Review of the Preparation and Application of Porous Materials for Typical Coal-Based Solid Waste
Abstract
:1. Introduction
2. Physicochemical Characteristics and the Recycling of Coal Gangue and Fly Ash
2.1. Physicochemical Characteristics of Coal Gangue
2.2. Physicochemical Characteristics of Fly Ash
2.3. General Processing Methods of Coal Gangue and Fly Ash
2.3.1. Extraction of Si and Reproduction
2.3.2. Mixing with Active Substances
2.3.3. Direct Modification
3. Application of Solid-Waste-Based Porous Materials in the Field of Energy and Environmental Protection
3.1. Application of Solid-Waste-Based Porous Materials in the Field of Wastewater Treatment
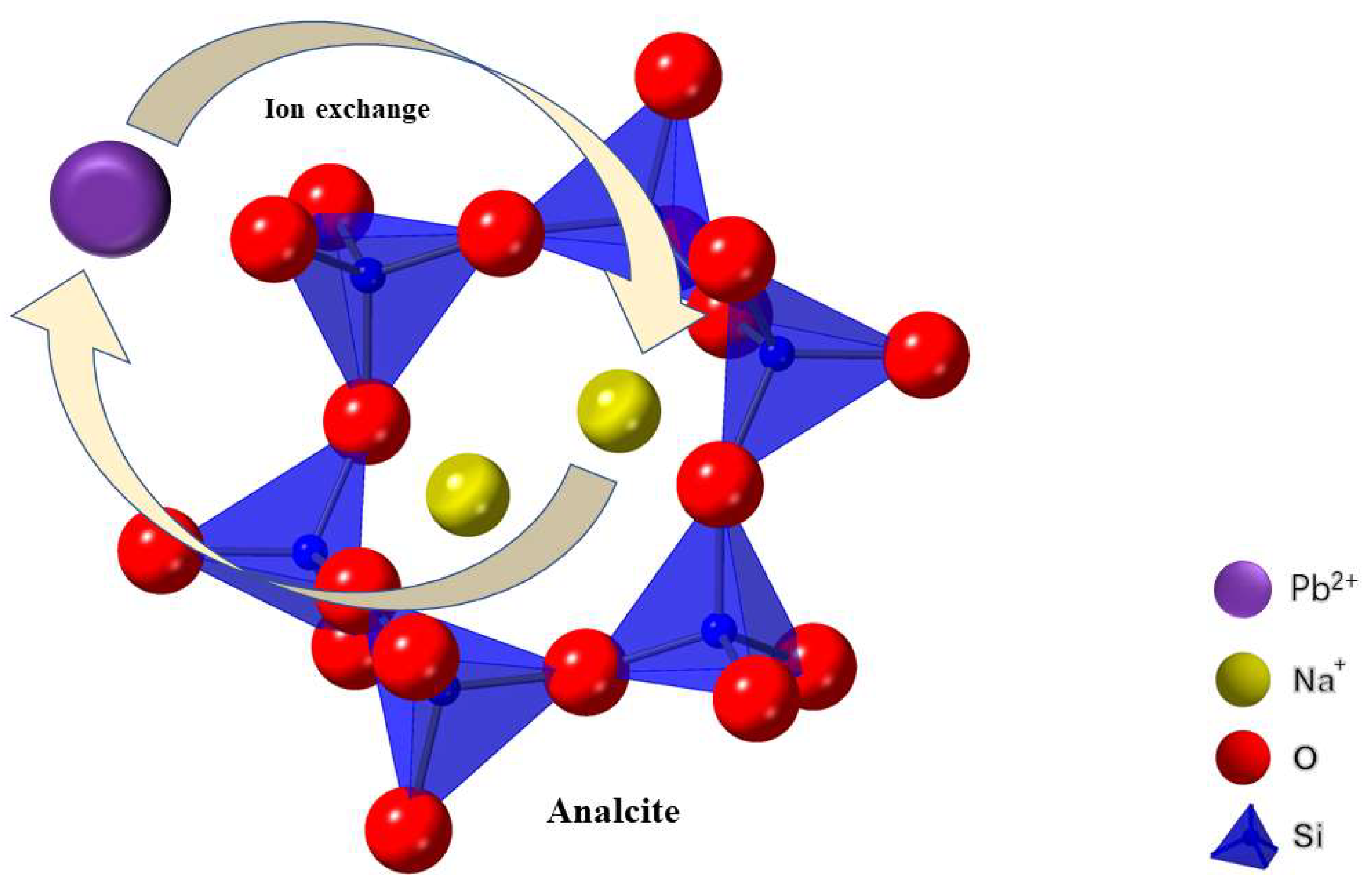
3.1.1. Adsorbing Heavy Metal Ions and NH4+ through Ion Exchange of Zeolites
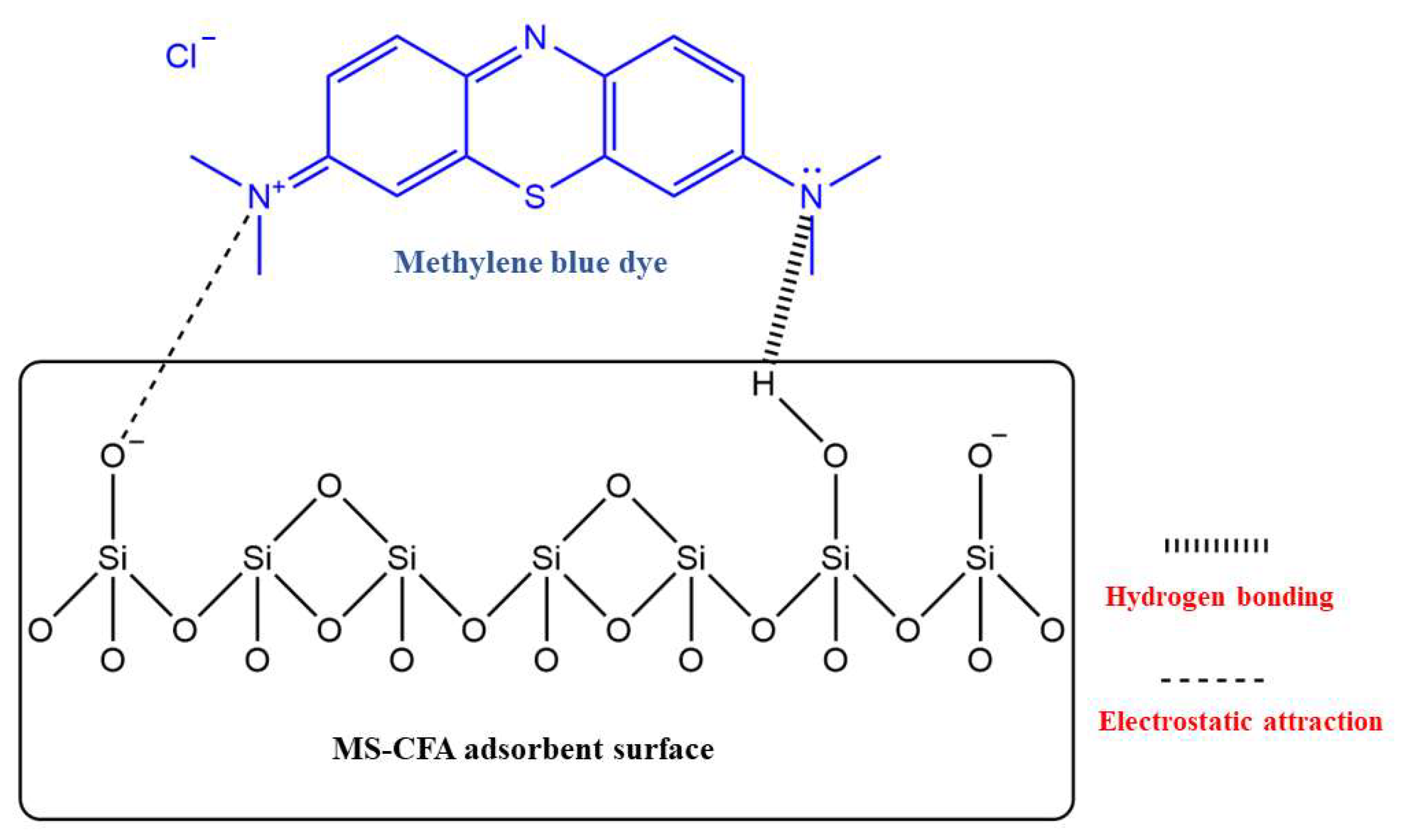
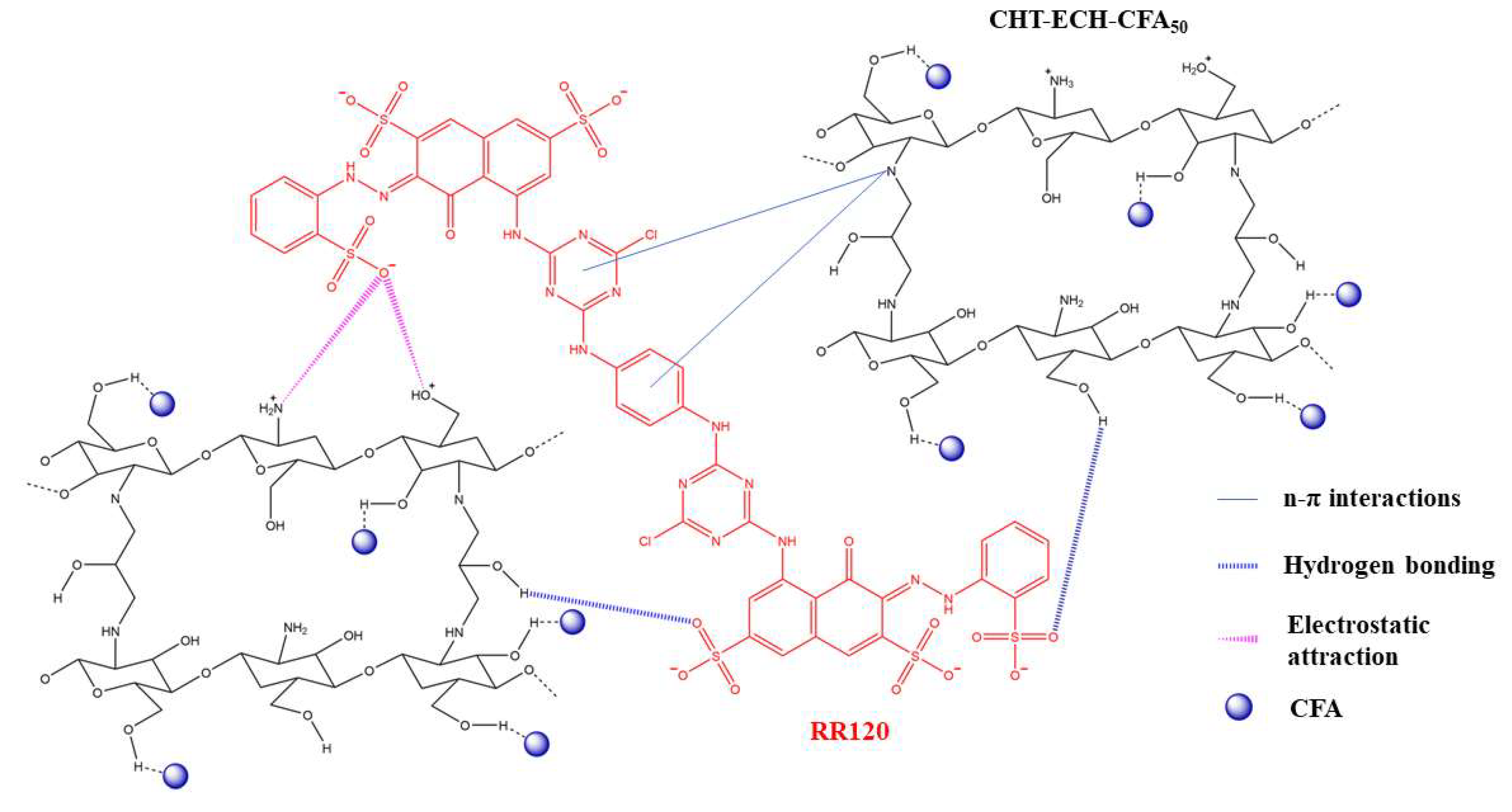
3.1.2. Adsorption of Metal Ions and Ionic Dyes Based on the Interaction of Hydroxyl Groups on the Material Surface
3.1.3. Precipitation and Adsorption of the Pollutants Based on the Alkali Supply Capacity of Materials
3.1.4. Loading Photocatalytic Materials for the Degradation of Organic Pollutants
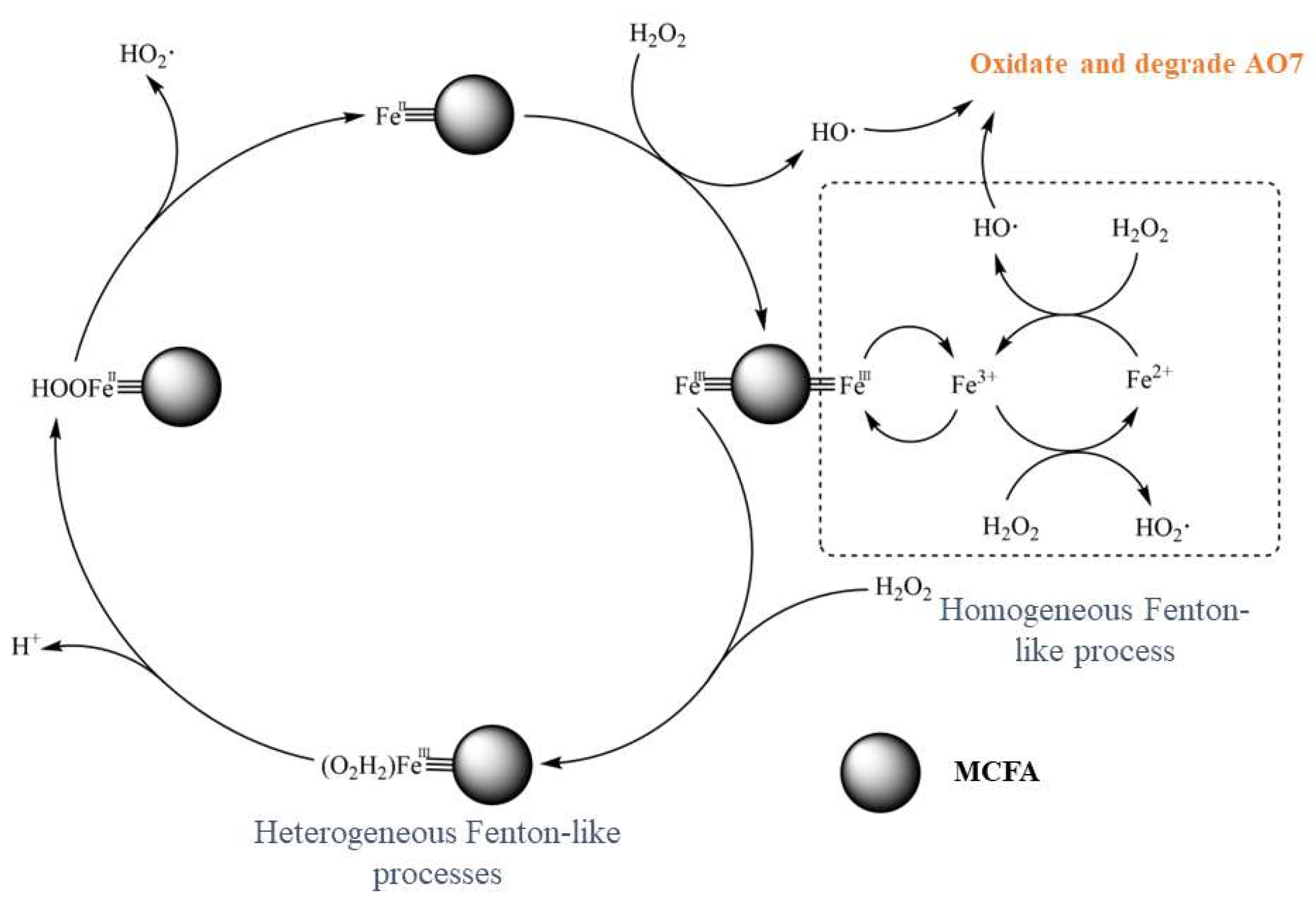
3.1.5. Preparing Heterogeneous Fenton-Like Catalysts for the Degradation of Organic Pollutants
3.2. The Application of Solid-Waste-Based Porous Materials in the Field of Waste Gas Treatment
3.2.1. The Adsorption of CO2 Based on Hydroxyl Hydrogen Bonding
3.2.2. Introducing Amino Groups by Chemical Modification for CO2 Adsorption
3.2.3. The Absorption of CO2 and SO2 Based on Alkaline Substances of Solid Waste
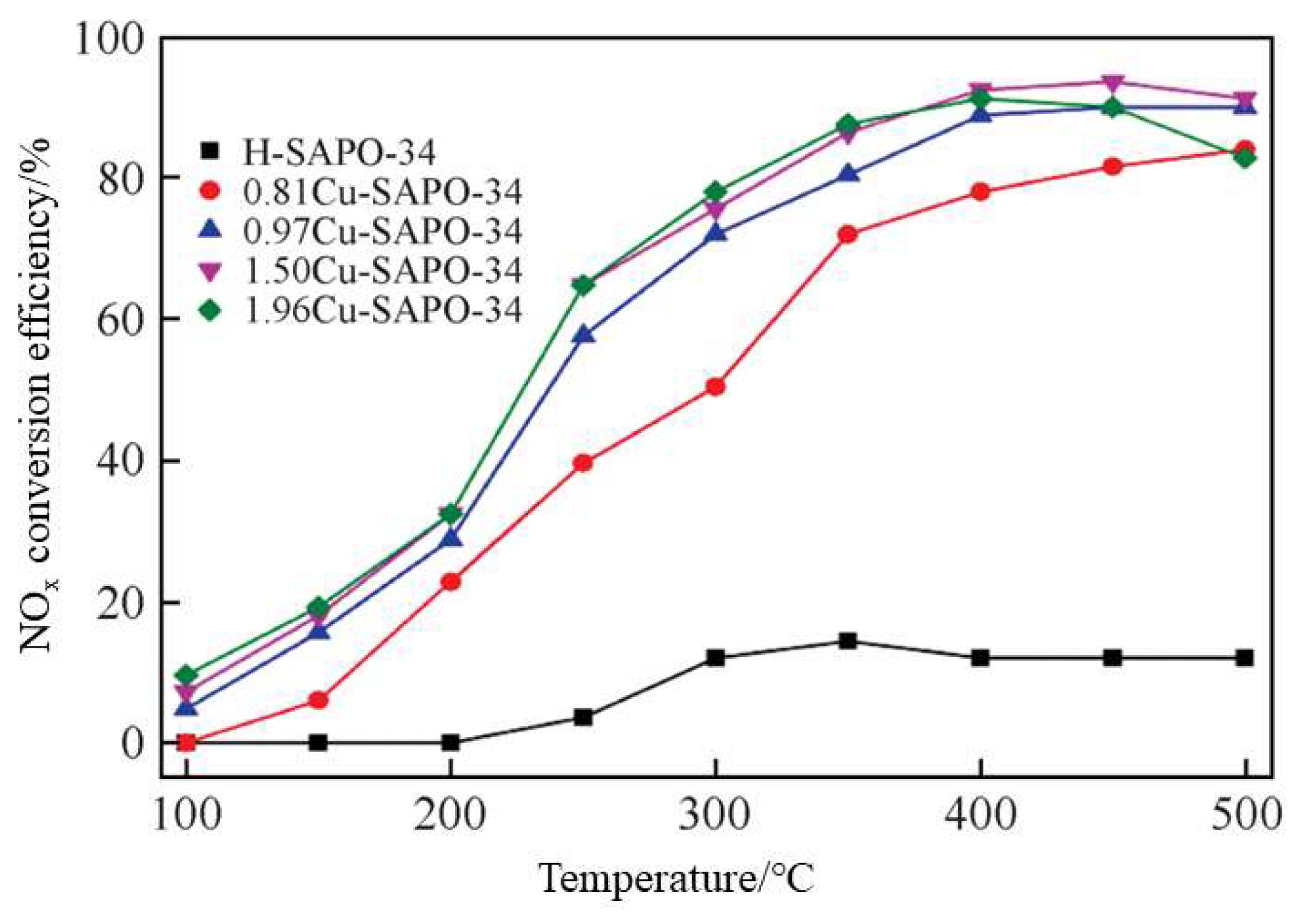
3.2.4. Denitration Based on NH3-SCR Technology
3.2.5. Absorption of VOCs
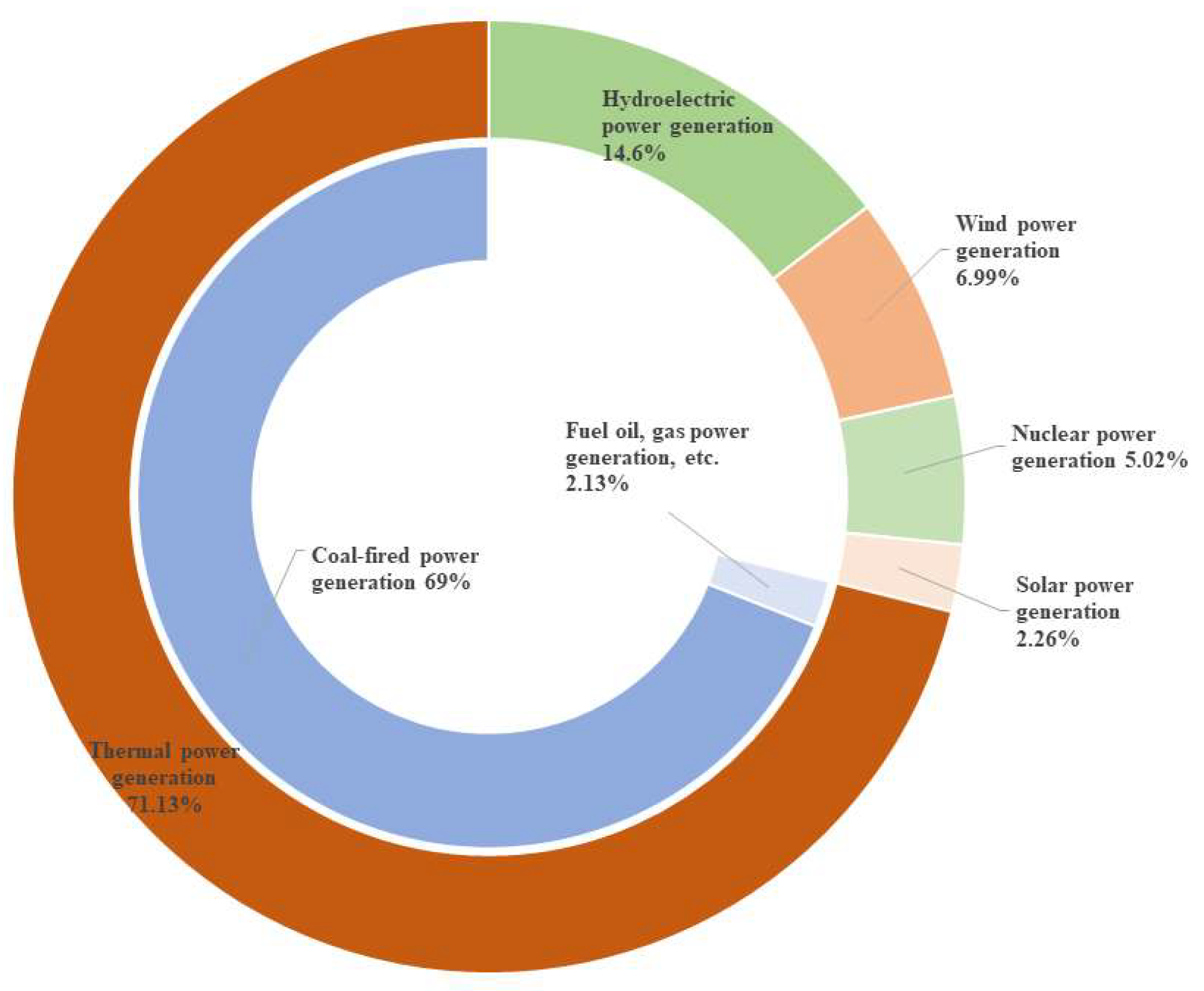
3.3. The Application of Solid-Waste-Based Porous Materials in Energy Regeneration
3.4. The Application of Solid-Waste-Based Porous Materials in Battery Manufacturing
4. Conclusions
- (1)
- In the field of wastewater treatment, adopt more efficient pretreatment methods (such as pressurized acid leaching, microwave acid leaching, complex acid leaching, etc.) before preparing water treatment materials to reduce heavy metal content in solid waste and focus research on materials with a more stable structure simultaneously, reducing the possibility of secondary pollution.
- (2)
- In the field of waste gas treatment, pre-treat waste gas with means of temperature control and dehumidification before using solid-waste-based gas adsorption materials, minimizing the adverse effects of external factors on the gas adsorption performance of the materials. Some gas adsorption materials that are difficult to regenerate can improve their gas diffusion performance by adding modified substances to form eutectic phases, thereby accelerating the regeneration rate.
- (3)
- In the field of energy regeneration, fully explore the mechanism of various effective components in solid-waste-based catalysts in the catalytic process to achieve efficient and controllable energy regeneration. By loading active metals, regulating the silicon–aluminum ratio, and improving the pore structure, this will improve catalyst energy regeneration efficiency and coking resistance.
- (4)
- In the field of battery manufacturing, the volume expansion problem of Si-based anode materials still requires further exploration of new nanostructures and composite systems to be solved, improving the cycling performance of electrode materials.
- (5)
- In the regeneration process of solid waste, apply technical methods (such as microwave-assisted, ultrasonic-assisted, solvent-free methods, etc.) that can effectively reduce energy consumption and increase productivity in the conversion preparation process of solid waste into functional porous materials, reducing the cost of reusing coal-based solid waste and promoting large-scale resource utilization of typical coal-based solid wastes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, W.; Zhang, J.; Li, M.; Yan, H.; Zhou, N.; Yao, Y.; Guo, Y. Underground Disposal of Coal Gangue Backfill in China. Appl. Sci. 2022, 12, 12060. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Ma, S.; Zheng, S.; Zhang, Y.; Chu, P. Utilization of coal fly ash in China: A mini-review on challenges and future directions. Environ. Sci. Pollut. Res. 2021, 28, 18727–18740. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, Z. Recycling utilization patterns of coal mining waste in China. Resour. Conserv. Recycl. 2010, 54, 1331–1340. [Google Scholar] [CrossRef]
- Babla, M.; Katwal, U.; Yong, M.; Jahandari, S.; Rahme, M.; Chen, Z.; Tao, Z. Value-added products as soil conditioners for sustainable agriculture. Resour. Conserv. Recycl. 2022, 178, 106079. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A. Characteristics of Some Selected Methods of Rare Earth Elements Recovery from Coal Fly Ashes. Metals 2021, 11, 142. [Google Scholar] [CrossRef]
- Querol, X.; Turiel, J.F.; Soler, A.L. The Behaviour of Mineral Matter During Combustion of Spanish Subbituminous and Brown Coals. Mineral. Mag. 1994, 58, 119–133. [Google Scholar] [CrossRef]
- Han, R.; Guo, X.; Guan, J.; Yao, X.; Hao, Y. Activation mechanism of coal gangue and its impact on the properties of geopolymers: A review. Polymers 2022, 14, 3861. [Google Scholar] [CrossRef]
- Hameed, A.; Alharbi, A.; Abdelrahman, E.; Mabrouk, E.; Hegazey, R.; Algethami, F.; Al-Ghamdi, Y.O.; Youssef, H.M. Facile Hydrothermal Fabrication of Analcime and Zeolite X for Efficient Removal of Cd(II) Ions From Aqueous Media and Polluted Water. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4117–4128. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, D.; Fang, J. Synthesis of silica aerogel microspheres by a two-step acid-base sol-gel reaction with emulsification technique. J. Porous Mater. 2015, 22, 621–628. [Google Scholar] [CrossRef]
- Han, L.; Song, J.; Zhang, Q.; Liu, T.; Luo, Z.; Lu, A. Synthesis, Structure and Properties of MgO-Al2O3-SiO2-B2O3 Transparent Glass-Ceramics. Silicon 2018, 10, 2685–2693. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Zhou, Y. Recent Progress on Synthesis, Multi-Scale Structure, and Properties of Y-Si-O Oxides. Int. Mater. Rev. 2014, 59, 357–383. [Google Scholar] [CrossRef]
- Sahoo, M.; Kale, P. Restructured porous silicon for solar photovoltaic: A review. Microporous Mesoporous Mater. 2019, 289, 109619. [Google Scholar] [CrossRef]
- Rad, L.R.; Anbia, M. Zeolite-based composites for the adsorption of toxic matters from water: A review. J. Environ. Chem. Eng. 2021, 9, 106088. [Google Scholar] [CrossRef]
- Oenema, J.; Harmel, J.; Vélez, R.; Meijerink, M.; Eijsvogel, W.; Poursaeidesfahani, A.; Vlugt, T.J.; Zečević, J.; de Jong, K.P. Influence of nanoscale intimacy and zeolite micropore size on the performance of bifunctional catalysts for n-heptane hydroisomerization. ACS Catal. 2020, 10, 14245–14257. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, N.; Shaghaleh, H.; Mao, X.; Shao, X.; Wang, Q.; Hamoud, Y.A. The Effect of Functional Ceramsite in a Moving Bed Biofilm Reactor and Its Ammonium Nitrogen Adsorption Mechanism. Water 2023, 15, 1362. [Google Scholar] [CrossRef]
- Xu, J.; Zha, X.; Wu, Y.; Ke, Q.; Yu, W. Fast and highly efficient SO2 capture by TMG immobilized on hierarchical micro-meso-macroporous AlPO-5/cordierite honeycomb ceramic materials. Chem. Commun. 2016, 52, 6367–6370. [Google Scholar] [CrossRef]
- Yakovenko, R.; Zubkov, I.; Bakun, V.; Papeta, O.; Savostyanov, A. Effects of SiO2/Al2O3 Ratio in ZSM-5 Zeolite on the Activity and Selectivity of a Bifunctional Cobalt Catalyst for Synthesis of Low-Pour-Point Diesel Fuels from CO and H2. Petrol. Chem+. 2022, 62, 101–111. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Ren, W.; Tan, Q.; Zhong, Z.; Su, F. Low-Cost Synthesis of Porous Silicon via Ferrite-Assisted Chemical Etching and Their Application as Si-Based Anodes for Li-Ion Batteries. Adv. Electron. Mater. 2015, 1, 1400059. [Google Scholar] [CrossRef]
- Li, N.; Han, B. Chinese research into utilisation of coal waste in ceramics, refractories and cements. Adv. Appl. Ceram. 2006, 105, 64–68. [Google Scholar] [CrossRef]
- Asl, S.M.H.; Ghadi, A.; Baei, M.S.; Javadian, H.; Maghsudi, M.; Kazemian, H. Porous catalysts fabricated from coal fly ash as cost-effective alternatives for industrial applications: A review. Fuel 2018, 217, 320–342. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Bai, C.; Yang, K.; Zheng, T.; Lu, S.; Li, H.; Qiao, Y.; Colombo, P. Porous alkali-activated material from hypergolic coal gangue by microwave foaming for methylene blue removal. J. Am. Ceram. Soc. 2023, 106, 1473–1489. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, J.; Hao, B.; Li, X.; Yan, M.; Liu, K. Feasible synthesis of coal fly ash based porous composites with multiscale pore structure and its application in Congo red adsorption. Chemosphere 2022, 298, 134136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, L.; Cheng, F.; Duan, X.; Chen, R. Hydrothermal Synthesis of SBA-15 Using Sodium Silicate Derived from Coal Gangue. J. Nanomater. 2013, 2013, 352157. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Hu, N.; Ye, Y.; Fu, F.; Lv, Y.; Jia, J.; Chen, D.; Ou, Z.; Li, J. A novel route for the fabrication of melilite-spinel porous ceramics with ultralow thermal conductivity and sufficient strength. Ceram. Int. 2022, 48, 37488–37491. [Google Scholar] [CrossRef]
- Zhang, C. New Technology for Comprehensive Utilization of Coal Gangue Resources; Chemical Industry Press: Beijing, China, 2008; pp. 20–29. (In Chinese) [Google Scholar]
- Zhou, C.; Liu, G.; Yan, Z.; Fang, T.; Wang, R. Transformation behavior of mineral composition and trace elements during coal gangue combustion. Fuel 2012, 97, 644–650. [Google Scholar] [CrossRef]
- Pan, J.; Long, X.; Zhang, L.; Shoppert, A.; Valeev, D.; Zhou, C.; Liu, X. The Discrepancy between Coal Ash from Muffle, Circulating Fluidized Bed (CFB), and Pulverized Coal (PC) Furnaces, with a Focus on the Recovery of Iron and Rare Earth Elements. Materials 2022, 15, 8494. [Google Scholar] [CrossRef]
- Seslija, M.; Rosic, A.; Radovic, N.; Vasic, M.; Dogo, M.; Jotic, M. Properties of fly ash and slag from the power plants. Geol. Croat. 2016, 69, 317–324. [Google Scholar] [CrossRef]
- Karayiğit, A.; Oskay, R.; Gayer, R. Mineralogy and geochemistry of feed coals and combustion residues of the Kangal power plant (Sivas, Turkey). Turk. J. Earth Sci. 2019, 28, 438–456. [Google Scholar] [CrossRef]
- Szatyłowicz, E.; Walendziuk, W. Analysis of Polycyclic Aromatic Hydrocarbon Content in Ash from Solid Fuel Combustion in Low-Power Boilers. Energies 2021, 14, 6801. [Google Scholar] [CrossRef]
- Zhou, C.; Li, C.; Li, W.; Sun, J.; Li, Q.; Wu, W.; Liu, G. Distribution and preconcentration of critical elements from coal fly ash by integrated physical separations. Int. J. Coal Geol. 2022, 261, 104095. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, L.; Peng, S.; Chou, C.; Wang, X.; Zhang, Y.; Li, D.; Sun, Y. Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar Power Plant, Inner Mongolia, China. Int. J. Coal Geol. 2010, 81, 320–332. [Google Scholar] [CrossRef]
- Miao, C.; Liang, L.; Zhang, F.; Chen, S.; Shang, K.; Jiang, J.; Zhang, Y.; Ouyang, J. Review of the fabrication and application of porous materials from silicon-rich industrial solid waste. Int. J. Miner. Met. Mater. 2022, 29, 424–438. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Zheng, S.; Di, Y.; Sun, Z. A review of the synthesis and application of zeolites from coal-based solid wastes. Int. J. Miner. Met. Mater. 2022, 29, 1–21. [Google Scholar] [CrossRef]
- Zhu, P.; Zheng, M.; Zhao, S.; Wu, J.; Xu, H. A novel environmental route to ambient pressure dried thermal insulating silica aerogel via recycled coal gangue. Adv. Mater. Sci. Eng. 2016, 2016, 9831515. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, O.D.; Piskin, S. A Novel Synthesis Method of Zeolite X From Coal Fly Ash: Alkaline Fusion Followed by Ultrasonic-Assisted Synthesis Method. Waste Biomass Valoriz. 2019, 10, 143–154. [Google Scholar] [CrossRef]
- Panitchakarn, P.; Laosiripojana, N.; Viriya-umpikul, N.; Pavasant, P. Synthesis of high-purity Na-A and Na-X zeolite from coal fly ash. J. Air Waste Manag. 2014, 64, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, H.; Zhang, F.; Li, D.; Wang, A.; Sun, D.; Oh, W.-C. Preparation of porous cordierite ceramic with acid-leached coal gangue. J. Korean Ceram. Soc. 2020, 57, 447–453. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, Z.; Zhang, Z.; Chu, Y.; Yuan, B.; Wei, Z. Development of highly porous mullite whisker ceramic membranes for oil-in-water separation and resource utilization of coal gangue. Sep. Purif. Technol. 2020, 237, 116483. [Google Scholar] [CrossRef]
- Zhang, X.; Mwamulima, T.; Wang, Y.; Song, S.; Gu, Q.; Peng, C. Preparation of Porous Pellets Based on Nano-Zero Valent Iron-Enhanced Fly Ash and Their Application for Crystal Violet Removal. Res. Environ. Sci. 2017, 30, 1295–1302. (In Chinese) [Google Scholar]
- Liu, B.; Zhang, L.; Meng, G.; Zheng, J. Research on Phosphorus Removal from Wastewater by TBX Porous Ceramisite Filter Media. Acta Sci. Nat. Univ. Pekin. 2010, 46, 389–394. (In Chinese) [Google Scholar]
- Mishra, D.P.; Das, S.K. A study of physico-chemical and mineralogical properties of Talcher coal fly ash for stowing in underground coal mines. Mater. Charact. 2010, 61, 1252–1259. [Google Scholar] [CrossRef]
- Xu, K.; Deng, T.; Liu, J.; Peng, W. Study on the phosphate removal from aqueous solution using modified fly ash. Fuel 2010, 89, 3668–3674. [Google Scholar] [CrossRef]
- Mazumder, N.; Rano, R. An efficient solid base catalyst from coal combustion fly ash for green synthesis of dibenzylideneacetone. J. Ind. Eng. Chem. 2015, 29, 359–365. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, X.; Zhang, L.; Sha, X.; Wang, Y.; Chen, J.; Luo, M.; Li, Y. Study on the Preparation of Plasma-Modified Fly Ash Catalyst and Its De–NOX Mechanism. Materials 2018, 11, 1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Li, Q.; Cui, H.; Pang, J.; Sun, L.; An, H.; Zhai, J. Adsorption of fluoride from aqueous solution on magnesia-loaded fly ash cenospheres. Desalination 2011, 272, 233–239. [Google Scholar] [CrossRef]
- Li, Q.; Lv, L.; Zhao, X.; Wang, Y.; Wang, Y. Cost-effective microwave-assisted hydrothermal rapid synthesis of analcime-activated carbon composite from coal gangue used for Pb2+ adsorption. Environ. Sci. Pollut. Res. 2022, 29, 77788–77799. [Google Scholar] [CrossRef]
- Yan, S.; Pan, Y.; Wang, L.; Liu, J.; Zhang, Z. Synthesis of low-cost porous ceramic microspheres from waste gangue for dye adsorption. J. Adv. Ceram. 2018, 7, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Qi, H.; Wei, X.; Liu, X.; Jiang, W. Photocatalytic activity of TiO2 supported SiO2-Al2O3 aerogels prepared from industrial fly ash. Chin. J. Catal. 2016, 37, 2025–2033. [Google Scholar] [CrossRef]
- Wang, N.; Hu, Q.; Hao, L.; Zhao, Q. Degradation of Acid Organic 7 by modified coal fly ash-catalyzed Fenton-like process: Kinetics and mechanism study. Int. J. Environ. Sci. Technol. 2019, 16, 89–100. [Google Scholar] [CrossRef]
- Khosravi, M.; Murthy, V.; Mackinnon, I.D.R. The Exchange Mechanism of Alkaline and Alkaline-Earth Ions in Zeolite N. Molecules 2019, 24, 3652. [Google Scholar] [CrossRef] [Green Version]
- Ankrah, A.; Tokay, B.; Snape, C. Heavy metal removal from aqueous solutions using fly-ash derived zeolite NaP1. Int. J. Environ. Res. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Li, R. Synthesis of Hierarchically Porous Na-P Zeotype Composites for Ammonium Removal. Environ. Eng. Sci. 2019, 36, 1089–1099. [Google Scholar] [CrossRef]
- Chen, X.; Song, H.; Guo, Y.; Wang, L.; Cheng, F. Converting waste coal fly ash into effective adsorbent for the removal of ammonia nitrogen in water. J. Mater. Sci. 2018, 53, 12731–12740. [Google Scholar] [CrossRef]
- Sahoo, P.; Tripathy, S.; Panigrahi, M.; Equeenuddin, S. Evaluation of the use of an alkali modified fly ash as a potential adsorbent for the removal of metals from acid mine drainage. Appl. Water. Sci. 2013, 3, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Sareen, S.; Kaur, S.; Mutreja, V.; Sharma, A.; Kansal, S.; Mehta, S. Coral-Reef Shaped Mesoporous Silica Obtained from Coal Fly Ash with High Adsorption Capacity. Top. Catal. 2022, 65, 1791–1810. [Google Scholar] [CrossRef]
- Mohammed, I.A.; Malek, N.N.A.; Jawad, A.H.; Mastuli, M.S.; ALOthman, Z.A. Box-Behnken Design for Optimizing Synthesis and Adsorption Conditions of Covalently Crosslinked Chitosan/Coal Fly Ash Composite for Reactive Red 120 Dye Removal. J. Polym. Environ. 2022, 30, 3447–3462. [Google Scholar] [CrossRef]
- Ou, C.; Dai, S.; Li, S.; Xu, J.; Qin, J. Adsorption performance and mechanism investigation of Mn2+ by facile synthesized ceramsites from lime mud and coal fly ash. Korean J. Chem. Eng. 2021, 38, 505–513. [Google Scholar] [CrossRef]
- Zhang, G.; Song, A.; Duan, Y.; Zheng, S. Enhanced photocatalytic activity of TiO2/zeolite composite for abatement of pollutants. Microporous Mesoporous Mater. 2018, 255, 61–68. [Google Scholar] [CrossRef]
- Xie, J.; Xia, R.; Zhao, S.; Li, M.; Xu, Y.; Du, H. Preparation and Properties of ZnO/Coal Gangue Composite Photocatalysts. Multipurp. Util. Miner. Resour. 2019, 4, 126–129. (In Chinese) [Google Scholar]
- Bai, C.; Fan, X.; Li, G.; Xu, Z.; Sheng, J.; Li, S. Preparation and photocatalytic degradation properties of TiO2/fly ash cenosphere composite materials. Environ. Pollut. Control 2017, 39, 735–739. (In Chinese) [Google Scholar]
- Song, Q.; Feng, Y.; Liu, G.; Lv, W. Degradation of the flame retardant triphenyl phosphate by ferrous ion-activated hydrogen peroxide and persulfate: Kinetics, pathways, and mechanisms. Chem. Eng. J. 2019, 361, 929–936. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J. Fe-based Fenton-like catalysts for water treatment: Catalytic mechanisms and applications. J. Mol. Liq. 2021, 332, 115755. [Google Scholar] [CrossRef]
- Xing, M.; Xu, W.; Dong, C.; Bai, Y.; Zheng, J.; Zhou, Y.; Zhang, J.; Yin, Y. Metal sulfides as excellent co-catalysts for H2O2 decomposition in advanced oxidation processes. Chem 2018, 4, 1359–1372. [Google Scholar] [CrossRef] [Green Version]
- Garza-Campos, B.R.; Guzmán-Mar, J.L.; Reyes, L.H.; Brillas, E.; Hernandez-Ramirez, A.; Ruiz-Ruiz, E.J. Coupling of solar photoelectro-Fenton with a BDD anode and solar heterogeneous photocatalysis for the mineralization of the herbicide atrazine. Chemosphere 2014, 97, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Du, D.-Y. Degradation of n-butyl xanthate using fly ash as heterogeneous Fenton-like catalyst. J. Cent. South. Univ. 2014, 21, 1448–1452. [Google Scholar] [CrossRef]
- Nadeem, N.; Zahid, M.; Rehan, Z.A.; Hanif, M.A.; Yaseen, M. Improved photocatalytic degradation of dye using coal fly ash-based zinc ferrite (CFA/ZnFe2O4) composite. Int. J. Environ. Sci. Technol. 2022, 19, 3045–3060. [Google Scholar] [CrossRef]
- Yan, Y.; Gao, Y.; Tang, W.; Li, Q.; Zhang, J. Characterization of high-alumina coal fly ash based silicate material and its adsorption performance to CO2. Korean J. Chem. Eng. 2016, 33, 1369–1379. [Google Scholar] [CrossRef]
- Chandrasekar, G.; Son, W.J.; Ahn, W.S. Synthesis of mesoporous materials SBA-15 and CMK-3 from fly ash and their application for CO2 adsorption. J. Porous Mater. 2009, 16, 545–551. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, B.; Lu, G.; Xiao, Y.; Yang, J.; Lu, J.; Li, G.; Zhou, J.; Wang, H.; Zhao, C. Preparation and performance of SAPO-34 based SCR catalyst derived from fly ash. Chem. Ind. Eng. Prog. 2020, 39, 4051–4060. (In Chinese) [Google Scholar]
- Abu Ghalia, M.; Dahman, Y. Development and Evaluation of Zeolites and Metal-Organic Frameworks for Carbon Dioxide Separation and Capture. Energy Technol. 2017, 5, 356–372. [Google Scholar] [CrossRef]
- Wu, D.; Jiang, W.; Liu, X.; Qiu, N.; Xue, Y. Theoretical study about effects of H2O and Na+ on adsorption of CO2 on kaolinite surfaces. Chem. Res. Chin. Univ. 2016, 32, 118–126. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.; Liu, K.; Li, F.; Pang, Y.; Zhang, J.; Si, H. Preparation of nano-sized Mg-doped copper silicate materials using coal gangue as the raw material and its characterization for CO2 adsorption. Korean J. Chem. Eng. 2020, 37, 1786–1794. [Google Scholar] [CrossRef]
- Zheng, F.; Tran, D.N.; Busche, B.J.; Fryxell, G.E.; Addleman, R.S.; Zemanian, T.S. Ethylenediamine-modified SBA-15 as regenerable CO2 sorbent. Ind. Eng. Chem. Res. 2005, 44, 3099–3105. [Google Scholar] [CrossRef]
- Du, H.; Ma, L.; Liu, X.; Zhang, F.; Yang, X.; Wu, Y.; Zhang, J. A Novel Mesoporous SiO2 Material with MCM-41 Structure from Coal Gangue: Preparation, Ethylenediamine Modification, and Adsorption Properties for CO2 Capture. Energy Fuels 2018, 32, 5374–5385. [Google Scholar] [CrossRef]
- Sanna, A.; Maroto-Valer, M.M. Potassium-based sorbents from fly ash for high-temperature CO2 capture. Environ. Sci. Pollut. Res. 2016, 23, 22242–22252. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Mao, Z. High adsorption activated calcium silicate enabling high-capacity adsorption for sulfur dioxide. New J. Chem. 2020, 44, 11879–11886. [Google Scholar] [CrossRef]
- Liu, S.; He, X. Researches in NH3-SCR technologies for removal of NOx from flue gas. Ind. Catal. 2021, 29, 7–12. (In Chinese) [Google Scholar]
- Mao, Y.; Wang, H.; Hu, P. Theoretical investigation of NH3-SCR processes over zeolites: A review. Int. J. Quantum Chem. 2015, 115, 618–630. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Guo, L.; Zheng, W.; Wang, G.; Zheng, S.; Wu, X. Research progress on NH3-SCR mechanism of metal-supported zeolite catalysts. J. Fuel Chem. Technol. 2021, 49, 1294–1315. (In Chinese) [Google Scholar] [CrossRef]
- Han, J.; Jin, X.; Song, C.; Bi, Y.; Li, Z. Rapid synthesis and NH3-SCR activity of SSZ-13 zeolite via coal gangue. Green Chem. 2020, 22, 219–229. [Google Scholar] [CrossRef]
- Isabella, N.; Enrico, T. Urea-SCR Technology for deNOx after Treatment of Diesel Exhausts; Springer: New York, NY, USA, 2014; pp. 507–550. [Google Scholar]
- Li, J.; Shi, Y.; Fu, X.; Huang, J.; Zhang, Y.; Deng, S.; Zhang, F. Hierarchical ZSM-5 based on fly ash for the low-temperature purification of odorous volatile organic compound in cooking fumes. React. Kinet. Catal. Lett. 2019, 128, 289–314. [Google Scholar] [CrossRef]
- Yuan, G.; Zhang, J.; Zhang, Y.; Yan, Y.; Ju, X.; Sun, J. Characterization of high-alumina coal fly ash based silicate material and its adsorption performance on volatile organic compound elimination. Korean J. Chem. Eng. 2015, 32, 436–445. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.; Lee, I.; Jeon, J.; Jung, S.; Chung, J.; Choi, W.G.; Park, Y.-K. Recent advances in the catalytic hydrodeoxygenation of bio-oil. Korean J. Chem. Eng. 2016, 33, 3299–3315. [Google Scholar] [CrossRef]
- Taufiqurrahmi, N.; Bhatia, S. Catalytic cracking of edible and non-edible oils for the production of biofuels. Energy Environ. Sci. 2011, 4, 1087–1112. [Google Scholar] [CrossRef]
- Qin, T.; Yuan, S. Research progress of catalysts for catalytic steam reforming of high temperature tar: A review—ScienceDirect. Fuel 2023, 331, 125790. [Google Scholar] [CrossRef]
- Gaurh, P.; Pramanik, H. Production of benzene/toluene/ethyl benzene/xylene (BTEX) via multiphase catalytic pyrolysis of hazardous waste polyethylene using low cost fly ash synthesized natural catalyst. Waste Manag. 2018, 77, 114–130. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, W.; Xu, K.; Liu, Y.; Wu, J.; Zhang, F. Understanding the Structure–Activity Relationships in Catalytic Conversion of Polyolefin Plastics by Zeolite-Based Catalysts: A Critical Review. ACS Catal. 2022, 12, 14882–14901. [Google Scholar] [CrossRef]
- Songip, A.R.; Masuda, T.; Kuwahara, H.; Hashimoto, K. Production of high-quality gasoline by catalytic cracking over rare-earth metal exchanged Y-type zeolites of heavy oil from waste plastics. Energy Fuels 1994, 8, 136–140. [Google Scholar] [CrossRef]
- Lai, J.; Meng, Y.; Yan, Y.; Lester, E.; Wu, T.; Pang, C. Catalytic pyrolysis of linear low-density polyethylene using recycled coal ash: Kinetic study and environmental evaluation. Korean J. Chem. Eng. 2021, 38, 2235–2246. [Google Scholar] [CrossRef]
- Inayat, A.; Fasolini, A.; Basile, F.; Fridrichova, D.; Lestinsky, P. Chemical recycling of waste polystyrene by thermo-catalytic pyrolysis: A description for different feedstocks, catalysts and operation modes. Polym. Degrad. Stab. 2022, 201, 109981. [Google Scholar] [CrossRef]
- Cocchi, M.; Angelis, D.D.; Mazzeo, L.; Nardozi, P.; Piemonte, V.; Tuffi, R.; Ciprioti, S.V. Catalytic Pyrolysis of a Residual Plastic Waste Using Zeolites Produced by Coal Fly Ash. Catalysts 2020, 10, 1113. [Google Scholar] [CrossRef]
- Al-Rahbi, A.S.; Williams, P.T. Waste ashes as catalysts for the pyrolysis-catalytic steam reforming of biomass for hydrogen-rich gas production. J. Mater. Cycles Waste Manag. 2019, 21, 1224–1231. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Dong, Y.; Guo, F.; Tian, B.; Mao, S.; Qian, L.; Xin, C. Preparation of high-activity coal char-based catalysts from high metals containing coal gangue and lignite for catalytic decomposition of biomass tar. Int. J. Hydrog. Energy 2021, 46, 14138–14147. [Google Scholar] [CrossRef]
- Du, S.; Shu, R.; Guo, F.; Mao, S.; Bai, J.; Qian, L.; Xin, C. Porous coal char-based catalyst from coal gangue and lignite with high metal contents in the catalytic cracking of biomass tar. Energy 2022, 249, 123640. [Google Scholar] [CrossRef]
- Du, S.; Mao, S.; Guo, F.; Dong, K.; Shu, R.; Bai, J.; Xu, L.; Li, D. Investigation of the catalytic performance of coal gangue char on biomass pyrolysis in a thermogravimetric analyzer and a fixed bed reactor. Fuel 2022, 328, 125216. [Google Scholar] [CrossRef]
- Manique, M.C.; Lacerda, L.V.; Alves, A.K.; Bergmann, C.P. Biodiesel production using coal fly ash-derived sodalite as a heterogeneous catalyst. Fuel 2016, 190, 268–273. [Google Scholar] [CrossRef]
- Yusuff, A.; Kumar, M.; Obe, B.; Mudashiru, L. Calcium Oxide Supported on Coal Fly Ash (CaO/CFA) as an Efficient Catalyst for Biodiesel Production from Jatropha curcas Oil. Top. Catal. 2021, 1–13. [Google Scholar] [CrossRef]
- Fotovat, F.; Kazemeini, M.; Kazemian, H. Novel Utilization of Zeolited Fly Ash Hosting Cobalt Nanoparticles as a Catalyst Applied to the Fischer-Tropsch Synthesis. Catal. Lett. 2009, 127, 204–212. [Google Scholar] [CrossRef]
- Sineva, L.V.; Asalieva, E.Y.; Mordkovich, V.Z. The role of zeolite in the Fischer–Tropsch synthesis over cobalt–zeolite catalysts. Russ. Chem. Rev. 2015, 84, 1176–1189. [Google Scholar] [CrossRef]
- Gupta, P.K.; Mahato, A.; Oraon, P.; Gupta, G.K.; Maity, S. Coal fly ash-derived mesoporous SBA as support material for production of liquid hydrocarbon through Fischer-Tropsch route. Asia-Pac. J. Chem. Eng. 2020, 15, 2471. [Google Scholar] [CrossRef]
- Feng, Z.; Peng, W.; Wang, Z.; Guo, H.; Li, X.; Yan, G.; Wang, J.-X. Review of silicon-based alloys for lithium-ion battery anodes. Int. J. Min. Met. Mater. 2021, 28, 1549–1564. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, C.; Song, L.; Zheng, Y.; Long, T. Research progress of nanosiliconbased materials and siliconcarbon composite anode materials for lithiumion batteries. J. Solid State Electrochem. 2022, 26, 1137. [Google Scholar] [CrossRef]
- Meng, G.; Yang, H.; Zheng, J.; Wang, C. Nanoscale Silicon as Anode for Li-ion Batteries: The Fundamentals, Promises, and Challenges. Nano Energy 2015, 17, 366–383. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Song, G.; Bhatt, A.I.; Wong, Y.C.; Wen, C. Nanostructured silicon anodes for high-performance lithiumion batteries. Adv. Funct. Mater. 2016, 26, 647–678. [Google Scholar] [CrossRef]
- Ryu, J.; Hong, D.; Lee, H.W.; Park, S. Practical considerations of Si-based anodes for lithium-ion battery applications. Nano Res. 2017, 10, 3970–4002. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Zhu, Y.; Zhao, J.; Chen, J.; Ye, H.; Wei, H.; Liu, Z. Trash to Treasure: Harmful Fly Ash Derived Silicon Nanoparticles for Enhanced Lithium-Ion Batteries. Silicon 2022, 14, 7983–7990. [Google Scholar] [CrossRef]
- Xing, A.; Zhang, J.; Wang, R.; Wang, J.; Liu, X. Fly ashes as a sustainable source for nanostructured Si anodes in lithium-ion batteries. SN Appl. Sci. 2019, 1, 181. [Google Scholar] [CrossRef] [Green Version]



| Chemical Composition | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 30~65 | 15~40 | 2~10 | 1~4 | 1~3 | 1~2 | 1~2 | 0.5~4.0 | 0.05~0.3 | 20~30 |
| Chemical Composition | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | Na2O | MgO | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Content | 40~60 | 17~35 | 2~15 | 1~10 | 0.5~4 | 0.5~4 | 0.5~2 | 0.1~2 | 1~26 |
| Regenerate Objects | Regeneration Effect | Catalytic Material | Preparation Method | Action Mechanism |
|---|---|---|---|---|
| Waste polyethylene (PE) | Reaction temperature: 700 °C, ratio of feed to catalyst: 20:1, maximum liquid product yield: 78.2%, with total aromatics yield reaching nearly 22%. | Modified fly ash | The coal fly ash was crushed, sieved, and heat-treated at 800 °C to modify [88]. | The fly ash catalyst with high specific surface area and high Si/Al ratio provides a large number of highly selective acidic sites, promoting the cracking of plastics and improving the yield of aromatics [89,90]. |
| Waste linear low-density polyethylene (LLDPE) | Catalyst dosage: 15 wt%, LLDPE’s cracking activation energy significantly decreased, and reaction rate and the yield of alicyclic hydrocarbons increased. | Modified fly ash | The coal fly ash was ground to a particle size of less than 150 μm to modify [91]. | Solid alkali components such as CaO in fly ash facilitate the formation of carbon anion intermediates, promoting the generation of alicyclic hydrocarbons and accelerating the reaction rate [92]. Transition metal oxide components such as Fe2O3 in fly ash also assist the plastic cracking process. |
| Plastic film residue (PFR) | Reaction temperature: 723 K. Use of catalyst inhibits tar and wax formation, reduces cracking temperature, and achieves a 44% product oil yield, with 70% composed of gasoline hydrocarbons. | HX/CFA zeolite | Coal fly ash (CFA) underwent alkali fusion and hydrothermal synthesis to produce NaX zeolite. After acidification, HX/CFA zeolite was obtained [93]. | Acidic sites on synthesized X-type zeolites (solid acid) promote the formation of carbocation intermediates in polymer chains, enabling high-quality and efficient cracking of waste plastics [89,90]. |
| Waste wood pellets | A 10 wt% Ni-loaded catalyst and waste wood pellets were steam reformed to obtain a 54.9% gas yield, with an H2 production of 7.29 mmol/g. | Ni-ash catalysts | Coal fly ash was impregnated in a Ni(NO3)2 · 6H2O aqueous solution after drying, calcining, reducing it in H2 atmosphere to prepare the Ni-ash catalyst [94]. | The hydrogenation and dehydrogenation functions of metal Ni, as well as the isomerization, cyclization, and hydrogenation cracking functions of acid components (Al2O3) in fly ash carrier occur through olefin intermediates during steam reforming. Metals such as Mg and Cu in fly ash also act as catalyst auxiliaries. |
| Pine sawdust biomass tar | Reaction temperature: 800 °C. The catalyst effectively promoted the decomposition of tar molecules, achieving a conversion rate of 93.5%, and significantly increasing the gas yield. | GC catalysts | The coal gangue (GC) was crushed and sieved, and then calcined at 800 °C for 1 h under N2 atmosphere to obtain the GC catalyst [95]. | The repeated oxidation and reduction of Fe2O3 in coal gangue effectively promotes the breaks of C-H bonds and dehydrogenation reactions in catalytic reactions, increasing the yield of H2 and CO. Alkali metals in coal gangue further facilitate tar decomposition. The formation of Fe0 during the process enhances the activity and lifespan of the catalyst [96,97]. |
| Soybean oil | Reaction temperature: 65 °C, catalyst concentration: 4%, molar ratio of methanol to oil: 12:1, reaction time: 2 h. The conversion rate of soybean oil methyl ester reached 95.5%. | Zeolite-type sodalite | Coal fly ash was added to an alkali solution and supplemented with an aluminate solution to form a gel. The zeolite-type sodalite was formed through hydrothermal crystallization at 100 °C for 24 h using the gel [98]. | Solid alkali components (Si-O-Na groups) in zeolite serve as active sites, promoting transesterification to produce biodiesel. |
| Jatropha curcas oil | Catalyst (40 wt% CaO loaded) dosage: 0.15 wt%, molar ratio of methanol to oil: 12:1, reaction temperature: 60 °C, and reaction time: 1 h. The maximum biodiesel yield was 94.72%. | CaO/CFA catalysts | Coal fly ash was activated by calcination at 400 °C for 5 h, impregnated with a calcium acetate solution and dried, then calcined at 750 °C for 4 h to obtain CaO/CFA catalyst [99]. | The appropriate amount of CaO loading improves the catalyst’s pore structure and increases the reaction rate. The introduced CaO (solid alkali) serves as an active site for transesterification, promoting the formation of biodiesel. Si and Al in fly ash act as catalyst carriers to improve catalyst stability. |
| CO and H2 | Reaction temperature: 523 K, air pressure: 20 bar, and H2/CO = 2. Zeolites carrying more Co particles have higher catalytic activity, resulting in a CO conversion rate of 53.2% and higher selectivity for producing liquid hydrocarbons (C5+). | Co/LTA and Co/FAU catalysts | LTA and FAU zeolites synthesized from high-silicon coal fly ash were used as carriers. The Co/LTA and Co/FAU catalysts were obtained by impregnating with Co(NO3)2 solutions and calcination in H2 atmosphere [100]. | CO and H2 are adsorbed on the surface of metal Co, activated through electron effects and interactions with transition metals, and then dissociated, leading to chain growth and termination to produce various hydrocarbons [101]. Fly-ash-based zeolite carriers plays a role in improving pore structure, enhancing catalyst stability, and increasing the activity of active components during the process. |
| CO and H2 | Reaction temperature: 220 °C and air pressure: 30 bar for FT synthesis. After 130 h of intake, the conversion rates of CO and H2 were 67% and 73%, respectively. The selectivity for liquid hydrocarbons (C5+) was 66.53%. Among C5+ hydrocarbons, the contents of gasoline (C5–C11), diesel (C12–C18), and high-carbon hydrocarbons (C19+) were 33.19%, 27.64%, and 5.7%, respectively. | Co-Fe/SBA-15 catalysts | Coal fly ash was activated by alkali fusion. A small amount of surfactant was added to hydrothermally synthesize mesoporous SBA-15 zeolite. The Co-Fe/SBA-15 catalyst was prepared by impregnating with Co(NO3)2 and Fe(NO3)3 solutions and calcining in H2 atmosphere [102]. | The loading of additional Fe in addition to Co makes the catalyst more advantageous for synthesizing low-carbon hydrocarbons, promoting the generation of gasoline-range products. This catalyst also has a wider CO/H2 range and higher poison resistance during F-T synthesis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Ma, A.; Wang, X.; Zheng, X. Review of the Preparation and Application of Porous Materials for Typical Coal-Based Solid Waste. Materials 2023, 16, 5434. https://doi.org/10.3390/ma16155434
Du J, Ma A, Wang X, Zheng X. Review of the Preparation and Application of Porous Materials for Typical Coal-Based Solid Waste. Materials. 2023; 16(15):5434. https://doi.org/10.3390/ma16155434
Chicago/Turabian StyleDu, Jinsong, Aiyuan Ma, Xingan Wang, and Xuemei Zheng. 2023. "Review of the Preparation and Application of Porous Materials for Typical Coal-Based Solid Waste" Materials 16, no. 15: 5434. https://doi.org/10.3390/ma16155434
APA StyleDu, J., Ma, A., Wang, X., & Zheng, X. (2023). Review of the Preparation and Application of Porous Materials for Typical Coal-Based Solid Waste. Materials, 16(15), 5434. https://doi.org/10.3390/ma16155434






