Development of Molecular Dynamics and Research Progress in the Study of Slag
Abstract
1. Introduction
2. General Situation Analysis of Molecular Dynamics
2.1. Development of Molecular Dynamics, Simulation Process, Common Algorithms and Boundary Conditions
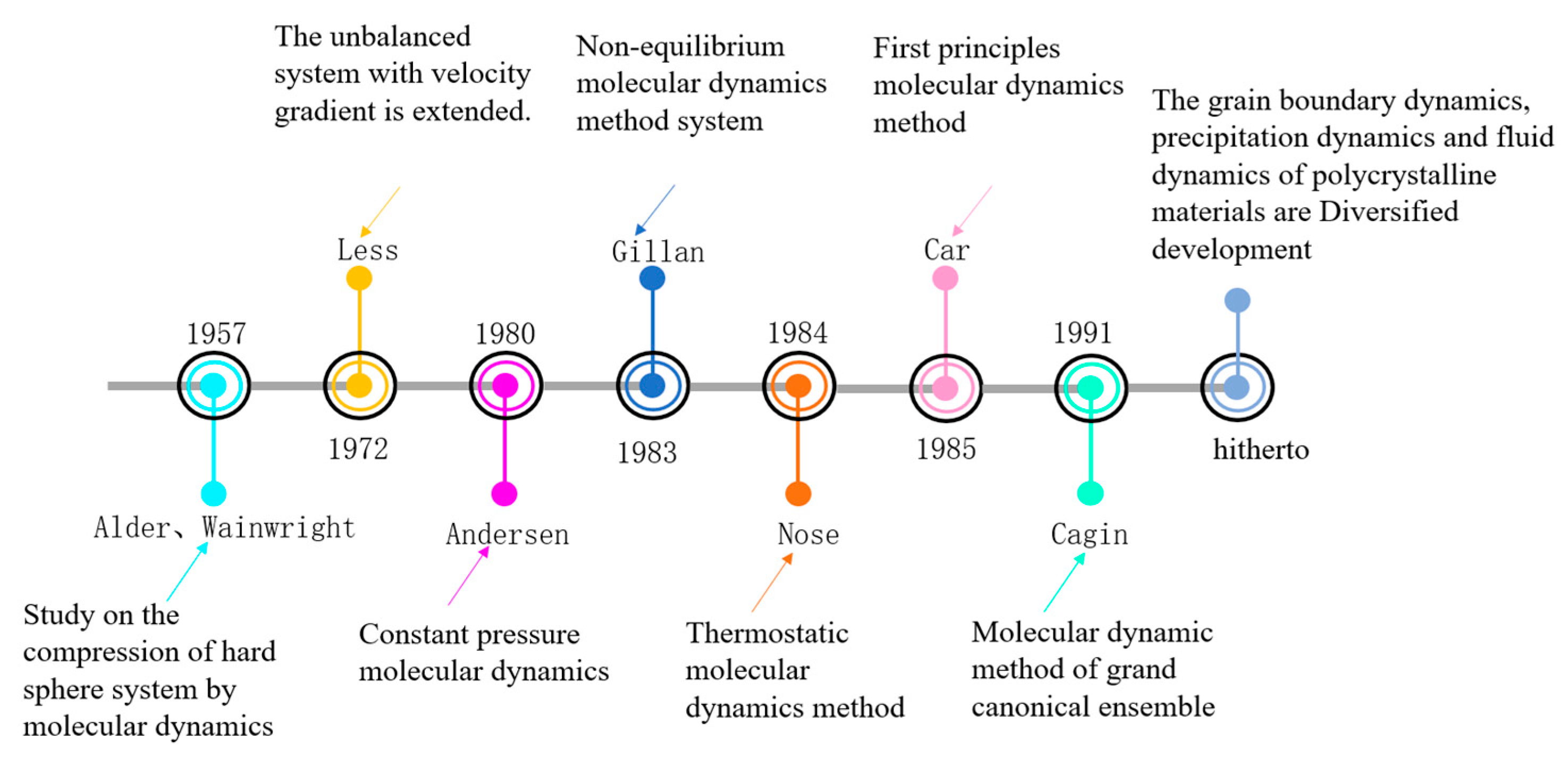
2.1.1. Development Profile Analysis
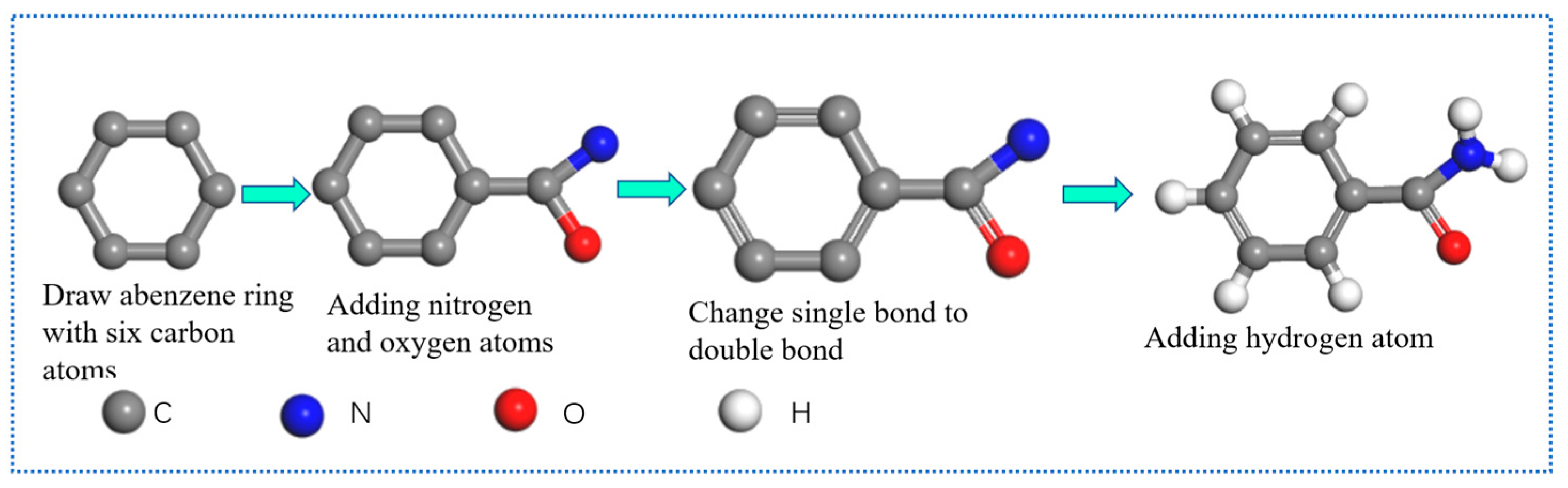
2.1.2. Overview Analysis of Simulation Steps
2.1.3. Overview Analysis of Common Algorithms
- 1.
- Verlet algorithm
- 2.
- Speed Verlet algorithm
- 3.
- Leapfrog algorithm
- 4.
- Beeman algorithm
- 5.
- Rahman algorithm
2.1.4. Boundary Conditions
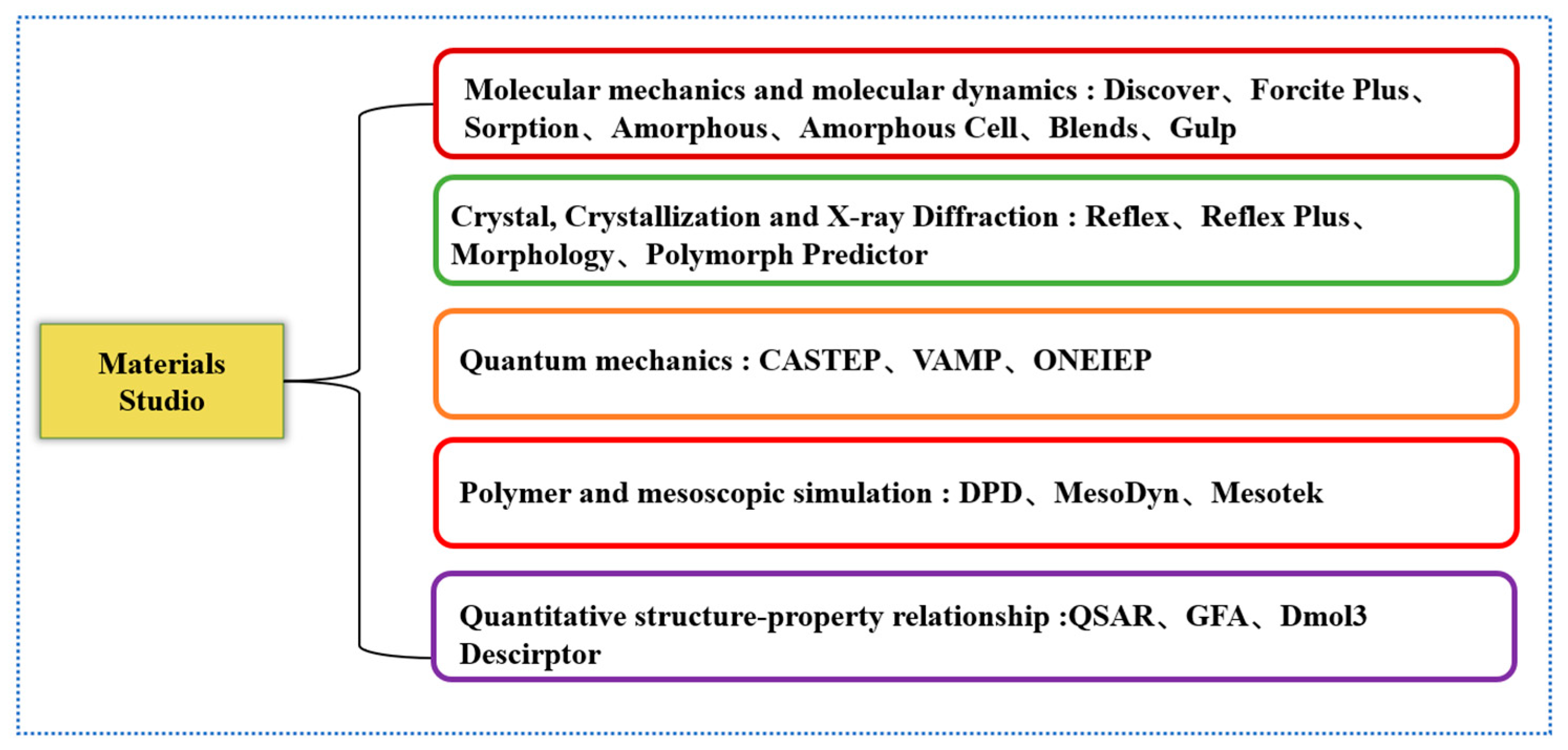
2.2. General Analysis of Common Software for Molecular Dynamics Simulation
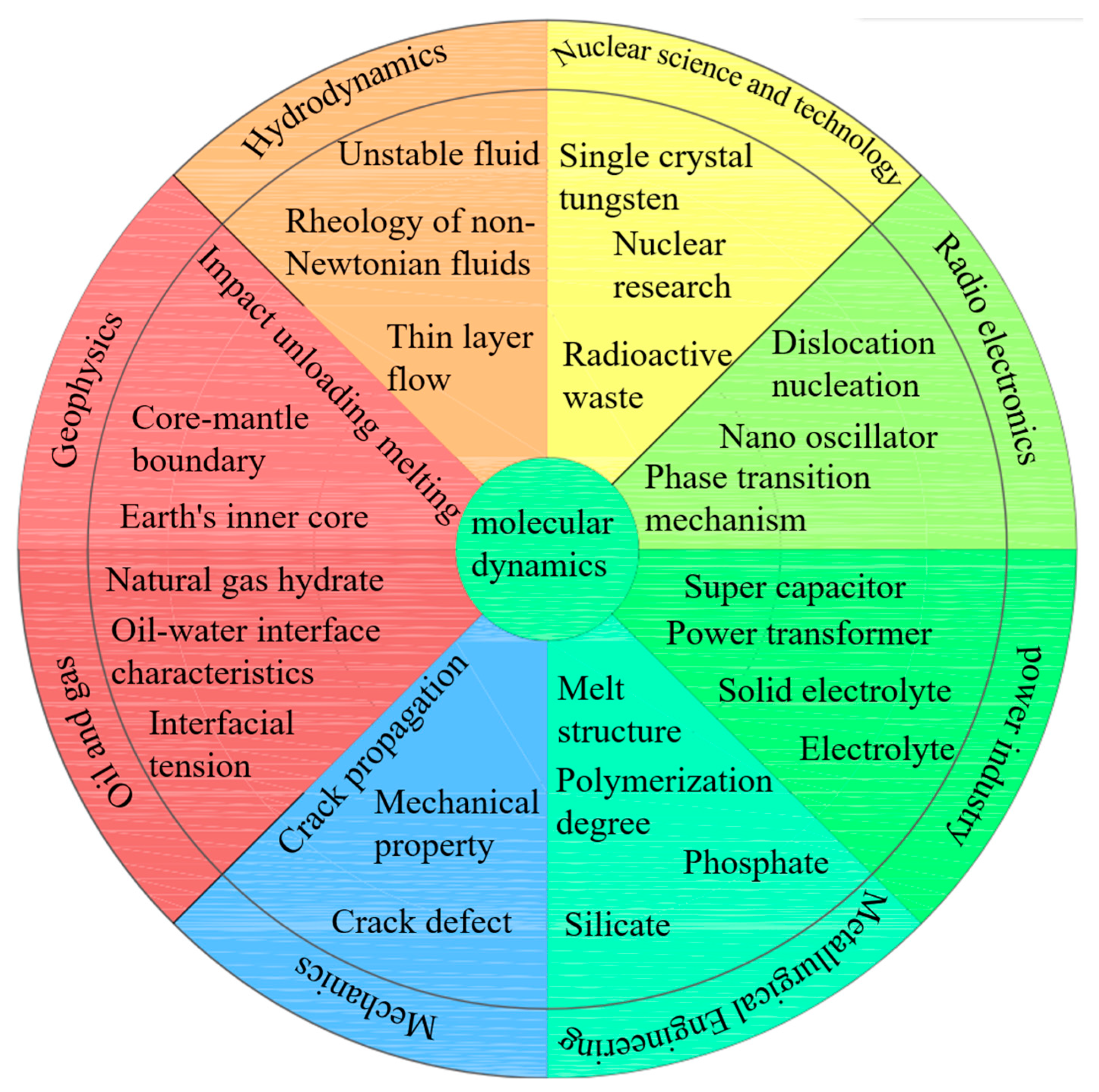
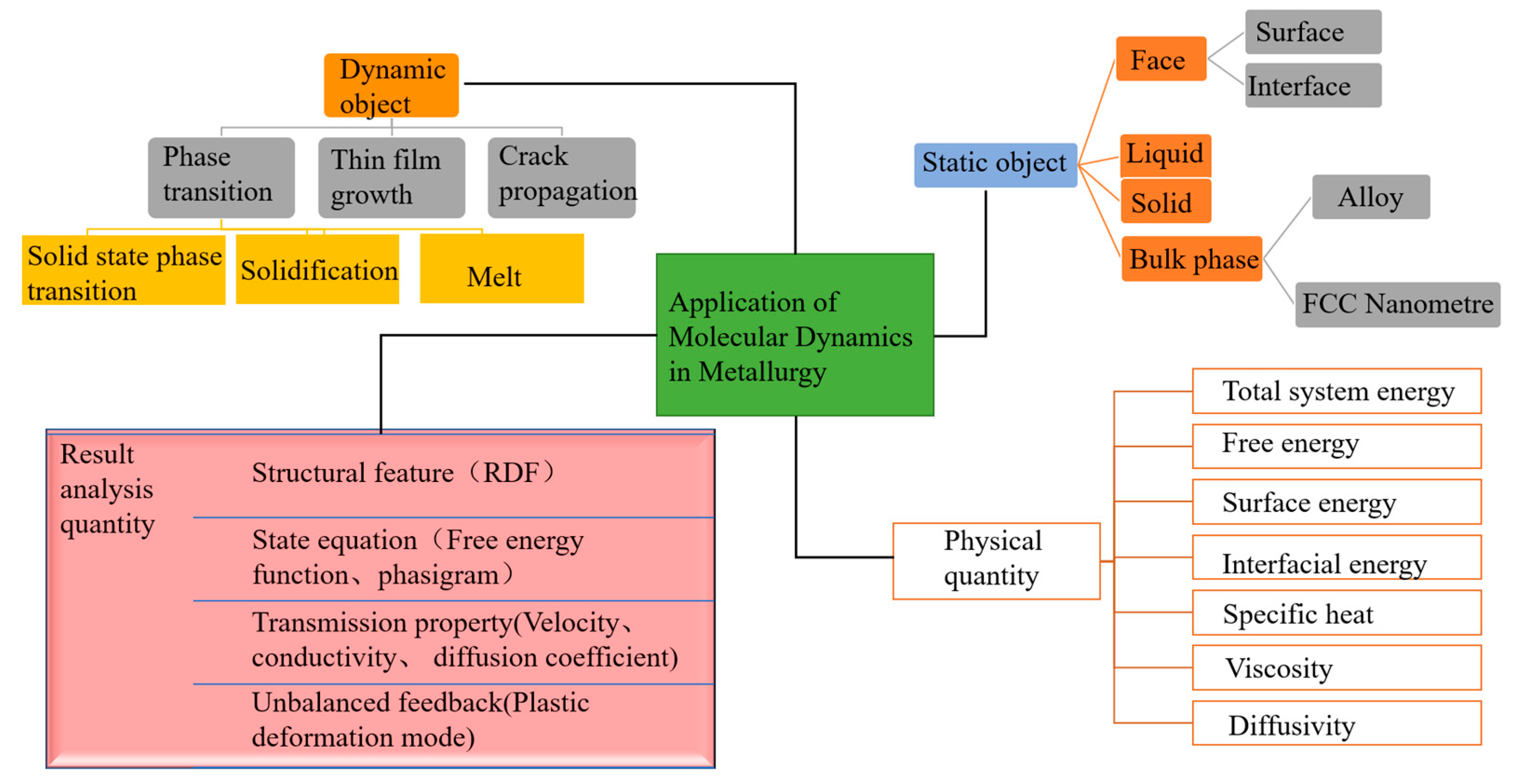
2.3. Overview of Molecular Dynamics Application Fields
3. Research and Application Analysis of Molecular Dynamics in Slag
3.1. Research Status and Application Analysis in Phosphate Residue
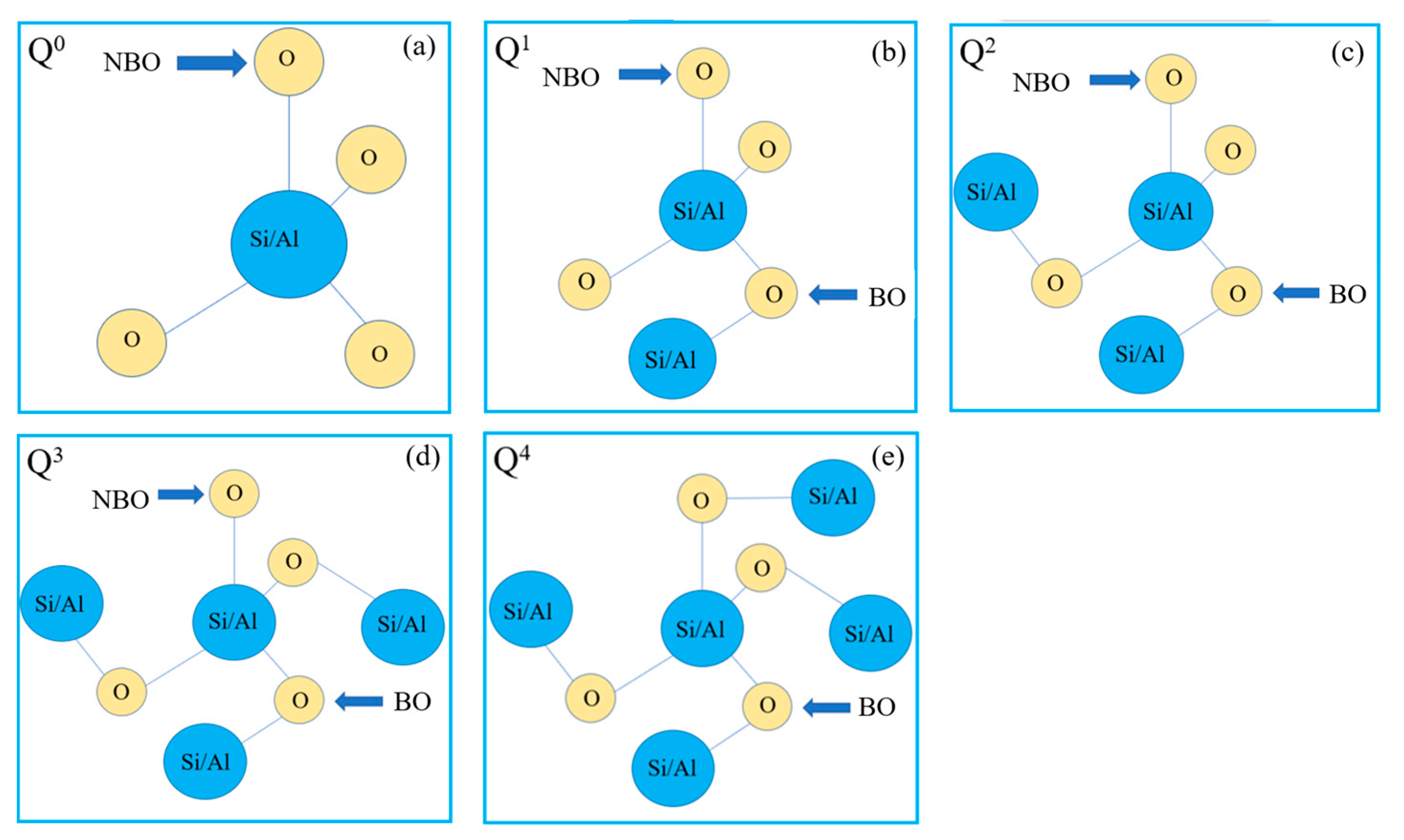
3.2. Research Status and Application Analysis in Silicate Slag
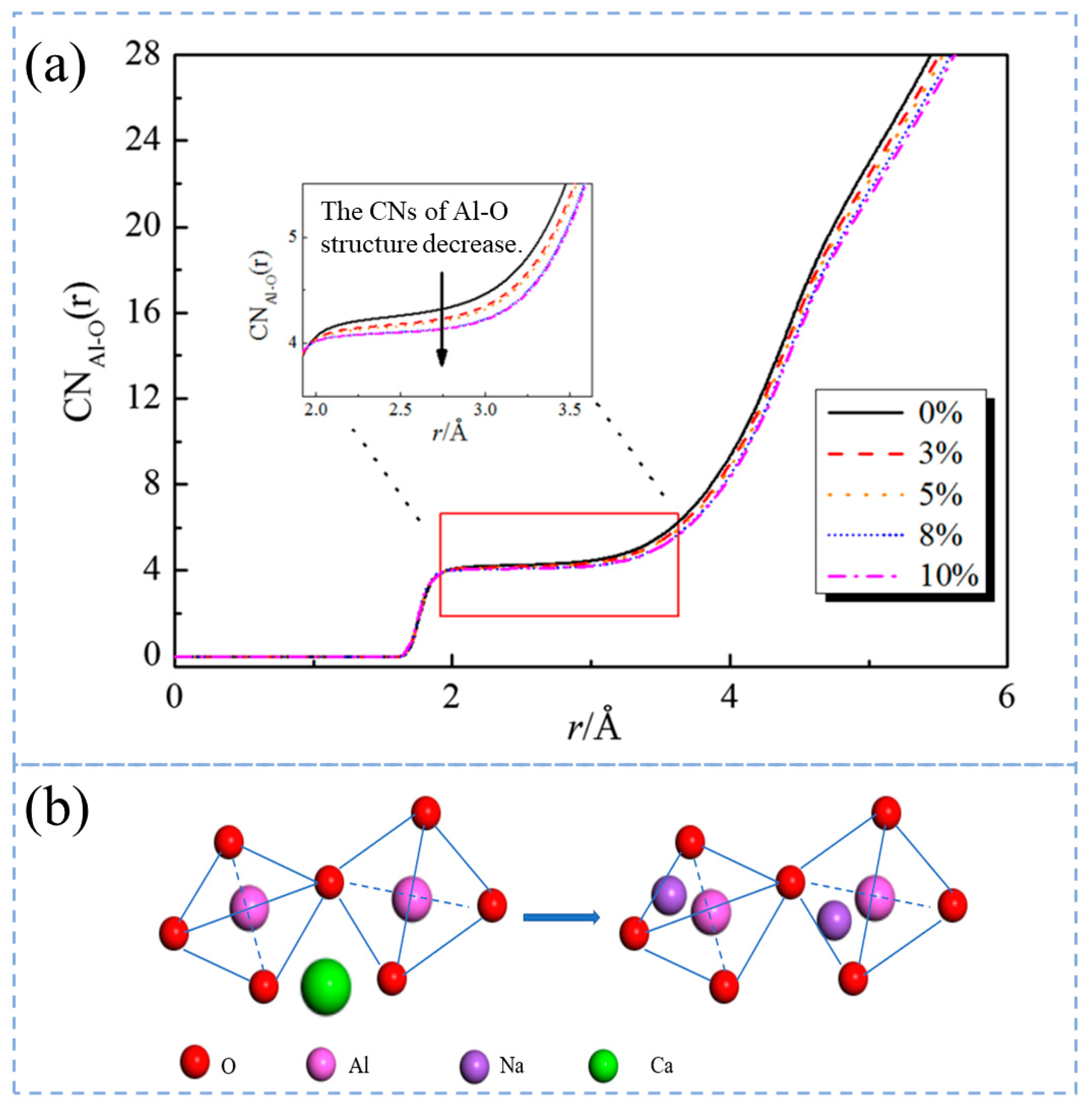
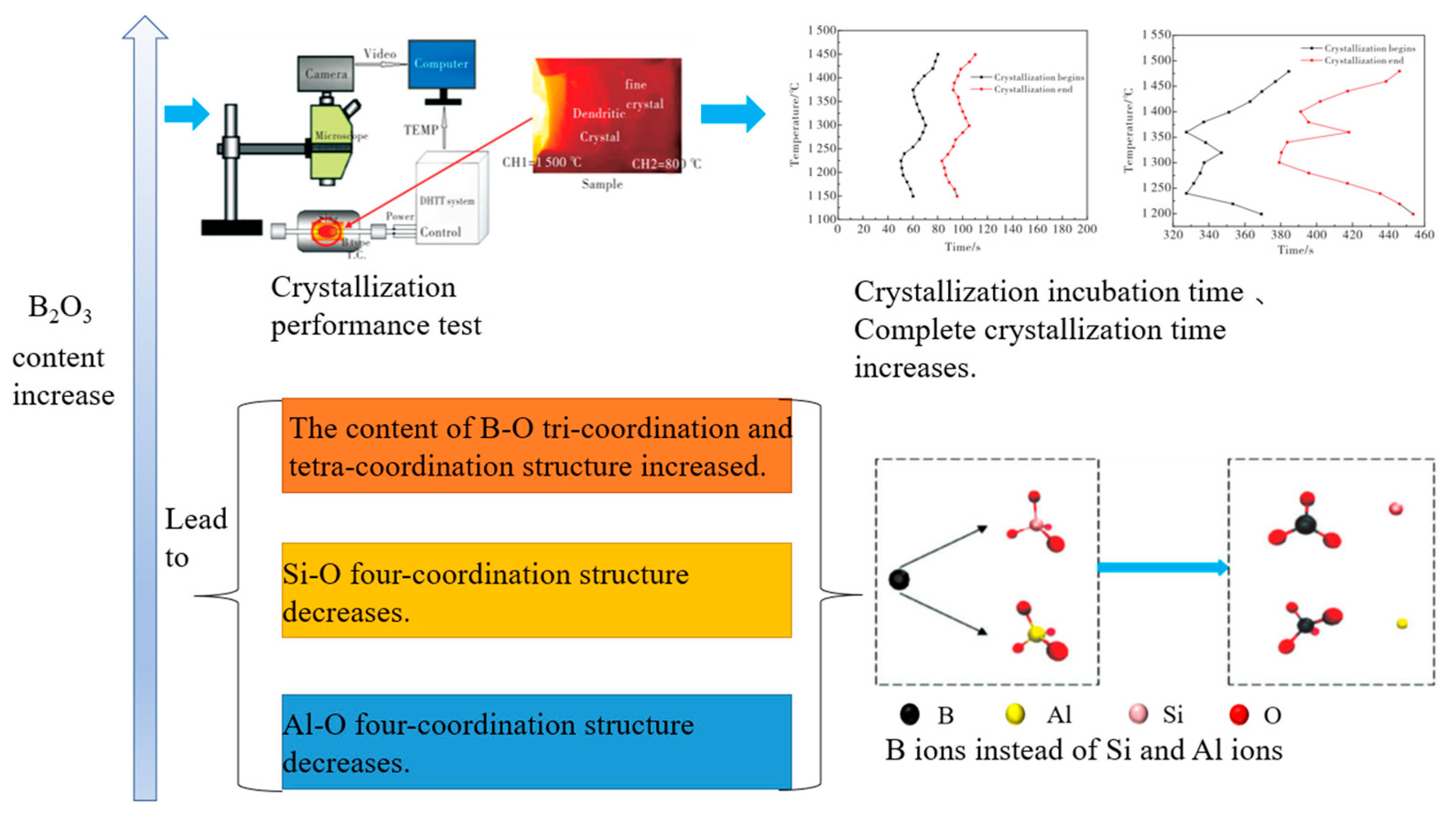
3.3. Research Status and Application Analysis in Aluminate Slag
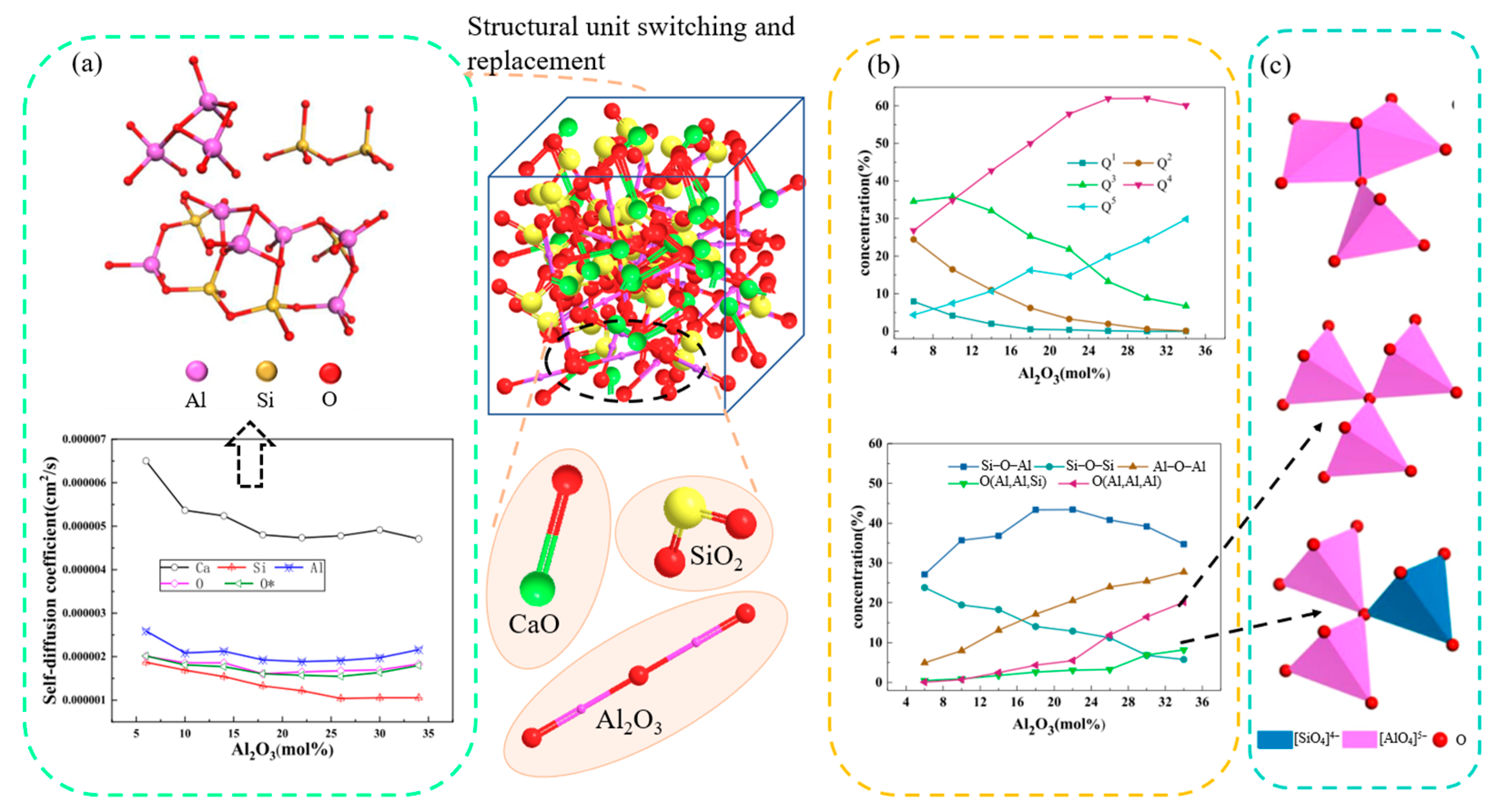
3.4. Research Status and Application Analysis in Aluminosilicate Slag
4. Conclusions and Outlook
- In the future, researchers will devote themselves to developing more targeted new potential functions to describe the behavior and characteristics of microstructures more accurately. This new potential function is used to solve problems in small-scale systems such as atoms and molecular clusters. There is a need for in-depth study and analysis of microstructure, design of more accurate and reliable models, and promotion of the research and application at atomic and molecular levels.
- In the future, efforts will be made to further integrate molecular dynamics simulation methods with experiments to jointly explore the viscosity and other macroscopic properties of slag systems, such as activity, thermodynamic properties, and oxidation. Through accurate simulation and experimental verification, the complex behavior and interaction of slag systems can be revealed, and the understanding of microstructure in slag can be improved.
- Most molecular dynamics simulation environments are usually assumed to be vacuum environments because vacuum environments can reduce the influence of intermolecular interaction and make the simulation simpler and faster. However, under the actual laboratory conditions, it is difficult to achieve a complete vacuum environment. Laboratories often choose to use inert gases, such as argon, and future research and technical development may create more accurate molecular dynamics simulations in different gas environments. By simulating the behavior of samples in different gas environments, the actual situation in the laboratory can be more accurately reflected, and the reliability and accuracy of the simulation results can be increased.
- In the phosphate slag system, important processes such as crystal growth and phase transformation are usually complex and long. These processes involve a series of microscopic changes such as interface reconstruction and energy redistribution. In future research, the simulation time of the phosphate slag system can be further increased to obtain a more comprehensive and detailed behavior evolution. By prolonging the simulation time, researchers observe the more complex and slow process, gain deeper insight, and understand the evolution process of the system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, K.; Zhang, Z.; Yang, F.; Sridhar, S. Molecular dynamics study of the structural properties of calcium aluminosilicate slags with varying Al2O3/SiO2 ratios. ISIJ Int. 2012, 52, 342–349. [Google Scholar] [CrossRef]
- Lin, G.; Guo, J.; Ji, P. Molecular dynamics study on the diffusion process of AuAgCuNiPd high-entropy alloy metallurgy induced by pulsed laser heating. Phys. Chem. Chem. Phys. 2021, 23, 19482–19493. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Li, K.; Jiang, C.; Zhang, J.; Ma, S.; Sun, M.; Wang, Z.; Li, H. Effects of B2O3 on the structure and properties of blast furnace slag by molecular dynamics simulation. J. Non-Cryst. Solids 2021, 551, 120412. [Google Scholar] [CrossRef]
- Yuelin, Q.; Hao, L.; Yanhua, Y. Structure evolution of blast furnace slag with high Al2O3 Content and 5 mass% TiO2 via molecular dynamics simulation and fourier transform infrared spectroscopy. Metall. Res. Technol. 2018, 115, 113. [Google Scholar] [CrossRef]
- Hei, B.; Schwartz, S.D. Classical molecular dynamics, transition path sampling, and umbrella sampling to investigate the structural, conformational, and proton transfer properties of glyonic liquids. Biophys. J. 2022, 121, 542a. [Google Scholar] [CrossRef]
- Kim, Y.; Han, Y.; Kim, S.; Jeon, H.S. Green extraction of lithium from waste lithium aluminosilicate glass-ceramics using a water leaching process. Process Saf. Environ. 2021, 148, 765–774. [Google Scholar] [CrossRef]
- Podda, O.; Tissandier, L.; Laplace, A.; Deloule, E. Solubility of uranium oxide in ternary aluminosilicate glass melts. J. Non-Cryst. Solids 2022, 595, 121845. [Google Scholar] [CrossRef]
- Lu, X.; Du, J. Quantitative structure-property relationship (QSPR) analysis of calcium aluminosilicate glasses based on molecular dynamics simulations. J. Non-Cryst. Solids 2020, 530, 119772. [Google Scholar] [CrossRef]
- Liang, J.J.; Cygan, R.T.; Alam, T.M. Molecular dynamics simulation of the structure and properties of lithium phosphate glasses. J. Non-Cryst. Solids 2000, 263, 167–179. [Google Scholar] [CrossRef]
- Xiang, Y.; Du, J.; Smedskjaer, M.M.; Mauro, J.C. Structure and properties of sodium aluminosilicate glasses from molecular dynamics simulations. J. Chem. Phys. 2013, 139, 044507. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, X.; Zhang, M.; Jia, X. Effect of CaF2 on viscosity, structure and properties of CaO-Al2O3-MgO-SiO2 slag glass ceramics. J. Non-Cryst. Solids 2018, 500, 310–316. [Google Scholar] [CrossRef]
- Rantitsch, G.; Bhattacharyya, A.; Schenk, J.; Lünsdorf, N.K. Assessing the quality of metallurgical coke by Raman spectroscopy. Int. J. Coal Geol. 2014, 130, 1–7. [Google Scholar] [CrossRef]
- Trucano, P.; Chen, R. Structure of graphite by neutron diffraction. Nature 1975, 258, 136–137. [Google Scholar] [CrossRef]
- Wang, G.C.; Fan, C.G.; Li, M.Z.; Zhang, L.F.; Fang, K.M. Application and prospect of molecular dynamics in iron and steel metallurgy. Mater. Rep. China 2013, 27, 134–138+151. [Google Scholar]
- Li, H.; Xu, F.; Li, B.; Sun, T.; Huang, X.; Zhu, J.; Peng, C.; Lin, J.; Chen, Z. Investigation on mechanical properties of excess-sulfate phosphogypsum slag cement: From experiments to molecular dynamics simulation. Constr. Build. Mater. 2022, 315, 125685. [Google Scholar] [CrossRef]
- Alder, B.J.; Wainwright, T.E. Phase Transition for a Hard Sphere System. J. Chem. Phys. 1957, 27, 1208–1209. [Google Scholar] [CrossRef]
- Lees, A.W.; Edwards, S.F. The computer study of transport processes under extreme conditions. J. Phys. C Solid State Phys. 1972, 5, 1921. [Google Scholar] [CrossRef]
- Andersen, H.C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef]
- Car, R.; Parrinello, M. Unified approach for molecular dynamics and density-functional theory. Phys Rev Lett. 1985, 55, 2471. [Google Scholar] [CrossRef]
- Cagin, T.; Pettitt, B.M. Grand molecular dynamics: A method for open systems. Mol. Simulat. 1991, 6, 5–26. [Google Scholar] [CrossRef]
- Han, J.; Thomas, S.L.; Srolovitz, D.J. Grain-boundary kinetics: A unified approach. Prog. Mater. Sci. 2018, 98, 386–476. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Y.; Brown, B.; Nesic, S.; Singer, M. Investigation of precipitation kinetics of FeCO3 by EQCM. Corros. Sci. 2018, 141, 195–202. [Google Scholar] [CrossRef]
- Chen, T.B.Y.; De Cachinho Cordeiro, I.M.; Yuen, A.C.Y.; Yang, W.; Chan, Q.N.; Zhang, J.; Cheung, S.C.P.; Yeoh, G.H. An investigation towards coupling molecular dynamics with computational fluid dynamics for modelling polymer pyrolysis. Molecules 2022, 27, 292. [Google Scholar] [CrossRef] [PubMed]
- Kalinichev, A.G.; Wang, J.; Kirkpatrick, R.J. Molecular dynamics modeling of the structure, dynamics and energetics of mineral–water interfaces: Application to cement materials. Cement Concrete Res. 2007, 37, 337–347. [Google Scholar] [CrossRef]
- Tang, Y.; Pan, H.; Li, D.Y. Contribution of cold-work to the wear resistance of materials and its limitation—A study combining molecular dynamics modeling and experimental investigation. Wear 2021, 476, 203642. [Google Scholar] [CrossRef]
- Daw, M.S.; Baskes, M.I. Embedded-atom method: Derivation and application to impurities, surfaces, and other defects in metals. Phys. Rev. B 1984, 29, 6443. [Google Scholar] [CrossRef]
- Li, Q.; Dong, Y.; Perez, D.; Martini, A.; Carpick, R.W. Speed dependence of atomic stick-slip friction in optimally matched experiments and molecular dynamics simulations. Phys. Rev. Lett. 2011, 106, 126101. [Google Scholar] [CrossRef]
- Fattebert, J.L.; Richards, D.F.; Glosli, J.N. Dynamic load balancing algorithm for molecular dynamics based on Voronoi cells domain decompositions. Comput. Phys. Commun. 2012, 183, 2608–2615. [Google Scholar] [CrossRef]
- Bekker, G.J.; Kamiya, N. N-Terminal-Driven Binding Mechanism of an Antigen Peptide to Human Leukocyte Antigen-A* 2402 Elucidated by Multicanonical Molecular Dynamic-Based Dynamic Docking and Path Sampling Simulations. J. Phys. Chem. B 2021, 125, 13376–13384. [Google Scholar] [CrossRef]
- Zepeda-Ruiz, L.A.; Stukowski, A.; Oppelstrup, T.; Bulatov, V.V. Probing the limits of metal plasticity with molecular dynamics simulations. Nature 2017, 550, 492–495. [Google Scholar] [CrossRef]
- Verlet, L. Computer “experiments” on classical fluids. I. Thermodynamical properties of Lennard-Jones molecules. Phys. Rev. 1967, 159, 98. [Google Scholar] [CrossRef]
- Swope, W.C.; Andersen, H.C.; Berens, P.H.; Wilson, K.R. A computer simulation method for the calculation of equilibrium constants for the formation of physical clusters of molecules: Application to small water clusters. J. Chem. Phys. 1982, 76, 637–649. [Google Scholar] [CrossRef]
- Hockney, R.W. The potential calculation and some applications. Methods Comput. Phys. 1970, 9, 136. [Google Scholar]
- Beeman, D. Some multistep methods for use in molecular dynamics calculations. J. Comput. Phys. 1976, 20, 130–139. [Google Scholar] [CrossRef]
- Rahman, A. Correlations in the motion of atoms in liquid argon. Phys. Rev. 1964, 136, A405. [Google Scholar] [CrossRef]
- Metropolis, N.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H.; Teller, E. Equation of state calculations by fast computing machines. J. Chem. Phys. 1953, 21, 1087–1092. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Yan, H.; Nie, B.; Peng, C.; Liu, P.; Wang, X.; Yin, F.; Gong, J.; Wei, Y.; Lin, S. Molecular Model Construction of Low-Quality Coal and Molecular Simulation of Chemical Bond Energy Combined with Materials Studio. Energy Fuel 2021, 35, 17602–17616. [Google Scholar] [CrossRef]
- Song, L.; Liu, W.; Xin, F.; Li, Y. “Materials Studio” Simulation Study of the Adsorption and Polymerization Mechanism of Sodium Silicate on Active Silica Surface at Different Temperatures. Int. J. Metalcast. 2021, 15, 1091–1098. [Google Scholar] [CrossRef]
- Siafakas, D.; Matsushita, T.; Jarfors, A.E.W.; Hakamada, S.; Watanabe, M. Viscosity of SiO2-CaO-Al2O3 Slag with Low Silica—Influence of CaO/Al2O3, SiO2/Al2O3 Ratio. ISIJ Int. 2018, 58, 2180–2185. [Google Scholar] [CrossRef]
- Wu, C.D.; Liao, B.W.; Chen, Y.L.; Pan, C.W.; Chen, P.Y.; Chan, P.L.; Shih, S.T. Molecular dynamics simulation of effects of microstructure and loading mode on mechanical properties of Au nanowires. Mol. Simulat. 2020, 46, 1291–1297. [Google Scholar] [CrossRef]
- Binder, K.; Horbach, J.; Kob, W.; Paul, W.; Varnik, F. Molecular dynamics simulations. J. Phys. Condens. Mat. 2004, 16, S429. [Google Scholar] [CrossRef]
- Colin-York, H.; Heddleston, J.; Wait, E.; Karedla, N.; DeSantis, M.; Khuon, S.; Chew, T.; Sbalzarini, L.F.; Fritzsche, M. Quantifying Molecular Dynamics within Complex Cellular Morphologies using LLSM-FRAP. Small Methods 2022, 6, 2200149. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, J.; Qiu, Z.; Cui, Z.; Li, N.; Li, X.; Wang, Y.; Zhang, H.; Zhao, C. Effects of polyethylene microplastics on cell membranes: A combined study of experiments and molecular dynamics simulations. J. Hazard. Mater. 2022, 429, 128323. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Z.; Yang, K.; Krishnan, N.A.; Smedskjaer, M.M.; Sant, G.; Bauchy, M. Rigidity theory of glass: Determining the onset temperature of topological constraints by molecular dynamics. J. Non-Cryst. Solids 2021, 554, 120614. [Google Scholar] [CrossRef]
- Hospital, A.; Goñi, J.R.; Orozco, M.; Gelpí, J.L. Molecular dynamics simulations: Advances and applications. Adv. Appl. Bioinform. Chem. 2015, 8, 37–47. [Google Scholar]
- Van Gunsteren, W.F.; Berendsen, H.J.C. Computer simulation of molecular dynamics: Methodology, applications, and perspectives in chemistry. Angew. Chem. Int. Ed. Engl. 1990, 29, 992–1023. [Google Scholar] [CrossRef]
- Guo, X.; Li, Q.; Liu, T.; Kang, R.; Jin, Z.; Guo, D. Advances in molecular dynamics simulation of ultra-precision machining of hard and brittle materials. Front. Mech. Eng. 2017, 12, 89–98. [Google Scholar] [CrossRef]
- Shao, C.D.; Ding, J.G.; Ni, H.H.; Yang, H.; Ding, Y. Molecular dynamics simulation of radial film stiffness of aerostatic spindle system of ultra-precision machining machine tool. J. Nanjing Univ. Sci. Technol. China 2018, 42, 142–147. [Google Scholar]
- Lourenco, T.C.; Ebadi, M.; Brandell, D.; Da Silva, J.L.; Costa, L.T. Correction to “Interfacial Structures in Ionic Liquid-Based Ternary Electrolytes for Lithium-Metal Batteries: A Molecular Dynamics Study”. J. Phys. Chem. B 2021, 125, 11337. [Google Scholar] [CrossRef]
- Liu, M.; Yang, J.F. Molecular dynamics simulation of electrolyte performance during charging and discharging of lithium-ion battery. J. Nanjing Normal Univ. Nat. Sci. Ed. China 2020, 43, 43–48. [Google Scholar]
- Galvez-Aranda, D.E.; Seminario, J.M. Li-Metal Anode in a Conventional Li-Ion Battery Electrolyte: Solid Electrolyte Interphase Formation using Ab Initio Molecular Dynamics. J. Electrochem. Soc. 2022, 169, 030502. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Y.; Huo, F.; He, H.; Zhang, S. Understanding Structural and Transport Properties of Dissolved Li2S8 in Ionic Liquid Electrolytes through Molecular Dynamics Simulations. ChemPhysChem 2021, 22, 419–429. [Google Scholar] [CrossRef]
- Belashchenko, D.K. Computer simulation of the structure and properties of non-crystalline oxides. Russ. Chem. Rev. 1997, 66, 733. [Google Scholar] [CrossRef]
- Belonoshko, A.B.; Dubrovinsky, L.S. Molecular and lattice dynamics study of the MgO-SiO2 system using a transferable interatomic potential. Geochim. Cosmochim. Acta 1996, 60, 1645–1656. [Google Scholar] [CrossRef]
- Tsuneyuki, S.; Tsukada, M.; Aoki, H.; Matsui, Y. First-principles interatomic potential of silica applied to molecular dynamics. Phys. Rev. Lett. 1988, 61, 869. [Google Scholar] [CrossRef]
- Shahbabaei, M.; Kim, D. Nanofluidics for Gas Separation Applications: The Molecular Dynamics Simulation Perspective. Sep. Purif. Rev. 2022, 51, 245–260. [Google Scholar] [CrossRef]
- Hawari, A.I. Modern Techniques for Inelastic Thermal Neutron Scattering Analysis. Nucl. Data Sheets 2014, 118, 172–175. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, S.L. Application of molecular dynamics simulation in tertiary oil recovery. Sci. Sin. Chim. China 2021, 51, 761–771. [Google Scholar] [CrossRef]
- Tong, R.T.; Zhang, X.; Zhang, T.; Du, J.T.; Liu, G. Molecular Dynamics Simulation on Friction Properties of Textured Surfaces in Nanoscale Rolling Contacts. J. Mater. Eng. Perform. 2022, 31, 5736–5746. [Google Scholar] [CrossRef]
- Yasbolaghi, R.; Khoei, A.R. Micro-structural aspects of fatigue crack propagation in atomistic-scale via the molecular dynamics analysis. Eng. Fract. Mech. 2020, 226, 106848. [Google Scholar] [CrossRef]
- Hansen-Dörr, A.C.; Wilkens, L.; Croy, A.; Dianat, A.; Cuniberti, G.; Kästner, M. Combined molecular dynamics and phase-field modelling of crack propagation in defective graphene. Comput. Mater. Sci. 2019, 163, 117–126. [Google Scholar] [CrossRef]
- Saitoh, K.I.; Takai, Y.; Sato, T.; Takuma, M.; Takahashi, Y. Optimization of LIB Electrolyte and Exploration of Novel Compounds via the Molecular Dynamics Method. Batteries 2022, 8, 27. [Google Scholar] [CrossRef]
- Yin, H.; Gao, G.; Liu, K.; Yang, Y.; Wu, G.; Ren, J. Decomposition properties of two phase immersion cooling medium C6F12O: A computational study. Chem. Phys. Lett. 2022, 794, 139505. [Google Scholar] [CrossRef]
- Walton, O.R. Application of molecular dynamics to macroscopic particles. Int. J. Eng. Sci. 1984, 22, 1097–1107. [Google Scholar] [CrossRef]
- Ikeshoji, T.; Hafskjold, B. Non-equilibrium molecular dynamics calculation of heat conduction in liquid and through liquid-gas interface. Mol. Phys. 1994, 81, 251–261. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Li, D. Molecular dynamics simulation of nanoscale liquid flows. Microfluid Nanofluid 2010, 9, 1011–1031. [Google Scholar] [CrossRef]
- Han, M. Thermophoresis in liquids: A molecular dynamics simulation study. J. Colloid Interface Sci. 2005, 284, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Do Hong, S.; Ha, M.Y.; Balachandar, S. Static and dynamic contact angles of water droplet on a solid surface using molecular dynamics simulation. J. Colloid Interface Sci. 2009, 339, 187–195. [Google Scholar] [CrossRef]
- Chen, J.; Huo, D.; Yeddu, H.K. Molecular dynamics study of phase transformations in NiTi shape memory alloy embedded with precipitates. Mater. Res. Express 2021, 8, 106508. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Shi, T. Molecular Dynamics Simulation on the Deposition Characteristics between Pt Cluster and Ni Substrate in Cold Gas Dynamic Spraying. Coatings 2022, 12, 142. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ishii, K. Molecular Dynamics Analysis of Three-dimensional Anionic Structures of Molten Al2O3-Na2O-SiO2 System. ISIJ Int. 2004, 44, 43–49. [Google Scholar] [CrossRef][Green Version]
- Zhu, M.; Wu, G.; Azarov, A.; Monakhov, E.; Tang, K.; Müller, M.; Safarian, J. Effects of La2O3 Addition into CaO-SiO2 Slag: Structural Evolution and Impurity Separation from Si-Sn Alloy. Metall. Mater. Trans. B 2021, 52, 3045–3063. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, F.; Wang, B.; Liu, Q.; Lu, X.; Hu, L.; Cai, F. The control and prediction of end-point phosphorus content during BOF steelmaking process. Steel Res. Int. 2014, 85, 599–606. [Google Scholar] [CrossRef]
- Yang, X.; Sun, F.M.; Yang, J.L.; Liu, F.; Cheng, K.S.; Wang, J.H. Optimization of low phosphorus steel production with double slag process in BOF. J. Iron Steel Res. Int. 2013, 20, 41–47. [Google Scholar] [CrossRef]
- Zhu, L.J.; Gu, Z.W.; Xu, H.; Lü, Y.; Chao, J. Modeling of microstructure evolution in 22MnB: Steel during hot stamping. J. Iron Steel Res. Int. 2014, 21, 197–201. [Google Scholar] [CrossRef]
- Zhou, C.G.; Yang, H.Z.; Ai, L.Q.; Wang, S.H.; Hu, J.Z.; Chen, H. Research status and prospect of recycling technology of converter slag containing phosphorus. Iron Steel China 2021, 56, 22–39. [Google Scholar]
- Shojaei, S.; Shahgholi, M.; Karimipour, A. The effects of atomic percentage and size of Zinc nanoparticles, and atomic porosity on thermal and mechanical properties of reinforced calcium phosphate cement by molecular dynamics simulation. J. Mech. Behav. Biomed. 2023, 141, 105785. [Google Scholar] [CrossRef]
- Mahjoory, M.; Shahgholi, M.; Karimipour, A. The effects of initial temperature and pressure on the mechanical properties of reinforced calcium phosphate cement with magnesium nanoparticles: A molecular dynamics approach. Int. Commun. Heat Mass Transf. 2022, 135, 106067. [Google Scholar] [CrossRef]
- Du, Y.; Yuan, Y.; Li, L.; Long, M.; Duan, H.; Chen, D. Insights into structure and properties of P2O5-based binary systems through molecular dynamics simulations. J. Mol. Liq. 2021, 339, 116818. [Google Scholar] [CrossRef]
- Yan, J.P.; Deng, L.; Hu, L.L. Research progress on the structure and properties of phosphate glass. J. Chin. Ceram. Soc. China 2022, 50, 1006–1021. [Google Scholar]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, S.; Zhang, M.; Guo, M.; Zhang, Z. Structural investigation of phosphorus in CaO-SiO2-P2O5 ternary glass. Metall. Mater. Trans. B 2017, 48, 1139–1148. [Google Scholar] [CrossRef]
- Boiko, G.G.; Andreev, N.S.; Parkachev, A.V. Structure of pyrophosphate 2ZnO·P2O5–2Na2O·P2O5 glasses according to molecular dynamics simulation. J. Non-Cryst. Solids 1998, 238, 175–185. [Google Scholar] [CrossRef]
- Goj, P.; Stoch, P. Molecular dynamics simulations of P2O5-Fe2O3-FeO-Na2O glasses. J. Non-Cryst. Solids 2018, 500, 70–77. [Google Scholar] [CrossRef]
- Rao, R.P.; Seshasayee, M. Molecular dynamics simulation of ternary glasses Li2O-P2O5-LiCl. Solid State Commun. 2004, 131, 537–542. [Google Scholar]
- Ainsworth, R.I.; Tommaso, D.D.; Christie, J.K.; de Leeuw, N.H. Polarizable force field development and molecular dynamics study of phosphate-based glasses. J. Chem. Phys. 2012, 137, 234502. [Google Scholar] [CrossRef]
- Wu, T.; He, S.; Liang, Y.; Wang, Q. Molecular dynamics simulation of the structure and properties for the CaO-SiO2 and CaO-Al2O3 systems. J. Non-Cryst. Solids 2015, 411, 145–151. [Google Scholar] [CrossRef]
- Yao, T.H. Simulation Study on Melting Structure and Properties of CaO-SiO2-TiO2 Slag System. Master’s Thesis, Chongqing University, Chongqing, China, 2017. [Google Scholar]
- Matsumiya, T.; Nogami, A.; Fukuda, Y. Applicability of molecular dynamics to analyses of refining slags. ISIJ Int. 1993, 33, 210–217. [Google Scholar] [CrossRef][Green Version]
- Asada, T.; Yamada, Y.; Ito, K. The estimation of structural properties for molten CaO-CaF2-SiO2 system by molecular dynamics simulations. ISIJ Int. 2008, 48, 120–122. [Google Scholar] [CrossRef]
- Fan, G.; Diao, J.; Jiang, L.; Zhang, Z.; Xie, B. Molecular dynamics analysis of the microstructure of the CaO-P2O5-SiO2 slag system with varying P2O5/SiO2 ratios. Mater. Trans. 2015, 56, 655–660. [Google Scholar] [CrossRef]
- Diao, J.; Zhang, Q.; Qiao, Y.; Jiang, L.; Xie, B. Computational Study of the Transport Properties of Molten CaO-SiO2-P2O5-FeO System. High Temp. Mater. Process. 2018, 37, 141–147. [Google Scholar] [CrossRef]
- Liang, X.P.; Lu, D.X.; Wang, Y.; Wu, J.Q. Computational simulation of coordination structure of CaO-B2O3-SiO2-TiO2 mold flux. J. Chongqing Univ. China 2015, 38, 135–141. [Google Scholar]
- Xiao, C. Molecular Dynamics Simulation of Melt Microstructure and Properties of CaO-SiO2-Al2O3-Na2O system. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2017. [Google Scholar]
- Wu, Y.Q.; You, J.L.; Jiang, G.C. Molecular dynamics simulation of the structure of calcium aluminate melt. J. Inorg. Mater. China 2003, 18, 619–626. [Google Scholar]
- Zhang, X.; Liu, C.; Jiang, M. Molecular Dynamics Simulations of Melt Structure Properties of CaO-Al2O3-Na2O Slag. Metall. Mater. Trans B 2021, 52, 2604–2611. [Google Scholar] [CrossRef]
- Zhang, X.B.; Liu, C.J.; Jiang, M.F. Molecular dynamics study on the effect of CaF2 on the structure of CaO-Al2O3-CaF2 melt. J. Northeast. Univ. China 2020, 41, 510–515. [Google Scholar]
- Yin, Q.; Lian, Y.D.; Wu, R.H.; Gao, L.Q.; Chen, S.Q.; Wen, Z.X. Effect of the potential function and strain rate on mechanical behavior of the single crystal Ni-based alloys: A molecular dynamics study. Chin. Phys. B 2021, 30, 080204. [Google Scholar] [CrossRef]
- Feng, G.P.; Feng, Y.H.; Feng, D.L.; Zhang, X.X. Molecular dynamics simulation of the structure and thermal conductivity of CaO-Al2O3-SiO2 slag. J. Eng. Therm. China 2021, 42, 718–723. [Google Scholar]
- Chen, Y.; Pan, W.; Jia, B.; Wang, Q.; Zhang, X.; Wang, Q.; He, S. Effects of the amphoteric behavior of Al2O3 on the structure and properties of CaO-SiO2-Al2O3 melts by molecular dynamics. J. Non-Cryst. Solids 2021, 552, 120435. [Google Scholar] [CrossRef]
- Wang, S. Simulation and Experimental Study on Melt Structure of CaO-SiO2-Al2O3-Li2O slag system. Master’s Thesis, Chongqing University, Chongqing, China, 2019. [Google Scholar]
- Jiang, C.; Li, K.; Zhang, J.; Qin, Q.; Liu, Z.; Liang, W.; Sun, M.; Wang, Z. Molecular dynamics simulation on the effect of MgO/Al2O3 ratio on structure and properties of blast furnace slag under different basicity conditions. Metall. Mater. Trans. B 2019, 50, 367–375. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Q.; Yu, C.; He, S. Structural and viscosity properties of CaO-SiO2-Al2O3-FeO slags based on molecular dynamic simulation. J. Non-Cryst. Solids 2016, 450, 23–31. [Google Scholar] [CrossRef]
- Wang, X.J.; Jin, H.B.; Zhu, L.G.; Piao, Z.L.; Wang, B.; Qu, S. Effect of B2O3 on properties and structure of CaO-Al2O3-SiO2-based mold flux for continuous casting. Mater. Rep. China 2019, 33, 1395–1400. [Google Scholar]
- Shimoda, K.; Saito, K. Detailed structure elucidation of the blast furnace slag by molecular dynamics simulation. ISIJ Int. 2007, 47, 1275–1279. [Google Scholar] [CrossRef]
- Mongalo, L.; Lopis, A.S.; Venter, G.A. Molecular dynamics simulations of the structural properties and electrical conductivities of CaO-MgO-Al2O3-SiO2 melts. J. Non-Cryst. Solids 2016, 452, 194–202. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Yang, S.; Liu, J.; Liu, W. Molecular Dynamics Study of Structural Properties of Refining Slag with Various CaO/Al2O3 Ratios. Minerals 2021, 11, 398. [Google Scholar] [CrossRef]





| Algorithm | Advantage | Disadvantage | References |
|---|---|---|---|
| Verlet algorithm | The calculation process is simple and easy to apply | It is not a self-starting algorithm, and its accuracy is inaccurate | [31] |
| Speed Verlet algorithm | The position, velocity and acceleration are given at the same time without reducing the accuracy | The calculation process is complicated | [32] |
| Leapfrog algorithm | With an explicit velocity term, there is no need to calculate the difference between two larger quantities 2r(t) and r(t − δ) | The position and speed cannot be synchronized | [33] |
| Beeman algorithm | Can maintain energy conservation | The amount of calculation is large | [34] |
| Rahman algorithm | Obtains a more accurate solution | Time consuming with a large amount of calculation | [35] |
| Research System | Research Content | Potential Function | Ensemble | References |
|---|---|---|---|---|
| CaO-P2O5/Na2O-P2O5 | Structural properties | BMH | NVT | [80] |
| CaO-SiO2-P2O5 | Organizational characteristics of the structure | BMH | NVT | [83] |
| 2ZnO·P2O5-2Na2O·P2O5 | Structural properties | BMH | — | [84] |
| P2O5-Fe2O3-FeO-Na2O | the effect of the addition of Na2O on the structure | Buckingham + Stillinger–Weber | NVT | [85] |
| Li2O–P2O5–LiCl | The effect of the addition of LiCl on the structure | BMH | — | [86] |
| P2O5-CaO-Na2O | Study the structure of phosphate glass | Buckingham + Born-Mayer | NVT | [87] |
| Research System | Research Content | Potential Function | Ensemble | References |
|---|---|---|---|---|
| CaO-SiO2 | Melt structure, viscosity, conductivity | BMH | NVT | [88] |
| CaO-SiO2-CaF2 | Effect of CaF2 on slag structure | BMH | — | [89] |
| CaO-SiO2-CaF2 | Melt structure | BMH + Buckingham + MiTra | — | [90] |
| CaO-SiO2-CaF2 | Melt structure property | BMH | — | [91] |
| CaO-P2O5-SiO2 | Melt structure | BMH | NVT | [92] |
| CaO-SiO2-P2O5-FeO | Melt structure and viscosity | BMH | NVT | [93] |
| CaO-B2O3-SiO2-TiO2 | Melt structure | BMH | — | [94] |
| Research System | Research Content | Potential Function | Ensemble | References |
|---|---|---|---|---|
| SiO2-Al2O3 | Melt structure | BMH | NVT | [95] |
| CaO-Al2O3 | Microstructure unit distribution, viscosity, conductivity | BMH | — | [96] |
| CaO-Al2O3-Na2O | Structural characteristics of slag | Buckingham | NVT | [97] |
| CaO-Al2O3-CaF2 | Melt structure and viscosity | Buckingham | — | [98] |
| Research System | Research Content | Potential Function | Ensemble | References |
|---|---|---|---|---|
| CaO-Al2O3-SiO2 | Electrical conductivity, structure | BMH | — | [100] |
| CaO-Al2O3-SiO2 | Melt structure and viscosity | BMH | NVT | [101] |
| CaO-SiO2-Al2O3-Li2O | Melt structure | BMH | NVT | [102] |
| SiO2-Al2O3-CaO-MgO | Slag structure | CMAS94 | NVT | [103] |
| CaO-SiO2-Al2O3-FeO | Structure and viscosity | Lennard-Jones | NVT | [104] |
| B2O3-CaO-Al2O3-SiO2 | Structure and viscosity | BMH | NVT | [105] |
| CaO-Al2O3-SiO2-MgO | Structure and viscosity | Morse + BMH | NPT | [106] |
| CaO-MgO-Al2O3-SiO2 | Structure, electrical conductivity | Morse | NVT | [107] |
| SiO2- Al2O3- CaO-MgO | Structure | BMH | NVT | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Li, J.; Wang, S.; Zhao, J.; Ai, L.; Chen, Q.; Chen, Q.; Zhao, D. Development of Molecular Dynamics and Research Progress in the Study of Slag. Materials 2023, 16, 5373. https://doi.org/10.3390/ma16155373
Zhou C, Li J, Wang S, Zhao J, Ai L, Chen Q, Chen Q, Zhao D. Development of Molecular Dynamics and Research Progress in the Study of Slag. Materials. 2023; 16(15):5373. https://doi.org/10.3390/ma16155373
Chicago/Turabian StyleZhou, Chaogang, Jinyue Li, Shuhuan Wang, Jingjing Zhao, Liqun Ai, Qinggong Chen, Qiya Chen, and Dingguo Zhao. 2023. "Development of Molecular Dynamics and Research Progress in the Study of Slag" Materials 16, no. 15: 5373. https://doi.org/10.3390/ma16155373
APA StyleZhou, C., Li, J., Wang, S., Zhao, J., Ai, L., Chen, Q., Chen, Q., & Zhao, D. (2023). Development of Molecular Dynamics and Research Progress in the Study of Slag. Materials, 16(15), 5373. https://doi.org/10.3390/ma16155373





