Enhancing the Electrochemical Stability of LiNi0.8Co0.1Mn0.1O2 Compounds for Lithium-Ion Batteries via Tailoring Precursors Synthesis Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of NCMO Precursors at Various Hydrothermal Temperatures
2.2. Preparation of LNCMO Cathode Materials Based on Precursors
2.3. Characterization of LNCMO Cathode Materials
3. Results and Discussion
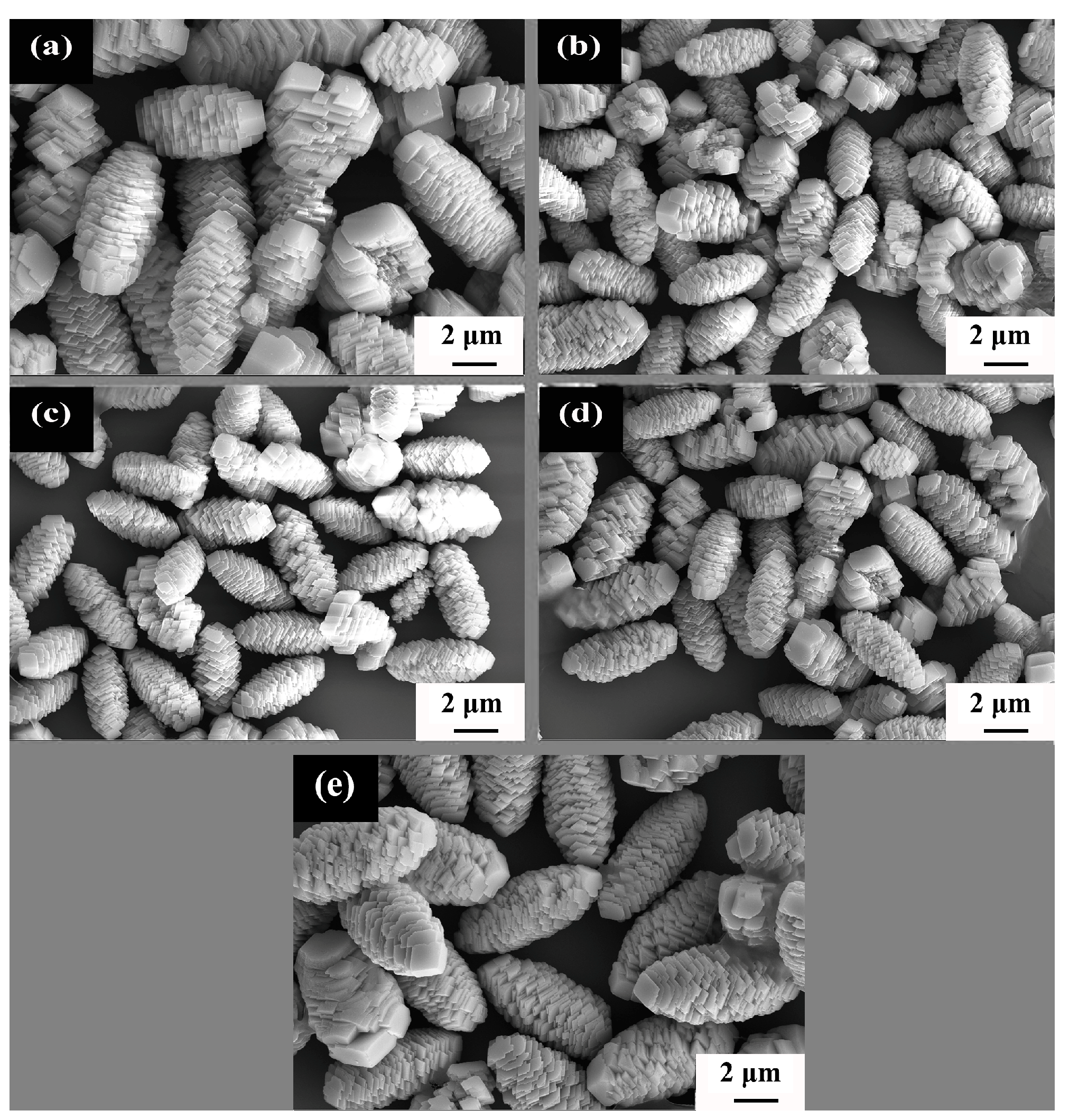
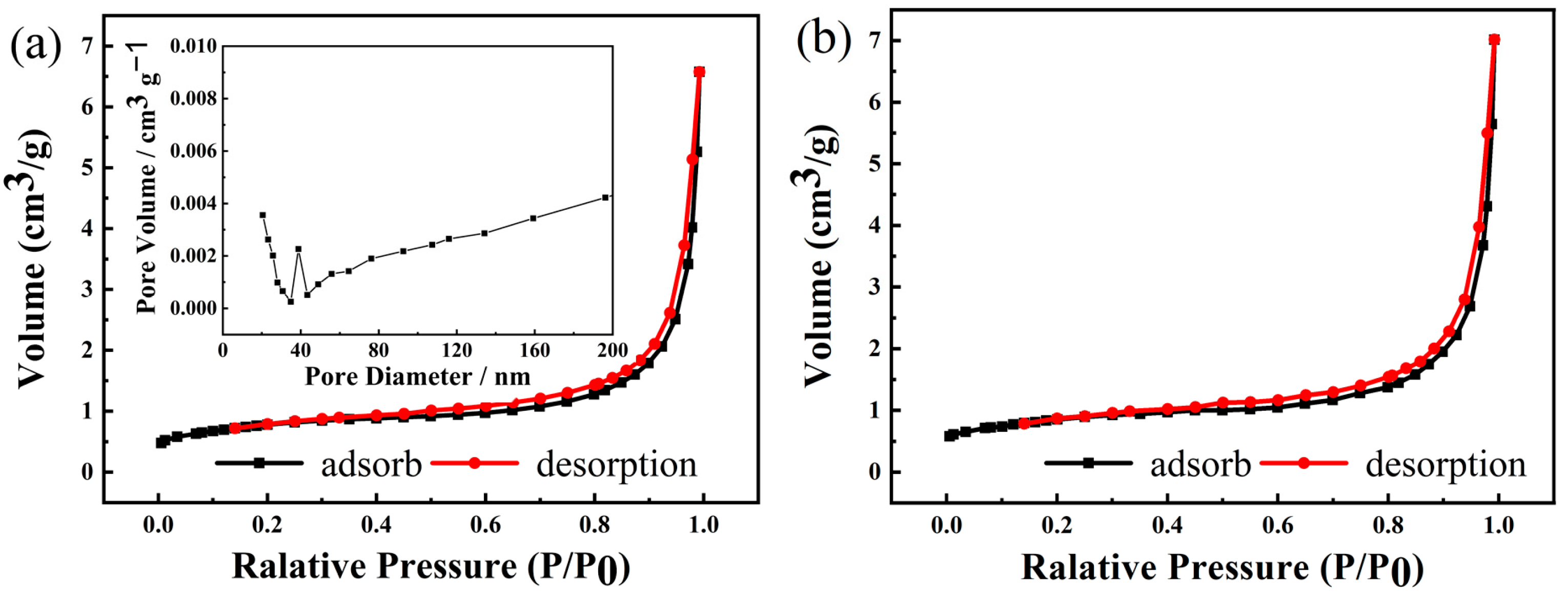
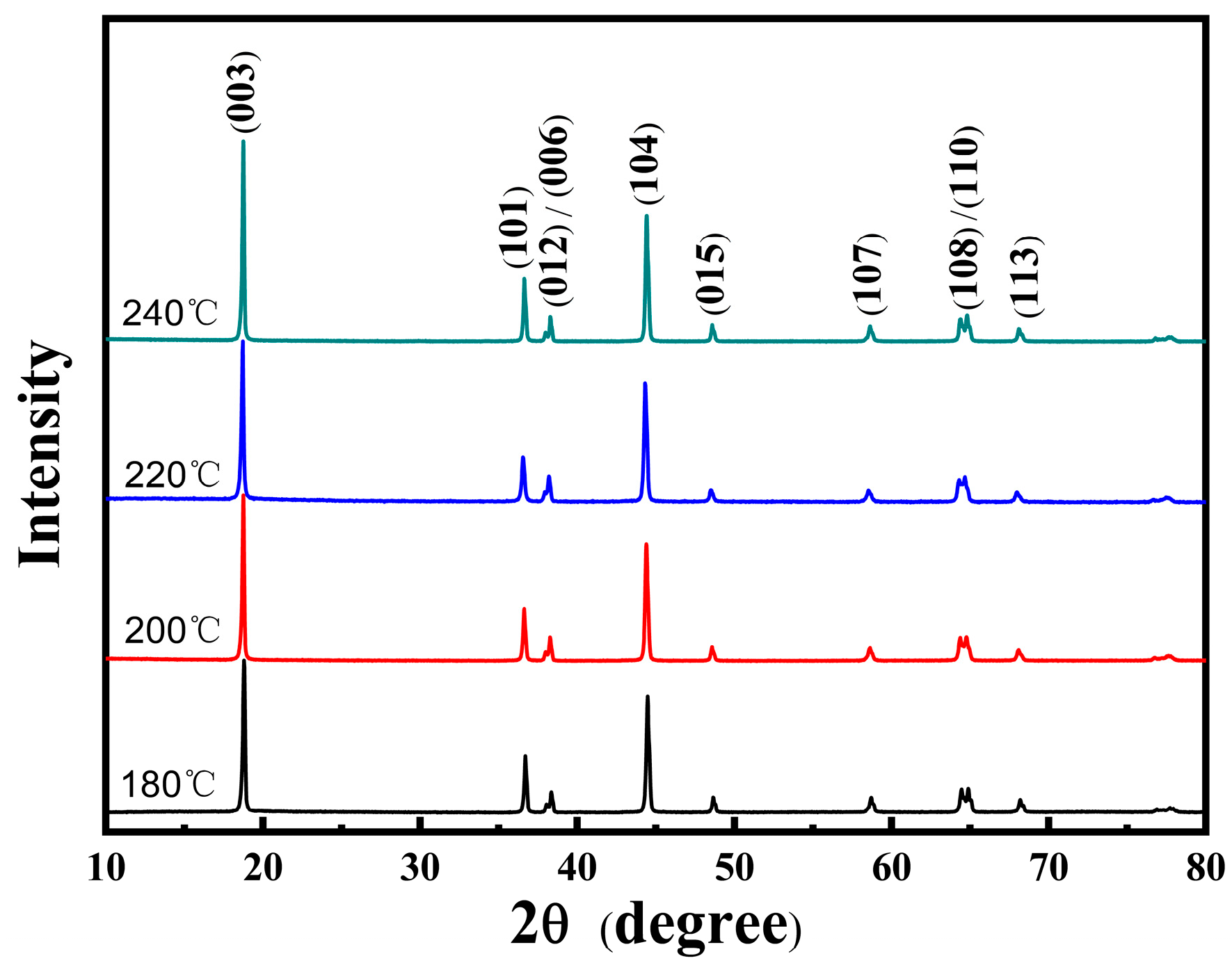
3.1. NCMO Precursors Synthesized at Various Temperatures
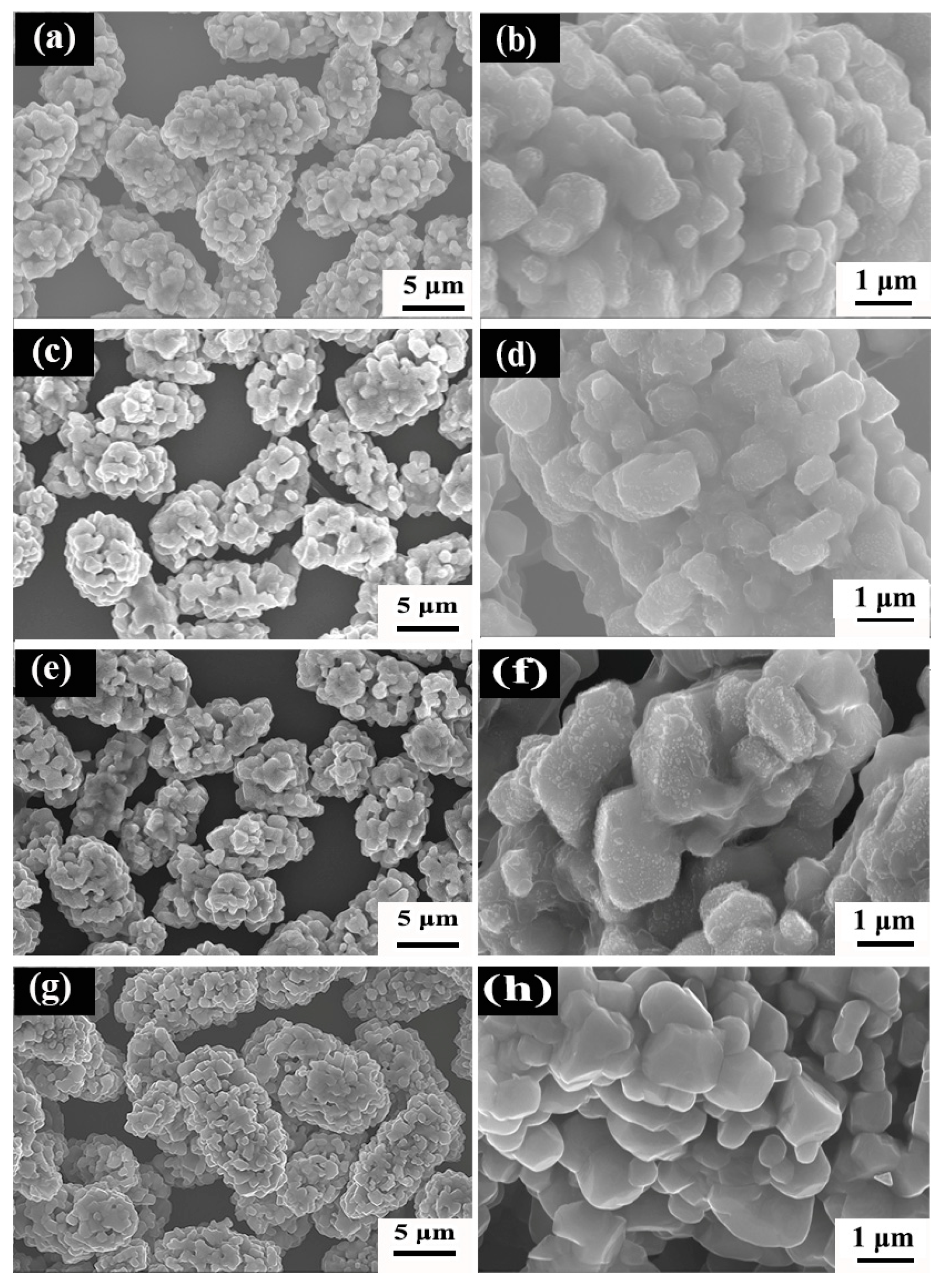
3.2. LNCMO Cathode Materials Based on Precursors Obtained at Different Temperatures
3.3. Electrochemical Performances of LNCMO Cathode Materials
3.3.1. Charge/Discharge Cycling Stability
3.3.2. Energy Density
3.3.3. Impedance and Morphology of LNCMO after Cycle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, J.M.; Yan, P.F.; Estevez, L.; Wang, C.; Zhang, J.-G. Effect of calcination temperature on the electrochemical properties of nickel-rich LiNi0.76Mn0.14Co0.10O2 cathodes for lithium-ion batteries. Nano Energy 2018, 49, 538–548. [Google Scholar] [CrossRef]
- Sun, Y.K.; Chen, Z.H.; Noh, H.J.; Lee, D.-J.; Jung, H.-G.; Ren, Y.; Wang, S.; Yoon, C.S.; Myung, S.-T.; Amine, K. Nanostructured high-energy cathode materials for advanced lithium batteries. Nat. Mater. 2012, 11, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Knight, J.C.; Myung, S.T.; Oh, S.-M.; Sun, Y.-K. Nickel-rich and lithium-rich layered oxide cathodes: Progress and perspectives. Adv. Energy Mater. 2016, 6, 1501010. [Google Scholar] [CrossRef]
- Luo, Y.H.; Wei, H.X.; Tang, L.B.; Huang, Y.-D.; Wang, Z.-Y.; He, Z.-J.; Yan, C.; Mao, J.; Dai, K.; Zheng, J.-C. Nickel-rich and cobalt-free layered oxide cathode materials for lithium ion batteries. Energy Storage Mater. 2022, 50, 274–307. [Google Scholar] [CrossRef]
- Moshtev, R.; Zlatilova, P.; Bakalova, I.; Vassilev, S. Synthesis, XRD characterization, and cycling performance of cobalt doped lithium nickelates. J. Power Source 2002, 112, 30–35. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.-J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef]
- Kim, N.Y.; Yim, T.; Song, J.H.; Yu, J.-S.; Lee, Z. Microstructural study on degradation mechanism of layered LiNi0.6Co0.2Mn0.2O2 cathode materials by analytical transmission electron microscopy. J. Power Source 2016, 307, 641–648. [Google Scholar] [CrossRef]
- Zhang, L.S.; Wang, H.; Wang, L.Z.; Cao, Y. High electrochemical performance of hollow corn-like LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries. Appl. Surf. Sci. 2018, 450, 461–467. [Google Scholar] [CrossRef]
- Noh, M.; Cho, J. Optimized synthetic conditions of LiNi0.5Co0.2Mn0.3O2 cathode materials for high rate lithium batteries via co-precipitation method. J. Electrochem. Soc. 2013, 160, A105–A111. [Google Scholar] [CrossRef]
- Kong, J.Z.; Zhai, H.F.; Ren, C.; Gao, M.-Y.; Zhang, X.; Li, H.; Li, J.-X.; Tang, Z.; Zhou, F. Synthesis and electrochemical performance of macroporous LiNi0.5Co0.2Mn0.3O2 by a modified sol-gel method. J. Alloys Compd. 2013, 577, 507–510. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, K.; Cao, Y.; Lu, Y.; Peng, Z.; Fan, J.; Li, L.; Xue, Z.; Su, H.; Hu, G. Hydrothermal preparing agglomerate LiNi0.8Co0.1Mn0.1O2 cathode material with submicron primary particle for alleviating microcracks. J. Power Source 2020, 477, 228701. [Google Scholar] [CrossRef]
- Ding, C.X.; Meng, Q.S.; Wang, L.; Chen, C.H. Synthesis, structure, and electrochemical characteristics of LiNi1/3Co1/3Mn1/3O2 prepared by thermal polymerization. Mater. Res. Bull. 2009, 44, 492–498. [Google Scholar] [CrossRef]
- Yang, C.N.; Xiao, W.C.; Ren, S.Z.; Li, Q.; Wang, J.; Li, Z.; Li, W.; Chen, B. Fabrication of LiNi0.8Co0.1Mn0.1O2 cathode materials via sodium dodecyl benzene sulfonate-assisted hydrothermal synthesis for lithium ion batteries. Int. J. Electrochem. Sci. 2022, 17, 221282. [Google Scholar] [CrossRef]
- Wang, M.M.; Shi, F.C.; Yang, H.Z.; Sun, C.; Gao, Y. Study on preparation and performance of LiNi0.8Co0.1Mn0.1O2 as cathode materials for lithium ion batteries. Int. J. Electrochem. Sci. 2020, 15, 9971–9980. [Google Scholar] [CrossRef]
- van Bommel, A.; Dahn, J.R. Analysis of the growth mechanism of coprecipitated spherical and dense nickel, manganese, and cobalt-containing hydroxides in the presence of aqueous ammonia. Chem. Mater. 2009, 21, 1500–1503. [Google Scholar] [CrossRef]
- Li, L.; Wang, D.; Xu, G.; Zhou, Q.; Ma, J.; Zhang, J.; Du, A.; Cui, Z.; Zhou, X.; Cui, G. Recent progress on electrolyte functional additives for protection of nickel-rich layered oxide cathode materials. J. Energy Chem. 2022, 65, 280–292. [Google Scholar] [CrossRef]
- Goonetilleke, D.; Riewald, F.; Kondrakov, A.O.; Janek, J.; Brezesinski, T.; Bianchini, M. Alleviating anisotropic volume variation at comparable Li utilization during cycling of Ni-rich, Co-free layered oxide cathode materials. J. Phys. Chem. C 2022, 126, 16952–16964. [Google Scholar] [CrossRef]
- Zhang, Y.; Xun, J.H.; Zhang, K.Y.; Zhang, B.; Xu, H. 2D-lamellar stacked Na3V2(PO4)(2)F-3@RuO2 as a high-voltage, high-rate capability and long-term cycling cathode material for sodium ion batteries. J. Mater. Chem. A 2022, 10, 11163–11171. [Google Scholar] [CrossRef]
- Mao, S.; Liu, C.; Wu, Y.; Xia, M.; Wang, F. Porous P, Fe-doped g-C3N4 nanostructure with enhanced photo-Fenton activity for removal of tetracycline hydrochloride: Mechanism insight, DFT calculation and degradation pathways. Chemosphere 2022, 291, 133039. [Google Scholar] [CrossRef]
- Zeeshan, A.; Shehzad, N.; Ellahi, R. Analysis of activation energy in Couette-Poiseuille flow of nanofluid in the presence of chemical reaction and convective boundary conditions. Results Phys. 2018, 8, 502–512. [Google Scholar] [CrossRef]
- Xiao, K.X.; Liu, H.; Li, Y.; Yang, G.; Wang, Y.; Yao, H. Excellent performance of porous carbon from urea-assisted hydrochar of orange peel for toluene and iodine adsorption. Chem. Eng. J. 2020, 382, 122997. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.; Kim, C.S.; Kwon, Y.M.; Kim, S.I.; Eom, J.-Y. Effect of particle morphology on the fast-charging properties of high-nickel cathode materials. Korean J. Chem. Eng. 2023, 40, 532–538. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yang, Z.; Liu, S.Z. Recent progress in single-crystalline perovskite research including crystal preparation, property evaluation, and applications. Adv. Sci. 2018, 5, 1700471. [Google Scholar] [CrossRef]
- Zhang, M.L.; Shen, J.T.; Li, J.; Zhang, D.; Yan, Y.; Huang, Y.; Li, Z. Effect of micron sized particle on the electrochemical properties of nickel-rich LiNi0.8Co0.1Mn0.1O2 cathode materials. Ceram. Int. 2020, 46, 4643–4651. [Google Scholar] [CrossRef]
- Liang, R.; Wu, Z.Y.; Yang, W.M.; Tang, Z.-Q.; Xiong, G.-G.; Cao, Y.-C.; Hu, S.-R.; Wang, Z.-B. A simple one-step molten salt method for synthesis of micron-sized single primary particle LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries. Ionics 2020, 26, 1635–1643. [Google Scholar] [CrossRef]
- Gao, P.; Wang, S.; Liu, Z.H.; Jiang, Y.; Zhou, W.; Zhu, Y. Experimental and mechanism research of gradient structured LiNi0.8Co0.1Mn0.1O2 cathode material for Li-ion batteries. Solid State Ion. 2020, 357, 115504. [Google Scholar] [CrossRef]
- Su, M.R.; Chen, Y.C.; Liu, H.J.; Li, J.; Fu, K.; Zhou, Y.; Dou, A.; Liu, Y. Storage degradation mechanism of layered Ni-rich oxide cathode material LiNi0.8Co0.1Mn0.1O2. Electrochim. Acta 2022, 422, 140559. [Google Scholar] [CrossRef]
- Oh, S.H.; Song, H.J.; Lee, Y.H.; Kim, J.; Yim, T. Facile interface functionalization of Ni-rich layered LiNi0.8Co0.1Mn0.1O2 cathode material by dually-modified phosphate and aluminum precursor for Li-ion batteries. Solid State Ion. 2021, 370, 115734. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.F.; Zhu, J.H.; Wang, Y.; Jiang, J.; Li, C.M. Which layered cathode suits more for nanosilica protection, Ni-rich LiNi0.8Co0.1Mn0.1O2 or Li-rich Li1.2Mn0.54Co0.13Ni0.13O2? ACS Appl. Energy Mater. 2021, 4, 11711–11719. [Google Scholar] [CrossRef]
- Feng, Y.H.; Xu, H.; Wang, B.; Tuo, K.; Wang, P.; Wang, S.; Liang, W.; Lu, H.; Li, S. Structural evolution of nickel-rich layered cathode material LiNi0.8Co0.1Mn0.1O2 at different current rates. Ionics 2021, 27, 517–526. [Google Scholar] [CrossRef]
- Lin, C.X.; Zhnag, X.X.; Liu, Y.C.; Chen, S.-J.; Wang, W.; Zhang, Y.-N. Effect of flow-rate induced cation mixing and particle size tuning on the structure and electrochemical properties of LiNi0.8Co0.1Mn0.1O2 synthesized by spray drying. Chin. J. Struct. Chem. 2020, 39, 164–173. [Google Scholar]
- Lin, C.-H.; Parthasarathi, S.-K.; Bolloju, S.; Abdollahifar, M.; Weng, Y.-T.; Wu, N.-L. Synthesis of micron-sized LiNi0.8Co0.1Mn0.1O2 and its application in bimodal distributed high energy density Li-ion battery cathodes. Energies 2022, 15, 8129. [Google Scholar] [CrossRef]
- Zha, G.J.; Hu, N.G.; Luo, Y.P.; Fu, X.; Jin, H.; Li, L.; Huang, Q.; Ouyang, C.; Hou, H. Dual conductive surface engineering of LiNi0.8Co0.1Mn0.1O2 cathode for high-energy density. Electrochim. Acta 2023, 451, 142284. [Google Scholar] [CrossRef]
- Li, L.J.; Li, X.H.; Wang, Z.X.; Wu, L.; Zheng, J.-C.; Li, J.-H. Synthesis of LiNi0.8Co0.1Mn0.1O2 cathode material by chloride co-precipitation method. Trans. Nonferrous Met. Soc. China 2010, 20, S279–S282. [Google Scholar] [CrossRef]
- Li, J.; Liang, G.; Zheng, W.; Zhang, S.; Davey, K.; Pang, W.K.; Guo, Z. Addressing cation mixing in layered structured cathodes for lithium-ion batteries: A critical review. Nano Mater. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Hwang, D.Y.; Lee, S.H. Improving electrochemical characteristics of Ni-rich cathode materials by Na doping. J. Ceram. Process. Res. 2021, 22, 615–619. [Google Scholar]


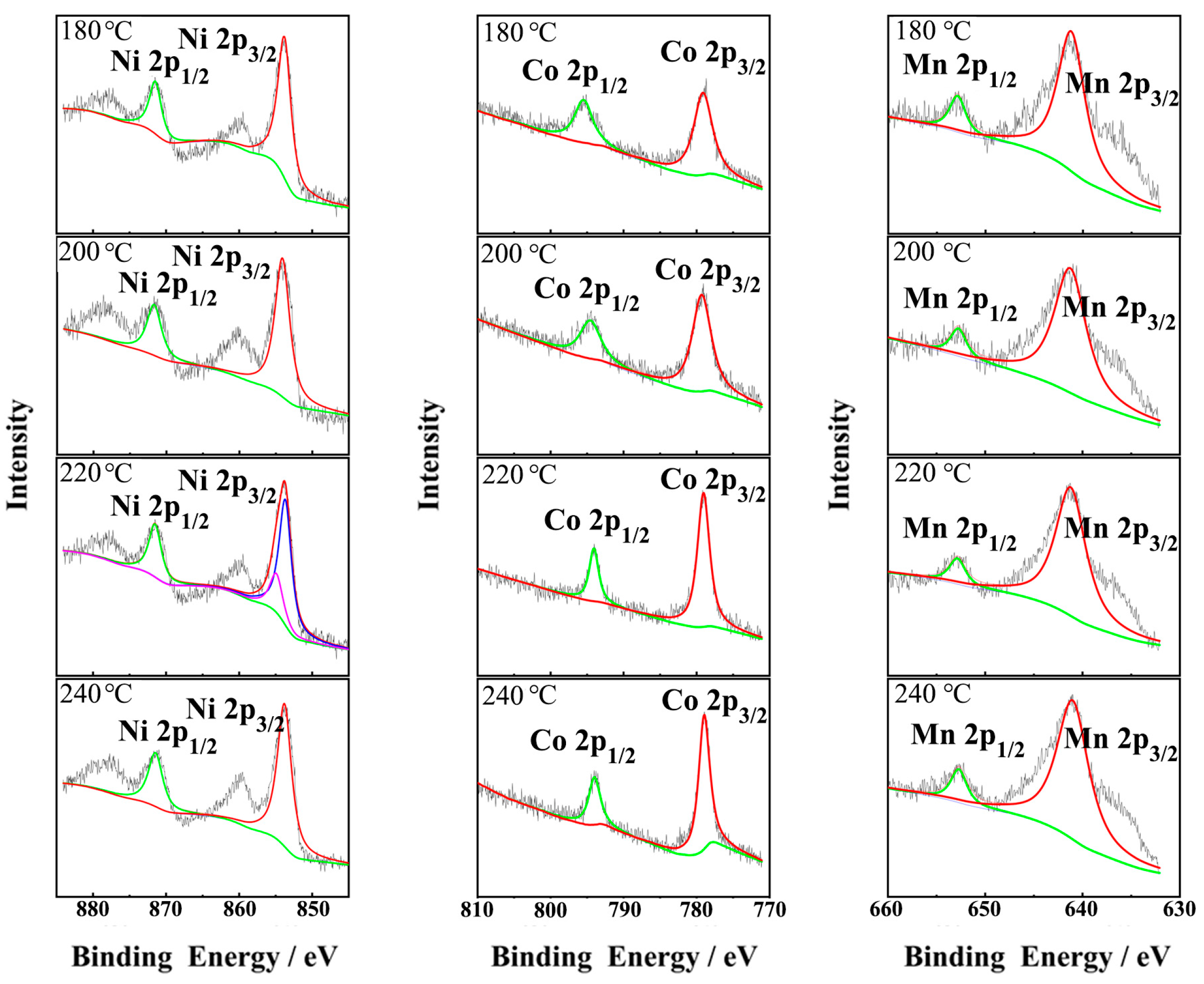
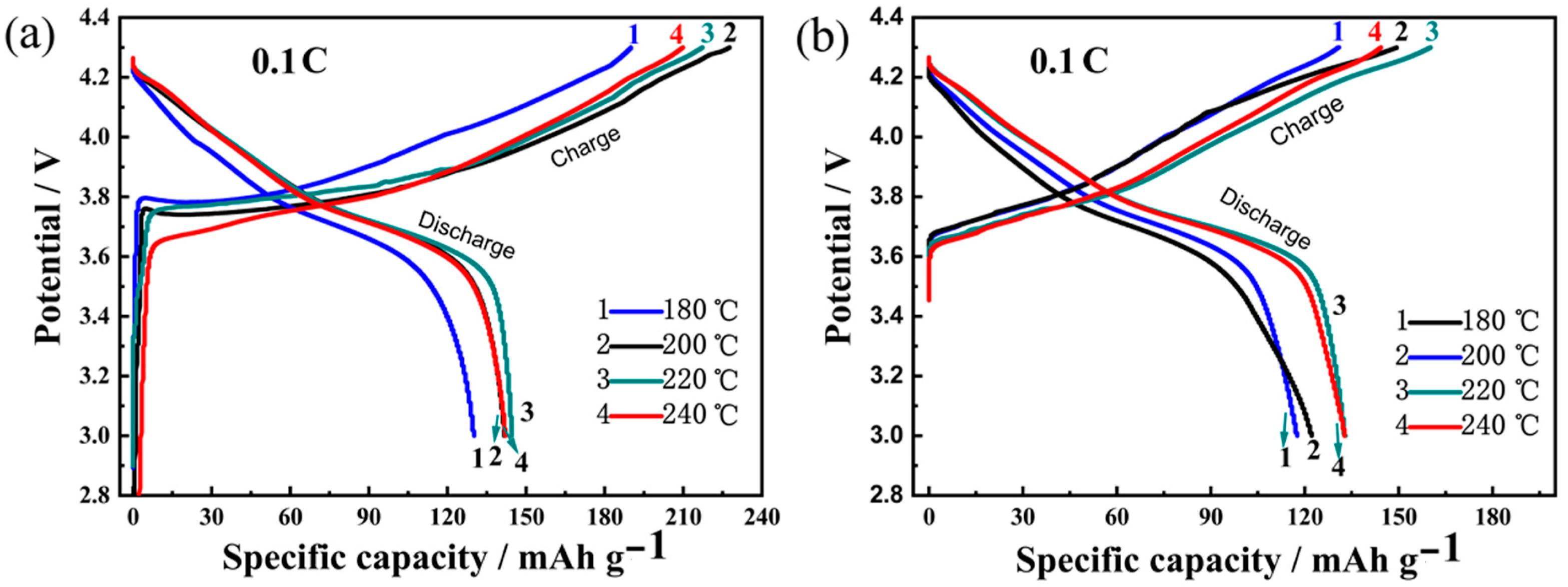

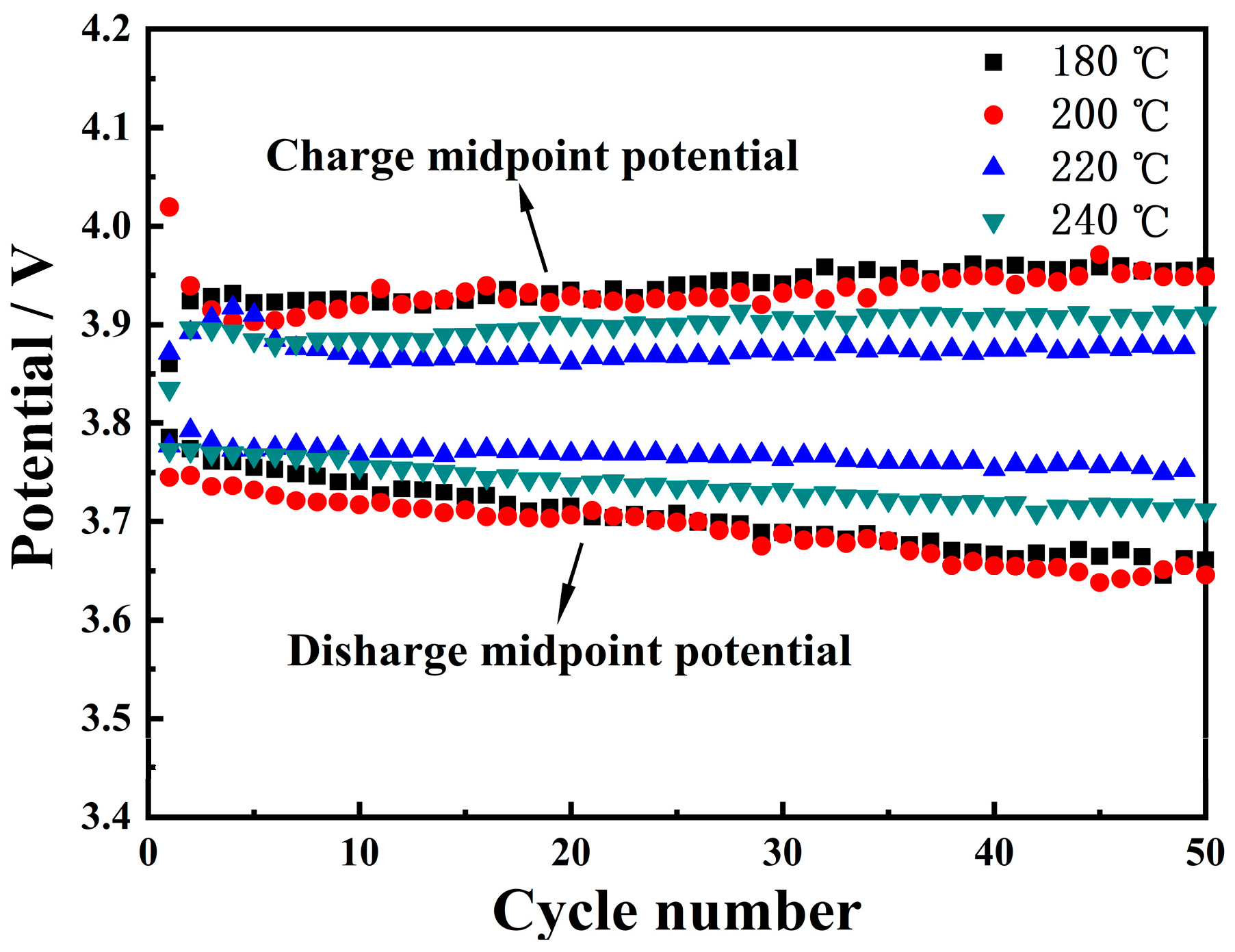

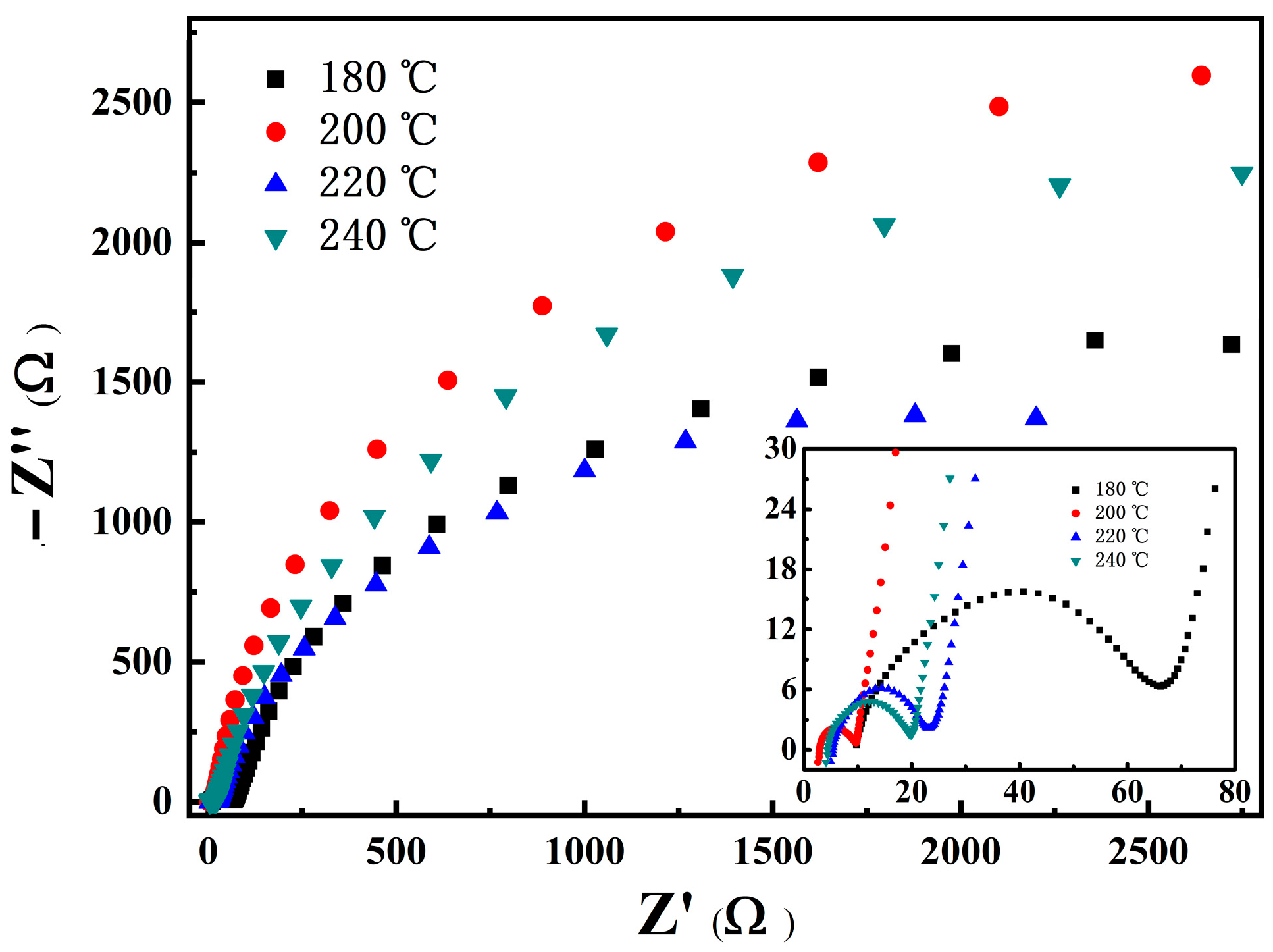

| Temperature (°C) | Ni (mmol/L) | Co (mmol/L) | Mn (mmol/L) | Ni:Co:Mn Ratio |
|---|---|---|---|---|
| 160 | 1.341 | 0.268 | 0.298 | 4.50:0.90:1 |
| 180 | 1.226 | 0.186 | 0.144 | 8.51:1.22:1 |
| 200 | 1.195 | 0.175 | 0.144 | 8.30:1.20:1 |
| 220 | 1.285 | 0.187 | 0.155 | 8.29:1.21:1 |
| 240 | 1.425 | 0.182 | 0.178 | 8.01:1.02:1 |
| Temperature (°C) | a (Å) | b (Å) | c (Å) | c/a | I(003)/I(104) |
|---|---|---|---|---|---|
| 180 | 2.875 | 2.875 | 14.217 | 4.945 | 1.20 |
| 200 | 2.874 | 2.874 | 14.200 | 4.941 | 1.58 |
| 220 | 2.873 | 2.873 | 14.207 | 4.945 | 1.94 |
| 240 | 2.875 | 2.875 | 14.207 | 4.942 | 1.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wang, H.; Yang, Z.; Xie, H.; Jia, Z.; Xiong, Y. Enhancing the Electrochemical Stability of LiNi0.8Co0.1Mn0.1O2 Compounds for Lithium-Ion Batteries via Tailoring Precursors Synthesis Temperatures. Materials 2023, 16, 5398. https://doi.org/10.3390/ma16155398
Zhang G, Wang H, Yang Z, Xie H, Jia Z, Xiong Y. Enhancing the Electrochemical Stability of LiNi0.8Co0.1Mn0.1O2 Compounds for Lithium-Ion Batteries via Tailoring Precursors Synthesis Temperatures. Materials. 2023; 16(15):5398. https://doi.org/10.3390/ma16155398
Chicago/Turabian StyleZhang, Guanhua, Hao Wang, Zihan Yang, Haoyang Xie, Zhenggang Jia, and Yueping Xiong. 2023. "Enhancing the Electrochemical Stability of LiNi0.8Co0.1Mn0.1O2 Compounds for Lithium-Ion Batteries via Tailoring Precursors Synthesis Temperatures" Materials 16, no. 15: 5398. https://doi.org/10.3390/ma16155398






