.; Kronberger, H.; Tobudic, S.; Windhager, R.; Holinka, J. Antimicrobial potential and osteoblastic cell growth on electrochemically modified titanium surfaces with nanotubes and selenium or silver incorporation. Sci. Rep. 2022, 12, 8298. [Google Scholar] [CrossRef]
- Liu, W.; Golshan, N.H.; Deng, X.; Hickey, D.J.; Zeimer, K.; Li, H.; Webster, T.J. Selenium nanoparticles incorporated into titania nanotubes inhibit bacterial growth and macrophage proliferation. Nanoscale 2016, 8, 15783–15794. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, D.; Su, J.; Zheng, R.; Ning, Z.; Zhao, M.; Zhu, B.; Li, Y. Selenium nanoparticles inhibited H1N1 influenza virus-induced apoptosis by ROS-mediated signaling pathways. RSC Adv. 2022, 12, 3862–3870. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Chen, D.; Zhao, M.; Lin, Z.; Guo, M.; Xu, T.; Chen, Y.; Hua, L.; Lin, T.; et al. The Inhibition of H1N1 Influenza Virus-Induced Apoptosis by Surface Decoration of Selenium Nanoparticles with β-Thujaplicin through Reactive Oxygen Species-Mediated AKT and p53 Signaling Pathways. ACS Omega 2020, 5, 30633–30642. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Gong, G.; Xia, Y.; Wang, C.; Chen, Y.; Hua, L.; Zhong, J.; Tang, Y.; Liu, X.; et al. Restriction of H1N1 influenza virus infection by selenium nanoparticles loaded with ribavirin via resisting caspase-3 apoptotic pathway. Int. J. Nanomed. 2018, 13, 5787–5797. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lin, Z.; Guo, M.; Zhao, M.; Xia, Y.; Wang, C.; Xu, T.; Zhu, B. Inhibition of H1N1 influenza virus-induced apoptosis by functionalized selenium nanoparticles with amantadine through ROS-mediated AKT signaling pathways. Int. J. Nanomed. 2018, 13, 2005–2016. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Xia, Y.; Hua, L.; Liu, X.; Xiao, M.; Xu, T.; Zhu, B.; Cao, H. Functionalized selenium nanoparticles enhance the anti-EV71 activity of oseltamivir in human astrocytoma cell model. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3485–3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Li, Y.; Xu, T.; Guo, M.; Wang, C.; Zhao, M.; Chen, H.; Kuang, J.; Li, W.; Zhang, Y.; et al. Inhibition of Enterovirus 71 by Selenium Nanoparticles Loaded with siRNA through Bax Signaling Pathways. ACS Omega 2020, 5, 12495–12500. [Google Scholar] [CrossRef]
- Makhlof, M.E.M.; Albalwe, F.M.; Al-Shaikh, T.M.; El-Sheekh, M.M. Suppression Effect of Ulva lactuca Selenium Nanoparticles (USeNPs) on HepG2 Carcinoma Cells Resulting from Degradation of Epidermal Growth Factor Receptor (EGFR) with an Evaluation of Its Antiviral and Antioxidant Activities. Appl. Sci. 2022, 12, 11546. [Google Scholar] [CrossRef]
- Touliabah, H.E.; El-Sheekh, M.M.; Makhlof, M.E.M. Evaluation of Polycladia myrica mediated selenium nanoparticles (PoSeNPS) cytotoxicity against PC-3 cells and antiviral activity against HAV HM175 (Hepatitis A), HSV-2 (Herpes simplex II), and Adenovirus strain 2. Front. Mar. Sci. 2022, 9, 1092343. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Guo, M.; Xia, Y.; Zhao, M.; Wang, C.; Xu, T.; Chen, T.; Zhu, B. Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int. J. Nanomed. 2017, 12, 5733–5743. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, Z.; Dai, C.; Wang, P.; Fan, S.; Yu, B.; Qu, Y. Antibacterial properties and mechanism of selenium nanoparticles synthesized by Providencia sp. DCX. Environ. Res. 2021, 194, 110630. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wang, L.; Zhang, M.; Ullah, M.; Liu, L.; Zhao, W.; Li, Y.; Ahmed, A.A.Q.; Cheng, H.; Shi, Z.; et al. In Situ Synthesized Selenium Nanoparticles-Decorated Bacterial Cellulose/Gelatin Hydrogel with Enhanced Antibacterial, Antioxidant, and Anti-Inflammatory Capabilities for Facilitating Skin Wound Healing. Adv. Healthc. Mater. 2021, 10, 2100402. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Boaretti, M.; Vandecandelaere, I.; Zonaro, E.; Coenye, T.; Lleo, M.M.; Lampis, S.; Vallini, G. Biogenic selenium nanoparticles synthesized by Stenotrophomonas maltophilia SeITE02 loose antibacterial and antibiofilm efficacy as a result of the progressive alteration of their organic coating layer. Microb. Biotechnol. 2018, 11, 1037–1047. [Google Scholar] [CrossRef] [Green Version]
- Abbas, H.S.; Abou Baker, D.H.; Ahmed, E.A. Cytotoxicity and antimicrobial efficiency of selenium nanoparticles biosynthesized by Spirulina platensis. Arch. Microbiol. 2021, 203, 523–532. [Google Scholar] [CrossRef]
- Pekarkova, J.; Gablech, I.; Fialova, T.; Bilek, O.; Fohlerova, Z. Modifications of Parylene by Microstructures and Selenium Nanoparticles: Evaluation of Bacterial and Mesenchymal Stem Cell Viability. Front. Bioeng. Biotechnol. 2021, 9, 782799. [Google Scholar] [CrossRef]
- Elakraa, A.; Salah Salem, S.; El-Sayyad, G.; Salah Attia, M. Cefotaxime incorporated bimetallic silver-selenium nanoparticles: Promising antimicrobial synergism, antibiofilm activity, and bacterial membrane leakage reaction mechanism. RSC Adv. 2022, 12, 26603–26619. [Google Scholar] [CrossRef]
- Galkina, K.V.; Zubareva, V.M.; Kashko, N.D.; Lapashina, A.S.; Markova, O.V.; Feniouk, B.A.; Knorre, D.A. Heterogeneity of Starved Yeast Cells in IF1 Levels Suggests the Role of This Protein in vivo. Front. Microbiol. 2022, 13, 816622. [Google Scholar] [CrossRef]
- Hyrslova, I.; Kaňa, A.; Kantorova, V.; Krausova, G.; Mrvikova, I.; Doskocil, I. Selenium accumulation and biotransformation in Streptococcus, Lactococcus, and Enterococcus strains. J. Funct. Foods 2022, 92, 105056. [Google Scholar] [CrossRef]
- Tendenedzai, J.T.; Chirwa, E.M.N.; Brink, H.G. Enterococcus spp. Cell-Free Extract: An Abiotic Route for Synthesis of Selenium Nanoparticles (SeNPs), Their Characterisation and Inhibition of Escherichia coli. Nanomaterials 2022, 12, 658. [Google Scholar] [CrossRef]
- Tran, P.A.; O’Brien-Simpson, N.; Reynolds, E.C.; Pantarat, N.; Biswas, D.P.; O’Connor, A.J. Low cytotoxic trace element selenium nanoparticles and their differential antimicrobial properties against S. aureus and E. coli. Nanotechnology 2016, 27, 045101. [Google Scholar] [CrossRef]
- Chandramohan, S.; Sundar, K.; Muthukumaran, A. Hollow selenium nanoparticles from potato extract and investigation of its biological properties and developmental toxicity in zebrafish embryos. IET Nanobiotechnol. 2019, 13, 275–281. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.A.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale 2019, 11, 14937–14951. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Zhou, R.; Webster, T. Green Synthesized BSA-Coated Selenium Nanoparticles Inhibit Bacterial Growth While Promoting Mammalian Cell Growth. Int. J. Nanomed. 2020, 15, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Kumari, S.; Herold, H.; Bargel, H.; Aigner, T.; Heath, D.; O’Brien-Simpson, N.; O’Connor, A.; Scheibel, T. Enhanced Antibacterial Activity of Se Nanoparticles Upon Coating with Recombinant Spider Silk Protein eADF4(κ16). Int. J. Nanomed. 2020, 2020, 4275–4288. [Google Scholar] [CrossRef]
- El-Sayyad, G.S.; El-Bastawisy, H.S.; Gobara, M.; El-Batal, A.I. Gentamicin-Assisted Mycogenic Selenium Nanoparticles Synthesized Under Gamma Irradiation for Robust Reluctance of Resistant Urinary Tract Infection-Causing Pathogens. Biol. Trace Elem. Res. 2020, 195, 323–342. [Google Scholar] [CrossRef]
- Guisbiers, G.; Wang, Q.; Khachatryan, E.; Mimun, L.; Mendoza-Cruz, R.; Larese-Casanova, P.; Webster, T.; Nash, K. Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int. J. Nanomed. 2016, 11, 3731–3736. [Google Scholar] [CrossRef] [Green Version]
- Shahmoradi, S.; Shariati, A.; Amini, S.M.; Zargar, N.; Yadegari, Z.; Darban-Sarokhalil, D. The application of selenium nanoparticles for enhancing the efficacy of photodynamic inactivation of planktonic communities and the biofilm of Streptococcus mutans. BMC Res. Notes 2022, 15, 84. [Google Scholar] [CrossRef]
- Hernández-Díaz, J.A.; Garza-García, J.J.; León-Morales, J.M.; Zamudio-Ojeda, A.; Arratia-Quijada, J.; Velázquez-Juárez, G.; López-Velázquez, J.C.; García-Morales, S. Antibacterial Activity of Biosynthesized Selenium Nanoparticles Using Extracts of Calendula officinalis against Potentially Clinical Bacterial Strains. Molecules 2021, 26, 5929. [Google Scholar] [CrossRef]
- Mansouri-Tehrani, H.A.; Keyhanfar, M.; Behbahani, M.; Dini, G. Synthesis and characterization of algae-coated selenium nanoparticles as a novel antibacterial agent against Vibrio harveyi, a Penaeus vannamei pathogen. Aquaculture 2021, 534, 736260. [Google Scholar] [CrossRef]
- Bagheri-Josheghani, S.; Bakhshi, B. Investigation of the Antibacterial and Antibiofilm Activity of Selenium Nanoparticles against Vibrio cholerae as a Potent Therapeutics. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3432235. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A. Antibacterial action of insect chitosan/gum Arabic nanocomposites encapsulating eugenol and selenium nanoparticles. J. King Saud Univ. Sci. 2022, 34, 102219. [Google Scholar] [CrossRef]
- Hosseini Bafghi, M.; Zarrinfar, H.; Darroudi, M.; Zargar, M.; Nazari, R. Green synthesis of selenium nanoparticles and evaluate their effect on the expression of ERG3, ERG11 and FKS1 antifungal resistance genes in Candida albicans and Candida glabrata. Lett. Appl. Microbiol. 2022, 74, 809–819. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Taha, T.F.; Najjar, A.A.; Zabermawi, N.M.; Nader, M.M.; AbuQamar, S.F.; El-Tarabily, K.A.; Salama, A. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J. Biol. Sci. 2021, 28, 6782–6794. [Google Scholar] [CrossRef]
- Safaei, M.; Mozaffari, H.R.; Moradpoor, H.; Imani, M.M.; Sharifi, R.; Golshah, A. Optimization of Green Synthesis of Selenium Nanoparticles and Evaluation of Their Antifungal Activity against Oral Candida albicans Infection. Adv. Mater. Sci. Eng. 2022, 2022, 1376998. [Google Scholar] [CrossRef]
- Lara, H.; Guisbiers, G.; Mendoza, J.; Mimun, L.; Vincent, B.; Lopez-Ribot, J.; Nash, K. Synergistic antifungal effect of chitosan-stabilized selenium nanoparticles synthesized by pulsed laser ablation in liquids against Candida albicans biofilms. Int. J. Nanomed. 2018, 13, 2697–2708. [Google Scholar] [CrossRef] [Green Version]
- Shahbaz, M.; Akram, A.; Raja, N.I.; Mukhtar, T.; Mehak, A.; Fatima, N.; Ajmal, M.; Ali, K.; Mustafa, N.; Abasi, F. Antifungal activity of green synthesized selenium nanoparticles and their effect on physiological, biochemical, and antioxidant defense system of mango under mango malformation disease. PLoS ONE 2023, 18, e0274679. [Google Scholar] [CrossRef]
- Lazcano-Ramírez, H.G.; Garza-García, J.J.O.; Hernández-Díaz, J.A.; León-Morales, J.M.; Macías-Sandoval, A.S.; García-Morales, S. Antifungal Activity of Selenium Nanoparticles Obtained by Plant-Mediated Synthesis. Antibiotics 2023, 12, 115. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Najjar, A.A.; Alzahrani, S.O.; Alkhatib, F.M.; Shafi, M.E.; Selem, E.; Desoky, E.-S.M.; Fouda, S.E.E.; El-Tahan, A.M.; et al. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021, 28, 4461–4471. [Google Scholar] [CrossRef]
- Bafghi, M.H.; Nazari, R.; Darroudi, M.; Zargar, M.; Zarrinfar, H. The effect of biosynthesized selenium nanoparticles on the expression of CYP51A and HSP90 antifungal resistance genes in Aspergillus fumigatus and Aspergillus flavus. Biotechnol. Prog. 2022, 38, e3206. [Google Scholar] [CrossRef]
- Shahbaz, M.; Fatima, N.; Mashwani, Z.-u.-R.; Akram, A.; Haq, E.U.; Mehak, A.; Abasi, F.; Ajmal, M.; Yousaf, T.; Raja, N.I.; et al. Effect of Phytosynthesized Selenium and Cerium Oxide Nanoparticles on Wheat (Triticum aestivum L.) against Stripe Rust Disease. Molecules 2022, 27, 8149. [Google Scholar] [CrossRef]
- Salem, M.F.; Abd-Elraoof, W.A.; Tayel, A.A.; Alzuaibr, F.M.; Abonama, O.M. Antifungal application of biosynthesized selenium nanoparticles with pomegranate peels and nanochitosan as edible coatings for citrus green mold protection. J. Nanobiotechnol. 2022, 20, 182. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.M.A.; Abd-Elsalam, K.A.; Khaleil, M.M. Bacillus megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants. J. Fungi 2021, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.M.; De Britto, S.; Jogaiah, S.; Ito, S.-I. Mycogenic Selenium Nanoparticles as Potential New Generation Broad Spectrum Antifungal Molecules. Biomolecules 2019, 9, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative Study of the Antimicrobial Activity of Selenium Nanoparticles with Different Surface Chemistry and Structure. Front. Bioeng. Biotechnol. 2021, 8, 624621. [Google Scholar] [CrossRef] [PubMed]
- Tuyen, N.N.K.; Huy, V.K.; Duy, N.H.; An, H.; Nam, N.T.H.; Dat, N.M.; Huong, Q.T.T.; Trang, N.L.P.; Anh, N.D.P.; Thy, L.T.M.; et al. Green synthesis of selenium nanorods using Muntigia calabura leaf extract: Effect of pH on characterization and bioactivities. Res. Sq. 2023, 1, 1–19. [Google Scholar] [CrossRef]
- Liu, P.; Ma, Y.; Cai, W.; Wang, Z.; Wang, J.; Qi, L.; Chen, D. Photoconductivity of single-crystalline selenium nanotubes. Nanotechnology 2007, 18, 205704. [Google Scholar] [CrossRef]
- Cheren’kaja, T.V.; Borisova, L.A.; Aleksandrova, I.V.; Kosolapov, D.A. The etiological structure of bacteremia and sepsis causative agents in patients with intensive care in an emergency hospital. Neotlozhnaya Meditsinskaja Pomos. 2013, 16, 33–36. (In Russian) [Google Scholar]
- Lesnichaya, M.V.; Malysheva, S.F.; Belogorlova, N.A.; Graskova, I.A.; Gazizova, A.V.; Perfilyeva, A.I.; Nozhkina, O.A.; Sukhov, B.G. Synthesis and antimicrobial activity of arabinogalactan-stabilized selenium nanoparticles from sodium bis(2-phenylethyl)diselenophosphinate. Russ. Chem. Bull. 2019, 68, 2245–2251. [Google Scholar] [CrossRef]
- Sahoo, B.; Leena Panigrahi, L.; Jena, S.; Jha, S.; Arakha, M. Oxidative stress generated due to photocatalytic activity of biosynthesized selenium nanoparticles triggers cytoplasmic leakage leading to bacterial cell death. RSC Adv. 2023, 13, 11406–11414. [Google Scholar] [CrossRef] [PubMed]
- Mühling, M.; Bradford, A.; Readman, J.W.; Somerfield, P.J.; Handy, R.D. An investigation into the effects of silver nanoparticles on antibiotic resistance of naturally occurring bacteria in an estuarine sediment. Mar. Environ. Res. 2009, 68, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Melotti, P.; Lleo, M.M.; Vallini, G. Biogenic selenium nanoparticles: Characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016, 9, 758–771. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.; Singh, C.; Pradhan, D.; Ghosh, G.; Rath, G. Topical application of nanoparticles integrated supramolecular hydrogels for the potential treatment of seborrhoeic dermatitis. Pharm. Dev. Technol. 2020, 25, 748–756. [Google Scholar] [CrossRef]
- Parsamehr, N.; Rezaie, S.; Khodavaisy, S.; Salari, S.; Hadizadeh, S.; Kord, M.; Ayatollahi Mousavi, S.A. Effect of biogenic selenium nanoparticles on ERG11 and CDR1 gene expression in both fluconazole-resistant and -susceptible Candida albicans isolates. Curr. Med. Mycol. 2017, 3, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Nagajyothi, P.C.; Sreekanth, T.V.M.; Tettey, C.O.; Jun, Y.I.; Mook, S.H. Characterization, antibacterial, antioxidant, and cytotoxic activities of ZnO nanoparticles using Coptidis Rhizoma. Bioorganic Med. Chem. Lett. 2014, 24, 4298–4303. [Google Scholar] [CrossRef]
- Rao, K.; Imran, M.; Jabri, T.; Ali, I.; Perveen, S.; Shafiullah; Ahmed, S.; Shah, M.R. Gum tragacanth stabilized green gold nanoparticles as cargos for Naringin loading: A morphological investigation through AFM. Carbohydr. Polym. 2017, 174, 243–252. [Google Scholar] [CrossRef]
- Song, Z.; Hrbek, J.; Osgood, R. Formation of TiO2 Nanoparticles by Reactive-Layer-Assisted Deposition and Characterization by XPS and STM. Nano Lett. 2005, 5, 1327–1332. [Google Scholar] [CrossRef]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef] [Green Version]
- Bell, N.C.; Minelli, C.; Tompkins, J.; Stevens, M.M.; Shard, A.G. Emerging Techniques for Submicrometer Particle Sizing Applied to Stöber Silica. Langmuir 2012, 28, 10860–10872. [Google Scholar] [CrossRef] [PubMed]
- Ehara, K.; Sakurai, H. Metrology of airborne and liquid-borne nanoparticles: Current status and future needs. Metrologia 2010, 47, S83. [Google Scholar] [CrossRef]
- Henriquez, R.R.; Ito, T.; Sun, L.; Crooks, R.M. The resurgence of Coulter counting for analyzing nanoscale objects. Analyst 2004, 129, 478–482. [Google Scholar] [CrossRef]
- Neville, F.; Broderick, M.J.F.; Gibson, T.; Millner, P.A. Fabrication and Activity of Silicate Nanoparticles and Nanosilicate-Entrapped Enzymes Using Polyethyleneimine As a Biomimetic Polymer. Langmuir 2011, 27, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Astashev, M.E.; Baimler, I.V.; Uvarov, O.V.; Voronov, V.V.; Simakin, A.V. Laser-Induced Optical Breakdown of an Aqueous Colloidal Solution Containing Terbium Nanoparticles: The Effect of Oxidation of Nanoparticles. J. Phys. Chem. B 2022, 126, 5678–5688. [Google Scholar] [CrossRef]
- Singh, V.; Shrivastava, A.; Wahi, N. Biosynthesis of silver nanoparticles by plants crude extracts and their characterization using UV, XRD, TEM and EDX. Afr. J. Biotechnol. 2015, 14, 2554–2567. [Google Scholar] [CrossRef] [Green Version]
- Naraginti, S.; Li, Y. Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J. Photochem. Photobiol. B Biol. 2017, 170, 225–234. [Google Scholar] [CrossRef]
- Zad, Z.R.; Davarani, S.S.H.; Taheri, A.; Bide, Y. A yolk shell Fe3O4@PA-Ni@Pd/Chitosan nanocomposite -modified carbon ionic liquid electrode as a new sensor for the sensitive determination of fluconazole in pharmaceutical preparations and biological fluids. J. Mol. Liq. 2018, 253, 233–240. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Sana, S.S.; Dogiparthi, L.K. Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity. Mater. Lett. 2018, 226, 47–51. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Natesh Kumar, B.; Prasad, C.H.; Venkateswarlu, P.; Jyothi, N.V.V. Bio-inspired green synthesis of Fe3O4 spherical magnetic nanoparticles using Syzygium cumini seed extract. Phys. B Condens. Matter 2014, 449, 67–71. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Serov, D.A.; Simakin, A.V.; Baimler, I.V.; Uvarov, O.V.; Gudkov, S.V. A Polytetrafluoroethylene (PTFE) and Nano-Al2O3 Based Composite Coating with a Bacteriostatic Effect against E. coli and Low Cytotoxicity. Polymers 2022, 14, 4764. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Hong, B.; Huang, W.; He, J. Selenium-Nanoparticles-Loaded Chitosan/Chitooligosaccharide Microparticles and Their Antioxidant Potential: A Chemical and In Vivo Investigation. Pharmaceutics 2020, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Shurygina, I.A.; Trukhan, I.S.; Dremina, N.N.; Shurygin, M.G. Selenium Nanoparticles. In Nanotechnology in Medicine; Wiley: Hoboken, NJ, USA, 2021; pp. 47–66. [Google Scholar]
- Kumar, C.M.V.; Karthick, V.; Inbakandan, D.; Kumar, V.G.; Rene, E.R.; Dhas, T.S.; Ravi, M.; Sowmiya, P.; Das, C.G.A. Effect of selenium nanoparticles induced toxicity on the marine diatom Chaetoceros gracilis. Process Saf. Environ. Prot. 2022, 163, 200–209. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci. 2021, 12, 141. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Hou, J.; Wang, X.; Hayat, T.; Wang, X. Ecotoxicological effects and mechanism of CuO nanoparticles to individual organisms. Environ. Pollut. 2017, 221, 209–217. [Google Scholar] [CrossRef]
- Tang, H.; Xu, M.; Luo, J.; Zhao, L.; Ye, G.; Shi, F.; Lv, C.; Chen, H.; Wang, Y.; Li, Y. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ. Sci. Eur. 2019, 31, 30. [Google Scholar] [CrossRef] [Green Version]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium Nanoparticles for Biomedical Applications: From Development and Characterization to Therapeutics. Adv. Healthc. Mater. 2021, 10, 2100598. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, C.H.; Sampath, K.S.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Biswas, J.; Sen, T.; Bhattacharya, S. Chemoprotective and chemosensitizing properties of selenium nanoparticle (Nano-Se) during adjuvant therapy with cyclophosphamide in tumor-bearing mice. Mol. Cell. Biochem. 2016, 424, 13–33. [Google Scholar] [CrossRef]
- Zambonino, M.C.; Quizhpe, E.M.; Mouheb, L.; Rahman, A.; Agathos, S.N.; Dahoumane, S.A. Biogenic Selenium Nanoparticles in Biomedical Sciences: Properties, Current Trends, Novel Opportunities and Emerging Challenges in Theranostic Nanomedicine. Nanomaterials 2023, 13, 424. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Wong, Y.-S.; Zheng, W.; Zhang, Y.; Cao, W.; Chen, T. Selenium Nanoparticles as a Carrier of 5-Fluorouracil to Achieve Anticancer Synergism. ACS Nano 2012, 6, 6578–6591. [Google Scholar] [CrossRef]
- Prasad, K.S.; Selvaraj, K. Biogenic Synthesis of Selenium Nanoparticles and Their Effect on As(III)-Induced Toxicity on Human Lymphocytes. Biol. Trace Elem. Res. 2014, 157, 275–283. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, X.; Chen, Z.; Zeng, X.; Yue, T.; Yuan, Y. Effects of Selenium Nanoparticles on Preventing Patulin-Induced Liver, Kidney and Gastrointestinal Damage. Foods 2022, 11, 749. [Google Scholar] [CrossRef]
- Deng, W.; Xie, Q.; Wang, H.; Ma, Z.; Wu, B.; Zhang, X. Selenium nanoparticles as versatile carriers for oral delivery of insulin: Insight into the synergic antidiabetic effect and mechanism. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1965–1974. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, S.; Liu, Y.; Wu, W.; Shen, Y.; Zhang, L.; Li, C.; Chen, H.; Liu, A.; Shen, L.; et al. Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol. 2018, 114, 632–639. [Google Scholar] [CrossRef]
- Abdel Moneim, A.; Al-Quraishy, S.; Dkhil, M.A. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int. J. Nanomed. 2015, 10, 6741–6756. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, W.; Chen, J.; Wang, N.; Zheng, G. A comparative study of resveratrol and resveratrol-functional selenium nanoparticles: Inhibiting amyloid β aggregation and reactive oxygen species formation properties. J. Biomed. Mater. Res. Part A 2018, 106, 3034–3041. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Shakibaie, M.; Tavakoli, R.; Jahanbakhsh, S.; Sharifi, I. In Vitro Study of Leishmanicidal Activity of Biogenic Selenium Nanoparticles against Iranian Isolate of Sensitive and Glucantime-Resistant Leishmania tropica. Iran. J. Parasitol. 2014, 9, 452–460. [Google Scholar]
- Wlodkowic, D.; Telford, W.; Skommer, J.; Darzynkiewicz, Z. Apoptosis and Beyond: Cytometry in Studies of Programmed Cell Death. Methods Cell Biol. 2011, 103, 55–98. [Google Scholar]
- Malekifard, F.; Tavassoli, M.; Vaziri, K. In Vitro Assessment Antiparasitic Effect of Selenium and Copper Nanoparticles on Giardia deodenalis Cyst. Iran. J. Parasitol. 2020, 15, 411. [Google Scholar] [CrossRef]
- Arafa, F.M.; Mogahed, N.M.F.H.; Eltarahony, M.M.; Diab, R.G. Biogenic selenium nanoparticles: Trace element with promising anti-toxoplasma effect. Pathog. Glob. Health 2023, 5, 1–16. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Fasihi Harandi, M.; Shakibaie, M.; Aflatoonian, M.R.; ZiaAli, N.; Makki, M.S.; Jahanbakhsh, S. Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int. J. Surg. 2014, 12, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, S.; Ali, A.A. Effect of selenium nanoparticles against protoscoleces of Echinococcus granulosus in vitro and hydatid cysts in mice. Iraqi J. Vet. Sci. 2022, 36, 195–202. [Google Scholar] [CrossRef]
- Sarhan, M.H.; Farghaly, A.; Abd El-Aal, N.F.; Mohammed Farag, S.; Ahmed Ali, A.; Farag, T.I. Egyptian propolis and selenium nanoparticles against murine trichinosis: A novel therapeutic insight. J. Helminthol. 2022, 96, e50. [Google Scholar] [CrossRef]
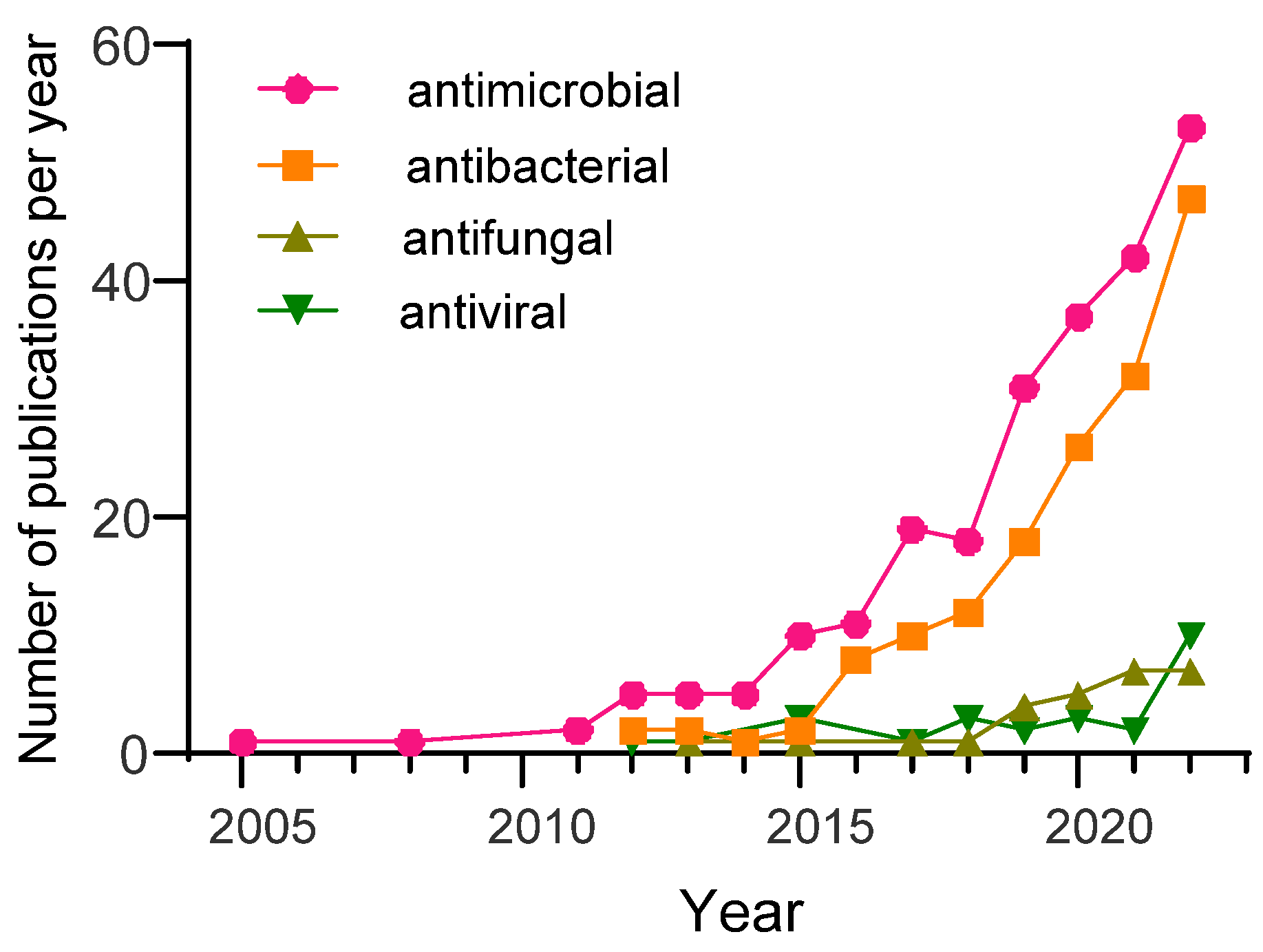
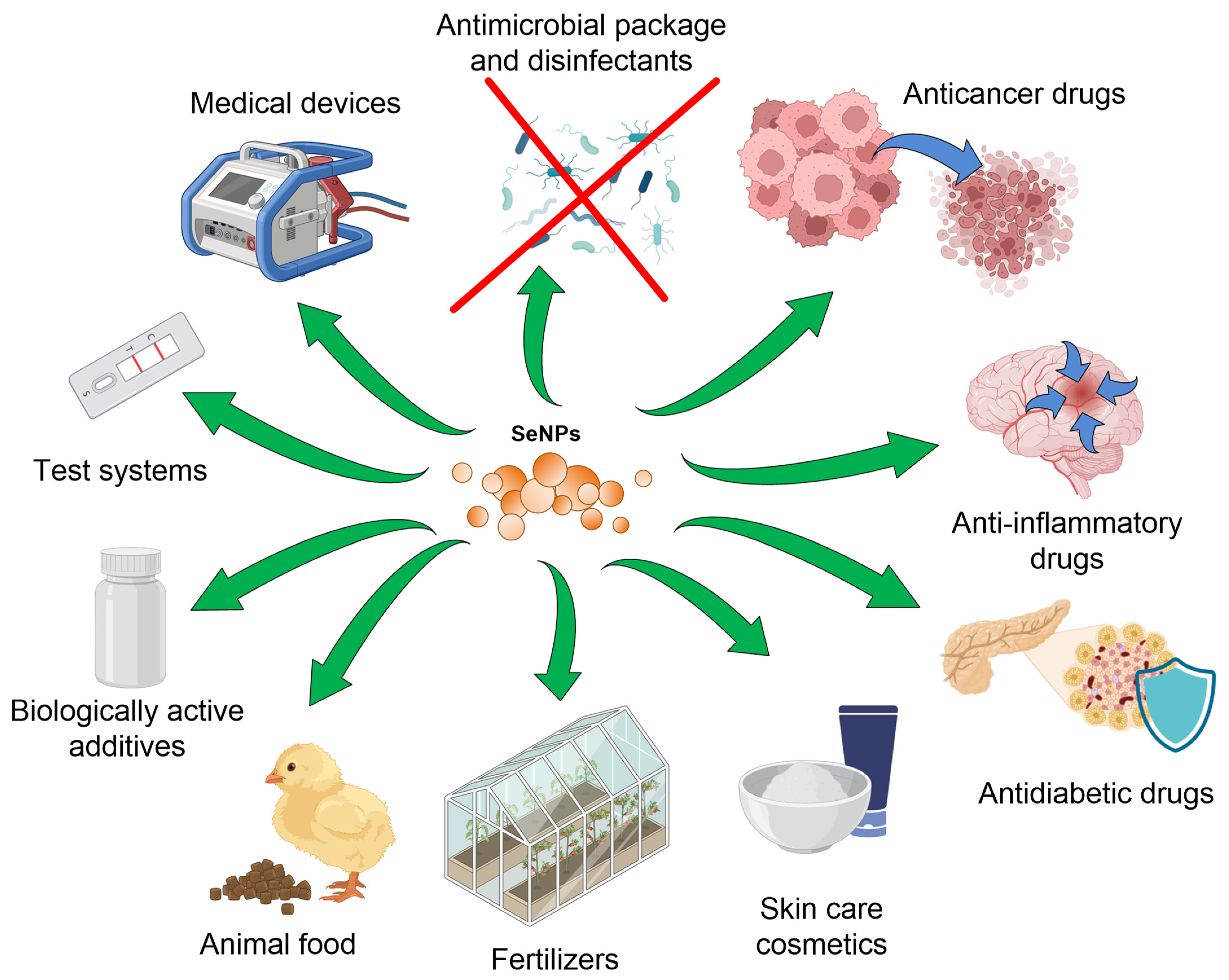
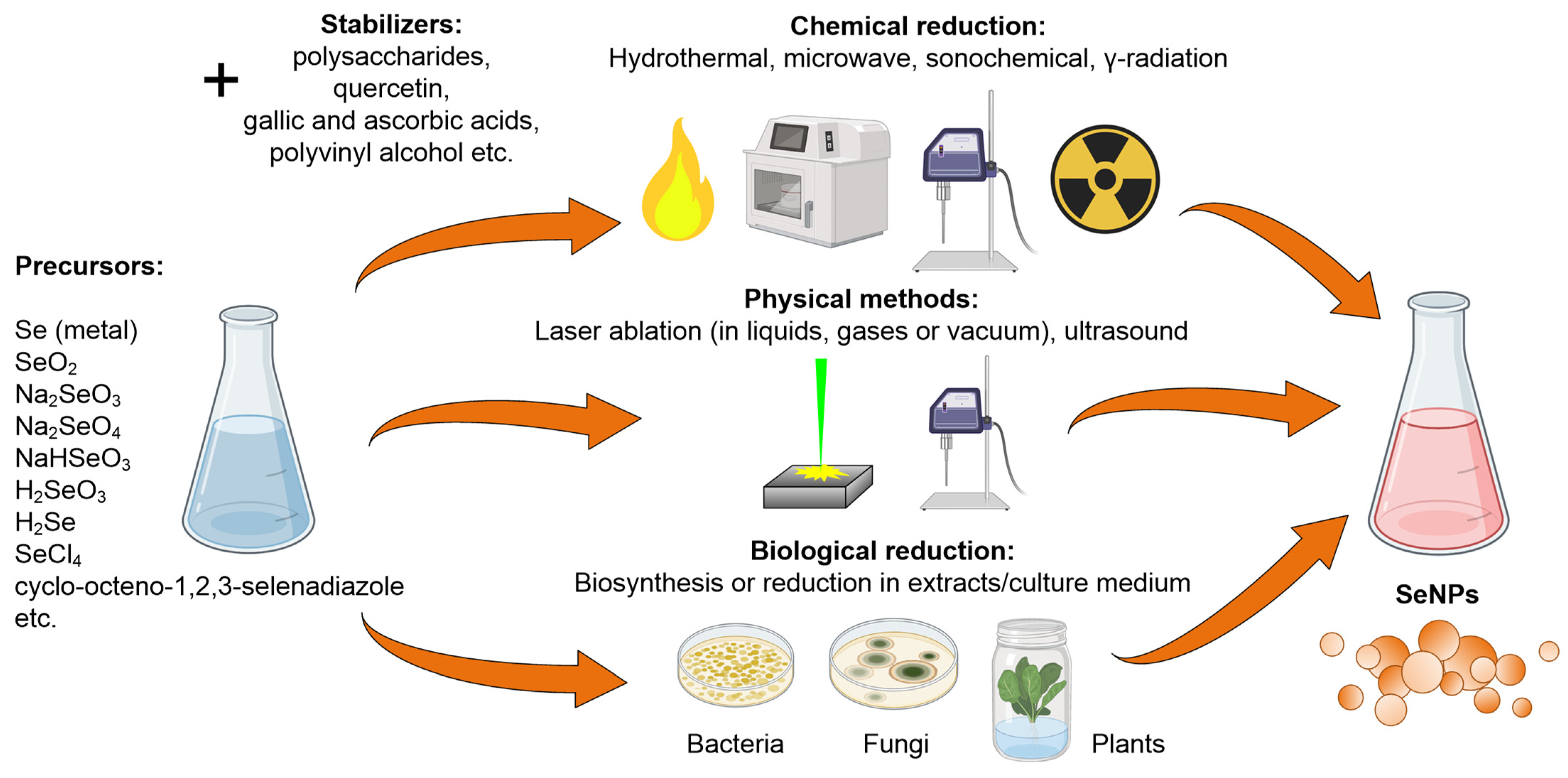
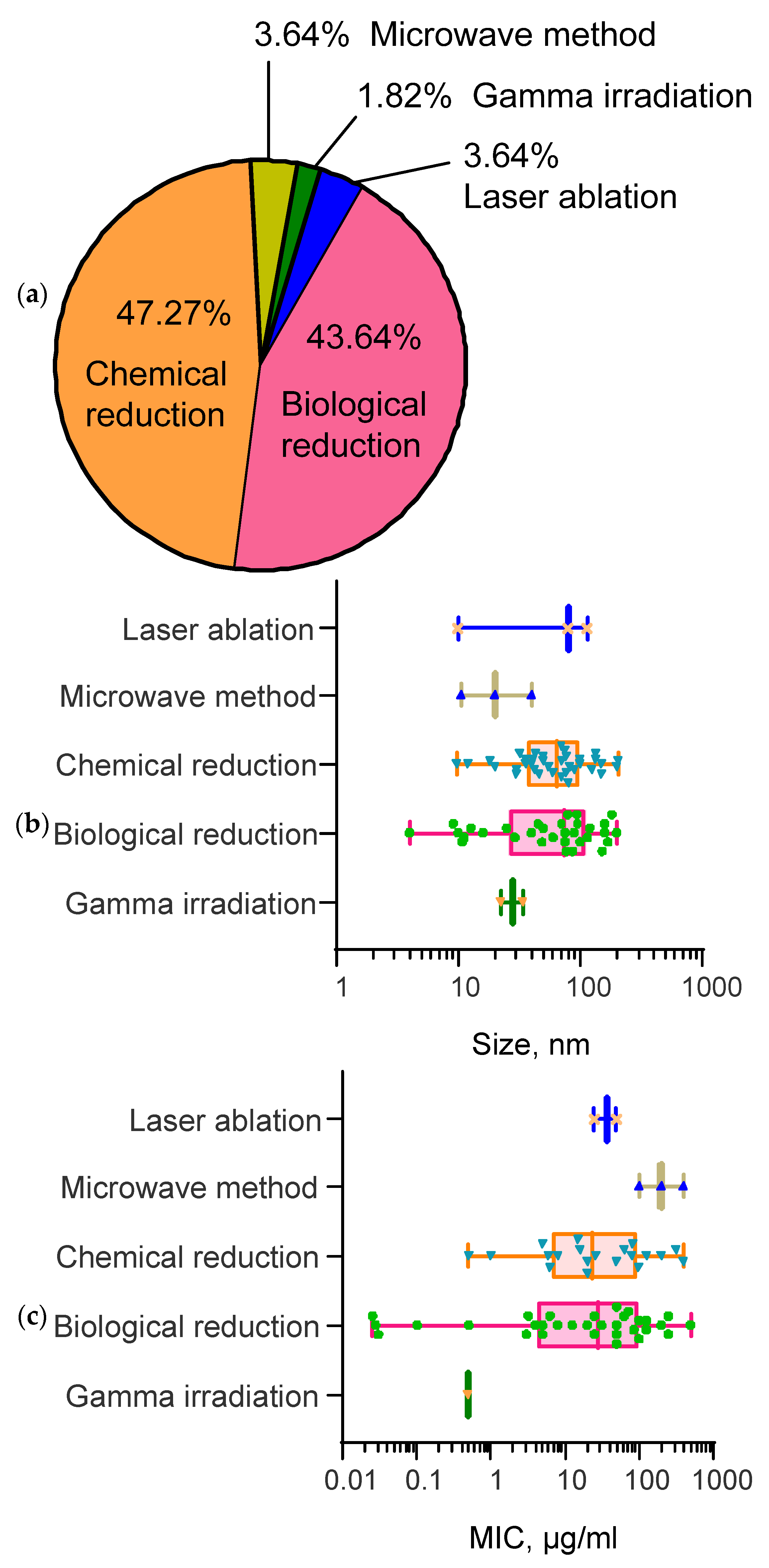
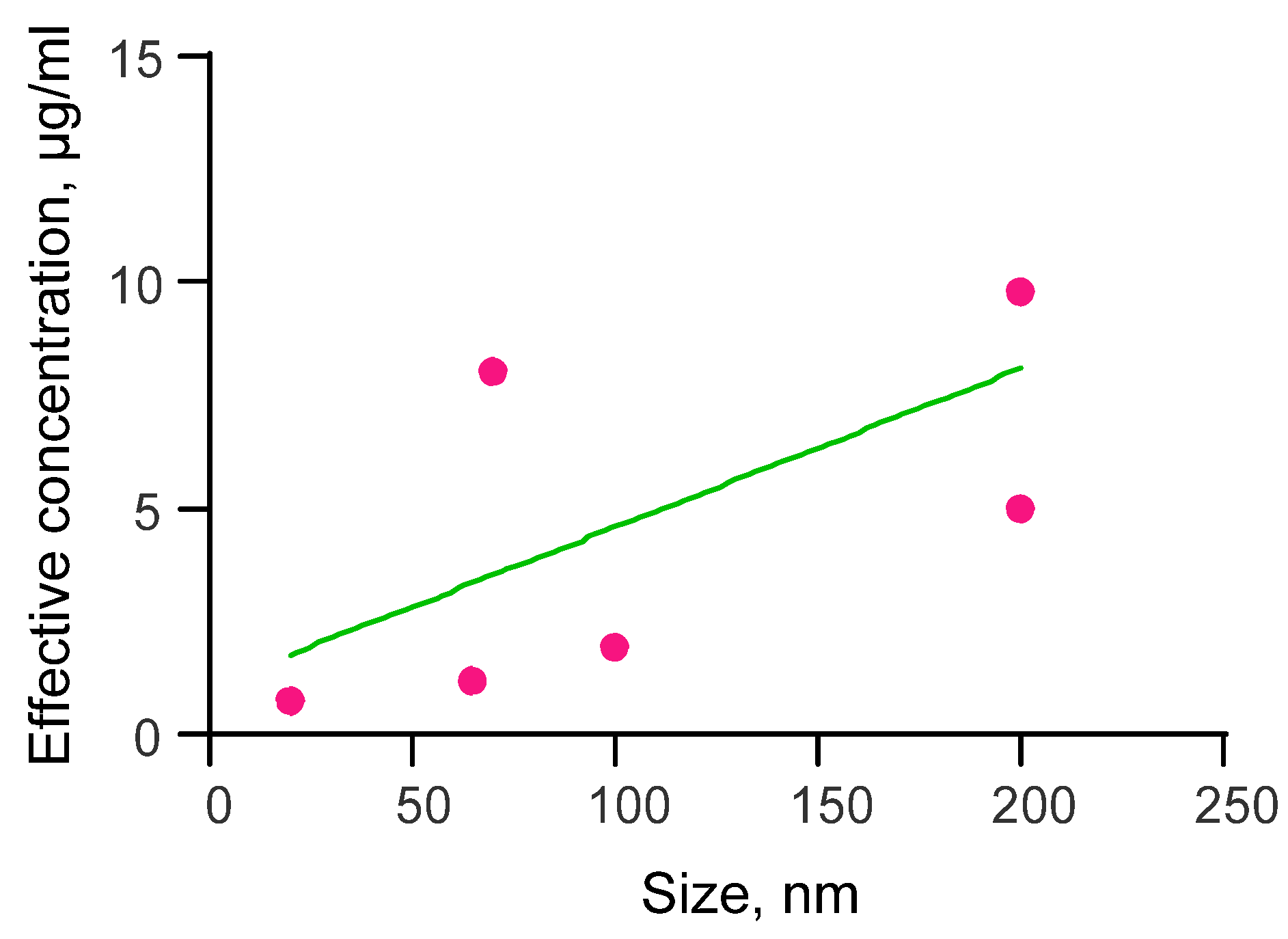
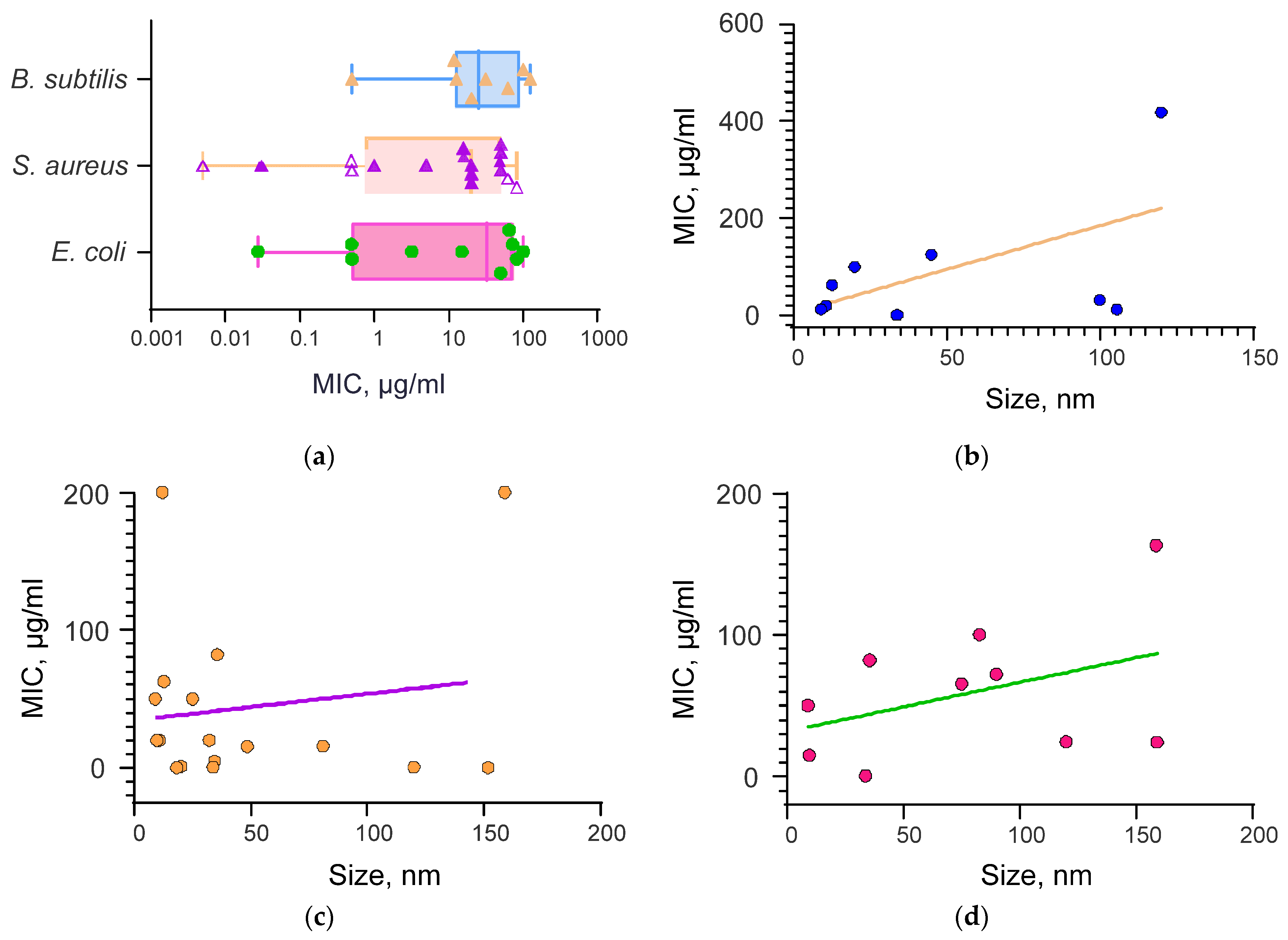
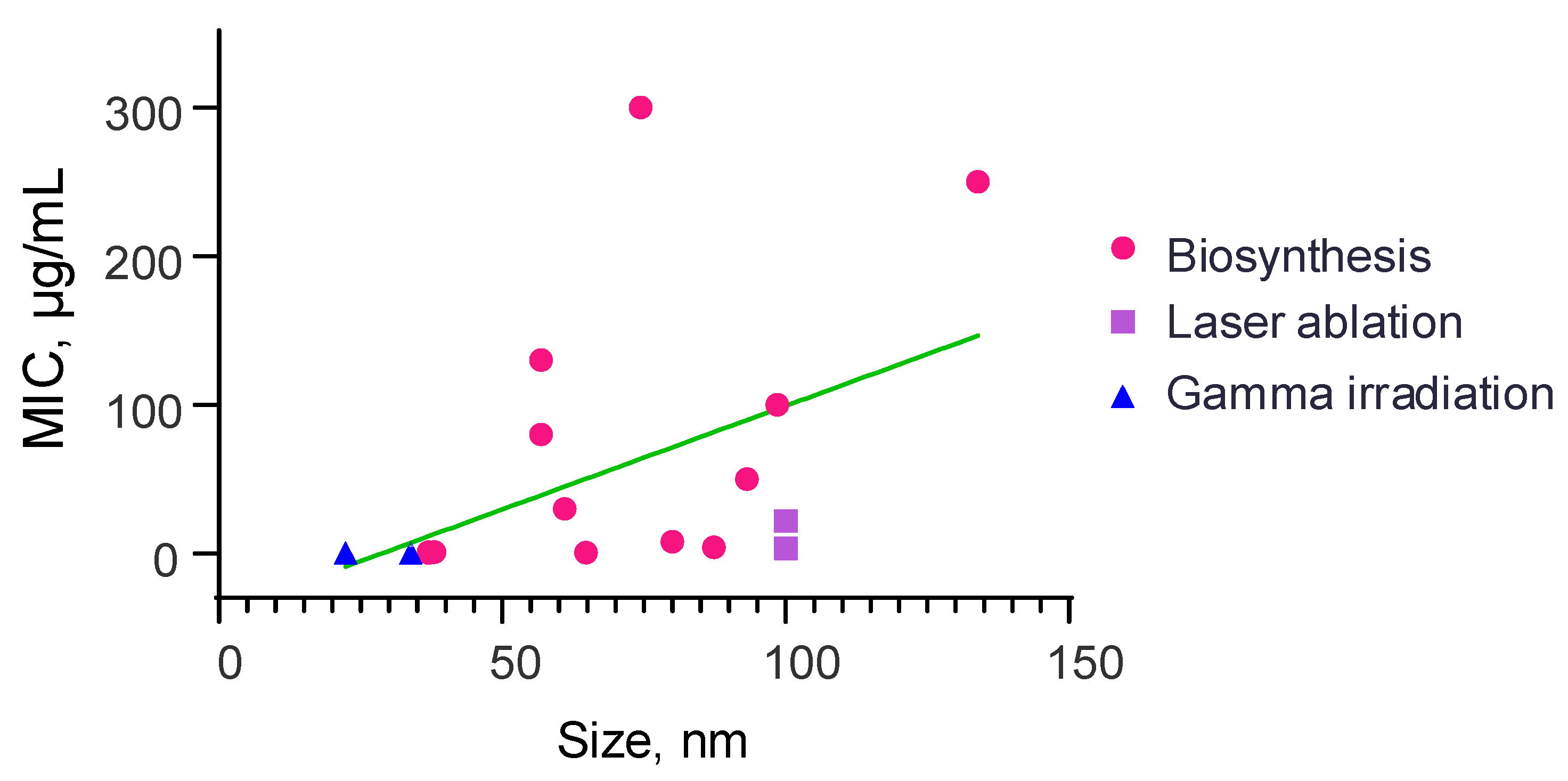
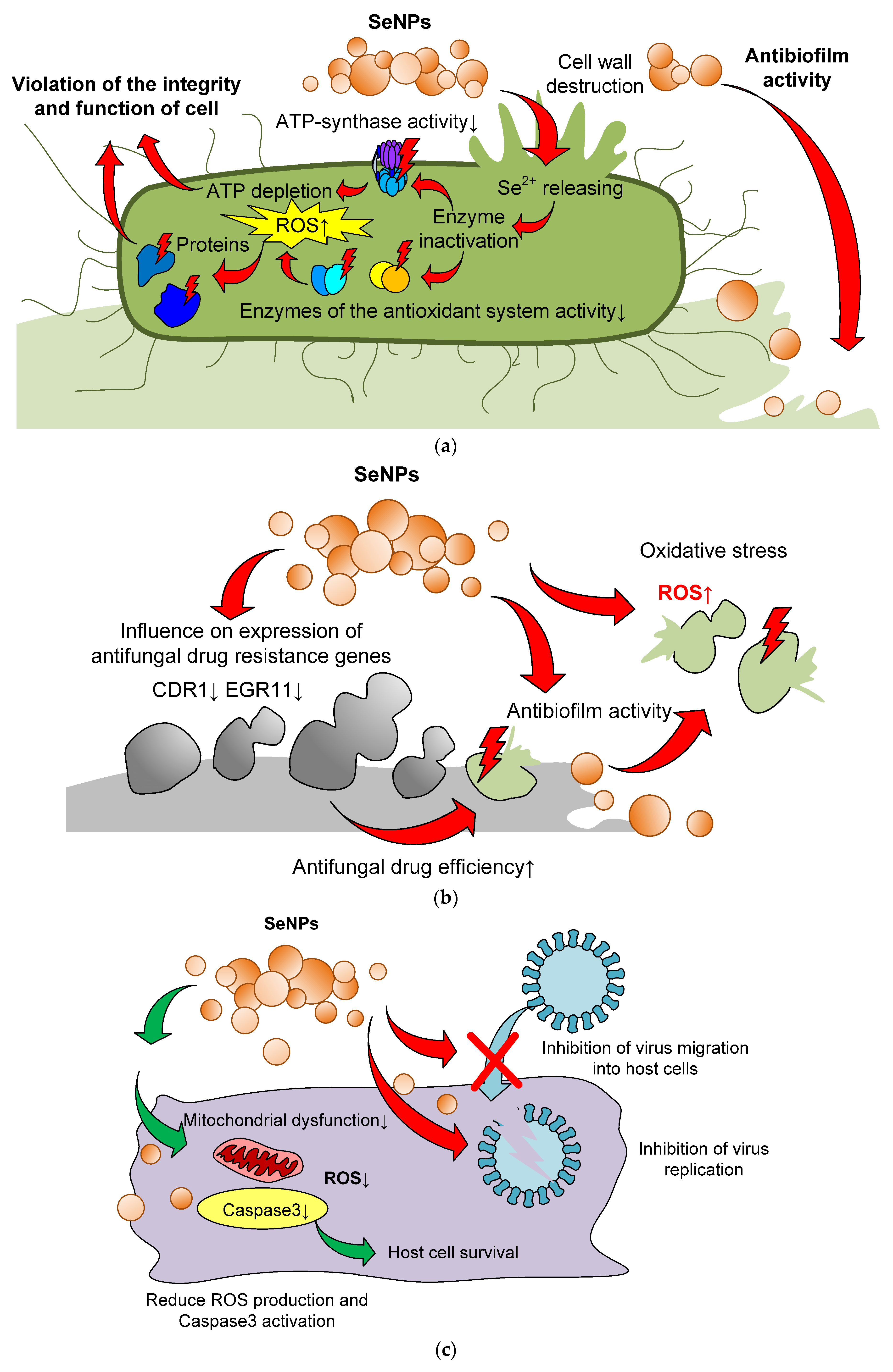
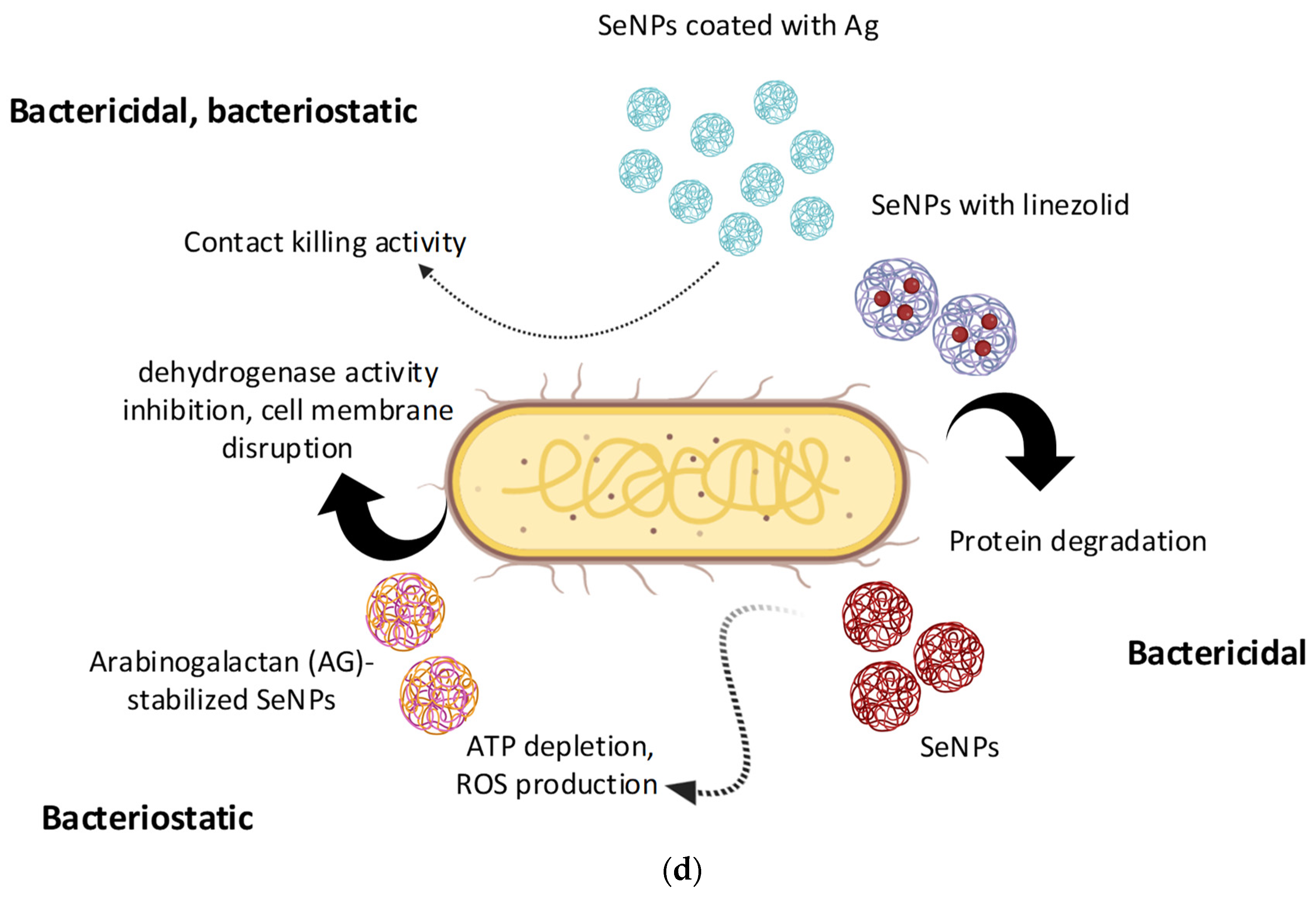
| № | Patent ID | Patent Name | Reference |
|---|---|---|---|
| 1 | US9624237B2 | Oridonin functionalized selenium nanoparticles and method of preparation thereof | [73] |
| 2 | US8445026B2 | Selenium nanoparticles with improved biological effects | [90] |
| 3 | US10807920B2 | Trichoderma-derived selenium nanoparticle foliar fertilizer for reducing crop fungal diseases and toxic contamination | [75] |
| 4 | US9259005B2 | Antipathogenic surfaces having selenium nanoclusters | [57] |
| 5 | CN111214460A | Folic acid-chitosan-nano-selenium tumor-targeted drug delivery system and preparation method thereof | [67] |
| 6 | RU2798268C1 | Method of obtaining a veterinary drug based on non-specific immunoglobulins and colloidal particles of selenium for the correction of the immune system | [52] |
| 7 | JP2011501977A | Method for producing hydrous tissue paper having antibacterial and antifungal functions | [56] |
| 8 | KR101120635B1 | Method for cultivating high quality and functional vegetable fruit | [80] |
| 9 | US20220339187A1 | Protein-bound nano-selenium and preparation method and application method thereof | [91] |
| 10 | US10875235B2 | Bactericidal surface patterns | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serov, D.A.; Khabatova, V.V.; Vodeneev, V.; Li, R.; Gudkov, S.V. A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles. Materials 2023, 16, 5363. https://doi.org/10.3390/ma16155363
Serov DA, Khabatova VV, Vodeneev V, Li R, Gudkov SV. A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles. Materials. 2023; 16(15):5363. https://doi.org/10.3390/ma16155363
Chicago/Turabian StyleSerov, Dmitry A., Venera V. Khabatova, Vladimir Vodeneev, Ruibin Li, and Sergey V. Gudkov. 2023. "A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles" Materials 16, no. 15: 5363. https://doi.org/10.3390/ma16155363
APA StyleSerov, D. A., Khabatova, V. V., Vodeneev, V., Li, R., & Gudkov, S. V. (2023). A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles. Materials, 16(15), 5363. https://doi.org/10.3390/ma16155363






