Review of the Hydrogen Permeation Test of the Polymer Liner Material of Type IV On-Board Hydrogen Storage Cylinders
Abstract
:1. Introduction
2. Hydrogen Permeation Mechanism in Polymer Liner Materials
3. Hydrogen Permeation Test Methods of the Polymer Liner Material
3.1. Sample Preparation
3.2. Sample Pretreatment
3.3. Test Device
3.4. Test Temperature and Pressure
3.5. Qualification Indicators
4. Discussion
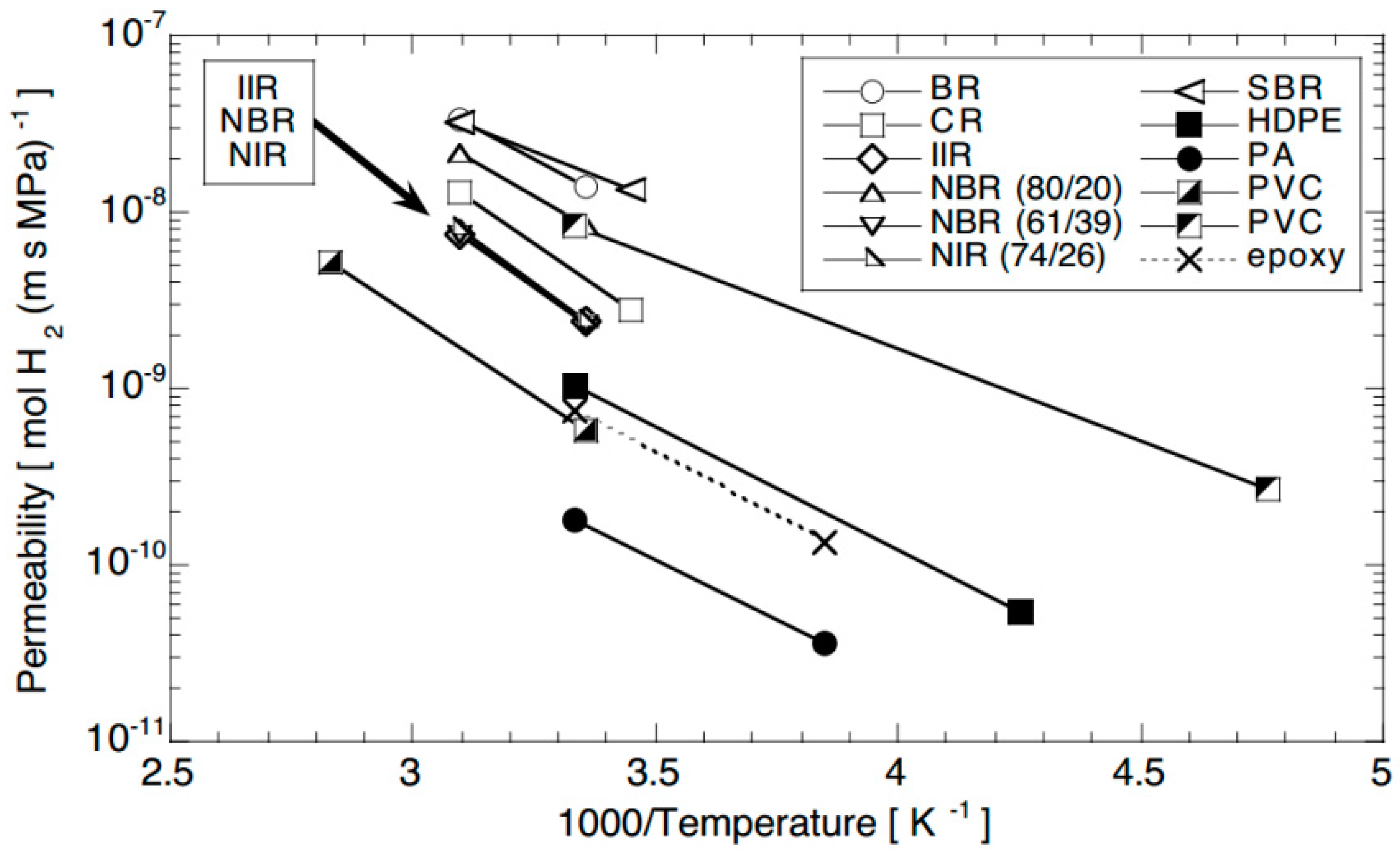
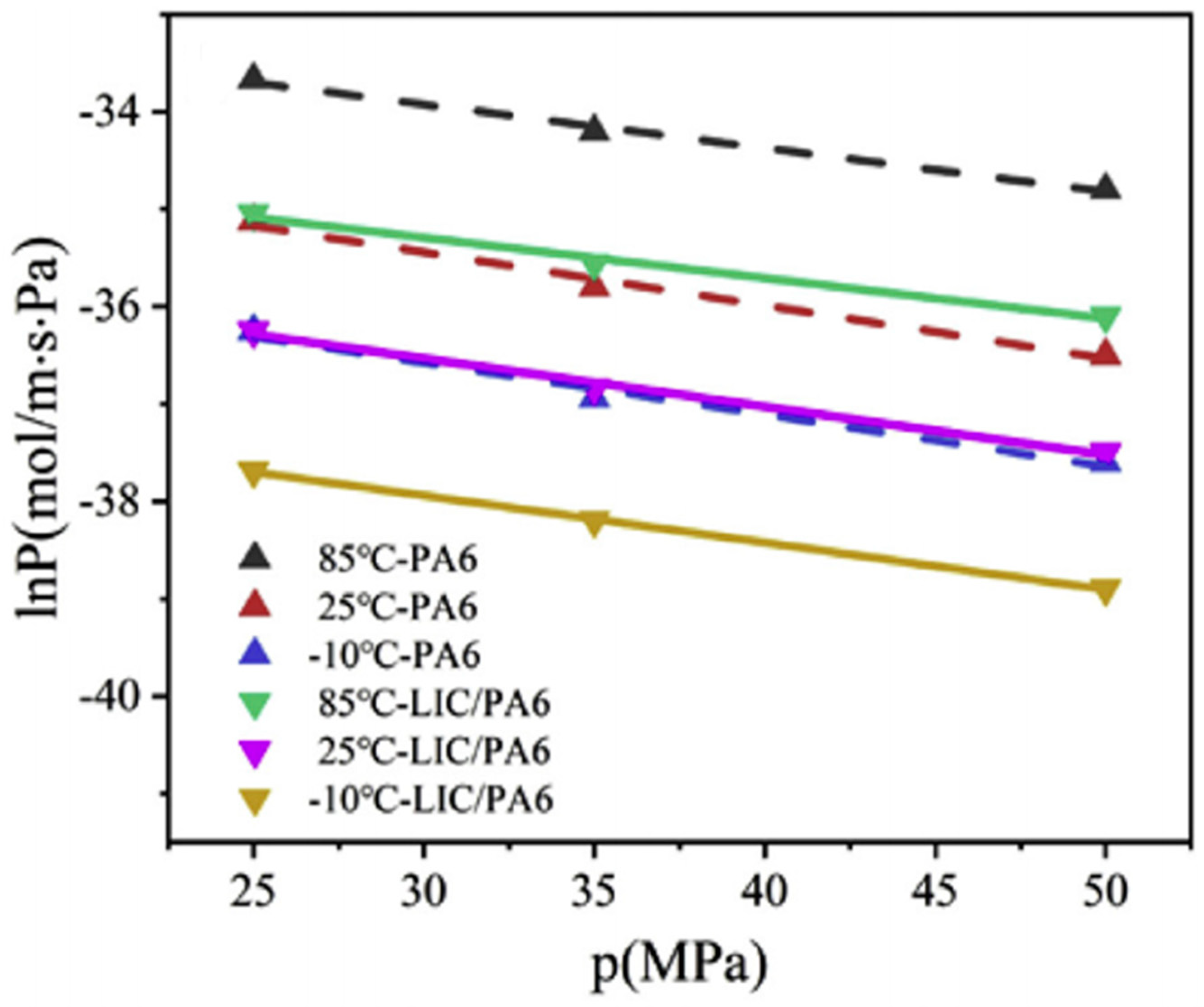
4.1. Test Temperature
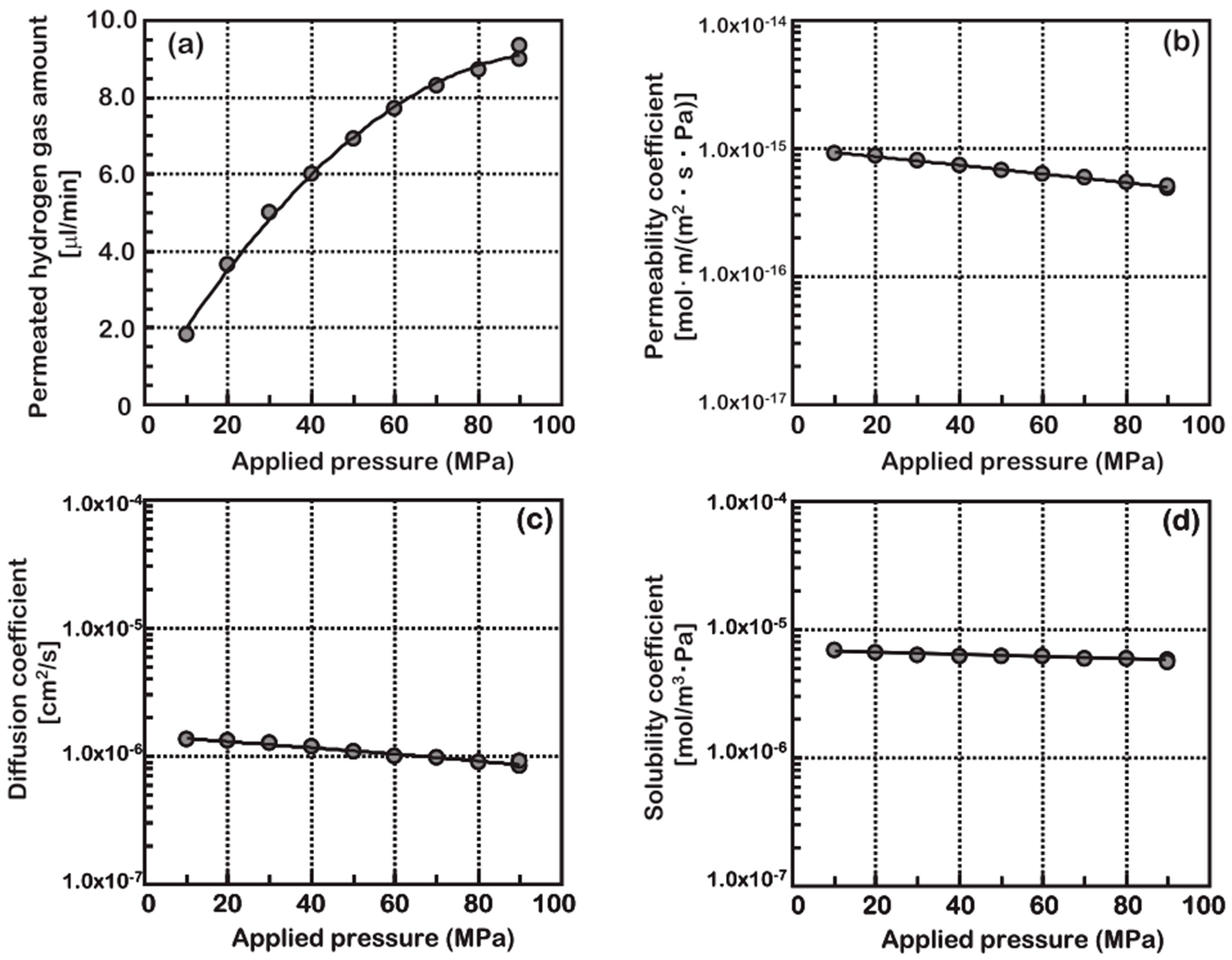
4.2. Test Pressure
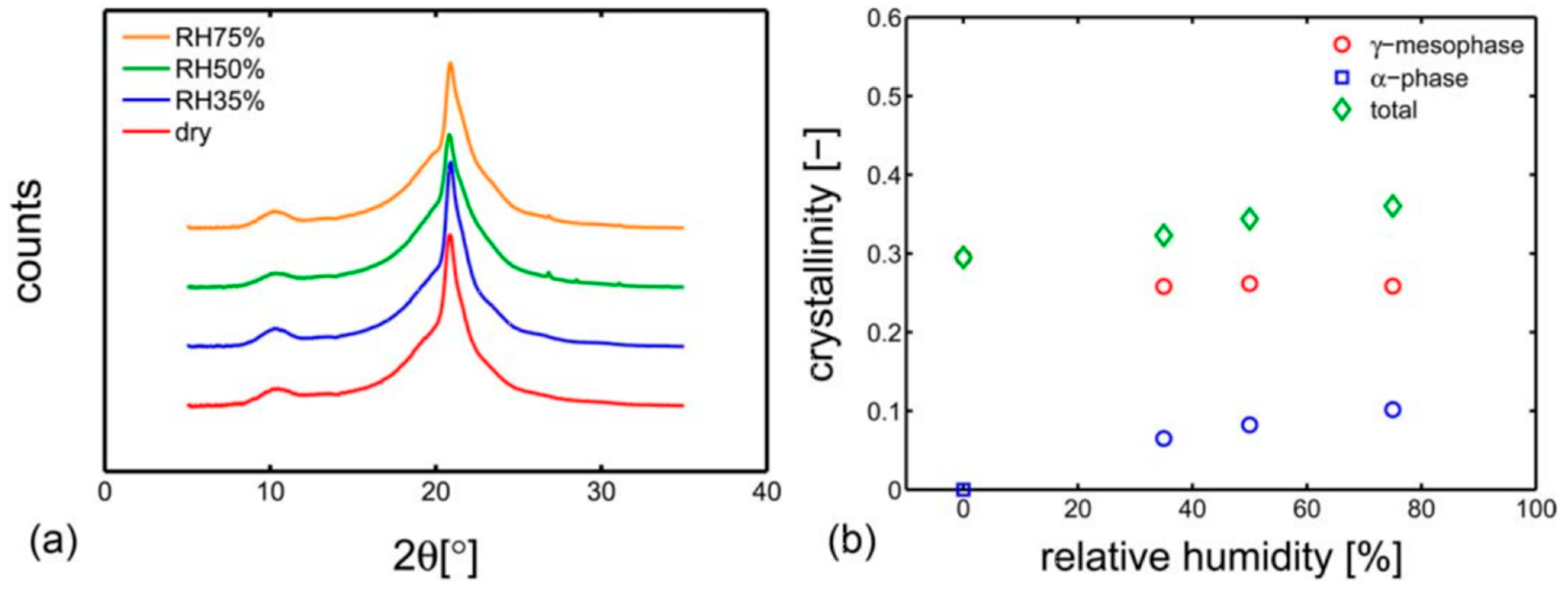
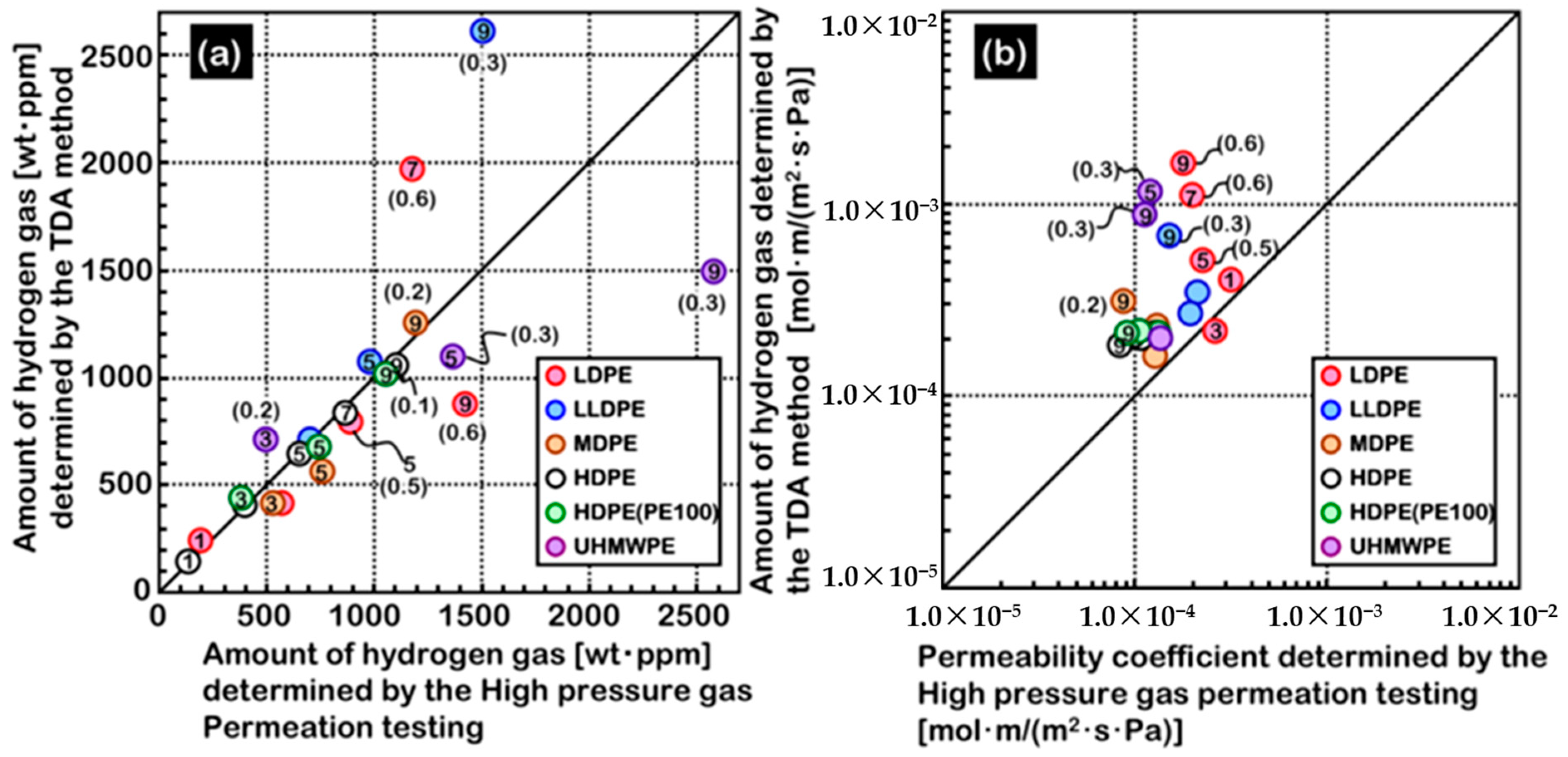
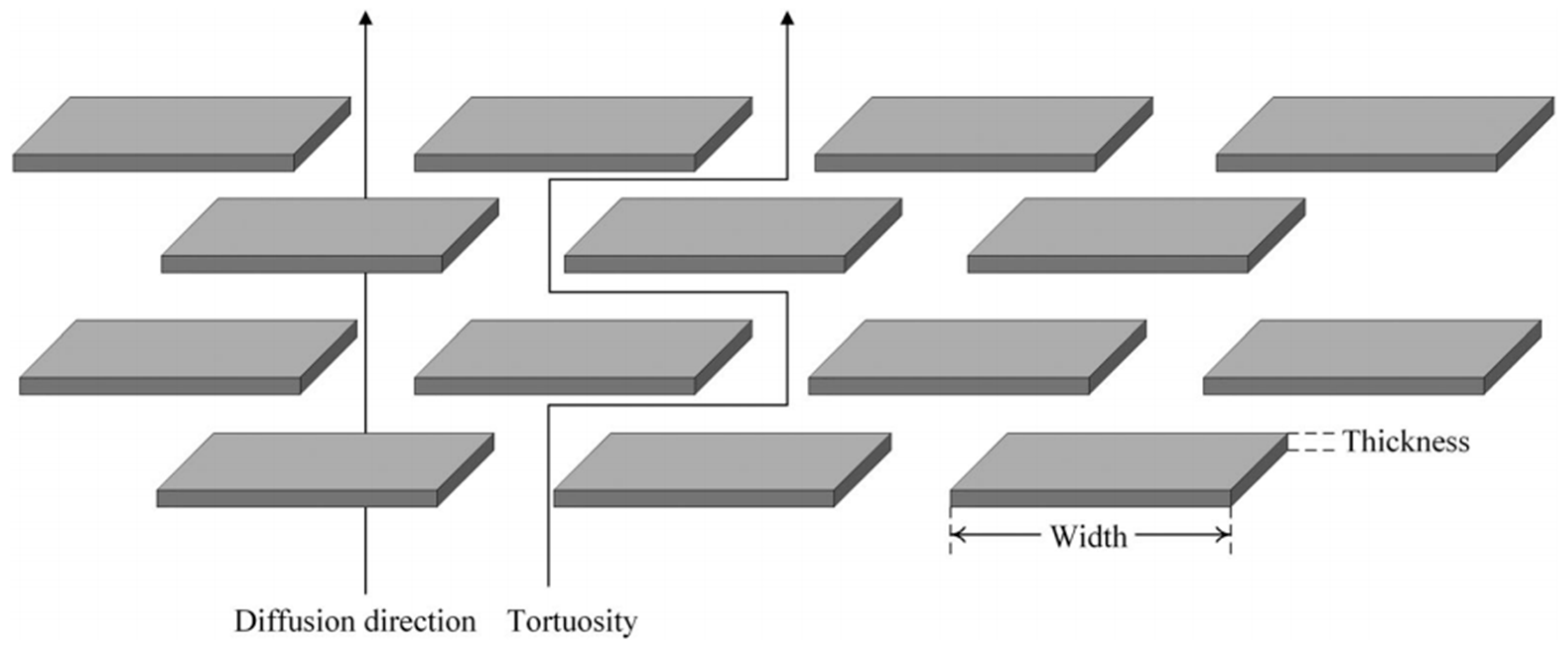
4.3. Polymer Material Properties
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Liu, J.; Zhao, B.; Wang, D.; Guo, S.; Song, J.; Li, X. Research on Temperature Rise of Type IV Composite Hydrogen Storage Cylinders in Hydrogen Fast-Filling Process. Energies 2023, 16, 2918. [Google Scholar] [CrossRef]
- Matsuo, Y.; Endo, S.; Nagatomi, Y.; Shibata, Y.; Komiyama, R.; Fujii, Y. A quantitative analysis of Japan’s optimal power generation mix in 2050 and the role of CO2-free hydrogen. Energy 2018, 165, 1200–1219. [Google Scholar] [CrossRef]
- Sgobbi, A.; Nijs, W.; De Miglio, R.; Chiodi, A.; Gargiulo, M.; Thiel, C. How far away is hydrogen? Its role in the medium and long-term decarbonisation of the European energy system. Int. J. Hydrogen Energy 2016, 41, 19–35. [Google Scholar] [CrossRef]
- Bakker, S.; van Lente, H.; Meeus, M.T. Credible expectations—The US Department of Energy’s Hydrogen Program as enactor and selector of hydrogen technologies. Technol. Forecast. Soc. Change 2012, 79, 1059–1071. [Google Scholar] [CrossRef]
- Jain, I. Hydrogen the fuel for 21st century. Int. J. Hydrogen Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- Jingyuan, L.; Qianghua, H. Technology trends of high pressure vehicle fuel tanks and challenges for China. Press. Vessel Technol. 2014, 31, 43–51. [Google Scholar] [CrossRef]
- Schurer, A.P.; Mann, M.E.; Hawkins, E.; Tett, S.F.B.; Hegerl, G.C. Importance of the pre-industrial baseline for likelihood of exceeding Paris goals. Nat. Clim. Chang. 2017, 7, 563–567. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, Z.; Hua, Z.; Guch, W.G.; Chen, L. Research status-in-situ and key challenges in hydrogen safety. J. Saf. Environ. 2020, 20, 106–115. [Google Scholar] [CrossRef]
- Hwang, H.T.; Varma, A. Hydrogen storage for fuel cell vehicles. Curr. Opin. Chem. Eng. 2014, 5, 42–48. [Google Scholar] [CrossRef]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef] [Green Version]
- Valencia, L.B.; Blanc-Vannet, P.; Domergue, D.; Heudier, L.; Jamois, D. Thermal History Resulting in the Failure of Lightweight Fully-Wrapped Composite Pressure Vessel for Hydrogen in a Fire Experimental Facility. Fire Technol. 2015, 52, 421–442. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Li, J.; Liu, Y.; Li, X.; Zhu, C. Overview of Standards on Pressure Cycling Test for On-Board Composite Hydrogen Storage Cylinders. In Proceedings of the ASME 2022 Pressure Vessels & Piping Conference, Las Vegas, NV, USA, 17–22 July 2022. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, B.; Li, J.; Sun, M.; Li, X.; Li, X. Research on Standard Comparison of Hydrogen Cycling Test Method for On-Board Composite Hydrogen Storage Cylinders. In Proceedings of the ASME 2022 Pressure Vessels & Piping Conference, Las Vegas, NV, USA, 17–22 July 2022. [Google Scholar] [CrossRef]
- Li, J.; Lv, R.; Gu, C.; Liu, Y.; Li, J.; Li, X. An Ageing Test Standards Analysis on Thermoplastic Liners of Type IV Composite Hydrogen Storage Tanks. Energies 2023, 16, 2818. [Google Scholar] [CrossRef]
- Ahluwalia, R.; Hua, T.; Peng, J. On-board and Off-board performance of hydrogen storage options for light-duty vehicles. Int. J. Hydrogen Energy 2012, 37, 2891–2910. [Google Scholar] [CrossRef]
- Pépin, J.; Lainé, E.; Grandidier, J.-C.; Benoit, G.; Mellier, D.; Weber, M.; Langlois, C. Replication of liner collapse phenomenon observed in hyperbaric type IV hydrogen storage vessel by explosive decompression experiments. Int. J. Hydrogen Energy 2018, 43, 4671–4680. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, H.; Kang, H.; Zhou, W.; Zhang, C. A literature review of failure prediction and analysis methods for composite high-pressure hydrogen storage tanks. Int. J. Hydrogen Energy 2019, 44, 25777–25799. [Google Scholar] [CrossRef]
- Castagnet, S.; Grandidier, J.-C.; Comyn, M.; Benoît, G. Hydrogen influence on the tensile properties of mono and multi-layer polymers for gas distribution. Int. J. Hydrogen Energy 2010, 35, 7633–7640. [Google Scholar] [CrossRef]
- Yersak, T.A.; Baker, D.R.; Yanagisawa, Y.; Slavik, S.; Immel, R.; Mack-Gardner, A.; Herrmann, M.; Cai, M. Predictive model for depressurization-induced blistering of type IV tank liners for hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 28910–28917. [Google Scholar] [CrossRef]
- Adams, P.; Bengaouer, A.; Cariteau, B.; Molkov, V.; Venetsanos, A. Allowable Hydrogen Permeation Rate from Road Vehicle Compressed Gaseous Storage Systems. Int. J. Hydrogen Energy 2011, 35, 2742–2749. [Google Scholar] [CrossRef]
- Gupta, S.; Brinster, J.; Studer, E.; Tkatschenko, I. Hydrogen related risks within a private garage: Concentration measurements in a realistic full scale experimental facility. Int. J. Hydrogen Energy 2009, 34, 5902–5911. [Google Scholar] [CrossRef]
- ISO 19881-2018; Gaseous Hydrogen—Land Vehicle Fuel Containers. International Organization for Standardization: Geneva, Switzerland, 2018.
- CSA/ANSI CHMC 2:2019; Test Methods for Evaluating Material Compatibility in Compressed Hydrogen Applications Polymers. Canadian Standards Association: Toronto, ON, Canada, 2019.
- ISO 11114-5: 2022; Gas Cylinders-Compatibility of Cylinder and Valve Materials with Gas Contents-Part 5: Test Methods for Evaluating Plastic Liners. International Organization for Standardization: Geneva, Switzerland, 2022.
- T/CATSI 02 007: 2020; Fully-Wrapped Carbon Fiber Reinforced Cylinder with a Plastic Liner for On-Board Storage of Compressed Hydrogen for Land Vehicles. China Association for Technical Supervision Information: Beijing, China, 2020.
- Castagnet, S.; Grandidier, J.-C.; Comyn, M.; Benoît, G. Mechanical Testing of Polymers in Pressurized Hydrogen: Tension, Creep and Ductile Fracture. Exp. Mech. 2011, 52, 229–239. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef] [Green Version]
- Habel, C.; Tsurko, E.S.; Timmins, R.L.; Hutschreuther, J.; Kunz, R.; Schuchardt, D.D.; Rosenfeldt, S.; Altstädt, V.; Breu, J. Lightweight Ultra-High-Barrier Liners for Helium and Hydrogen. ACS Nano 2020, 14, 7018–7024. [Google Scholar] [CrossRef]
- Klopffer, M.-H.; Berne, P.; Espuche, E. Development of Innovating Materials for Distributing Mixtures of Hydrogen and Natural Gas. Study of the Barrier Properties and Durability of Polymer Pipes. Oil Gas Sci. Technol. 2014, 70, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, K.; Kuo, A.-T.; Hirai, T.; Miyajima, T.; Urata, S.; Terazono, S.; Okazaki, S.; Shinoda, W. Hydrogen Permeation in Hydrated Perfluorosulfonic Acid Polymer Membranes: Effect of Polymer Crystallinity and Equivalent Weight. J. Phys. Chem. C 2019, 123, 20628–20638. [Google Scholar] [CrossRef]
- Briottet, L.; Moro, I.; Escot, M.; Furtado, J.; Bortot, P.; Tamponi, G.; Solin, J.; Odemer, G.; Blanc, C.; Andrieu, E. Fatigue crack initiation and growth in a CrMo steel under hydrogen pressure. Int. J. Hydrogen Energy 2015, 40, 17021–17030. [Google Scholar] [CrossRef] [Green Version]
- Hua, Z.; Zhang, X.; Zheng, J.; Gu, C.; Cui, T.; Zhao, Y.; Peng, W. Hydrogen-enhanced fatigue life analysis of Cr–Mo steel high-pressure vessels. Int. J. Hydrogen Energy 2017, 42, 12005–12014. [Google Scholar] [CrossRef]
- Yamabe, J.; Itoga, H.; Awane, T.; Matsunaga, H.; Hamada, S.; Matsuoka, S. Fatigue-Life and Leak-Before-Break Assessments of Cr-Mo Steel Pressure Vessels with High-Pressure Gaseous Hydrogen. In Proceedings of the ASME 2014 Pressure Vessels and Piping Conference, Anaheim, CA, USA, 20–24 July 2014. [Google Scholar] [CrossRef]
- Barth, R.R.; Simmons, K.L.; Marchi, C.W.S. Polymers for Hydrogen Infrastructure and Vehicle Fuel Systems; Sandia National Lab.: Livermore, CA, USA, 2013. [CrossRef] [Green Version]
- Balasooriya, W.; Clute, C.; Schrittesser, B.; Pinter, G. A Review on Applicability, Limitations, and Improvements of Polymeric Materials in High-Pressure Hydrogen Gas Atmospheres. Polym. Rev. 2021, 62, 175–209. [Google Scholar] [CrossRef]
- Zhang, D.; Li, H.; Qi, D.; Ding, N.; Shao, X.; Wei, B.; Cai, X. Gas Permeation behaviors of high-density polyethylene as a liner material of flexible pipes. Nat. Gas Ind. 2017, 37, 104–109. [Google Scholar] [CrossRef]
- Humpenöder, J. Gas permeation of fibre reinforced plastics. Cryogenics 1998, 38, 143–147. [Google Scholar] [CrossRef]
- Klopffer, M.H.; Flaconneche, B. Transport Properdines of Gases in Polymers: Bibliographic Review. Oil Gas Sci. Technol. 2001, 56, 223–244. [Google Scholar] [CrossRef]
- Neogi, P. Diffusion in Polymers; CRC Press: Boca Raton, FL, USA, 1996; Volume 32, pp. 290–291. [Google Scholar]
- Su, Y.; Lv, H.; Zhou, W.; Zhang, C. Review of the Hydrogen Permeability of the Liner Material of Type IV On-Board Hydrogen Storage Tank. World Electr. Veh. J. 2021, 12, 130. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, H.; Zhou, W.; Zhang, C. Research on hydrogen permeability of polyamide 6 as the liner material for type Ⅳ hydrogen storage tank. Int. J. Hydrogen Energy 2020, 45, 24980–24990. [Google Scholar] [CrossRef]
- Zhihui, G.; Shufeng, G.; Hailong, S. Analysis and research on key technologies of non-metallic liner fully wound gas cylinder. Auto Manuf. Eng. 2022, 1, 21–27. [Google Scholar] [CrossRef]
- Wu, L.; Huang, Q.; Shi, F.; Zhang, Z.; Liu, Y. Research process on the inner liner material of carbon fiber fully-reinforced composite tank with plastic liner. New Chem. Mater. 2022, 50, 262–265. [Google Scholar] [CrossRef]
- Lee, J.K.; Yao, S.X.; Li, G.; Jun, M.B.G.; Lee, P.C. Measurement Methods for Solubility and Diffusivity of Gases and Supercritical Fluids in Polymers and Its Applications. Polym. Rev. 2017, 57, 695–747. [Google Scholar] [CrossRef]
- Macher, J.; Hausberger, A.; Macher, A.E.; Morak, M.; Schrittesser, B. Critical review of models for H2-permeation through polymers with focus on the differential pressure method. Int. J. Hydrogen Energy 2021, 46, 22574–22590. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ono, H.; Onoue, K.; Nishimura, S. High-pressure gaseous hydrogen permeation test method -property of polymeric materials for high-pressure hydrogen devices (1). Int. J. Hydrogen Energy 2020, 45, 29082–29094. [Google Scholar] [CrossRef]
- Prewitz, M.; Gaber, M.; Müller, R.; Marotztke, C.; Holtappels, K. Polymer coated glass capillaries and structures for high-pressure hydrogen storage: Permeability and hydrogen tightness. Int. J. Hydrogen Energy 2018, 43, 5637–5644. [Google Scholar] [CrossRef]
- Parodi, E.; Peters, G.W.M.; Govaert, L.E. Prediction of plasticity-controlled failure in polyamide 6: Influence of temperature and relative humidity. J. Appl. Polym. Sci. 2018, 135, 45942. [Google Scholar] [CrossRef] [Green Version]
- Barrer, R.M.; Rideal, E.K. Permeation, diffusion and solution of gases in organic polymers. Trans. Faraday Soc. 1939, 35, 628–643. [Google Scholar] [CrossRef]
- Brubaker, D.W.; Kammermeyer, K. Flow of Gases through Plastic Membranes. Ind. Eng. Chem. 1953, 45, 1148–1152. [Google Scholar] [CrossRef]
- van Amerongen, G.J. The Permeability of Different Rubbers to Gases and Its Relation to Diffusivity and Solubility. Rubber Chem. Technol. 1947, 20, 494–514. [Google Scholar] [CrossRef]
- Yamabe, J.; Nishimura, S. Influence of fillers on hydrogen penetration properties and blister fracture of rubber composites for O-ring exposed to high-pressure hydrogen gas. Int. J. Hydrogen Energy 2009, 34, 1977–1989. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ono, H.; Ohyama, K.; Kasai, M.; Kaneko, F.; Nishimura, S. Hydrogen permeation under high pressure conditions and the destruction of exposed polyethylene-property of polymeric materials for high-pressure hydrogen devices (2). Int. J. Hydrogen Energy 2021, 46, 11832–11848. [Google Scholar] [CrossRef]
- Stern, S.A.; Fang, S.M.; Jobbins, R.M. Permeation of gases at high pressures. J. Macromol. Sci. Part B 1971, 5, 41–69. [Google Scholar] [CrossRef]
- SAE J2579: 2018; Technical Information Report for Fuel Systems in Fuel Cell and Other Hydrogen Vehicles. Society of Automotive Engineers: Warrendale, PA, USA, 2018.
- SAE J2601: 2020; Fueling Protocols for Light Duty Gaseous Hydrogen Surface Vehicles. Society of Automotive Engineers: Warrendale, PA, USA, 2020.
- HFCV-GTR: 2013; Global Technical Regulation for Hydrogen Fuel Cell Vehicles. United Nations Economic Commission for Europe: Geneva, Switzerland, 2013.
- Maus, S.; Hapke, J.; Na Ranong, C.; Wüchner, E.; Friedlmeier, G.; Wenger, D. Filling procedure for vehicles with compressed hydrogen tanks. Int. J. Hydrogen Energy 2008, 33, 4612–4621. [Google Scholar] [CrossRef]
- Flaconneche, B.; Martin, J.; Klopffer, M.H. Transport Properties of Gases in Polymers: Experimental Methods. Oil Gas Sci. Technol. 2001, 56, 245–259. [Google Scholar] [CrossRef] [Green Version]
- Naito, Y.; Mizoguchi, K.; Terada, K.; Kamiya, Y. The effect of pressure on gas permeation through semicrystalline polymers above the glass transition temperature. J. Polym. Sci. Part B Polym. Phys. 1991, 29, 457–462. [Google Scholar] [CrossRef]
- Rogers, C.E. Permeation of Gases and Vapours in Polymers. In Polymer Permeability; Springer: Berlin, Germany, 1985; pp. 11–73. [Google Scholar] [CrossRef]
- Eberle, U.; Müller, B.; von Helmolt, R. Fuel cell electric vehicles and hydrogen infrastructure: Status 2012. Energy Environ. Sci. 2012, 5, 8780–8798. [Google Scholar] [CrossRef]
- Hua, T.Q.; Ahluwalia, R.K.; Peng, J.-K.; Kromer, M.; Lasher, S.; McKenney, K.; Law, K.; Sinha, J. Technical assessment of compressed hydrogen storage tank systems for automotive applications. Int. J. Hydrogen Energy 2011, 36, 3037–3049. [Google Scholar] [CrossRef] [Green Version]
- Flaconneche, B.; Martin, J.; Klopffer, M.H. Permeability, Diffusion and Solubility of Gases in Polyethylene, Polyamide 11 and Poly (Vinylidene Fluoride). Oil Gas Sci. Technol. 2001, 56, 261–278. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhou, J.; Hu, S.; Dong, S.; Wei, P. Investigations of filling mass with the dependence of heat transfer during fast filling of hydrogen cylinders. Int. J. Hydrogen Energy 2014, 39, 4380–4388. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kanagaraj, G. Investigation on Mechanical and Tribological Behaviors of PA6 and Graphite-Reinforced PA6 Polymer Composites. Arab. J. Sci. Eng. 2016, 41, 4347–4357. [Google Scholar] [CrossRef]
- Vasudeo, R.A.; Vk, A.; Vr, U.; Samarth, N.; Jadhav, S.; Patil, S.; Vr, A.; Narute, S. Development in Air Permeability of Natural Rubber Tire Tube Compound by Adding Variable Dosage of Nanoclay. Macromol. Symp. 2016, 361, 34–41. [Google Scholar] [CrossRef]
- Li, N.N.; Henley, E.J. Permeation of gases through polyethylene films at elevated pressures. AIChE J. 1964, 10, 666–670. [Google Scholar] [CrossRef]
- Li, N.N.; Long, R.B. Permeation through plastic films. AIChE J. 1969, 15, 73–80. [Google Scholar] [CrossRef]
- Stern, S.A.; Mullhaupt, J.T.; Gareis, P.J. The effect of pressure on the permeation of gases and vapors through polyethylene. Usefulness of the corresponding states principle. AIChE J. 1969, 15, 64–73. [Google Scholar] [CrossRef]
- Stern, S.A.; Fang, S.-M.; Frisch, H.L. Effect of pressure on gas permeability coefficients. A new application of “free volume” theory. J. Polym. Sci. Part A-2 Polym. Phys. 1972, 10, 201–219. [Google Scholar] [CrossRef]
- Naito, Y.; Bourbon, D.; Terada, K.; Kamiya, Y. Permeation of high-pressure gases in poly(ethylene-co-vinyl acetate). J. Polym. Sci. Part B Polym. Phys. 1993, 31, 693–697. [Google Scholar] [CrossRef]
- Fumitoshi, K.; Hirotada, F.; Shin, N. Influence of high-pressure hydrogen gas on crystalline polymers. In Proceedings of the 33rd Polymer Degradation Discussion Group, St. Julians, Malta, 1–5 September 2019. [Google Scholar]
- Pepin, J.; Lainé, E.; Grandidier, J.-C.; Castagnet, S.; Blanc-Vannet, P.; Papin, P.; Weber, M. Determination of key parameters responsible for polymeric liner collapse in hyperbaric type IV hydrogen storage vessels. Int. J. Hydrogen Energy 2018, 43, 16386–16399. [Google Scholar] [CrossRef]
- Smith, B.; Anovitz, L.M. IV.F.1 Lifecycle Verification of Polymeric Storage Liners. Available online: https://www.hydrogen.energy.gov/pdfs/progress13/iv_f_1_smith_2013.pdf (accessed on 14 July 2021).
- Condé-Wolter, J.; Ruf, M.G.; Liebsch, A.; Lebelt, T.; Koch, I.; Drechsler, K.; Gude, M. Hydrogen permeability of thermoplastic composites and liner systems for future mobility applications. Compos. Part A Appl. Sci. Manuf. 2023, 167, 107446. [Google Scholar] [CrossRef]



| Standard | CSA ANSI CHMC 2 | ISO 11114-5 | T/CATSI 02 007 |
|---|---|---|---|
| Sample source | Sample from polymer liner or prepared from polymer liner produced using same molding process as the product | Same as CSA ANSI CHMC 2 | Sample from polymer liner |
| Sampling position | Unspecified | Unspecified | Seamless liner: middle of cylinder Welded liner: middle of cylinder away from the welding seam |
| Diameter (mm) | ≥25 mm (recommend 78) | Between 40 mm and 80 mm | 78 mm |
| Thickness (mm) | 1 ≤ liner thickness ≤ 6 (recommend liner thickness) | Liner thickness | Liner thickness |
| Standard | CSA ANSI CHMC 2 | ISO 11114-5 | T/CATSI 02 007 |
|---|---|---|---|
| Drying temperature | Recommend 60 °C | Recommend 65 °C | Unspecified |
| Vacuum pressure | Unspecified | Recommend 10–50 mbar | Unspecified |
| Vacuum drying finish conditions | Mass loss rate within 48 h or 1 h of drying is <0.5% | The mass loss rate within 24 h of drying is <0.1% | Unspecified |
| Standard | CSA ANSI CHMC 2 | ISO 11114-5 | T/CATSI 02 007 |
|---|---|---|---|
| Device structure diagram |  |  | Unspecified |
| Test Method | HPHP method | HPHP method | HPHP method |
| Sintered metal plate porosity | Porosity reaches grade 2 | Unspecified | Unspecified |
| Wire mesh size | 140 μm | 150 μm | Unspecified |
| Hydrogen exposure zone diameter | ≥di mm | ≥di mm | ≥di mm |
| Standard | CSA ANSI CHMC 2 | ISO 11114-5 | T/CATSI 02 007 |
|---|---|---|---|
| Test temperature and pressure | (85 ± 1) °C, 1.25 NWP, (15 ± 1) °C, 1.25 NWP, (85 ± 1) °C, 0.1 NWP, (15 ± 1) °C, 0.1 NWP. | Test should be carried out at a certain pressure and temperature | (55 ± 1) °C, 1.15 NWP, (15 ± 1) °C, NWP, (55 ± 1) °C, 0.1 MPa, (15 ± 1) °C, 0.1 MPa. |
| Standard | CSA ANSI CHMC 2 | ISO 11114-5 | T/CATSI 02 007 |
|---|---|---|---|
| Qualification indicators | The steady-state gas transmission rate measured at 15 °C for a sample with a diameter of 78 mm is divided into six rating values: 10: ≤0.8 Ncm³/h, 8: >0.8–1.5 Ncm³/h, 6: >1.5–3 Ncm³/h, 4: >3–6 Ncm³/h, 2: >6–16 Ncm³/h, and 0: >16 Ncm³/h. | Unspecified | At 15 °C, Pe ≤ 1 × 10−13 cm3·cm/(cm2·s·Pa), At 55 °C, Pe ≤ 1 × 10−12 cm3·cm/(cm2·s·Pa) |
| Polymer | Pressure/Bar | Pe/mol H2·m/(m2·s) |
|---|---|---|
| Injection-molded HDPE | 134 | 2.08 × 10−10 |
| Extrusion-molded HDPE | 104 | 5.51 × 10−11 |
| Rotomolded HDPE | 345 | 2.58 × 10−10 |
| Extrusion-molded PA6 | 137 | 3.38 × 10−11 |
| Compression-molded TLCP | 136 | 7.09 × 10−13 |
| Blow-molded PET | 137 | 6.71 × 10−12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Huang, Q.; Liu, Y.; Zhao, B.; Li, J. Review of the Hydrogen Permeation Test of the Polymer Liner Material of Type IV On-Board Hydrogen Storage Cylinders. Materials 2023, 16, 5366. https://doi.org/10.3390/ma16155366
Li X, Huang Q, Liu Y, Zhao B, Li J. Review of the Hydrogen Permeation Test of the Polymer Liner Material of Type IV On-Board Hydrogen Storage Cylinders. Materials. 2023; 16(15):5366. https://doi.org/10.3390/ma16155366
Chicago/Turabian StyleLi, Xiang, Qianghua Huang, Yitao Liu, Baodi Zhao, and Jiepu Li. 2023. "Review of the Hydrogen Permeation Test of the Polymer Liner Material of Type IV On-Board Hydrogen Storage Cylinders" Materials 16, no. 15: 5366. https://doi.org/10.3390/ma16155366
APA StyleLi, X., Huang, Q., Liu, Y., Zhao, B., & Li, J. (2023). Review of the Hydrogen Permeation Test of the Polymer Liner Material of Type IV On-Board Hydrogen Storage Cylinders. Materials, 16(15), 5366. https://doi.org/10.3390/ma16155366







